Abstract
Purpose of review:
An intronic G4C2 expansion mutation in C9orf72 is the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia (C9-ALS/FTD). While there are currently no treatments for this insidious, fatal disease, intense research has led to promising therapeutic strategies, which will be discussed here.
Recent findings:
Therapeutic strategies for C9-ALS/FTD have primarily focused on reducing the toxic effects of mutant expansion RNAs or the dipeptide repeat proteins (DPRs). The pathogenic effects of G4C2 expansion transcripts have been targeted using approaches aimed at promoting their degradation, inhibiting nuclear export or silencing transcription. Other promising strategies include immunotherapy to reduce the DPRs themselves, reducing RAN translation, removing the repeats using DNA or RNA editing and manipulation of downstream disease-altered stress granule pathways. Finally, understanding the molecular triggers that lead to pheno-conversion may lead to opportunities that can delay symptomatic disease onset.
Summary:
A large body of evidence implicates RAN-translated DPRs as a main driver of C9-ALS/FTD. Promising therapeutic strategies for this devastating disease are being rapidly developed with several approaches already in or approaching clinical trials.
Keywords: C9orf72-ALS/FTD, therapeutic strategies, gene therapy, symptomatic management, small molecule inhibitors
Introduction
A GGGGCC hexanucleotide repeat expansion in the first intron of C9ORF72 causes the most common forms of familial amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (1, 2), genetically linking these two clinically distinct adult-onset neurodegenerative disorders. C9ORF72 ALS, FTD or both ALS and FTD can occur in individual patients and within families (3). C9-ALS/FTD patients typically have hundreds to thousands of G4C2•G2C4 repeats while shorter tracts of 2–24 repeats are present in unaffected people (1, 2). ALS is characterized by motor neuron loss, muscle atrophy, progressive paralysis and usually death within 2–5 years of onset (4). In FTD, degeneration of neurons in the frontal and anterior temporal lobes can result in personality changes such as apathy, loss of empathy, disinhibition and executive function deficits (4). Therapeutic options are limited and there are no current treatment options that substantially change the course C9orf72 ALS or FTD. The standard of care includes the anti-glutamatergic drug riluzole for ALS (5) and the anti-depressant fluoxetine or a related compound for FTD (6). In 2017, the free-radical scavenger drug edaravone was approved for use in ALS patients (7). Unfortunately, none of these treatments improve motor or cognitive deficits, however riluzole and edaravone have been shown to modestly slow disease progression in some ALS patients.
Similar to other microsatellite expansion disorders (8, 9), the C9orf72 G4C2•G2C4 mutation is bi-directionally transcribed and sense G4C2 and antisense G2C4 expansion transcripts form RNA foci and produce repeat associated non-AUG (RAN) proteins (10–15). Proposed disease mechanisms include: (i) toxic effects of the expansion RNAs (1, 16); (ii) toxic effects of sense (poly-GA, GR, GP) and antisense (poly-PR, PA, GP) dipeptide RAN proteins (10, 12–14) and (iii) haploinsufficiency of the C9ORF72 protein (17, 18). Overall, RAN proteins, particularly GA, GR and PR, have been shown to be toxic in a number of cell culture and animal models (for reviews (19, 20)).
The discovery of the C9ORF72 expansion as the most common genetic cause of ALS and FTD (1, 2) has fueled an interdisciplinary worldwide research effort to understand the mechanisms and develop therapies for this disorder. C9ORF72 expansions cause ~40% of familial and 6–8% of sporadic ALS cases and ~18% of familial and 6% of sporadic FTD cases (21, 22*) in European populations but are relatively rare in Asia (23). The relatively large numbers of C9-ALS/FTD patients worldwide, combined with multiple emerging therapeutic strategies have positioned C9-ALS/FTD well for breakthrough therapy development. Emerging therapeutic strategies include: targeting and removing the expanded repeats; degrading, or preventing expression of expansion transcripts; reducing toxic RAN proteins: and modulating downstream affected pathways including nucleocytoplasmic transport and stress granules (Figure 1). These strategies and additional efforts to understand key molecular and physiological changes that trigger disease may provide insights that will lead to better disease management and help stratify the inclusion of the most informative patients for clinical trials.
