Abstract
Serious hypoglycemia is a major adverse event associated with insulin secretagogues. Previous studies have suggested a potential relationship between angiotensin-converting enzyme inhibitors (ACEIs) used with sulfonylureas and serious hypoglycemia, and widely used drug compendia warn of this potential drug-drug interaction. We investigated the association between serious hypoglycemia and concomitant use of ACEIs in patients receiving insulin secretagogues, using the self-controlled case series design and Medicaid claims data from 5 US states linked to Medicare claims from 1999–2011. The exposure of interest was active prescription for ACEIs during insulin secretagogue or metformin (negative control object drug) episodes. The outcome was hospital presentation for serious hypoglycemia, identified by discharge diagnosis codes in inpatient and emergency department claims (positive predictive value ~78–89%). We calculated confounder-adjusted rate ratios (RRs) and 95% confidence internals (CIs) of outcome occurrence during ACEI-exposed versus ACEI-unexposed time using conditional Poisson regression. The RRs for ACEIs were not statistically elevated during observation time of glipizide (RR, 1.06; CI, 0.98–1.15), glyburide (RR, 1.05; CI, 0.96–1.15), repaglinide (RR, 1.15; CI, 0.94–1.41), or metformin (RR, 1.02; CI, 0.97–1.06); but was modestly elevated with glimepiride (RR, 1.23; CI, 1.11–1.37) and modestly reduced with nateglinide (RR, 0.73; CI, 0.56–0.96). The overall pattern of results do not suggest that ACEIs used with insulin secretagogues were associated with increased rates of serious hypoglycemia, with the possible exception of glimepiride.
Keywords: angiotensin-converting enzyme inhibitors, insulin secretagogues, sulfonylureas, metformin, serious hypoglycemia, drug-drug interaction, pharmacoepidemiology, Medicaid
INTRODUCTION
Insulin secretagogues—oral antihyperglycemic agents that include sulfonylureas and meglitinides—lower blood glucose levels by stimulating the release of insulin from the pancreas. Although these drugs are widely used for type 2 diabetes, serious hypoglycemia associated with their use (especially sulfonylureas) is among the most common, clinically significant, and preventable of all adverse drug events.1,2 Angiotensin-converting enzyme inhibitors (ACEIs) are widely prescribed for cardiovascular diseases including hypertension and heart failure, which are among the most common comorbidities among diabetes patients,4,5 for patients with hypertension and a history of diabetes,6 and to slow the progression of renal impairment in diabetes patients.7 There have been case reports8,9 and nested case-control studies10,11 suggesting that the use of ACEIs in combination with sulfonylureas or other oral antidiabetes drugs might be associated with an increased risk of hypoglycemic events. Suggested potential mechanisms involve enhanced insulin sensitivity and glucose disposal rate by ACEIs.8,12 The risk of hypoglycemia with concomitant use of ACEIs and sulfonylureas is listed as a potential drug-drug interaction (DDI) in some widely used drug compendia,13,14 and this drug-drug pair is among the most common interruptive drug class-based DDI alert groups.15 We investigated whether concomitant use of insulin secretagogues with ACEIs is associated with an increased rate of serious hypoglycemia compared to insulin secretagogues use without concomitant ACEIs.
METHODS
Study design and data
We conducted a population-based epidemiologic study using the self-controlled case series (SCCS) design,16–18 with insulin secretagogues as object drugs (i.e., affected drugs)19 and ACEIs as potential precipitant drugs (i.e., affecting drugs).19 Because we know of no mechanism by which metformin and ACEIs could interact to affect hypoglycemia risk, we used metformin as a negative control object drug.19 Our methods were similar to those we used for a previous study.20 We used Medicaid claims data from five US states (California, Florida, New York, Ohio, and Pennsylvania) from 1 January 1999 to 31 December 2011, linking to Medicare claims data and Medicare Part D Event File for Medicaid-Medicare dual-enrollees, and the Social Security Death Master file to ascertain dates of death. These states constitute about 40% of the nationwide Medicaid population.21 We chose the SCCS design because it inherently controls for all time-invariant confounders, measured and unmeasured, and it does not require a separate control group, which can help reduce a potential bias in estimation that may be caused by selection of an inappropriate comparison group that has different underlying risk.
We constructed object drug (i.e., antidiabetes drug)-specific samples (Figure 1) using dispensing date and days’ supply data from pharmacy claims. Eligible persons were adults 18 years of age or older who experienced at least one serious hypoglycemic event during observation time, defined as a period covered by object drug prescriptions, i.e., object drug episodes, and had continuous enrollment in Medicaid for at least 180 days immediately before the first observation time (i.e., the baseline period), free of an enrollment gap and of dispensing of that object drug. The rationale for requiring a continuous-enrollment baseline period was to reduce potential bias from not being able to reliably identify the start date of an object drug episode and/or the concomitant use of a precipitant drug.
Figure 1.
