Abstract
Background:
Some older adults show exaggerated responses to drugs that act on the brain. The brain’s response to anesthetic drugs is often measured clinically by processed electroencephalogram (EEG) indices. Thus, we developed a processed EEG based-measure of the brain’s resistance to volatile anesthetics, and hypothesized that low scores on it would be associated with postoperative delirium risk.
Methods:
We defined the Duke Anesthesia Resistance Scale (DARS) as the average bispectral index (BIS) divided by the quantity (2.5 minus the average age-adjusted end-tidal MAC (aaMAC) inhaled anesthetic fraction). The relationship between DARS and postoperative delirium was analyzed in 139 older surgical patients (age ≥65) from Duke (N=69) and Mt Sinai (N=70). Delirium was assessed by geriatrician interview at Duke, and by research staff utilizing the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) instrument at Mt Sinai. We examined the relationship between DARS and delirium, and used the Youden index to identify an optimal low DARS threshold (for delirium risk), and 95% bootstrap confidence bounds for it. We used multivariable logistic regression to examine the relationship between low DARS and delirium risk.
Results:
The relationship between DARS and delirium risk was non-linear, with higher delirium risk at low DARS scores. A DARS threshold of 28.755 maximized the Youden index for the association between low DARS and delirium, with a bootstrap 95% confidence bounds of 26.18 and 29.80. A low DARS (<28.755) was associated with increased delirium risk in multivariable models adjusting for site (OR (95% CI) 4.30 (1.89, 10.01), p=0.001), or site plus patient risk factors (OR (95% CI) 3.79 (1.63, 9.10), p=0.003). These associations with postoperative delirium risk remained significant when using the 95% bootstrap confidence bounds for the low DARS threshold (p<0.05 for all). Further, a low DARS (<28.755) was associated with delirium risk after accounting for opioid, midazolam, propofol, phenylephrine and ketamine dosage as well as site (OR (95% CI) 4.21 (1.80, 10.16), p=0.002). This association between low DARS and postoperative delirium risk after controlling for these other medications, remained significant (p<0.05) when using either the lower or the upper 95% bootstrap confidence bounds for the low DARS threshold.
Conclusions:
These results demonstrate that an intraoperative processed EEG-based measure of lower brain anesthetic resistance (i.e. low DARS) is independently associated with increased postoperative delirium risk in older surgical patients.
Keywords: Age factors, Anesthesia, methods, Delirium, surgery/prevention & control, Electroencephalography, drug effects
INTRODUCTION
Postoperative delirium is a common complication in older surgical patients and has been associated with increased length of stay, functional decline, and 1 year postoperative mortality risk.1–3 Recent guidelines call for or at least encourage 4,5 electroencephalography (EEG)-based anesthetic management to reduce delirium rates. In research settings, intraoperative anesthetized raw EEG features such as burst suppression and alpha band power have been associated with postoperative delirium 6–9 and preoperative cognitive impairment,10,11 respectively.
However, processed EEG measures are used more frequently than raw EEG for intraoperative brain monitoring in current anesthesiology practice, and there is equipoise regarding whether processed EEG measures are associated with postoperative delirium. Processed EEG values measure the brain’s response to anesthetic drug dosage (as well as surgical stress), though the anesthetic dose required to produce specific processed EEG values or raw EEG states varies across patients. Further, these individual differences in anesthetic dose-dependent EEG responses are not completely explained by age, gender, or weight. Fritz et al demonstrated that increased anesthetic sensitivity (as indicated by EEG burst suppression at lower anesthetic dosage) is associated with increased postoperative delirium risk.12 On the other hand, anesthetic resistance is the concept that some individuals may require higher or lower anesthetic dosage to produce the EEG patterns seen at typical anesthetic doses in most patients.
Here we studied an anesthetic resistance index based on processed EEG (i.e. bispectral index, or BIS) values and age-adjusted end tidal volatile anesthetic concentrations. Then we tested the hypothesis that low values of this brain anesthetic resistance index would be associated with increased postoperative delirium risk in older surgical patients. In essence, we theorized that lower BIS values in response to relatively lower anesthetic doses would serve as a marker of decreased neurophysiologic resistance of the brain to the sedative/hypnotic effects of gamma amino butyric acid (GABA)-ergic anesthetic drugs, similar to the way that significant sedation in response to small amounts of alcohol is commonly viewed as a marker of lower alcohol “tolerance”. We theorized that just as reduced alcohol tolerance can serve as a marker of decreased neurocognitive function,13 decreased anesthetic brain resistance would be associated with a perioperative neurocognitive disorder (e.g. postoperative delirium).
MATERIALS AND METHODS
Patient Population
All Duke perioperative optimization of senior health clinic14 patients seen from June 24, 2013 to September 25, 2015 were screened for inclusion into this study (N=278, see supplemental methods for additional details). The Duke University Medical Center Institutional Review Board (IRB) approved this study and waived the informed consent requirement. These data were combined with data prospectively collected from patients enrolled in an observational cohort study approved by the Mt. Sinai Medical Center IRB and registered with clinicaltrials.gov (NCT02650687). All Mt. Sinai patients underwent informed consent prior to participation, and were enrolled between November 2015 and 2018. The Mt. Sinai observational cohort study was primarily focused on postoperative cognitive dysfunction, though it also obtained postoperative delirium data. The Mount Sinai IRB waived the requirement for patients in this study to provide additional informed consent for the inclusion of their de-identified data in this manuscript. We obtained intraoperative data including BIS values, intraoperative end-tidal anesthetic concentrations and additional baseline medication information for both Duke and Mt. Sinai patients. Data were saved directly from operating room monitors onto secure servers at both institutions.
Duke and Mt Sinai patients were included in this study if they had surgery for >1 hour duration, and had end tidal anesthetic gas values and BIS data available for more than 50% of the case minutes. To exclude total intravenous anesthetic cases, we excluded any case in which the patient received >500 mg/hr of propofol. Anesthetic case length was defined by the case start and end times documented by the anesthesia provider.
