Abstract
Background:
The aim of this study was to evaluate the efficacy and safety of bismuth pectin capsules and bismuth pectin granules in the first-line quadruple treatment of Helicobacter pylori (H. pylori).
Methods:
This study was a multicenter, randomized, open-labelled controlled clinical trial. Patients with a H. pylori infection were randomized into 4 groups (1:1:1:1) and treated with a 14-day bismuth-containing quadruple therapy. The 4 groups received either bismuth potassium citrate capsules (220 mg), colloidal bismuth pectin capsules (200 mg), bismuth pectin granules (150 mg), or bismuth pectin granules (300 mg). The primary outcome was the eradication rate of H. pylori. The secondary outcomes included symptom improvement, patient compliance, and incidence of adverse events. This study was registered at ClinicalTrials.gov (NCT04209933).
Result(s):
A total of 240 patients were included in this study, and 211 patients completed the follow-up. An intention-to-treat analysis showed that the H. pylori eradication rates of the 4 groups were 73.3%, 76.7%, 75.0%, and 71.7%, respectively. The per-protocol analysis showed that the H. pylori eradication rates of the 4 groups were 86.3%, 82.1%, 83.3%, and 86.0%. There was no significant difference among the 4 groups in the H. pylori eradication rate (P > .05). There were also no significant differences in the symptom improvement rate, overall adverse reaction rate, or patient compliance among the 4 groups.
Conclusion(s):
Bismuth pectin capsules and bismuth pectin granules had similar efficacy and safety for H. pylori eradication compared to bismuth potassium citrate. These data suggest that bismuth pectin can be an alternative to bismuth potassium citrate to eradicate H. pylori when using bismuth-containing quadruple therapy.
Keywords: bismuth pectin, Helicobacter pylori, initial treatment, potassium bismuth citrate
1. Introduction
Helicobacter pylori (H. pylori) is a bacterial pathogen in the human stomach.[1] A global epidemiological survey showed that the H. pylori infection rate in adults is greater than 50%, and the infection rate is higher in developing countries. H. pylori infection is closely related to the occurrence and development of a variety of digestive diseases, including chronic gastritis, peptic ulcer disease, gastric mucosal-associated tissue lymphoma, and gastric cancer.[2–4] Eradication of H. pylori is conducive to the recovery from chronic gastritis, the healing of upper gastrointestinal ulcers, and the reduction of the incidence of ulcer complications and may reduce the risk of gastric cancer.[5–9] Therefore, the Kyoto global consensus report and the Fifth Chinese National Consensus both recommend that all known patients with H. pylori infection should receive eradication therapy.[10]
The main regimen of H. pylori eradication is a combination of proton-pump inhibitors (PPIs) and antibiotics,[11,12] and the widely used antibiotics include amoxicillin, clarithromycin, metronidazole, levofloxacin, tetracycline, and furazolidone.[3] However, the eradication of H. pylori using standard triple therapy fails in approximately 20% to 30% of patients, which is mainly attributed to the increase in microbial drug resistance to metronidazole, clarithromycin, and levofloxacin in various countries and regions.[13–17] In 2017, the Maastricht V Consensus suggested that in areas with high resistance rates (>15%) to both clarithromycin and metronidazole, quadruple therapy should be used as a first-line eradication regimen.[3] Considering the current high resistance rate of H. pylori to metronidazole, clarithromycin and levofloxacin in China, bismuth-containing quadruple therapy (a bismuth compound combined with PPI and 2 antibiotics) was recommended as the only empirical treatment for H. pylori eradication by The Fifth Chinese National Consensus on the management of H. pylori infection.[18]
An increasing number of studies have shown that bismuth agents can increase the sensitivity of H. pylori to antibiotics, thus improving the eradication success rate.[19–21] Bismuth agents commonly used clinically include bismuth citrate and bismuth pectin.[22] A previous pharmaceutical study reported that bismuth citrate is a medium molecular weight bismuth agent, while bismuth pectin is a high molecular weight bismuth agent. As bismuth pectin is rarely absorbed into the blood after oral administration, its blood drug concentration is significantly lower than that of bismuth citrate, suggesting that it has higher safety.[23] The standard bismuth agent recommended in the Fifth Chinese National Consensus report on the management of H. pylori infection is bismuth potassium citrate.[18] The consensus also indicates that bismuth pectin can be used for the eradication of H. pylori, but the optimal dose of bismuth pectin remains to be determined. Therefore, the purpose of this study was to conduct a multicenter, randomized controlled, open-labelled, parallel trial to investigate the efficacy and safety of different doses and forms of bismuth pectin in H. pylori eradication.
