Abstract
Differentiation of oligodendrocyte progenitor cells (OPC) into myelination capable mature oligodendrocytes is essential for proper function of the central nervous system (CNS). OPCs are tissue resident stem cells that populate all regions of the CNS and exist beyond development into adulthood. Disorders that lead to disruption of this critical cell state change cause devastating myelin diseases that are often associated with shortened lifespan. Recent findings have also provided support for a newly appreciated contribution of perturbed OPC differentiation to neurodegenerative and psychiatric diseases. These findings emphasize the need for a more complete understanding of OPC differentiation in health and disease. Here we review recent molecular and functional findings revealing new roles of OPCs. It is our hope that this review provides readers with an enticing snapshot of current OPC research and highlights the potential of controlling OPC fate and function to treat diseases of the brain.
Keywords: Oligodendrocyte progenitor cell, Differentiation, Oligodendrocyte, Learning and Memory, Myelin Plasticity
Introduction
Oligodendrocytes in the central nervous system (CNS) produce myelin, a multilayered lipid membrane structure that wraps axons to enhance the speed of action potential propagation and provide axons with metabolic trophic support [1]. During development, and throughout adulthood, myelinating oligodendrocytes are formed through terminal differentiation of oligodendrocyte progenitor cells (OPCs). OPCs are CNS resident stem cells that originate in brain and spinal cord ventricular zones before proliferating and migrating to populate the CNS [2]. Unlike most progenitor cell populations, OPCs remain present throughout life. This has led glial biologists to wonder why have adult OPCs? Are they present solely to replace lost oligodendrocytes? Do OPCs have functions that are independent of differentiation? For decades, glial biologists have been able to grow and differentiate OPCs in culture providing direct access to study this process. This has led to a detailed understanding of molecular regulators of OPC differentiation into oligodendrocytes. Broad mechanisms that are known to regulate OPC differentiation include transcription factors, chromatin regulators, protein post translational modifications, miRNAs, and external cues including oxygen saturation and mechanotransduction. Molecular regulation of OPC differentiation by these mechanisms has been addressed in excellent reviews and we would direct readers there for more detail [3–7]. Nevertheless, there are still many questions that surround OPC biology. New molecular mechanisms that govern OPC differentiation during development continue to be discovered. Evidence that OPCs are involved in learning and memory is accumulating. New OPC disease cell states and their functions are being uncovered. Moreover, there is a growing appreciation for the role of OPCs in the pathophysiology of neurodegenerative and psychiatric diseases. In this review we provide a series of short vignettes that highlight recent work that expand our understanding of OPCs. We hope that this engenders in the reader the same excitement that we have for these dynamic cells and the role they play in the normal function and pathology of the CNS.
New regulator of OPC differentiation during development
Appropriate regulation of OPC differentiation is critical for the proper development and function of the CNS. Important recent work has shown that OPC differentiation is regulated by N6-methyladenosine (m6A) modifications on mRNA. This is the most common internal modification of mRNA, and its discovery adds an additional method of post-transcriptional gene regulation [8]. Modifications to mRNA are placed by “writers”, protein complexes like the m6A methyltransferase that adds m6A marks to mRNA [9]. “Readers” then recognize mRNA modifications and translate them to a functional outcome, for example some readers stabilize modified mRNA [10]. Finally, mRNA modifications can be removed by “erasers” [11]. Recent work from two groups has identified a role for an m6A “reader” and “writer” in OPC differentiation (Fig 1). These components allow for a dynamic system that can ultimately affect gene and protein levels in cells. Wu et al., showed that Proline rich coiled-coil 2A (Prrc2a) binds to m6A to stabilize the oligodendrocyte lineage specifying transcription factor oligodendrocyte transcription factor (Olig2), and that Prrc2a conditional deletion in the oligodendrocyte lineage decreased oligodendrocyte formation and caused hypomyelination [12]. In addition, Xu et al. showed that blocking m6A modification specifically in the oligodendrocyte lineage through conditional deletion of methyltransferase like 14 (Mettl14), a core component of the m6A methyltransferase complex, decreased OPC differentiation leading to hypomyelination [13]. Xu et al. also performed m6A-seq in OPCs and oligodendrocytes and found thousands of transcripts with dynamic m6A marks suggesting that this mRNA modification may have additional roles to play within the oligodendrocyte lineage. These findings also show that our understanding of OPC differentiation mechanisms during development is incomplete. In addition, there is not yet evidence of disease associated changes in m6A modified mRNA within the oligodendrocyte lineage. Determining whether perturbations in this added layer of regulatory complexity contributes to OPC dysfunction in disease and whether it can be leveraged as a therapeutic target should be a prioritized focus of future studies.