Intraoperative Anesthetic Dosage
End-tidal anesthetic concentration (ETAC) was recorded continuously from 5 minutes after incision until 5 minutes prior to the end of surgery, in order to capture the anesthetic “plateau phase” of the case.15 Using a previously described method 16 to avoid data artifacts, the end-tidal minimum alveolar concentration (MAC) fraction was recorded once per minute, and the median value over each 5-minute case epoch was obtained. The mean of these median values was then calculated to determine the overall end-tidal MAC fraction. Next, we used MAC values at age 40 from our recent meta-regression analysis of age-related changes in MAC in published studies17 to calculate the age-adjusted end-tidal MAC fraction (aaMAC), again using the mean of median values obtained from each 5-minute case epoch.
| (1) |
MAC-hours was defined as the product of the case duration (in hours) and the aaMAC value from equation (1).
Bispectral Index (BIS) Value
Bispectral Index (BIS) (Covidien, Mansfield, MA) processed EEG values from electrodes placed on the left forehead were utilized for all cases at both institutions. The Duke operating rooms utilized 2 channel unilateral BIS electrodes connected via an E-BIS module to display the BIS index and raw waveform on the anesthesia GE (General Electric) monitors. The Mt. Sinai cases utilized BIS Vista monitors (Covidien, Mansfield, MA).
At both sites, the BIS proprietary algorithm transformed raw EEG data to a number from 0–100, with >90 indicating an awake state, <60 indicating unconsciousness and general anesthesia, and <40 indicating deep sedation. BIS values were obtained from 5 minutes after incision until 5 minutes before the “end of surgery” time stamp, again to target the anesthetic “plateau phase”. The BIS index was recorded once per minute, and the median value from each 5-minute case epoch was used to calculate a case average, as described.15
Inhaled Anesthetic Resistance Measurement
To gauge the appropriateness of BIS index values for a given aaMAC dose, and to measure the degree of BIS index drop for a given aaMAC dose, we developed the Duke Anesthesia Resistance Scale (DARS), defined as:
| (2) |
Here, BIS = mean of the median BIS readings during the case. The constant term of 2.5 represents the highest aaMAC value given in over 17,000 cases performed at a single academic center over a roughly two year period,15 and approximates the highest aaMAC value used in typical adult anesthesiology practice. The DARS denominator thus measures the difference between the highest volatile anesthetic dose possible in clinical practice and the actual dose received by a given patient. A high DARS value could thus result from a high BIS reading and/or a large aaMAC, and vice versa.
Delirium Evaluation and Diagnosis
All Duke patients in this study were followed daily after their index surgery by fellowship-trained geriatricians,14 all of whom underwent detailed training during their geriatrics fellowships on the standard Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition (DSM-V) clinical criteria for diagnosing delirium. These attending geriatricians closely examined patients for delirium and then coded it (if present) with one of the following International Classification of Disease, ninth edition (ICD9) codes in the patient’s chart: 290.11, 290.3, 290.41, 291.0, 292.81, 293.0, 293.1, 298.2, 348.3, 348.31, 348.39, 349.82, 437.2, 572.2, 768.7, 768.71, 768.72, 768.73, 780.09, or 780.97.13 Duke patients were defined as having postoperative delirium if any of these ICD9 codes was present in their patient record at any point during their postoperative index hospitalization. No inter-rater delirium reliability assessments were conducted between attending geriatricians in the Duke cohort, as attending-level geriatrician or psychiatrist assessments are already considered the “gold standard” for the evaluation of delirium.18
For Mt. Sinai patients, delirium assessments were performed twice daily by research staff using the confusion assessment method for the intensive care unit (CAM-ICU) instrument. Delirium assessment training for Mt. Sinai study staff was performed by the neuropsychiatry team; inter-rater reliability for Mt Sinai staff delirium assessments was not performed because the CAM-ICU questions have very little subjectivity.19 Mt. Sinai patients were considered to have postoperative delirium if they had a positive delirium assessment at any point during hospitalization after their index surgery.
At both Duke and Mt Sinai, in order to avoid potential bias in delirium assessments, individuals performing the delirium assessments were blinded (i.e. not given access) to intraoperative DARS scores.
Anticholinergic cognitive burden (ACB) score
The ACB assigns prescribed medications with known anticholinergic activity a score from 0–3 based on degree of predicted cognitive impairment in older adults, based on a multi-disciplinary consensus opinion validated to predict adverse outcomes such as delirium.20,21
Statistical analysis
Analysis was conducted on the combined patient cohort (i.e. from Duke and Mt Sinai). Categorical and numeric patient characteristics were summarized and compared between patients with versus without delirium using Pearson Chi-Square, t-tests, Wilcoxon rank sum, or Fisher’s exact test as appropriate. Normality was assessed via Shapiro-Wilks tests; non-parametric statistics were used when evidence of non-normality was found.
We compared DARS values between patients with and without delirium via a Wilcoxon ranked sum test and utilizing restricted cubic splines in univariable logistic regression. We found evidence of a threshold effect for DARS in the log-odds results for delirium; thus, we used the Youden Index22 to identify the cut point for DARS that maximizes the sensitivity and specificity associated with delirium risk. We determined the univariable association of the dichotomized “low” DARS variable with delirium using chi-square test and odds ratio estimation from logistic regression controlling for institutional site. Subsequently we performed multivariable logistic regression for delirium based on low DARS while adjusting for potential confounding from a priori known delirium risk factors, e.g. age, procedure duration, and anticholinergic burden score, as well as institutional site and intraoperative medications administered (opioids, midazolam, propofol, ketamine, phenylephrine, dexmedetomidine, and N2O use). Since our observed delirium incidence was low, we used Firths penalized likelihood in our multivariable logistic regression analysis to control for multiple potential confounding factors. Finally, to generate risk ratios we also analyzed the relationship between low DARS and delirium via log-linear binomial regression.
This was an exploratory study designed to develop a composite processed EEG-based measure of brain resistance to volatile anesthetics and evaluate the relationship between this measure and postoperative delirium. At the time the project was conceived, there was no prior literature describing either a processed EEG measure of brain anesthetic resistance, or the relationship between such a measure and postoperative delirium risk. Nonetheless, we reasoned that this study would likely have sufficient power, as prior studies relating other EEG metrics to other neurocognitive variables have often had slightly smaller sample sizes10,23 than the cohort studied here. A power analysis demonstrates that a sample size of 139 patients with a 25% delirium incidence and a 30% incidence of low DARS scores would provide 80% power with α=0.05 to detect an odds ratio of ≥3.0 for an association between low DARS and postoperative delirium risk.
