PURPOSE
Early-onset (EO) colorectal cancer (CRC, age < 50 years) incidence is increasing. Decisions on optimal adjuvant therapy should consider treatment adherence, adverse events, and expected outcomes in a population with life expectancy longer than later-onset (LO) CRC (age ≥ 50 years).
MATERIALS AND METHODS
Individual patient data from six trials in the International Duration Evaluation of Adjuvant Chemotherapy database were analyzed. Characteristics, treatment adherence, and adverse events in stage II or III EO-CRC and LO-CRC were compared. To reduce confounders of non–cancer-related deaths because of age or comorbidities, time to recurrence (3-year relapse-free rate) and cancer-specific survival (5-year cancer-specific mortality rate) were considered.
RESULTS
Out of 16,349 patients, 1,564 (9.6%) had EO-CRC. Compared with LO-CRC, EO-CRC had better performance status (86% v 80%, P < .01), similar T stage (% T1-3/T4: 76/24 v 77/23, P = .97), higher N2 disease rate (24% v 22%, P < .01), more likely to complete the planned treatment duration (83.2% v 78.2%, P < .01), and received a higher treatment dose intensity, especially with 6-month regimens. Gastrointestinal toxicity was more common in EO-CRC; hematologic toxicity was more frequent in LO-CRC. Compared with LO-CRC, significantly worse cancer-specific outcomes were demonstrated especially in high-risk stage III EO-CRC: lower 3-year relapse-free rate (54% v 65%; hazard ratio [HR] 1.33; 95% CI, 1.14 to 1.55; P value < .001) and higher 5-year cancer-specific mortality rate (24% v 20%; HR 1.21; 95% CI, 1.00 to 1.47; P value < .06). In this subgroup, no difference was observed with 3 or 6 months of therapy, with equally poor disease-free survival rates (57% v 56%; HR 0.97; 95% CI, 0.73 to 1.29; P value = .85).
CONCLUSION
Young age is negatively prognostic in high-risk stage III CRC and associated with significantly higher relapse rate; this is despite better treatment adherence and higher administered treatment intensity, suggesting more aggressive disease biology.
INTRODUCTION
Colorectal cancer (CRC) incidence and mortality have significantly increased in young individuals over the past 2 decades, in particular in Western countries.1,2 Putative causes include increased exposure to risk factors like Western-style diet, obesity, physical inactivity, and prenatal and childhood antibiotic usage.3 Although biologic characteristics such as distal tumor location, poor differentiation, and advanced stage at diagnosis seem more prevalent, stage-specific survival was reported to be comparable with older patients.4 However, the current knowledge mainly derives from retrospective analyses and population-based cancer registries. Whether outcomes under the condition of specific contemporary interventions are different between early-onset (EO)-CRC and later-onset (LO)-CRC is largely unknown. Also, there is uncertainty whether EO-CRC should be managed with different approaches compared with LO-CRC. Limited evidence from randomized control trials is available in view of the relatively small number of cases compared with older population.
CONTEXT
Key Objective
To compare clinical characteristics, treatment adherence, adverse events, and outcomes of patients with early-onset (EO) colorectal cancer (CRC) to those of patients with later-onset CRC included in the International Duration Evaluation of Adjuvant Chemotherapy pooled analysis.
Knowledge Generated
Patients with EO-CRC experience disease recurrence more frequently than patients with later-onset CRC despite receiving a higher adjuvant treatment intensity; cancer-specific mortality rate is higher in high-risk stage III EO-CRC.
Relevance
Early-onset colorectal cancer has a unique and generally more aggressive biology compared with late-onset disease, potentially warranting different adjuvant and surveillance strategies for this subgroup of patients.
The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) Collaboration published a prospective, preplanned pooled analysis of six randomized phase III trials of 3 versus 6 months of adjuvant fluoropyrimidine and oxaliplatin.5 The noninferiority of a 3-month treatment was not confirmed in stage III patients. However, subgroup analyses demonstrated that 3 months of oxaliplatin and capecitabine (CAPOX) was as effective as 6 months in low-risk patients.
Here, we exploited the IDEA pooled analysis database to compare characteristics, treatment adherence, adverse events, and outcomes of patients with EO-CRC to those of patients with LO-CRC in the adjuvant setting.5
MATERIALS AND METHODS
Patients
Individual patient data from stage II and III patients from the IDEA database were included. Clinical characteristics of all randomly assigned patients were analyzed according to two age groups: EO-CRC with age at random assignment lower than 50 years and LO-CRC with age ≥ 50 years.
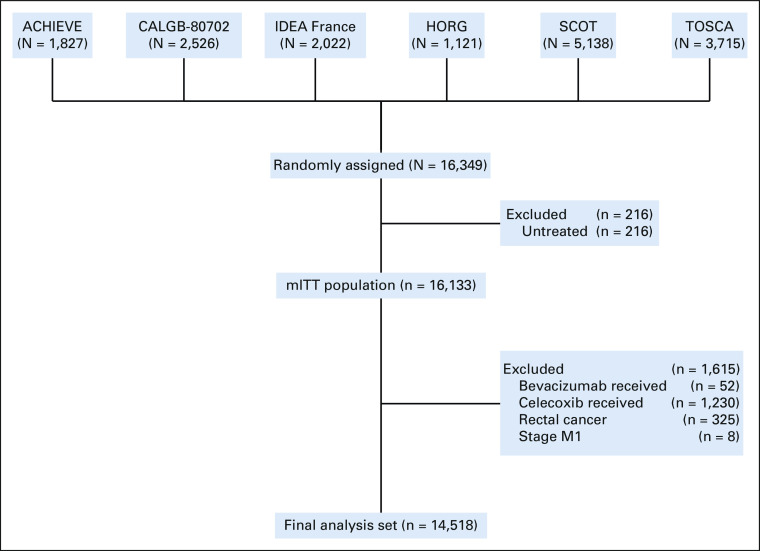
To reduce possible confounders, patients who did not start treatment, patients with metastatic disease at trial entry, or who received bevacizumab or celecoxib together with chemotherapy and/or with rectal cancer (possibly not treatment-naive) were excluded for treatment adherence, adverse events, and survival analyses (Appendix Fig A1, online only).
Objectives
The primary objectives of this work were to determine whether potential differences between EO-CRC and LO-CRC existed in terms of (1) clinical characteristics; (2) treatment adherence; (3) adverse events; and (4) survival outcomes.
First, disease-free survival (DFS) was analyzed, since this was previously chosen as the primary end point in the IDEA pooled analysis. To reduce the confounder of non–cancer-related deaths because of age or comorbidities, time to recurrence (TTR) and cancer-specific mortality (CSM) were evaluated. Overall survival (OS), overall mortality (1 – OS), mortality after recurrence (ar), and ar-CSM were secondary end points.
Duration (3 v 6 months) and type of regimen (CAPOX or infusional fluorouracil, leucovorin, and oxaliplatin) of adjuvant treatment in age groups were evaluated in an exploratory analysis.
Statistical Analysis
Clinical characteristics, treatment adherence, and adverse events were analyzed with descriptive statistics. T stage and N stage were described both independently and combined in a TN variable to define three risk groups: high-risk stage II (T1-4, N0), low-risk stage III (T1-3, N1) and high-risk stage III (T4, N any; T any, N2).
DFS and TTR end points (with associated 3-year DFS rate and 3-year relapse-free rate [3y-RF rate]) by age groups and stage were estimated with direct adjusted Kaplan-Meier curves, stratified by clinical trials, and compared using log-rank test and multivariable Cox models stratified by studies. Cancer-specific survival was compared by Gray k-sample test and multivariate competing risk models stratified by studies. Five-year CSM (5y-CSM) rate were estimated by adjusted cumulative incidence function.
The association of baseline characteristics and survival outcomes was assessed using univariate Cox analyses; variables with P values < 0.05 were included into multivariable Cox regression models with backward selection. Tumor location and risk groups were not included in multivariable models as already considered in other variables (sidedness and T and N stages).
The prognostic effect of age with respect to DFS, OS, and survival ar was assessed in various subgroups using univariate Cox models and visualized in forest plots including interaction P values (type-3 likelihood ratio test of the interaction term). Three subgroups (other race, mixed race, and rectum) were excluded because of limited number of patients with consequent model instability.
