PURPOSE:
Patients with cancer frequently encounter financial hardship, yet systematic strategies to identify at-risk patients are not established in care delivery. We assessed sensitivity of distress-based screening to identify patients with cancer-related financial hardship and associated care delivery outcomes.
METHODS:
A survey of 225 patients at a large cancer center assessed cancer-related financial hardship (0-10 Likert scale; highest quintile scores ≥ 5 defined severe hardship). Responses were linked to electronic medical records identifying patients’ distress screening scores 6 months presurvey (0-10 scale) and outcomes of missed cancer care visits and bad debt charges (unrecovered patient charges) within 6 months postsurvey. A positive screen for distress was defined as score ≥ 4. We analyzed screening test characteristics for identifying severe financial hardship within 6 months and associations between financial hardship and outcomes using logistic models.
RESULTS:
Although patients with positive distress screens were more likely to report financial hardship (odds ratio [OR], 1.21; 1.08-1.37; P < .001), a positive distress screen was only 48% sensitive and 70% specific for identifying severe financial hardship. Patients with worse financial hardship scores were more likely to miss oncology care visits within 6 months (for every additional point in financial hardship score from 0 to 10, OR, 1.28; 1.12-1.47; P < .001). Of patients with severe hardship, 72% missed oncology visits versus 35% without severe hardship (P = .006). Patients with worse hardship were more likely to incur any bad debt charges within 6 months (OR, 1.32; 1.13-1.54; P < .001).
CONCLUSION:
Systematic financial hardship screening is needed to help mitigate adverse care delivery outcomes. Existing distress-based screening lacks sensitivity.
BACKGROUND
Patients with cancer frequently encounter financial hardship throughout their disease trajectory, including costly medical bills and depleted savings, along with lost income and unemployment.1,2 Such cancer-related financial hardship is associated with worse quality of life and psychologic stress.3,4 It is estimated that approximately 50% of patients with cancer experience medical financial hardship.5 Despite increasingly wide recognition of this high prevalence and adverse impact of financial hardship on patients,1,6,7 a systematic strategy for early proactive identification of at-risk patients is not yet established in care delivery. Such a strategy is needed to enhance timely referral to financial hardship interventions for high-risk patients during care delivery in oncology practices and health care systems.
Findings from a recent survey of National Cancer Institute–designated cancer centers revealed that most centers acknowledged financial hardship as a problem frequently encountered by patients during their cancer care trajectory. More than 80% of centers in this sample reported using a distress screening–based strategy for identifying patients with financial hardship and providing assistance.8 In another survey of 17 National Comprehensive Cancer Center member institutions, 50% used distress-based screening to identify patients with financial hardship, whereas only 19% implemented a dedicated financial hardship measure.9 The actual effectiveness of screening approaches, however, for identifying patients at risk for financial hardship is unclear.
An effective screening approach to systematically identify patients and prompt intervention is needed, since patients with cancer report frequent barriers to or discomfort with initiating financial discussions with their oncology care teams.4,8,10 Yet it remains unknown whether commonly used psychosocial distress–based screening strategies are adequate. Although evidence supports that widely adopted general psychosocial distress screening in cancer care delivery effectively identifies patients needing psychosocial interventions,11 whether distress screening can also systematically identify patients with cancer with financial hardship is not known.
Identifying gaps in screening for financial hardship among patients with cancer and ascertaining associated adverse care delivery outcomes are presently needed steps to help direct emerging financial hardship intervention strategies.10,12 Therefore, to inform these knowledge gaps in financial hardship screening and outcomes, we conducted an analysis of care delivery variables related to financial hardship reported in a survey of patients with cancer and cancer survivors who received care at a large metropolitan cancer center. The specific objectives of this study were (1) to assess the sensitivity of distress-based screening to identify patients with cancer-related financial hardship and (2) to identify the impact of patients' financial hardship on cancer care delivery outcomes, namely, missed oncology visits and accumulation of bad debt charges (the unpaid or unrecovered patient responsible portion of bills for cancer care).
METHODS
Data Sources and Patient Sample
This study was approved by the Institutional Review Board at The M.D. Anderson Cancer Center. We surveyed adult patients (age ≥ 18 years) with a confirmed diagnosis of cancer presenting for care at a large metropolitan comprehensive cancer center (including study sites at the main hub and a community-based regional oncology clinic) between March 2019 and September 2019. Individuals eligible for the survey study were age ≥ 18 years with a pathologically confirmed diagnosis of cancer, undergoing ambulatory cancer care in 14 voluntarily participating medical, surgical, or radiation oncology clinics in this cross-sectional study of patients with a spectrum of disease sites.13,14 Of 364 patients identified through convenience sampling from the participating clinics, on the basis of research team and patient availability for in-person approach, 232 agreed to enroll (63.7%). Excluded from this analysis were patients who declined review of their medical record for study abstraction (n = 2); did not answer > 50% survey questions (n = 1); or had no additional clinical follow-up after the survey date to assess care delivery outcomes (n = 4), for a final sample of 225 patients.