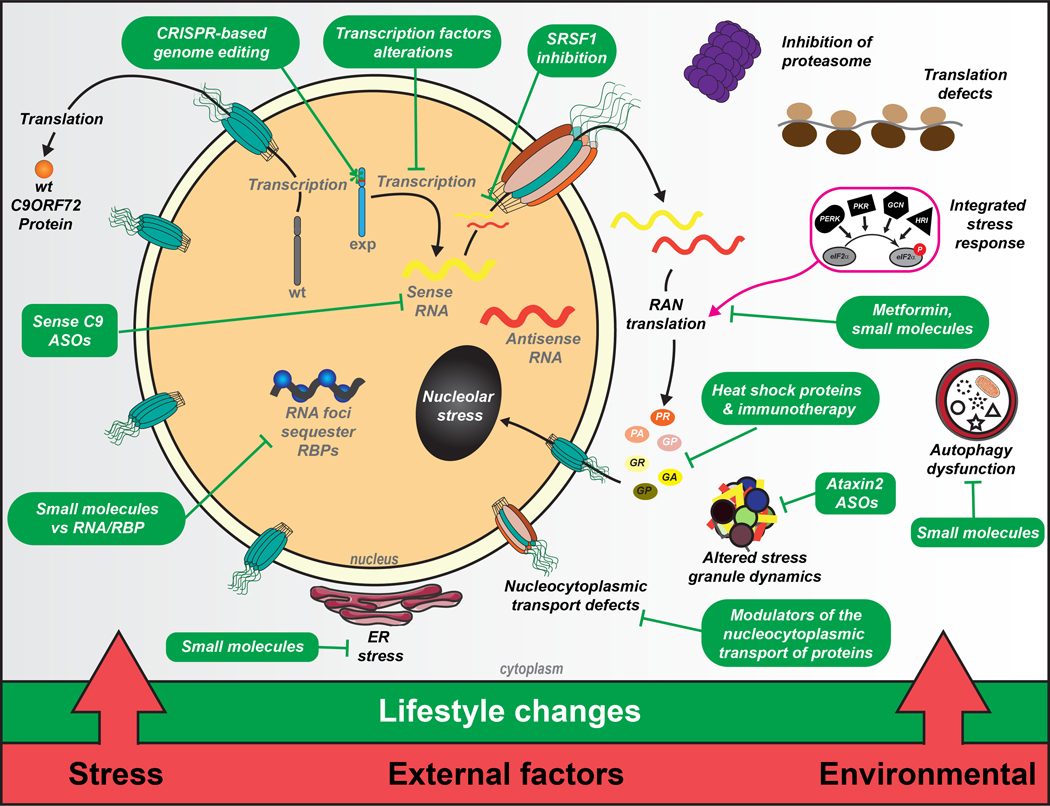
Figure. Cellular consequences and therapeutic approaches for C9orf72-ALS/FTD.
Expression of sense and antisense expanded C9 transcript RNA and dipeptide repeat (DPR) proteins affect a wide array of downstream cellular pathways including ER stress, nucleocytoplasmic defects, altered stress granule dynamics, autophagy dysfunction, translational defects, proteasome inhibition, RAN translation, nucleolar stress, RNA foci formation. Different therapeutic approaches (green boxes) target the C9 RNAs and RAN DPRs directly as well as the downstream pathways. Additionally, external factors, such as patient lifestyle, stress, comorbid diseases and environmental factors (bottom red bar) can influence cellular events with exercise and lifestyle changes offering mixed therapeutic potential. Some content modified from Servier Medical Art (smart.servier.com) under creative common license.
Targeting expansion transcripts for degradation
Sense C9ORF72-repeat transcripts.
Almost immediately following the discovery of the C9orf72 expansion mutation efforts to develop antisense oligonucleotide (ASO) drugs to knock-down the repeat expansion RNAs began. These nucleic acid-based drugs are chemically modified and in some applications take advantage of the nuclear RNase H1 pathway to degrade double-stranded sequences that form when ASOs bind to targeted gene transcripts (24**). For C9ORF72, specific ASOs were shown to selectively reduce sense G4C2 RNA foci in patient cells without reducing the levels of C9ORF72 mRNA (11, 16). BAC transgenic mice treated with single-dose ASOs that selectively target sense expansion RNAs but not mRNAs encoding C9orf72 protein, decreased sense RNA foci and sense DPRs and improved behavioral abnormalities (25). Together these results paved the way for a phase I clinical trial to test the safety, tolerability, and pharmacokinetics of the Ionis/Biogen BIIB078 ASO in adults with C9ORF72 ALS (NCT03626012).
More recently, stereopure ASOs were shown to increase RNAse H activity in vitro and in vivo compared to stereorandom ASOs (26). A lead candidate stereopure ASO showed selective degradation of sense G4C2 expansion containing transcripts without reducing the variant 2 isoform which lacks the repeat. Treatment of BAC transgenic mice with these ASOs reduced RNA foci and RAN GP proteins but not C9ORF72 protein levels. Additionally, these oligonucleotides selectively protected motor neurons harboring C9ORF72-expansion mutations from glutamate-induced toxicity (27**). In an alternative approach, miRNAs targeting sense C9ORF72-repeat transcripts using adeno-associated virus serotype 5 (AAV5) delivery, which reduced levels of sense expansion transcripts and RNA foci in FTD iPSC-derived frontal brain-like neurons and BAC transgenic mice (28**). Interestingly, AAV-mediated miRNA-depletion of SOD1 was recently reported in 2 patients with SOD1-ALS (29**), providing proof of concept data that intrathecally delivered microRNAs can be used as a potential treatment strategy for ALS.
Inhibiting transcription of C9-expansion transcripts.
Several different strategies are being actively pursued to decrease the transcription of C9-repeat expansion transcripts. For example, SUPT4H, SUPT5H and RNA polymerase II associated factor 1 complex (PAF1C) are transcription factors that play important roles in the elongation of RNAs containing expanded repeats (30, 31). Decreased expression of SPT4 encoded by SUPT4H was shown to decrease the levels of sense and antisense C9orf72 expansion transcripts and GP-RAN proteins in C. elegans, Drosophila and iPSC-derived models of C9ORF72-ALS/FTD and to ameliorate neurodegenerative phenotypes in Drosophila (32). Similarly, depletion of PAF1C reduced expression of G4C2 expansion RNAs and poly(GR) in a Drosophila model (31). Although interesting, the therapeutic potential of these strategies may be limited by off-target effects (33*).
