Abstract
The rising incidence of tuberculosis worldwide means an increasing burden on diagnostic facilities, so tests simpler than Ziehl-Neelsen staining are needed. Such tests should be objective, reproducible, and have at least as good a detection limit as 104 bacteria/ml. A capture enzyme-linked immunosorbent assay (ELISA) was developed for detection of lipoarabinomannan (LAM) in human sputum samples. As a capture antibody, we used a murine monoclonal antibody against LAM, with rabbit antiserum against Mycobacterium tuberculosis as a source of detector antibodies. The sensitivity of the capture ELISA was evaluated by using purified LAM and M. tuberculosis whole cells. We were able to detect 1 ng of purified LAM/ml and 104 M. tuberculosis whole cells/ml. LAM could also be detected in culture filtrate of a 3-week-old culture of M. tuberculosis. The culture filtrate contained approximately 100 μg of LAM/ml. The detection limit in sputum pretreated with N-acetyl-l-cysteine and proteinase K was 104 M. tuberculosis whole cells per ml. Thirty-one (91%) of 34 sputum samples from 18 Vietnamese patients with tuberculosis (32 smear positive and 2 smear negative) were positive in the LAM detection assay. In contrast, none of the 25 sputum samples from 21 nontuberculous patients was positive. This specific and sensitive assay for the detection of LAM in sputum is potentially useful for the diagnosis of tuberculosis.
It is estimated that the incidence of tuberculosis worldwide and the number of cases attributable to coexisting human immunodeficiency virus (HIV) infection will increase substantially during the next decade (16). Most of this burden occurs among the low-income countries of the world, particularly those in South East Asia and sub-Saharan Africa. The usual means of diagnosing tuberculosis in resource-poor countries where culture facilities are not available is by the detection of acid-fast bacteria (AFB) in sputum by direct microscopy.
Sputum smear-positive patients are the most potent sources of transmission in the community. Therefore, the presence of AFB in sputum is an important marker of infectiousness. When done properly, approximately 60 to 70% of all adults with pulmonary tuberculosis can be identified with the current direct microscopy test using Ziehl-Neelsen staining (ZN). In practice, however, this proportion is around 40 to 60% at best (18). This reduced sensitivity is related to problems associated with the stringent requirements of the test (7). For example, if the need for multiple samples and multiple patient visits is ignored, then fewer smear-positive cases will be identified and treated. The International Union against Tuberculosis and Lung Disease recommends on average 20 slides per technician per working day. Due to overloading of the diagnostic facilities and lack of staff, most laboratory workers, especially in developing countries, process an excessive number of slides or have to combine smear examination with other diagnostic procedures, resulting in a lower quality of the diagnostic service. Patients coinfected with HIV are more likely to have negative sputum AFB smears (15).
The challenge is to develop a simple and inexpensive test—with at least as good a detection limit as that of direct microscopy (104 bacteria/ml)—that can reduce the workload of laboratory personnel.
Most assays developed so far are based on the detection of specific circulating antibodies. The serodiagnosis of tuberculosis has been the subject of investigation for a long time, but we still lack a test with widespread clinical utility. The available tests have both a sensitivity and specificity of around 80% (3). In HIV seropositive patients coinfected with tuberculosis, the sensitivity of antibody tests is much lower, between 10 and 40% (2, 12, 19). More efforts should be directed toward developing assays based on the detection of antigens in body fluids. Such tests could be useful for the diagnosis and follow-up of patients during treatment.
Mycobacterial antigens have been detected by enzyme-linked immunosorbent assay (ELISA) in sputum (22) and cerebrospinal fluid (13) and by latex agglutination assay in cerebrospinal fluid (10). Lipoarabinomannan (LAM), a major component of the mycobacterial cell wall, has been detected in the serum (14) and sputum (4) of patients with tuberculosis. None of these tests to detect mycobacterial antigens has achieved widespread use for the diagnosis of active tuberculosis.
In this study, we have developed a specific and sensitive assay for the detection of LAM, which can be used for the diagnosis of tuberculosis. The test is based on a capture ELISA using as a capture antibody a monoclonal antibody against LAM with a rabbit antiserum against Mycobacterium tuberculosis bacteria as a source of detector antibodies.
MATERIALS AND METHODS
Patients.
We used sputum samples from nontuberculous patients that had been spiked with M. tuberculosis suspension to develop the capture assay. Two Sudanese smear-positive pulmonary tuberculosis patients provided large volumes of sputum to determine the optimal test conditions. The test was then evaluated with the sputum samples as described below.
(i) Patients with pulmonary tuberculosis from Vietnam.
