Abstract
Coordinated spatiotemporal expression of large sets of genes is required for the development and homeostasis of organisms. To achieve this goal, organisms use myriad strategies where they form operons, utilize bidirectional promoters, cluster genes, share enhancers among genes by DNA looping, and form topologically associated domains and transcriptional condensates. Coexpression achieved by these different strategies is hypothesized to have functional importance in minimizing gene expression variability, establishing dosage balance to ensure stoichiometry of protein complexes, and minimizing accumulation of toxic intermediate metabolites. By combining gene-editing tools with computational modeling, recent studies tested the advantages of adjacent genes located in pairs and clusters. We propose that with the advancement of gene editing, single-cell sequencing and imaging tools, one could readily test the functional importance of different coexpression strategies in a variety of biological processes.
Different strategies to achieve gene coexpression
The positions of genes across the genome are not completely random, with functionally related genes frequently located in close spatial proximity. One extreme example of this phenomenon is observed in bacterial double-stranded DNA viruses, where not only are functionally related genes grouped together, but they are also organized in highly specific orders, with genome packaging, portal and capsid-related genes the mostly tightly clustered followed by tail-related genes in large groups of viruses [1]. In higher order organisms, grouping genes based on their functionality is also common, with genes organized into operons, clusters, and pairings. When genes are located in close spatial proximity to each other their expression is more easily coordinated, and such coordination could provide a fitness advantage for the survival of an organism. In this review, we start from the most extreme mechanism to achieve gene coexpression – operons, which lead to almost perfect coexpression of multicistronic genes. Then we will review multiple complex phenomena that physically bring intra- or inter-chromosomal genes in close spatial proximity to coordinate their coexpression (Figure 1A). We will next describe recent studies utilizing gene editing, single-cell sequencing and imaging tools to test the significance of gene pairs and clusters and the mechanisms controlling 3D organization of coexpressed genes.
Fig.1. Myriad strategies to achieve gene coexpression.
a ∣ An overview of gene coexpression strategies reviewed in this manuscript. As the distance between two coexpressed genes gets longer, the mechanism to achieve gene coexpression gets more complex. b ∣ The lac operon contains three genes – lacZ, lacY, lacA. These three genes share the same promoter, and they transcribe into one mRNA thus ensure perfect coexpression. c ∣ Divergently-paired genes (DPGs) are two adjacent genes that are transcribed toward the opposite direction. d ∣ Gene clusters are large number of genes within genomic neighborhoods. e ∣ The percentage of pathways displaying gene clustering is species-specific. f ∣ Topologically associated domains (TADs) boundaries are marked by the insulator binding protein CTCF/Cohesin. g ∣ Transcription factors condensate in 3D, which drive coexpression of multiple genes located on different chromosomes. h ∣ Transvection is the paring of two alleles of one gene on homologous chromosomes; the pairing is stabilized by the interaction between insulators (purple ovals). Transcription factors (cyan and blue ovals), co-activators (gray oval) and Pol II complexes (yellow ovals) assemble to drive coexpression in g and h.
Organizing genes in operons leads to almost perfect coexpression
An operon utilizes a single promoter to initiate transcription of multiple genes transcribed into a single mRNA. Thus, organizing genes in operons leads to almost perfect coexpression. The best-studied operon is the E. coli lactose metabolism biosynthesis operon [2]. The lac operon contains three genes – lacZ, lacY, lacA – coding proteins involved in lactose uptake and metabolism (Figure 1B). While prevalent in many prokaryotic organisms, in eukaryotes, operons are less common, but are found in Caenorhabditis elegans [3], trypanosomes [4], tunicate Ciona [5], flatworms [6], and chloroplasts in plants [7]. In C. elegans, approximately 20% of genes are organized into operons throughout their genome [3]. Genes within an operon are functionally related, for example genes coding for mitochondrial proteins have a strong tendency to be grouped into operons [3]. Like bacterial operons, operons in C. elegans might ensure coordinated expression of genes. Neighboring genes, even when they are not in the same operon, still benefit from adjacency. A study in C. elegans showed that two neighboring genes not belonging to an operon have much stronger coexpression compared to two genes selected at random [8]. Multiple mechanisms could explain this phenomenon, as neighboring genes may be regulated by shared regulatory elements or opening of the chromatin structure [8]. Thus, operons can mostly be replaced by chromatin-level regulation in higher eukaryotes.