In other approaches, CRISPR-Cas9 deletion of the promoter region driving expression of the C9ORF72 repeat-containing transcript isoforms 1 and 3 led to the efficient reduction in expression levels of all three sense DPRs in C9-ALS patient-derived motor neurons (34*). Pinto et al., showed targeting C9ORF72 expansions at the DNA level using deactivated Cas9 (dCas9) efficiently inhibits transcription and reduced the levels of GP RAN protein in reporter cells (35). Similarly, use of RNA-targeting deactivated-Cas9 (RCas9) allows degradation of C9ORF72 expansion transcripts in cell models (36), although it is possible that this strategy also blocks transcription by binding of the dCas9-PIN-gRNA to the DNA repeats as in dCas9 strategy.
While there is great hope that decreasing the levels of G4C2 expansion transcripts will be sufficient to improve disease, several studies suggest that it will be important to increase efforts to also target antisense transcripts. For example, antisense RNA foci preferentially accumulate in regions of pathology in a C9ORF72 BAC transgenic mouse model suggesting antisense expansion RNAs or RAN proteins may be more toxic than their corresponding sense products (37). ASOs that target sense C9ORF72 expansion transcripts did not correct widespread transcriptomic defects found in patient-derived cells (11), suggesting strategies targeting both transcripts may offer the best outcomes.
Immunotherapy strategies targeting RAN proteins
There is strong evidence that RAN DPRs, particularly GA, GR and PR, are one of the main drivers of disease (for reviews (19, 20)) and hence decreasing their levels is an attractive therapeutic approach. Several immunotherapy approaches have focused on the GA RAN proteins. In patient fibroblasts and primary neurons, α-GA antibodies reduced GA aggregate formation and blocked aggregate seeding activity of cerebellar extracts from C9ORF72 autopsy tissue (38). Vaccination of GA-overexpression mice with ovalbumin-(GA)10 peptides elicited the production of α-GA antibodies, lowered GA protein levels and prevented microglial activation and motor deficits (39**). Using BAC transgenic mice that express sense and antisense transcripts and multiple types of RAN proteins, Nguyen et al., (40**) showed passive immunotherapy with α-GA antibodies improved behavioral deficits, increased survival and decreased neuroinflammation and motor neuron loss. These peripherally injected antibodies crossed the blood brain barrier and co-localized with GA protein aggregates. Glycosylation of the Fc antibody region was important for cell entry and GA proteins were reduced in a TRIM-21-, and proteosome-, and autophagy-dependent manner (40**). In addition to reducing GA, the α-GA1 treatment surprisingly also reduced GP and GR proteins, likely through increased proteosome function. No changes in sense or antisense RNA levels or foci were observed in α-GA1 treated mice providing strong support that RAN proteins drive C9-ALS/FTD (40**).
Decreasing RAN protein levels
Several groups have shown that activation of the integrated stress response (ISR) and increased p-eIF2α levels increase RAN translation (41-44, 45**). Zu et al. showed G4C2 and other repeat expansion RNAs activate ISR protein kinase R (PKR) and that PKR inhibition dramatically reduces RAN protein levels (45**). Zu et al., went on to show Inhibition of PKR using AAV-delivered dominant negative PKR-K296R or the FDA-approved drug metformin decreased p-PKR and RAN protein levels and improved behavior and neuropathology in C9 BAC transgenic mice (45**) without changing sense or antisense transcript levels. There is an active clinical trial to test safety of metformin in C9ORF72 ALS patients and its effects on RAN protein levels (NCT04220021). Inhibition of the SRSF1-dependent nuclear export of both sense and antisense C9ORF72-repeat transcripts and subsequent RAN translation was also reported as a promising gene therapy approach in preclinical models including patient-derived motor neurons and Drosophila models of disease (46).
Other strategies to reduce RAN protein levels include stimulating their clearance. For example, heat shock protein family B member 8 (HSPB8) has been shown to promote autophagy-mediated removal of several misfolded C9 RAN proteins from motoneurons (47). Although the therapeutic potential of this approach is uncertain, clearance of protein aggregates could have applications for a wide variety of neurodegenerative diseases. Taken together, these data support the therapeutic potential of targeting RAN translation and RAN proteins for C9-ALS/FTD as well as other RAN protein associated disorders.
Targeting the genomic C9ORF72 hexanucleotide-repeats
Correcting the GGGGCC•GGCCCC repeat expansion mutation should theoretically address all deleterious mutation effects, including effects from sense and antisense RNAs and DPRs. Current gene editing techniques have focused primarily on clustered regular interspaced short palindromic repeats (CRISPR)-associated (Cas) systems, although application to C9-ALS/FTD has so far been limited (48, 49*). In iPSC-cells CRISPR/Cas9 editing and homology-directed repair (HDR) replacement of the expansion with a wildtype repeat resulted in restoration of C9ORF72 gene expression and methylation and reduced intron retention and downstream pathogenic phenotypes (49*). In iPSC-derived motor neurons, CRISPR/Cas9 correction abolished GluA1 AMPA receptor (AMPAR) mediated excitotoxicity (48). Targeting regions outside the repeat or the entire C9ORF72 gene have also been tested with varying degrees of success. For example, deletion of a portion of the upstream C9ORF72 promoter prevents the production of exon 1a expansion containing transcripts and the activation of neurodegenerative pathways (34*). Unfortunately, this approach does not prevent expression of antisense RNA and associated antisense DPRs, which likely contribute to disease. While correcting the expansion mutation seems to be a straight-forward idea, adequate delivery to affected tissues/cell types and the accuracy of emerging CRISPR based approaches will be critical for effective therapy development.