Analyses were performed using SAS software (version 9.4M7; SAS Institute Inc, Cary, NC).
RESULTS
Population Characteristics
A total of 16,349 patients enrolled in six clinical trials were included in this analysis; 1,564 patients with EO-CRC (9.6%) and 14,785 with LO-CRC (90.4%) (Appendix Fig A1 and Data Supplement [online only]).
Overall, patients with EO-CRC were more frequently male (51.3%), although the proportion of male patients was lower in EO-CRC than in LO-CRC (57%); most patients with EO-CRC had a performance status (PS) of 0 and a distal, low-grade tumor; a higher number of lymph nodes was examined. The stage distribution between EO-CRC and LO-CRC was similar but with a 3% more high-risk stage II young patients.
Treatment Adherence
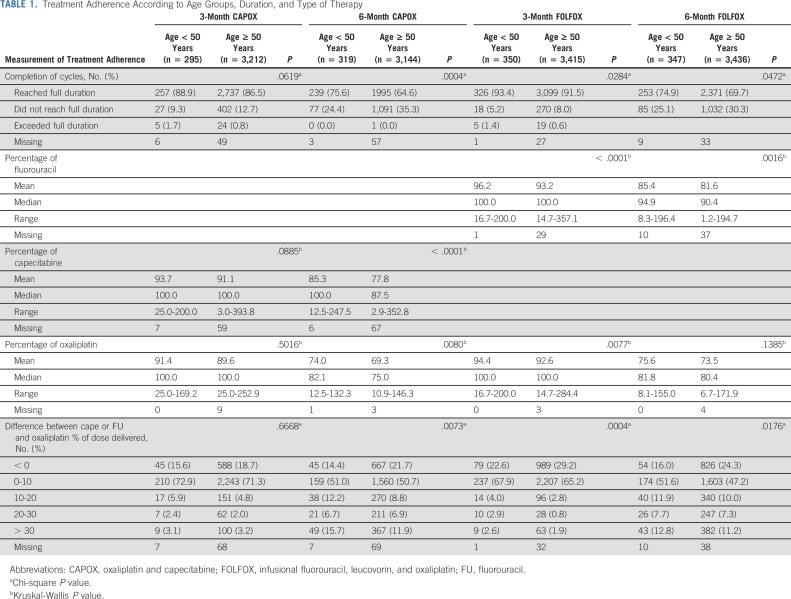
Overall, 14,518 patients were included in the following analyses: 1,311 patients with (9%) EO-CRC and 13,207 patients with (91%) LO-CRC. A significantly higher proportion of patients with EO-CRC was able to complete the planned duration of treatment compared with older patients with LO-CRC; of note, in the 6-month CAPOX group, 76% of EO-CRC reached full therapy duration versus 65% of older patients (P value < .001; Table 1). Conversely, this difference was less substantial among patients who received 3 months of treatment (89% EO-CRC v 87% LO-CRC, P = .06 with CAPOX; 93% EO-CRC v 92% LO-CRC, P = .03 with infusional fluorouracil, leucovorin, and oxaliplatin).
TABLE 1.
Treatment Adherence According to Age Groups, Duration, and Type of Therapy
Dose intensity was significantly different between age groups; this may suggest that it was more likely for patients with EO-CRC to continue at least the fluoropyrimidine to complete the preplanned number of cycles, rather than discontinue the treatment. This was more substantial in the 6-month CAPOX therapy group, where 16% of patients with EO-CRC versus 12% of patients with LO-CRC had > 30% difference between fluoropyrimidine and oxaliplatin dose delivered.
Adverse Events
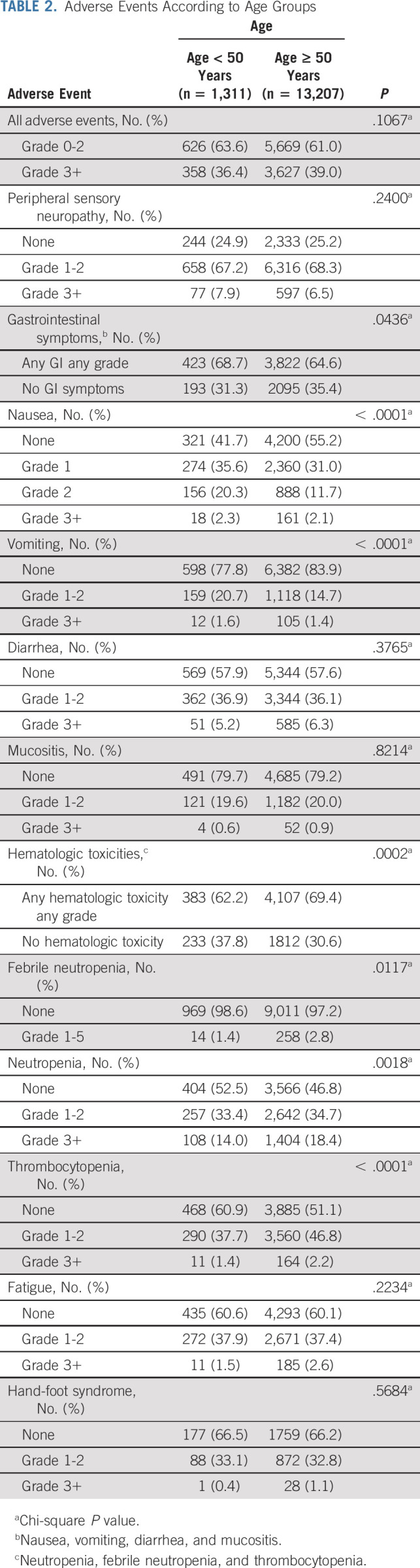
Overall, the number of adverse events was not significantly different between age groups (Table 2). Grade ≥ 3 adverse events were 36% and 39% in EO-CRC and LO-CRC groups, respectively. One patient with EO-CRC (0.1%) and 16 patients with LO-CRC (0.2%) died during treatment. Peripheral neuropathy incidence and degrees were similar in the overall comparison and also within comparisons of different treatment regimens (Data Supplement). The incidence of gastrointestinal symptoms, specifically nausea and vomiting but not diarrhea and mucositis, was higher in patients with EO-CRC. Conversely, hematologic toxicities were significantly more frequent in LO-CRC (62% for EO-CRC and 69% LO-CRC, P value < .001). Rates of febrile neutropenia were 1.4% in EO-CRC and 2.8% in LO-CRC. Fatigue and hand-foot syndrome were similar.
TABLE 2.
Adverse Events According to Age Groups
Survival
DFS and TTR.
At a median follow-up of 69 (EO-CRC) and 72 (LO-CRC) months, no significant difference in DFS was demonstrated in the overall population (3-year rates: 76% [95% CI, 73 to 78] for EO-CRC and 78% [95% CI, 77 to 79] for LO-CRC [hazard ratio (HR), 1.01; 95% CI, 0.91 to 1.13; P value = .81]; Fig 1A). Similarly, no significant DFS difference was demonstrated in stage II and stage III subgroups (Figs 1B and 1C). However, by only examining disease relapse, a significantly lower 3y-RF rate was demonstrated overall (75% [95% CI, 72 to 77] v 79% [95% CI, 78 to 80] in LO-CRC; HR 1.13 [95% CI, 1.01 to 1.27]; P value = .04) and in stage III EO-CRC compared with older patients (69% [95% CI, 66 to 73] v 76% [95% CI, 75 to 77] in LO-CRC; HR 1.21 [95% CI, 1.07 to 1.37]; P value = .003; Figs 1D-1F).
FIG 1.