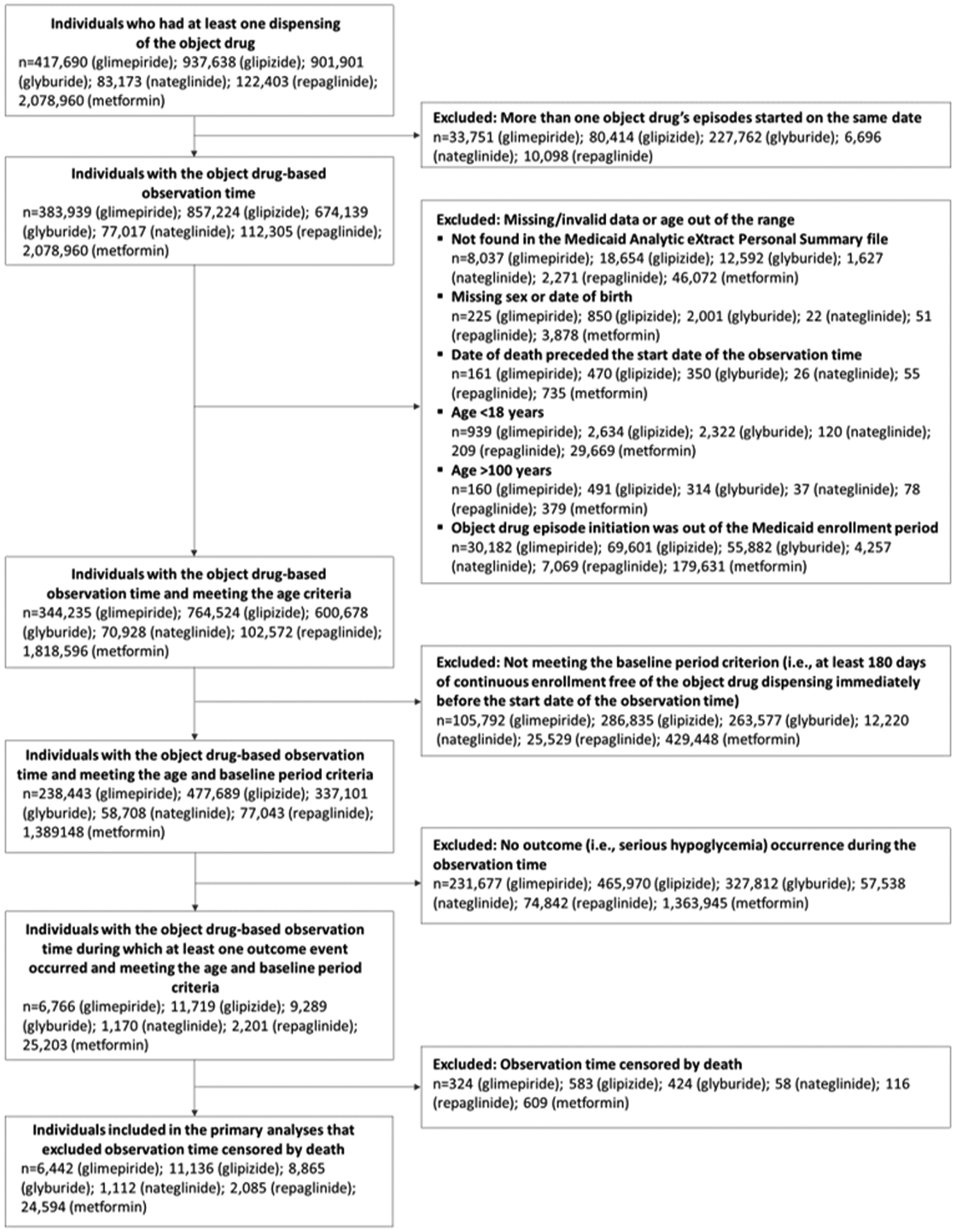
Identification of study samples
Object drug-specific study samples were constructed separately. Object drugs (glimepiride, glipizide, glyburide, nateglinide, repaglinide, and metformin) are indicated in the parentheses.
Exposure and outcome of interest
The exposure of interest was use of an ACEI concomitantly with either an insulin secretagogue or metformin during observation time, as described in detail below. Insulin secretagogues analyzed were glimepiride, glipizide, glyburide, nateglinide, and repaglinide. ACEIs analyzed include all ACEIs in our claims data, i.e., benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, and trandolapril.
The outcome of interest was hospital presentation with serious hypoglycemia. We used a validated outcome ascertainment algorithm (with a positive predictive value of 89%22 in emergency department claims and 78%23 in inpatient claims) that used the following International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes appearing in the principal position in inpatient claims or in any position in emergency department claims: 251.0 (hypoglycemic coma); 251.1 (other specific hypoglycemia); 251.2 (hypoglycemia, unspecified); or 250.8X (diabetes with other specified manifestations), unless accompanied by one of the exclusionary diagnosis codes suggesting manifestations other than hypoglycemia (Table S1).
Observation time
Object drug episodes in which at least one outcome event occurred served as observation time (Figure S1). A drug episode was defined as a unit of continuous prescriptions that allowed 7 days to account for potential incomplete adherence between contiguous prescriptions, and at the end of the last prescription. Days’ supply of an object drug was censored the day before a different object drug was dispensed, with an exception, i.e., when the object drug was an insulin secretagogue and metformin (negative control object drug) was subsequently dispensed before the object drug’s end of days’ supply, we controlled for metformin use (yes/no) in the statistical analysis without censoring the object drug’s days’ supply. An object drug episode began at the first dispensing date of that episode and ended by end of days’ supply of the episode (including a 7-day grace period), Medicaid disenrollment, or end of dataset (31 December 2011), whichever occurred first. A precipitant drug episode was allowed to begin on, before, or after the start date of the object drug episode. More than one precipitant drug episode was allowed within an object drug’s observation time. Within the observation time, we considered the period following the end of a precipitant-exposed time (i.e., object-precipitant concomitant use time) to be indeterminate time, i.e., neither precipitant-exposed nor precipitant-unexposed. This indeterminate time lasted up to 30 days, or until another prescription for the precipitant was dispensed. Individuals were allowed to contribute multiple object drug episodes if inclusion criteria and baseline period requirement were met. We included any object drug episodes during which the outcome of interest occurred at least once (i.e., we did not restrict to incident events). We did not censor object drug episodes with the occurrence of serious hypoglycemia on the basis of the assumptions underlying the SCCS design, and we excluded any observation period that was censored by death in the primary analysis.16 One of the sensitivity analyses we performed (described below) included observation periods that were censored by death.