2. Methods
2.1. Subjects and study design
This is a multicenter, randomized, open-labelled, parallel, controlled clinical trial. The study was carried out in the Department of Outpatients at the Xijing Hospital (Xi’an, Shaanxi Province, China), the First Hospital of Shanxi Medical University (Taiyuan, Shanxi Province, China), and the Gansu Provincial Hospital (Lanzhou, Gansu Province, China) from May 2020 to December 2020. The trial was approved by the Ethics Committee of each participating center, and all participants signed a written informed consent prior to enrollment. The study was conducted according to the Declaration of Helsinki 2013 and the CONSORT statement for randomized controlled studies. This study was registered at ClinicalTrials.gov (NCT04209933).
2.2. Inclusion and exclusion criteria
Treatment-naive patients with H. pylori infection were included in this trial. Inclusion criteria were: Adult participants aged between 18 and 70 years old and both genders; Patients with a definite H. pylori infection who have not previously received eradication therapy; Patients desired H. pylori eradication therapy; Women were eligible if they were not pregnant or nursing, and if they had the potential for childbearing, they were required to use medically acceptable contraception during the study and 30 days thereafter.
Exclusion criteria were: Contraindications to the study drugs or being allergic to the study drugs; Substantial organ impairment, severe or unstable cardiopulmonary disease, or endocrine disease; Constant use of PPI within 2 weeks before screening and constant use of antibiotics or bismuth complexes within 1 month before screening; Diagnosed with gastric mucosal-associated tissue lymphoma; Pregnant and lactating women; Patients who underwent upper gastrointestinal surgeries; Patients with a history of malignancy; Patients with a history of drug or alcohol abuse within the past year; Systemic use of corticosteroids, non-steroidal anti-inflammatory drugs, anticoagulants, or platelet aggregation inhibitors (except the use of aspirin for less than 100 mg/d); Patients who had psychological problems or poor compliance; Enrolled in other clinical trials within the past 3 months; Refusal to sign an informed consent.
2.3. Grouping and medication
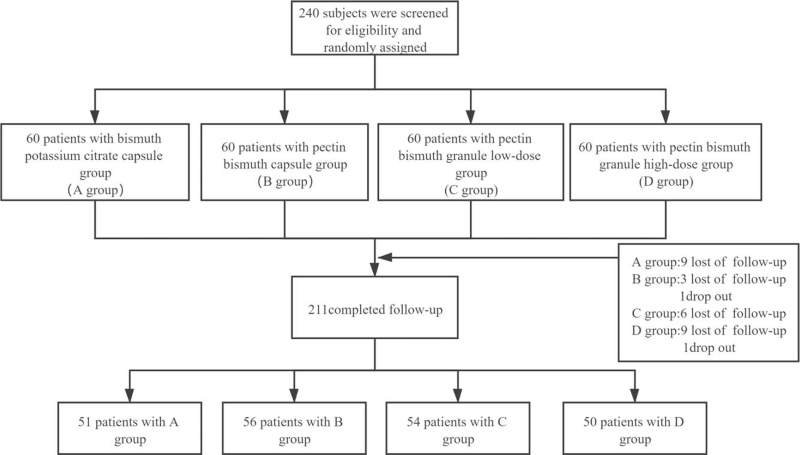
The overview of this study is shown in Figure 1. Two hundred forty patients who met the inclusion and exclusion criteria were randomly assigned to the bismuth potassium citrate capsule group (group A), bismuth pectin capsule group (group B), bismuth pectin granule low-dose group (group C), and bismuth pectin granule high-dose group (group D) at a ratio of 1:1:1:1. All patients were treated with rabeprazole (10 mg each dose), amoxicillin (1000 mg each dose), clarithromycin (500 mg each dose), and bismuth. The 4 drugs were administered twice daily for a course of 14 days. The bismuth agent in group A was a bismuth potassium citrate capsule at 220 mg for each dose, group B was a bismuth pectin capsule at 200 mg for each dose, group C was bismuth pectin granules given at 150 mg each dose, and group C was bismuth pectin granules give at 300 mg each dose.
