PURPOSE:
Availability of targeted oral anticancer agents (OAAs) has transformed care for patients with metastatic renal cell carcinoma (mRCC). Our objective was to identify patterns and predictors of OAA use within 12 months after mRCC was detected to understand real-world adoption of OAAs.
METHODS:
We used a novel, North Carolina cancer registry–linked multipayer claims data resource to examine patterns of use of five oral therapies among patients with mRCC diagnosed in 2006-2015, with claims through 2016. Patients were required to have 12 months of continuous enrollment before metastatic index date. Log-Poisson models estimated unadjusted and adjusted risk ratios (RRs) for associations between patient characteristics and OAA use. In sensitivity analyses, we used a competing risk framework to estimate adjusted risk differences in OAA use.
RESULTS:
Our population-based study of 713 patients demonstrated low (37%) OAA use during the first year after metastatic index date among both publicly and privately insured patients, with shifting patterns of use consistent with regulatory approvals over time. Compared with patients age 18-49 years, patients age 70-74 years were half likely to use OAAs (95% confidence limit [CL], 0.34 to 0.78) and patients age 80+ years were 71% less likely to use OAAs (95% CL, 0.17 to 0.50). Patients with two comorbidities (RR, 0.73; 95% CL, 0.55 to 0.98) and those with 3+ comorbidities (RR, 0.68; 95% CL, 0.50 to 0.91) were less likely to receive OAA than those without comorbidities. Patients with higher frailty also had lower OAA utilization (RR, 0.67; 95% CL, 0.52 to 0.85).
CONCLUSION:
These findings suggest a need to better understand the system-level and provider-level drivers of OAA underuse, as well as OAA adherence and associated survival.
INTRODUCTION
Renal cell carcinoma (RCC) is the ninth most common malignancy diagnosed in the United States,1 with an estimated 73,750 new cases diagnosed and 14,830 deaths in 2020.2 RCC outcomes in the United States have improved over the past 40 years, in large part due to increased imaging and incidental detection and targeted and immune-activating therapeutics that extend survival. The 5-year survival increased from approximately 50% in 1977 to approximately 75% in 2015, with survival strongly associated with stage at diagnosis.1,3,4 Those diagnosed with stage IV disease—representing approximately one third of all incident RCC cases—experience a median 5-year survival of < 15%. In addition to those diagnosed with incident metastatic disease (ie, synchronous metastases), another 20%-50% of all patients develop metastatic disease after having been originally diagnosed with locoregional disease (ie, metachronous metastases).1 Like many other cancers in the United States, age and Black race are associated with poorer survival from RCC, the latter of which is believed to be caused by differences in socioeconomic status, access to care, structural racism, and poorer health status, rather than biologic factors.1,5
Because deaths from RCC and from all causes are strongly associated with measures of poorer health, such as smoking, obesity, and hypertension,1 it is important to understand the extent to which rapidly emerging new therapies are used among patients with RCC and complex health care needs. It is also important to understand how these therapies diffuse across, and can improve outcomes within, real-world populations. Real-world populations include age groups and racial groups not well-represented in clinical trials, such as people older than age 65 years and Black patients, as well as those who are medically complex or are insured by different payers whose benefit plan designs may promote or inhibit clinical trial participation and real-world use of expensive therapies.6-8 Multipayer claims data linked to cancer registries can enable real-world studies by providing both health care service utilization information and cancer-specific clinical information.9
In particular, metastatic RCC (mRCC) represents an opportunity to investigate these questions fully about patterns and predictors of oral anticancer agent (OAA) use in real-world populations, given that the introduction of new, targeted OAAs to the mRCC landscape has transformed the way that care is delivered among those with advanced disease.4,10,11 The availability of these new, targeted OAAs has the potential to substantially alter the disease course and improve survival outcomes—but only if those therapies are accessible to and being used by patients with mRCC, particularly among younger, racially and ethnically diverse, and medically complex patients. Therefore, our objective was to identify patterns and predictors of use of an approved OAA (sorafenib, sunitinib, pazopanib, everolimus, and axitinib) within the 12 months after mRCC detection in a diverse cohort.
METHODS
Study Population
We conducted a retrospective cohort study of patients with a diagnosis of mRCC between 2006 and 2015. The data were obtained from the University of North Carolina Lineberger Comprehensive Cancer Center's Cancer Information Population Health Resource (CIPHR), which links state cancer registry data to private health insurance plans, Medicare and Medicaid fee-for-service claims data in North Carolina.9 CIPHR includes data on patient demographics and area-level contextual characteristics, tumor characteristics, prescription drugs and emergency, and inpatient and outpatient services.