Financial Hardship Outcome Measure
To characterize patient-reported financial hardship, patients completed a survey with 15 items addressing the severity of cancer-related financial burden in the past 30 days, including (1) direct material burdens such as amount of spending on medical bills, lost savings, and accumulation of debt; (2) psychologic burdens, such as stress from cancer-related financial hardships; and (3) exhaustion of coping resources such as income and work benefits and aid from formal (organizations) and informal (family and friends) sources.13,14 These items were derived from the Economic Strain and Resilience in Cancer survey study13,14 and address the established theoretical domains of material, psychologic, and coping financial hardship identified in previous studies comprising cancer-related financial burden.2,5 Each item was scored on a 0 to 10 Likert scale (a score of 10 indicated most severe financial burden). The overall financial hardship score was then calculated as the arithmetic average of items (re-weighted for missing items), with an overall score range from 0 to 10. This approach was previously demonstrated to be valid and reliable with concurrent validity with other indicators of cancer-related financial toxicity, such as the COST tool.3,14 In statistical analyses, the continuous score for financial hardship from 0 to 10 was tested as well as a categorical variable was defined by a threshold for any hardship as score > 1 and severe hardship scored as score ≥ 5 (defined a priori by the highest quintile of scores in this sample).
Psychosocial Distress Screening and Care Delivery Workflow
To assess distress, patients' psychosocial distress screening assessments as a part of their routine oncology care were abstracted from the medical chart. Patient-reported psychosocial distress was captured in the medical chart during routine oncology care visits before and after the survey date, using an adapted version of the National Comprehensive Cancer Center Distress Thermometer and Problem List for Patients.15 Distress screening assessments followed the standard institutional workflow that asks patients whether they have experienced distress in the past 7 days from physical, mental, social, or spiritual factors using nurse-based assessment in routine clinical encounters. Screening results standardly facilitate automated reminders for repeat screening and/or social worker intervention. Standard distress screening is repeated if no screening was performed within the past 30 days and/or if there was a major care transition (eg, initiation of new treatment, postsurgical, or transfer to emergency care), coordinated with routine clinical encounters. The distress screen provides an overall psychosocial distress score, reported by patients on a 0-10 Likert scale (score of 10 indicates most severe distress). A positive distress screen score in this study was defined at the threshold distress score ≥ 4, the usual care threshold that triggers distress intervention in the institution's practice, including intervention for financial issues if needed. In statistical analyses, if a patient reported any score within the analytic time frame (6 months), which met the threshold distress score of ≥ 4, the patient was categorized as having had a positive distress screen.
Care Delivery Outcomes
Outcomes were abstracted from the medical chart. Missed oncology visits were defined as oncology appointments that were scheduled but where the patient failed to appear. The percentage of missed appointments was calculated as the number of appointments missed within 6 months after the survey date divided by the total number of scheduled appointments from 6 months before to 6 months after the survey date. The date range encompassing the denominator count of appointments was selected to account for the potential misclassification of patients who had only a single scheduled appointment in the 6-month follow-up after the survey date and missed, who would be classified as having a high (100%) frequency of missed visits within the postsurvey period. A sensitivity analysis was also performed using this more restrictive definition, by calculating the percentage of missed appointments using solely the denominator count of appointments in the 6 months after the survey date. Per inclusion criteria, patients must have had at least a single scheduled appointment during the follow-up period for inclusion in analysis. In statistical analyses, the dichotomous outcome was classified as having the highest quintile of missed visit frequency (≥ 3% when the denominator of appointments was from 6 months before to 6 months after survey and in the sensitivity analysis, ≥ 8% when the denominator of appointments was 6 months after survey).
Clinical encounters that resulted in bad debt within 6 months of the survey date were identified from billing records. Baseline bad debt was identified for the 6 months before the survey date. Cumulative hospital or technical charges during the follow-up period were obtained from billing records. Bad debt charges represented the charge amount for which there was an unpaid patient-responsible portion after standard collection efforts and thus remained unrecovered typically after 120 days. In statistical analyses, patients' outcomes were coded dichotomously for the outcome of postsurvey accumulation of new bad debt as having any versus no bad debt encounters within a 6-month follow-up period.