Figure 1.
Flow diagram of the study.
The drug information is as follows: rabeprazole (Eisai (China) Pharmaceutical, Suzhou, Jiangsu Province, China), amoxicillin (Zhuhai United Laboratories, Zhuhai, Guangdong Province, China), clarithromycin (Jiangbo Pharmaceutical, Laiyang, Shandong Province, China), bismuth potassium citrate capsules (Sinopharm Shantou Jinshi Pharmaceutical, Shantou, Guangdong Province, China), pectic bismuth capsule (Shanxi Xinbaoyuan Pharmaceutical, Datong, Shanxi Province, China), and pectic bismuth particles (Shanxi Xinbaoyuan Pharmaceutical, Datong, Shanxi Province, China).
2.4. Diagnosis of H. pylori infection
The diagnosis of H. pylori infection was confirmed when any one of the following tests was positive: 13C/14C urea breath test, H. pylori stool antigen test, histological confirmation of H. pylori, or the H. pylori rapid urease test.
Successful H. pylori eradication was determined with negative 13C/14C urea breath test, negative H. pylori stool antigen test, or rapid urease test at 4 to 8 weeks after the completion of therapy.
2.5. Evaluation of symptoms
The patients were assessed for various symptoms, including nausea, vomiting, abdominal pain, early satiety, abdominal distension, diarrhea, constipation, belching, hiccups, bitter taste, headache, dizziness, fever, cough, acid reflux, and heartburn, before treatment, at the end of treatment, and at 4 weeks after completing treatment. Both the severity and frequency of symptoms were classified between 0 to 3 (0, 1, 2, and 3 represent none, mild, moderate, and severe, respectively). The score of each symptom was calculated as follows: symptom score = frequency score × severity score. The total symptom score was calculated as the sum of all symptoms. At least a 50% reduction in the total score after treatment was defined as symptom improvement.
2.6. Outcomes
The primary outcome of this study was the H. pylori eradication rate at least 4 weeks after the completion of the therapy. The secondary outcomes were the overall rates of adverse events, the symptom improvement rate (at the end of treatment and 4 weeks after treatment), and the patient compliance (good compliance was defined by taking more than 80% of the prescribed medication).
2.7. Statistical analysis
All statistical analyses were performed using IBM SPSS statistics ver. 25.0 software (SPSS, Inc). Continuous variables are expressed as the mean ± standard deviation () and were compared using the Student t test or analysis of variance. The classification variables were expressed as rates and evaluated by the chi-square test or Fisher exact probability method. Eradication rates were calculated using both intention-to-treat (ITT) analysis and per-protocol (PP) analysis. A P value of less than .05 (P < .05) was considered statistically significant.
3. Results
3.1. Baseline characteristics of the participants
A total of 240 patients who met the inclusion criteria were enrolled in the study. Among the participants, 211 patients completed follow-up, and 29 were lost to follow-up and dropped out (9 in group A, 4 in group B, 6 in group C, and 10 in group D). An ITT analysis was performed on 240 enrolled subjects, and a PP analysis was performed on 211 subjects who completed follow-up. As shown in Table 1, there were no significant differences in age, gender, body mass index, ethnicity, smoking, drinking, or other aspects of baseline characteristics among the 4 groups (P > .05).
Table 1.
