Abstract
Background:
Severe trauma is associated with severe systemic inflammation and neuroendocrine activation that is associated with erythroid progenitor growth suppression and refractory anemia. Although distinct transcriptional profiles have been detected in numerous tissue types after trauma, no study has yet characterized this within the bone marrow. This study sought to identify a unique bone marrow transcriptomic response following trauma.
Methods:
In a prospective observational cohort study, bone marrow was obtained from severely injured trauma patients with a hip or femur fracture (n=52), elective hip replacement patients (n=33) and healthy controls (n=11). RNA was isolated from bone marrow using a Purelink RNA mini kit. Direct quantification of mRNA copies was performed by NanoString Technologies on a custom gene panel.
Results:
Trauma patients displayed an upregulation of genes encoding receptors known to have inhibitory downstream effects on erythropoiesis, including ferroportin, interleukin-6 (IL-6) receptor, transforming growth factor-beta (TGF-β) receptor, and IL-10, as well as genes involved in innate immunity including toll-like receptor 4 (TLR4)-mediated signaling factors. In contrast, hip replacement patients had downregulated transcription of IL-1β, IL-6, TGF-β, tumor necrosis factor alpha (TNFα), and the HAMP gene with no change in TLR4-mediated signaling factors.
Conclusions:
A unique transcriptomic response within the bone marrow was identified following severe trauma compared to elective hip replacement. These transcriptomic differences were related to the innate immune response as well as known inhibitors of erythropoiesis. Although confined to just one time point, this differential transcriptional response may be linked to refractory anemia and inflammation after injury.
Keywords: erythropoiesis, innate immunity, toll-like receptor, ferroportin, IL-6
Introduction
Approximately one fourth of all hospitalized trauma patients spend time in the intensive care unit (ICU) (1). Among those critically ill patients, anemia is nearly universal and treatment typically involves transfusions of packed red blood cells which are independently associated with nosocomial infections, increased ICU length of stay, and in-hospital mortality (2–9). To date, no successful therapeutic agents have been shown to prevent bone marrow dysfunction after trauma in reproducible clinical trials (10–13). Chronic inflammation along with a hyperadrenergic response while in the ICU has been shown to play a role in bone marrow dysfunction but the underlying mechanisms are not fully understood (14–16). It is not known which transcriptional changes or inflammatory pathways are responsible for hindering adequate erythroid recovery after injury and hemorrhage.
Erythropoiesis is the crucial process by which hematopoietic stem cells differentiate into erythroid progenitors which mature into enucleated reticulocytes to form blood erythrocytes. Rodent models of trauma, hemorrhage, and stress demonstrate an inflammatory response that leads to decreased erythroid progenitor cell growth, increased erythropoietin with decreased erythropoietin receptor expression in the bone marrow, and reduced iron bioavailability (17, 18). Low reticulocyte counts despite elevated erythropoietin levels, along with increased hepcidin concentrations, have also been shown following trauma (19, 20). Human data offers evidence of increased inflammatory cytokines and an abnormal erythropoietin response in critically ill injured patients (21–23). Others have shown genome-wide transcriptional changes after trauma, including increased inflammatory signaling and alterations in innate and adaptive immune pathways (24).
Despite decades of research into the postinjury inflammatory response, hematopoietic studies have been limited by the invasive nature of bone marrow sampling or reliance on circulating peripheral bone marrow cells (25). To overcome the limitations of peripheral blood samples, this study examined intraoperative bone marrow samples of patients after severe injury as well as patients undergoing elective hip replacement. The aim of this study was to quantify changes in key inflammatory and erythropoietic modulatory genes within the bone marrow to identify foci of transcriptional reprioritization after trauma. We hypothesized that the post-traumatic human bone marrow transcriptomic response would be uniquely altered compared to elective hip replacement patients and healthy controls, with an upregulation of inflammatory activity and downregulation of basal erythropoietic activity.
Materials and Methods
Study Population
We performed a prospective observational cohort study comparing three populations: severely injured blunt trauma patients (n=52), patients undergoing elective total hip replacement (n=33), and bone marrow from healthy donors (n=11). Patients undergoing elective total hip replacement were enrolled to serve as a control for anesthesia exposure, blood loss, and orthopedic manipulation at time of bone marrow collection. Trauma patients from a Level 1 trauma center were screened on admission for inclusion in the study, with prior approval by the Institutional Review Board (IRB201601386). Inclusion criteria for the blunt trauma cohort were: age ≥ 18, lower extremity long bone or pelvic fracture requiring open reduction and internal fixation, hemorrhagic shock at time of admission (defined by systolic blood pressure ≤ 90 mmHg, or mean arterial pressure ≤ 65 mmHg, or base deficit ≥ 5 mEq/L or lactate ≥ 2 mmol/L) and an injury severity score (ISS) ≥ 15. Exclusion criteria included: survival < 48 hours, incarceration, pregnancy, patients receiving chronic corticosteroids or immunosuppressive therapies, previous bone marrow transplantation, or end stage renal disease. The elective hip replacement cohort were age ≥ 18 years and underwent an elective total hip replacement with the same exclusion criteria as the trauma cohort.