A total of 34 sputum samples were obtained from the Pham Ngoc Thach TB and Lung Disease Center, Ho Chi Minh City, Vietnam. These included sputum samples from 18 Vietnamese patients, for whom the diagnosis was based on positive culture results for M. tuberculosis. Seventeen of 18 patients had proven, untreated active pulmonary tuberculosis, with ZN-positive sputum smears (ranging from weak positive to strong positive, according to the following reporting scale: ZN−, no AFB per 300 fields; ZN±, 1 to 9 AFB per 100 fields; ZN+, 10 to 99 AFB per 100 fields; ZN 2+, 1 to 10 AFB per field in at least 50 fields; ZN 3+, more than 10 AFB per field in at least 20 fields). In the remaining Vietnamese patient, the sputum smear was negative. The patient had been treated for pulmonary tuberculosis 8 months before, but treatment failed because of patient noncompliance.
A single sputum sample was obtained from 4 patients, two samples were obtained from 12 patients, and three sputum samples were obtained from 2 patients. When multiple samples were collected, they were produced on the same day. All patients were HIV negative.
(ii) Microscopy and culture for M. tuberculosis.
Direct microscopy (17) was performed in Vietnam on a purulent part of the same sputum sample sent to The Netherlands for testing in a capture assay. Decontaminated sputum samples were cultured on two Löwenstein-Jensen slants. Cultures were examined weekly for growth for a total of 8 weeks.
(iii) Control group from Vietnam with a diagnosis other than tuberculosis.
A total of nine sputum samples were obtained from five Vietnamese patients (Pham Ngoc Thach TB and Lung Disease Center) who were initially suspected of having pulmonary tuberculosis, but were finally diagnosed as having bronchitis (n = 3), asthma (n = 1), or chronic obstructive pulmonary disease (n = 1). All patients had negative sputum smears and negative culture results for M. tuberculosis. Two patients had a previous medical history of tuberculosis, and two others had a history of contact with tuberculosis within the household. From four of these patients, two samples were obtained (both samples produced on the same day), and from one patient, one sample was obtained. All patients were HIV negative.
(iv) Control group from The Netherlands with a diagnosis other than tuberculosis.
Sixteen sputum samples were obtained from 16 Dutch subjects who were admitted to the Division of Pulmonary Diseases of the Academic Medical Center in Amsterdam because of chronic obstructive pulmonary disease (n = 5), cystic fibrosis (n = 4), asthma (n = 2), bronchitis (n = 1), idiopathic pulmonary fibrosis (n = 1), heart failure (n = 1), breast cancer (n = 1), and chronic tonsillitis (n = 1). Microorganisms were isolated from the sputum of 10 of these patients and included Pseudomonas aeruginosa (4 patients), Haemophilus influenzae (3 patients), Streptococcus pneumoniae (1 patient), Branhamella catarrhalis (1 patient), and Staphylococcus aureus (1 patient). None of the sputum samples contained AFB.
Sample storage.
Sputum samples were collected and frozen on the same day and stored at −20°C until use.
Pretreatment of the sputum samples.
Five hundred microliters of the sputum sample was transferred with a positive displacement pipette (Microman M250; Gilson, Villiers-le-Bel, France) to a 2-ml vial (Sarstedt, Nümbrecht, Germany) containing 5 μl of 100-mg/ml N-acetyl-l-cysteine (NALC) in 2.0 M sodium phosphate (pH 8). After mixing, 5 μl of 10 mg of proteinase K/ml was added (end concentration, 0.1 mg/ml). The sample was rotated for 6 h at 50°C in a hybridization oven. The vials were then placed in boiling water for 10 min to inactivate the proteinase K and centrifuged for 10 min at 12,000 × g at room temperature. The supernatant was tested in the capture ELISA as described below.
Antibodies used.
As capture antibodies, we used our own mouse immunoglobulin M (IgM) monoclonal antibody (F30-5) against LAM (21). A New Zealand rabbit (Ra-8.106) was immunized with 0.2 ml of a sonicate of M. tuberculosis strain H37Ra, containing 0.3 mg of protein, mixed with 0.2 ml of incomplete Freund adjuvant. Half of the dose was given intramuscularly, and the other half was given in two subcutaneous injections on the back of the rabbit. After 5 weeks, a booster injection was given in the same way. Two weeks later, blood was collected from the ear vein. The serum was used as a source of detector antibodies in the capture ELISA.
Specificity of the capture antibody and detection antibody.