All statistical analyses were performed using SAS v 9.4 (SAS Inc., Cary, NC), or R v 3.6.1 (R Foundation, Vienna, Austria) with the rms, OptimalCutpoints, and boot packages, and p < 0.05 was considered statistically significant.
RESULTS
We identified 69 Duke patients and 70 Mt Sinai patients who met our inclusion criteria (Fig. 1). Baseline and intraoperative characteristics for patients with vs without delirium are shown in Tables 1A and 1B, respectively. Postoperative delirium occurred in 35 of the 139 patients, i.e. in ~25% of the patients (Table 1A). Patients with versus without postoperative delirium were generally similar (Table 1). Neither aaMAC (median [Q1, Q3] 0.86 [0.76, 0.98] vs. 0.96 [0.80, 1.17]; p=0.074) nor BIS values (mean (SD) 46.72 (8.89) vs. 48.88 (8.77); p=0.212) differed significantly among patients with vs without postoperative delirium. DARS scores were significantly lower in patients with vs without delirium (median of 27.92 [24.85, 36.74] vs. 32.88 [28.95, 38.15], p=0.015; Table 1B); see methods for the DARS equation. Patients who developed postoperative delirium also received higher intraoperative phenylephrine dosage than those who did not develop delirium (1.74 [0.63, 5.32] vs 0.75 [0.25, 3.33] mg; p=0.038).
Figure 1:
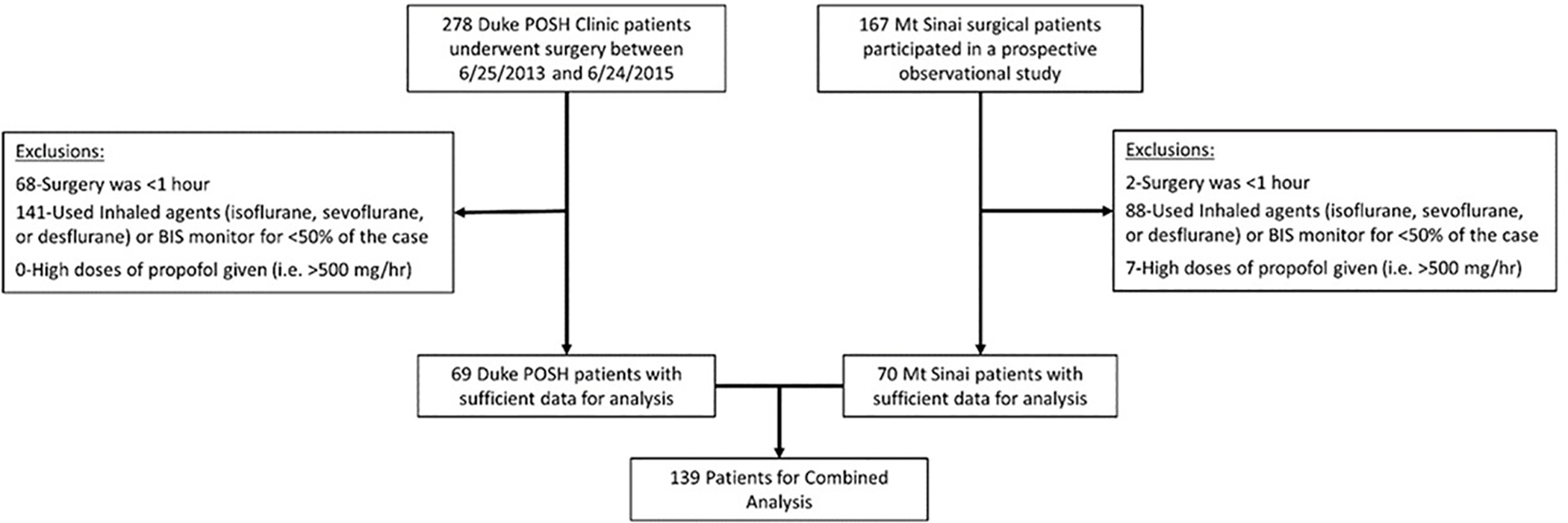
STROBE Diagram for the Analysis Cohort Formulation.
Table 1A.
Cohort baseline characteristics by delirium status. Factors summarized as count (%) for categorical variables and mean (SD) or median [Q1, Q3] for numeric variables.
| Overall (N=139) | No Delirium (n=104) | Delirium (n=35) | P Value | |
|---|---|---|---|---|
| Site | 0.0711 | |||
| Duke | 69 (49.6%) | 47 (45.2%) | 22 (62.9%) | |
| Mt Sinai | 70 (50.4%) | 57 (54.8%) | 13 (37.1%) | |
| Age | 73 (6) | 73 (6) | 74 (7) | 0.1792 |
| Gender (Male) | 60 (43.2%) | 46 (44.2%) | 14 (40.0%) | |
| BMI(kg/m^2) | 29.1 (7.7) | 29.0 (7.7) | 29.3 (8.1) | 0.8372 |
| ACBScore* | 0 [0, 1] | 0 [0, 1] | 0 [0, 2] | 0.4793 |
| ASAStatus | 0.6613 | |||
| 2 | 28 (20.1%) | 23 (22.1%) | 5 (14.3%) | |
| 3 | 106 (76.3%) | 76 (73.1%) | 30 (85.7%) | |
| 4 | 5 (3.6%) | 5 (4.8%) | 0 (0.0%) | |
| SurgeryCategory | 0.1244 | |||
| General | 59 (42.4%) | 48 (46.2%) | 11 (31.4%) | |
| Orthopedic | 50 (36.0%) | 32 (30.8%) | 18 (51.4%) | |
| Thoracic | 16 (11.5%) | 14 (13.5%) | 2 (5.7%) | |
| Urologic | 14 (10.1%) | 10 (9.6%) | 4 (11.4%) |
ACB score not available for 2 patients 1 with delirium and one without.
P-value key:
=chi-square
=t-test
=Wilcoxon rank sum
=fisher exact
Table 1B.