Gene pairing and clustering is commonly used to achieve coexpression in fungi, plants and animals
One widespread mechanism for achieving coexpression is through the adjacent pairing genes on the same chromosome. Adjacent genes, regardless of orientation, are significantly coexpressed with the distance between the paired genes being a critical factor for their coexpression [9]. Divergently-paired genes (DPGs) were initially classified as two adjacent genes that are transcribed toward the opposite direction with their transcription start sites (TSSs) less than 1,000 base pairs apart (Figure 1C) [10]. With this stringent classification, DPGs make up about half of the yeast, 32% of fruit fly and 10% of human genome [10-14]. DPGs are often coexpressed throughout eukaryotic species, from yeasts through vertebrates [13-16]. DPGs often fall into the same functional categories, many encoding for essential or housekeeping proteins. In the human genome, DPGs transcribed from a bidirectional regulatory region are overrepresented to encode for proteins that function in DNA-repair, ribosome biogenesis, chaperones, mitochondria, and RNA-helicases processes [17]. Defining DPGs based on a fixed 1 kb distance might be suitable for small yeast genome. However, genome sizes are significantly larger in higher organisms, and as such the number of DPGs in eukaryotes are underestimated by current standard. For example, human CYP1A1 and CYP1A2 genes are separated by a 23 kb intergenic region but are still coregulated through common regulatory elements [18]. Similarly, zebrafish her1 and her7 are separated by a 12 kb intergenic spacer region but are mostly coexpressed in the paraxial mesoderm [19]. Given the close proximity, some of the DPGs could be controlled by bidirectional promoters. In other cases, shared enhancers may activate two genes simultaneously, causing simultaneous cofiring of their expressions in individual cells. For example, in Drosophila, a single enhancer can co-activate two convergently paired synthetic reporter genes whose TSSs are separated by 15 kb [20]. Alternatively, as the distance between TSSs of two genes increases, activation of one gene could trigger activation of an adjacent gene most probably owing to opening of the chromatin. For example, two synthetic reporters are more coexpressed when they are next to each other on the same chromosome than they are on separate chromosomes in both yeast and mammalian cells [21-23].
Beyond gene pairs, coexpression gene clusters contain many genes within genomic neighborhoods in eukaryotes [17,24] (Figure 1D). In yeast, genes with similar functions tend to be positioned adjacently along the chromosomes; gene clusters containing up to 30 genes (100 kb) [9,25]. A significant fraction of yeast genes coding for essential proteins, such as subunits of proteasome, ribosome, nucleosome, mitochondria, DNA and RNA polymerases, are located within 10–30 kb of each other [26,27]. In Arabidopsis, coexpression clusters contain up to 20 genes with an overall median cluster size of 100 kb. Although genes in the same pathway are coexpressed, this is not a major cause for the coexpression of neighboring genes [28]. Coexpression clusters in D. melanogaster are composed of both large clusters of functionally unrelated housekeeping genes and small clusters of functionally related genes [29]. In mouse, coexpression of neighboring genes is long-range, extending to millions of bases apart [30,31]. As for human, approximately 16% of genes are present in functional clusters [32]. Housekeeping genes show strong clustering also in the human genome [33]. Overall, between 98% and 30% of pathways exhibit significant gene clustering in descending order from Saccharomyces cerevisiae, Homo sapiens, Caenorhabditis elegans, Arabidopsis thaliana, to Drosophila melanogaster (Figure 1E) [34]. Clustering of genes, which encode subunits of protein complexes, may ensure better coregulation and keep the right stoichiometry of complexes [26,27]. Even though genes belonging to similar gene ontology exhibit clustering in budding and pathogenic yeasts, the identity of the individual pairings are not conserved [16]. Therefore, pathway clustering exhibits a species-specific pattern probably due to adaptations that are unique to each species [34].
3D organization of genomes drive coexpression of large sets of genes
As the distance between genes grows, complex DNA-chromatin interactions group genes on the same chromosome into topologically associated domains (TADs) (Figure 1F) [31]. TAD boundaries are enriched by the insulator binding proteins, such as CTCF and Cohesin in vertebrates [35]. With the advance of single-cell sequencing technology, one can quantify genome wide coexpression at single-cell resolution. A single-cell sequencing study showed that genes present on the same chromosome display high covariations in mouse embryonic stem cells: genes that are less than 5 Mb apart on the chromosome are enriched approximately 4-fold in covariations, while genes that are separated more than 50 Mb are enriched approximately 2-fold. On the other hand, genes located within the same TAD are 15-fold more likely to covary [31]. Similarly, genes on different chromosomes but proximal within three-dimensional nuclear space also show substantial covariation, suggesting transcription factories forming among multiple chromosomes. Importantly, there appear to be a hierarchy in the regulation of gene covariation in mESCs. Intrachromosomal proximity is the most influential factor at achieving coexpression. Transcription factors come a close second due to the size of the target pool. Though gene covariation is less affected by interchromosomal proximity, it is still substantial [31].