Statistical analysis
We calculated occurrence rate ratios (RRs) with 95% confidence intervals (CIs) for serious hypoglycemia during use of an insulin secretagogue or metformin with versus without an ACEI (i.e., precipitant-exposed time versus precipitant-unexposed time within the observation time), using conditional Poisson regression.16,17 The SCCS design inherently eliminates confounding by measured and unmeasured factors that are time-invariant within person during the observation time, including genetic characteristics, chronic diseases or conditions, overall health status, and lifestyle and stable health behaviors. We additionally controlled for the following time-varying potential confounders that were ascertained on the person-day level (Table S2): drugs that may be associated with hypoglycemia; drugs that may be associated with hyperglycemia; drugs that may interact with insulin secretagogues; major non-chronic conditions that may be associated with hypoglycemia, i.e., acute infections (identified by ICD-9-CM diagnosis codes in any position in claims; codes are listed in Table S3); and others, i.e., age, nursing home residence status, Medicaid-Medicare dual-enrollment status, and concomitant metformin use when the object drug was an insulin secretagogue.
We also conducted sensitivity analyses by (a) including observation periods censored by death; and (b) excluding observation time during which individuals might have had incomplete data (defined operationally as individuals with a managed care plan, a private health insurance, restricted benefits, or among Medicaid-Medicare dual-enrollees, individuals enrolled in a group health organization or a Medicare Advantage plan for which the Centers for Medicare and Medicaid Services does not process provider claims).
We used SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). This study was approved by the institutional review board of the University of Pennsylvania, which waived the requirement for informed consent.
RESULTS
We identified 6442, 11136, 8865, 1112, 2085, and 24594 individuals who experienced serious hypoglycemia at least once while receiving glimepiride, glipizide, glyburide, nateglinide, repaglinide, and metformin, respectively, and met all inclusion criteria. The number of serious hypoglycemia events during observation time was 8159, 14353, 11129, 1595, 2901, and 32639 for glimepiride, glipizide, glyburide, nateglinide, repaglinide, and metformin, respectively. Median age at the start of observation time ranged 63.2–72.8 years, and the proportion of female patients ranged 63.8–67.7%. Table 1 shows characteristics of the study samples by object drug.
Table 1.
Characteristics of the study samples by object drug
| Object drugs | |||||||
|---|---|---|---|---|---|---|---|
| glimepiride | glipizide | glyburide | nateglinide | repaglinide | metformin | ||
| Persons, person-days, and outcome occurrence | |||||||
| Number of Persons | 6,442 | 11,136 | 8,865 | 1,112 | 2,085 | 24,594 | |
| Person-days of observation time, median per individual (Q1, Q3) | 128.0 (60.0, 290.0) | 127.0 (60.0, 289.0) | 104.0 (42.0, 234.0) | 92.0 (38.0, 205.0) | 93.0 (38.0, 196.0) | 206.0 (97.0, 440.0) | |
| Person-days of observation time, total | 1,524,588 | 2,604,469 | 1,799,555 | 203,173 | 359,615 | 8,707,447 | |
| Number of outcome occurrence during observation time | 8,159 | 14,353 | 11,129 | 1,595 | 2,901 | 32,639 | |
| Demographic characteristics | |||||||
| Age in years at start of observation time, median (Q1, Q3) | 71.4 (60.8, 79.8) | 68.4 (56.6, 78.1) | 69.7 (58.1, 79.2) | 70.5 (59.7, 79.1) | 72.8 (63.2, 80.8) | 63.2 (50.0, 73.8) | |