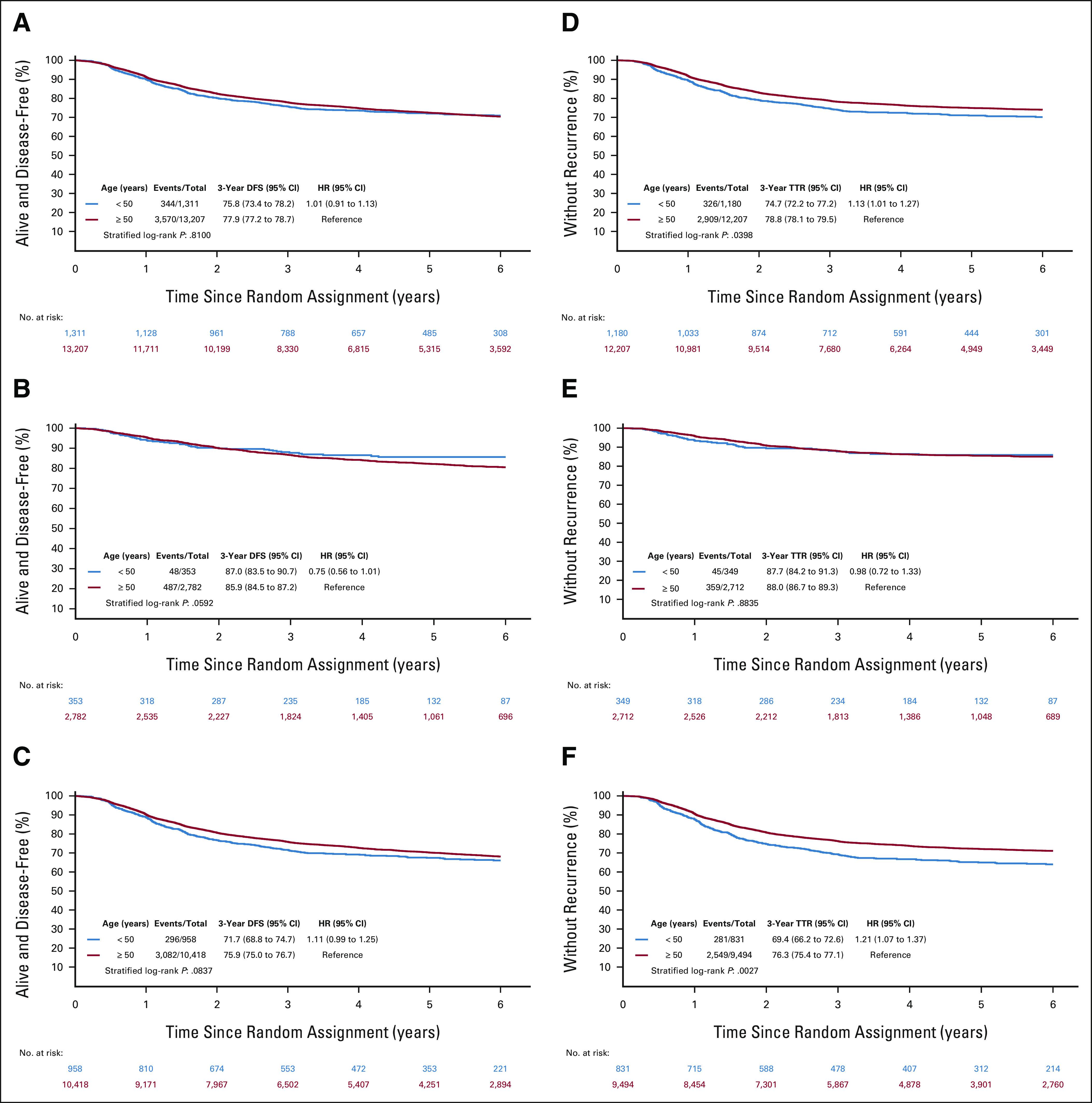
DFS and TTR according to age groups and disease stage. DFS in (A) all stages, (B) high-risk stage II patients, and (C) stage III patients. TTR in (D) all stages, (E) high-risk stage II patients, and (F) stage III patients. DFS, disease-free survival; HR, hazard ratio; TTR, time to recurrence.
OS and CSM.
In keeping with fitter patients, OS was significantly higher in EO-CRC (5y-OS rates: 86% [95% CI, 84 to 88] v 83% [95% CI, 83 to 84]; HR 0.84 [95% CI, 0.73 to 0.97; P value 0.02]). No difference was demonstrated in stage III patients with cancer, whereas OS difference was significantly evident in stage II disease (Figs 2A-2C). However, when considering CSM, opposite results were demonstrated: no difference was observed overall and in stage II patients; conversely, a significantly higher CSM rate was demonstrated in stage III EO-CRC compared with older patients (5y-CSM rates: 15% [95% CI, 12 to 17] v 12% [95% CI, 12 to 13]; HR 1.20 [95% CI, 1.02 to 1.42; P value = .03]; Figs 2D-2F).
FIG 2.
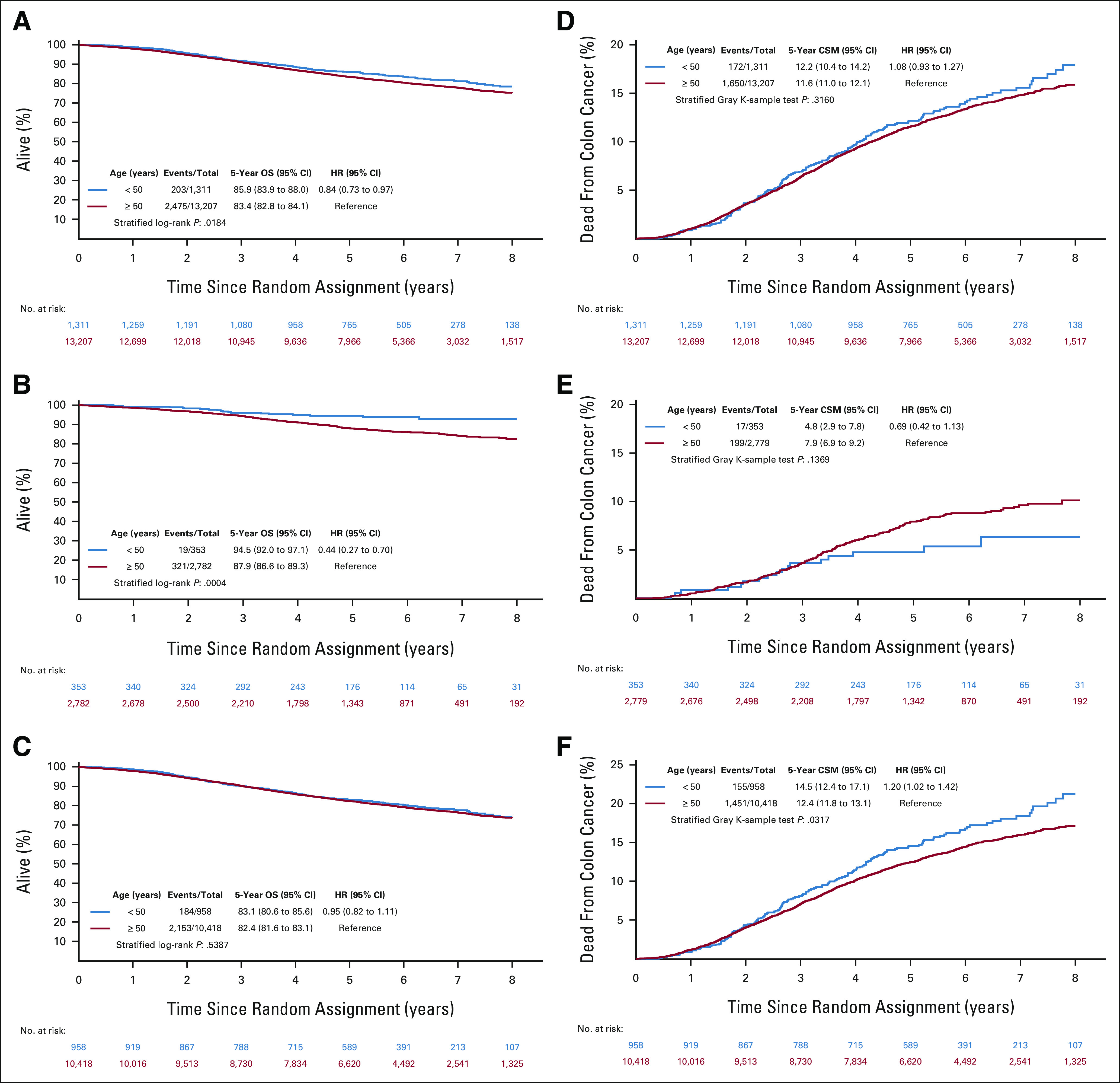
OS and cancer-specific survival according to age groups and disease stage. OS in (A) all stages, (B) high-risk stage II patients, and (C) stage III patients. CSM in (D) all stages, (E) high-risk stage II patients, and (F) stage III patients. CSM, cancer-specific mortality; HR, hazard ratio; OS, overall survival.