Although genes in TADs are often coexpressed, recent studies have raised the question as to whether TADs play a major role in leading gene coexpression in Drosophila. The first study combined RNA fluorescence in situ hybridization (RNA-FISH) and Oligopaint-FISH methods to simultaneously detect transcriptional status and chromatin structure [36]. The second study used mutants which affect dorsoventral patterning in Drosophila and performed single-cell RNA-seq and ChIP-seq (for H3K27ac and H3K27me3) experiments, combining them with Hi-C data [37]. Both studies showed that enhancer-promoter interactions are established before the formation of TADs in Drosophila. Furthermore, TADs did not differ much among cells committed to different lineages, suggesting that TADs are not the primary determinants of which gene sets to be expressed in each lineage, rather they constitute a baseline genome structure on which further regulatory mechanisms will be built to control coordinated gene expression and cell differentiation. The third study showed that TADs are not needed for enhancer promoter interactions within a domain but facilitates interaction of distal enhancers with target promoters located in different TADs [38]. The fourth study showed that rearranged chromosomes disrupting several TAD domains did not affect expression of majority of genes in Drosophila [39]. Besides TADs, another study identified coexpression domains (CODs), which are intrachromosomal regions containing highly coexpressed collinear genes in human breast tissue [40]. They showed that although COD genes often coexpress with other genes localized in different CODs, CODs do not overlap with TAD boundaries. Although there was a significant enrichment of housekeeping genes in CODs, most COD genes (78.3%) are not housekeeping genes [40]. In human breast tissue, 37.4% of genes were found to be split into 500 CODs. In addition, the breast cancer COD gene composition is different from that of normal tissue, with less coregulation between CODs [40]. In conclusion, it is not clear whether formation of TADs is a prerequisite for gene coexpression and whether TADs define all coexpression domains in genomes.
Another means by which cells control the expression of large sets of genes is through the maintenance of special sub-nuclear compartments. One of the well-known sub-nuclear compartments is the nucleolus, where the rDNA is transcribed and rRNAs are incorporated into pre-ribosomal particles [41]. Similarly, increasing local concentrations of transcriptional regulators in phase-separated biomolecular condensates could also drive coexpression of multiple genes. Formation of these condensates is dependent on intrachromosomal or interchromosomal interactions, which could be detected by chromosome conformation capture technologies (3C and derivations including Hi-C) [35,42-46]. Common upstream transcriptional regulators or histone modifiers could be enriched in these compartments and simultaneously regulate transcription of many target genes by local 3D diffusion (Figure 1G). A unique case is transvection, which refers to the interaction of two alleles of one gene on homologous chromosomes. Proximity to insulator elements stabilizes the efficiency of homolog pairing and thereby transvection in Drosophila (Figure 1H) [47]. During mouse ESC differentiation, transvection-like interaction causes allelic pairing and increases repressive H3K9me2 marks at Oct4 enhancers [48].
How to achieve correlated gene expression when genomes get bigger? One study found average distance of correlated expression scaled with genome size. For example, the average distance of correlation decay is 1 kb in S. cerevisiae, this distance grows to ~10 kb in C. elegans and D. melanogaster, and further grows to ~350 kb in M. musculus and H. sapiens. These expression correlations are beyond any gene ontology class [49]. Similarly, enhancer promoter interactions often scale consistent with the distance of correlation decay in different species [49]. Thus, proximities of genes enable similar chromatin states, making them accessible to similar enhancer action, which causes activation of multiple neighboring genes by activation of an enhancer in mammalian cells [50]. Thus, the transcription level of a given gene could be controlled by the combined contributions from multiple shared enhancers [49].
Proposed advantages of gene pairing and clustering
One of the benefits of coexpression of genes is to achieve dosage balance among proteins that form a complex. Accordingly, any imbalance in the concentration of the subunits of a complex can be harmful: both over- and under-expression of complex subunits lower fitness in yeast (Saccharomyces cerevisiae) [51]. This is because making these complexes, such as the ribosome, consume significant energetic reserves. Especially when dealing with the production of large macromolecular complexes, dosage balance on stoichiometric levels of proteins is necessary.