Baseline characteristics of the participants.
| Ethnicity n (%) | ||||||||
| Age y (mean ± SD) | Sex n (male/female) | BMI (kg/m2) | Han | Others | Smoking n/N (%) | Drinking n/N (%) | ||
| PP analysis | Group A (N = 51) | 44.22 ± 12.51 | 25/26 | 23.00 ± 2.93 | 49 (96.1%) | 2 (3.9%) | 11/51 (21.6%) | 12/51 (23.5%) |
| Group B (N = 56) | 40.71 ± 12.30 | 34/22 | 22.39 ± 2.87 | 56 (100%) | 0 (0%) | 11/56 (19.6%) | 16/56 (28.6%) | |
| Group C (N = 54) | 41.52 ± 13.75 | 23/31 | 22.59 ± 3.34 | 53 (98.1%) | 1 (1.9%) | 9/54 (16.7%) | 13/54 (24.1%) | |
| Group D (N = 50) | 42.93 ± 13.00 | 25/25 | 22.63 ± 3.02 | 48 (96.0%) | 2 (4.0%) | 9/50 (18.0%) | 14/50 (28.0%) | |
| P value | .542 | .292 | .780 | .470 | .928 | .905 | ||
| ITT analysis | Group A (N = 60) | 44.27 ± 12.87 | 27/33 | 22.98 ± 2.87 | 58 (96.7%) | 2 (3.3%) | 12/60 (20.0%) | 15/60 (25.0%) |
| Group B (N = 60) | 41.10 ± 13.41 | 35/25 | 22.23 ± 1.85 | 60 (100%) | 0 (0%) | 11/60 (18.3%) | 16/60 (26.7%) | |
| Group C (N = 60) | 42.87 ± 12.87 | 25/35 | 22.38 ± 3.29 | 59 (98.3%) | 1 (1.7%) | 10/60 (16.7%) | 15/60 (25.0%) | |
| Group D (N = 60) | 43.87 ± 11.32 | 29/31 | 22.48 ± 2.92 | 58 (96.7%) | 2 (3.3%) | 11/60 (18.3%) | 15/60 (25.0%) | |
| P value | .625 | .291 | .798 | .523 | .974 | .996 | ||
Group A: bismuth potassium citrate capsule group; Group B: bismuth pectin capsule group; Group C: pectin bismuth granule low-dose group; Group D: pectin bismuth granule high-dose group.
BMI = body mass index, ITT = intention-to-treat, PP = per-protocol, SD = standard deviation.
3.2. H. pylori eradication rate
As shown in Table 2, according to the ITT analysis, the eradication rates of groups A, B, C, and D were 73.3%, 76.7%, 75.0%, and 71.7%, respectively, and there was no significant difference among the 4 groups (P > .05). The results of the PP analysis showed that the eradication rates of groups A, B, C, and D were 86.3%, 82.1%, 83.3%, and 86.0%, respectively, and there was no statistically significant difference among the 4 groups (P > .05).
Table 2.
Helicobacter pylori eradication rates.
| ITT analysis | Relative rate | PP analysis | Relative rate | |
| Group A | 44/60 (73.3%) | A/A (1.000) | 44/51 (86.3%) | A/A (1.000) |
| Group B | 46/60 (76.7%) | B/A (1.046) | 46/56 (82.1%) | B/A (0.951) |
| Group C | 45/60 (75.0%) | C/A (1.023) | 45/54 (83.3%) | C/A (0.965) |
| Group D | 43/60 (71.7%) | D/A (0.978) | 43/50 (86.0%) | D/A (0.996) |
| P value | PAB = .559 | — | PAB = .673 | — |
| PAC = .675 | — | PAC = .835 | — | |
| PAD = .968 | — | PAD = .838 | — | |
| PBC = .869 | — | PBC = .831 | — | |
| PBD = .589 | — | PBD = .532 | — | |
| PCD = .706 | — | PCD = .680 | — |
Group A: bismuth potassium citrate capsule group; Group B: bismuth pectin capsule group; Group C: pectin bismuth granule low-dose group; Group D: pectin bismuth granule high-dose group.
ITT = intention-to-treat, PP = per-protocol.