In univariate analyses, young age was not associated with poorer outcomes (Data Supplement). In the adjusted model, female patients, patients with PS = 0, low T stage, no lymph node involvement, with appropriate number of lymph nodes resected (> 12), and patients who completed adjuvant treatment had better DFS and OS.
Mortality and ar-CSM.
ar–EO-CRC had a significantly lower mortality (median time to death 3.2 years [95% CI, 2.7 to 3.8] v 2.5 years [95% CI, 2.4 to 2.6]; HR 0.82 [95% CI, 0.71 to 0.96]; P value = .01). Similar to OS outcomes, ar-mortality difference was no longer observed when considering ar-CSM (Appendix Figs A2A-A2F, online only). After adjustment, PS, sidedness, and time to recurrence remained significantly associated with survival ar (Data Supplement).
Prognostic Effect of Age in Patient Subgroups
No interaction for DFS nor for OS (Appendix Fig A3, online only) was observed between age groups and sex, race, PS, sidedness, histologic grade, node examined, and treatment regimen. However, age had different prognostic effects on the basis of risk groups (interaction P value for DFS = 0.009, for OS < 0.001). The positive prognostic value of young age in stage II disease (HRs for DFS = 0.75, for OS = 0.44) shrunk in low-risk stage III (HRs for DFS = 0.92, for OS = 0.73) and completely reverted into a negative or no prognostic value in the high-risk stage III subgroup (T4 and/or N2 disease; HRs for DFS = 1.22, for OS = 1.04).
No significant difference in 3y-RF rates and 5y-CSM rates was observed in stage II and low-risk stage III patients; conversely, high-risk stage III EO-CRC had significantly lower 3y-RF rate (54% v 65%, HR 1.33 [95% CI, 1.14 to 1.15], P value < .001) and higher 5y-CSM rate (24% v 20%, HR 1.21 [95% CI, 1.00 to 1.47], P value < .06) compared with older patients (Figs 3A and 3B).
FIG 3.
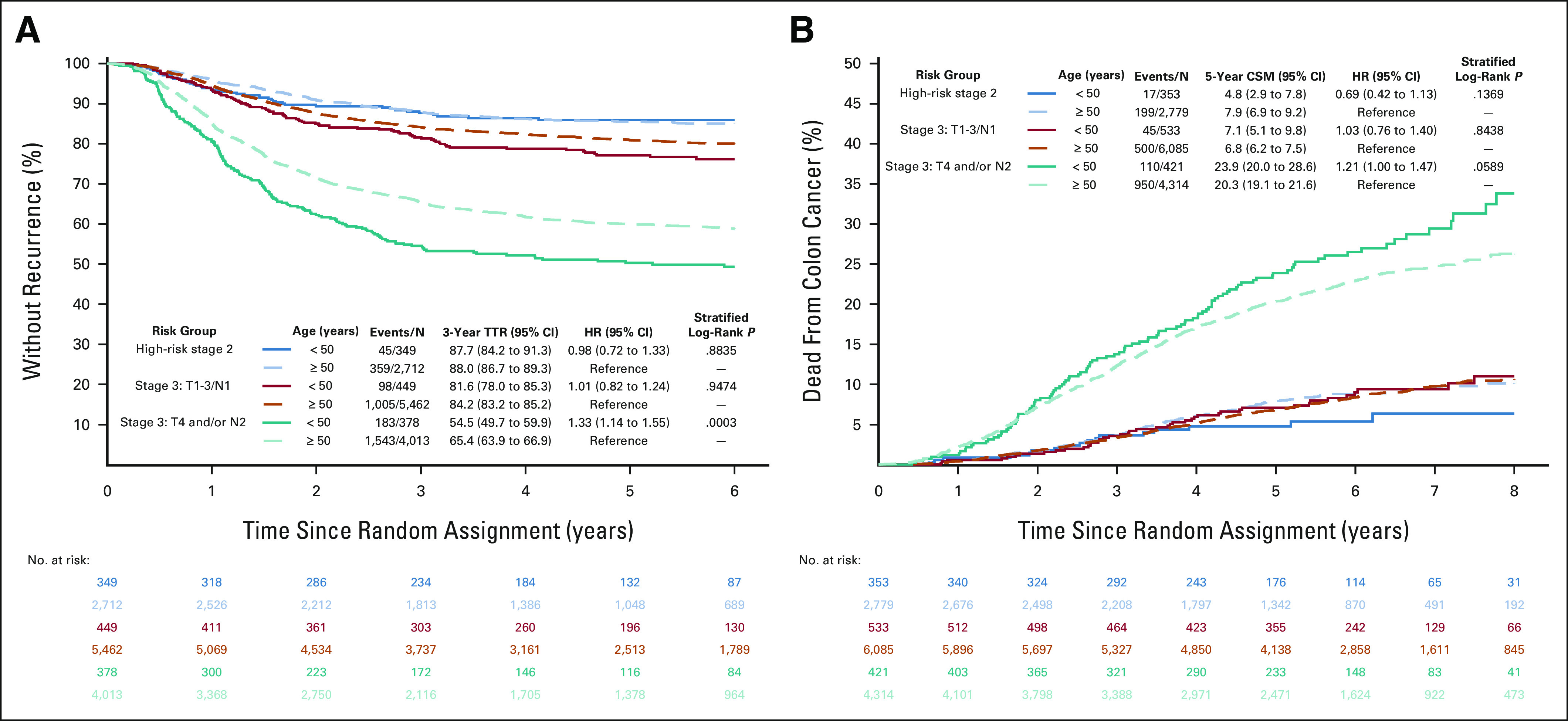
(A) TTR and (B) cancer-specific survival according to age and risk groups. CSM, cancer-specific mortality; HR, hazard ratio; TTR, time to recurrence.
Effect of Treatment Duration in Age Groups
In this exploratory analysis, the impact of 3 versus 6 months of adjuvant therapy in stage III disease on DFS was assessed separately in the two age groups and according to low-risk or high-risk stage. DFS was chosen as the end point for this analysis, given this was the primary end point of IDEA and also because this is a comparison within same age groups, thus not affected by non–cancer-related deaths confounders. In the LO-CRC group, the results were in line with the previous findings.5 Conversely, low-risk stage III EO-CRC had significantly lower 3y-DFS rate with 3 months of treatment compared with 6 months (81% v 87%; HR 1.49 [95% CI, 1.00 to 2.20], P value < .05); in the high-risk stage III EO-CRC group, no difference was observed, with equally poor DFS rates of 57% and 56% (HR 0.97 [95% CI, 0.73 to 1.29; P value = .85]) with 3 and 6 months, respectively (Fig 4). The Data Supplement includes 3y-DFS and 5y-CSM comparisons in EO-CRC and LO-CRC according to TN stages and chemotherapy regimen.
FIG 4.