Cohort intraoperative measures by Delirium status. Data are summarized as count (%) for categorical variables and mean (SD) or median [Q1, Q3] for numeric variables.
| Overall (N=139) | No Delirium (n=104) | Delirium (n=35) | P Value | |
|---|---|---|---|---|
| Procedure Length (min) | 151 [110, 213] | 144 [110, 190] | 177 [110, 244] | 0.0991 |
| Fentanyl or Hydromorphone Used/ Dose (ME) | 135 (97.1%)/35 [25, 49] | 102 (98.1%)/35 [25, 50] | 33 (94.3%)/33.3 [25, 45] | 0.4481 |
| Midazolam Used/ Dose (mg) | 41 (29.5%)/2 [1, 2] | 28 (26.9%)/2 [2, 2] | 13 (37.1%)/1 [1, 2] | 0.5611 |
| Propofol Used / Dose (mg) | 85 (61.2%)/130 [100, 190] | 63 (60.6%%)/120 [100, 200] | 22 (62.9%)/142 [120, 190] | 0.4481 |
| Ketamine Used / Dose (mg) | 46 (33.1%)/71 [50, 100] | 33 (31.7%)/61 [35, 87] | 13 (37.1%)/85 [68, 106] | 0.2861 |
| Phenylephrine Used / Dose (mg) | 103 (74.1%)/1.10 [0.30, 4.18] | 75 (72.1%)/0.75 [0.25, 3.33] | 28 (80.0%)/1.74 [0.63, 5.32] | 0.0381 |
| Dexmedetomidine Used / Dose (mcg) | 6 (%)/19 [16, 20] | 4 (3.8%)/17 [12, 19] | 2 (5.7%)/34 [20, 48] | 0.6051 |
| N2O used | 4(2.9%) | 4 (3.9%) | 0 (0.0%) | 0.5722 |
| Primary Gas Used | 0.0643 | |||
| Desflurane | 42 (30.2%) | 28 (26.9%) | 14 (40.0%) | |
| Isoflurane | 56 (40.3%) | 40 (38.5%) | 16 (45.7%) | |
| Sevoflurane | 41 (29.5%) | 36 (34.6%) | 5 (14.3%) | |
| Case Average aaMAC | 0.94 [0.79, 1.15] | 0.96 [0.80, 1.17] | 0.86 [0.76, 0.98] | 0.0741 |
| aaMAC Hours | 2.17 [1.53, 3.59] | 2.11 [1.53, 3.52] | 2.74 [1.46, 3.74] | 0.5081 |
| Case Average BIS | 48.33 (8.82) | 48.88 (8.77) | 46.72 (8.89) | 0.2124 |
| Case Average BIS <45 | 50 (36.0%) | 36 (34.6%) | 14 (40.0%) | 0.5663 |
| DARS | 31.76 [27.03,37.94] | 32.88 [28.95, 38.15] | 27.92 [24.85, 36.74] | 0.0151 |
P-value key:
=Wilcoxon rank sum
=Fisher exact
=chi-square
=t-test
Lower DARS values were non-linearly associated with increased delirium risk (Fig. 2A), and more than 75% of all patients had DARS values <40 (see bar and whisker plot of DARS distribution across patients, bottom of Fig 2A). To determine an optimal threshold for defining a low DARS range associated with delirium, we used the Youden index. A threshold of 28.755 maximized the combined sensitivity and specificity of low DARS with postoperative delirium risk. To investigate the robustness of associations between low DARS status and delirium we calculated a bootstrap 95% confidence interval (26.18, 29.80) for this low DARS threshold (Fig 2A). The distribution of DARS scores in patients with vs without delirium is shown in Fig 2B.
Figure 2:
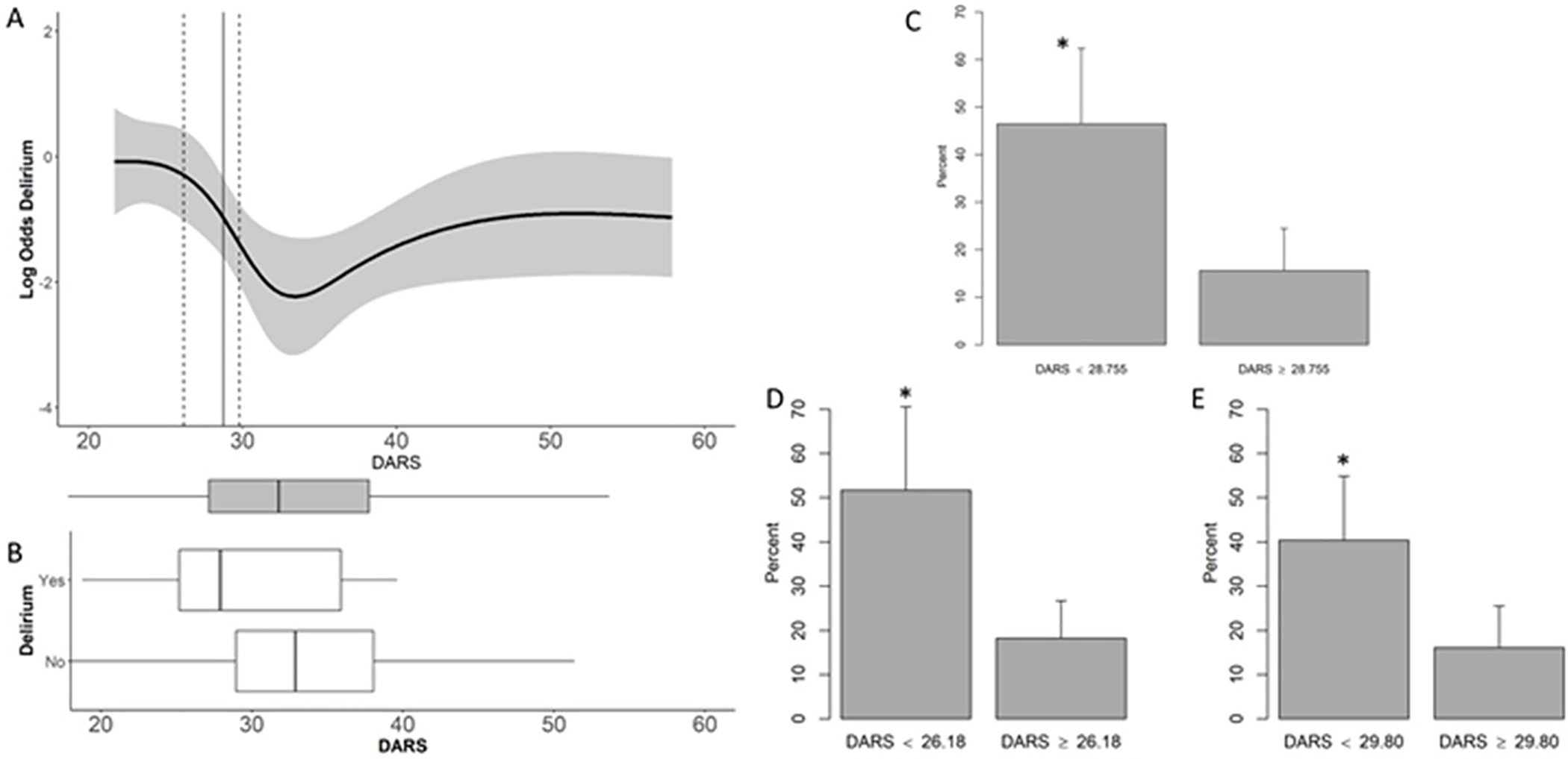
Low DARS and delirium. Panel A shows the relationship between DARS scores (x axis) and delirium risk (y axis) using a spline fit. The vertical straight line depicts the threshold for defining the low DARS range that maximizes the Youden index; the dashed vertical lines show the 95% bootstrap confidence lower and upper bounds for this threshold. The bar and whisker plot underneath Panel A shows the DARS score distributions among all patients- the center line within the shaded region is the median DARS score, the edges of the shaded box represent the lower and upper quartile boundaries, and the whiskers represent 1.5 times the interquartile range, with points beyond that range shown as individual dots. Panel B displays the DARS score distributions among patients with vs without delirium, using the same format as in the bottom of Panel A. Panels C-E display delirium rates in patients with versus without a low DARS score, using the optimum bound (C), lower bound (D), or upper bound (E) threshold to define the low DARS range. Error bars in C-E represent the 95% confidence interval. *indicates p<0.05 from the logistic regression model controlling for site.
