Abstract
Background:
Heart failure (HF) after kidney transplantation is a significant but understudied problem. Pre-transplant dialysis modality could influence incident HF risk through differing cardiac stressors. However, whether pre-transplant dialysis modality is associated with the development of post-transplant HF is unknown.
Methods:
We used the US Renal Data System to assemble a cohort of 27,701 patients who underwent their first kidney transplant in the United States between the years 2005 and 2012 and who had Medicare fee-for-service coverage for >6 months preceding their transplant date. Patients with any HF diagnosis prior to transplant were excluded. Detailed baseline patient characteristics and comorbidities were abstracted. The outcome of interest was de novo post-transplant HF. Pre-transplant dialysis modality was defined as the dialysis modality used at the time of transplant. We conducted time-to-event analyses using Cox regression. Death was treated as a competing risk in the study’s primary analysis. Graft failure was included as a time-varying covariate.
Results:
Among eligible patients, 81% were treated with hemodialysis prior to transplant, and hemodialysis patients were more likely to be male, had a shorter dialysis vintage, and had more diabetes and vascular disease diagnoses. When adjusted for all available demographic and clinical data, pre-transplant treatment with hemodialysis (vs. peritoneal dialysis) was associated with a 19% increased risk in de novo post-transplant HF, with sub-distribution HR 1.19 (CI 1.09-1.29).
Conclusions:
Our results suggest that choice of pre-transplant dialysis modality may impact the development of post-transplant HF.
Keywords: heart failure, end-stage renal disease, hemodialysis, peritoneal dialysis, kidney transplantation, outcomes, cohort study, USRDS
Introduction
For many patients with end stage kidney disease (ESKD), kidney transplantation offers the best outcome in terms of survival and quality of life [1,2]. However, survival of patients with a kidney transplant is still reduced compared to that of the general population, an effect attributable, at least in part, to an excess risk of cardiovascular disease in the kidney transplant population [3]. Heart failure (HF) following kidney transplant is a particularly important problem. Post-transplant HF is the most frequent cardiovascular cause for hospital admission in the two years following transplant and is associated with reduced patient and graft survival [4,5].
Patients with ESKD are exposed to a myriad of both traditional and non-traditional risk factors for HF [6-12]. Chronic intermittent hemodialysis (vs. peritoneal dialysis) exposes patients to a number of unique cardiac stressors including 1) frequent and rapid intravascular volume shifts, 2) myocardial stunning, and 3) the presence of AV shunts that usually remain in situ after they receive a kidney transplant. We therefore hypothesized that those patients undergoing hemodialysis (vs. peritoneal dialysis) would be at higher risk for post-transplant heart failure. Herein, we formally examine the association between pre-transplant dialysis modality and the incidence of de novo post-transplant HF using a large, population-based U.S. ESKD registry.
Methods
Study Cohort
We used the United States Renal Data System (USRDS) dataset to identify adult patients who underwent their first kidney transplant in the United States between January 1 2005 and September 30 2012. We required that patients have at least 6 months of Medicare Parts A and B coverage prior to their kidney transplant. Prior diagnoses of HF were identified using International Classification of Diseases, 9th revision (ICD-9), codes of 428.xx, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91 and 404.93. We excluded patients with any inpatient or outpatient HF claims in up to 2 years prior to kidney transplant. We additionally excluded 1) patients for whom data on pre-transplant dialysis modality was missing, 2) patients that received a simultaneous kidney-pancreas transplant, and 3) patients for whom there were no Medicare claims visible in the 2 years prior to transplant.
Exposure
The exposure of interest was last pre-transplant dialysis modality (hemodialysis vs. peritoneal dialysis) as reported in the USRDS treatment history files.
Outcome
The outcome was de novo post-transplant HF. HF was identified using ICD-9 claims. To ascertain de novo HF we required either one post-transplant inpatient HF claim or one outpatient HF claim followed by another either inpatient or outpatient HF claim within 30 days of the first. For outpatient HF diagnosis the date of the first outpatient claim was the date used for de novo HF. Patients were censored at the end of the study (September 30 2015), loss of Medicare Parts A and B coverage or at 3 years post-transplant (as many patients lose Medicare coverage at this point).