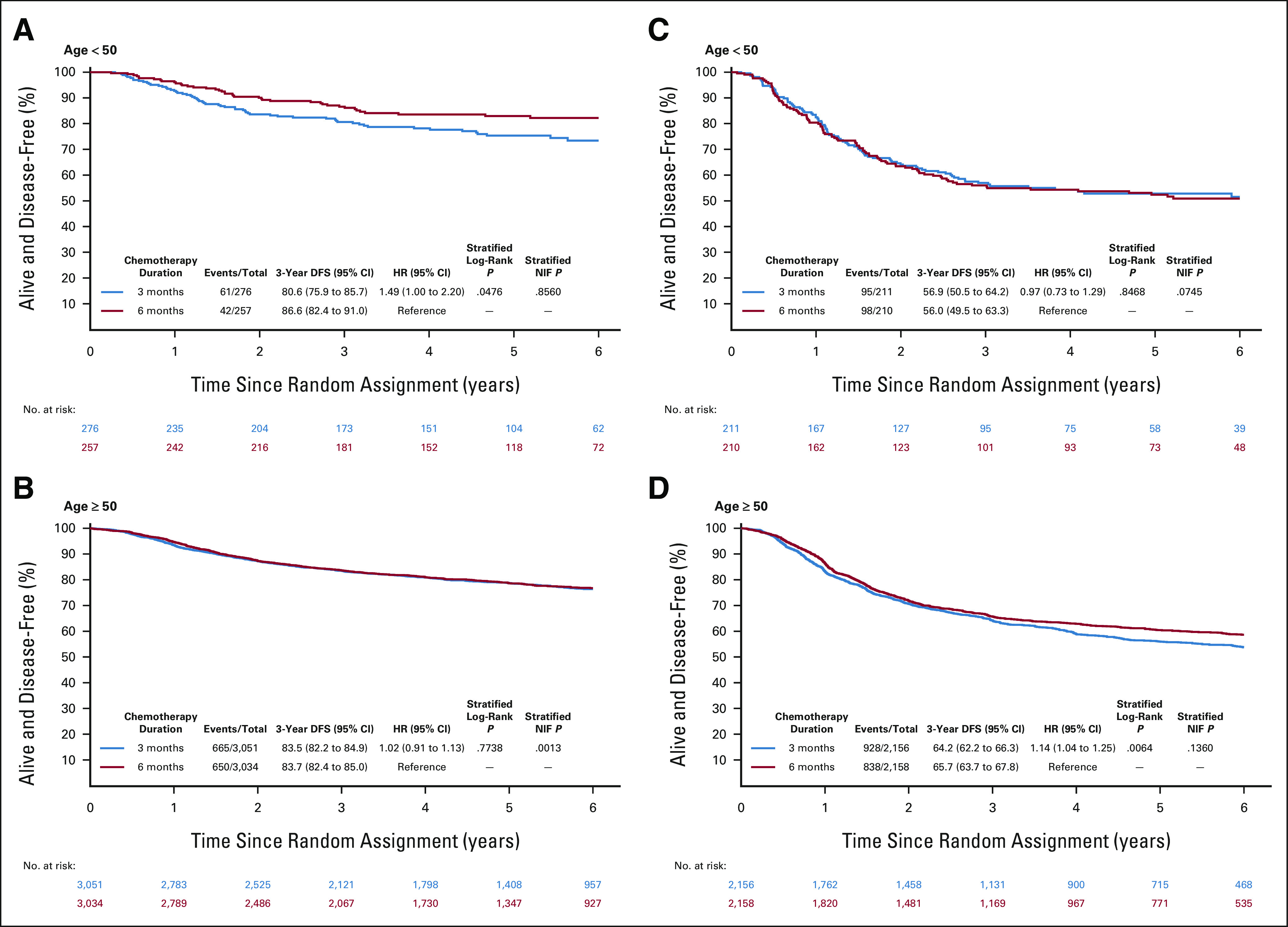
DFS in stage III low and high risk according to age groups and treatment duration. DFS in (A) low-risk stage III patients age < 50 years, (B) low-risk stage III patients age ≥ 50 years, (C) high-risk stage III patients age < 50 years, and (D) high-risk stage III patients age ≥ 50 years. DFS, disease-free survival; HR, hazard ratio; NIF, noninferiority.
DISCUSSION
In this comprehensive analysis including six multicenter, randomized clinical trials of adjuvant chemotherapy, patients with EO-CRC have been evaluated and compared with older patients for the first time.
Clinically, young patients have better PS and more frequently a distal tumor, in line with known higher prevalence of proximal and BRAF-mutant/MMRd tumors in older patients.6
A better PS and likely lower comorbidities in young patients may allow for a more aggressive treatment approach, suggested here by the higher number of stage II young patients included, higher number of lymph nodes examined, and higher chemotherapy dose intensity.
This is in line with previous evidence highlighting how young patients are more likely to receive more intense treatment approaches.7
A higher incidence of nausea and vomiting in young patients was observed; this confirmed similar findings previously reported in patients with CRC included in the ACCENT database and also in other cancer types.8-10 Whether these are anticipatory, acute or delayed nausea, and vomiting is not documented in the IDEA database and should be prospectively investigated for treatment optimization. Conversely, the lower incidence of hematologic toxicities despite higher chemotherapy doses received may open opportunities for further treatment intensification. Information on whether granulocyte colony-stimulating factors (GCSFs) were more frequently used in EO-CRC to maintain dose intensity is not available; upfront GCSFs are not standard with fluoropyrimidine and oxaliplatin, thus the incidence of neutropenic events should well represent the reality, while lower dose reductions compared with older patients may also be explained by a different GCSF usage. Although peripheral neuropathy rates looked similar between age groups, the long-term impact and persistency of low-grade neurotoxicity were not assessed in this study. Similarly, cardiovascular, psychologic, and gonadal toxicities were not documented; these should be prospectively assessed in cancer survivors.
In terms of survival outcomes, in this work, we highlight the importance of evaluating cancer-specific outcomes instead of traditional end points when comparing groups with significantly different life expectancies. In fact, young patients had similar DFS and longer OS than older patients; however, these results may be confounded by an expected higher mortality rate for competing causes in older patients. Cancer-specific outcomes highlighted how young patients have the same risk of dying of cancer than older patients overall, whereas in high-risk stage III disease, younger age is a significant poor prognostic factor. This might be explained by a more aggressive disease biology. Interestingly, comprehensive genomic profiling demonstrated only minor genomic differences between younger and older patients with CRC, with TP53 and CTNNB1 more frequently found in young patients, and APC, BRAF, and KRAS more frequently altered in older patients.11,12 Whether post-transcriptional differences exist or meaningful differences can be found in the tumor microenvironment, leading to more immune escape in younger patients, needs to be assessed.
After disease recurrence, the risk of dying of cancer in young patients was not different compared with that of older patients, despite higher likelihood of receiving more aggressive treatments including more intense chemotherapy, wider use of surgery for metastatic disease, or more frequent inclusion in clinical trials.7
Treatment duration should be carefully considered in patients with EO-CRC. On the basis of the inferiority of 3 versus 6 months shown in the IDEA pooled analysis in high-risk stage III disease, 3 months of therapy should not be standard in this specific group of patients.5 Importantly, this study further highlights poor overall completion rates of adjuvant therapy, especially with 6-month regimens even in young clinical trial patients with expected excellent PS. Here, in patients with EO-CRC with high-risk stage III cancer, the benefit of 6 months of treatment is more likely overcome by the overall poor outcome of this subgroup, with nearly half of the patients experiencing disease recurrence with either 3 or 6 months of treatment. This highlights the unmet need for early detection and possibly completely novel strategies to be developed in this group of patients.
Three months of adjuvant fluoropyrimidine and oxaliplatin therapy is now standard in low-risk stage III disease5; the current analysis demonstrates a significant 6% incremental benefit in 3y-DFS rate in patients assigned to 6 months of treatment over 3 months of treatment in patients with EO-CRC, despite completion rates in 6-month groups being lower than in 3-month groups. However, this age group–specific analysis was ad hoc with a relatively small sample size. Thus, cautions are needed to interpret the results, especially in conjunction with toxicity data. A minimum of 3 months of treatment is recommended in all stage III patients with cancer; longer duration of therapy may have a minimal effect in this subgroup at a clear cost of increased toxicity.
Limitations of our analysis are mainly because of its post hoc nature and the absence of important prognostic biomarkers like microsatellite instability (MSI), including whether germline (Lynch syndrome) or sporadic status. Recently, the effect of MSI status was explored in a similar setting in the ACCENT pooled analysis of 12 adjuvant trials.13 Up to 11% of patients had an MSI-high tumor; however, the germline status was not available. The higher prevalence of MSI-high in older patients with associate positive prognostic value in early-stage disease may further explain the poorer outcomes observed in patients with EO-CRC. Although a higher incidence of Lynch syndrome could be expected in younger patients, it is not clear whether the prognostic value of germline or sporadic MSI is significantly different. This information should be systematically and prospectively collected in future studies.
Previous findings showed how young patients are more likely to have a cancer of the rectum and metastatic disease at diagnosis compared with older patients; these patients were not included in the current analysis. Finally, an arbitrary cutoff at 50 years was used to define the two age groups. This cutoff has been consistently used in recent epidemiologic studies and identifies a population of patients diagnosed before screening programs.1,2 Although different age cutoffs or age as continuous variable could be further explored, statistical power could be lost in smaller subgroup analyses. Our pragmatic approach may help informing decisions related to changes of surveillance strategies.