3.3. Rates of adverse events, compliance, and symptom improvement
As shown in Table 3, the overall frequencies of adverse events were 49.0%, 26.8%, 37.0%, and 36.0% for groups A, B, C, and D, respectively. The statistical analysis revealed that the adverse event rate in group A was significantly higher than that in group B (P < .05). The most common adverse event was having a bitter taste, which was reported by 22.0% to 31.4% of participants. However, there was no statistical significance among the 4 groups regarding having a bitter taste (P > .05). Other adverse events included nausea, abdominal pain, diarrhea, abdominal distension, and loss of appetite. The incidence of diarrhea in group D was higher than that in group B, and the difference was statistically significant (P < .05). The incidence of loss of appetite in group A was higher than that in group B, and there was a significant difference between those 2 groups (P < .05).
Table 3.
Rates of adverse events (n/N, %).
| Groups | Overall adverse events | Bitterness | Nausea | Diarrhea | Abdominal pain | Abdominal distension | Loss of appetite |
| A | 25/51 (49.0%) | 16/54 (31.4%) | 1/51 (2.0%) | 1/51 (2.0%) | 3/51 (5.9%) | 0 (0%) | 4/51 (7.8%) |
| B | 15/56 (26.8%) | 13/56 (23.2%) | 0/56 (0%) | 0 (0%) | 1/56 (1.8%) | 1/56 (1.8%) | 0 (0%) |
| C | 20/54 (37.0%) | 13/54 (24.1%) | 1/54 (1.9%) | 3/54 (5.6%) | 1/54 (1.9%) | 1/54 (1.9%) | 1/54 (1.9%) |
| D | 18/50 (36.0%) | 11/50 (22.0%) | 0/50 (0%) | 5/50 (10.0%) | 1/50 (2.0%) | 0 (0%) | 1/50 (2.0%) |
| P value | PAB = .018 | PAB = .343 | PAB = .292 | PAB = .292 | PAB = .265 | PAB = .338 | PAB = .033 |
| PAC = .215 | PAC = .403 | PAC = .967 | PAC = .336 | PAC = .281 | PAC = .329 | PAC = .150 | |
| PAD = .186 | PAD = .287 | PAD = .320 | PAD = .087 | PAD = .317 | — | PAD = .176 | |
| PBC = .249 | PBC = .915 | PBC = .306 | PBC = .074 | PBC = .979 | PBC = .979 | PBC = .306 | |
| PBD = .306 | PBD = .881 | — | PBD = .015 | PBD = .935 | PBD = .342 | PBD = .288 | |
| PCD = .916 | PCD = .802 | PBD = .334 | PCD = .395 | PCD = .956 | PCD = .334 | PCD = .956 |
Group A: bismuth potassium citrate capsule group; Group B: bismuth pectin capsule group; Group C: pectin bismuth granule low-dose group; Group D: pectin bismuth granule high-dose group.
As shown in Table 4, there were no significant differences in the symptom improvement rate among the 4 groups at the end of treatment and at 4 weeks after the treatment (P > .05), and there was no significant difference in patient compliance (P > .05).
Table 4.
Compliance and symptom improvement at the end of treatment and 4 weeks after treatment (n/N, %).
| Groups | Symptom improvement rate at the end of treatment | Symptom improvement rate 4 wks after treatment | Compliance |
| A | 43/51 (84.3%) | 49/51 (96.1%) | 51/60 (85.0%) |
| B | 48/56 (85.7%) | 53/56 (94.6%) | 56/60 (93.3%) |
| C | 48/54 (88.9%) | 53/54 (98.1%) | 54/60 (90.0%) |
| D | 41/50 (82.0%) | 48/50 (96.0%) | 50/60 (83.3%) |
| P value | PAB = .839 PAC = .491 PAD = .756 PBC = .617 PBD = .603 PCD = .318 | PAB = .725 PAC = .525 PAD = .984 PBC = .326 PBD = .742 PCD = .513 | PAB = .142 PAC = .408 PAD = .803 PBC = .509 PBD = .088 PCD = .283 |
Group A: bismuth potassium citrate capsule group; Group B: bismuth pectin capsule group; Group C: pectin bismuth granule low-dose group; Group D: pectin bismuth granule high-dose group.