A second benefit of coexpression of genes is to minimize gene expression variability (i.e., noise) [52]. Development and homeostasis depend on precisely controlled gene expression, which is intrinsically variable [53]. Random fluctuations in abundance of essential proteins can be deleterious. Essential gene clusters are associated with low nucleosome occupancy likely to minimize their expression noise by eliminating transcriptional bursting due to chromatin remodeling [52]. In Drosophila, two convergently paired synthetic transgenes are coexpressed due to a common enhancer [20]. In zebrafish, two divergently paired segmentation clock genes, her1 and her7, are also coexpressed [19]. Thus, by sharing common cis-regulatory sites, gene pairs may suppress the uncorrelated expression variability between the two genes. However, two genes located even on different chromosomes can be regulated by sharing the same transcription factors. Therefore, the selective advantage of organizing coregulated genes in pairs or clusters in the genome has to be tested [23].
Potential disadvantages of gene pairing and clustering
While gene pairs and clusters achieve coexpression by sharing chromosomal neighborhood, they also bear increased mutational burdens in maintaining their normal functions. In human diseases, when mutations occur in intergenic regulatory regions, sometimes multiple genes might be affected rather than only one. For instance, simultaneous disruption of functionally related genes is significantly observed in de novo copy number variants (CNVs) in patients with developmental disorders [32]. Enhancers shared by neighboring genes could be utilized differentially in cancer versus normal tissues due to disruptions in insulated genomic neighborhoods in cancer [54,55]. Another disadvantage of gene pairs and clusters is that the regulatory signal of one gene can stochastically leak to the other [23]. If these two genes function in different biological processes, this undesired coordination could cost energy resources for cells and potentially reduce fitness.
There are mechanisms to counteract transcriptional leakage such as insulator elements [56,57]. Furthermore, undesired transcriptional correlations could be further buffered by post-transcriptional and translational control mechanisms [31,58]. Finally, multi-cellular organisms could utilize cell competition to eliminate the unfit cells displaying transcriptional leakage. Altogether, these strategies could minimize the damages of transcriptional leakage generated due to proximity of genes in the genome.
Gene editing technology enables testing the functional significance of gene pairing and clustering
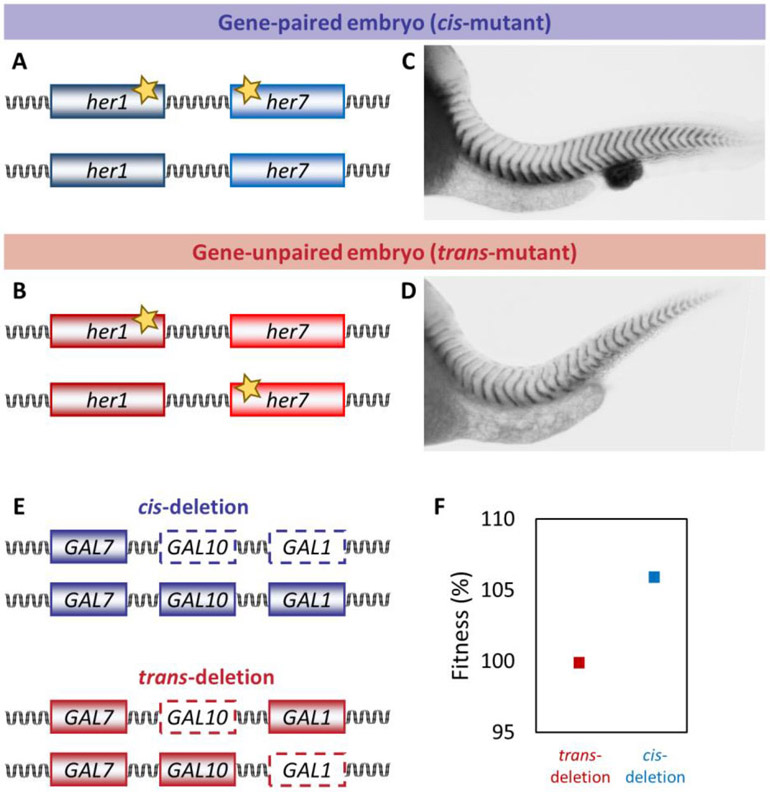
Advancement of gene editing technology provides unprecedented opportunity to investigate the advantages of gene pairing or clustering. A recent study in zebrafish tested the advantages of gene pairing in the context of developmental pattern formation [59]. The anterior–posterior axis of vertebrate embryos is sequentially divided into a fixed number of somites, which are the precursors of the vertebral column. Approximately every 30 min in zebrafish, around 200 cells segment to form a new somite. The tempo of segmentation is set by the period of a genetic oscillator (i.e., the segmentation clock). Disruptions of oscillations lead to vertebral segmentation defects (i.e., congenital scoliosis in humans). The zebrafish segmentation clock relies on a transcriptional negative-feedback loop in which Her protein dimers repress transcription of her1 and her7 – a divergent gene pair separated by a 12 kb regulatory intervening region [60-62]. Single-molecule fluorescence in situ hybridization experiments showed that the probability of transcriptional co-firing of paired her1 and her7 genes on the same chromosome is significantly higher than the two unpaired her1 genes on homolog chromosomes [59]. This finding demonstrated