Patient Characteristics
For each patient we abstracted demographic (age at time of transplant, sex, race [white, black or other]), dialysis (cause of ESKD [diabetes, hypertension, glomerulonephritis or other], total dialysis vintage, duration of last pre-transplant dialysis modality and body mass index) and transplant variables (blood type, calculated panel reactive antibody, donor-recipient human leukocyte antigen match, cold ischemia time, donor age, donor sex, donor type and any history of prior non-kidney solid organ transplant).
We also identified a wide-array of pre-transplant comorbidities using ICD-9 codes in claims-based algorithm which required the presence of either 1 inpatient or two outpatient claims (not on the same day). The pre-transplant comorbidities abstracted were alcohol dependence, arrhythmia, cancer, cerebrovascular disease, chronic pulmonary disease, coronary artery disease, diabetes, hypertension, liver disease, peripheral arterial disease, tobacco use, and valvular heart disease (see Supplemental Table 1).
We also abstracted information regarding pre-transplant skilled nursing facility admissions, inpatient hospital days, and non-nephrology outpatient visits in the 6 months prior to transplant. Graft failure, defined as the need for dialysis or re-transplant, was identified from the USRDS patient file and was treated as a time-varying covariate.
Statistical Analysis
Baseline characteristics were tabulated for all patients as well as separately for the pre-transplant hemodialysis and peritoneal dialysis groups. Continuous variables were presented as either means with standard deviations or medians with interquartile ranges where appropriate. Categorical variables were expressed as percentages. We presented cumulative incidence function (CIF) plots to compare 3-year cumulative incidence of heart failure and death by pre-transplant modality type.
We estimated unadjusted and incrementally adjusted sub-distribution or cause-specific hazard ratios for de novo post-transplant heart failure by pre-transplant dialysis modality (with peritoneal dialysis being the referent). All models were stratified by era of transplant; 2005-06, 2007-08, 2009-10 and 2011-12. Models 1-4 were incrementally adjusted as follows; model 1 - time-varying graft failure; model 2 – model 1 plus age at time of transplant, sex, race, body mass index, cause of ESRD, dialysis vintage, and duration of last pre-transplant dialysis modality; model 3 – model 2 plus comorbidities, health care utilization metrics (nursing home stay, number of hospital days, number of non-nephrology clinic visits) and prior solid organ transplant status and; model 4 – model 3 plus transplant characteristics. The primary analysis treated death as a competing risk and generated sub-distribution hazard ratios using the Kaplan-Meier multiple imputation (KMI) method [13,14]. A secondary analysis treated death as a censoring event. Both analyses used extended Cox models. We tested for proportional hazards by looking at the correlation of the scaled Schoenfeld residuals with time and found no evidence that the log-hazard ratio changed with follow-up time for any of the covariates that were included in the model. The p-value for the global test was 0.49. We additionally tested a number of pre-transplant patient characteristics as effect modifiers of pre-transplant dialysis modality (hemodialysis vs. peritoneal dialysis) on de novo post-transplant heart failure in the primary analysis. The pre-transplant characteristics that were tested were 1) age at time of transplant, 2) race, 3) dialysis vintage, 4) dialysis modality vintage, 5) body mass index (BMI), 6) the presence of coronary artery disease and 7) the presence of diabetes mellitus.
In our cohort of 27,701 patients, 6,842 (24.7%) had at least one variable missing. The variables most frequently missing were calculated panel reactive antibody (15%) and cold ischemia time (8%). Data were assumed to be missing at random. Missing data were handled using multiple imputation by fully conditional specification (FCS) as implemented in SAS, and 25 imputed datasets were obtained for the primary outcome. In addition to the exposure and all covariates included in the analysis model, the imputation model also included the event indicator and the Nelson–Aalen estimator of the cumulative marginal hazard. Imputation models were run separately for the main analysis (to calculate sub-distribution HR). Imputation models were stratified by treatment modality.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA), R version 3.1.2, and Stata MP, version 13.1 (StataCorp, College Station, TX).