To summarize our cohort eligibility criteria, we selected patients who were diagnosed with American Joint Committee on Cancer 6th edition clinical stage I-IV RCC using International Classification of Diseases (ICD) for Oncology, Third edition codes C641, C642, and C649. Stage IV patients were included if they had a claims code for RCC within 2 months of the diagnosis date recorded in the registry. Stage I-III patients were included if they had claims for a secondary malignant neoplasm (using ICD-9-CM codes 198.XX and ICD-10-CM codes C641, C642, and C649) on two separate days at any time after their initial RCC diagnosis in the cancer registry. The study's index mRCC diagnosis date was defined as the date of diagnosis for stage IV patients and the date of the first of the two separate claims for stage I-III patients.
Because our intent was to examine OAA use after the metastatic index date, patients were excluded if their RCC diagnosis was identified from death certificate or autopsy, if tumor histology was classified as a sarcoma, medulloblastoma, leiomyosarcoma, or malignant rhabdoid tumor, or if they had a diagnosis of additional primary malignancies at sites other than the kidney during the 12 months before their metastatic index date. Patients were further excluded if they were younger than age 18 years at the time of their metastatic index date or if they did not have 12 months of continuous insurance enrollment before the index date. Moreover, they were required to have 12 months of continuous enrollment, including prescription drug coverage, following their metastatic index date, with the exception of death; patients who died within the 12 month postindex period contributed person-time up to the date of death. Observations with missing or incomplete residential data at the time of RCC diagnosis were excluded. This study was approved by the Duke University Health System Institutional Review Board (#Pro00101962).
Dependent Variable
The study's primary outcome of OAA initiation was an OAA prescription drug fill within the 12 months following the metastatic index date from patient prescription drug files, pharmacy claims, and Medicare Part D claims, identified by searching through brand names, generic names, and National Drug Codes for sorafenib, sunitinib, pazopanib, everolimus, axitinib, cabozantinib, and lenvatinib, noting the US Food and Drug Administration (FDA) approval date for this indication for each OAA.
Independent Variables
Patient-level characteristics in our analyses included sex (male and female), age (18-49, 50-64, 65-69, 70-74, 75-79, and 80+ years), race or ethnicity (non-Hispanic White, non-Hispanic Black, and Others), patient location (rural and nonrural—defined from the registry on the basis of the revised 2010 Rural Urban Commuting Area code categorizations for census tracts12), insurance provider (any Medicaid, private, and Medicare only), and clinical cancer characteristics, all assessed at or near the metastatic index date. In addition, the number and types of comorbid conditions and frailty were examined in the 12 months before metastatic index date, given that comorbidity burden and medical frailty are conceptually distinct medical complexity constructs. We defined comorbidities using claims on the basis of conditions included in the Charlson Comorbidity Index, identified during the 12 months before the metastatic index date.13 Frailty was measured from claims as a predicted probability of frailty using the Faurot algorithm for predicting dependency in daily living activities.14 Additional clinical covariates included histology (clear cell and others), stage at initial diagnosis, year of metastatic diagnosis, and radical or partial nephrectomy in the claims during the 12 months before metastatic diagnosis (yes and no). Finally, we included two census tract-level socioeconomic variables for area-level education and poverty: percentage with bachelor's degree and percentage living below the poverty level, using 5-year estimates from the 2008-2012 American Community Survey.
Statistical Analysis
Categorical variable distributions were compared by OAA utilization using χ2 and Fisher's exact tests. Continuous variables were presented using medians and interquartile ranges and were compared using the Mann-Whitney U-test. We estimated the proportion of patients and yearly trends in OAA initiation for (1) any OAA and (2) across individual OAAs (eg, sunitinib, pazopanib, sorafenib, and everolimus/axitinib) using Cochrane-Armitage trend tests. Everolimus and axitinib were combined because of small sample sizes.
We used log-Poisson models15 to estimate unadjusted and adjusted risk ratios (RRs) and 95% confidence limits (CLs) for associations between patient characteristics and utilization of OAAs within 12 months following metastatic diagnosis. Multicollinearity was assessed using a variance inflation factor threshold of 5. As a sensitivity analysis, we conducted a time-to-event analysis using Fine and Gray's model where death was considered a competing event to the time to initiate OAAs. We estimated adjusted risk differences and corresponding 95% CLs at 12 months after the metastatic index date from the cumulative incidence function (data not shown). All analyses were conducted using SAS v9.4 (Cary, NC).