Figure 1. Advances in our understanding of OPC physiology.
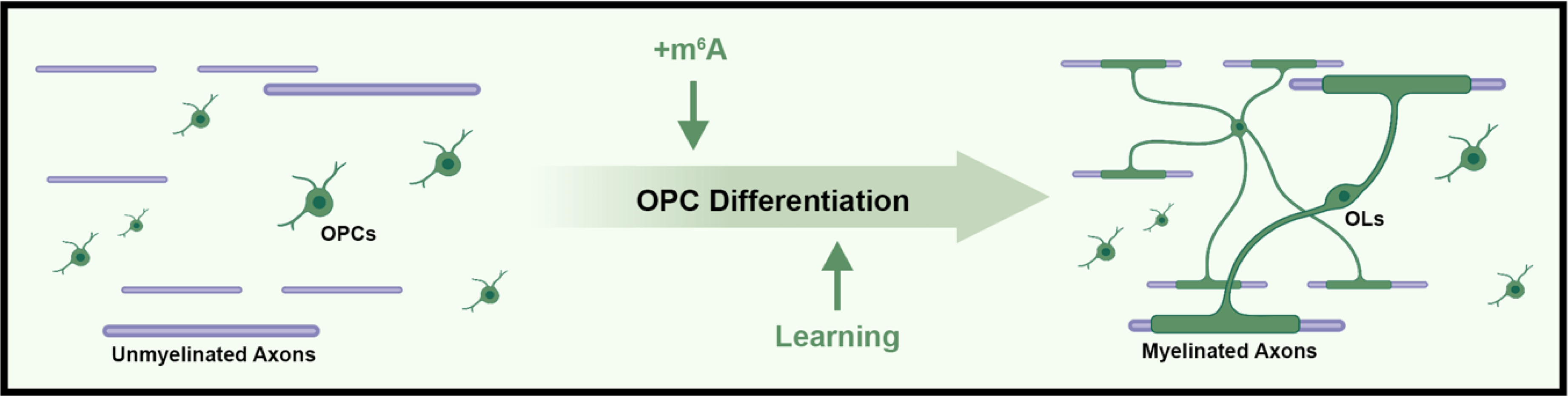
m6A modifications on mRNA are positive regulators of OPC differentiation. Deletion of the m6A reader Prrc2a, or the m6A writer Mettl14, leads to decreased mature oligodendrocytes and hypomyelination. Learning in multiple paradigms promotes OPC differentiation and myelination that is required for consolidating those learned behaviors into memory. Image created with BioRender.com
New evidence of a role for OPCs in learning and memory
Memory and learning require the synchronization of action potentials, and this requires tuning the speed of actional potential propagation. One mechanism to tune actional potential speed is myelin plasticity, which is the addition or removal of myelinated segments along an axon [14]. In humans, magnetic resonance imaging studies show that learning a new task correlates with increased myelin in brain regions associated with that task [15]. Exciting new research has shed more light on this connection and shown that myelination is required for learning and memory (Fig 1). Steadman et al. utilized transgenic mice where tamoxifen treatment excises a transcription factor, specifically in OPCs, that is required for OPC differentiation into oligodendrocytes. These transgenic mice allowed the authors to block the myelination from new oligodendrocytes and they used this system to show that de-novo myelination is required for spatial learning and memory consolidation in the water maze behavioral assay [16]. In agreement with these findings, Pan et al. used the same transgenic approach to block oligodendrocyte formation and myelination during a contextual fear conditioning paradigm. In this paradigm mice are exposed to contextual cues followed by foot shocks which induces fear causing the mouse to freeze when exposed to those same contextual cues either 24hrs or 30 days after the initial fear training [17]. Without the ability to form new oligodendrocytes and myelin, Pan et al. showed that mice were still able to recall the fear memory and froze 24hrs after initial training but did not consolidate the fear memory and did not freeze 30 days after the initial training. What drives OPCs to differentiate into myelin forming oligodendrocytes in response to these learning and memory tasks? OPCs form synapses with neurons and respond to neuronal activity directly through these connections [18] and neuronal activity causes increased OPC proliferation and differentiation ultimately leading to increased myelination [19]. These exciting findings strengthen the connection between OPC function and learning and memory, indicating that interventions which promote OPC differentiation may be used to enhance learning and memory.