that gene pairing augments correlated transcription of the two clock genes by triggering transcriptional co-firing [19,59]. This study tested the functional benefit of gene pairing by comparing cis and trans double heterozygous mutants generated by CRISPR/Cas9. The cis heterozygous mutants carried two mutant genes in one chromosome and two wild-type genes on the homologous chromosome (Figure 2A). In contrast, the trans mutants carried a mutant her1 gene adjacent to a wild-type her7 gene on one chromosome, and a wild-type her1 gene adjacent to a mutant her7 gene on the other chromosome (Figure 2B). Hence, cis and trans heterozygous mutant fish had the same functional gene dose. Under low temperature rearing conditions, the amplitude of oscillations decreased in gene-unpaired embryos compared to gene-paired embryos. Therefore, gene-unpaired embryos had less success in somite segmentation as compared to gene-paired embryos (Figure 2C,D). These results revealed that keeping paired genes in the genome is beneficial for robust pattern formation during embryonic development [59].
Fig 2. Testing the functional significance of gene pairing and clustering.
a, b ∣ Genotype of her1 and her7 gene-paired and gene-unpaired embryos. c, d ∣ Segmentation phenotypes of gene-paired and gene-unpaired zebrafish embryos. Gene-paired embryos have less segmentation defect compare to gene-unpaired embryos. e ∣ Genotype of yeast cis and trans deletion for GAL gene cluster. f ∣ In a medium containing galactose, the fitness of the cis-deletion strain was approximately 6% higher than that of the trans-deletion strain.
Another benefit of coexpression of genes is to minimize accumulation of toxic intermediate compounds in metabolic pathways. Some human metabolic disorders are triggered when an intermediate compound is not used as a substrate by a downstream enzyme in the metabolic pathway. A study showed that gene pairs are enriched in metabolic pathways likely to minimize toxic intermediate compounds in 100 fungal genomes [63]. This correlation-based hypothesis has recently been experimentally tested using the S. cerevisiae GAL cluster comprising three chromosomally adjacent GAL1, GAL7, and GAL10 genes [64]. Using CRISPR-Cas9, cis- and trans-deletion diploid yeast cells with only one intact GAL1 allele and one intact GAL10 allele were generated. Two genes were located on the same chromosome in cis-deletion yeast but on the two homologous chromosomes in trans-deletion cells (Figure 2E). Concentration of Galactose-1-P, a toxic intermediate, was significantly lower in the cis- than in the trans-deletion strain. In a medium containing galactose, the fitness of the cis-deletion strain was approximately 6% higher than that of the trans-deletion strain. This fitness benefit was not observed when glucose was the carbon source (Figure 2F) [64]. Thus, chromosomal clustering of genes encoding enzymes in the same metabolic pathway promotes their coexpression, and thereby decreases accumulation of toxic intermediates and promotes growth [64,65].
Concluding Remarks
Metazoans utilize diverse strategies to achieve coordinated expression of large sets of genes in a spatiotemporally coordinated manner. Combination of modern tools, such as CRISPR/Cas9 and other gene editing mechanisms, with computational modeling will elucidate the functional benefits and selective advantages of different gene pairs and clusters in different organisms [59,64] (see Outstanding Questions). Furthermore, single-cell imaging and high-throughput methods should elucidate the hierarchy among different layers of 3D chromatin structure and in the long run, possibly, lead to detailed understanding of gene regulatory networks and their roles in embryonic development and homeostasis.
OUTSTANDING QUESTIONS BOX.
Are there other mechanisms, besides operons, gene clusters, TADs, and transcriptional condensates, organisms utilize to achieve gene coexpression?