This study was registered at ClinicalTrials.gov (NCT02577731). All elective hip replacement patients and non-intubated trauma patients gave written informed consent for the research procedures. For those critically ill trauma patients who were not able to provide written informed consent before tissue sampling, written informed consent was obtained from their surrogate decision maker, and written informed consent was also obtained from the patient if and when they regained decision-making capacity.
Clinical data regarding patient demographics and operative management were obtained from the electronic medical record by a physician reviewer and stored in an encrypted database. Documented values included age, sex, admission vital signs, ICU and hospital length of stay, and complete blood count (CBC) results. Blood loss during the time of hip replacement or fracture fixation surgery was documented as estimated operative blood loss. Total hospital blood loss was calculated as the sum of documented blood loss from any surgeries or procedures that occurred during admission.
Bone Marrow Collection and Processing
For the trauma patients, bone marrow samples were obtained using an 11-gauge bone marrow biopsy needle (Argon Medical Devices, Frisco, TX) from either the ilium, femur, or tibial plateau under general anesthesia during their scheduled open reduction and internal fixation of their pelvis, acetabulum, femur, or tibia. During total hip replacement surgery, bone marrow was collected from the femoral head. A physician investigator was present in the operating room for collection of all bone marrow samples, which were obtained from the sterile field by an orthopedic surgeon to ensure consistency of the sample collection. A minimum 2 mL liquid bone marrow was obtained from each patient for purposes of this study. Healthy control bone marrow samples obtained via iliac crest biopsy under local anesthesia were purchased from Lonza (Basel, Switzerland).
Each bone marrow sample was passed through a 70 μm cell strainer into a 50 mL conical tube. Erythrocytes were then lysed by adding 3 volumes of ammonium-chloride-potassium lysis buffer (Gibco, ThermoFisher Scientific, Waltham, MA). The mixture of bone marrow and lysis buffer was gently mechanically agitated for five minutes and then centrifuged for 8 minutes at 400 g. The supernatant was decanted. This process was repeated until a white pellet was formed and suspended in 1 mL Iscove’s Modified Dulbecco’s Medium.
Blood Collection and Processing
Blood samples were obtained from the trauma and hip replacement patients on day of surgery and collected in a fully filled heparinized 6 mL tube (Becton Dickinson, Franklin Lakes, NJ). Blood samples from the same healthy control bone marrow donors were not available. Plasma from trauma and hip replacement patients was collected and stored in a −80°C freezer. Plasma hepcidin was measured by enzyme linked immunosorbent assay according to manufacturer’s protocol (R&D Systems, Minneapolis, MN).
RNA isolation and direct mRNA quantification
Bone marrow RNA was isolated using the RNeasy Mini Kit (Qiagen, Germantown, MD) and stored at −80°C. 100ng total RNA per sample was analyzed for the expression of 34 key inflammation- and erythropoiesis-related genes on the nCounter MAX Analysis System (NanoString Technologies, Seattle, WA) to determine mRNA copy number (26). The panel was chosen to focus on a select sampling of genes involved in erythropoietic and inflammatory pathways. Raw data were normalized using the nSolver Analysis Software (Version 4.0).
Statistical Analysis
Statistical analysis for gene expression was performed using nSolver Analysis Software Version 4.0 (NanoString Technologies, Seattle, WA) and graphed in GraphPad Prism Version 9 (GraphPad Software, La Jolla, CA). Geometric means of healthy controls were used to normalize the housekeeping genes, which were then used to normalize the samples. All normalization and gene data analysis were performed using nSolver Analysis Software Version 4.0. The cutoff for significance was set to *p<0.05 for differentially expressed genes. Associations between mRNA transcript numbers, age, and time to fracture fixation surgery (i.e. time elapsed from injury to bone marrow collection) were assessed using GraphPad Prism Version 9.0 by means of simple linear regression; two-tailed unpaired t-tests comparing age greater or less than 65 or time to surgery greater or less than 1 day after admission; or ANOVA with Tukey’s post hoc test for multiple comparisons comparing age by decade of life or 0–2, 3–5, and 6–9 days from admission to surgery. Data analysis for plasma hepcidin levels was performed in GraphPad Prism Version 9.0 using a two-tailed unpaired t-test to determine significance or using simple linear regression to determine correlations with gene transcription. All data were reported as mean ± standard error.
Results
Patient characteristics
There was a significant difference in age across all three groups (Table 1). Patients in the elective hip replacement cohort were significantly older, being an average 21 and 34 years older than the trauma cohort and healthy controls, respectively. The trauma cohort included a larger proportion of males than operative or healthy controls (59% vs. 42.4% and 45.4%, respectively). Trauma patients included in the study had a mean ISS of 23.7 ± 1.5. As anticipated, the trauma cohort was more anemic than the elective hip replacement cohort on admission (11.6 g/dL vs. 13.7 g/dL) and had suffered an indeterminately large amount of prehospital blood loss. Intraoperative blood loss was similar during hip replacement and fracture fixation surgery, although the trauma cohort went on to undergo on average two additional surgeries and ultimately sustained cumulative in-hospital blood losses nearly triple that of hip replacement cohort (Table 1). Average time from admission to fracture fixation surgery and bone marrow collection was 2.9 days (median 2 days, range 0–9 days). Fifty percent of trauma patients underwent fracture fixation surgery within the first four days of hospitalization. Among trauma patients, there were no statistically significant associations between time from admission to fracture fixation surgery (i.e. time since injury) and number of differentially expressed mRNA transcripts using any of the three statistical methods employed. Among all cohorts, there were no statistically significant associations identified between age and number of mRNA transcripts.