New OPC cell state in multiple sclerosis.
Multiple sclerosis (MS) is an autoimmune disorder caused by inflammatory attacks against myelin in the CNS. Traditionally OPCs have not been considered to play a role in the etiology of MS. Instead, the role of OPCs in repair of demyelinated lesions has been the focus. This has led to important work on remyelinating therapies aimed at enhancing the inherent remyelinating capabilities of OPCs to repair MS lesions with the goal of halting or even reversing the progressive neurological decline that occurs as MS advances. However, recent findings show that OPCs undergo a state change to acquire immune cell functions that contribute to disease pathogenesis and inhibit repair by impeding the ability of OPCs to differentiate and replace lost oligodendrocytes and myelin (Fig 2). Using single-cell RNAseq to analyze oligodendrocyte heterogeneity in the MS mouse model, experimental autoimmune encephalomyelitis, Falcão et al. identified oligodendrocyte lineage cells that express genes associated with immune cells including major histocompatibility complex class I and II [20]. The authors confirmed in vitro that interferon-gamma (IFNγ) can drive OPCs to an immune-like state with immune-like functions including phagocytosis and activation of T-cells via MHC-II. Importantly, Falcão et al. confirmed that oligodendrocyte lineage cells in human MS lesions express MHC-II. The presence of immune-like OPCs in human MS lesions was further confirmed by single-nuclei analysis of oligodendrocytes from patients with primary progressive MS [21]. Jäkel and Agirre et al. found a distinct population of oligodendrocyte lineage cells in human MS that also take on an immune-like state. Independent validation of these findings was published by Kirby et al., showing again that IFNγ can drive OPCs to an immune like state that includes expression of MHC-I and MHC-II, and the immunoproteasome [22]. Importantly, this work also showed that immune-like OPCs could cross-present antigen to become targets of cytotoxic CD8 T cells and that transition to this immune-like state driven by IFNγ inhibits differentiation of OPCs. These two findings provide additional mechanisms for failed OPC differentiation and remyelination in MS lesions. Therapeutics that inhibit this OPC state change may provide an additional avenue to slow lesion formation and support remyelination.
Figure 2. Advances in our understanding of OPC fate and function in disease.