Stress granules and nucleocytoplasmic transport
TDP43 plays important roles in transcriptional regulation, alternative splicing of pre-mRNAs, axonal transport of mRNAs, translational regulation and miRNA processing. TDP-43 also associates with stress granules (50), which constitute dynamic membrane-less organelles that promote cell survival by halting translation of non-essential mRNAs in response to cellular stress (51). Stress granules are composed of RNA and RNA-binding proteins with low complexity domains (LCDs) that mediate liquid-liquid phase separation (LLPS). Mutations in the LCDs domains of TDP43, Ataxin-2 and other RNA-binding proteins involved in ALS/FTLD stimulate their self-assemblies leading to the formation of persistent cytoplasmic stress granules leaving aggregated proteins which contribute to disease pathogenesis (52). Interestingly, arginine-rich C9ORF72 DPRs impair stress granules assembly dynamics by undergoing LLPS and further inducing the phase separation of stress granule proteins (53) and promote nucleocytoplasmic transport disruption by stimulating the recruitment of nucleocytoplasmic transport proteins to stress granules (54). TDP-43 proteinopathy, aggregation of stress granule proteins (G3BP1, ataxin-2), nucleocytoplasmic defects, neuronal loss and motor/cognitive deficits were observed in an AAV-driven overexpression mouse model of expanded G4C2 repeats (55). DPRs also promote nucleocytoplasmic transport disruption by stimulating the recruitment of nucleocytoplasmic transport proteins to stress granules (56). In fact, many nucleocytoplasmic transport factors are localized to stress granules when exposed to stressors or mutant proteins implicated in ALS pathogenesis, leading to impaired nucleocytoplasmic transport (56).
Targeting nucleocytoplasmic transport deficits
In 2015, Zhang et al. demonstrated increased nuclear export in C9orf72 ALS iPSN model shown as abnormal cytoplasmic RanGTPase accumulation (57). RanGTPase is important in nucleocytoplasmic protein transport (reviewed in (58)). Abnormal expression and localization of nuclear pore proteins found in C9ORF72 autopsy tissue and patient-derived iPSNs (57, 59). Modulating the expression of nuclear pore proteins or transport associated proteins affects G4C2 expansion transcripts and arginine containing RAN protein toxicity (57, 60, 61). Overexpression of importin or inhibition of nuclear export with RNA inhibition of (Exportin 1) XPO1 or pharmacologically ablating XPO1 function using KPT-276 rescued C9orf72 toxicity in the C9orf72 fly model (57). Another XPO1 inhibitor, KPT-350, designed by Karyopharm Therapeutics and acquired by Biogen (BIIB100) has been used in pre-clinical studies of many neurological diseases and demonstrated neuroprotective and anti-inflammatory roles (62-64*, 65).
Trophic support supplementation
Neurotrophic factors (NTFs), a family of biomolecules that support neuronal growth, survival and differentiation, have been explored for decades as therapeutic strategy for neurodegenerative diseases (31428042), including ALS. Various neurotrophic factors have been tested in preclinical rodent models of SOD1-ALS, including Brain-derived Neurotrophic Factor (BDNF), Insulin-like Growth Factor 1 (IGF-1) and Vascular Endothelial Growth Factor (VEGF). Small molecule agonist of the BDNF receptor (66), VEGF injections (67, 68), lentiviral and AAV-mediated delivery of NTFs (69–72) and stem cell therapy of NTF secreting cells (73–81) have shown promise in SOD ALS models. Despite these promising results, including several ongoing clinical trials (82*), there has been little to no direct studies looking at NTF therapeutics in C9-ALS/FTD. While some NTF clinical trials in ALS likely include C9-ALS/FTD participants it is important, given the unique disease mechanisms of C9-ALS/FTD, to directly examine NTF specifically in the C9 context.
Prevention and functional management
For C9-ALS/FTD there is a relatively long-period of apparent good health prior to disease onset, often in the fourth or fifth decade of life and extending this period of good health has been gaining considerable attention. Both preclinical animal studies and human studies demonstrate that moderate exercise regimens improve functionality and ameliorate disease symptoms for ALS in general (reviewed in (83)). However, the role of exercise is complex. In a retrospective study, patient-reported exercise history was inversely correlated with age-of-onset in C9orf72 but not other forms of ALS (84), although additional studies that examine the impact of specific types of exercise will be important. Targeted training may be key. In a randomized, sham-controlled clinical trial, Plowman et al. showed, expiratory muscle-strength training is well tolerated in ALS patients and improved bulbar function in a longitudinal C9orf72 case-study and also in larger cohorts of genetically undefined ALS patients (85, 86**). Longitudinal studies suggest that lifestyle modifications (e.g. smoking cessation, maintaining a healthy body-mass index) at a younger age may lower the risk of developing ALS (87**). Recent studies have identified C9ORF72 expansions in1.6% (n = 8/487) of cases with possible idiopathic normal pressure hydrocephalus (iNPH) but no controls (n = 0/432) >65 years. Clinically significant shunt response was detected in 6 out of 7 shunted C9ORF72 expansion carriers. Additional studies are needed to understand the frequency of NPH in C9ORF72 expansion carriers and the potential utility of shunts to drain excess cerebrospinal fluid in these patients. Dietary studies specific to C9-ALS/FTD are rare, although a larger study focused on ALS in general has demonstrated that increasing fruit and vegetable associated fiber, antioxidants and carotenes was associated with improved function (88). The results of many of these broader ALS studies are complicated by the complex genetics and phenotypic presentation of ALS, increasing the call for preventative and lifestyle studies that focus on C9orf72 or other single ALS mutations.