Statistical Analysis
Univariate associations between continuous financial hardship scores and categorical patient variables were evaluated using Kruskal-Wallis test or Wilcoxon Rank Sum test, and Spearman's correlation coefficient for continuous variables (with median scores and interquartile range [IQR] presented). The unadjusted association between financial hardship survey scores and presurvey distress screen scores was tested using the Wilcoxon Rank Sum test. The sensitivity of any positive distress screen (score ≥ 4 in the 6 months before the survey date) for identifying severe financial hardship (score ≥ 5) was calculated on the basis of the number of patients who had severe financial hardship who had a positive distress screen divided by the total number who had severe financial hardship. Specificity was calculated as the number of patients without severe financial hardship (score < 5) who had a negative distress screen divided by the total number without severe financial hardship. Log-log plots for these dichotomized outcomes of high frequency of missed oncology visits and incurring any bad debt were generated, with financial hardship scores in these plots binned by quintile to represent the unadjusted associations across the spectrum of scores. Logistic modeling identified associations between financial hardship and the dichotomous outcomes of excess missed oncology visits and incurring bad debt for clinical encounters within 6 months of patient-reported financial hardship, unadjusted and adjusted for covariates. Covariates included age (at survey), sex, race, ethnicity, cancer histology and stage, medical insurance abstracted from the medical record, patient educational attainment, household income, and other sources of medical insurance self-reported in the survey and tested in multivariate models. A parsimonious final model was selected to reduce collinearity and include a priori clinically relevant covariates. Analyses were conducted using SAS Enterprise Guide version 7.11 (Cary, NC). Statistical tests were two-sided with the P value < .05 considered statistically significant.
RESULTS
Patient Characteristics and Financial Hardship
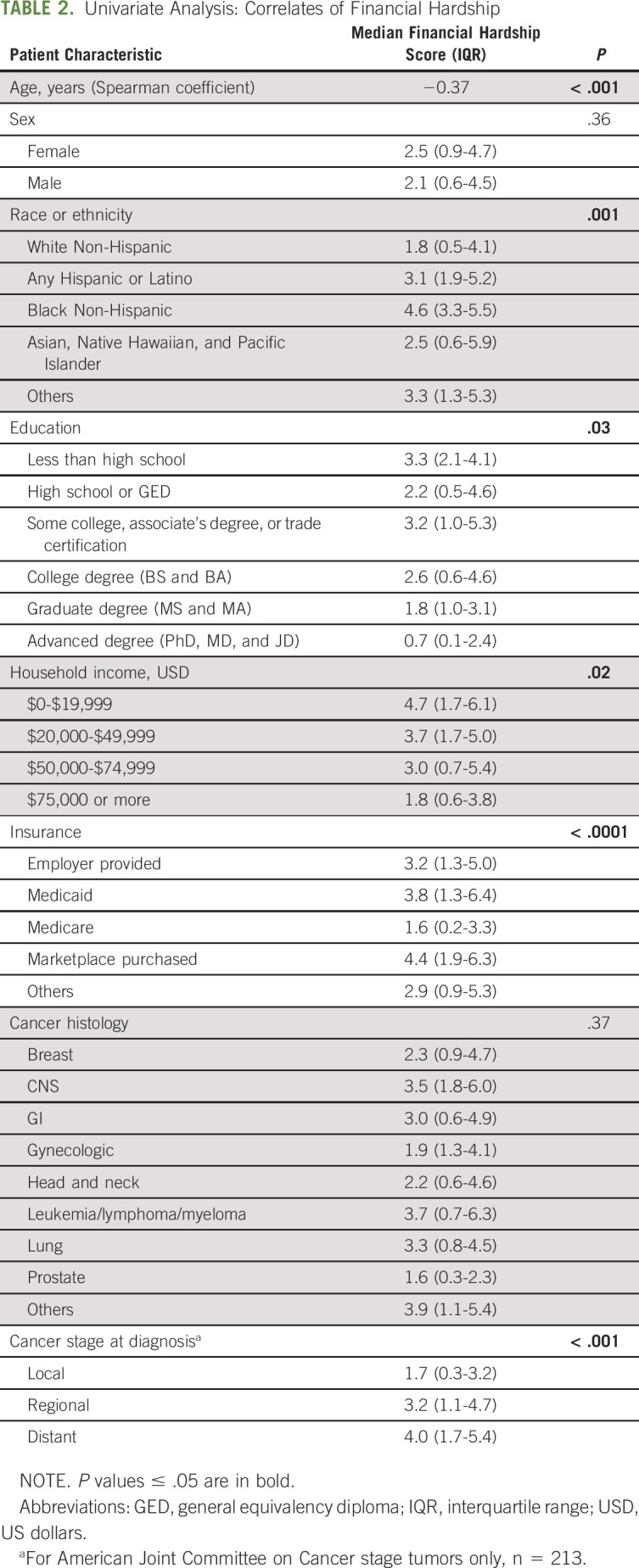
Among 225 patients, the median age was 63 years (IQR 53-70), 44.4% were men, 81.8% were White, and 8.4% reported an annual household income ≤ $20,000 US dollars (USD). The most frequent cancer diagnosis types were 36.4% of patients with breast and 12.4% with prostate cancer. A total of 54.5% of patients had regional or distant disease at the time of survey (Table 1). The median time from cancer diagnosis to financial hardship assessment was 0.62 years (IQR, 0.28-1.10). A total of 72% (n = 163) of patients reported any financial hardship (score ≥ 1) and 22% (n = 50) severe financial hardship (score ≥ 5). Adverse socioeconomic factors (ie, lower household income and lower educational attainment) and advanced cancer were associated with more severe hardship (Table 2).