Will it be beneficial to engineer a synthetic circuit by grouping genes functioning in the same biological process?
Are there other advantages for maintaining gene pairs and clusters in genomes?
Are there any benefits of pairing coding and non-coding genes together?
What kind of technological developments are required to systematically test the functionality of gene pairs and clusters?
Recent studies questioned the role of TADs in gene coexpression in Drosophila. Are TADs needed for gene coexpression at all? Does the conclusion from Drosophila hold true in other species?
Will the hierarchy among coexpression strategies revealed by single-cell sequencing in mouse embryonic stem cells hold true in other cell types and species?
Do different tissues or cell types have preferences for different mechanisms to achieve coordinated gene expression?
Key figure.
Myriad strategies to achieve gene coexpression
HIGHLIGHTS.
During development and homeostasis, organisms use diverse strategies such as operons, gene clusters, topological associated domains, and transcriptional condensates to coordinate spatiotemporal expression of large sets of genes.
With recent development in gene-editing tools and computational modeling, recent studies have revealed the advantages of adjacently locating genes in pairs and clusters.
Single-cell imaging and high-throughput methods will elucidate the hierarchy among different strategies to achieve coordinated gene expression.
Organizing genes in pairs and clusters, while providing some benefits, might also increase mutational burdens where a single mutation could influence expression of multiple neighboring genes.
Acknowledgements
We thank Laurence Hurst, James Arnone, Eileen Furlong and Makiko Iwafuchi for discussions. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM140805 to E.M.Ö. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- Bidirectional regulatory region
intergenic region of DNA that regulates transcription towards opposite directions
- Chromosome conformation capture technologies
a molecular biology method to analyze contact frequencies between genomic sites in a cell.
- Cis heterozygous mutants
mutation of two genes on one of the homolog chromosomes
- Coexpression domains (CODs)
intrachromosomal genomic region that have a high tendency to be coexpressed
- Coexpression gene clusters
large number of genes that show similar expression pattern
- Copy number variants (CNVs)
number of copies of a section of the genome varying among individuals
- Divergently-paired genes (DPGs)
two adjacent genes that are transcribed from a bidirectional regulatory region
- Gene coexpression
genes that display coordinated expression
- Genetic oscillator
a gene that has a rhythmic expression pattern
- Interchromosomal proximity
clustering of genes from different chromosomes
- Intrachromosomal proximity
clustering of genes within the same chromosome
- Multicistronic
containing multiple coding areas
- Nucleolus
a membrane-less dense structure where ribosomal RNA (rRNA) synthesis and ribosome biogenesis occurs in nucleus
- Operons
genes that under the control of a single promoter which initiates single transcription.
- Segmentation clock
a molecular oscillator controlling sequential segmentation of somites.
- Single-molecule fluorescence in situ hybridization
a molecular biology technique, which is used to detect and count individual RNA molecules in single cells.
- Topologically associated domains (TADs)
megabase-scale genomic regions that contain coregulated gene clusters within insulatory boundaries
- Toxic intermediate compounds
toxic products that are formed between two consecutive metabolic reactions during cellular metabolism
- Trans heterozygous mutant
mutation of two genes on alternative homolog chromosomes
- Transcriptional bursting
occurrence of transcription in bursts or pulses
- Transvection
interaction of two alleles of one gene on homologous chromosomes
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mahmoudabadi G and Phillips R (2018) A comprehensive and quantitative exploration of thousands of viral genomes. Elife 7. 10.7554/eLife.31955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob F and Monod J (1961) Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 3, 318–356. 10.1016/s0022-2836(61)80072-7 [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal T et al. (2002) A global analysis of Caenorhabditis elegans operons. Nature 417, 851–854. 10.1038/nature00831 [DOI] [PubMed] [Google Scholar]