RESULTS
Analytic Sample
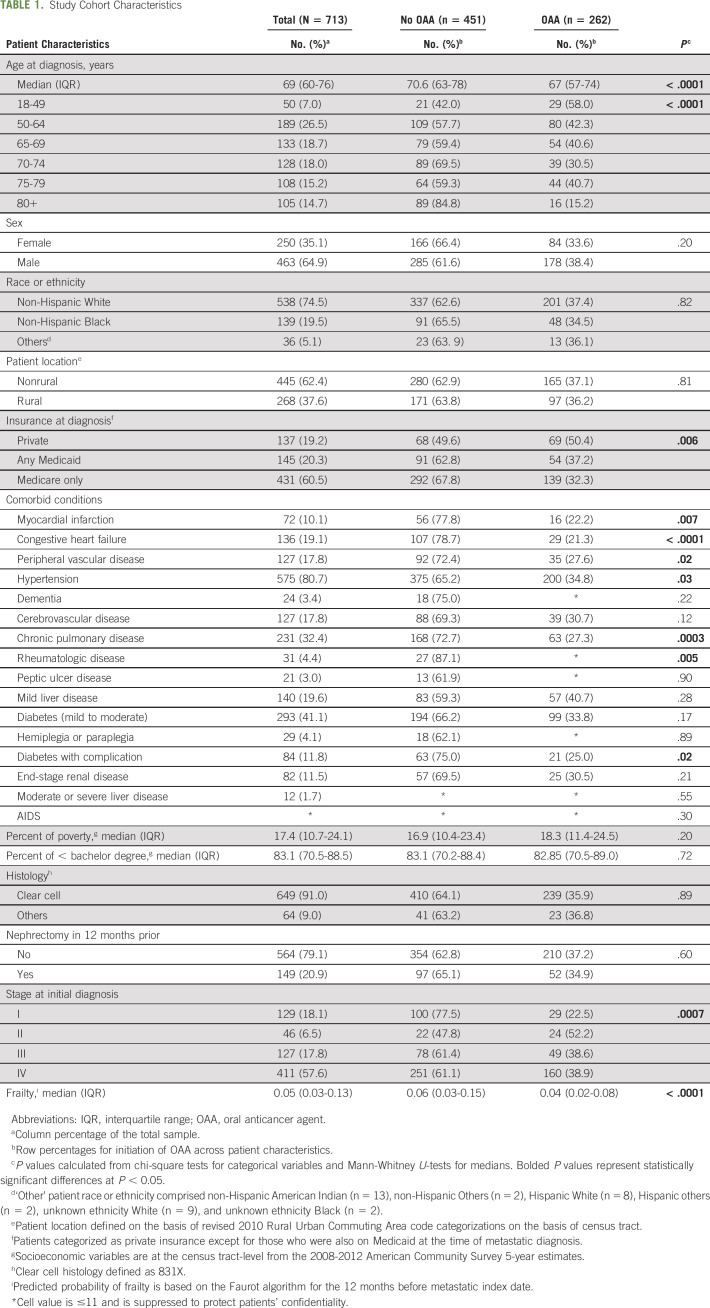
Our analytic sample included 713 patients (Table 1). The median age at metastatic diagnosis was 69 years. The sample was predominantly male (65%), non-Hispanic White (75%), nonrural (62%), and Medicare-insured at diagnosis (60%). The most common comorbid conditions present were hypertension (81%) and diabetes (41%). More than half (58%) of the sample was stage IV at diagnosis, and a majority did not have a nephrectomy in the 12 months before metastasis (79%).
TABLE 1.
Study Cohort Characteristics
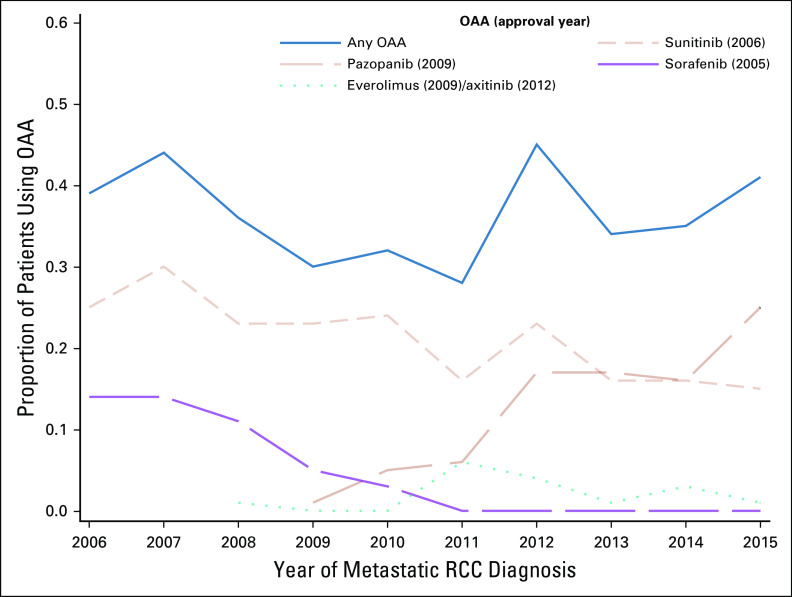
During the study period, 37% of patients used OAAs. Patient utilization of any OAA varied across years, with no consistent trend over time (Cochran-Armitage trend test P value = .79), despite an increase in FDA-approved medications during the study period (Fig 1). Utilization ranged from 28% in 2011 to 45% in 2012. Utilization of sunitinib and sorafenib gradually declined over time to approximately 15% and < 5%, respectively, by 2015. By contrast, since its FDA approval, pazopanib utilization increased from 1% in 2009 to 25% in 2015.