Results
A total of 27,701 adults who underwent their first kidney transplant satisfied all inclusion criteria and were included in the study (Figure 1). Of these, 81% of patients were treated with hemodialysis prior to transplant while the remaining 19% were treated with peritoneal dialysis for a median 3.2 and 3.7 years, respectively. The baseline characteristics of the study cohort stratified by pre-transplant dialysis modality are shown in Table 1. Patients treated with hemodialysis prior to transplant were more likely to be male, African American, had a shorter dialysis vintage, and had a greater burden of diabetes and vascular disease.
Figure 1.
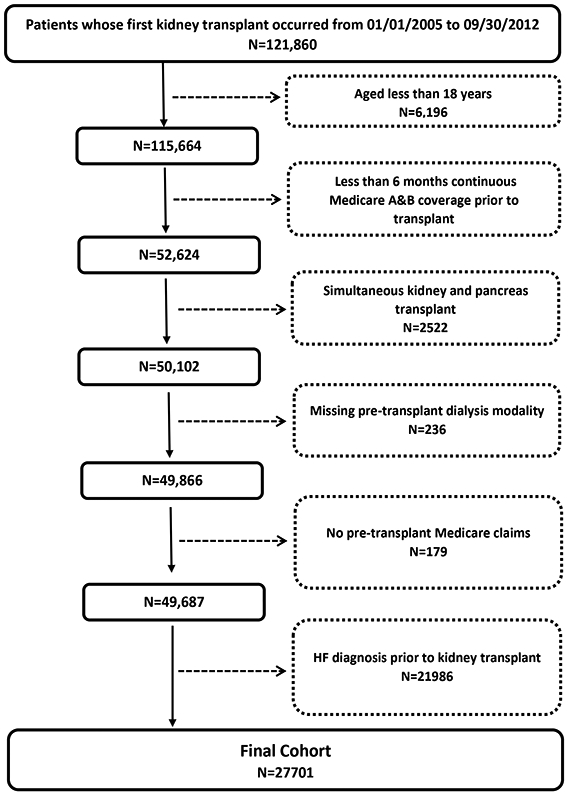
Cohort Flow Chart
Table 1.
Baseline characteristics of US patients who underwent their first kidney transplant between 2005 and 2012, altogether and stratified by pre-transplant dialysis modality.
| Baseline characteristics | All | HD | PD |
|---|---|---|---|
| N=27,701 | N=22,375 | N=5326 | |
| Female (%) | 10,878 (39.3%) | 8268 (37.0%) | 2610 (49.0%) |
| Age (years) | |||
| Mean (SD) | 47 (14) | 47 (14) | 46 (15) |
| Median (IQR) | 48 (36, 58) | 48 (37, 58) | 47 (35, 58) |
| Race | |||
| White | 16,313 (58.9%) | 12,760 (57.0%) | 3553 (66.7%) |
| Black | 8909 (32.2%) | 7692 (34.4%) | 1217 (22.9%) |
| Other | 2440 (8.8%) | 1891 (8.5%) | 549 (10.3%) |
| Cause of ESKD | |||
| Diabetes | 6991 (25.2%) | 6042 (27.0%) | 949 (17.8%) |
| Hypertension | 7187 (25.9%) | 5439 (24.3%) | 1748 (32.8%) |
| Glomerulonephritis | 6905 (24.9%) | 5704 (25.5%) | 1201 (22.5%) |
| Other | 6519 (23.5%) | 5111 (22.8%) | 1408 (26.4%) |
| Year of ESKD | |||
| 2005-2006 | 7199 (26.0%) | 5801 (25.9%) | 1398 (26.2%) |
| 2007-2008 | 7068 (25.5%) | 5809 (26.0%) | 1259 (23.6%) |
| 2009-2010 | 7130 (25.7%) | 5754 (25.7%) | 1376 (25.8%) |
| 2011-2012 | 6304 (22.8%) | 5011 (22.4%) | 1293 (24.3%) |
| BMI at transplant (kg/m2) | |||
| Mean (SD) | 27.9 (5.2) | 27.6 (5.0) | 28.0 (5.2) |
| Median (IQR) | 27.6 (24.0, 31.6) | 27.6 (24.0, 31.7) | 27.3 (23.8, 31.1) |
| <18.5 | 530 (1.9%) | 399 (1.8%) | 131 (2.5%) |
| 18.5-24.9 | 8164 (29.5%) | 6560 (29.3%) | 1604 (30.1%) |
| 25-29.9 | 9370 (33.8%) | 7498 (33.5%) | 1872 (35.1%) |
| ≥30 | 9172 (33.1%) | 7517 (33.6%) | 1655 (31.1%) |
| Dialysis Vintage (time since initiation of dialysis, yr) | |||
| Mean (SD) | 4.0 (2.7) | 3.5 (2.3) | 4.1 (2.7) |
| Median (IQR) | 3.6 (2.1, 5.2) | 3.2 (1.9, 4.6) | 3.7 (2.2, 5.3) |
| < 2.5 | 8416 (30.4%) | 6546 (29.3%) | 1870 (35.1%) |
| 2.5-5 | 11755 (42.4%) | 9384 (41.9%) | 2371 (44.5%) |
| 5-9 | 6308 (22.8%) | 5347 (23.9%) | 961 (18.0%) |
| ≥ 9 | 1222 (4.4%) | 1098 (4.9%) | 124 (2.3%) |
| Duration of last dialysis modality (yr) | |||