The specificity of the monoclonal antibody against LAM (F30-5) and rabbit anti-M. tuberculosis serum (Ra-8.106) was tested by direct ELISA. Sonicates of E. coli, H. influenzae, S. pneumoniae, S. aureus, C. albicans, Nocardia asteroides, and 18 mycobacterial species (Table 1) were used to coat polystyrene flat-bottom microtiter plates (High Binding; Greiner Labortechnik, Nürtingen, Germany) in a concentration of 5 μg of protein per ml in phosphate-buffered saline (PBS [pH 8.0]). The plates were incubated overnight at 37°C in a water bath and washed three times with washing buffer containing 0.15 M NaCl, 1.2 mM KH2PO4, 4.8 mM Na2HpO4, and 0.05% (wt/vol) Tween 80 (pH 7.3). After blocking of the plates for 1 h at room temperature (20°C) with 1% bovine serum albumin (BSA) in PBS (pH 8.0) at 150 μl/well, the plates were washed again three times, and 100 μl of F30-5 (dilution 1:32,000) or 100 μl of Ra-8.106 (dilution 1:32,000) was added to each well. The dilution buffer contained 0.1 M Tris, 0.15 M NaCl, 1% BSA, and 0.05% Tween 80 (pH 8.0). The plates were incubated for 1 h at 37°C in a water bath. After the plates had been washed four times, wells incubated with murine antibody were filled with 100 μl of peroxidase-labeled sheep anti-mouse IgG (heavy and light chain) (Sanofi Diagnostics, Pasteur, Marne la Coquette, France) in a 1:2,000 dilution in dilution buffer; wells incubated with rabbit antisera were filled with 100 μl of goat anti rabbit IgG (heavy and light chain) (Sanofi Diagnostics, Pasteur) in a 1:4,000 dilution in dilution buffer. The plates were incubated for 1 h at 37°C in a water bath and washed again four times, followed by addition of 100 μl of tetramethylbenzidine (TMB) substrate solution (0.04% TMB and 0.04% urea-peroxide in 0.1 M sodium acetate citric acid buffer [pH 4.0]).
TABLE 1.
Bacterial strains used for determining the specificity of the antibodies used in the LAM detection assay
| KIT codea | Sourceb | Other designation(s) and/or source |
|---|---|---|
| Non-Mycobacterium | ||
| E. coli 2 | UvA | MC4100, ATCC 35695 |
| H. influenzae 1 | AMC | Clinical isolate |
| S. pneumoniae 1 | AMC | Clinical isolate |
| S. aureus 1 | AMC | Clinical isolate |
| C. albicans 1 | AMC | Clinical isolate |
| N. asteroides 1 | AMC | Clinical isolate |
| Mycobacterium | ||
| M. tuberculosis 1 | RIVM | myc4514 |
| M. bovis 2 | RIVM | ATCC 19210 |
| M. avium 1 | RIVM | myc3875 |
| M. africanum 1 | RIVM | myc5544 |
| M. microti 1 | ITG | 1287 |
| M. vaccae 1 | RIVM | ATCC 25949 |
| M. kansasii 1 | RIVM | myc1012 |
| M. marinum 3 | ITG | L66 |
| M. nonchromogenicum 1 | RIVM | ATCC 25145 |
| M. fortuitum 1 | RIVM | ATCC 6841 |
| M. smegmatis 1 | RIVM | ATCC 14468 |
| M. intracellulare 1 | ITG | 6997, ATCC 15985 |
| M. gordonae 3 | ITG | 8960 |
| M. terrae 2 | RIVM | SCS74/14 |
| M. xenopi 1 | ITG | 7003 |
| M. scrofulaceum 1 | RIVM | myc3442 |
| M. duvalii 1 | ITG | Clinical isolate |
| M. leprae 13 | IEBM | A54 |
Species were determined by biochemical means by the supplier of the strain. KIT, Royal Tropical Institute, Amsterdam, The Netherlands.
AMC, Academic Medical Center, Amsterdam, The Netherlands; IEBM, Institute for Experimental Biology and Medicine, Borstel, Germany; ITG, Institute for Tropical Medicine, Antwerp, Belgium; RIVM, National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands; UvA, University of Amsterdam, Amsterdam, The Netherlands.
After 30 min of incubation in the dark at room temperature, the reaction was stopped by the addition of 100 μl of 0.5 M H2SO4 to each well. The A450 was measured with a microtiter plate reader (Bio-kinetics reader; Bio-tec Instruments, Winooski, Vt.).
M. tuberculosis suspension for spiking experiments.
M. tuberculosis was cultured in Sauton medium as previously described (20). Briefly, the suspension was heated for 15 min at 80°C and centrifuged at 2,000 × g for 20 min. The pellet was resuspended in PBS containing 0.1% BSA and 0.05% (vol/vol) Tween 20 and sonicated for 30 s with a cell disruptor (Branson 250-Sonifier; Branson Ultrasonics Corporation, Danbury, Conn.) at 100 W. The A420 was adjusted to 0.15, which corresponds to 108 bacteria/ml (21). Serial dilutions in PBS containing 0.005% (vol/vol) Tween 20 were prepared by using a positive displacement pipette (Microman M250) with a new tip for every dilution.
Capture assay.