| Sex, female | 4,362 (67.7%) | 7,100 (63.8%) | 5,668 (63.9%) | 731 (65.7%) | 1,404 (67.3%) | 15,891 (64.6%) | |
| Race | White | 2,683 (41.6%) | 4,036 (36.2%) | 3,192 (36.0%) | 471 (42.4%) | 778 (37.3%) | 9,388 (38.2%) |
| Black | 1,128 (17.5%) | 2,759 (24.8%) | 1,987 (22.4%) | 208 (18.7%) | 470 (22.5%) | 5,607 (22.8%) | |
| Hispanic/Latino | 1,271 (19.7%) | 2,236 (20.1%) | 1,919 (21.6%) | 204 (18.3%) | 308 (14.8%) | 5,106 (20.8%) | |
| Other/unknown | 1,360 (21.1%) | 2,105 (18.9%) | 1,767 (19.9%) | 229 (20.6%) | 529 (25.4%) | 4,493 (18.3%) | |
| State of residence | California | 3,176 (49.3%) | 5,318 (47.8%) | 5,060 (57.1%) | 561 (50.4%) | 828 (39.7%) | 12,181 (49.5%) |
| Florida | 660 (10.2%) | 1,320 (11.9%) | 727 (8.2%) | 112 (10.1%) | 207 (9.9%) | 2,294 (9.3%) | |
| New York | 1,248 (19.4%) | 2,578 (23.2%) | 1,696 (19.1%) | 226 (20.3%) | 690 (33.1%) | 5,677 (23.1%) | |
| Ohio | 949 (14.7%) | 1,155 (10.4%) | 956 (10.8%) | 148 (13.3%) | 121 (5.8%) | 2,792 (11.4%) | |
| Pennsylvania | 409 (6.3%) | 765 (6.9%) | 426 (4.8%) | 65 (5.8%) | 239 (11.5%) | 1,650 (6.7%) | |
| Calendar year at start of observation time | 1999 | 169 (2.6%) | 436 (3.9%) | 494 (5.6%) | 0 (0.0%) | 91 (4.4%) | 1,213 (4.9%) |
| 2000 | 351 (5.4%) | 946 (8.5%) | 857 (9.7%) | 0 (0.0%) | 133 (6.4%) | 2,406 (9.8%) | |
| 2001 | 371 (5.8%) | 902 (8.1%) | 748 (8.4%) | 87 (7.8%) | 169 (8.1%) | 2,408 (9.8%) | |
| 2002 | 457 (7.1%) | 910 (8.2%) | 747 (8.4%) | 110 (9.9%) | 165 (7.9%) | 2,011 (8.2%) | |
| 2003 | 581 (9.0%) | 908 (8.2%) | 789 (8.9%) | 124 (11.2%) | 170 (8.2%) | 2,012 (8.2%) | |
| 2004 | 564 (8.8%) | 899 (8.1%) | 795 (9.0%) | 131 (11.8%) | 151 (7.2%) | 1,994 (8.1%) | |
| 2005 | 575 (8.9%) | 993 (8.9%) | 783 (8.8%) | 180 (16.2%) | 147 (7.1%) | 2,065 (8.4%) | |
| 2006 | 784 (12.2%) | 1,260 (11.3%) | 956 (10.8%) | 122 (11.0%) | 243 (11.7%) | 2,605 (10.6%) | |
| 2007 | 668 (10.4%) | 936 (8.4%) | 693 (7.8%) | 102 (9.2%) | 215 (10.3%) | 1,826 (7.4%) | |
| 2008 | 520 (8.1%) | 769 (6.9%) | 572 (6.5%) | 85 (7.6%) | 205 (9.8%) | 1,651 (6.7%) | |
| 2009 | 534 (8.3%) | 907 (8.1%) | 542 (6.1%) | 74 (6.7%) | 142 (6.8%) | 1,874 (7.6%) | |
| 2010 | 516 (8.0%) | 755 (6.8%) | 538 (6.1%) | 56 (5.0%) | 145 (7.0%) | 1,530 (6.2%) | |
| 2011 | 352 (5.5%) | 515 (4.6%) | 351 (4.0%) | 41 (3.7%) | 109 (5.2%) | 999 (4.1%) | |
| Medicare dual-enrollment at start of observation time | 5,165 (80.2%) | 8,202 (73.7%) | 6,531 (73.7%) | 908 (81.7%) | 5,165 (80.2%) | 15,839 (64.4%) | |
| Nursing home resident at start of observation time | 801 (12.4%) | 1,648 (14.8%) | 1,148 (12.9%) | 202 (18.2%) | 801 (12.4%) | 2,261 (9.2%) | |
| Exposure to Precipitant Drugs (person-days exposed) | |||||||
| Any ACEIs | 616,833 (40.5%) | 1,104,652 (42.4%) | 738,981 (41.1%) | 71,654 (35.3%) | 143,972 (40.0%) | 3,996,889 (45.9%) | |
| benazepril | 127,045 (8.3%) | 193,718 (7.4%) | 141,777 (7.9%) | 12,161 (6.0%) | 22,150 (6.2%) | 761,136 (8.7%) | |
| captopril | 16,818 (1.1%) | 41,448 (1.6%) | 27,646 (1.5%) | 5,072 (2.5%) | 4,578 (1.3%) | 108,096 (1.2%) | |
| enalapril | 91,649 (6.0%) | 185,060 (7.1%) | 132,107 (7.3%) | 9,027 (4.4%) | 29,020 (8.1%) | 661,992 (7.6%) | |
| fosinopril | 12,368 (0.8%) | 38,107 (1.5%) | 28,204 (1.6%) | 2,001 (1.0%) | 7,364 (2.0%) | 145,584 (1.7%) | |
| lisinopril | 275,978 (18.1%) | 525,269 (20.2%) | 328,056 (18.2%) | 30,765 (15.1%) | 55,815 (15.5%) | 1,863,778 (21.4%) | |
| moexipril | 2,142 (0.1%) | 1,252 (0.0%) | 2,276 (0.1%) | 252 (0.1%) | 302 (0.1%) | 13,649 (0.2%) | |
| perindopril | 157 (0.0%) | 1,087 (0.0%) | 677 (0.0%) | 334 (0.2%) | 398 (0.1%) | 3,145 (0.0%) | |
| quinapril | 23,055 (1.5%) | 47,659 (1.8%) | 33,560 (1.9%) | 1,571 (0.8%) | 8,850 (2.5%) | 162,811 (1.9%) | |