| Mean (SD) | 3.5 (2.5) | 2.7 (1.9) | 3.7 (2.5) |
| Median (IQR) | 3.2 (1.7, 4.7) | 2.4 (1.2, 3.7) | 3.4 (1.8, 4.9) |
| < 2.5 | 10729 (38.7%) | 7967 (35.6%) | 2762 (51.9%) |
| 2.5-5 | 10929 (39.5%) | 8985 (40.2%) | 1944 (36.5%) |
| 5-9 | 5196 (18.8%) | 4619 (20.6%) | 577 (10.8%) |
| ≥ 9 | 847 (3.1%) | 804 (3.6%) | 43 (0.8%) |
| Comorbidities | |||
| Diabetes mellitus | 11229 (40.5%) | 9624 (43.0%) | 1605 (30.1%) |
| Alcohol Dependence | 451 (1.6%) | 400 (1.8%) | 51 (1.0%) |
| CAD | 6835 (24.7%) | 5779 (25.8%) | 1056 (19.8%) |
| COPD | 3940 (14.2%) | 3226 (14.4%) | 714 (13.4%) |
| CVD | 1903 (6.9%) | 1623 (7.3%) | 280 (5.3%) |
| Cerebral bleed | 256 (0.9%) | 222 (1.0%) | 34 (0.6%) |
| Cancer | 1839 (6.6%) | 1543 (6.9%) | 296 (5.6%) |
| Hypertension | 25536 (92.2%) | 20797 (92.9%) | 4739 (89.0%) |
| VHD | 3388 (12.2%) | 2890 (12.9%) | 498 (9.4%) |
| PVD | 5233 (18.9%) | 4731 (21.1%) | 502 (9.4%) |
| Liver disease | 4496 (16.2%) | 3834 (17.1%) | 662 (12.4%) |
| Tobacco use | 2397 (8.7%) | 2010 (9.0%) | 387 (7.3%) |
| Arrhythmia | 1463 (5.3%) | 1252 (5.6%) | 211 (4.0%) |
| Previous solid organ transplant | 527 (1.9%) | 472 (2.1%) | 55 (1.0%) |
| Patient Blood Type | |||
| O | 13249 (47.8%) | 10706 (47.8%) | 2543 (47.7%) |
| A | 9158 (33.1%) | 7360 (32.9%) | 1798 (33.8%) |
| B | 3965 (14.3%) | 3225 (14.4%) | 740 (13.9%) |
| AB | 1116 (4.0%) | 900 (4.0%) | 216 (4.1%) |
| Donor Type | |||
| Living | 5881 (21.2%) | 4644 (20.8%) | 1237 (23.2%) |
| Deceased | 17779 (64.2%) | 14357 (64.2%) | 3422 (64.3%) |
| Expanded Criteria | 3868 (14.0%) | 3225 (14.4%) | 643 (12.1%) |
| Donor Characteristics | |||
| Age (years) | |||
| Mean (SD) | 39 (16) | 39 (16) | 38 (16) |
| Median (IQR) | 41 (27, 51) | 41.0 (27, 51) | 40.0 (26, 51) |
| Female Donor | 12290 (44.4%) | 9882 (44.2%) | 2408 (45.2%) |
| HLA mismatch | |||
| 0 | 2099 (7.6%) | 1680 (7.5%) | 419 (7.9%) |
| 1-3 | 6413 (23.2%) | 5111 (22.8%) | 1302 (24.4%) |
| 4-6 | 18668 (67.4%) | 15137 (67.7%) | 3531 (66.3%) |
| Panel-reactive antibody titer, mean (SD) | 14.7 (26.8) | 14.5 (26.7) | 15.3 (27.3) |
| Median (IQR) | 0.0 (0.0, 14.0) | 0.0 (0.0, 14.0) | 0.0 (0.0, 16.0) |
| Cold ischemia time (hrs), mean (SD) | 15.0 (10.6) | 15.2 (10.6) | 14.3 (10.3) |
| Median (IQR) | 14.5 (7.5, 21.0) | 14.8 (8.0, 21.1) | 14.0 (6.1, 21.0) |
| Nursing Home Stay | 205 (0.7%) | 185 (0.8%) | 20 (0.4%) |
| Hospital Days, Mean (SD) | 3 (4) | 3 (5) | 3 (4) |
| Hospital Days, median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Non-nephrology clinic visits, Mean (SD) | 13 (12) | 13 (12) | 12 (12) |
| Non-nephrology clinic visits, median (IQR) | 10 (5, 18) | 10 (5, 18) | 9 (5, 17) |