4. Discussion
With antibiotic resistance increasing worldwide, the eradication rate of H. pylori is rapidly declining. In particular, the drug resistance rates of clarithromycin, metronidazole, and levofloxacin are increasing, which leads to the failure of standard triple therapy to achieve an acceptable eradication rate of 80% in most studies.[3,10,18] Because bismuth agents have good antibiotic sensitization effects, both domestic and foreign consensus recommend bismuth quadruple therapy as the first-line treatment for H. pylori eradication.[3,18] A large number of clinical studies have also confirmed that the eradication rate of H. pylori in bismuth-containing quadruple therapy around the world has reached 85% to 95%.[24–28]
Bismuth agents commonly used in clinical practice include bismuth potassium citrate, colloidal bismuth pectin, colloidal bismuth tartrate, bismuth subsalicylate, and so on.[29] Due to the potential neurotoxicity of bismuth agents, blood bismuth levels are usually recommended not to exceed 100 ng/mL.[30] Colloidal bismuth pectin is a compound formed after the replacement of inorganic and small molecular organic acid radicals by high molecular organic acid radicals. It can form a stable colloidal dispersion system in water and acidic gastric juice, and it can be deposited on a wound surface to form a film which strengthens its protective effect on cells.[31] Due to its larger molecular weight, the largest plasma concentration of bismuth pectin is usually no more than 40 ng/mL. Therefore, pectic bismuth is thought to have a higher safety margin than bismuth potassium citrate.[19,30] The main component of the pectin bismuth capsules and pectin bismuth particles used in our study is colloidal pectin bismuth. Pectin bismuth particles have a better tissue preparation and a stronger affinity for damaged epithelial cells in the gastrointestinal mucosa than pectin bismuth capsules. In addition, the particle form increases the contact area of the colloidal pectin bismuth with the inflammatory and bacterial infection sites and promotes the penetration of the wrapped drugs into H. pylori cells.[32] However, the efficacy, safety, and optimal dosage of bismuth pectin for H. pylori eradication have not been determined.
Previous studies also found that compared with the standard triple regimen, the combined regimen which includes bismuth pectin can significantly improve the success of H. pylori eradication.[33] Other studies have reported that compared with potassium bismuth citrate, the eradication success rate of the bismuth pectin capsule quadruple regimen is comparable.[34] The results of our experiment confirmed that the eradication rates of 200 mg bismuth pectin capsules, 150 mg bismuth pectin particles, and 300 mg bismuth pectin particles were not significantly different from the eradication rate of 220 mg bismuth potassium citrate capsules. It is suggested that both bismuth pectin capsules and bismuth pectin granules can be used in bismuth-containing quadruple therapy for H. pylori eradication, and 150 mg bismuth pectin granules can achieve a satisfactory eradication effect. This study also showed that the overall incidence of adverse events was high in the 4 bismuth-containing regimens. Among them, bitterness of the medication was the most common adverse reaction, which was speculated to be related to the bismuth agent. However, most of the bitterness of the treatment experienced by patients disappeared 1 day to 2 days after drug withdrawal, which was similar to previous reports.[33] In addition, the incidence of diarrhea in the 300 mg bismuth pectin granule group was 10.0%, which was significantly higher than that in the bismuth pectin capsule group (2.0%). Previous studies have also found that large doses of bismuth pectin have a higher incidence of diarrhea.[35,36] We speculate that because bismuth pectin is less absorbed into the blood, a large dose of bismuth pectin stays in the intestinal tract and stimulates the intestinal and induces diarrhea. For the occurrence of abdominal pain and loss of appetite, we have considered that these may be associated with bismuth therapy, but studies have demonstrated that no toxic effects occur when blood bismuth concentrations are below 100 ng/mL and that only 2 weeks of H. pylori eradication treatment causes a lower incidence of serious adverse events.[37]
In this study, more than 80% of patients achieved satisfactory symptom improvement at the end of 2 weeks of eradication therapy. The symptom improvement rate reached more than 90% 4 weeks after treatment. Although there was no statistically significant difference in the rate of symptom improvement among the 4 regimens, the rate of symptom improvement in the 150 mg bismuth pectin granule group was relatively high. In addition, the patient compliances of the 4 groups were all more than 80%, and the compliances of the 200 mg bismuth pectin capsule group and the 150 mg bismuth pectin granule group were more than 90%. These results indicated that both bismuth pectin capsules and bismuth pectin granules could achieve eradication success rates comparable to that of potassium bismuth citrate.