In conclusion, in this post hoc analysis of the IDEA database, we demonstrated how age is prognostic with significant negative value for early-onset colon cancer in stage III. Young patients with high-risk stage III cancer experience disease recurrence more frequently than older patients despite receiving a higher adjuvant treatment intensity. These findings should inform the design of future clinical trials and the optimization of surveillance strategies in light of the challenging, increased incidence of CRC in young adults.
ACKNOWLEDGMENT
This work is dedicated to the memory of Daniel J. Sargent. Dan was one of the world's foremost experts in biostatistics and oncology who brought together disparate investigators and established data sharing across academia and industry internationally. His groundbreaking initiatives of integrating large collections of databases enabled research to answer questions otherwise beyond statistical possibility, to design important new clinical studies, to make regulatory observations, and to set new standards. He pushed these innovations farther to prospectively plan internationally combined analyses that answered questions previously believed to be impossible. The world of oncology statistics and analysis will not be the same without him, but his legacy continues.
The authors acknowledge all the authors who contributed to the clinical trials included in the IDEA academic collaboration and the Gastrointestinal Track Cancer Group at the European Organisation for Research and Treatment of Cancer (EORTC) for funding.
APPENDIX
FIG A1.
Flow diagram. CALGB, Cancer and Leukemia Group B; HORG, The Hellenic Oncology Research Group; IDEA, International Duration Evaluation of Adjuvant Chemotherapy; mITT, modified intention-to-treat; SCOT, 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer; TOSCA, The Three or Six Colon Adjuvant.
FIG A2.
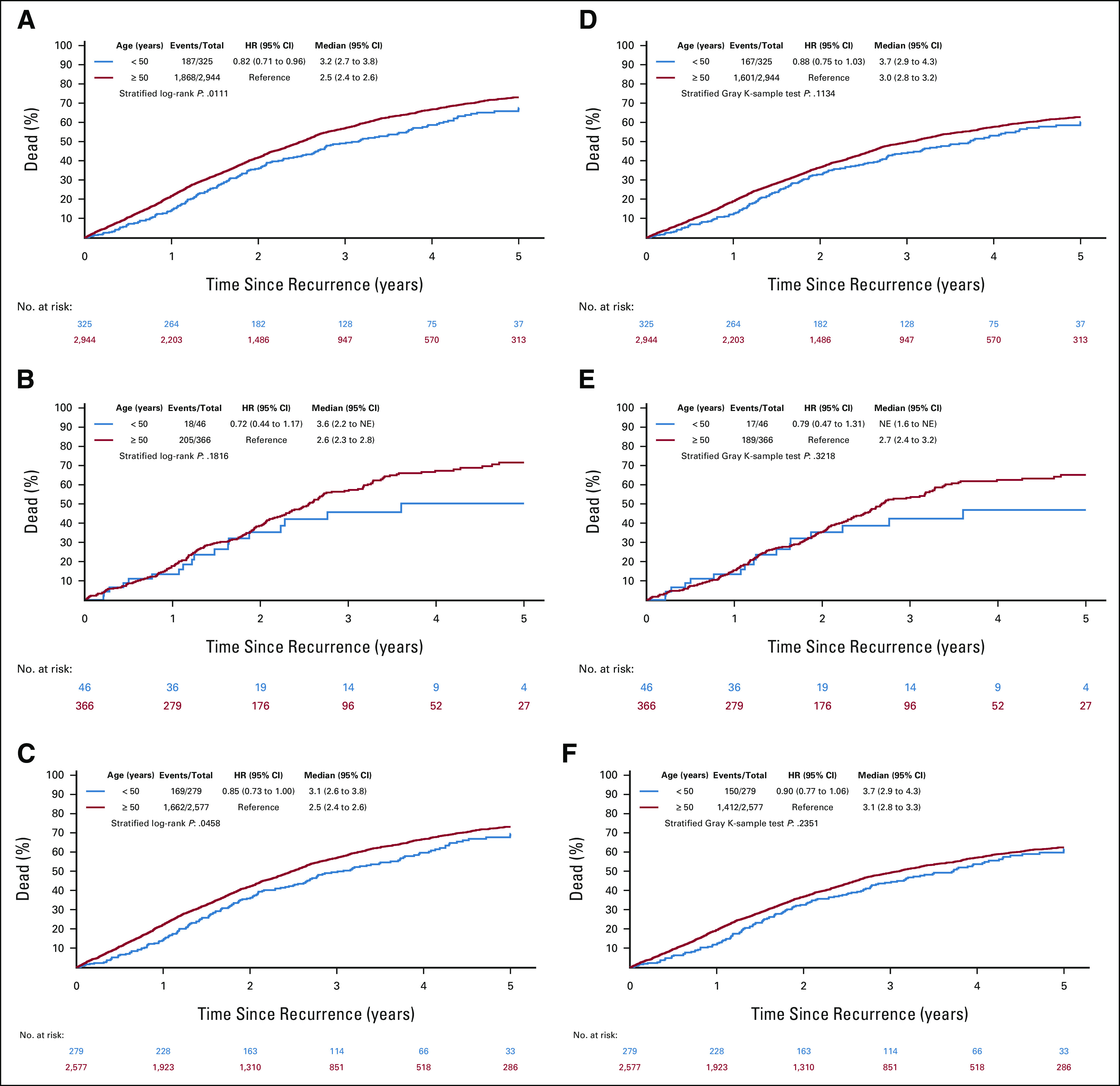
Mortality and ar-CSM according to age groups and disease stage at diagnosis. Overall mortality in (A) all stages, (B) high-risk stage II patients, and (C) stage III patients. Cancer-specific mortality in (D) all stages, (E) high-risk stage II patients, and (F) stage III patients. ar-CSM, cancer-specific mortality after recurrence; ar-CSS, cancer-specific survival after recurrence; ar-OM, overall mortality after recurrence; HR, hazard ratio; NE, not evaluable.
FIG A3.
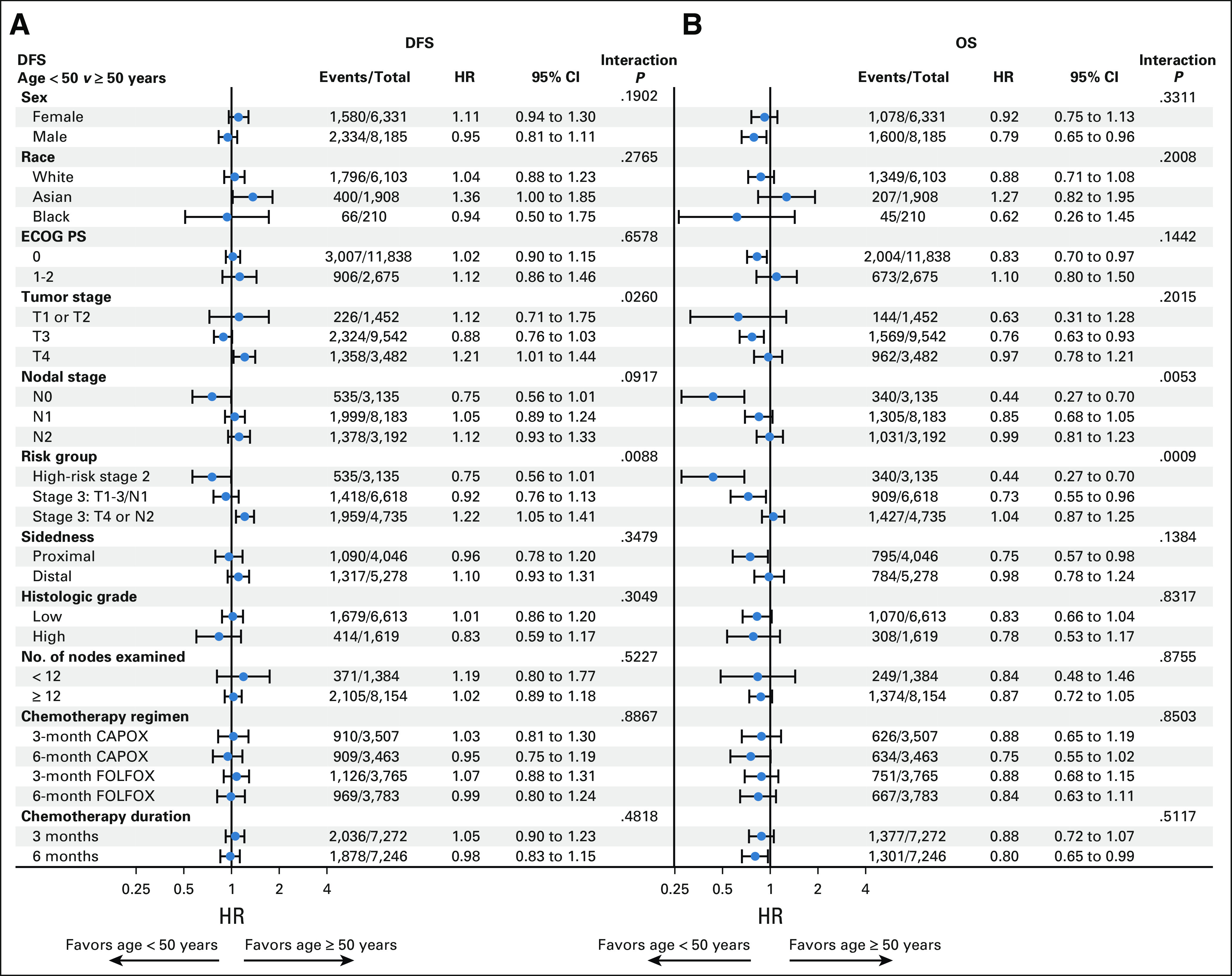
Forest plot for age group effect on (A) DFS and (B) OS by baseline factors. CAPOX, oxaliplatin and capecitabine; DFS, disease-free survival; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; OS, overall survival.