IQR, interquartile range; SD, standard deviation; ESKD, end-stage kidney disease; CVD, cerebrovascular disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease; VHD, valvular heart disease; HLA, human leukocyte antigen.
Several variables had incomplete data, specifically the proportion of observations missing the following variables were: sex (<0.1%); race (0.1); cause of ESKD (0.4); body mass index (1.7%); blood type (0.8%); donor type (0.6%); donor age (0.8%); donor sex (0.8%); HLA mismatch (1.9%); calculated panel-reactive antibody titer (15.5%); cold ischemia time (8.5%).
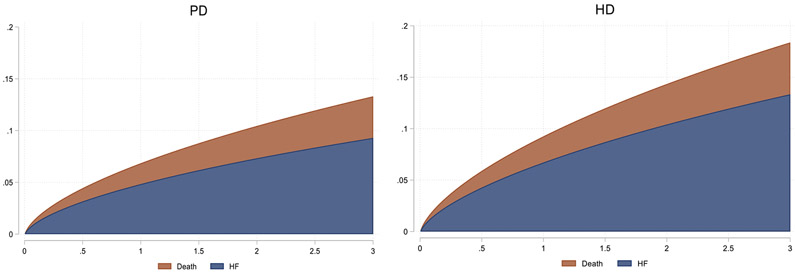
Over a mean follow-up of 2.33 years 3,283 patients, (11.9%) were diagnosed with de novo HF, with a median time from transplant surgery to a de novo HF diagnosis of 9.7months (interquartile range, 2.13-22.03); among those previously treated with hemodialysis, 2809 (12.6%) developed de novo HF compared with 8.8% of those previously treated with PD.
Pre-transplant hemodialysis (vs. peritoneal dialysis) treatment was associated with an increased risk of post-transplant HF (Figure 2, Table 2). The unadjusted and model 4-adjusted sub-distribution HRs for de novo HF for hemodialysis vs. peritoneal dialysis were 1.36 (CI 1.27, 1.46) and 1.19 (CI 1.09 to 1.28), respectively. Results were similar when death was treated as a censoring event. Complete results for both models that use death as a competing risk and death-censoring models are shown in Table 2. There was a significant interaction identified for BMI (p-value for interaction = 0.03) and diabetes mellitus (p-value for interaction = 0.008), where higher BMI and the presence of pre-transplant diabetes mellitus were synergistically associated with an increased risk of de novo post-transplant HF in those patients treated pre-transplant with hemodialysis (vs. peritoneal dialysis). More specifically, in patients with a pre-transplant BMI of ≥30 kg/m2, hemodialysis (vs. peritoneal dialysis) treatment was associated with a 36% (95% CI, 13%-64%) increase in the sub-distribution hazard of de novo HF in those who were event free or died. However, there was no difference in the sub-distribution hazard between patients treated with hemodialysis vs. peritoneal dialysis in other strata of pre-transplant BMI below 30 kg/m2. We also found that among patients with pre-transplant diabetes mellitus, hemodialysis (vs. peritoneal dialysis) treatment was associated with a 38% (95% CI, 16%-65%) increase in the sub-distribution hazard of de novo HF in those who were event free or died. However, there was no significant difference observed in the sub-distribution hazard of de novo HF in patients without diabetes mellitus treated with hemodialysis vs. peritoneal dialysis. Full results for the interaction analysis are shown in Figure 3.