- 4.Vanhamme L and Pays E (1995) Control of gene expression in trypanosomes. Microbiol Rev 59, 223–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satou Y et al. (2006) Genomic overview of mRNA 5'-leader trans-splicing in the ascidian Ciona intestinalis. Nucleic Acids Res 34, 3378–3388. 10.1093/nar/gkl418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boroni M et al. (2018) Landscape of the spliced leader trans-splicing mechanism in Schistosoma mansoni. Sci Rep 8, 3877. 10.1038/s41598-018-22093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y et al. (2020) The Role of Chloroplast Gene Expression in Plant Responses to Environmental Stress. Int J Mol Sci 21. 10.3390/ijms21176082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lercher MJ et al. (2003) Coexpression of neighboring genes in Caenorhabditis elegans is mostly due to operons and duplicate genes. Genome Res 13, 238–243. 10.1101/gr.553803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen BA et al. (2000) A computational analysis of whole-genome expression data reveals chromosomal domains of gene expression. Nat Genet 26, 183–186. 10.1038/79896 [DOI] [PubMed] [Google Scholar]
- 10.Yang L and Yu J (2009) A comparative analysis of divergently-paired genes (DPGs) among Drosophila and vertebrate genomes. Bmc Evol Biol 9. Artn 55 10.1186/1471-2148-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnone JT et al. (2012) The adjacent positioning of co-regulated gene pairs is widely conserved across eukaryotes. Bmc Genomics 13. Artn 546 10.1186/1471-2164-13-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi N and Lieber MR (2002) Bidirectional gene organization: a common architectural feature of the human genome. Cell 109, 807–809. 10.1016/s0092-8674(02)00758-4 [DOI] [PubMed] [Google Scholar]
- 13.Trinklein ND et al. (2004) An abundance of bidirectional promoters in the human genome. Genome Res 14, 62–66. 10.1101/gr.1982804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kensche PR et al. (2008) Conservation of divergent transcription in fungi. Trends Genet 24, 207–211. 10.1016/j.tig.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Herr DR and Harris GL (2004) Close head-to-head juxtaposition of genes favors their coordinate regulation in Drosophila melanogaster. FEBS letters 572, 147–153. 10.1016/j.febslet.2004.07.026 [DOI] [PubMed] [Google Scholar]
- 16.Asfare S et al. (2021) Systematic Analysis of Functionally Related Gene Clusters in the Opportunistic Pathogen, Candida albicans. Microorganisms 9. 10.3390/microorganisms9020276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalak P (2008) Coexpression, coregulation, and cofunctionality of neighboring genes in eukaryotic genomes. Genomics 91, 243–248. 10.1016/j.ygeno.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Ueda R et al. (2006) A common regulatory region functions bidirectionally in transcriptional activation of the human CYP1A1 and CYP1A2 genes. Mol Pharmacol 69, 1924–1930. 10.1124/mol.105.021220 [DOI] [PubMed] [Google Scholar]
- 19.Keskin S et al. (2018) Noise in the Vertebrate Segmentation Clock Is Boosted by Time Delays but Tamed by Notch Signaling. Cell reports 23, 2175–2185 e2174. 10.1016/j.celrep.2018.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukaya T et al. (2016) Enhancer Control of Transcriptional Bursting. Cell 166, 358–368. 10.1016/j.cell.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj A et al. (2006) Stochastic mRNA synthesis in mammalian cells. Plos Biology 4, 1707–1719. ARTN e309 DOI 10.1371/journal.pbio.0040309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becskei A et al. (2005) Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nature genetics 37, 937–944. 10.1038/ng1616 [DOI] [PubMed] [Google Scholar]
- 23.Yan C et al. (2016) Regulation of cell-to-cell variability in divergent gene expression. Nature communications 7, 11099. 10.1038/ncomms11099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nutzmann HW et al. (2018) Metabolic Gene Clusters in Eukaryotes. Annu Rev Genet 52, 159–183. 10.1146/annurev-genet-120417-031237 [DOI] [PubMed] [Google Scholar]
- 25.Lercher MJ and Hurst LD (2006) Co-expressed yeast genes cluster over a long range but are not regularly spaced. J Mol Biol 359, 825–831. 10.1016/j.jmb.2006.03.051 [DOI] [PubMed] [Google Scholar]
- 26.Teichmann SA and Veitia RA (2004) Genes encoding subunits of stable complexes are clustered on the yeast chromosomes: an interpretation from a dosage balance perspective. Genetics 167, 2121–2125. 10.1534/genetics.103.024505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal C and Hurst LD (2003) Evidence for co-evolution of gene order and recombination rate. Nat Genet 33, 392–395. 10.1038/ng1111 [DOI] [PubMed] [Google Scholar]