| ramipril | 64,706 (4.2%) | 67,519 (2.6%) | 42,970 (2.4%) | 9,831 (4.8%) | 15,008 (4.2%) | 261,894 (3.0%) | |
| trandolapril | 2,915 (0.2%) | 3,533 (0.1%) | 1,708 (0.1%) | 640 (0.3%) | 487 (0.1%) | 14,804 (0.2%) | |
| Time-varying covariates (person-days exposed) * | |||||||
| Acute infection in prior 15 days | 210,315 (13.8%) | 372,181 (14.3%) | 242,328 (13.5%) | 35,813 (17.6%) | 59,448 (16.5%) | 959,180 (11.0%) | |
| Atypical antipsychotics in prior 30 days | 156,683 (10.3%) | 336,643 (12.9%) | 210,480 (11.7%) | 32,602 (16.0%) | 35,983 (10.0%) | 1,655,554 (19.0%) | |
| Calcineurin inhibitors in prior 30 days | 4,738 (0.3%) | 16,929 (0.6%) | 7,770 (0.4%) | 2,381 (1.2%) | 1,513 (0.4%) | 10,735 (0.1%) | |
| Corticosteroids in prior 30 days | 91,295 (6.0%) | 151,595 (5.8%) | 106,712 (5.9%) | 15,557 (7.7%) | 25,074 (7.0%) | 399,114 (4.6%) | |
| CYP2C9 inhibitors in prior 30 days | 192,273 (12.6%) | 322,629 (12.4%) | 201,423 (11.2%) | 32,490 (16.0%) | 43,151 (12.0%) | 1,034,732 (11.9%) | |
| CYP3A4 inhibitors in prior 30 days | 288,421 (18.9%) | 474,634 (18.2%) | 276,337 (15.4%) | 39,587 (19.5%) | 80,354 (22.3%) | 1,726,004 (19.8%) | |
| Furosemide in prior 30 days | 421,501 (27.6%) | 681,447 (26.2%) | 428,724 (23.8%) | 59,318 (29.2%) | 97,680 (27.2%) | 1,399,156 (16.1%) | |
| Insulin in prior 30 days | 362,443 (23.8%) | 608,649 (23.4%) | 304,662 (16.9%) | 83,309 (41.0%) | 137,844 (38.3%) | 2,323,606 (26.7%) | |
| Non-insulin other anti-diabetics in prior 30 days | 391,065 (25.7%) | 449,326 (17.3%) | 260,728 (14.5%) | 56,785 (27.9%) | 74,295 (20.7%) | 1,924,117 (22.1%) | |
| Other drugs that can cause hypoglycemia in prior 30 days | 31,284 (2.1%) | 79,789 (3.1%) | 51,982 (2.9%) | 5,572 (2.7%) | 7,190 (2.0%) | 297,895 (3.4%) | |
| Other anti-infectives in prior 15 days | 136,410 (8.9%) | 242,749 (9.3%) | 157,311 (8.7%) | 21,522 (10.6%) | 31,760 (8.8%) | 746,558 (8.6%) | |
| Protease inhibitors in prior 30 days | 2,404 (0.2%) | 12,988 (0.5%) | 7,995 (0.4%) | 1,312 (0.6%) | 1,171 (0.3%) | 53,009 (0.6%) | |
| Quinolones in prior 15 days | 59,887 (3.9%) | 93,014 (3.6%) | 63,653 (3.5%) | 9,499 (4.7%) | 15,689 (4.4%) | 241,389 (2.8%) | |
| Salicylates in prior 30 days | 191,960 (12.6%) | 316,864 (12.2%) | 210,910 (11.7%) | 33,891 (16.7%) | 45,884 (12.8%) | 1,185,306 (13.6%) | |
| Thiazide diuretics in prior 30 days | 223,688 (14.7%) | 343,242 (13.2%) | 227,188 (12.6%) | 28,332 (13.9%) | 53,142 (14.8%) | 1,390,700 (16.0%) | |
CYP: cytochrome P450 enzyme.
Exposure to pre-specified time-varying covariates: assessed at the person-day level during the observation time. Details on each time-varying covariate are presented in Table S2.
Figure 2 presents confounder-adjusted RRs and 95% CIs of serious hypoglycemia with the concomitant use of ACEIs, along with the number of outcome occurrences during ACEI-exposed and unexposed time separately for each object drug. The RR was not statistically significantly elevated with glipizide (RR, 1.06; CI, 0.98 to 1.15; this means that the point estimate for the outcome occurrence rate during ACEI-exposed time was 6% higher compared to the rate during ACEI-unexposed time when other factors are held constant, with the 95% confidence interval including the null value), glyburide (RR, 1.05; CI, 0.96 to 1.15), repaglinide (RR, 1.15; CI, 0.94 to 1.41), or metformin (RR, 1.02; CI, 0.97 to 1.06), but was modestly elevated with glimepiride (RR, 1.23; CI, 1.11 to 1.37) and modestly reduced with nateglinide (RR, 0.73; CI, 0.56 to 0.96). In sensitivity analyses, the patterns of these associations were not substantially different, although statistical significance differed (Tables S4 and S5).
Figure 2.