Table 1.
Summary of enrolled patient characteristics. Data presented as mean ± SEM. Operative blood loss reflects losses sustained during fracture fixation or total hip replacement surgery only. Total hospital blood loss reflects both phlebotomy and cumulative operative blood loss. Preoperative and postoperative refer to before and after fracture fixation or total hip replacement.
| Healthy Control | Hip Replacement | Trauma | |
|---|---|---|---|
| Number of patients | 11 | 33 | 52 |
| Age (years) | 29 ± 2# | 63 ± 2 * | 42 ± 2#, * |
| Male (%) | 45.4 | 42.4 | 59 |
| Hispanic (%) | 36.4 | 0 * | 0 * |
| Total PRBCs (units) | - | 0 | 5.1 ± 1.0# |
| Total operations | - | 1 | 3.0 ± 0.3# |
| Hospital length of stay (days) | - | 1.3 ± 0.2 | 14.2 ± 1.1# |
| ICU length of stay (days) | - | 0 | 7.6 ± 1.0# |
| Anesthesia | Local | General | General |
| Tibial plateau | - | - | 12% |
| Operative blood loss (mL) | - | 508 ± 43 | 526 ± 77 |
| Total hospital blood loss (mL) | - | 520 ± 43 | 1430 ± 181# |
| Admission hemoglobin (g/dL) | - | 13.7 ± 0.2 | 11.6 ± 0.3# |
| Preoperative hemoglobin (g/dL) | - | 13.7 ± 0.2 | 9.8 ± 0.3# |
| Postoperative hemoglobin (g/dL) | - | 11.1 ± 0.3 | 9.3 ± 0.2# |
| Discharge hemoglobin (g/dL) | - | 10.6 ± 0.2 | 9.8 ± 0.2# |
ICU=intensive care unit; PRBCs=packed red blood cells;
p<0.05 vs. elective total hip replacement;
p<0.05 vs. healthy controls.
Dysfunctional erythropoiesis and iron regulation following trauma
Out of the 34 genes assayed (including 29 target genes), 25 showed significant differential expression between trauma patients and operative controls undergoing elective hip replacement (Supplemental Table 1). Genes encoding erythropoietin were not significantly altered in the bone marrow of trauma patients or elective hip replacement patients as compared to healthy controls. There was a significant differential expression of erythropoietin from trauma patients compared to elective hip replacement (Figure 1). There was also significant reduction in the bone marrow hepcidin gene (HAMP) following elective hip replacement which was not seen after trauma (Figure 1). This occurred in conjunction with increased plasma hepcidin levels found in trauma patients. Plasma hepcidin levels were more than double that found in elective hip replacement patients (66±4 vs. 29±3 ng/mL, p<0.001). This increase in plasma hepcidin coincided with an increase in ferroportin gene copies within the bone marrow of trauma patients (Figure 1). There was significant but weak correlation between hepcidin and increased toll-like receptor 4 (TLR4) and IL-10 transcription in the bone marrow of trauma patients (r2 = 0.103 and 0.144, p = 0.025 and 0.0079, respectively).
Figure 1.