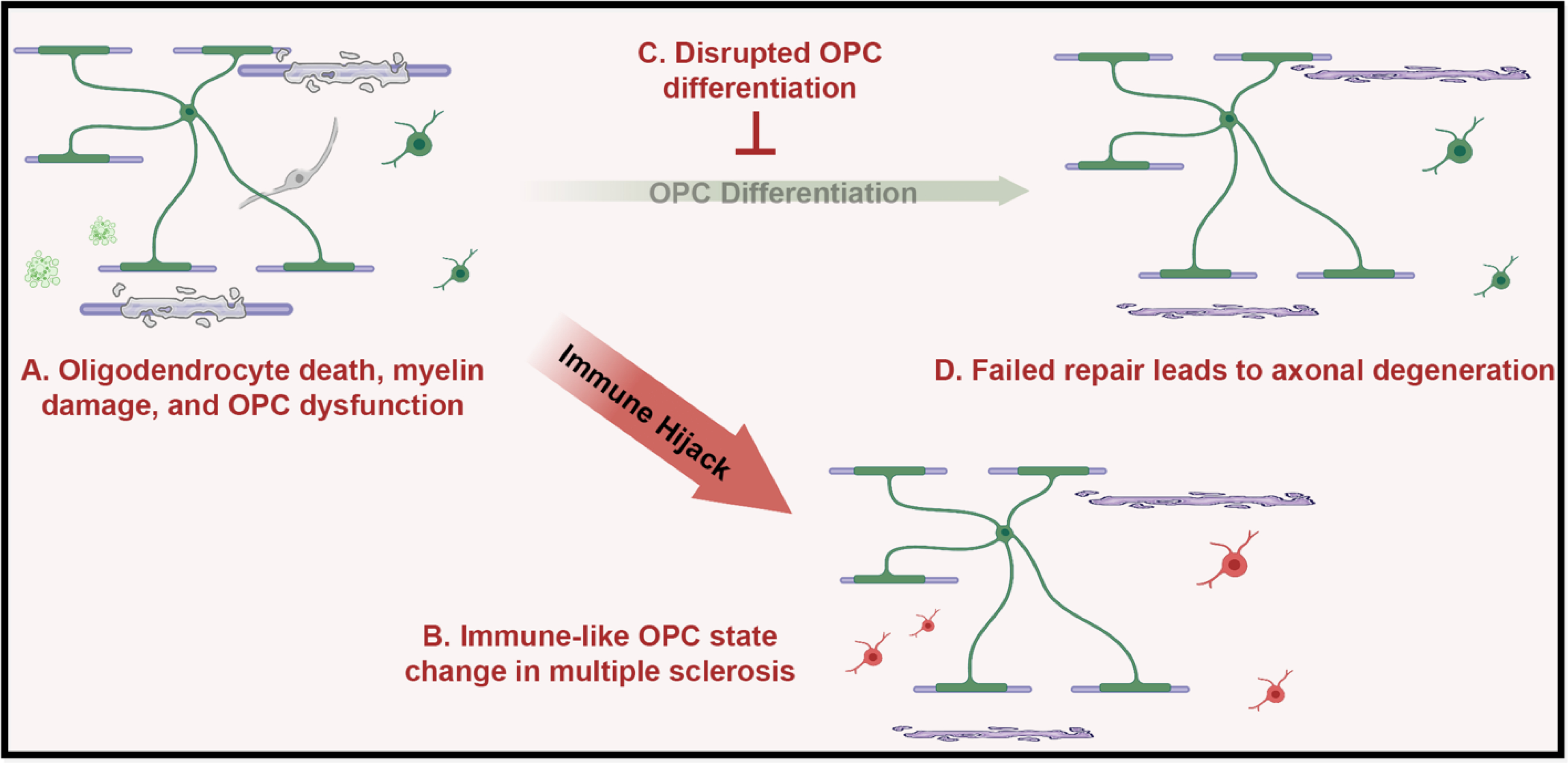
A) Oligodendrocyte death, myelin damage, and OPC dysfunction occur, not only in myelin diseases like multiple sclerosis, but in neurodegenerative and psychiatric disease as well. In disease repair by endogenous OPCs is disrupted either by B) OPC cell state change to immune-like cells in multiple sclerosis, or by C) perturbed OPC differentiation in neurodegenerative and psychiatric diseases. D) This failure of OPCs to differentiate and replace lost myelin leads to axonal degeneration causing cognitive and motor deficits. Image created with BioRender.com
New appreciation for OPC dysfunction in neurodegenerative and psychiatric disease.