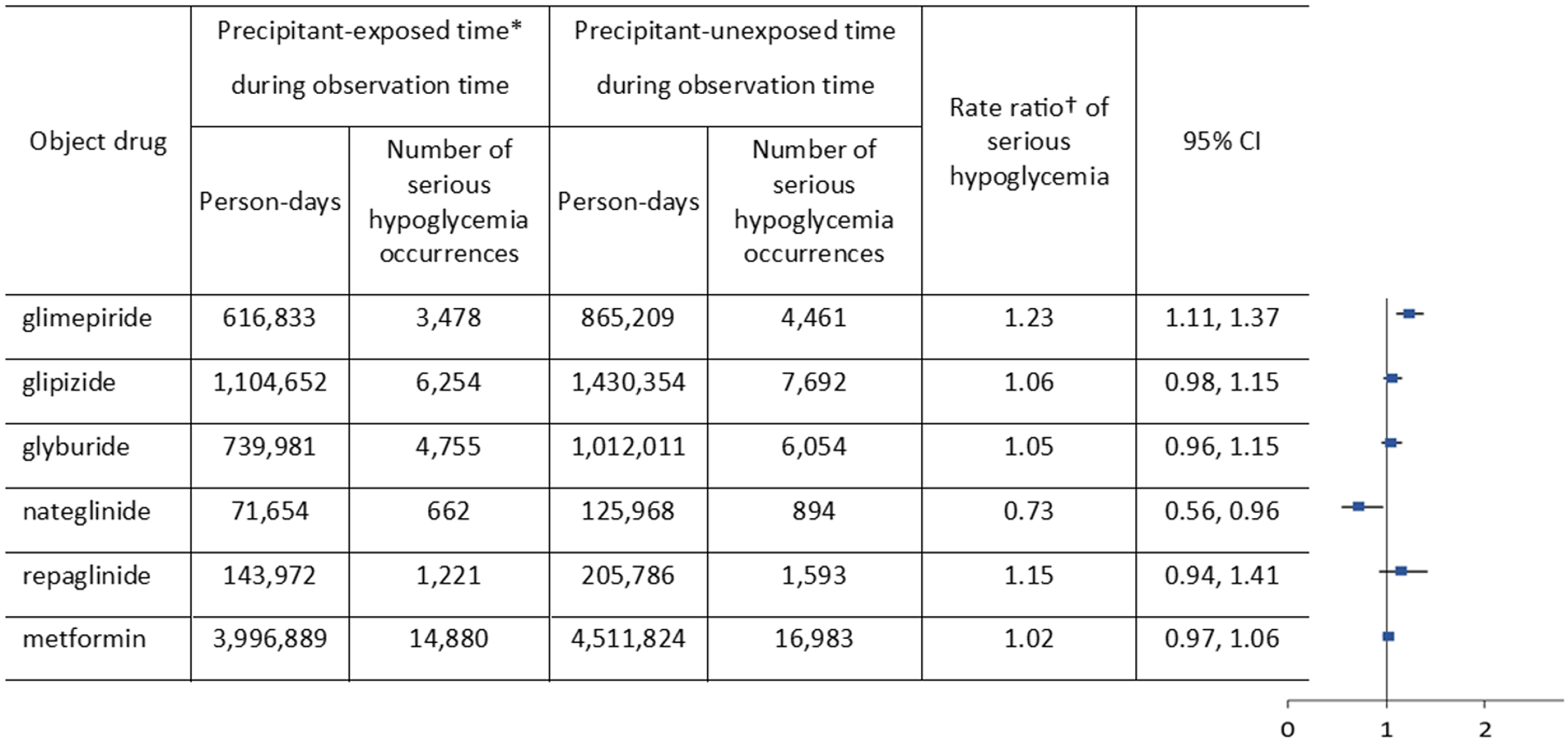
Rate ratios of serious hypoglycemia occurrence from the use of insulin secretagogues or metformin with versus without concomitant angiotensin-converting enzyme inhibitors
CI: confidence interval (based on a two-tailed test). *Precipitant-exposed time: days of concomitant use with a precipitant drug (angiotensin-converting enzyme inhibitor) during observation time since the initiation of the concomitant use. †Rate ratio: [(outcome occurrence rate during precipitant-exposed time)/(outcome occurrence rate during precipitant-unexposed time)]; confounder-adjusted.
DISCUSSION
The overall pattern of results does not suggest that concomitant use of ACEIs in users of insulin secretagogues or metformin is associated with an increased rate of serious hypoglycemia. Given the overall pattern of RRs and lack of known underlying mechanisms, the modestly elevated RR observed with glimepiride and ACEIs (RR, 1.23; CI, 1.11 to 1.37) should be investigated further to confirm whether it represents a true relationship that differs from other insulin secretagogues and ACEIs combinations. If glimepiride is truly an exception, a possible mechanism is that the plasma concentration of its active metabolites, which are mainly renally cleared, is more likely to increase when the renal clearance is reduced by the use of ACEIs. Although ACEIs have beneficial effects of improving renal hemodynamics, it is known that ACEIs can cause deterioration of renal function under certain conditions.24 Because angiotensin II is necessary for glomerular filtration rate (GFR) to be maintained during volume depletion, inhibition of angiotensin II via ACEIs can reduce GFR, and thereby reduce clearance of renally eliminated drugs.24 For glimepiride, ~99% of the dose is excreted as metabolized products, and ~60% of that 99% is excreted in urine. One of the major metabolites of glimepiride has ~36% of the activity of the parent drug.25 Glipizide’s renally-excreted metabolites are inactive.26 Glyburide is renally excreted (~50%) less than other sulfonylureas, and one of its major metabolites has ~15% of the activity of the parent drug.27
Prior studies of whether ACEIs increase the risk of hypoglycemia in sulfonylureas or oral antidiabetes drugs have produced inconsistent results. Two nested case-control studies suggested a potential association between serious hypoglycemia and ACEIs used in diabetes patients taking insulin or oral antidiabetes drugs,10,11 but could have been affected by between-person confounding. In contrast, an observational study in older patients with type 2 diabetes including sulfonylurea users found that ACEIs were not associated with an increased risk of hypoglycemia.28 Potential mechanisms suggested to explain possible association between ACEIs and hypoglycemia risk include increased insulin sensitivity and glucose utilization or disposal rate by ACEIs,8,12 which assume that angiotensin II reduces insulin sensitivity and glucose utilization. However, several studies have found that angiotensin II is associated with increased insulin sensitivity and increased glucose utilization in individuals with or without diabetes.29–31
The absolute risk of serious hypoglycemia cannot be drawn from this case-only design. However, the age- and sex-standardized occurrence rates (95% CI) of serious hypoglycemia per 1,000 person-years with oral antidiabetes monotherapy were reported in a prior study: glimepiride, 52.9 (48.9 to 56.9); glipizide, 49.6 (47.2 to 51.9); glyburide, 68.0 (64.9 to 71.2); nateglinide, 23.2 (15.6 to 30.7); repaglinide, 44.4 (34.7 to 54.1); and metformin, 11.9 (11.3 to 12.5).32
Our findings, together with lack of compelling mechanistic data indicative of ACEI-associated increased risk of hypoglycemia in sulfonylurea users, suggest that widely used drug compendia that warn of potential drug-drug interaction between sulfonylureas and ACEIs and electronic medical record systems generating interruptive alerts on this potential DDI should consider updating their current advice, which was based primarily on relatively small case-control studies and on case reports.