FIG 1.
Patterns over time of OAA initiation within 12 months of year of index metastatic RCC diagnosis, with FDA approval dates noted. FDA, US Food and Drug Administration; OAA, oral anticancer agent; RCC, renal cell carcinoma.
Associations With OAA Utilization
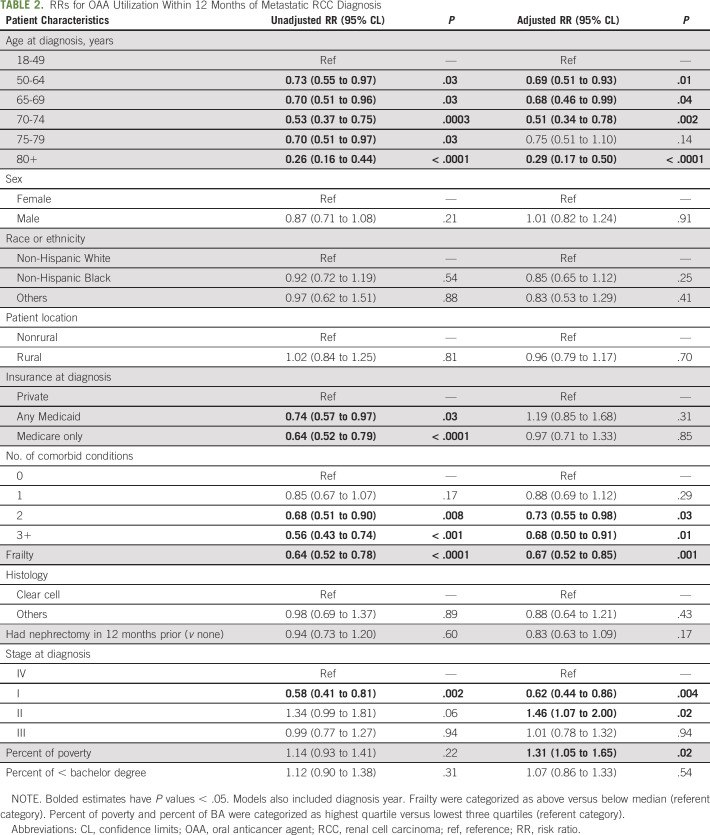
In adjusted models (Table 2), older age, higher number of comorbid conditions, frailty, and being stage I at initial diagnosis (v stage IV) were associated with decreased OAA utilization (Table 2). Specifically, compared with the youngest age category (age 18-49 years), patients age 70-74 years were half likely to use OAAs (95% CL, 0.34 to 0.78) and patients age 80 years and above were 71% less likely to use OAAs (95% CL, 0.17 to 0.50). Patients with two comorbidities (RR, 0.73; 95% CL, 0.55 to 0.98) and those with three or more comorbidities (RR, 0.68; 95% CL, 0.50 to 0.91) were less likely to receive OAA than those who had no comorbidities. Patients with higher-than-median probability of frailty also had lower OAA utilization (RR, 0.67; 95% CL, 0.52 to 0.85). Patients initially diagnosed as stage I were less likely than those diagnosed at stage IV to use OAAs (RR, 0.62; 95% CL, 0.44 to 0.86). Other patient-level characteristics, such as sex, race or ethnicity, rurality, and insurance type, were not significant predictors of OAA use.
TABLE 2.
RRs for OAA Utilization Within 12 Months of Metastatic RCC Diagnosis
Figure 2 presents our sensitivity analysis as differences, by age, frailty, and comorbidity, in cumulative incidence functions using a competing risk time-to-event framework. We observed a trend of increasing differences in cumulative incidence functions across older age categories, up to approximately 90 days postmetastatic detection, after which the difference stabilizes (Figs 2A-2C). Adjusted models indicate that the risk difference in OAA initiation was 14-52 percentage points lower for age groups ranging from 50-64, 64-69, 70-74, 75-79, and age 80+ years, compared with those of age 18-49 years (data not shown). Similarly, the difference in the initiation of OAA use between more frail and less frail patients increased over time, up to 90 days after metastatic diagnosis, after which the difference stabilizes (Fig 2D), with OAA initiation 19 percentage points lower among more frail patients (data not shown). Finally, with regard to comorbidities, within the first 50 days of metastatic diagnosis, patients with three or more comorbidities were more likely to use OAAs than patients with no comorbidities, but after 50 days, the trend reverses (Fig 2E). At 12 months, however, the initiation of OAAs for people with three or more comorbidities was 14 percentage points lower, relative to those with no comorbidities (data not shown).
FIG 2.