TABLE 1.
Patient Characteristics
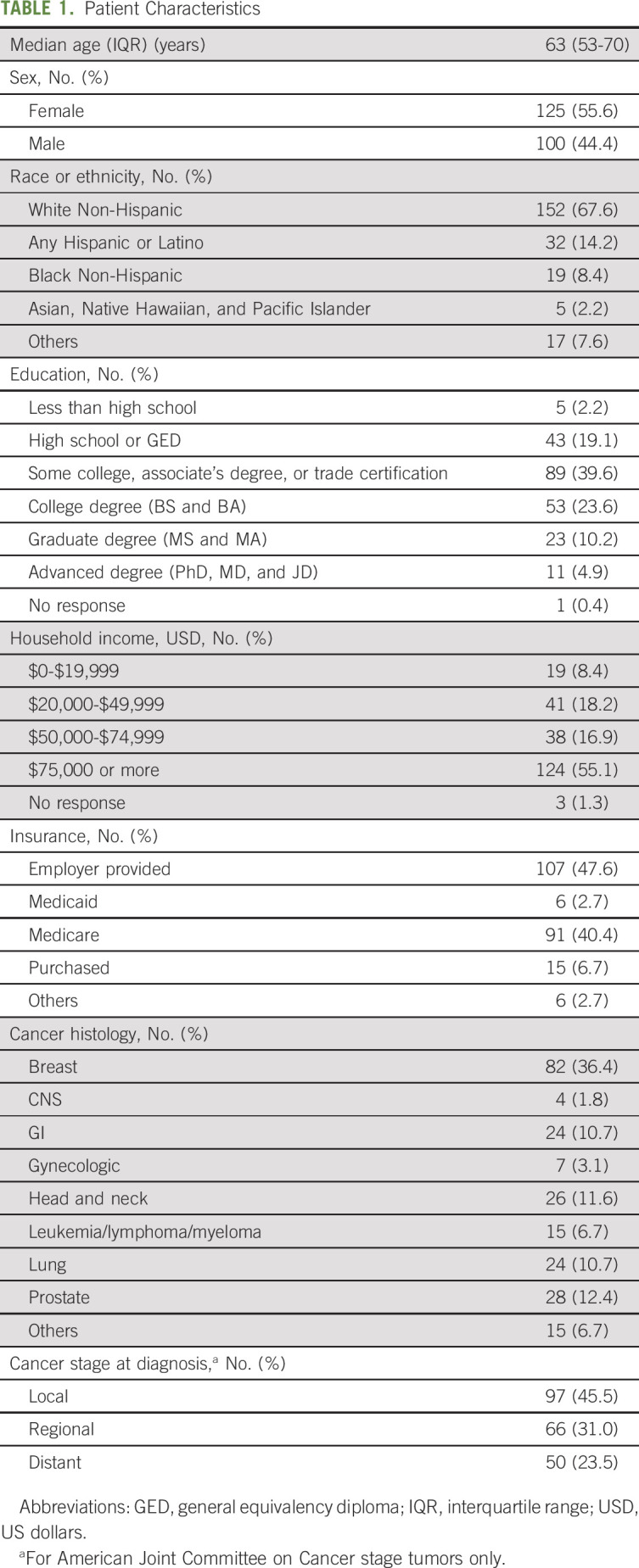
TABLE 2.