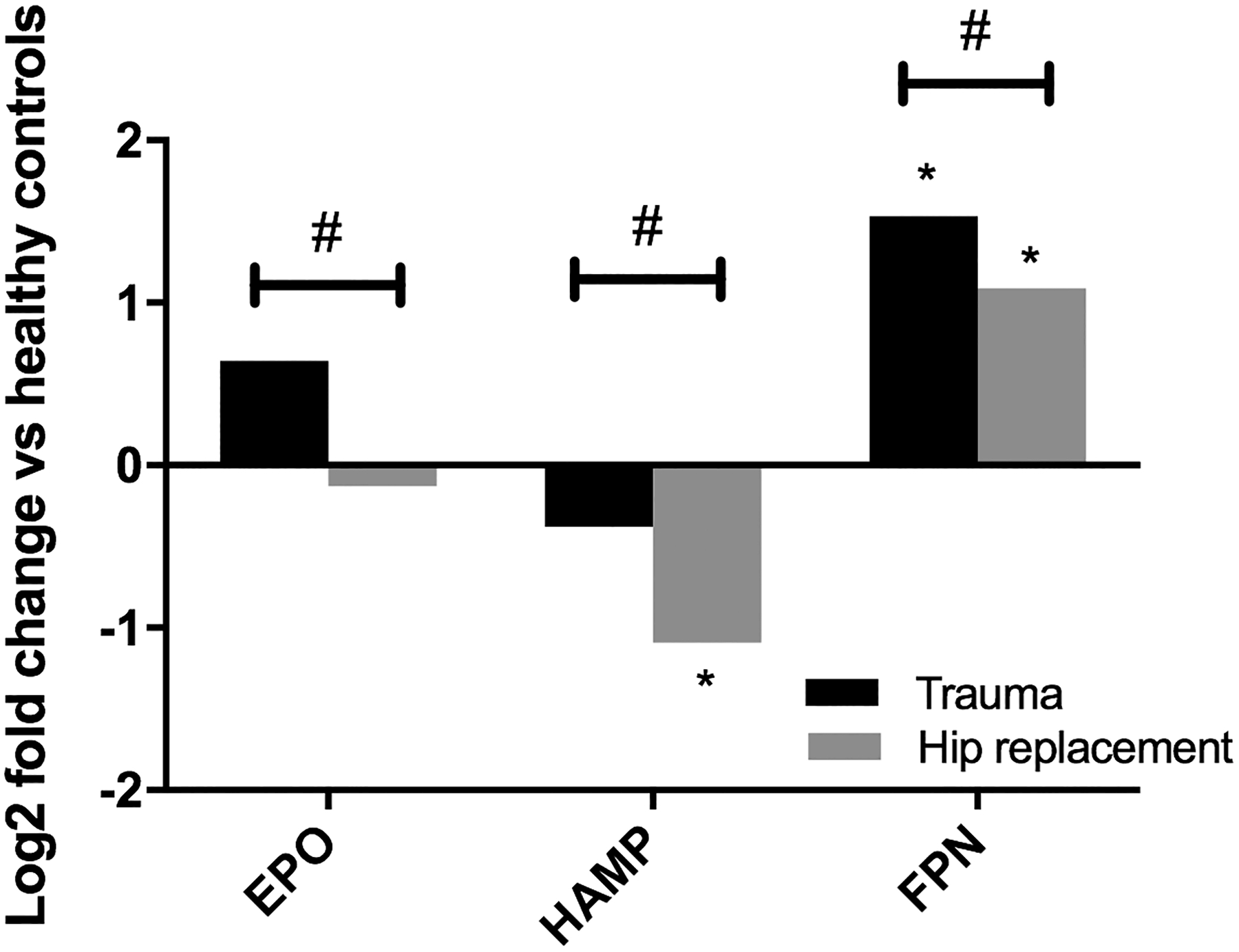
Presented as fold change relative to healthy controls, which were normalized to a baseline of zero. EPO = erythropoietin; HAMP = hepcidin; FPN = ferroportin; *p<0.05 vs. healthy controls; #p<0.05 elective hip replacement vs. trauma patients
Upregulated toll-like receptor proinflammatory pathways following trauma
Following trauma, there was a significant increase in transcription of genes associated with TLR4 signaling (Figure 2). This included the genes encoding TLR4 itself as well as adaptor and mediator proteins involved in canonical and non-canonical MyD88-dependent signaling pathways. Significantly increased copy numbers of TLR4, MyD88 and genes encoding downstream products interleukin (IL)-6 receptor and IL-1β were shown after trauma (Figure 2). Nuclear factor kappa B (NFκB), the downstream transcription factor responsible for canonical upregulation of IL-6, IL-1β, and tumor necrosis factor alpha (TNFα), was also present in significantly greater amounts in the trauma patients compared to elective hip replacement, though neither values significantly differed from healthy controls. Elective hip replacement was not associated with proinflammatory TLR4 pathway activation. Furthermore, IL-6, TNFα, and IL-1β were all significantly downregulated following elective hip replacement (Figure 2).
Figure 2.

Changes in Toll-like receptor 4 (TLR4) signaling in canonical myeloid differentiation primary response 88 (MyD88)-mediated signaling pathways. Presented as fold change relative to healthy controls, which were normalized to a baseline of zero. NFκB = nuclear factor kappa B; IL-6 = interleukin 6; IL-6R = interleukin 6 receptor; TNFa = tumor necrosis factor alpha; IL-1b = interleukin 1 beta; *p<0.05 vs. healthy controls; #p<0.05 elective hip replacement vs. trauma patients
The mitogen-activated protein kinase (MAPK)-mediated pathway was also activated following trauma (Figure 3). There were significant increases in TLR4, MAPK 1, 3, and 8, which encode extracellular signal-related kinases 1 and 2 (ERK1, ERK2) and c-Jun N-terminal kinase (JNK), respectively, following trauma (Figure 3). Bone marrow interferon-gamma (IFNγ), a downstream product of the MyD88-independent pathway, significantly decreased in both trauma patients and elective hip replacement patients compared to healthy controls.
Figure 3.