In addition to new roles in traditional myelin disorders, there is also a growing appreciation for the role that OPCs play in the pathogenesis of neurodegenerative diseases like Alzheimer’s disease. White matter pathology in Alzheimer’s disease patients can occur prior to the onset of cognitive decline and prior to the appearance of amyloid-ß plaques and neurofibrillary tangles of hyperphosphorylated tau that are hallmarks of the disease [23,24]. Oligodendrocytes also expression high levels of Alzheimer’s risk genes including bridging integrator 1 (BIN1) [25], and human post-mortem studies show demyelination and OPC dysfunction [23]. Two recent studies leveraging single-nuclei RNAseq to identify cell type specific gene changes in Alzheimer’s disease provide further support for oligodendrocyte lineage cells, including OPCs, playing an important role in Alzheimer’s disease pathology (Fig 2). Both studies by Mathys et al. and Grubman et al. found perturbed expression in genes associated with oligodendrocyte differentiation and myelination [26,27]. Both studies also identified leucine rich repeat and Ig domain containing 1 (LINGO1), a negative regulator of OPC differentiation [28], as a gene significantly upregulated in Alzheimer’s tissue in multiple cell types including neurons and oligodendrocytes [26,27]. These data support the intriguing hypothesis that Alzheimer’s disease pathology is, at least in part, driven by myelin loss followed by failed remyelination in old-age that leads to axonal damage and ultimately neuronal death [29]. Evidence of OPC involvement is also present for Parkinson’s and Huntington’s disease. In Parkinson’s disease single-nuclei RNAseq of the substantia nigra combined with GWAS data showed that genetic risk for Parkinson’s was associated with OPCs and oligodendrocytes [30] In Huntington’s, Osipovitch et al. injected human embryonic stem cell derived glial progenitor cells (a corollary to OPCs) from Huntington’s patients into mice that lack myelin [31]. These human glia chimeric mouse models showed that cells from Huntington’s patients had a significantly lower ability to generate myelinating oligodendrocytes than cells from healthy donors. Importantly, this deficit could be rescued by forced expression of transcription factors that drive OPC differentiation [31]. More studies that examine cell-autonomous OPC dysfunction are needed to provide mechanistic understanding of the molecular pathways that drive OPC dysfunction in neurodegenerative diseases and thereby represent promising approaches for OPC-targeted therapies.
OPC dysfunction is also suspected to contribute to psychiatric disorders (Fig 2). In schizophrenia multiple lines of evidence point to disrupted OPC differentiation in patients. These include studies that report lower myelin signal, decreased oligodendrocyte numbers, and decreased levels of oligodendrocyte genes and proteins in schizophrenia patients [32]. Two recent studies have leveraged schizophrenia patient derived induced pluripotent stem (iPSCs) to provide further evidence for cell-autonomous OPC dysfunction as a contributor to schizophrenia pathology. Using the same approach as described for Huntington’s above, Windrem et al. generated iPSCs derived glial progenitor cells from patients with childhood onset schizophrenia and healthy controls [33]. Compared to controls, these schizophrenia patient derived cells failed to myelinate the brains of myelin deficient mice [33]. In another study utilizing iPSC derived OPCs from two siblings with schizophrenia and one control sibling, de Vrij et al. similarly showed that OPCs from the schizophrenia patients had abnormal morphology, lower viability, and decreased differentiation and myelination of slice cultures from hypomyelinated mouse [34]. Evidence also supports a role for OPC dysfunction in major depressive disorder. In the brains of major depressive disorder patients there are fewer oligodendrocytes, the corpus callosum is thinned, and expression of myelin associated genes are decreased in patients with major depressive disorder [35–37]. Single-nuclei RNAseq has also been used to show cell autonomous defects in gene expression in OPCs from patients with major depressive disorder [38]. Nagy et al. showed that OPCs from major depressive disorder patients had the greatest number of differentially expressed genes compared to control, and further showed through pseudotime analysis that while major depressive disorder patient OPCs differentiate they dysregulated genes associated with apoptosis [38]. This suggests that perturbed OPC differentiation may contribute to major depressive disorder. In addition, Nagy et al. identified a population of oligodendrocyte lineage cells that had overlap in gene expression with OPCs in MS that acquire immune functions. Although more direct examination of OPCs in the pathogenesis of major depressive disorder is needed, these exciting results suggest that promoting effective OPC differentiation may be beneficial for this and other neuropsychiatric diseases.