The ascites fluid F30-5 was diluted 1:1,000 in PBS (pH 7.2). A polystyrene flat-bottom microtiter plate (High Binding) was coated with the diluted monoclonal antibody F30-5 (50 μl/well) and incubated overnight at 4°C. The plate was then washed twice with PBS and blocked with 300 μl of 1% (wt/vol) skim milk in PBS per well for 1 h at 37°C. After two washes with PBS, 100 μl of the supernatant from the pretreated sputum sample was added to each well. Each sample was tested in duplicate (unless otherwise stated). The plate was incubated on a rotary shaker (100 rpm) overnight at room temperature and then washed five times with 0.05% (vol/vol) Tween 20 in PBS (pH 7.2) (PBST). Rabbit anti-M. tuberculosis serum was added (100 μl/well) at a 1:1,000 dilution in 1% (wt/vol) skim milk in PBST. After incubation for 1 h at 37°C, the plate was washed again five times with PBST, and 100 μl of peroxidase-labeled goat anti-rabbit IgG (heavy and light chains) (Sanofi Diagnostics, Pasteur) was added to each well in a 1:4,000 dilution in 1% (wt/vol) skim milk in PBST. The plate was incubated for 1 h at 37°C and washed 5 times with PBST, followed by the addition of 100 μl of TMB substrate solution as described above. After precisely 30 min of incubation in the dark at room temperature, the A630 was measured. The reaction was then stopped by the addition of 100 μl of 0.5 M H2SO4 to each well, and the A450 was measured. By measuring at two wavelengths (630 and 450 nm), we were able to increase the detection range of LAM. High concentrations of LAM could be detected by measuring the A630, and low concentrations of LAM could be detected by measuring the A450. The relationship between the two is A450/A630 = 3. To correct for day-to-day and plate-to-plate variation, M. tuberculosis culture filtrate (20), passed through a 0.2-μm-pore-size filter and frozen in small aliquots at −20°C, was used as a positive control. All values were corrected by multiplying the A450 of the unknown samples by the correction factor (A450 of culture filtrate at day 0/A450 of culture filtrate at day of testing). To control for the background reaction (conjugate control), four wells were filled with PBST. The final results were expressed as the mean A450 of the duplicates, after subtraction of the background A450. Values above the cutoff value, 0.150, were considered to be positive. The cutoff value was slightly higher than the mean A450 plus two times the standard deviation (SD), 0.126, of the sputum samples from the 16 Dutch subjects with diseases other than tuberculosis as mentioned above.
Statistical analysis.
Kruskal-Wallis one-way analysis of variance was used to establish differences in ELISA signals between groups of sputum samples with different ZN scores. A P value of ≤0.05 was considered significant.
The coefficient of variation was used when studying the reproducibility of the assay and the effect of individual variation in sputum on LAM detection, to establish whether differences in ELISA signals were acceptable or not.
RESULTS
Specificity of the capture antibody and detection antibody.
There was no cross-reactivity between the antibody against LAM (F30-5) and sonicates of the common pulmonary pathogens H. influenzae, S. pneumoniae, S. aureus, and B. catarrhalis or with N. asteroides, E. coli, and C. albicans. As expected, F30-5 cross-reacted with sonicates of all 18 mycobacterial species. The rabbit anti-M. tuberculosis serum (Ra-8.106) contained antibodies which reacted slightly with sonicates of E. coli, H. influenzae, and N. asteroides. However, E. coli, H. influenzae, and N. asteroides did not give a positive result in the final capture ELISA (data not shown).
Detection of solutions containing purified LAM, LAM in M. tuberculosis culture filtrate, and LAM in M. tuberculosis suspensions.
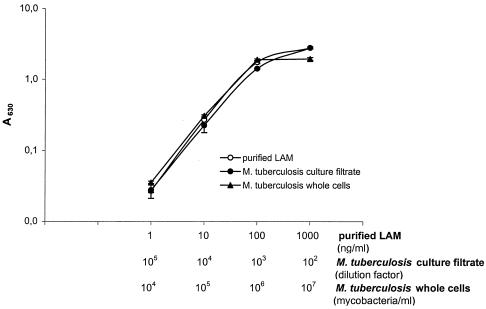
A calibration curve was made by using purified LAM (kindly provided by Patrick Brennan) diluted in PBS containing 0.005% Tween 20 (Fig. 1). It was possible to detect as little as 1 ng of purified LAM per ml (measurements of A450 or A630 gave the same detection limit).
FIG. 1.
Detection of purified LAM, LAM in culture filtrate from a 3-week-old culture of M. tuberculosis, and M. tuberculosis whole cells. The A630 values (y axis), the concentration of LAM/M. tuberculosis whole cells, and the dilution factor of the culture filtrate (x axis) were plotted on a log scale. As the capture antibody, an IgM monoclonal antibody against LAM (F30-5) was used to coat ELISA plates, and a rabbit anti-M. tuberculosis serum (Ra-8.106) was used as the detector antibody. Results are given as the mean A630 minus the plate background ± SD.
LAM could be detected by the capture ELISA in the culture filtrate of a 3-week-old culture of M. tuberculosis. The culture filtrate contained approximately 100 μg of LAM/ml (Fig. 1). As a positive control for day-to-day and plate-to-plate variations in the capture ELISA, we used the M. tuberculosis culture filtrate with 0.005% (vol/vol) Tween 20 in PBS as a dilution buffer.