There are some limitations in our study. First, although this study is a multicenter study, the sample size of our study is small, which may lead to inevitable bias. Therefore, the results of this study need to be verified by further clinical studies with larger sample sizes. In addition, an H. pylori antibiotic sensitivity test was not conducted in this study. It was impossible to compare the clinical efficacy of individualized therapy based on past antibiotic use with the individualized therapy based on antibiotic sensitivity tests.
In summary, the present study demonstrated that pectic bismuth granules, pectic bismuth particles, and bismuth potassium citrate had comparable efficacy and safety in bismuth-containing quadruple therapy for H. pylori eradication. Both pectic bismuth granules and pectic bismuth particles could be recommended as alternatives to bismuth potassium citrate in bismuth-containing quadruple therapy.
Acknowledgments
We thank the study participants and the clinical teams. We also thank the Good Clinical Practice center of each center.
Author contributions
Conceptualization: Jian Zhang, Yuan Liu, Yongquan Shi.
Investigation: Yaping Cao, Lifeng Zhang, Lu Wang, Jie Wang, Ying Qi, Huanhuan Lv, Juan Liu, Lijuan Huo, Xiaoguo Wei, Yongquan Shi.
Methodology: Yaping Cao, Jian Zhang, Yuan Liu, Lu Wang, Yongquan Shi.
Project administration: Yongquan Shi.
Resources: Lijuan Huo.
Writing – original draft: Yaping Cao.
Writing – review & editing: Jian Zhang, Lifeng Zhang, Ying Qi, Huanhuan Lv, Juan Liu, Yongquan Shi.
Footnotes
Abbreviations: BMI = body mass index, H. pylori = Helicobacter pylori, ITT = intention-to-treat, PP = per-protocol, PPIs = proton-pump inhibitors, SD = standard deviation.
How to cite this article: Cao Y, Zhang J, Liu Y, Zhang L, Wang L, Wang J, Qi Y, Lv H, Liu J, Huo L, Wei X, Shi Y. The efficacy and safety of different bismuth agents in Helicobacter pylori first-line eradication: a multicenter, randomized, controlled clinical trial. Medicine. 2021;100:50(e27923).
YC, JZ, and YL contributed equally to this work.
The study was financially supported by the Shaanxi Province Innovation Ability Team Support Plan (2018TD-003).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology 2009;136:1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014;59:1698–709. [DOI] [PubMed] [Google Scholar]
- [3].Malfertheiner P, Megraud F, O’morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:06–30. [DOI] [PubMed] [Google Scholar]
- [4].De Brito BB, Da Silva FAF, Soares AS, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol 2019;25:5578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol 2004;99:1833–55. [DOI] [PubMed] [Google Scholar]
- [6].Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150:1113–24.e5. [DOI] [PubMed] [Google Scholar]
- [7].Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
- [8].Fang JY, Du YQ, Liu WZ, et al. Chinese consensus on chronic gastritis (2017, Shanghai). J Dig Dis 2018;19:182–203. [DOI] [PubMed] [Google Scholar]
- [9].Mathew J. H pylori eradication therapy reduces gastric cancer in patients with or without gastric neoplasia. Ann Intern Med 2020;173:JC32. [DOI] [PubMed] [Google Scholar]
- [10].Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Morain NR, Dore MP, O’Connor AJP, Gisbert JP, O’Morain CA. Treatment of Helicobacter pylori infection in 2018. Helicobacter 2018;23: (Suppl 1): e12519. [DOI] [PubMed] [Google Scholar]
- [12].O’Connor A, Lamarque D, Gisbert JP, O’Morain C. Treatment of Helicobacter pylori infection 2017. Helicobacter 2017;22: (Suppl 1): e12410. [DOI] [PubMed] [Google Scholar]
- [13].Gao W, Cheng H, Hu F, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 2010;15:460–6. [DOI] [PubMed] [Google Scholar]