Stratification and efficiency in clinical trials
While there are multiple therapeutic approaches for C9-ALS/FTD in the pre-clinical pipeline, there are only two C9orf72-ALS/FTD specific clinical trials registered in ClinicalTrials.gov: (1) an antisense oligonucleotide (ASO)-based clinical trial of BIIB078 (Biogen), which targets C9orf72 expansion containing transcripts for ASO-induced RNase H-mediated degradation (NCT03626012) and; (2) a clinical trial (NCT04220021), to test the safety and tolerability of metformin in C9orf72 ALS patients and the drug’s ability to decrease RAN protein levels. Several additional trials are open to but not specific to C9-ALS/FTD patients. These include a phase 1 clinical trial of BIIB100 to reduce excessive nuclear export (NCT03945279) in C9 and other ALS patients, to assess safety, tolerability, pharmacokinetics, and pharmacodynamics. A phase 2 clinical trial examining the safety, tolerability, PK and PD of AL001, a recombinant human anti-human sortilin (SORT1) monoclonal IgG1 in FTD patients with either granulin or C9orf72 mutations (NCT03987295). Sortilin is a type I membrane glycoprotein involved in proganulin trafficking that is expressed in the central nervous system (89). Frontotemporal degeneration can be caused by mutation in the progranulin (GRN) gene or the C9orf72 hexanucleotide expansion repeat and there are rare patients with mutations in both (90). More recently, Wave Life Sciences has been reported to seek regulatory approval for WVE-004, an investigational stereopure ASO targeting the expansion transcript of C9-ALS/FTD (27**). It is interesting to note the current batch of clinical trials, which focus on different pathogenic pathways, could potentially be used together.
Conclusions
Despite the mechanistic and clinical complexity of C9ORF72 ALS/FTD, intense research efforts over the 10 years since the expansion mutation was identified have led to a remarkable number of novel therapeutic approaches in pre-clinical and clinical trial stages. The breadth and diversity of these approaches provide hope for C9-ALS/FTD patients, who currently have limited therapeutic options focused on supportive care. The pace of research focused on the root causes of this disease has been remarkable and is likely to accelerate and uncover additional new therapeutic targets and treatment strategies that will significantly impact C9-ALS/FTD and the larger family of repeat expansion disorders.
Summary.
Hexanucleotide repeat expansions in C9ORF72 gene are the most common known genetic cause of ALS and FTD, a spectrum of debilitating and incurable neurodegenerative diseases.
Repeat associated non-AUG (RAN) translation which leads to the production of dipeptide repeat proteins is a major driver of neuronal injury and disease.
A number of therapeutic strategies aimed at reducing the impact of expansion transcripts, RAN proteins, nuclear transport deficits or stress granule biology have shown efficacy in preclinical models of disease.
Approaches aimed at modifying environmental factors and lifestyle are promising complementary avenues for improving the primary care of patients.
Intense research efforts have resulted in several clinical trials and others are expected to start soon.
Acknowledgements
LPWR gratefully acknowledges funding from the National Institutes of Health (RO1 NS098819, R37NS040389 and PO1-NS058901), Target ALS, ALS Association, Packard Center, Myotonic Dystrophy Foundation, Department of Defense (W81XWH1910654), and Muscular Dystrophy Association for support. GMH thanks the Medical Research Council (MRC) New Investigator research grant MR/R024162/1 and the Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/S005277/1. LPWR and GMH express their gratitude to ALS patients and their families for participating in research and for donating biological samples to the Center for NeuroGenetics at the University of Florida or the NIHR Sheffield Biomedical Research Centre: Translational Neuroscience for chronic neurological disorders (IS-BRC-1215-20017).
Footnotes
Competing interests
GMH is an inventor on patents granted in the USA (US10801027B2) and Europe (EP3430143B1) for the use of inhibitors of SRSF1 to treat neurodegenerative disorders (WO2017207979A1). LPWR is an inventor on patents and pending patents related to repeat associated non-AUG (RAN) translation and the use of metformin and PKR inhibition to treat RAN protein disorders. The authors declare no other relationships, conditions or circumstances that present a potential conflict of interest.