Changes in Toll-like receptor 4 (TLR4) signaling in noncanonical myeloid differentiation primary response 88 (MyD88)-mediated and MyD88 independent signaling pathways. Presented as fold change relative to healthy controls, which were normalized to a baseline of zero. MAPK8 = mitogen activated protein kinase 8/c-Jun N-terminal kinase (JNK); MAPK1 = mitogen activated protein kinase 1/extracellular signal-related kinase 2 (ERK2); MAPK3 = mitogen activated protein kinase 3/extracellular signal-related kinase 1 (ERK1); NFKB = nuclear factor kappa B; IFNG = interferon gamma; *p<0.05 vs. healthy controls; #p<0.05 elective hip replacement vs. trauma patients
Alterations in anti-inflammatory cytokines following trauma
Pro-inflammatory disorders have been shown to be associated with an increase in the compensatory anti-inflammatory response. Trauma patients demonstrated upregulation in bone marrow transforming growth factor beta (TGF-β) receptor and IL-10 genes which was not seen following elective hip replacement (Figure 4). In comparison to healthy controls, elective hip replacement led to decreased transcription of TGF-β and IL-10 (Figure 4).
Figure 4.

Transcriptomic changes in anti-inflammatory cytokines following trauma. Presented as fold change relative to healthy controls, which were normalized to a baseline of zero. TGFb =transforming growth factor beta; TGFbR1 = transforming growth factor beta receptor 1; IL-10 = interleukin 10; *p<0.05 vs. healthy controls; #p<0.05 elective hip replacement vs. trauma patients
Discussion
The primary goal of this work was to describe the transcriptomic changes in human bone marrow following severe injury. This study identified a number of differentially expressed inflammatory genes in the bone marrow of trauma patients when compared to elective hip replacement and nonoperative healthy controls. Despite the presence of anemia following both elective hip replacement and severe trauma, the trauma cohort demonstrated markedly increased transcription of inflammatory mediators, particularly of genes involved in TLR4 pathway-mediated responses, which included intracellular mediators of canonical and non-canonical MyD88-dependent signaling pathways (Figure 5). In addition, trauma patients also had significant upregulation of the genes encoding receptors known to have inhibitory downstream effects on erythropoiesis, including ferroportin, IL-6 receptor, and TGF-β receptor.
Figure 5.

Overview of TLR4 signaling pathway. TIRAP (Toll/interleukin-1 receptor domain-containing adaptor protein); MyD88 (myeloid differentiation primary response 88); IRAK (interleukin-1 receptor associated kinase); TRAF (tumor necrosis factor receptor associated factor); IKK (IkappaB kinase); NFκB (nuclear factor kappa B); TAK1 (transforming growth factor-beta-activated kinase 1); JNK (c-Jun N-terminal kinase); ERK (extracellular signal-related kinase); AP-1 (activator protein 1); TRAM (translocation associated membrane protein); TRIF (Toll-interleukin-1 receptor domain-containing adapter protein inducing interferon beta); IRF3 (interferon regulatory transcription factor 3); TNFα (tumor necrosis factor alpha); IFNγ (interferon gamma); HSC (hematopoietic stem cell); CFU-GEMM (colony forming unit-granulocyte erythrocyte monocyte megakaryocyte); BFU-E (burst forming unit-erythroid); CFU-E (colony forming unit-erythroid).
Following trauma, increased IL-6 and persistent inflammation have been shown to increase hepcidin (HAMP) expression within the liver (17). Hepcidin, secreted by hepatocytes, binds to ferroportin to sequester iron, leaving less iron available for use in producing erythrocytes. In this study, genes encoding erythropoietin and ferroportin were present in greater number in the bone marrow of trauma patients. There was a downregulation of HAMP in the bone marrow of trauma patients. Since the majority of hepcidin is produced by the liver, the significance of genes like HAMP in the bone marrow is much less clear. IL-6 has been linked to paracrine signaling within the bone marrow (27, 28). Similarly, a small but meaningful amount of endogenous erythropoietin is believed to be produced by progenitor cells in the bone marrow for autocrine regulation of erythropoiesis (29). This study did demonstrate that plasma hepcidin was significantly elevated as previously shown (15). The positive correlation between plasma hepcidin and IL-10 transcription is consistent with previously described iron restriction induced by IL-10 (30). While not necessarily causative, these findings implicate hepcidin and relative iron restriction as a cause of anemia following trauma.
TLR4 is present on hematopoietic stem and progenitor cells and responds to a variety of pathogen associated molecular patterns by releasing inflammatory cytokines and promoting hematopoietic differentiation favoring a myeloid lineage (31–34). Following trauma, there were greater mRNA copy numbers of both TLR4 and its main adaptor protein MyD88, along with canonical MyD88-dependent transcription factor NFκB and its target genes the proinflammatory cytokines IL-6, TNFα, and IL-1β compared to hip replacement. Elective hip replacement patients exhibited an overall reduction in IL-6 and TGFβ mRNA copies compared to healthy controls. Previous studies showed increased TLR activity and circulating inflammatory cytokines like IL-1, TNFα, IL-6, and IFN-γ in the peripheral blood after injury and critical illness (15, 24, 35–37). Increased IL-1β has been shown following trauma, along with reductions in TLR3 and TLR7 gene transcription, with more dramatic reductions in TLR transcription identified in the patients who developed a more complicated hospital course (24). Similar to our study, a recent study of sepsis patients identified inverse correlations between circulating inflammatory cytokines in the plasma and hemoglobin on day 14, including IL-6 and TNFα (16). TNFα’s negative impact on erythropoiesis is multifactorial involving inhibition of erythroid transcriptional activity, reduction in erythropoietin- and stem cell factor-induced colony formation, suppression of erythroid progenitor cell proliferation and maturation, and the promotion of progenitor apoptosis (38–42). IL-1β has been shown to force myeloid differentiation and reduce erythroid colony formation (41).