- [14].Camargo MC, Garcia A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol 2014;109:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rasheed F, Campbell BJ, Alfizah H, et al. Analysis of clinical isolates of Helicobacter pylori in Pakistan reveals high degrees of pathogenicity and high frequencies of antibiotic resistance. Helicobacter 2014;19:387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fischbach W, Malfertheiner P. Helicobacter pylori infection. Dtsch Arztebl Int 2018;115:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018;155:1372–82.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018;23:e12475. [DOI] [PubMed] [Google Scholar]
- [19].Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. [DOI] [PubMed] [Google Scholar]
- [20].Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther 2018;16:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu L, Luo L, Long X, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: a randomized trial. Helicobacter 2019;24:e12596. [DOI] [PubMed] [Google Scholar]
- [22].Alkim H, Koksal A, Boga S, Sen I, Alkim C. Role of bismuth in the eradication of Helicobacter pylori. Am J Ther 2017;24:e751–7. [DOI] [PubMed] [Google Scholar]
- [23].Lambert JR, Midolo P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 1997;11: (Suppl 1): 27–33. [DOI] [PubMed] [Google Scholar]
- [24].Ko SW, Kim YJ, Chung WC, Lee SJ. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter 2019;24:e12565. [DOI] [PubMed] [Google Scholar]
- [25].Kim SE, Roh JH, Park MI, et al. Effect of 7-day bismuth quadruple therapy versus 14-day moxifloxacin triple therapy for second-line Helicobacter pylori eradication therapy. Korean J Gastroenterol 2019;73:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zagari RM, Romiti A, Ierardi E, et al. The “three-in-one” formulation of bismuth quadruple therapy forHelicobacter pylorieradication with or without probiotics supplementation: efficacy and safety in daily clinical practice. Helicobacter 2018;23:e12502. [DOI] [PubMed] [Google Scholar]
- [27].Fiorini G, Zullo A, Saracino IM, Gatta L, Pavoni M, Vaira D. Pylera and sequential therapy for first-line Helicobacter pylori eradication. Eur J Gastroenterol Hepatol 2018;30:621–5. [DOI] [PubMed] [Google Scholar]
- [28].Chen L, He J, Wang L, et al. Efficacies of different proton pump inhibitor-based 14-day bismuth–furazolidone quadruple regimens for the initial eradication of Helicobacter pylori in the southeast coastal region of China: an open-label, randomized clinical trial. Clin Exp Med 2018;18:569–76. [DOI] [PubMed] [Google Scholar]
- [29].Wang JL, Liu XY, Nie Y, et al. Thinking about the advantages and disadvantages of bismuth agent in clinical application. Chin J Internal Med 2012;51:932–4. [Google Scholar]
- [30].Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics 2012;4:239. [DOI] [PubMed] [Google Scholar]
- [31].Yang XJ, Chen SB. Adverse reactions of colloidal acid. New Drugs Clin Remedies 1992;03:178–80. [Google Scholar]
- [32].Qi CF. Clinical efficacy and pharmaceutical toxicology of colloid pectin bismuth capsule. Heilongjiang Traditi Chin Med 2008;11:47. [Google Scholar]
- [33].Mcnicholl AG, Bordin DS, Lucendo A, et al. Combination of bismuth and standard triple therapy eradicates Helicobacter pylori infection in more than 90% of patients. Clin Gastroenterol Hepatol 2020;18:89–98. [DOI] [PubMed] [Google Scholar]
- [34].Lu XD. Effect of colloidal bismuth pectin 200 mg bid 10-day quadruple therapy on helicobacter pylori eradication. World Latest Med Inf 2019;19:212–8. [Google Scholar]
- [35].Zhang QY, Li Y. Efficacy of three bismuth-containing quadruple therapy for eradication of Helicobacter pylori. Chin J Gastroenterol Hepatol 2019;28:886–9. [Google Scholar]
- [36].Fu LH. Comparison of efficacy of triple therapy with bismuth-containing quadruple therapy and sequential therapy in the treatment of Helicobacter pylori infection. Chin J Clin Rational Drug Use 2020;13:57–8. [Google Scholar]
- [37].Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol 2008;14:7361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]