References:
- 1.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrell JR, Halliday GM, Kril JJ, Ittner LM, Gotz J, Kiernan MC, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388(10047):919–31. [DOI] [PubMed] [Google Scholar]
- 4.Abramzon YA, Fratta P, Traynor BJ, Chia R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front Neurosci. 2020;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–91. [DOI] [PubMed] [Google Scholar]
- 6.Mendez MF. Frontotemporal dementia: therapeutic interventions. Front Neurol Neurosci. 2009;24:168–78. [DOI] [PubMed] [Google Scholar]
- 7.Writing G, Edaravone ALSSG. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–12. [DOI] [PubMed] [Google Scholar]
- 8.Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38(7):758–69. [DOI] [PubMed] [Google Scholar]
- 9.Cho DH, Thienes CP, Mahoney SE, Analau E, Filippova GN, Tapscott SJ. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell. 2005;20(3):483–9. [DOI] [PubMed] [Google Scholar]
- 10.Gendron TF, Bieniek KF, Zhang YJ, Jansen-West K, Ash PE, Caulfield T, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A. 2013;110(51):E4968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339(6125):1335–8. [DOI] [PubMed] [Google Scholar]
- 15.Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108(1):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Mistry NA, Vidensky S, et al. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron. 2013;80(2):415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rourke JG, Bogdanik L, Yáñez A, Lall D, Wolf AJ, Muhammad AK, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science. 2016;351(6279):1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burberry A, Suzuki N, Wang JY, Moccia R, Mordes DA, Stewart MH, et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med. 2016;8(347):347ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balendra R, Isaacs AM. C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat Rev Neurol. 2018;14(9):544–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang J, Ravits J. Pathogenic Mechanisms and Therapy Development for C9orf72 Amyotrophic Lateral Sclerosis/Frontotemporal Dementia. Neurotherapeutics. 2019;16(4):1115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol. 2020;27(10):1918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roggenbuck J. C9orf72 and the Care of the Patient With ALS or FTD: Progress and Recommendations After 10 Years. Neurol Genet. 2021;7(1):e542. * Excellent review on C9ORF72-ALS/FTD disease etiology, clinical challenges and available care for patients regarding testing and counselling.
- 23.Ogaki K, Li Y, Atsuta N, Tomiyama H, Funayama M, Watanabe H, et al. Analysis of C9orf72 repeat expansion in 563 Japanese patients with amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33(10):2527 e11–6. [DOI] [PubMed] [Google Scholar]
- 24. Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673–94. ** Comprehensive review on the use and delivery of advanced therapeutics including RNA interference, RNase H-mediated degradation, transcription/ splicing modulation and genome editing technologies.
- 25.Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis-Downes M, Seelman A, et al. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron. 2016;90(3):535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwamoto N, Butler DCD, Svrzikapa N, Mohapatra S, Zlatev I, Sah DWY, et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat Biotechnol. 2017;35(9):845–51. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Dodart JC, Tran H, Berkovitch S, Braun M, Byrne M, et al. Variant-selective stereopure oligonucleotides protect against pathologies associated with C9orf72-repeat expansion in preclinical models. Nat Commun. 2021;12(1):847. ** Latest improved iterations of antisense oligonucleotides targeting all isoforms of pathological sense C9ORF72-repeat transcripts with increased RNase H-mediated activity. Non-viral gene therapeutic therapy approach requiring repeat injections over time.
- 28. Martier R, Liefhebber JM, Garcia-Osta A, Miniarikova J, Cuadrado-Tejedor M, Espelosin M, et al. Targeting RNA-Mediated Toxicity in C9orf72 ALS and/or FTD by RNAi-Based Gene Therapy. Mol Ther Nucleic Acids. 2019;16:26–37. ** Use of adeno-associated virus serotype 5 (AAV5) expressing a microRNA targeting C9ORF72 intron-1 upstream of the repeat expansion in patient-derived neurons and a C9ORF72-ALS BAC transgenic mouse model. Viral gene therapeutic approach which requires a single dose injection.
- 29. Mueller C, Berry JD, McKenna-Yasek DM, Gernoux G, Owegi MA, Pothier LM, et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N Engl J Med. 2020;383(2):151–8. **First In ALS-Man adminsitration of adeno-associated virus rh10 expressing a microRNA targeting human SOD1 in two SOD1-linked ALS patients.
- 30.Liu CR, Chang CR, Chern Y, Wang TH, Hsieh WC, Shen WC, et al. Spt4 is selectively required for transcription of extended trinucleotide repeats. Cell. 2012;148(4):690–701. [DOI] [PubMed] [Google Scholar]
- 31.Goodman LD, Prudencio M, Kramer NJ, Martinez-Ramirez LF, Srinivasan AR, Lan M, et al. Toxic expanded GGGGCC repeat transcription is mediated by the PAF1 complex in C9orf72-associated FTD. Nat Neurosci. 2019;22(6):863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer NJ, Carlomagno Y, Zhang YJ, Almeida S, Cook CN, Gendron TF, et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science. 2016;353(6300):708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naguib A, Sandmann T, Yi F, Watts RJ, Lewcock JW, Dowdle WE. SUPT4H1 Depletion Leads to a Global Reduction in RNA. Cell Rep. 2019;26(1):45–53 e4. * siRNA interference-mediated of the RNA polymerase II transcriptional elongation SUPT4H1 subunit in several human cell models highligting a global genome-wide alteration of RNA expression levels and therefore a limitation of the depletion of SUPT4H1 as a therapeutic strategy.
- 34. Krishnan G, Zhang Y, Gu Y, Kankel MW, Gao FB, Almeida S. CRISPR deletion of the C9ORF72 promoter in ALS/FTD patient motor neurons abolishes production of dipeptide repeat proteins and rescues neurodegeneration. Acta Neuropathol. 2020;140(1):81–4. * Therapeutic strategy based on the CRISPS-Cas9 medaited chromosomal deletion of the promoter region of pathological sense V1 and V3 isoforms of C9ORF72-repeat transcripts in patient-derived motor neurons.