Downstream of MyD88, genes involved in the non-canonical MAPK signaling cascade were also significantly increased following trauma (Figure 5). There were greater copies of MAPK1, MAPK3 and MAPK8 which encode for ERK2, ERK1, and JNK, respectively. All three protein kinases have been shown to modulate and inhibit proliferation, maturation, and self-renewal of erythroid progenitor cells and reduce the erythrocyte life span (43, 44). Although this study did not directly address the MyD88-independent pathway, its downstream target IFNγ was less downregulated following trauma compared to elective hip replacement. Elevated levels of IFNγ have been shown to suppress erythropoiesis with reduced proliferation of erythroid progenitors (41). TLR4 on the hepatocellular membrane is also capable of promoting HAMP expression in the liver via the MyD88-dependent MAPK signaling cascade (45). Interestingly, although there was a correlation between bone marrow TLR4 mRNA expression and plasma hepcidin levels among trauma patients, no such correlation was identified in MyD88 or MAPK gene transcription. This finding may suggest that TLR4-mediated HAMP upregulation is not a dominant mechanism of hepcidin regulation in the bone marrow.
Dysfunctional TGFβ signaling, including increased TGFβ−1 receptor transcription shown in this study, has been linked to erythroid dysfunction (46). TGFβ promotes more rapid erythroid maturation and differentiation at the cost of reduced progenitor proliferation (47). Anti-TGFβ−1 antibodies can partially reverse the inhibitory effects of trauma plasma in vitro on erythroid colony formation of healthy bone marrow, an effect that appears to be the result of intermediary bone marrow stromal cells (48, 49). These findings were not demonstrated in the patients undergoing elective hip replacement.
IL-10 is upregulated in most inflammatory disorders and has been shown to alter iron metabolism and induce anemia (30). IL-1β is a potent inducer of IL-10. Tilg et al. (30) demonstrated that IL-10 induced anemia in a dose dependent fashion in Crohn’s disease patients and that this anemia was associated with hyperferritinemia and limited iron availability for erythroid progenitor cells. In our study, traumatic injury was followed by an increase in bone marrow gene copies of both IL-1β and IL-10. This again demonstrates the similar findings between the anemia seen following trauma and the anemia of inflammation.
Although expressed in greater quantities relative to operative controls, expression of genes encoding inflammatory cytokines like TNFα, IL-1β, and IL-6 were not elevated compared to healthy controls. This suggests additional mechanisms at play within the bone marrow. One such mechanism may be catecholamine activity. Preclinical and clinical models have demonstrated that trauma and hemorrhagic shock are associated with prolonged elevation of catecholamines, reduced bone marrow cellularity, and reduced erythroid progenitor growth (50, 51). Similarly, previous studies with adrenergic receptor blockers (e.g. propranolol) and centrally acting alpha-2 agonists that reduce sympathetic outflow (i.e. clonidine) have shown an improvement in post-traumatic erythropoietic function in the bone marrow (50). Catecholamines, in particular beta-adrenergic stimulation, have been shown to inhibit production of inflammatory cytokines like TNFα, IL-6, and IL-1 in a number of cell populations in humans and mice, while concurrently increasing plasma IL-10 (50). Our findings of overall reduced TNFα in both operative groups and increased IL-10 transcription after trauma may indirectly reflect increased adrenergic stimulation after injury and/or surgery. However, this hypothesis was not directly tested in this study, and it does not fully explain our constellation of findings, including the marked upregulation of IL-1β transcription seen after trauma. Competing effects between immunosuppressive catecholamines and proinflammatory (e.g. TLR) signaling within unique cell populations may be partly responsible for the inconsistent changes in differential gene expression noted in this study. It can also be hypothesized that age, the presence of chronic conditions like arthritis, anesthesia, or time since the physiologic insult occurred may have all played a role in the unique expression patterns seen in injured patients versus those undergoing hip replacement. In our study, neither age nor time to surgery demonstrated any correlation with gene transcription in the bone marrow. Anesthetic agent was not documented during this study.
Age of enrolled subjects was a significant limitation in this study. Using elective total hip replacement as our operative control group was inherently associated with an older patient population as compared to the younger trauma patient population and even younger healthy controls (52). In addition, trauma patients had already undergone substantial blood loss resulting in hemorrhagic shock at time of arrival at the hospital, with additional variability present in time to bone marrow collection and anatomic biopsy site. It is an inherent challenge to control for preoperative blood loss, transfusion requirement, and time to OR. This study sought to investigate trends in bone marrow gene transcription, and as such individual cell populations were not isolated or assayed separately. Future transcriptomic analyses include cell sorting with or without the use of flow-assisted or magnetic-activated cell sorting and single cell RNA sequencing. This study also lacked follow up bone marrow samples, restricting characterization of the transcriptomic response to a single varied timepoint within the first week after injury, and does not address the trajectory of anemia, which remains a poorly understood phenomenon after injury and critical illness (16, 22, 53).