A serial dilution of M. tuberculosis bacteria in PBS containing 0.005% (vol/vol) Tween 20 (see Materials and Methods) was tested in the capture ELISA (Fig. 1). The detection limit was 104 M. tuberculosis bacteria/ml (measurements of A450 or A630 gave the same detection limit). Figure 1 shows that the amount of LAM found in 105 mycobacteria/ml equals 10 ng of purified LAM/ml. Assuming that all LAM was extracted from the mycobacteria, we can then estimate that one M. tuberculosis bacterium contains approximately 100 fg of LAM (10 ng = 107 fg; 107 fg/105 mycobacteria = 100 fg of LAM/mycobacterium).
Detection of LAM in spiked sputum.
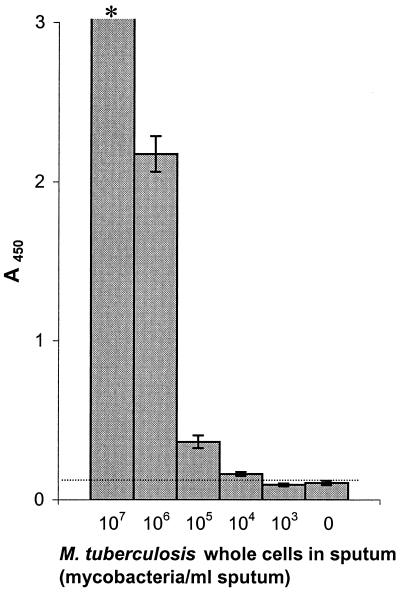
Sputum samples from 10 Dutch patients not suspected of having tuberculosis were pooled and spiked with M. tuberculosis whole cells. We found that treatment of purified LAM and M. tuberculosis culture filtrate with 0.25 M sodium hydroxide in combination with 15 mM NALC resulted in a 50% reduction of the signal. Sputum therefore was not treated with NaOH-NALC. Instead, we used NALC (1 mg/ml) in combination with proteinase K (0.1 mg/ml). The detection limit in sputum treated this way was 104 mycobacteria/ml (Fig. 2).
FIG. 2.
Detection of LAM in sputum spiked with a serial dilution of M. tuberculosis (107 to 103 cells/ml of sputum) and unspiked (0 cells/ml of sputum). The sputum was pooled and contained equal portions of sputum from 10 Dutch patients with a diagnosis other than tuberculosis. Results are given as the mean A450 minus the plate background. •••••, ELISA value of unspiked sputum +2 SDs (0.121); ∗, A450 of >3. Error bars show ± 2 SDs. The detection limit was 104 M. tuberculosis bacteria/ml sputum.
Reproducibility of the assay.
We tested the reproducibility of the assay, including pretreatment of the sputum with NALC and proteinase K on 3 consecutive days, by using samples of sputum from four patients (Table 2). Two patients had a disease other than tuberculosis, and two Sudanese patients with tuberculosis had ZN-positive sputa. The SD of the daily differences was small (Table 2). For the sputum samples from both tuberculous patients, the coefficient of variation was 5%.
TABLE 2.
Reproducibility of the assay
| Parameter | Result for sputum from patient groupa:
|
|||
|---|---|---|---|---|
| Nontuberculousb
|
Tuberculousc
|
|||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Day 1 | 0.009 | 0.021 | 0.220 | 1.792 |
| Day 2 | 0.019 | 0.019 | 0.223 | 1.759 |
| Day 3 | 0.019 | 0.023 | 0.203 | 1.918 |
| Mean | 0.016 | 0.021 | 0.215 | 1.823 |
| SD | 0.005 | 0.002 | 0.011 | 0.084 |
| CV (%)d | 31.3 | 9.5 | 5.1 | 4.6 |
Results are given as the mean A630 of the duplicates in each test minus the plate background, after correction for day-to-day and plate-to-plate variation of the detection assay.
The patient 1 sample was sputum from a patient from The Netherlands with idiopathic pulmonary fibrosis, with no suspicion of tuberculosis. From the sputum sample, S. aureus was cultured. The patient 2 sample was sputum of a patient from The Netherlands with chronic obstructive pulmonary disease, with no suspicion of tuberculosis.
The patient 3 and 4 samples were ZN-positive sputa from two patients from Sudan with pulmonary tuberculosis.
CV, coefficient of variation.
Effect of individual variation in sputum on LAM detection.
To explore whether variation in sputum contents or consistency could influence the detection of LAM, six sputum samples from six Dutch patients with a diagnosis other than tuberculosis were tested both unspiked and spiked with 5 × 106 M. tuberculosis bacteria/ml sputum (Table 3). The sputum samples differed in optical appearance and viscosity. All sputum samples were treated by the NALC-proteinase K method described in Materials and Methods. No great difference was found between the ELISA signals (coefficient of variation, 6%).
TABLE 3.