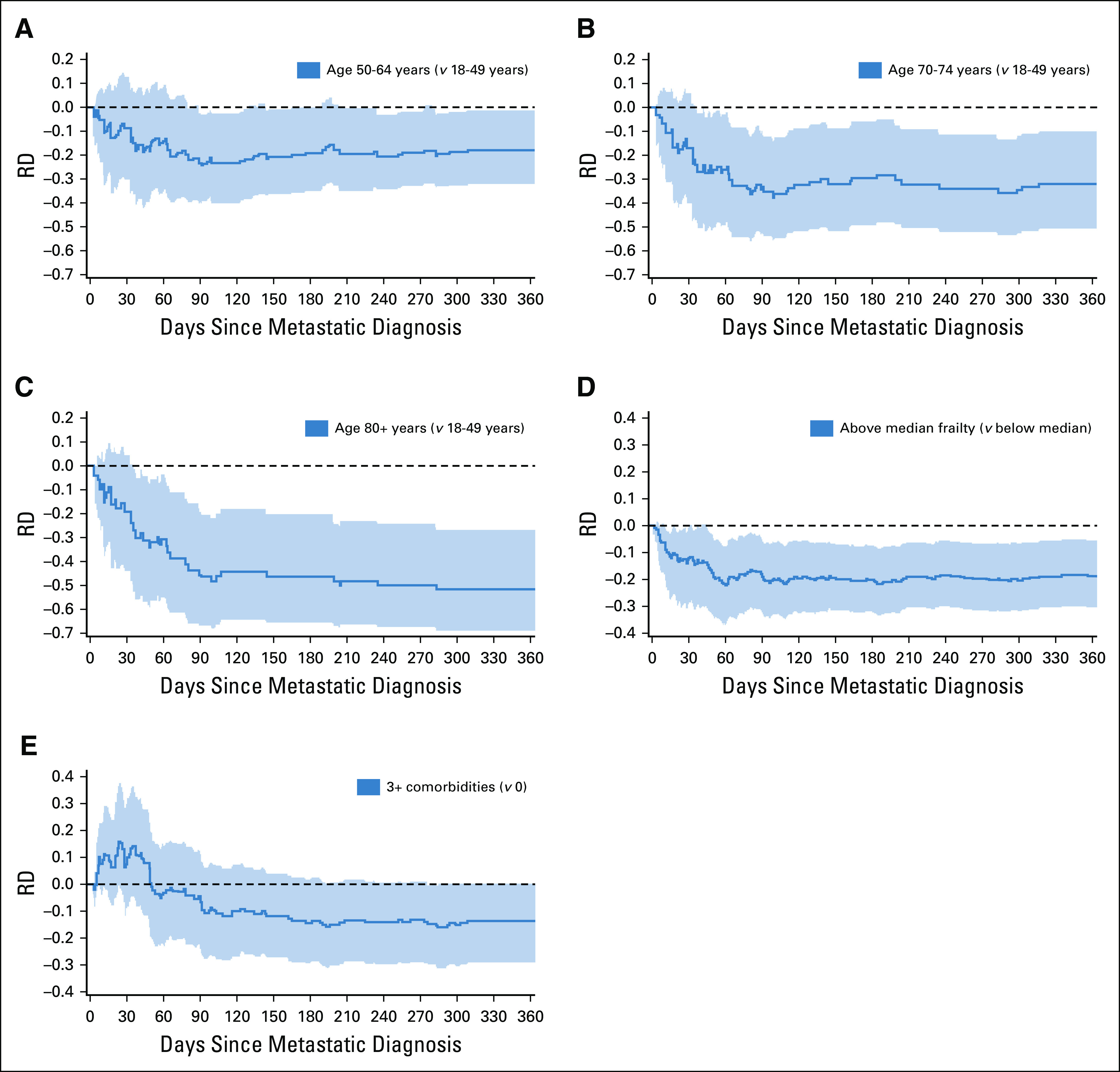
RDs in OAA utilization in 12 months following metastatic RCC for age (A-C), frailty (D), and comorbid conditions (E). Curves below the dotted reference line indicate that the comparison group has lower OAA utilization. Black dashed lines represent the RDs, and shaded areas represent 95% CIs (on the basis of 2,000 bootstraps). OAA, oral anticancer agent; RCC, renal cell carcinoma; RD, risk difference.