The effects of inflammation on erythropoietic dysfunction are well described, but the mechanisms involved following trauma and acute blood loss remain incompletely understood (14, 39, 54). Following hemorrhage and severe injury, patients demonstrated upregulated bone marrow transcription of inflammatory pathways, particularly those pertaining to TLR4 signaling. There was also an increase in gene transcription of receptors and intracellular mediators that are known to inhibit erythropoiesis. This constellation of transcriptomic responses may be instrumental in development of anemia after traumatic injury. Future assessment of serial bone marrow samples will be needed to correlate these transcriptomic changes with the time course and resolution of inflammation and anemia in these injured patients.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Nanostring Technologies for their assistance with RNA analysis and Jillianne Brakenridge, Ruth Davis, Ashley McCray, and Jennifer Lanz for their work in support of this study.
Conflicts of Interest and Source of Funding:
The authors declare that they have no relevant conflicts of interests. This research was supported by the National Institutes of Health. AMM was supported by NIH NIGMS grant R01 GM105893. LSK, DBD, and BPF were supported by postgraduate training grant T32 GM-008721 in burns, trauma, and perioperative injury by NIGMS. PAE was supported by P30 AG028740 from the National Institute on Aging and by the NIH NIGMS grant R01 GM113945. Finally, AMM and PAE were supported by NIH NIGMS grant P50 GM111152.
References
- 1.Nathens AB, Maier RV, Jurkovich GJ, Monary D, Rivara FP and Mackenzie EJ: The delivery of critical care services in US trauma centers: is the standard being met? J Trauma 60(4):773–83; disucssion 783–4, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM and Napolitano LM: Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma 54(5):898–905, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Greenburg AG: Benefits and risks of blood transfusion in surgical patients. World J Surg 20(9):1189–1193, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D and Investigators AAaBTiCC: Anemia and blood transfusion in critically ill patients. JAMA 288(12):1499–1507, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J, Jensen L, Nahirniak S and Gibney RT: Anemia and blood transfusion practices in the critically ill: a prospective cohort review. Heart Lung 39(3):217–25, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS and Shapiro MJ: The CRIT Study: anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med 32(1):39–52, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro MJ, Gettinger A, Corwin HL, Napolitano L, Levy M, Abraham E, Fink MP, MacIntyre N, Pearl RG and Shabot MM: Anemia and blood transfusion in trauma patients admitted to the intensive care unit. J Trauma 55(2):269–73; discussion 273–4, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Charles A, Shaikh AA, Walters M, Huehl S and Pomerantz R: Blood transfusion Is an independent predictor of mortality after blunt trauma. Am Surg 73(1):1–5, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Athar MK, Puri N and Gerber DR: Anemia and blood transfusions in critically ill patients. J Blood Transfus 2012:629204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH and Mohr AM: Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg 77(1):54–60; discussion 59–60, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, et al. : Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 357(10):965–976, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Pieracci FM, Stovall RT, Jaouen B, Rodil M, Cappa A, Burlew CC, Holena DN, Maier R, Berry S, Jurkovich J, et al. : A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness. Crit Care Med 42(9):2048–57, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal MD, Bala T, Wang Z, Loftus T and Moore F: Chronic Critical Illness Patients Fail to Respond to Current Evidence-Based Intensive Care Nutrition Secondarily to Persistent Inflammation, Immunosuppression, and Catabolic Syndrome. JPEN J Parenter Enteral Nutr, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macdougall IC and Cooper AC: Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant 17:39–43, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Loftus TJ, Mira JC, Miller ES, Kannan KB, Plazas JM, Delitto D, Stortz JA, Hagen JE, Parvataneni HK, Sadasivan KK, et al. : The postinjury inflammatory state and the bone marrow response to anemia. Am J Respir Crit Care Med 198(5):629–638, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus TJ, Mira JC, Stortz JA, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Brumback BA, Ungaro RF, Bihorac A, Leeuwenburgh C, et al. : Persistent inflammation and anemia among critically ill septic patients. J Trauma Acute Care Surg 86(2):260–267, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alamo IG, Kannan KB, Smith MA, Efron PA and Mohr AM: Characterization of erythropoietin and hepcidin in the regulation of persistent injury-associated anemia. J Trauma Acute Care Surg 81(4):705–12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB and Mohr AM: Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg 79(1):91–6; discussion 96–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaziri ND, Eltorai IM, Segal J, Winer RL, Gonzales E, Brunnemann S and Elmzadeh M: Erythropoietin profile in spinal cord injured patients. Arch Phys Med Rehabil 74(1):65–67, 1993. [PubMed] [Google Scholar]