Elisa Fontana
Employment: HCA/Sarah Cannon
Consulting or Advisory Role: Astellas Pharma (I), Celgene (I), Servier (I), Bristol Myers Squibb (I)
Patents, Royalties, Other Intellectual Property: Patent No: 1716712.3 pending (I)
Travel, Accommodations, Expenses: Bristol Myers Squibb (I), Servier (I)
Alberto Sobrero
Stock and Other Ownership Interests: Bayer
Consulting or Advisory Role: Merck Serono, Servier, Sanofi, Celgene, Amgen, Bayer, Menarini
Speakers' Bureau: Sanofi, Merck Serono, Takeda, Roche, Bayer, Amgen, Celgene, Lilly, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Bayer, Merck Serono, Roche, Takeda
Timothy Iveson
Honoraria: Servier
Consulting or Advisory Role: Servier, Bristol Myers Squibb, Pierre Fabre, MSD Oncology
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Esperas Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Julien Taieb
Consulting or Advisory Role: Roche, Merck KGaA, Amgen, Servier, MSD, Pierre Fabre, Novartis
Speakers' Bureau: Servier, Amgen, Roche/Genentech, Sanofi, Merck, Lilly, MSD, Pierre Fabre
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, Lilly, Taiho Pharmaceutical
Research Funding: Chugai Pharma (Inst), MSD (Inst), Daiichi Sankyo Company, Limited (Inst), PAREXEL International Inc (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst)
Ioannis Souglakos
Consulting or Advisory Role: Roche (Inst), Servier (Inst), Ipsen (Inst), Pierre Fabre, MSD, Bristol Myers Squibb-Ono Pharmaceutical
Speakers' Bureau: Sanofi, Merck KGaA (Inst), Roche (Inst), MSD
Research Funding: Amgen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Merck Serono, Amgen, Sanofi, Roche, Servier
Elizabeth C. Smyth
Employment: HCA International (I)
Honoraria: Servier, Novartis, Bristol Meyer Squibb, Pfizer
Consulting or Advisory Role: Servier, Amal Therapeutics, BeiGene, Zymeworks, Novartis, Bristol Myers Squibb, Merck Serono, Roche, AstraZeneca, Amgen
Research Funding: Bristol Myers Squibb (Inst)
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech AG, Servier, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamedup!
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Servier, Zymeworks, Amgen
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Markus Moehler
Honoraria: Amgen, Roche/Genentech, Merck Serono, MSD Oncology, Bristol Myers Squibb, AstraZeneca/MedImmune, Servier, Pierre Fabre, Sanofi
Consulting or Advisory Role: Bayer, MSD, Merck Serono, Amgen, Taiho Pharmaceutical, Pfizer, Roche, Lilly, Servier, BeiGene, BMS
Research Funding: Amgen (Inst), Leap Therapeutics (Inst), Merck Serono (Inst), AstraZeneca (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Amgen, Merck Serono, Roche, Bayer, ASCO, German Cancer Society, MSD, ESMO
Roberto Labianca
Consulting or Advisory Role: Roche, Lilly, Sanofi, Merck Serono
Travel, Accommodations, Expenses: Roche, Merck Serono, Servier
Jeffrey Meyerhardt
Honoraria: Cota Healthcare, Taiho Pharmaceutical
Research Funding: Boston Biomedical (Inst)
Thierry André
Honoraria: Roche/Genentech, Bristol Myers Squibb, Servier, Bayer, Sanofi, Amgen, Pierre Fabre, Ventana Medical Systems, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, HalioDX, MSD Oncology, Servier, Bayer, AstraZeneca/MedImmune, Tesaro, Clovis Oncology, Gastrointestinal Cancers Advice, Pierre Fabre, GamaMabs Pharma, Astellas Pharma, Kaleido Biosciences, Gritstone Oncology, GlaxoSmithKline, Seagen, Transgene
Travel, Accommodations, Expenses: Roche/Genentech, Amgen, Bristol Myers Squibb, MSD Oncology, Roche, Ventana Medical Systems
Ioannis Boukovinas
Employment: Pierre Fabre (I)
Honoraria: Roche, MSD, Bristol Myers Squibb, Pfizer, Novartis, Merck, AstraZeneca, LEO Pharma, Servier
Consulting or Advisory Role: Roche, Sanofi, AstraZeneca, Bristol Myers Squibb, LEO Pharma, MSD, Novartis, Ipsen, Genesis Pharma
Research Funding: Roche, Novartis, Bristol Myers Squibb, MSD, Regeneron, Boehringer Ingelheim, Lilly, Pfizer
Travel, Accommodations, Expenses: MSD, Roche, Pfizer, Bristol Myers Squibb, Servier, Ipsen
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck Serono, Lilly, Servier, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, MSD
Speakers' Bureau: Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen
Research Funding: Amgen, Merck Serono, Bayer (Inst), Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Mark Saunders
Honoraria: Servier, Merck Serono
Travel, Accommodations, Expenses: Servier
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, Incyte, Fondazione Smith Kline, Invectys, AC Biotech
Travel, Accommodations, Expenses: MSD
Eiji Oki
Speakers' Bureau: Chugai Pharma, Lilly Japan, Takeda, Ono Pharmaceutical, Bayer Yakuhin, Bristol Myers Squibb Japan
Vassilis Georgoulias
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca
Qian Shi
Stock and Other Ownership Interests: Amgen, Johnson & Johnson, Merck
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO 2021 annual meeting as poster discussion in the colorectal session, June 4-8, 2021, virtual.
SUPPORT
Supported by European Organisation for Research and Treatment of Cancer EORTC-GITCG-RP 2009 Prof Irit Ben-Aharon; Japanese Foundation for Multidisciplinary Treatment of Cancer and Yakult Konsha Co Ltd ACHIEVE clinical Trial; National Cancer Institute NCI, CALGB/SWOG 80702 clinical trial, U10CA180821, U10CA180835, UG1CA233163, U10CA180882, and U10CA180888; HORG Foundation HORG clinical trial; Institut National du Cancer and Programme Hospitalier de Recherche Clinique en Cancérologie, IDEA clinical trial, PHRC2009; Agenzia Italiana del Farmaco and AIRC TOSCA, clinical trial, FARM 5RWTWZ and AIRC IG21742-2018; National Institute for Health Research, Efficacy and Mechanism Evaluation, the National Institute for Health Research, Health Technology Assessment, and Cancer Research United Kingdom SCOT clinical trial, EME 09/800/34 and C1348/A15960.