DISCUSSION
Our data, drawn from a novel statewide, multipayer cohort of patients with mRCC over a ten-year period, illuminate real-world patterns and predictors of use of new, targeted OAAs in an era of rapid expansion of FDA approvals for and proliferation of these drugs. Given that overall RCC mortality has decreased significantly as a result of the introduction of these drugs,4,11 but that mortality disparities remain, it is critical to understand whether novel OAAs available to treat mRCC are, in fact, being equally received by diverse populations. Our findings illustrate three key takeaways: (1) Initiation of novel OAAs remained low over this study period—roughly one-in-three patients with mRCC received any OAA, with no real increase observed over time. (2) Patient-level characteristics, including older age, higher comorbidity burden, higher frailty, and stage I, at initial diagnosis were each inversely associated with OAA use, which may represent more conservative and clinically appropriate judgment in treating patients with either increased competing nononcologic risks with skewed OAA risk-benefit tradeoffs or patient preferences or provider biases against using targeted therapies with older patients. (3) Patient-level characteristics that may reflect disparities in treatment access, including sex, race or ethnicity, rurality, insurance type, and area-level socioeconomic status, were not associated with OAA use in adjusted analyses; although this is reassuring, it must be confirmed in other studies, given that these groups historically have faced worse RCC outcomes.
Our finding that only 37% of patients with mRCC used OAAs during this time period is similar to a national study of Medicare enrollees, which found that 32% of patients with mRCC received OAA during the same time period. In both studies, sunitinib and pazopanib accounted for the majority of OAA use.16 Given that our cohort included younger patients insured by payers other than Medicare, we would have expected to see a slight increase in the overall proportion of patients using OAAs in our multigenerational, multipayer cohort. Other studies from commercial claims data indicate similarly shifting patterns of use of various OAAs in mRCC, with sunitinib and pazopanib among the most commonly used agents and patient age and comorbidities being major drivers of treatment choice.17 Importantly, the burden of comorbidity in our population was high, and the observed trends in OAA use by comorbidity count may reflect clinically appropriate practice consistent with clinical contraindications to OAA treatment, severity of comorbid illness, and patients' ability to access and tolerate therapy.
Although omission of OAAs may be clinically appropriate among the oldest, sickest, and most frail patients, given that clinical trials and corresponding guidelines less often address these groups' needs,18,19 foregoing OAAs in two of three patients with mRCC, including two of five 18-49 year old patients, may suggest a missed opportunity to extend survival. Because some evidence suggests that combinations of OAAs may be beneficial for mRCC,20 it is critical to improve monotherapy access and ensure adherence among healthy patients, to realize the potential full benefits of combination approaches. Fortunately, evidence from commercial and Medicare Advantage members suggests that adherence to oral therapies among patients with mRCC is high,21,22 but evidence is needed from less-advantaged patients as well.
These results point to several future research and practice opportunities: (1) Real-world health care delivery in the context of mRCC evidence is difficult because mRCC is diagnosed in much older and sicker populations in the real world than what is represented in clinical trials. Given this disconnect, providers may be hesitant to prescribe OAAs for older, frailer, and sicker patients, which likely contributes to overall underuse of OAAs. Nevertheless, providers should continue to evaluate on a per-patient basis whether OAAs may have potential benefits, particularly among those groups that may not have been well-represented in trials. (2) Additional research is needed to understand how physicians and patients make decisions about OAA use when patients are older, have multiple comorbidities, and/or are frail. Better understanding of physician and patient decision making around OAA use in mRCC requires different data and methods than registry-linked claims data provide. Opportunities exist to gather this information through patient-provider interaction observations and collecting provider data on decision making using real-world vignettes. (3) Future research should seek to clarify on a population level with greater precision in which populations OAAs have clear benefits (including among older people with comorbidity) and to develop interventions to promote and support OAA initiation and adherence among those populations.
Although our study did not uncover racial or ethnic, rural, or insurance-related disparities in OAA access, significantly lower OAA use among older patients may reflect social and structural vulnerabilities that warrant attention. Lack of social support, inability to access care, and inability to pay out-of-pocket expenses associated with OAAs, including both medical and nonmedical expenses, may be significant barriers to using OAAs among older patients. The economic burden of mRCC is significant,23 increasingly so in the OAA era, and other studies have previously demonstrated that cost is a major barrier to accessing RCC OAAs.11 For example, one study comparing Medicare beneficiaries with very low cost sharing for their initial oral prescription (< $6.60 US dollars) via low-income subsidy with those without a low-income subsidy (with initial oral prescription costs > $2,800 US dollars) found significantly delayed or reduced use of targeted OAAs with higher out-of-pocket cost.11
As with all retrospective studies of secondary registry-linked claims databases, our study has several limitations to consider. First, our CIPHR data represent patients with mRCC from multiple payers; however, these patients were diagnosed and received care in North Carolina, a large, socioeconomically, and racially diverse state, which may not be reflective of care patterns elsewhere. Second, our claims-derived definition of metastasis is limited to registry-reported data and observed clinical encounters for which a claim was processed, both of which may be incomplete. Third, our analyses are limited to those patients who had continuous insurance enrollment during the study period, which might have excluded individuals who lost insurance during this period, never had insurance, had a different insurance provider, or did not have prescription drug coverage. Furthermore, because our study period ended in 2016, more recent OAA uptake may be different. Nevertheless, our study represents one of the largest and most comprehensive real-world evaluations of OAA use in the mRCC setting, given its multipayer structure and 10-year analytic window, presenting a unique opportunity to examine trends in OAA use among these patients. Importantly, this study sets the historical OAA use rates as the first-line therapeutic landscape in mRCC changed in 2017, and future studies on OAA use in combination with immunotherapeutics will be enlightening to evaluate fluctuating delivery of effective first-line therapies in mRCC.