Patients with vs without low DARS (as defined by a threshold 28.755) generally had similar characteristics, except that patients with low DARS scores not surprisingly tended to receive lower case average aaMAC (mean (SD) 0.80 (0.17) vs 1.11 (0.36), p<0.001) and had lower case average BIS values (mean (SD) 42 (6) vs 51 (8), p<0.001; Supplemental Table 1). In a logistic regression analysis, adjusting for site, low DARS status (i.e. <28.755) was associated with a nearly 4-fold increase in the odds of delirium (Fig 2C; OR=4.30, 95% CI: 1.89, 10.01; p=0.001). Similarly, logistic regression analysis, adjusting for site, using the lower or upper 95% confidence bound for the low DARS range also found a roughly 4-fold increased odds of delirium (Fig 2D–E, OR (95% CI) 4.64 (1.92, 11.39) p=0.001, and 3.24 (1.46, 7.40) p=0.004, respectively).
In multivariable models controlling for institutional site, patient age, anticholinergic burden (ACB) score, procedure duration, gender and ASA Status, a low DARS was associated with increased odds of postoperative delirium whether it was defined as <26.18 (OR (95% CI) 3.74 (1.52, 9.32); p=0.005), <28.755 (OR (95% CI) 3.79 (1.63, 9.10); p=0.003), or <29.80 (OR (95% CI) 3.16 (1.37, 7.55); p=0.009). Finally, even after controlling for other intraoperative medications and institutional site, a low DARS was associated with increased odds of postoperative delirium whether a low DARS was defined as <26.18 (OR (95% CI) 4.57 (1.84, 11.58); p=0.002), <28.755 (OR (95% CI) 4.21 (1.80, 10.16); p=0.002, or <29.80 (OR (95% CI) 3.44 (1.47, 8.34); p=0.006). These 9 different multiple variable models are summarized in Table 2 and Figure 3; full model details are provided in Supplemental Table 2.
Table 2.
Summary of Associations between low DARS and Postoperative Delirium Risk Using the three different cut point locations for low DARS, in four different multiple variable logistic regression models. Risk Factors modelled include age, anticholinergic burden (ACB) score, procedure duration, gender and ASA Physical Status Classification. Medications modelled include opioids (in morphine equivalents), midazolam, propofol, ketamine, phenylephrine, dexmedetomidine, and nitrous oxide use.
| Model | Low DARS Effect Conservative Cut Point (26.18) | Low DARS Effect Optimimum Cut Point (28.755) | Low DARS Effect Liberal Cut Point (29.80) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| 1. DARS +Site | 4.64 (1.92, 11.39) | 0.001 | 4.30 (1.89, 10.01) | 0.001 | 3.24 (1.46, 7.40) | 0.004 |
| 2. DARS + Site + Risk Factors | 3.74 (1.52, 9.32) | 0.005 | 3.79 (1.63, 9.10) | 0.003 | 3.16 (1.37, 7.55) | 0.009 |
| 3. DARS + Site + Medications | 4.57 (1.84, 11.58) | 0.002 | 4.21 (1.80, 10.16) | 0.002 | 3.44 (1.47, 8.34) | 0.006 |
| 4. DARS + Site + Risk factors + Meds | 3.75 (1.45, 9.87) | 0.009 | 3.64 (1.49, 9.25) | 0.007 | 3.12 (1.28, 7.96) | 0.017 |
Figure 3.