Conclusion and perspective
Discovering therapies that promote OPC differentiation and enhance remyelination has long been a clinical goal for myelin disorders like multiple sclerosis. With increasing evidence that OPC dysfunction also contributes to neurodegenerative and psychiatric disorders, the potential for OPC targeted therapies to impact disease is even greater. OPCs generated from stem cells can be expanded in culture to generate millions of cells [39]. In addition to their regenerative potential as a cellular source for transplantation medicine, numerous labs have leveraged OPC platforms to perform large scale drug screens and identify small-molecules that enhance the transition of OPCs to oligodendrocytes [40–43]. The majority of small-molecules identified in these screens converge on a single unifying mechanism of action to drive oligodendrocyte formation by inhibiting specific enzymes in the cholesterol biosynthesis pathway [44]. This work suggests that sterol modulating medicines might provide a powerful approach to stimulate the regeneration of new oligodendrocytes from CNS resident OPCs in vivo. What remains unknown is whether such approaches will be universally regenerative across various neurological and neuropsychiatric diseases or whether disease- or context-specific screens will be required for effective therapies.
In close, OPCs are more than passive support cells waiting to engage in remyelination following loss of mature oligodendrocytes. These are dynamic cells with many critical functions that continue to be discovered. Here we have highlighted a sample of the exciting new discoveries that expand our understanding of OPC fate and function in health and disease. There are still more questions to be answered and we look forward to the many important discoveries to come.
Highlights.
Oligodendrocyte progenitor cells are dynamic resident stem cells of the CNS with diverse functions in health and disease.
m6A modifications on RNA is a newly discovered molecular regulator of OPC differentiation.
OPCs undergo a cell state change and become immune-like in multiple sclerosis.
Accumulating evidence for OPC dysfunction contributing to neurodegenerative and psychiatric diseases.
Acknowledgements
Current work in the Tesar laboratory is supported by the National Institutes of Health (R35NS116842, R61NS117774).
Footnotes
Conflict of interest statement
P.J.T. and B.L.L.C. are listed as inventors on issued and pending patent claims covering compositions and methods of enhancing glial function. P.J.T. is a co-founder and consultant for Convelo Therapeutics, which has licensed some of these claims and patents from Case Western Reserve University (CWRU). P.J.T. and CWRU retain equity in Convelo Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore S, Meschkat M, Ruhwedel T, Trevisiol A, Tzvetanova ID, Battefeld A, Kusch K, Kole MHP, Strenzke N, Möbius W, et al. : A role of oligodendrocytes in information processing. Nat Commun 2020, 11:5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson WD, Kessaris N, Pringle N: Oligodendrocyte wars. Nature Reviews. Neuroscience 2006, 7:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbaz B, Popko B: Molecular Control of Oligodendrocyte Development. Trends Neurosci 2019, 42:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry K, Wang J, Lu QR: Epigenetic regulation of oligodendrocyte myelination in developmental disorders and neurodegenerative diseases. F1000Res 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galloway DA, Moore CS: miRNAs As Emerging Regulators of Oligodendrocyte Development and Differentiation. Front Cell Dev Biol 2016, 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery B, Lu QR: Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb Perspect Biol 2015, 7:a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler NA, Fuss B: Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp Neurol 2016, 283:512–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y, Dominissini D, Rechavi G, He C: Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet 2014, 15:293–306. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. : A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 2014, 10:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. : N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, et al. : N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011, 7:885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu R, Li A, Sun B, Sun JG, Zhang J, Zhang T, Chen Y, Xiao Y, Gao Y, Zhang Q, et al. : A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res 2019, 29:23–41. ••Identifies Prrc2a as a novel m6A reader required for oligodendrocyte differentiation. Loss of Prrc2a specifically in the oligodendrocyte lineage leads to decreased mature oligodendrocyte numbers and hypomeylination.