In conclusion, our population-based study demonstrated low (37%) OAA use among publicly and privately insured patients with mRCC, with substantial variation in OAA initiation, by age, comorbidities, and frailty, but not sex, race, rurality, type of insurance, or area socioeconomic status. These data underscore the importance of clarifying, with greater precision, the benefits of OAAs in real-world populations and distinguishing clinically appropriate from potentially poor-quality care. Additional studies should also seek to understand in more depth the system-level and provider-level drivers of OAA initiation and adherence and to develop interventions to support OAA use where appropriate.
Stephanie B. Wheeler
Research Funding: Pfizer
Travel, Accommodations, Expenses: Pfizer
Christopher D. Baggett
Employment: Rho Inc
Lauren E. Wilson
Research Funding: AstraZeneca
Melissa A. Greiner
Research Funding: Novartis, Abbott Laboratories, Philips Healthcare
Deborah R. Kaye
Employment: Blue Cross Blue Shield North Carolina
Research Funding: Blue Cross Blue Shield of Michigan
Travel, Accommodations, Expenses: MedReviews
Tian Zhang
Leadership: Capio Biosciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: Exelixis, Genentech/Roche, MJH Life Sciences, Pacific Genuity
Consulting or Advisory Role: Janssen, Genentech/Roche, Sanofi, Exelixis, AstraZeneca, Pfizer, Bristol Myers Squibb, Foundation Medicine, Pharmacyclics, Amgen, Merck, Seattle Genetics, Dendreon, Calithera Biosciences
Speakers' Bureau: Exelixis, Genentech/Roche, Genomic Health, Sanofi/Aventis
Research Funding: Janssen, Acerta Pharma, Pfizer, Merrimack, Stem CentRx, Novartis, OmniSeq, Personal Genome Diagnostics, Regeneron, Merck, Mirati Therapeutics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology, Prochelators as Targeted Prodrugs for Prostate Cancer
Travel, Accommodations, Expenses: Acerta Pharma, Genomic Health, AstraZeneca
Daniel George
Leadership: Capio Biosciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Vizuri, Constellation Pharmaceuticals, Physicians' Education Resource, Propella Therapeutics
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis, Janssen Oncology, Novartis, Pfizer, Astellas Pharma, Bristol Myers Squibb, Acerta Pharma, Bayer, Dendreon, Innocrin Pharma, Calithera Biosciences, Sanofi/Aventis
Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroToday
Charles D. Scales Jr
Research Funding: Pfizer, Exelixis, Merck, Bristol Myers Squibb-Ono Pharmaceutical
Jessica E. Pritchard
Research Funding: St Jude Medical, Pfizer, Boston Scientific
Michaela A. Dinan
Research Funding: AstraZeneca
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Cancer Institute (NCI R01-CA226842-02; PI: Dinan). The database infrastructure used for this project was supported through the University of North Carolina Clinical and Translational Science Award (UL1TR001111) and the UNC Lineberger Comprehensive Cancer Center, University Cancer Research Fund via the State of North Carolina.