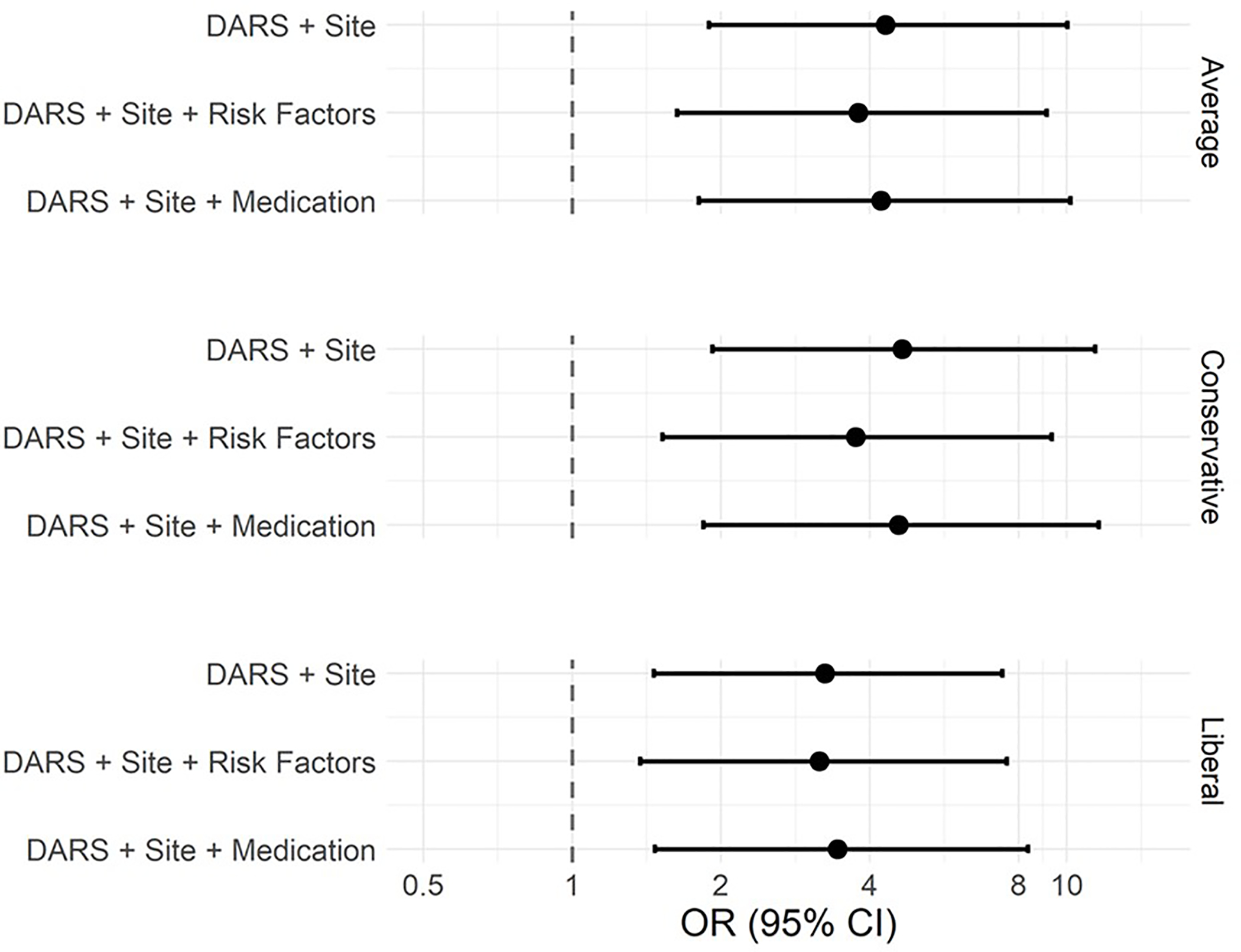
Forest plot for log of the odds ratio (and 95% CI) results from three models at the 3 different thresholds/cut points for the low DARS range.
Similarly, a log-linear model controlling for site showed a significant risk ratio (RR) for postoperative delirium in patients with a low DARS (RR (95% CI) 2.77 (1.55, 4.96); p=0.006). This result remained significant whether using the lower or upper confidence bound to define a low DARS (p<0.001 and p=0.006, respectively; Supplemental Table 3).
DISCUSSION
We found that low values of a processed EEG measure of brain resistance to volatile anesthetics (i.e. DARS) was independently associated with postoperative delirium risk. Compared to patients with a DARS of 28.755 or higher, those with a DARS <28.755 had a roughly four times higher odds of developing postoperative delirium. Although neither BIS nor aaMAC alone was associated with postoperative delirium risk alone, a low DARS was associated with postoperative delirium risk. The DARS effectively scales the BIS score by the difference between the maximum aaMAC fraction likely used in clinical practice (2.5) minus the actual aaMAC fraction received by the patient. A lower DARS thus implies a less active brain than would be expected for a given inhaled anesthetic dose (i.e. aaMAC fraction), i.e. decreased brain resistance to anesthetic-induced decreases in brain activity. Since low DARS scores were associated with increased postoperative delirium risk, low DARS scores could potentially be used clinically to direct scarce resources (such as geriatrics consultations) towards patients at relatively higher delirium risk.
These data complement the finding that increased anesthetic sensitivity (i.e. burst suppression divided by a composite anesthetic dosage measure) is associated with postoperative delirium risk.12 BIS values <30 are linearly (and inversely) related to burst suppression ratio.24 Thus, the relationship identified here between lower DARS and increased delirium risk may partly reflect an association between increased burst suppression at lower inhaled anesthetic doses and increased postoperative delirium risk.12 Further, the fact that there is a linear relationship between burst suppression ratio and BIS index numbers only below a BIS index value of 30 may help explain why there is a non-linear threshold relationship between the DARS and delirium risk. Indeed, the BIS index number also shows non-continuous threshold associations with other raw EEG parameters, such as spectral edge frequency 95%.25 Although the BIS index produces numbers from 0 to 100, it was never intended to be used as a linear index of sedation state. Instead, the manufacturer has suggested that there is a threshold relationship between the BIS index and intraoperative awareness with explicit recall, and recommends that clinicians maintain patients undergoing general anesthesia at a BIS value below 60 to reduce the risk of intraoperative awareness with explicit recall.26 Thus, given all of these non-linear threshold relationships between the BIS index numbers and both raw EEG parameters and clear clinical outcomes (such as awareness with explicit recall), it is unsurprising that there is also a non-linear threshold relationship between an anesthetic resistance index based on BIS values (the DARS) and postoperative delirium risk.
Our data also demonstrate that a low DARS was associated with increased delirium risk even after controlling for other drugs that can affect the EEG (i.e. opioids,27,28 ketamine,29 neuromuscular blockers,30 nitrous oxide,31 etc). This suggests that lower brain anesthetic resistance in response to volatile anesthetics is relatively greater in magnitude than the EEG effects of these other anesthetic drug adjuncts, at least at the doses in which they were used here. These results suggest that a low DARS identifies core neurologic features of a brain at risk for postoperative delirium independent of specific intraoperative anesthetic drug administration, at least in cases based primarily upon volatile anesthetics.