Figure 2.
3-year cumulative incidence of post-transplant heart failure and death in patients treated pre-transplant with peritoneal dialysis (PD) and hemodialysis (HD)
Table 2.
Risk of de novo post-transplant heart failure in patients treated pre-transplant with hemodialysis versus peritoneal dialysis
| Models | Sub-distribution HR (95% CI)* | Cause-specific HR (95% CI)* |
|---|---|---|
| Model 1 | 1.36 (1.27-1.46) | 1.37 (1.28-1.46) |
| Model 2 | 1.24 (1.14-1.34) | 1.25 (1.15-1.34) |
| Model 3 | 1.18 (1.09-1.28) | 1.19 (1.09-1.29) |
| Model 4 | 1.19 (1.09-1.28) | 1.20 (1.10-1.29) |
CI – confidence interval; HR – hazard ratio
Model 1 - calendar year and graft failure
Model 2 – model 1 + age at time of transplant, sex, race, BMI, cause of ESRD and modality duration and dialysis vintage
Model 3 – model 2 + comorbidities, health care utilization metrics and prior solid organ transplant status
Model 4 – model 3 + transplant characteristics
Graft failure was treated as a time-varying covariate. All models stratified by incidence year categories (2005-2006, 2007-2008, 2009-2010, and 2011-2012)
Sub-distribution hazard ratio treats death as a competing event; cause-specific hazard ratio treats death as a censoring event.
Figure 3.
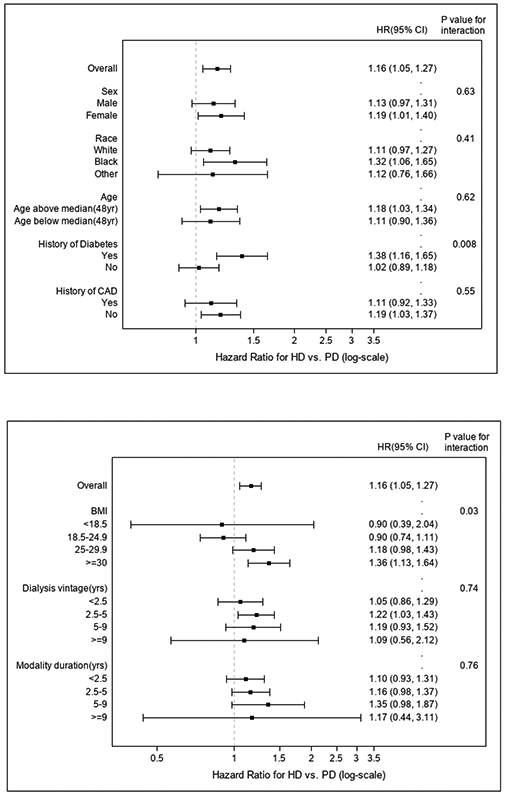
Test for interactions between selected patient characteristics, pre-transplant dialysis modality and the risk of de novo post-transplant heart failure
Discussion
In this study of US patients with ESKD on dialysis who received a first kidney-only transplant we found that pre-transplant treatment with hemodialysis (vs. peritoneal dialysis) was associated with de novo post-transplant HF. After adjustment for numerous potential confounders those patients treated with hemodialysis before undergoing transplant had an almost 20% higher risk of being diagnosed with HF in the 3 years after transplant compared to those patients who were treated with peritoneal dialysis. The increased risk of post-transplant HF associated with receipt of pre-transplant hemodialysis therapy was greater in those patients with a high body mass index and in those with pre-existing diabetes mellitus. Chronic cardiac volume and pressure overload may complicate both peritoneal dialysis and hemodialysis therapies and can result in the development of left ventricular hypertrophy, which is a precursor of both diastolic and systolic cardiac dysfunction. Both modalities also have distinct (modality-specific) features that are relevant to the development of structural cardiac disease and warrant discussion.