AUTHOR CONTRIBUTIONS
Conception and design: Stephanie B. Wheeler, Lisa P. Spees, Christopher D. Baggett, Lauren E. Wilson, Melissa A. Greiner, Deborah R. Kaye, Tian Zhang, Daniel George, Charles D. Scales Jr, Michaela A. Dinan
Administrative support: Jessica E. Pritchard
Collection and assembly of data: Stephanie B. Wheeler, Christopher D. Baggett, Michaela A. Dinan
Data analysis and interpretation: Stephanie B. Wheeler, Lisa P. Spees, Bradford E. Jackson, Christopher D. Baggett, Lauren E. Wilson, Melissa A. Greiner, Deborah R. Kaye, Daniel George, Charles D. Scales Jr, Jessica E. Pritchard, Michaela A. Dinan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patterns and Predictors of Oral Anticancer Agent Use in Diverse Patients With Metastatic Renal Cell Carcinoma
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephanie B. Wheeler
Research Funding: Pfizer
Travel, Accommodations, Expenses: Pfizer
Christopher D. Baggett
Employment: Rho Inc
Lauren E. Wilson
Research Funding: AstraZeneca
Melissa A. Greiner
Research Funding: Novartis, Abbott Laboratories, Philips Healthcare
Deborah R. Kaye
Employment: Blue Cross Blue Shield North Carolina
Research Funding: Blue Cross Blue Shield of Michigan
Travel, Accommodations, Expenses: MedReviews
Tian Zhang
Leadership: Capio Biosciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: Exelixis, Genentech/Roche, MJH Life Sciences, Pacific Genuity
Consulting or Advisory Role: Janssen, Genentech/Roche, Sanofi, Exelixis, AstraZeneca, Pfizer, Bristol Myers Squibb, Foundation Medicine, Pharmacyclics, Amgen, Merck, Seattle Genetics, Dendreon, Calithera Biosciences
Speakers' Bureau: Exelixis, Genentech/Roche, Genomic Health, Sanofi/Aventis
Research Funding: Janssen, Acerta Pharma, Pfizer, Merrimack, Stem CentRx, Novartis, OmniSeq, Personal Genome Diagnostics, Regeneron, Merck, Mirati Therapeutics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology, Prochelators as Targeted Prodrugs for Prostate Cancer
Travel, Accommodations, Expenses: Acerta Pharma, Genomic Health, AstraZeneca
Daniel George
Leadership: Capio Biosciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Vizuri, Constellation Pharmaceuticals, Physicians' Education Resource, Propella Therapeutics
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis, Janssen Oncology, Novartis, Pfizer, Astellas Pharma, Bristol Myers Squibb, Acerta Pharma, Bayer, Dendreon, Innocrin Pharma, Calithera Biosciences, Sanofi/Aventis
Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroToday
Charles D. Scales Jr
Research Funding: Pfizer, Exelixis, Merck, Bristol Myers Squibb-Ono Pharmaceutical
Jessica E. Pritchard
Research Funding: St Jude Medical, Pfizer, Boston Scientific
Michaela A. Dinan
Research Funding: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Padala SA, Barsouk A, Thandra KC, et al. : Epidemiology of renal cell carcinoma. World J Oncol 11:79-87, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCI SEER Program : Kidney and Renal Pelvis Cancer. https://seer.cancer.gov/statfacts/html/kidrp.html [Google Scholar]
- 3.Capitanio U, Bensalah K, Bex A, et al. : Epidemiology of renal cell carcinoma. Eur Urol 75:74-84, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gad MM, Saad AM, Al-Huseini MJ, et al. : Incidence and mortality of renal cell carcinoma in the U.S.: A SEER-based study investigating trends over the last four decades. J Clin Oncol 36, 2018. (suppl; abstr 604) [Google Scholar]
- 5.Bailey ZD, Krieger N, Agenor M, et al. : Structural racism and health inequities in the USA: Evidence and interventions. Lancet 389:1453-1463, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Felder TM, Pena GD, Chapital BF: Disparities in cancer clinical trials: An analysis of comprehensive cancer control plans. Prev Chronic Dis 6:A116, 2009 [PMC free article] [PubMed] [Google Scholar]
- 7.Kaldany A, Blum KA, Paulucci DJ, et al. : An evaluation of race, ethnicity, age, and sex-based representation in phase I to II renal cell carcinoma clinical trials in the United States. Urol Oncol 36:363.e361-363.e366, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Paul K, Sathianathen N, Dahm P, et al. : Variation in accrual and race/ethnicity reporting in urological and nonurological related cancer trials. J Urol 202:385-391, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Meyer AM, Olshan AF, Green L, et al. : Big data for population-based cancer research: The integrated cancer information and surveillance system. N C Med J 75:265-269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppin C, Kollmannsberger C, Le L, et al. : Targeted therapy for advanced renal cell cancer (RCC): A Cochrane systematic review of published randomised trials. BJU Int 108:1556-1563, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Li P, Wong YN, Armstrong K, et al. : Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med 5:169-181, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Department of Agriculture Economic Research Service : Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Faurot KR, Jonsson Funk M, Pate V, et al. : Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 24:59-66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou G: A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702-706, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Wilson LE, Spees L, Pritchard J, et al. : Real-world utilization of oral anticancer agents costs in older adults with metastatic renal cell carcinoma in the United States. Kidney Cancer (in press) [DOI] [PMC free article] [PubMed]
- 17.Pal S, Gong J, Mhatre SK, et al. : Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer 19:548, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722-731, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan AR, Wang J, Yates TJ, et al. : Molecular targeting of renal cell carcinoma by an oral combination. Oncogenesis 9:52, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackshaw MD, Nagar SP, Parks DC, et al. : Persistence and compliance with pazopanib in patients with advanced renal cell carcinoma within a U.S. administrative claims database. J Manag Care Spec Pharm 20:603-610, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller LA, Stemkowski S, Saverno K, et al. : Patterns of care in patients with metastatic renal cell carcinoma among a U.S. Payer Population with Commercial or Medicare advantage membership. J Manag Care Spec Pharm 22:219-226, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta K, Miller JD, Li JZ, et al. : Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev 34:193-205, 2008 [DOI] [PubMed] [Google Scholar]