Lower brain anesthetic resistance (i.e. low DARS) was more closely associated than age with delirium risk, similar to the finding that neurophysiologic brain age is more closely related to delirium than is chronologic age,6 and consistent with the idea that the variance in organ function increases with age.32 There are age-dependent changes in EEG responses to inhaled anesthetics and propofol,33,34 yet chronological age can clearly be disassociated from biological age.35–37 In fact, markers of “brain age” are more closely associated than chronologic age with postoperative delirium risk, perhaps due to biological changes within the brain that occur at variable rates across people.6 Biological age has also been associated with increased inflammation,36,38 and inflammation increases anesthetic sensitivity in cultured neurons and whole animals.39 Thus, increased brain inflammation at baseline and/or in response to surgical stress40,41 could make the brain less resistant (or more sensitive) to anesthesia, resulting in lower DARS values. Future studies should examine the relationship between neuro-inflammation, brain anesthetic resistance (i.e. DARS values), and postoperative delirium.
This study has several limitations. First, we studied a brain anesthetic resistance (i.e. DARS) measure based on case summary BIS and aaMAC data. It is unclear whether brain anesthetic resistance measures based on shorter epochs would be similarly associated with postoperative delirium risk; this is an important topic for larger, future studies.
Second, the cohort studied here is of moderate size. We performed bootstrapping to define the 95% confidence bounds for a low DARS threshold for delirium risk to determine if the associations were robust to a potentially cohort specific cut point, and found consistent relationships between low DARS and delirium across the bootstrap interval. However, given the modest number of total delirium events and incidence of DARS < 27 in our cohort, future prospective studies with sufficient size are warranted to provide a more precise point estimate for both the odds ratio for delirium among patients with a low DARS threshold, and to more precisely define this low DARS threshold.
Third, the relationship between DARS and delirium observed here was studied in older adults who are at increased risk for postoperative delirium. Thus, the extent to which the association demonstrated here between low DARS scores and postoperative delirium risk can be extrapolated to younger patients is unclear. Future studies should evaluate the extent to which low DARS scores are associated with delirium in young and middle aged adults.
Fourth, delirium was assessed here via two different methods: geriatrician interviews (based on DSM-V criteria) at Duke, versus CAM-ICU assessments performed by research staff at Mt. Sinai. The use of these two different delirium assessment methods in our cohort may thus have increased statistical error, although both geriatrician interview and CAM-ICU assessments by well-trained staff are highly sensitive and specific for identifying delirium.42
Fifth, the DARS utilizes the BIS index, which is based on an unpublished algorithm,43,44 and BIS values are likely erroneously high by several points in older adults.15 Yet, despite this issue, these results demonstrate that BIS values in older adults are still sufficient for finding an association with increased postoperative delirium risk when used together with aaMAC in the DARS equation. Without an “ideal” anesthetic EEG monitor,45,46 the DARS offers a way to utilize BIS data and end tidal anesthetic dosage to evaluate postoperative delirium risk. Future studies should compare the association strength with delirium of the DARS versus other EEG parameters (e.g., burst suppression, alpha power, etc), and to examine relationships between these measures and other postoperative outcomes.
Nonetheless, the association between low DARS scores and increased delirium risk is a step forwards based on the current state of the field, as we are unaware of any other anesthetic resistance index utilizing routinely available intraoperative monitor data that is associated with postoperative delirium risk. The DARS is the first such equation to be studied, and we expect further refinements of it or other such equations will help improve the utility of these measures for clinical practice.
In summary, these data demonstrate that a processed EEG-based measure of brain anesthetic resistance is independently associated with delirium risk in older surgical patients. Future studies should attempt to replicate these findings, to define more precisely the threshold for a low DARS, to evaluate its use as an intraoperative real-time delirium risk stratification tool, to understand the neurobiological basis of low brain anesthetic resistance, and to study whether altering intraoperative care in patients with low DARS scores could help prevent delirium.
Supplementary Material
Key Points Summary.
Question: Is a processed EEG based-brain anesthetic resistance index associated with postoperative delirium risk in older surgical patients?
Findings: An intra-operative processed EEG-based measure of lower brain anesthetic resistance (i.e. DARS < 28.755) was independently associated with postoperative delirium risk in a combined patient cohort from two different institutions.
Meaning: A processed EEG-based measure of brain anesthetic resistance could potentially be used to identify older adults at increased risk for postoperative delirium
Acknowledgements:
We thank Austin Traylor (Duke Anesthesiology Department) for obtaining intraoperative electronic data from the Epic medical record system, Dr Stacey Chung (Duke Anesthesiology Department) for literature search assistance, and Dr. Carl Pieper for thoughtful comments on the manuscript. The Duke Anesthesia Resistance Scale, including the equation, and all related documentation and software, are owned by Duke University. (c) Copyright 2020. Duke University. All Rights Reserved. Developed by Duke University School of Medicine, Duke University.
Funding Statement: Dr. Berger acknowledges support from a mentored research grant from the International Anesthesia Research Society; NIH grants R03-AG050918 and K76-AG057022, and additional support from NIH grants T32-GM08600 and P30AG028716; a small project grant from the American Geriatrics Society; a Jahnigen Scholar Award from the Foundation for Anesthesia Education and Research and the American Geriatrics Society; and the Duke Private Diagnostic Clinic ENABLE program and the Duke Anesthesiology Department. Dr. Whitson’s contributions were supported by the Duke Claude D. Pepper Older American Independence Centre [P30AG028716]; the Physical Resilience Indicators and Mechanisms in the Elderly (PRIME) Collaborative [UH2AG056925]; and National Center for Advancing Translational Sciences of the National Institutes of Health [UL1TR002553]. Dr. Deiner acknowledges support from the American Federation of Aging Beeson Program and NIH K23 AG048332.
Conflicts of Interest: No authors or any close family members have any financial stake, stock, or other interest in Medtronic or Covidien. Dr. Deiner has served as a consultant for Medtronic Boulder CO and Merck as has served as an expert legal witness. MB acknowledges receiving material support (monitors) from Masimo for another study unrelated to this manuscript, and private consulting income from two legal cases regarding postoperative cognition.