- 20.Deitch EA and Sittig KM: A serial study of the erythropoietic response to thermal injury. Ann Surg 217(3):293–299, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D and Pearl RG: Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 16(1):36–41, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA and Rameshwar P: Bone marrow failure following severe injury in humans. Ann Surg 238(5):748–53, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogiers P, Zhang H, Leeman M, Nagler J, Neels H, Mélot C and Vincent JL: Erythropoietin response is blunted in critically ill patients. Intensive Care Medicine 23(2):159–162, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, Gentile LF, Nacionales DC, Cuenca AL, Bihorac A, et al. : Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg 76(1):21–9; discussion 29–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, et al. : Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367(16):1487–96, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, et al. : Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26(3):317–25, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC and DeClerck YA: Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res 69(1):329–37, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durie BG, Vela EE and Frutiger Y: Macrophages as an important source of paracrine IL6 in myeloma bone marrow. Curr Top Microbiol Immunol 166:33–6, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Maekawa T, Watanabe S, Tsuji K and Nakahata T: Erythroid progenitors differentiate and mature in response to endogenous erythropoietin. J Clin Invest 106(2):263–70, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilg H, Ulmer H, Kaser A and Weiss G: Role of IL-10 for induction of anemia during inflammation. J Immunol 169(4):2204–9, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K and Kincade PW: Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24(6):801–12, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanez A, Flores A, Murciano C, O’Connor JE, Gozalbo D and Gil ML: Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol 12(1):114–28, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Sioud M, Floisand Y, Forfang L and Lund-Johansen F: Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol 364(5):945–54, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Kumar H, Kawai T and Akira S: Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388(4):621–5, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. : Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care 19:77, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenz A, Franklin GA and Cheadle WG: Systemic inflammation after trauma. Injury 38(12):1336–45, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Polk HC, Jr G, C. D, Wellhausen SR, Cost K, Davidson PR, Regan MP and Borzotta AP: A systematic study of host defense processes in badly injured patients. Annals of surgery 204(3):282–299, 1986. [PMC free article] [PubMed] [Google Scholar]
- 38.Rusten LS and Jacobsen SE: Tumor necrosis factor (TNF)-alpha directly inhibits human erythropoiesis in vitro: role of p55 and p75 TNF receptors. Blood 85(4):989–996, 1995. [PubMed] [Google Scholar]
- 39.Morceau F, Dicato M and Diederich M: Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediators Inflamm 2009:405016, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buck I, Morceau F, Cristofanon S, Heintz C, Chateauvieux S, Reuter S, Dicato M and Diederich M: Tumor necrosis factor alpha inhibits erythroid differentiation in human erythropoietin-dependent cells involving p38 MAPK pathway, GATA-1 and FOG-1 downregulation and GATA-2 upregulation. Biochem Pharmacol 76(10):1229–39, 2008. [DOI] [PubMed] [Google Scholar]
- 41.De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U and Peschle C: Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 401(6752):489–493, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Raducka-Jaszul O, Boguslawska DM, Jedruchniewicz N and Sikorski AF: Role of Extrinsic Apoptotic Signaling Pathway during Definitive Erythropoiesis in Normal Patients and in Patients with beta-Thalassemia. Int J Mol Sci 21(9), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W and Liu H: MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research 12:9–18, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Geest CR and Coffer PJ: MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 86(2):237–50, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Lee YS, Kim YH, Jung YS, Kim KS, Kim DK, Na SY, Lee JM, Lee CH and Choi HS: Hepatocyte toll-like receptor 4 mediates lipopolysaccharide-induced hepcidin expression. Exp Mol Med 49(12):e408, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge J, Apicella M, Mills JA, Garcon L, French DL, Weiss MJ, Bessler M and Mason PJ: Dysregulation of the Transforming Growth Factor beta Pathway in Induced Pluripotent Stem Cells Generated from Patients with Diamond Blackfan Anemia. PLoS One 10(8):e0134878, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zermati Y, Fichelson S, Valensi F, Freyssinier JM, Rouyer-Fessard P, Cramer E, Guichard J, Varet B and Hermine O: Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Experimental hematology 28(8):885–894, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Wu JC, Livingston DH, Hauser CJ, Deitch EA and Rameshwar P: Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Annals of Surgery 234(2):224–232, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P and Livingston DH: Adrenergic Modulation of Erythropoiesis Following Severe Injury Is Mediated Through Bone Marrow Stroma. Surgical Infections 5(4):385–393, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Loftus TJ, Efron PA, Moldawer LL and Mohr AM: beta-Blockade use for Traumatic Injuries and Immunomodulation: A Review of Proposed Mechanisms and Clinical Evidence. Shock 46(4):341–51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apple CG, Miller ES, Loftus TJ, Kannan KB, Parvataneni HK, Hagen JE, Efron PA and Mohr AM: Impact of Injury Severity on the Inflammatory State and Severe Anemia. J Surg Res 248:109–116, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Probst C, Pape HC, Hildebrand F, Regel G, Mahlke L, Giannoudis P, Krettek C and Grotz MR: 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury 40(1):77–83, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Bateman AP, McArdle F and Walsh TS: Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med 37(6):1906–12, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Macdougall IC and Cooper AC: Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. European Journal of Clinical Investigation 35:32–35, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