AUTHOR CONTRIBUTIONS
Conception and design: Elisa Fontana, Alberto Sobrero, Anthony F. Shields, Julien Taieb, Takayuki Yoshino, Markus Moehler, Anne Giraut, Ioannis Boukovinas, Irit Ben-Aharon, Qian Shi
Administrative support: Anne Giraut, Qian Shi
Provision of study materials or patients: Timothy Iveson, Anthony F. Shields, Julien Taieb, Ioannis Souglakos, Florian Lordick, Thierry André, Sara Lonardi, Eiji Oki, Qian Shi
Collection and assembly of data: Jeff Meyers, Anthony F. Shields, Julien Taieb, Takayuki Yoshino, Ioannis Souglakos, Markus Moehler, Andrea Harkin, Roberto Labianca, Thierry André, Ioannis Boukovinas, Sara Lonardi, Mark Saunders, Dewi Vernerey, Eiji Oki, Vassilis Georgoulias, Qian Shi
Data analysis and interpretation: Elisa Fontana, Jeff Meyers, Alberto Sobrero, Timothy Iveson, Julien Taieb, Takayuki Yoshino, Ioannis Souglakos, Elizabeth C. Smyth, Florian Lordick, Markus Moehler, Roberto Labianca, Jeffrey Meyerhardt, Thierry André, Ioannis Boukovinas, Mark Saunders, Qian Shi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Early-Onset Colorectal Adenocarcinoma in the IDEA Database: Treatment Adherence, Toxicities, and Outcomes With 3 and 6 Months of Adjuvant Fluoropyrimidine and Oxaliplatin
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elisa Fontana
Employment: HCA/Sarah Cannon
Consulting or Advisory Role: Astellas Pharma (I), Celgene (I), Servier (I), Bristol Myers Squibb (I)
Patents, Royalties, Other Intellectual Property: Patent No: 1716712.3 pending (I)
Travel, Accommodations, Expenses: Bristol Myers Squibb (I), Servier (I)
Alberto Sobrero
Stock and Other Ownership Interests: Bayer
Consulting or Advisory Role: Merck Serono, Servier, Sanofi, Celgene, Amgen, Bayer, Menarini
Speakers' Bureau: Sanofi, Merck Serono, Takeda, Roche, Bayer, Amgen, Celgene, Lilly, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Bayer, Merck Serono, Roche, Takeda
Timothy Iveson
Honoraria: Servier
Consulting or Advisory Role: Servier, Bristol Myers Squibb, Pierre Fabre, MSD Oncology
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Esperas Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Julien Taieb
Consulting or Advisory Role: Roche, Merck KGaA, Amgen, Servier, MSD, Pierre Fabre, Novartis
Speakers' Bureau: Servier, Amgen, Roche/Genentech, Sanofi, Merck, Lilly, MSD, Pierre Fabre
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, Lilly, Taiho Pharmaceutical
Research Funding: Chugai Pharma (Inst), MSD (Inst), Daiichi Sankyo Company, Limited (Inst), PAREXEL International Inc (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst)
Ioannis Souglakos
Consulting or Advisory Role: Roche (Inst), Servier (Inst), Ipsen (Inst), Pierre Fabre, MSD, Bristol Myers Squibb-Ono Pharmaceutical
Speakers' Bureau: Sanofi, Merck KGaA (Inst), Roche (Inst), MSD
Research Funding: Amgen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Merck Serono, Amgen, Sanofi, Roche, Servier
Elizabeth C. Smyth
Employment: HCA International (I)
Honoraria: Servier, Novartis, Bristol Meyer Squibb, Pfizer
Consulting or Advisory Role: Servier, Amal Therapeutics, BeiGene, Zymeworks, Novartis, Bristol Myers Squibb, Merck Serono, Roche, AstraZeneca, Amgen
Research Funding: Bristol Myers Squibb (Inst)
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech AG, Servier, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamedup!
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Servier, Zymeworks, Amgen
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Markus Moehler
Honoraria: Amgen, Roche/Genentech, Merck Serono, MSD Oncology, Bristol Myers Squibb, AstraZeneca/MedImmune, Servier, Pierre Fabre, Sanofi
Consulting or Advisory Role: Bayer, MSD, Merck Serono, Amgen, Taiho Pharmaceutical, Pfizer, Roche, Lilly, Servier, BeiGene, BMS
Research Funding: Amgen (Inst), Leap Therapeutics (Inst), Merck Serono (Inst), AstraZeneca (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Amgen, Merck Serono, Roche, Bayer, ASCO, German Cancer Society, MSD, ESMO
Roberto Labianca
Consulting or Advisory Role: Roche, Lilly, Sanofi, Merck Serono
Travel, Accommodations, Expenses: Roche, Merck Serono, Servier
Jeffrey Meyerhardt
Honoraria: Cota Healthcare, Taiho Pharmaceutical
Research Funding: Boston Biomedical (Inst)
Thierry André
Honoraria: Roche/Genentech, Bristol Myers Squibb, Servier, Bayer, Sanofi, Amgen, Pierre Fabre, Ventana Medical Systems, GlaxoSmithKline
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, HalioDX, MSD Oncology, Servier, Bayer, AstraZeneca/MedImmune, Tesaro, Clovis Oncology, Gastrointestinal Cancers Advice, Pierre Fabre, GamaMabs Pharma, Astellas Pharma, Kaleido Biosciences, Gritstone Oncology, GlaxoSmithKline, Seagen, Transgene
Travel, Accommodations, Expenses: Roche/Genentech, Amgen, Bristol Myers Squibb, MSD Oncology, Roche, Ventana Medical Systems
Ioannis Boukovinas
Employment: Pierre Fabre (I)
Honoraria: Roche, MSD, Bristol Myers Squibb, Pfizer, Novartis, Merck, AstraZeneca, LEO Pharma, Servier
Consulting or Advisory Role: Roche, Sanofi, AstraZeneca, Bristol Myers Squibb, LEO Pharma, MSD, Novartis, Ipsen, Genesis Pharma
Research Funding: Roche, Novartis, Bristol Myers Squibb, MSD, Regeneron, Boehringer Ingelheim, Lilly, Pfizer
Travel, Accommodations, Expenses: MSD, Roche, Pfizer, Bristol Myers Squibb, Servier, Ipsen
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck Serono, Lilly, Servier, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, MSD
Speakers' Bureau: Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen
Research Funding: Amgen, Merck Serono, Bayer (Inst), Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Mark Saunders
Honoraria: Servier, Merck Serono
Travel, Accommodations, Expenses: Servier
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, Incyte, Fondazione Smith Kline, Invectys, AC Biotech
Travel, Accommodations, Expenses: MSD
Eiji Oki
Speakers' Bureau: Chugai Pharma, Lilly Japan, Takeda, Ono Pharmaceutical, Bayer Yakuhin, Bristol Myers Squibb Japan
Vassilis Georgoulias
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca
Qian Shi
Stock and Other Ownership Interests: Amgen, Johnson & Johnson, Merck
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Torre LA, Soerjomataram I, et al. : Global patterns and trends in colorectal cancer incidence in young adults. Gut 68:2179-2185, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA 318:572-574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akimoto N, Ugai T, Zhong R, et al. : Rising incidence of early-onset colorectal cancer—A call to action. Nat Rev Clin Oncol 18:230-243, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahnen DJ, Wade SW, Jones WF, et al. : The increasing incidence of young-onset colorectal cancer: A call to action. Mayo Clin Proc 89:216-224, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Sobrero AF, Shields AF, et al. : Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378:1177-1188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran B, Kopetz S, Tie J, et al. : Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117:4623-4632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kneuertz PJ, Chang GJ, Hu CY, et al. : Overtreatment of young adults with colon cancer: More intense treatments with unmatched survival gains. JAMA Surg 150:402-409, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Jin Z, Dixon JG, Parekh HD, et al. : Clinicopathological and molecular biological characteristics of early-onset stage II/III colorectal adenocarcinoma: An analysis of 25 studies with 47,184 patients (pts) in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol 38, 2020. (15_suppl; abstr 4099) [Google Scholar]
- 9.Hesketh PJ, Aapro M, Street JC, et al. : Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: Analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171-1177, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Dong ST, Butow PN, Costa DS, et al. : Symptom clusters in patients with advanced cancer: A systematic review of observational studies. J Pain Symptom Manag 48:411-450, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Lieu CH, Golemis EA, Serebriiskii IG, et al. : Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin Cancer Res 25:5852-5858, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang MJ, Ping J, Li Y, et al. : The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep 5:10645, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen R, Taieb J, Fiskum J, et al. : Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An accent pooled analysis of 12 adjuvant trials. J Clin Oncol 39:642-651, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]