Hemodialysis is associated with large, intermittent, and ‘unphysiological’ volume shifts. Large interdialytic weight gains have been shown to be associated with the development of left ventricular hypertrophy while more frequent hemodialysis (compared to standard frequency) appears to protect against the development of left ventricular hypertrophy [15-17]. Hemodialysis-induced myocardial stunning/ischemia is a now well-described phenomenon which also likely results in incremental myocardial injury [18]. The use of arteriovenous shunts for hemodialysis access result in an obligate increase in cardiac output and the development of left ventricular hypertrophy. In patients with high-flow arteriovenous fistulas, high output cardiac failure may sometimes ensue. Some (although not all) observational studies show that arteriovenous fistula ligation is associated with favorable left ventricular structural and functional changes [12,19-21]; a randomized trial in 64 patients found that arteriovenous fistula ligation caused significant reductions in left ventricular mass and size [22].
Peritoneal dialysis which offers smooth and continuous volume removal should better mimic normal physiology. However, volume control with peritoneal dialysis may be imperfect [23,24]. Peritoneal dialysis may also predispose to an unfavorable metabolic and inflammatory milieu which may contribute adversely to cardiac pathophysiology [25]. Sparse data comparing left ventricular hypertrophy prevalence in peritoneal dialysis versus hemodialysis patients yield conflicting results [26,27]. In patients with existing heart failure and ESKD, peritoneal dialysis is often favored over hemodialysis as being potentially better hemodynamically tolerated; however, this is practice is not supported by the results of a large (albeit retrospective and potentially confounded) study [28].
Our study has several strengths. Our cohort is large and from a relatively recent era. By using the USRDS and linked Medicare fee-for-service claims data, we are able to ascertain richly detailed patient demographic and clinical characteristics. However, as with all retrospective claims-based studies residual confounding through either incorrectly or uncollected covariates is a possibility. We do not have access to cardiac investigations, dialysis prescriptions, medication history, or laboratory results including post-transplant glomerular filtration rate. We also do not have information regarding the type of pre-transplant hemodialysis access.
Post-transplant heart failure is an important problem and is associated with reduced graft and patient survival [5]. Minimizing cardiac stress and injury should be an important focus of pre-transplant management in patients with chronic kidney disease. Ideally, all patients with ESKD should undergo pre-emptive kidney transplant, which is associated with the best post-transplant outcomes [29,30]. However, long deceased donor waiting times and insufficient numbers of living donors mean that pre-emptive kidney transplant is not an option for the majority of Americans with ESKD. Therefore, identifying modifiable dialysis-related risk factors for the development of post-transplant heart disease that may help to extend the lives of kidney transplant recipients is imperative. Our findings suggest that further study regarding the impact of pre-transplant dialysis modality on post-transplant cardiac function is warranted.
Supplementary Material
Acknowledgements
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government. None of the authors report a significant financial interest regarding the topic of this study.
Funding Sources
This work was supported a Mentored Clinical and Population Research Program Grant from the American Heart Association Western States Affiliate (to Dr. Lenihan) and grant R01DK095024 from the National Institute of Diabetes, Digestive, and Kidney Diseases
Footnotes
Statement of Ethics
This is a retrospective cohort study and the Internal Review Boards at the Stanford University School of Medicine and Baylor College of Medicine approved the study.
Conflict of Interest Statement
The authors declare no conflicts of interest.