Effect of individual variation in sputum on LAM detection
| Sputum samplea | Result by LAM detection assayb
|
|
|---|---|---|
| Unspiked | Spiked with 5 × 106 mycobacteria/ml | |
| A | 0.080 | 1.814 |
| B | 0.062 | 1.953 |
| C | 0.002 | 1.813 |
| D | 0.000 | 1.740 |
| E | 0.016 | 1.861 |
| F | 0.006 | 1.618 |
| Mean ± SD | 1.800 ± 0.113c | |
None of the patients had any clinical or radiological sign of M. tuberculosis infection. A, sputum sample from a patient who was admitted to the hospital because of hematemesis; B, sputum sample from a patient with pneumonia, from which no organisms (including AFB) were cultured; C, sputum sample from a patient with bronchitis, from which H. influenzae was cultured; D, sputum sample from a patient with chronic obstructive pulmonary disease, from which no organisms were cultured; E, sputum sample from a patient with recurrent respiratory tract infections, from which no organisms were cultured; F, sputum sample from a patient with acute bronchitis, from which S. pneumoniae was cultured.
Results are given as the mean A630 of the duplicates in each test minus the plate background.
Coefficient of variation = 6%.
Detection of LAM in sputum of patients with pulmonary tuberculosis.
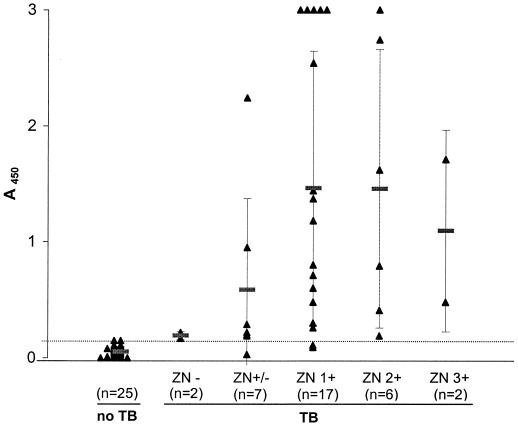
In total, 59 sputum samples from 39 subjects were tested in the LAM detection assay: 34 sputum samples from 18 tuberculous patients from Vietnam, 9 sputum samples from 5 nontuberculous patients from Vietnam, and 16 sputum samples from 16 nontuberculous patients from The Netherlands (see Materials and Methods). Arbitrarily, we chose a cutoff value just above the mean A450 plus two times the SD of the group of sputum samples from the 16 nontuberculous Dutch patients. Figure 3 shows that 31 of 34 (91%) of the sputum samples from the patients with tuberculosis had an optical density value above the cutoff value, whereas all sputum samples from the patients with a diagnosis other than tuberculosis had an optical density value below the cutoff (91% sensitivity and 100% specificity).
FIG. 3.
LAM detection assay results according to the ZN score. TB, tuberculosis. ZN−, no AFB per 300 fields; ZN+/−, 1 to 9 AFB per 100 fields; ZN+, 10 to 99 AFB per 100 fields; ZN 2+, 1 to 10 AFB per field in at least 50 fields; ZN 3+, more than 10 AFB per field in at least 20 fields. •••••, cutoff value for positivity (A450 = 0.150). The cutoff value is slightly higher than the A450 + 2 SDs of the sputum samples from 16 nontuberculous Dutch patients (0.126). n, number of sputum samples in each group.
Figure 3 shows the results of the LAM detection assay according to the ZN score. There was a significant difference between the ELISA signals in the group of sputum samples from tuberculous patients and the group of sputum samples from nontuberculous patients (Kruskal-Wallis one-way analysis of variance, P < 0.001). No significant difference in ELISA signals was found between groups of sputum samples with different ZN scores.
Table 4 shows the correlation between the results of the LAM detection assay and the disease status of the patient (tuberculous versus nontuberculous). A patient was called positive if at least one sputum sample was positive in the LAM detection assay. Seventeen of 18 patients with tuberculosis (including the partially treated, smear-negative tuberculosis patient) were positive, whereas all of the patients with other diseases were negative, resulting in a sensitivity of 94% and a specificity of 100%.
TABLE 4.
Correlation between the results of the LAM detection assay and disease status
| Result by LAM detection assaya | No. of patients with status:
|
|
|---|---|---|
| Proven TBb | Non-TBc | |
| Positive | 17 | 0 |
| Negative | 1 | 21 |
| Total | 18 | 21 |
A patient was positive if in the LAM detection assay the A450 of at least one sputum sample was higher than the cutoff value (0.150).
Pulmonary tuberculosis (TB) patients from Vietnam with a positive sputum culture.
Five patients from Vietnam and 16 patients from The Netherlands with a disease other than tuberculosis.
DISCUSSION
The assay for detection of LAM in sputum is promising and is a potential candidate to replace direct microscopy.
Preliminary results have been published on new diagnostic tests for tuberculosis based on detection of mycobacterial antigens in cerebrospinal fluid (10, 13), serum (11, 14), and sputum (4, 22). The authors reported sensitivity rates of 45 to 88% and specificity rates of 91 to 100%. However, since then, little has been published on this subject, suggesting that these tests did not live up to their original promise.