- 13. Xu H, Dzhashiashvili Y, Shah A, Kunjamma RB, Weng YL, Elbaz B, Fei Q, Jones JS, Li YI, Zhuang X, et al. : m(6)A mRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination. Neuron 2020, 105:293–309.e295. ••Oligodendrocyte lineage specific excision of Mettl14, a critical component of the methyltransferase complex, leads to decreased oligodendrocyte numbers and hypomyelination. Further, identifies transcriptome white m6A targets that change during OPC differentiation.
- 14.Fields RD: Myelination: an overlooked mechanism of synaptic plasticity? The Neuroscientist 2005, 11:528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholz J, Klein MC, Behrens TE, Johansen-Berg H: Training induces changes in white-matter architecture. Nat Neurosci 2009, 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW: Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020, 105:150–164.e156. ••Refernces 16 and 17 show that learning in two distinct behavioral paradigms, spatial and fear, promotes OPC differnetiation and that inhibiting OPC differentiation and therfore myelination during learning perturbs memory consolidation in these paradigms.
- 17. Pan S, Mayoral SR, Choi HS, Chan JR, Kheirbek MA: Preservation of a remote fear memory requires new myelin formation. Nat Neurosci 2020, 23:487–499. ••Refernces 16 and 17 show that learning in two distinct behavioral paradigms, spatial and fear, promotes OPC differnetiation and that inhibiting OPC differentiation and therfore myelination during learning perturbs memory consolidation in these paradigms.
- 18.Bergles DE, Roberts JD, Somogyi P, Jahr CE: Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 2000, 405:187–191. [DOI] [PubMed] [Google Scholar]
- 19.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. : Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344:1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcao AM, van Bruggen D, Marques S, Meijer M, Jakel S, Agirre E, Samudyata Floriddia EM, Vanichkina DP, Ffrench-Constant C, et al. : Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med 2018, 24:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakel S, Agirre E, Mendanha Falcao A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, Ffrench-Constant C, Williams A, Castelo-Branco G: Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566:543–547. ••Single-nuclei analysis of primary progressive multiple sclerosis patient tissue identifies an OPC state change into immune-like cells.
- 22. Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J, et al. : Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 2019, 10:3887. ••OPCs exposed to IFNγ take on immune-like properties including upregulation of the immunoproteasome and MHC class I molecules.
- 23.Butt AM, De La Rocha IC, Rivera A: Oligodendroglial Cells in Alzheimer’s Disease. Adv Exp Med Biol 2019, 1175:325–333. [DOI] [PubMed] [Google Scholar]
- 24.Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, Sager MA, Asthana S, Johnson SC, Alexander AL, et al. : Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS One 2017, 12:e0173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Rossi P, Buggia-Prévot V, Clayton BLL, Vasquez JB, van Sanford C, Andrew RJ, Lesnick R, Botté A, Deyts C, Salem S, et al. : Predominant expression of Alzheimer’s disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Molecular Neurodegeneration 2016, 11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, et al. : Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570:332–337. ••Referencs 26 and 27 leverage single-nuclei RNAseq of Alzheimer’s disease patient tissue to identify transcriptional changes in the oligodendrocyte lineage, including increased expression of LINGO-1.