Univariate Analysis: Correlates of Financial Hardship
Sensitivity of Distress Screening in Care Delivery to Identify Downstream Financial Hardship
All patients (100%) underwent at least 1 psychosocial distress screen during routine cancer care delivery encounters within the 6 months before the financial hardship survey, and 34% had a positive screen for distress. Patients who had an initial positive screen for psychosocial distress were more likely to later report worse financial hardship (odds ratio [OR], 1.21; 95% CI, 1.08 to 1.37; P = .001). Still, the sensitivity of a positive distress screen before financial hardship was only 48% for identifying the patients who would later report experiencing the most severe financial hardship (score ≥ 5) within the next 6 months. The specificity was 70%. As a secondary definition, even when all distress screen scores from 6 months before to 6 months after patients' financial hardship survey were included, and any positive distress screen over the entire time frame was included, screening was 68% sensitive for identifying financial hardship.
Impact of Financial Hardship on Care Delivery Outcomes
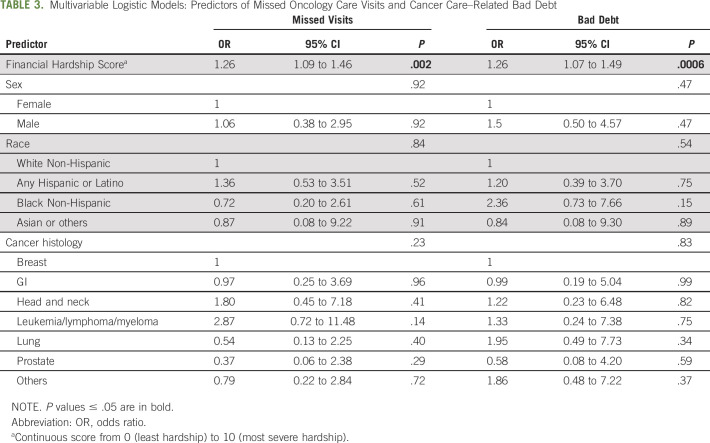
Patients who reported higher severity of financial hardship had higher likelihood of missed oncology visits (unadjusted OR, 1.28; 1.12 to 1.47; P < .001) and even after adjusting for sex, race, and cancer type (OR, 1.26; 1.09 to 1.46; P = .002) (Table 3). A total of 72% of patients with severe hardship subsequently missed oncology visits versus 35% of patients without severe hardship (P = .006). In the sensitivity analysis that defined missed oncology visits on the basis of the denominator of appointments 6 months after the survey, the results were similar (adjusted OR, 1.18; 1.02 to 1.36; P = .03).
TABLE 3.
Multivariable Logistic Models: Predictors of Missed Oncology Care Visits and Cancer Care–Related Bad Debt
In the 6 months before the survey, 20.4% of patients had incurred any bad debt. Patients with severe financial hardship at the time of survey also had higher likelihood of incurring subsequent cancer care–related bad debt in the 6 months after the survey (unadjusted OR, 1.32; 1.13 to 1.54; P < 0.001), even after adjusting for covariates (adjusted OR, 1.26; 1.07 to 1.49; P = .006) (Table 3). The mean cumulative bad debt charges per patient in the cohort during the 6-month follow-up was $5,989 USD. Among the 32 patients with any bad debt, the median bad debt in this subset was $9,645 USD (IQR $2,096-$69,466 USD). Log-log plots demonstrated that patients within the highest quintile of financial hardship scores had highest risks for missed visits and bad debt (Appendix Fig A1, online only).
DISCUSSION
The high prevalence of financial hardship in patients with cancer is increasingly recognized,1,6 along with its impact on patient quality of life and long-term adherence to treatments such as oral anticancer therapies and downstream difficulties in maintaining day-to-day living expenses, meeting basic needs, and accumulating debt and leading to bankruptcy.7,16 This study further delineated significant associations of financial hardship in patients with cancer with care delivery outcomes measured as missed patient oncology visits and bad debt related to oncology care. These outcomes not only adversely affect individual patients but also are costly for oncology practices and health care systems.
Across the spectrum of patient-reported financial hardship, worse hardship in this study was associated with increased risk of adverse outcomes. There appeared to be meaningfully highest risk of outcomes for the top quintile of patient-reported financial hardship scores—the most severe levels. Therefore, the results from this analysis help to identify that high-risk patients could potentially experience greatest benefits from patient- and system-level interventions (eg, social work support and financial navigation services). Identifying and intervening on the highest-risk group for adverse and costly outcomes are especially needed when financial navigation resources are constrained.