Any test which is to replace direct microscopy must offer advantages in terms of speed and ease of use and preferably have a higher sensitivity. In high-prevalence areas of tuberculosis, the greatest need is for new diagnostic tools which result in a reduction of laboratory workload. Antigen detection assays are promising in this regard, since they enable the analyst to test many samples at once.
In antigen detection assays, sample processing is an important, but often laborious and time-consuming, step. The number of nonspecific reactions can be high because of cross-reacting substances present in untreated human specimens. Mycobacterial antigens have been found as components of circulating immune complexes (1), so it may be necessary to dissociate the immune complexes to achieve a higher sensitivity in the immunoassay (5). Sada et al. (14), who detected LAM in serum, used an ethanol precipitation method (6) to reduce nonspecific reactions. Their detection system, a simple coagulation technique, is suitable for routine use. However, the described method for sample processing is still too laborious for daily use in laboratories in areas where tuberculosis is endemic, where more than 100 specimens per day have to be examined.
Yáñez et al. (22) and Cho et al. (4), who detected mycobacterial antigens and LAM in sputum, respectively, used NaOH for pretreatment of sputum. We found that treatment of LAM with sodium hydroxide, even for a short time at room temperature, resulted in a dramatic decrease in the LAM detection assay. LAM is alkali labile (8), so treatment with NaOH could destroy the epitope which is recognized by the capture antibody. For the LAM detection assay, we have developed an effective pretreatment method for sputum (NALC-proteinase K method), avoiding the use of NaOH. By this method, the results of the LAM detection assay were reproducible when a single sputum sample was pretreated and tested on 3 consecutive days. The NALC-proteinase K method can be further simplified, since we have found that it is possible to reduce the incubation time at 50°C (unpublished results).
We could detect as little as 1 ng of purified LAM per ml and 104 M. tuberculosis whole cells per ml by using the capture ELISA. In spiked sputum, the detection limit was also 104 M. tuberculosis whole cells. We have shown that there was a direct correlation between the LAM detection assay results and the numbers of whole mycobacteria in sputum spiked with different amounts of M. tuberculosis (Fig. 2). On the other hand, we found that there was no significant correlation between the LAM detection assay results and ZN score of the sputum samples from patients with tuberculosis. An explanation is that, for direct microscopy, the sample was selected from the purulent portion of the sputum, while for the LAM detection assay, a homogenate of the remaining portion of the sample was taken. Thus, the LAM detection assay may be more representative and give a more accurate indication of the bacterial load in the sputum. It could become a tool in the follow-up of patients during therapy.
Since LAM is present in all mycobacteria, the assay could be used for the diagnosis of any mycobacterial disease, including disease caused by the Mycobacterium avium-Mycobacterium intracellulare complex. The methods used then to identify the infecting mycobacteria will depend upon local circumstances (e.g., culture or multiplex PCR) (9). However, the LAM detection assay has been designed for use particularly in developing countries, where tuberculosis is by far the most common mycobacterial disease.
Our results with the LAM detection assay showed that 91% of the sputum specimens in the group of tuberculous patients were positive, and all of the specimens in the group of nontuberculous patients were negative. Based on the definition that a patient was positive in the LAM detection assay if at least one sputum specimen had an A450 value higher than the cutoff value, 94% of the tuberculous patients were found to be positive, whereas all of the nontuberculous patients were negative. Our results are promising, but the assay needs further evaluation in the field to determine its sensitivity and specificity, since we have tested only a small study population so far.
ACKNOWLEDGMENTS
L.M.P. was supported by a grant from the Amsterdam Society and Research Fund for Prevention and Cure of Tuberculosis.