- 35.Pinto BS, Saxena T, Oliveira R, Mendez-Gomez HR, Cleary JD, Denes LT, et al. Impeding Transcription of Expanded Microsatellite Repeats by Deactivated Cas9. Mol Cell. 2017;68(3):479–90 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batra R, Nelles DA, Pirie E, Blue SM, Marina RJ, Wang H, et al. Elimination of Toxic Microsatellite Repeat Expansion RNA by RNA-Targeting Cas9. Cell. 2017;170(5):899–912 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, et al. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 2016;90(3):521–34. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q, Lehmer C, Michaelsen M, Mori K, Alterauge D, Baumjohann D, et al. Antibodies inhibit transmission and aggregation of C9orf72 poly-GA dipeptide repeat proteins. EMBO Mol Med. 2017;9(5):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Q, Mareljic N, Michaelsen M, Parhizkar S, Heindl S, Nuscher B, et al. Active poly-GA vaccination prevents microglia activation and motor deficits in a C9orf72 mouse model. EMBO Mol Med. 2020;12(2):e10919. ** Administration of poly-GA in mice to investigate a vaccine approach as a novel therapeutic strategy for neuroportection in mice expressing poly-GA.
- 40. Nguyen L, Montrasio F, Pattamatta A, Tusi SK, Bardhi O, Meyer KD, et al. Antibody Therapy Targeting RAN Proteins Rescues C9 ALS/FTD Phenotypes in C9orf72 Mouse Model. Neuron. 2020;105(4):645–62 e11. ** Development of human anti polyGA antibodies as a novel therapeutic strategy which improved behavior, motor neuron survival and extended lifespan in C9ORF72-ALS BAC transgenic mice.
- 41.Green KM, Glineburg MR, Kearse MG, Flores BN, Linsalata AE, Fedak SJ, et al. RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat Commun. 2017;8(1):2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W, Wang S, Mestre AA, Fu C, Makarem A, Xian F, et al. C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2alpha phosphorylation. Nat Commun. 2018;9(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westergard T, McAvoy K, Russell K, Wen X, Pang Y, Morris B, et al. Repeat-associated non-AUG translation in C9orf72-ALS/FTD is driven by neuronal excitation and stress. EMBO Mol Med. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonobe Y, Ghadge G, Masaki K, Sendoel A, Fuchs E, Roos RP. Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol Dis. 2018;116:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zu T, Guo S, Bardhi O, Ryskamp DA, Li J, Khoramian Tusi S, et al. Metformin inhibits RAN translation through PKR pathway and mitigates disease in C9orf72 ALS/FTD mice. Proc Natl Acad Sci U S A. 2020;117(31):18591–9. ** Showed PKR activation is a key pathway for RAN translation and PKR inhibition with dominant negative AAV PKR-K296R or repurposed FDA-approved metformin inhibit RAN translation and improve behavior and neurodegeneration in C9ORF72-ALS BAC mice.
- 46.Hautbergue GM, Castelli LM, Ferraiuolo L, Sanchez-Martinez A, Cooper-Knock J, Higginbottom A, et al. SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nat Commun. 2017;8:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristofani R, Crippa V, Vezzoli G, Rusmini P, Galbiati M, Cicardi ME, et al. The small heat shock protein B8 (HSPB8) efficiently removes aggregating species of dipeptides produced in C9ORF72-related neurodegenerative diseases. Cell Stress Chaperones. 2018;23(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selvaraj BT, Livesey MR, Zhao C, Gregory JM, James OT, Cleary EM, et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca(2+)-permeable AMPA receptor-mediated excitotoxicity. Nat Commun. 2018;9(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ababneh NA, Scaber J, Flynn R, Douglas A, Barbagallo P, Candalija A, et al. Correction of amyotrophic lateral sclerosis related phenotypes in induced pluripotent stem cell-derived motor neurons carrying a hexanucleotide expansion mutation in C9orf72 by CRISPR/Cas9 genome editing using homology-directed repair. Hum Mol Genet. 2020;29(13):2200–17. * CRISPR-Cas9-mediated chromosomal deletion of C9ORF72-ALS/FTD repeat expansion rescues altered genome-wide gene expression and methylation of the C9ORF72 promoter in patient-derived motor neurons.
- 50.Khalfallah Y, Kuta R, Grasmuck C, Prat A, Durham HD, Vande Velde C. TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Scientific reports. 2018;8(1):7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Protter DSW, Parker R. Principles and Properties of Stress Granules. Trends in Cell Biology. 2016;26(9):668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154(4):727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boeynaems S, Bogaert E, Kovacs D, Konijnenberg A, Timmerman E, Volkov A, et al. Phase Separation of C9orf72 Dipeptide Repeats Perturbs Stress Granule Dynamics. Mol Cell. 2017;65(6):1044–55 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y-J, Gendron TF, Ebbert MTW, O&apo ;Raw AD, Yue M, Jansen-West K et al. Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. Nature Medicine. 2018;24(8):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew J, Cook C, Gendron TF, Jansen-West K, Del Rosso G, Daughrity LM, et al. Aberrant deposition of stress granule-resident proteins linked to C9orf72-associated TDP-43 proteinopathy. Molecular neurodegeneration. 2019;14(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang K, Daigle JG, Cunningham KM, Coyne AN, Ruan K, Grima JC, et al. Stress Granule Assembly Disrupts Nucleocytoplasmic Transport. Cell. 2018;173(4):958–71 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525(7567):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2(10):a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coyne AN, Zaepfel BL, Hayes L, Fitchman B, Salzberg Y, Luo EC, et al. G4C2 Repeat RNA Initiates a POM121-Mediated Reduction in Specific Nucleoporins in C9orf72 ALS/FTD. Neuron. 2020;107(6):1124–40 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525(7567):129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovicic A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci. 2015;18(9):1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haines JD, Herbin O, de la Hera B, Vidaurre OG, Moy GA, Sun Q, et al. Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination. Nat Neurosci. 2015;18(4):511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94(1):93–107 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hightower RM, Reid AL, Gibbs DE, Wang Y, Widrick JJ, Kunkel LM, et al. The SINE Compound KPT-350 Blocks Dystrophic Pathologies in DMD Zebrafish and Mice. Mol Ther. 2020;28(1):189–201. * KPT-350, an inhibitor of the nuclear export protein XPO1 involved in the nuclear export of proteins and non-coding RNAs including rRNA and U snRNAs, mitigates Duchenne muscular dystrophy pathologies in zebrafish and mouse models of disease.