This analysis also suggested that general psychosocial distress screening to identify patients with financial hardship—a commonly practiced approach in many centers11,17,18—had only low to moderate sensitivity to identify patients with highest risk for financial hardship. Although our study validated that psychosocial distress was strongly associated with higher levels of financial hardship, also reported in previous studies,19 in this study cohort, as many as half of patients who developed severe financial hardship within a 6-month follow-up period still did not indicate psychologic distress in screening. Even if distress scores through the 6 months after survey were included, more than one quarter of patients with severe hardship would not be identified.
Therefore, the results of this study support the concept, also raised by Khera et al,10,12 that a critical gap in oncology care delivery exists, with lacking systematic identification of patients with financial hardship to accurately direct financial navigation interventions. Systematic identification of other cancer patient–reported outcomes in care delivery, for example, physical symptoms and psychologic distress, has resulted in effective interventions that enhance patients' quality of life and survival11 and reduce costly acute care utilization.11,20 Evidence supports that financial navigation can similarly improve patient and health care systems level outcomes.21,22 Thus, the present study provides initial data suggesting that creating an effective systematic screening approach to optimally identify is a foundational need for advancing financial hardship intervention in oncology care. Such intervention at the patient level and care delivery level may synergize with system and policy level changes, such as newly implemented price transparency policies, to improve cancer-related financial hardship.
Ideal frequency and timing of financial hardship screening and assessment in care delivery also need future study, especially for individuals at high risk for cancer-related financial hardship such as younger (working age) and lower-income patients.16,23 In the present study, a clinical factor associated with financial hardship was the presence of distant disease. This finding raises a hypothesis that a critical time for financial intervention assessment or re-assessment may be at development of advanced or distant disease.
This analysis has several limitations. Temporal direction of the association between distress and financial hardship is not available in these data, as longitudinal outcome was not assessed. Also, only systematic distress screening assessments were analyzed, and therefore, other ad hoc triggers for financial navigation (eg, a conversation with the physician requesting financial help for care during a clinical visit) were not examined. Finally, sensitivity or specificity of the adapted distress thermometer measure in this analysis may not represent sensitivity or specificity of other patient-reported outcome measures of distress or financial hardship, and such measures will require future study. This study was based on patients and facilities within one metropolitan region. Despite the heterogeneous study sample, the results need to be validated in other care delivery settings, for example, where there are a high percentage of uninsured or underinsured patients. Additionally, associations between patient-reported financial hardship and downstream missed visits and bad debt may not be causal. Prospective studies are needed to quantify the actual impact of financial hardship intervention on modifying these care delivery outcomes.
In conclusion, in this cohort of patients with cancer, the commonly used approach of psychosocial distress–based screening to identify patients at risk for financial hardship was only moderately sensitive for systematically recognizing patients at highest risk for financial hardship and the downstream adverse outcomes. Developing more sensitive—but brief and practical—financial hardship screening is needed to overcome barriers to proactively identifying vulnerable patients in cancer care settings. Implementing a robust financial navigation delivery workflow that is effective widely within cancer care delivery systems, to appropriately intervene once patients are identified, is a further challenge, but needed to improve costly care delivery outcomes that affect patients and health care systems.
Appendix
FIG A1.
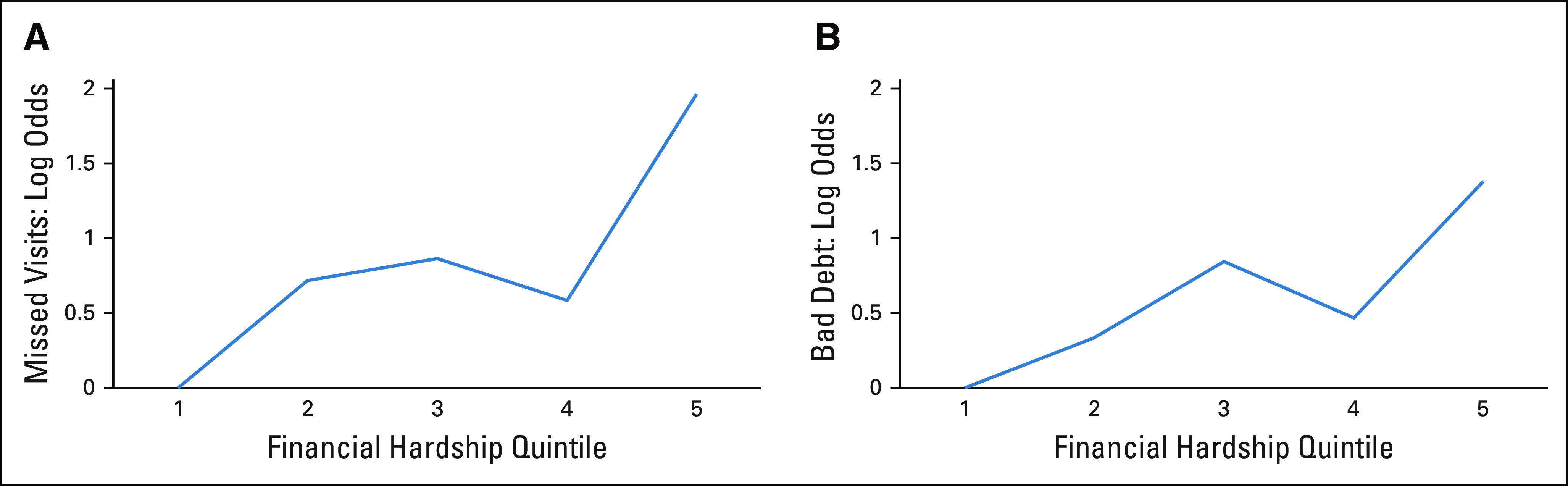
Log-odds plots: (A and B) Quintile of financial hardship score and odds of missed oncology visits within the subsequent 6 months and odds of cancer care–related bad debt within the subsequent 6 months.