The results presented in this paper have not been published previously in whole or part, except in abstract format
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996;50:235–42. [DOI] [PubMed] [Google Scholar]
- 3.van Walraven C, Manuel DG, Knoll G. Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 2014;63:491–9. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 2014;63:A7. [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Schnitzler MA, Abbott KC, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis 2005;46:720–33. [DOI] [PubMed] [Google Scholar]
- 6.Jablonski KL, Chonchol M. Vascular calcification in end-stage renal disease. Hemodial Int 2013;17Suppl 1:S17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999;34:125–34. [DOI] [PubMed] [Google Scholar]
- 8.Gupta J, Dominic EA, Fink JC, et al. Association between Inflammation and Cardiac Geometry in Chronic Kidney Disease: Findings from the CRIC Study. PLoS One 2015;10:e0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel RK, Oliver S, Mark PB, et al. Determinants of left ventricular mass and hypertrophy in hemodialysis patients assessed by cardiac magnetic resonance imaging. Clin J Am Soc Nephrol 2009;4:1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 1995;5:2024–31. [DOI] [PubMed] [Google Scholar]
- 12.Movilli E, Viola BF, Brunori G, et al. Long-term effects of arteriovenous fistula closure on echocardiographic functional and structural findings in hemodialysis patients: a prospective study. Am J Kidney Dis 2010;55:682–9. [DOI] [PubMed] [Google Scholar]
- 13.Ruan PK, Gray RJ. Analyses of cumulative incidence functions via non-parametric multiple imputation. Stat Med 2008;27:5709–24. [DOI] [PubMed] [Google Scholar]
- 14.Allignol, Kaplan-Meier A. Multiple Imputation for the Analysis of Cumulative Incidence Functions in the Competing Risks Setting. R package version 0.5.3, 2017. [Google Scholar]
- 15.Paglialonga F, Consolo S, Galli MA, Testa S, Edefonti A. Interdialytic weight gain in oligoanuric children and adolescents on chronic hemodialysis. Pediatr Nephrol 2015;30:999–1005. [DOI] [PubMed] [Google Scholar]
- 16.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol 2005;16:2778–88. [DOI] [PubMed] [Google Scholar]
- 17.Chan CT, Greene T, Chertow GM, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging 2012;5:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009;4:914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger P, Velez-Roa S, Wissing KM, Hoang AD, van de Borne P. Regression of left ventricular hypertrophy after arteriovenous fistula closure in renal transplant recipients: a long-term follow-up. Am J Transplant 2004;4:2038–44. [DOI] [PubMed] [Google Scholar]
- 20.Unger P, Wissing KM. Arteriovenous fistula after renal transplantation: utility, futility or threat? Nephrol Dial Transplant 2006;21:254–7. [DOI] [PubMed] [Google Scholar]
- 21.De Lima JJ, Vieira ML, Molnar LJ, Medeiros CJ, Ianhez LE, Krieger EM. Cardiac effects of persistent hemodialysis arteriovenous access in recipients of renal allograft. Cardiology 1999;92:236–9. [DOI] [PubMed] [Google Scholar]
- 22.Rao NN, Stokes MB, Rajwani A, et al. Effects of Arteriovenous Fistula Ligation on Cardiac Structure and Function in Kidney Transplant Recipients. Circulation 2019;139:2809–18. [DOI] [PubMed] [Google Scholar]
- 23.Koc M, Toprak A, Tezcan H, Bihorac A, Akoglu E, Ozener IC. Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol Dial Transplant 2002;17:1661–6. [DOI] [PubMed] [Google Scholar]
- 24.Wang MC, Tseng CC, Tsai WC, Huang JJ. Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit Dial Int 2001;21:36–42. [PubMed] [Google Scholar]
- 25.Wu CK, Huang YT, Lin HH, et al. Dissecting the mechanisms of left ventricular diastolic dysfunction and inflammation in peritoneal dialysis patients. PLoS One 2013;8:e62722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai S, Molfino A, Russo GE, et al. Cardiac, Inflammatory and Metabolic Parameters: Hemodialysis versus Peritoneal Dialysis. Cardiorenal Med 2015;5:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian JP, Wang T, Wang H, et al. The prevalence of left ventricular hypertrophy in Chinese hemodialysis patients is higher than that in peritoneal dialysis patients. Ren Fail 2008;30:391–400. [DOI] [PubMed] [Google Scholar]
- 28.Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 2003;64:1071–9. [DOI] [PubMed] [Google Scholar]
- 29.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001;344:726–31. [DOI] [PubMed] [Google Scholar]
- 30.Davis CL. Preemptive transplantation and the transplant first initiative. Curr Opin Nephrol Hypertens 2010;19:592–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.