Strengths of this study include use of a large healthcare database, a design that eliminates confounding by time-invariant factors, adjustment for time-varying potential confounders, use of validated outcome ascertainment algorithm with high positive predictive values, performance of sensitivity analyses, and study of a vulnerable population who may be most likely to show an adverse effect if one is present. Limitations include lack of information on actual intake of drugs, diet, exercise, and other health behaviors that could affect hypoglycemia risk. However, such factors could have introduced bias only if they varied within person and were temporally associated with both ACEI use and serious hypoglycemia.
In summary, concomitant use of ACEIs overall was not associated with an elevated rates of serious hypoglycemia in users of insulin secretagogues, with the possible exception of glimepiride. Further research is warranted to understand any underlying mechanisms.
Supplementary Material
STUDY HIGHLIGHTS.
-
What is the current knowledge on the topic?
Serious hypoglycemia is a major adverse event associated with insulin secretagogues and among the most common, clinically significant, and preventable of all adverse drug events. Previous studies suggested a potential relationship between angiotensin-converting enzyme inhibitors (ACEIs) used with sulfonylureas and serious hypoglycemia, and widely used drug compendia warn of this potential risk.
-
What question did this study address?
This study investigated whether concomitant use of insulin secretagogues with ACEIs is associated with an increased rate of serious hypoglycemia compared to insulin secretagogue use without concomitant ACEIs, using real-world health care claims data from the US Medicaid population.
-
What does this study add to our knowledge?
Concomitant use of ACEIs overall was found not to be associated with an increased rate of serious hypoglycemia in users of insulin secretagogues, with the possible exception of glimepiride.
-
How might this change clinical pharmacology or translational science?
Widely used drug compendia that warn of potential drug-drug interaction between sulfonylureas and ACEIs and electronic medical record systems generating interruptive alerts on this potential drug interaction should consider updating the information that they provide.
ACKNOWLEDGMENTS
The authors thank Ms. Qing Liu and Ms. Min Du of the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, for their assistance with biostatistics computer programming.
Source of Funding:
This study was supported by the National Institutes of Health (grants R01DK102694, R01AG025152, R01AG060975, R01AG064589, R01DA048001) and the Patient-Centered Outcomes Research Institute (CER-2017C3-9230). These organizations had no role in the design and conduct of the study, data collection and analysis, interpretation of the results, writing and review of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interests: C.E.L. is an Executive Committee member of and S.H. directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training that receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. C.E.L. recently received honoraria from the American College of Clinical Pharmacy Foundation and the University of Florida College of Pharmacy, unrelated to this submitted work. S.H. has received support through his employer from Pfizer, Sanofi, and Johnson and Johnson, and has consulted for Merck, the Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca, GlaxoSmithKline, Eli Lilly), and Eli Lilly, all unrelated to this submitted work. All other authors declared no competing interests for this work.
Approval of human subject research: The study was approved by the institutional review board of the University of Pennsylvania.
Data availability:
Medicaid and Medicare claims data are available via a data use agreement with the Centers for Medicare and Medicaid Services (CMS) (https://www.cms.gov/). The authors did not have any special access privileges that others would not have. The procedures to obtain access to these data are described in the CMS website (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/Researchers) and the Research Data Assistance Center (ResDAC) website (https://www.resdac.org/research-identifiable-files-rif-requests).
REFERENCES
- 1.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316:2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. 2014. Available at https://health.gov/hcq/pdfs/ade-action-plan-508c.pdf. Accessed 6 May 2019.