Ying-Shiuan Chen
Stock and Other Ownership Interests: Novavax, Inovio Pharmaceuticals, Trevena, Vaxart, Heat Biologics, Agenus
Matthew P. Banegas
Employment: VIR Biotechnology
Stock and Other Ownership Interests: VIR Biotechnology
Research Funding: AstraZeneca
Chang Shine
Leadership: IBS Corp
Stock and Other Ownership Interests: Anthem Inc, Walgreens
Reshma Jagsi
Employment: University of Michigan
Stock and Other Ownership Interests: Equity Quotient
Consulting or Advisory Role: Amgen, Vizient
Research Funding: AbbVie, Genentech
Expert Testimony: Baptist Health/Dressman Benziger Lavalle Law
Travel, Accommodations, Expenses: Amgen
Other Relationship: JAMA Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/373670/summary
Robert J. Volk
Research Funding: AstraZeneca
Ya-Chen T. Shih
Consulting or Advisory Role: Pfizer, AstraZeneca
Research Funding: Novartis
Grace L. Smith
Research Funding: Varian Medical Systems
Other Relationship: Oncora Medical
No other potential conflicts of interest were reported.
SUPPORT
G.L.S. was supported by the National Cancer Institute (NIH/NCI K07CA211804) and by the Andrew Sabin Family Fellowship. SHG was supported by CPRIT PR160674 and Komen SAC150061. Y-.C.T.S. was supported by NCI R01CA207216, R01CA225647, and Health Care Services Corporation of BCBSTX. R.J.V. was supported by NIH/NCI under award number P30CA016672 and used the Shared Decision Making Core. This research was supported in part by the MD Anderson Cancer Center grant P30 CA016672.
AUTHOR CONTRIBUTIONS
Conception and design: J. Alberto Maldonado, Chiara Acquati, K. Robin Yabroff, Shine Chang, Rena M. Conti, Susan K. Peterson, Reshma Jagsi, Grace L. Smith
Administrative support: Cristina M. Checka
Provision of study materials or patients: Grace L. Smith
Collection and assembly of data: J. Alberto Maldonado, Chiara Acquati, Rena M. Conti, Cristina M. Checka, Pragati Advani, Grace L. Smith
Data analysis and interpretation: J. Alberto Maldonado, Shuangshuang Fu, Ying-Shiuan Chen, Chiara Acquati, K. Robin Yabroff, Matteo P. Banegas, Shine Chang, Rena M. Conti, Cristina M. Checka, Susan K. Peterson, Pragati Advani, Kimberly Ku, Reshma Jagsi, Sharon H. Giordano, Robert J. Volk, Ya-Chen T. Shih
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sensitivity of Psychosocial Distress Screening to Identify Cancer Patients at Risk for Financial Hardship During Care Delivery
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ying-Shiuan Chen
Stock and Other Ownership Interests: Novavax, Inovio Pharmaceuticals, Trevena, Vaxart, Heat Biologics, Agenus
Matthew P. Banegas
Employment: VIR Biotechnology
Stock and Other Ownership Interests: VIR Biotechnology
Research Funding: AstraZeneca
Chang Shine
Leadership: IBS Corp
Stock and Other Ownership Interests: Anthem Inc, Walgreens
Reshma Jagsi
Employment: University of Michigan
Stock and Other Ownership Interests: Equity Quotient
Consulting or Advisory Role: Amgen, Vizient
Research Funding: AbbVie, Genentech
Expert Testimony: Baptist Health/Dressman Benziger Lavalle Law
Travel, Accommodations, Expenses: Amgen
Other Relationship: JAMA Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/373670/summary
Robert J. Volk
Research Funding: AstraZeneca
Ya-Chen T. Shih
Consulting or Advisory Role: Pfizer, AstraZeneca
Research Funding: Novartis
Grace L. Smith
Research Funding: Varian Medical Systems
Other Relationship: Oncora Medical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Smith GL, Lopez-Olivo MA, Advani PG, et al. : Financial burdens of cancer treatment: A systematic review of risk factors and outcomes. J Natl Compr Canc Netw 17:1184-1192, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yabroff KR, Dowling EC, Guy GP, et al. : Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J Clin Oncol 34:259-267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Souza JA, Yap BJ, Wroblewski K, et al. : Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST): Measuring Financial Toxicity. Cancer 123:476-484, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagsi R, Ward KC, Abrahamse PH, et al. : Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer 124:3668-3676, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, Jemal A, Han X, et al. : Medical financial hardship among cancer survivors in the United States. Cancer 125:1737-1747, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Altice CK, Banegas MP, Tucker-Seeley RD, et al. : Financial hardships experienced by cancer survivors: A systematic review. JNCI J Natl Cancer Inst 109:djw205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey SD, Bansal A, Fedorenko CR, et al. : Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 34:980-986, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croyle RT, Jacobsen P, de Moor JS: Survey of Financial Navigation Services and Research: Summary Report. Bethesda, MD, National Cancer Institute, 2019 [Google Scholar]
- 9.Khera N, Sugalski J, Krause D, et al. : Current practices for screening and management of financial distress at NCCN member institutions. J Natl Compr Canc Netw 18:825-831, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Khera N, Holland JC, Griffin JM: Setting the stage for universal financial distress screening in routine cancer care: Financial Distress Screening in Cancer. Cancer 123:4092-4096, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Deal AM, Dueck AC, et al. : Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318:197-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrera PM, Kantarjian HM, Blinder VS: The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin 68:153-165, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GL, Lowenstein LM, Peterson SK, et al. : Financial toxicity of cancer care: Defining pathways of decline vs resilience. J Clin Oncol 36, 2018. (15 suppl; e22150) [Google Scholar]
- 14.Smith GL, Volk RJ, Lowenstein LM, et al. : ENRICH: Validating a multidimensional patient-reported financial toxicity measure. J Clin Oncol 37, 2019. (27 suppl; abstr 153) [Google Scholar]
- 15.Distress Thermometer and Problem List Information. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2020. https://www.nccn.org/about/permissions/thermometer.aspx [Google Scholar]
- 16.Han X, Zhao J, Zheng Z, et al. : Medical financial hardship intensity and financial sacrifice associated with cancer in the United States. Cancer Epidemiol Prev Biomark 29:308-317, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acquati C, Kayser K: Addressing the psychosocial needs of cancer patients: A retrospective analysis of a distress screening and management protocol in clinical care. J Psychosoc Oncol 37:287-300, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Zebrack B, Kayser K, Sundstrom L, et al. : Psychosocial distress screening implementation in cancer care: An analysis of adherence, responsiveness, and acceptability. J Clin Oncol 33:1165-1170, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Chan RJ, Gordon LG, Tan CJ, et al. : Relationships between financial toxicity and symptom burden in cancer survivors: A systematic review. J Pain Symptom Manage 57:646-660.e1, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Zebrack B, Kayser K, Bybee D, et al. : A practice-based evaluation of distress screening protocol adherence and medical service utilization. J Natl Compr Cancer Netw JNCCN 15:903-912, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Yezefski T, Steelquist J, Watabayashi K, et al. : Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care 24:S74-S79, 2018. (5 suppl) [PubMed] [Google Scholar]
- 22.Bedard L, Corp S, Doherty D, et al. : Financial Navigation for People Undergoing Cancer Treatment. Lansing, MI, Michigan Cancer Consortium, 2018. https://www.michigancancer.org/PDFs/Publications_Products/MCCExclusiveProd/FinancialNavigationReport_FinalAccessible2018.pdf [Google Scholar]
- 23.Mohammadi I, Wu H, Turkcan A, et al. : Data analytics and modeling for appointment no-show in community health centers. J Prim Care Community Health 9:2150132718811692, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]