- 27. Grubman A, Chew G, Ouyang JF, Sun G, Choo XY, McLean C, Simmons RK, Buckberry S, Vargas-Landin DB, Poppe D, et al. : A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat Neurosci 2019, 22:2087–2097. ••Referencs 26 and 27 leverage single-nuclei RNAseq of Alzheimer’s disease patient tissue to identify transcriptional changes in the oligodendrocyte lineage, including increased expression of LINGO-1.
- 28.Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, et al. : LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 2005, 8:745–751. [DOI] [PubMed] [Google Scholar]
- 29.Bartzokis G: Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 2011, 32:1341–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal D, Sandor C, Volpato V, Caffrey TM, Monzon-Sandoval J, Bowden R, Alegre-Abarrategui J, Wade-Martins R, Webber C: A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat Commun 2020, 11:4183. ••Single-nuclei RNAseq shows that Parkinson’s disease genetic risk is associated with OPC and oligodendrocytes and that LRRK2, a known Parkinson’s gene, is most highly expressed in OPCs.
- 31. Osipovitch M, Asenjo Martinez A, Mariani JN, Cornwell A, Dhaliwal S, Zou L, Chandler-Militello D, Wang S, Li X, Benraiss SJ, et al. : Human ESC-Derived Chimeric Mouse Models of Huntington’s Disease Reveal Cell-Intrinsic Defects in Glial Progenitor Cell Differentiation. Cell Stem Cell 2019, 24:107–122.e107. ••When injected into hypomyelinated mice, Huntington’s patient derived glial progenitor cells reveals a cell autonomous defect in the ability of Huntington’s cells to differentiate into oligodendrocytes.
- 32.Raabe FJ, Slapakova L, Rossner MJ, Cantuti-Castelvetri L, Simons M, Falkai PG, Schmitt A: Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, et al. : Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 2017, 21:195–208.e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Vrij FM, Bouwkamp CG, Gunhanlar N, Shpak G, Lendemeijer B, Baghdadi M, Gopalakrishna S, Ghazvini M, Li TM, Quadri M, et al. : Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol Psychiatry 2019, 24:757–771. ••Glial progenitor cells from schizophrenia patients with CSPG4 mutations show cell autonomous dysfunction leading to decreaesed myelination potential when injected into hypomeylinated mice.
- 35.Williams MR, Sharma P, Macdonald C, Pearce RKB, Hirsch SR, Maier M: Axonal myelin decrease in the splenium in major depressive disorder. Eur Arch Psychiatry Clin Neurosci 2019, 269:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamidi M, Drevets WC, Price JL: Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry 2004, 55:563–569. [DOI] [PubMed] [Google Scholar]
- 37.Aston C, Jiang L, Sokolov BP: Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 2005, 10:309–322. [DOI] [PubMed] [Google Scholar]
- 38. Nagy C, Maitra M, Tanti A, Suderman M, Théroux JF, Davoli MA, Perlman K, Yerko V, Wang YC, Tripathy SJ, et al. : Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci 2020, 23:771–781. ••Single-nuclei RNAseq analysis of tissue from patients with major depressive disorder show that gene expression changes predominantly occur in excitatory neurons and immature OPCs.
- 39.Najm FJ, Zaremba A, Caprariello AV, Nayak S, Freundt EC, Scacheri PC, Miller RH, Tesar PJ: Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nature Methods 2011, 8:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, et al. : Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015, 522:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mei F, Fancy SPJ, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, et al. : Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 2014, 20:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lariosa-Willingham KD, Rosler ES, Tung JS, Dugas JC, Collins TL, Leonoudakis D: A high throughput drug screening assay to identify compounds that promote oligodendrocyte differentiation using acutely dissociated and purified oligodendrocyte precursor cells. BMC Res Notes 2016, 9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, et al. : A regenerative approach to the treatment of multiple sclerosis. Nature 2013, 502:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubler Z, Allimuthu D, Bederman I, Elitt MS, Madhavan M, Allan KC, Shick HE, Garrison E, M TK, Factor DC, et al. : Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 2018, 560:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]