- 3.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. [DOI] [PubMed] [Google Scholar]
- 4.Weng W, Tian Y, Kong SX, Ganguly R, Hersloev M, Brett J, Hobbs T. The prevalence of cardiovascular disease and antidiabetes treatment characteristics among a large type 2 diabetes population in the United States. Endocrinol Diab Metab. 2019;2:e0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantalone KM, Hobbs TM, Wells BJ, Kong SX, Kattan MW, Bouchard J, Chagin KM, Yu C, Sakurada B, Milinovich A, Weng W, Bauman J, Zimmerman RS. Changes in characteristics and treatment patterns of patients with newly diagnosed type 2 diabetes in a large United States integrated health system between 2008 and 2013. Clin Med Insights Endocrinol Diabetes. 2016;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman LL, Padala SA, Annamaraju P, Bashir K. Angiotensin Converting Enzyme Inhibitors (ACEI) [Updated 2020 Jun 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available at https://www.ncbi.nlm.nih.gov/books/NBK431051. Accessed 17 November 2020. [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. [DOI] [PubMed] [Google Scholar]
- 8.Rett K, Wicklmayr M, Dietze GJ, Schwabing K, Diabetes Study Group. Hypoglycemia in hypertensive diabetic patients treated with sulfonylureas, biguanides, and captopril. N Engl J Med. 1988;319(24):1609. [DOI] [PubMed] [Google Scholar]
- 9.Arauz-Pacheo C, Ramirez LC, Rios JM, Raskin P. Hypoglycemia induced by angiotensin-converting enzyme inhibitors in patients with non-insulin-dependent diabetes receiving sulfonylurea therapy. Am J Med. 1990;89(6):811–813. [DOI] [PubMed] [Google Scholar]
- 10.Herings RMC, de Boer A, Stricker BHC, Leufkens HGM, Porsius A. Hypoglycemia associated with use of inhibitors of angiotensin converting enzyme. Lancet. 1995;345(8959):1195–1198. [DOI] [PubMed] [Google Scholar]
- 11.Morris AD, Boyle DIR, McMahon AD, Pearce H, Evans JMM, Newton RW, Jung RT, MacDonald TM. ACE inhibitor use is associated with hospitalization for severe hypoglycemia in patients with diabetes. Diabetes Care. 1997;20(9):1363–1367. [DOI] [PubMed] [Google Scholar]
- 12.Rave K, Flesch S, Nikolaus Kühn-Velten W, Hompesch BC, Heinemann L, Heise T. Enhancement of blood glucose lowering effect of a sulfonylurea when coadministered with an ACE inhibitor: results of a glucose-clamp study. Diabetes Metab Res Rev. 2005;21:459–464. [DOI] [PubMed] [Google Scholar]
- 13.Facts & Comparisons®. Wolters Kluwer Health, Conshohocken, Pennsylvania, USA. Available at http://online.factsandcomparisons.com. Accessed 17 November 2020. [Google Scholar]
- 14.Micromedex® (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA. Available at https://www.micromedexsolutions.com. Accessed 17 November 2020. [Google Scholar]
- 15.Phansalkar S, van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, Middleton B, Bates DW. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: The self-controlled case series method. Stat Med. 25, 1768–1797 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 18, 7–26 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 354, i4515 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Hennessy S, Leonard CE, Gatne JJ, Flory JH, Han X, Brensinger CM, Bilker WB. Pharmacoepidemiologic methods for studying the health effects of drug-drug interactions. Clin Pharmacol Ther. 2016;99(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam YH, Brensinger CM, Bilker WB, Leonard CE, Han X, Hennessy S. Serious hypoglycemia and use of warfarin in combination with sulfonylureas or metformin. Clin Pharmacol Ther. 2019;105(1):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser Family Foundation. Medicaid State Fact Sheets. 2017. Available at https://www.kff.org/interactive/medicaid-state-fact-sheets/. Accessed 19 January 2018.
- 22.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. Doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88(2):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal considerations in angiotensin converting enzyme inhibitor therapy: A Statement for Healthcare Professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104:1985–1991. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkranz B, Profozic V, Metelko Z, Mrzljak V, Lange C, Malerczyk V. Pharmacokinetics and safety of glimepiride at clinically effective doses in diabetic patients with renal impairment. Diabetologia. 1996;39:1617–1624.. [DOI] [PubMed] [Google Scholar]
- 26.Balant L, Zahnd G, Gorgia A, Schwarz R, Fabre J. Pharmacokinetics of glipizide in man: influence of renal insufficiency. Diabetologia. 1973:331–338. [DOI] [PubMed] [Google Scholar]
- 27.Pearson JG, Antal EJ, Raehl CI, Gorsch HK, Graig WA, Albert KS, Welling PG. Pharmacokinetic disposition of 14C-glyburide in patients with varying renal function. Clin Pharmacol Ther. 1986;39:318–324. [DOI] [PubMed] [Google Scholar]
- 28.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. JAMA. 1997;278(1):40–43. [PubMed] [Google Scholar]
- 29.Petrie JR, Morris AD, Ueda S, Elliott HL, Connell JM, Small M, Donnelly R. Do ACE inhibitors improve insulin sensitivity? Lancet. 1995;346(8974):583–584. [DOI] [PubMed] [Google Scholar]
- 30.Morris AD, Petrie JR, Ueda S, Connell JM, Elliott HL, Small M, Donnelly R. Pressor and subpressor doses of angiotensin II increase insulin sensitivity in NIDDM: dissociation of metabolic and blood pressure effects. Diabetes. 1994;43(12):1445–1449. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan TA, Thawani H, Kades W, Modrall JG, Weaver FA, Laurel C, Poppiti R, Xiang A, Hsueh W. Angiotensin II increases glucose utilization during acute hyperinsulinemia via a hemodynamic mechanism. J Clin Invest. 1993;92(2):720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard CE, Han X, Brensinger CM, Bilker WB, Cardillo S, Flory JH, Hennessy S. Comparative risk of serious hypoglycemia with oral antidiabetic monotherapy: A retrospective cohort study. Pharmacoepidemiol Drug Saf. 2018;27(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Medicaid and Medicare claims data are available via a data use agreement with the Centers for Medicare and Medicaid Services (CMS) (https://www.cms.gov/). The authors did not have any special access privileges that others would not have. The procedures to obtain access to these data are described in the CMS website (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/Researchers) and the Research Data Assistance Center (ResDAC) website (https://www.resdac.org/research-identifiable-files-rif-requests).