Glossary of Terms:
- aaMAC
age adjusted end-tidal MAC fraction
- ACB
Anti-Cholinergic Burden
- ASA
American Society of Anesthesiology
- BIS
BiSpectral Index
- CAM
Confusion Assessment Method
- CAM-ICU
Confusion Assessment Method for the Intensive Care Unit
- CI
Confidence Interval
- DARS
Duke Anesthesia Resistance Scale
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EEG
Electroencephalogram
- ETAC
End Tidal Anesthetic Concentration
- ICD-9
International Classification of Diseases, 9th edition
- IRB
Institutional Review Board
- MAC
Minimum Alveolar Concentration
- OR
Odds Ratio
- RR
Risk Ratio
Footnotes
Clinical Trial Number and Registry URL: N/A
Prior presentations: N/A
References
- 1.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. [DOI] [PubMed] [Google Scholar]
- 2.American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger M, Mark JB, Kreuzer M. Of Parachutes, Speedometers, and EEG: What Evidence Do We Need to Use Devices and Monitors? Anesth Analg. 2020;130(5):1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan MTV, Hedrick TL, Egan TD, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on the Role of Neuromonitoring in Perioperative Outcomes: Electroencephalography. Anesth Analg. 2020;130(5):1278–1291. [DOI] [PubMed] [Google Scholar]
- 6.Hesse S, Kreuzer M, Hight D, et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2019;122(5):622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedemonte JC, Plummer GS, Chamadia S, et al. Electroencephalogram Burst-suppression during Cardiopulmonary Bypass in Elderly Patients Mediates Postoperative Delirium. Anesthesiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz BA, Kalarickal PL, Maybrier HR, et al. Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg. 2016;122(1):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giattino CM, Gardner JE, Sbahi FM, et al. Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front Syst Neurosci. 2017;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch S, Feinkohl I, Chakravarty S, et al. Cognitive Impairment Is Associated with Absolute Intraoperative Frontal alpha-Band Power but Not with Baseline alpha-Band Power: A Pilot Study. Dement Geriatr Cogn Disord. 2019;48(1–2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritz BA, Maybrier HR, Avidan MS. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth. 2018;121(1):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14(1):69–89. [DOI] [PubMed] [Google Scholar]
- 14.McDonald SR, Heflin MT, Whitson HE, et al. Association of Integrated Care Coordination With Postsurgical Outcomes in High-Risk Older Adults: The Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018;153(5):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni K, Cooter M, Gupta DK, et al. Paradox of age: older patients receive higher age-adjusted minimum alveolar concentration fractions of volatile anaesthetics yet display higher bispectral index values. Br J Anaesth. 2019;123(3):288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cleve WC, Nair BG, Rooke GA. Associations Between Age and Dosing of Volatile Anesthetics in 2 Academic Hospitals. Anesth Analg. 2015. [DOI] [PubMed] [Google Scholar]
- 17.Cooter M, Ni K, Thomas J, et al. Age-dependent decrease in minimum alveolar concentration of inhaled anaesthetics: a systematic search of published studies and meta-regression analysis. Br J Anaesth. 2020;124(1):e4–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184(3):340–344. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 20.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Puglia MP, Lapointe AP, et al. Age-Related Changes in Cortical Connectivity During Surgical Anesthesia. Front Aging Neurosci. 2019;11:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruhn J, Bouillon TW, Shafer SL. Bispectral index (BIS) and burst suppression: revealing a part of the BIS algorithm. J Clin Monit Comput. 2000;16(8):593–596. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto Y, Hagihira S, Koizumi Y, Ishida K, Matsumoto M, Sakabe T. The relationship between bispectral index and electroencephalographic parameters during isoflurane anesthesia. Anesth Analg. 2004;98(5):1336–1340, table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Medtronic I. MONITORING CONSCIOUSNESS Using the Bispectral Index™ (BIS™) brain monitoring system during anesthesia. https://www.medtronic.com/content/dam/covidien/library/us/en/product/brain-monitoring/bis-complete-4-channel-monitoring-consciousness-during-anesthesia-brochure.pdf. Accessed April 19, 2021, 2021.
- 27.Cox EH, Kuipers JA, Danhof M. Pharmacokinetic-pharmacodynamic modelling of the EEG effect of alfentanil in rats: assessment of rapid functional adaptation. Br J Pharmacol. 1998;124(7):1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien CB, Baghdoyan HA, Lydic R. Computer-based Multitaper Spectrogram Program for Electroencephalographic Data. J Vis Exp. 2019(153). [DOI] [PubMed] [Google Scholar]
- 29.Akeju O, Song AH, Hamilos AE, et al. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127(6):2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuller PJ, Newell S, Strickland PA, Barry JJ. Response of bispectral index to neuromuscular block in awake volunteers. Br J Anaesth. 2015;115 Suppl 1:i95–i103. [DOI] [PubMed] [Google Scholar]
- 31.Pavone KJ, Akeju O, Sampson AL, Ling K, Purdon PL, Brown EN. Nitrous oxide-induced slow and delta oscillations. Clin Neurophysiol. 2016;127(1):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger M, Acker L, Deiner SG. Miller’s Anesthesia. Elsevier; 2019. [Google Scholar]
- 33.Schultz A, Grouven U, Zander I, Beger FA, Siedenberg M, Schultz B. Age-related effects in the EEG during propofol anaesthesia. Acta Anaesthesiol Scand. 2004;48(1):27–34. [DOI] [PubMed] [Google Scholar]
- 34.Purdon PL, Pavone KJ, Akeju O, et al. The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115 Suppl 1:i46–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkle A, Moreno-Villanueva M, Bernhard J, et al. MARK-AGE biomarkers of ageing. Mech Ageing Dev. 2015;151:2–12. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. [DOI] [PubMed] [Google Scholar]
- 39.Avramescu S, Wang DS, Lecker I, et al. Inflammation Increases Neuronal Sensitivity to General Anesthetics. Anesthesiology. 2016;124(2):417–427. [DOI] [PubMed] [Google Scholar]
- 40.Berger M, Ponnusamy V, Greene N, et al. The Effect of Propofol vs. Isoflurane Anesthesia on Postoperative Changes in Cerebrospinal Fluid Cytokine Levels: Results from a Randomized Trial. Front Immunol. 2017;8:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch J, Vacas S, Terrando N, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 43.Avidan MS, Jacobsohn E, Glick D, et al. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365(7):591–600. [DOI] [PubMed] [Google Scholar]
- 44.Mashour GA, Shanks A, Tremper KK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology. 2012;117(4):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berger M, Schenning KJ, Brown CHt, et al. Best Practices for Postoperative Brain Health: Recommendations From the Fifth International Perioperative Neurotoxicity Working Group. Anesth Analg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitlock EL, Villafranca AJ, Lin N, et al. Relationship between bispectral index values and volatile anesthetic concentrations during the maintenance phase of anesthesia in the B-Unaware trial. Anesthesiology. 2011;115(6):1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