- 65.Archbold HC, Jackson KL, Arora A, Weskamp K, Tank EMH, Li X, et al. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Scientific reports. 2018;8(1):4606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korkmaz OT, Aytan N, Carreras I, Choi J-K, Kowall NW, Jenkins BG, et al. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neuroscience Letters. 2014;566:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng C, Nennesmo I, Fadeel B, Henter J-I. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Annals of Neurology. 2004;56(4):564–7. [DOI] [PubMed] [Google Scholar]
- 68.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano M-P, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nature neuroscience. 2005;8(1):85–92. [DOI] [PubMed] [Google Scholar]
- 69.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–7. [DOI] [PubMed] [Google Scholar]
- 70.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(12):2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Duan W, Wang W, Wen D, Liu Y, Liu Y, et al. scAAV9-VEGF prolongs the survival of transgenic ALS mice by promoting activation of M2 microglia and the PI3K/Akt pathway. Brain research. 2016;1648(Pt A):1–10. [DOI] [PubMed] [Google Scholar]
- 72.Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science (New York, NY). 2003;301(5634):839–42. [DOI] [PubMed] [Google Scholar]
- 73.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82(7):865–75. [DOI] [PubMed] [Google Scholar]
- 74.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. The Journal of comparative neurology. 2009;514(4):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang DH, Lee HJ, Park IH, Seok JI, Kim BG, Joo IS, et al. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Therapy. 2009;16(10):1234–44. [DOI] [PubMed] [Google Scholar]
- 76.Knippenberg S, Rath KJ, Böselt S, Thau-Habermann N, Schwarz SC, Dengler R, et al. Intraspinal administration of human spinal cord-derived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. Journal of tissue engineering and regenerative medicine. 2017;11(3):751–64. [DOI] [PubMed] [Google Scholar]
- 77.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiology of Disease. 2008;31(3):395–405. [DOI] [PubMed] [Google Scholar]
- 78.Uccelli A, Milanese M, Principato MC, Morando S, Bonifacino T, Vergani L, et al. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Molecular medicine (Cambridge, Mass). 2012;18(5):794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boido M, Piras A, Valsecchi V, Spigolon G, Mareschi K, Ferrero I, et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2014;16(8):1059–72. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16(12):2002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21(8):1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gouel F, Rolland AS, Devedjian JC, Burnouf T, Devos D. Past and Future of Neurotrophic Growth Factors Therapies in ALS: From Single Neurotrophic Growth Factor to Stem Cells and Human Platelet Lysates. Front Neurol. 2019;10:835. * Excellent review on the use of neurotrophific factors and potential regenerative strategy approaches in neurodegeneration.
- 83.Tsitkanou S, Della Gatta P, Foletta V, Russell A. The Role of Exercise as a Non-pharmacological Therapeutic Approach for Amyotrophic Lateral Sclerosis: Beneficial or Detrimental? Front Neurol. 2019;10:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Julian TH, Glascow N, Barry ADF, Moll T, Harvey C, Klimentidis YC, et al. Physical exercise is a risk factor for amyotrophic lateral sclerosis: Convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine. 2021;68:103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robison R, Tabor-Gray LC, Wymer JP, Plowman EK. Combined respiratory training in an individual with C9orf72 amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2018;5(9):1134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Plowman EK, Tabor-Gray L, Rosado KM, Vasilopoulos T, Robison R, Chapin JL, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: Results of a randomized, sham-controlled trial. Muscle Nerve. 2019;59(1):40–6. ** Important study showing simple and well-tolerated expiratory muscle training improves respiratory strength and swallowing function in ALS patients.
- 87. Westeneng HJ, van Veenhuijzen K, van der Spek RA, Peters S, Visser AE, van Rheenen W, et al. Associations between lifestyle and amyotrophic lateral sclerosis stratified by C9orf72 genotype: a longitudinal, population-based, case-control study. Lancet Neurol. 2021;20(5):373–84. ** Identified modifiable disease-promoting lifestyle factors that may provide opportunities to lower risk of developing neurodegenerative disease.
- 88.Nieves JW, Gennings C, Factor-Litvak P, Hupf J, Singleton J, Sharf V, et al. Association Between Dietary Intake and Function in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016;73(12):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lashley T, Rohrer JD, Mahoney C, Gordon E, Beck J, Mead S, et al. A pathogenic progranulin mutation and C9orf72 repeat expansion in a family with frontotemporal dementia. Neuropathol Appl Neurobiol. 2014;40(4):502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]