REFERENCES
- 1.Bhattacharya A, Ranadive S N, Kale M, Battacharya S. Antibody-based enzyme-linked immunosorbent assay for determination of immune complexes in clinical tuberculosis. Am Rev Respir Dis. 1986;134:205–209. doi: 10.1164/arrd.1986.134.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Boggian K, Fierz W, Vernazza P L The Swiss HIV Cohort Study. Infrequent detection of lipoarabinomannan antibodies in human immunodeficiency virus-associated mycobacterial disease. J Clin Microbiol. 1996;34:1854–1855. doi: 10.1128/jcm.34.7.1854-1855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang I-H, Suo J, Bai K-J, Lin T-P, Luh K-T, Yu C-J, Yang P-C. Serodiagnosis of tuberculosis: a study comparing three specific mycobacterial antigens. Am J Respir Crit Care Med. 1997;156:906–911. doi: 10.1164/ajrccm.156.3.9607122. [DOI] [PubMed] [Google Scholar]
- 4.Cho S N, Shin J S, Kim J D, Chong Y. Production of monoclonal antibodies to lipoarabinomannan-B and use in the detection of mycobacterial antigens in sputum. Yonsei Med J. 1990;31:333–338. doi: 10.3349/ymj.1990.31.4.333. [DOI] [PubMed] [Google Scholar]
- 5.De Jonge N, Fillié Y E, Deelder A M. A simple and rapid treatment (trichloroacetic acid precipitation) of serum samples to prevent non-specific reactions in the immunoassay of a proteoglycan. J Immunol Methods. 1987;99:195–197. doi: 10.1016/0022-1759(87)90127-x. [DOI] [PubMed] [Google Scholar]
- 6.Doskeland S O, Berdal B P. Bacterial antigen detection in body fluids: methods for rapid antigen concentration and reduction of nonspecific reactions. J Clin Microbiol. 1980;11:380–384. doi: 10.1128/jcm.11.4.380-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulds J, O'Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998;2:778–783. [PubMed] [Google Scholar]
- 8.Hunter S W, Gaylord H, Brennan P J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 9.Kox L F F, Jansen H M, Kuijper S, Kolk A H J. Multiplex PCR assay for immediate identification of the infecting species in patients with mycobacterial disease. J Clin Microbiol. 1997;35:1492–1498. doi: 10.1128/jcm.35.6.1492-1498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krambovitis E, McIllmurray M B, Lock P E, Hendrickse W, Holzel H. Rapid diagnosis of tuberculous meningitis by latex particle agglutination. Lancet. 1984;ii:1229–1231. doi: 10.1016/s0140-6736(84)92792-2. [DOI] [PubMed] [Google Scholar]
- 11.Krambovitis E, Harris M, Hughes D T D. Improved serodiagnosis of tuberculosis using two assay test. J Clin Pathol. 1986;39:779–785. doi: 10.1136/jcp.39.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratanasuwan W, Kreiss J K, Nolan C M, Schaeffler B A, Suwanagool S, Tunsupasawasdikul S, Chuchottaworn C, Dejsomritrutai W, Foy H M. Evaluation of the MycoDot test for the diagnosis of tuberculosis in HIV seropositive and seronegative patients. Int J Tuberc Lung Dis. 1997;1:259–264. [PubMed] [Google Scholar]
- 13.Sada E, Ruiz-Palacios G M, López-Vidal Y, Ponce de León S. Detection of mycobacterial antigens in cerebrospinal fluid of patients with tuberculous meningitis by enzyme-linked immunosorbent assay. Lancet. 1983;ii:651–652. doi: 10.1016/s0140-6736(83)92532-1. [DOI] [PubMed] [Google Scholar]
- 14.Sada E, Aguilar D, Torres M, Herrera T. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J Clin Microbiol. 1992;30:2415–2418. doi: 10.1128/jcm.30.9.2415-2418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samb B, Sow P S, Kony S, Maynart-Badiane M, Diouf G, Cissokho S, Bâ D, Sané M, Klotz F, Faye-Niang M A, Mboup S, Ndoye I, Delaporte E, Hane A A, Samb A, Coulaud J-P, Coll-Seck A M, Larouzé B, Murray J F. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV seroprevalence. Int J Tuberc Lung Dis. 1999;3:330–336. [PubMed] [Google Scholar]
- 16.Schürmann D, Nightingale S D, Bergmann F, Ruf B. Tuberculosis and HIV infection: a review. Infection. 1997;25:274–280. doi: 10.1007/BF01720396. [DOI] [PubMed] [Google Scholar]
- 17.Smithwick R W. Laboratory manual for acid-fast microscopy. 2nd ed. Atlanta, Ga: Centers for Disease Control and Prevention; 1976. [Google Scholar]
- 18.Van Deun A, Portaels F. Limitations and requirements for quality control of sputum smear microscopy for acid-fast bacilli. Int J Tuberc Lung Dis. 1998;2:756–765. [PubMed] [Google Scholar]
- 19.Verbon A, Weverling G J, Kuijper S, Speelman P, Jansen H M, Kolk A H J. Evaluation of different tests for the serodiagnosis of tuberculosis and the use of likelihood ratios in serology. Am Rev Respir Dis. 1993;148:378–384. doi: 10.1164/ajrccm/148.2.378. [DOI] [PubMed] [Google Scholar]
- 20.Verbon A, Kuijper S, Jansen H M, Speelman P, Kolk A H J. Antigens in culture supernatant of M. tuberculosis: epitopes defined by monoclonal and human antibodies. J Gen Microbiol. 1990;136:955–964. doi: 10.1099/00221287-136-5-955. [DOI] [PubMed] [Google Scholar]
- 21.Verstijnen C P H J, Ly H M, Polman K, Richter C, Smits S P, Maselle S Y, Peerbooms P, Rienthong D, Montreewasuwat N, Koanjanart S, Trach D D, Kuijper S, Kolk A H J. Enzyme-linked immunosorbent assay using monoclonal antibodies for identification of mycobacteria from early cultures. J Clin Microbiol. 1991;29:1372–1375. doi: 10.1128/jcm.29.7.1372-1375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yáñez M A, Coppola M P, Russo D A, Delaha E, Chaparas S D, Yeager H., Jr Determination of mycobacterial antigens in sputum by enzyme immunoassay. J Clin Microbiol. 1986;23:822–825. doi: 10.1128/jcm.23.5.822-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]