- 28.Williams EJ and Bowles DJ (2004) Coexpression of neighboring genes in the genome of Arabidopsis thaliana. Genome Res 14, 1060–1067. 10.1101/gr.2131104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber CC and Hurst LD (2011) Support for multiple classes of local expression clusters in Drosophila melanogaster, but no evidence for gene order conservation. Genome Biol 12, R23. 10.1186/gb-2011-12-3-r23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun M and Zhang J (2019) Chromosome-wide co-fluctuation of stochastic gene expression in mammalian cells. PLoS genetics 15, e1008389. 10.1371/journal.pgen.1008389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarbier M et al. (2020) Nuclear gene proximity and protein interactions shape transcript covariations in mammalian single cells. Nature communications 11, 5445. 10.1038/s41467-020-19011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews T et al. (2015) The clustering of functionally related genes contributes to CNV-mediated disease. Genome Res 25, 802–813. 10.1101/gr.184325.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lercher MJ et al. (2002) Clustering of housekeeping genes provides a unified model of gene order in the human genome. Nat Genet 31, 180–183. 10.1038/ng887 [DOI] [PubMed] [Google Scholar]
- 34.Lee JM and Sonnhammer EL (2003) Genomic gene clustering analysis of pathways in eukaryotes. Genome Res 13, 875–882. 10.1101/gr.737703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon JR et al. (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380. 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinola SM et al. (2021) Cis-regulatory chromatin loops arise before TADs and gene activation, and are independent of cell fate during early Drosophila development. Nat Genet 53, 477–486. 10.1038/s41588-021-00816-z [DOI] [PubMed] [Google Scholar]
- 37.Ing-Simmons E et al. (2021) Independence of chromatin conformation and gene regulation during Drosophila dorsoventral patterning. Nat Genet 53, 487–499. 10.1038/s41588-021-00799-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoshi M et al. (2020) Visualizing the Role of Boundary Elements in Enhancer-Promoter Communication. Mol Cell 78, 224–235 e225. 10.1016/j.molcel.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 39.Ghavi-Helm Y et al. (2019) Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat Genet 51, 1272–1282. 10.1038/s41588-019-0462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soler-Oliva ME et al. (2017) Analysis of the relationship between coexpression domains and chromatin 3D organization. PLoS Comput Biol 13, e1005708. 10.1371/journal.pcbi.1005708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fromont-Racine M et al. (2003) Ribosome assembly in eukaryotes. Gene 313, 17–42. 10.1016/s0378-1119(03)00629-2 [DOI] [PubMed] [Google Scholar]
- 42.Sexton T and Cavalli G (2015) The role of chromosome domains in shaping the functional genome. Cell 160, 1049–1059. 10.1016/j.cell.2015.02.040 [DOI] [PubMed] [Google Scholar]
- 43.Hnisz D et al. (2017) A Phase Separation Model for Transcriptional Control. Cell 169, 13–23. 10.1016/j.cell.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibn-Salem J et al. (2017) Co-regulation of paralog genes in the three-dimensional chromatin architecture. Nucleic Acids Res 45, 81–91. 10.1093/nar/gkw813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabari BR et al. (2020) Biomolecular Condensates in the Nucleus. Trends Biochem Sci 45, 961–977. 10.1016/j.tibs.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furlong EEM and Levine M (2018) Developmental enhancers and chromosome topology. Science 361, 1341–1345. 10.1126/science.aau0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim B et al. (2018) Visualization of Transvection in Living Drosophila Embryos. Mol Cell 70, 287–296 e286. 10.1016/j.molcel.2018.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan MS et al. (2015) Transient pairing of homologous Oct4 alleles accompanies the onset of embryonic stem cell differentiation. Cell stem cell 16, 275–288. 10.1016/j.stem.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintero-Cadena P and Sternberg PW (2016) Enhancer Sharing Promotes Neighborhoods of Transcriptional Regulation Across Eukaryotes. G3 (Bethesda) 6, 4167–4174. 10.1534/g3.116.036228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebisuya M et al. (2008) Ripples from neighbouring transcription. Nat Cell Biol 10, 1106–1113. 10.1038/ncb1771 [DOI] [PubMed] [Google Scholar]
- 51.Papp B et al. (2003) Dosage sensitivity and the evolution of gene families in yeast. Nature 424, 194–197. 10.1038/nature01771 [DOI] [PubMed] [Google Scholar]
- 52.Batada NN and Hurst LD (2007) Evolution of chromosome organization driven by selection for reduced gene expression noise. Nat Genet 39, 945–949. 10.1038/ng2071 [DOI] [PubMed] [Google Scholar]
- 53.Ozbudak EM et al. (2002) Regulation of noise in the expression of a single gene. Nat Genet 31, 69–73. 10.1038/ng869 ng869 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Bradner JE et al. (2017) Transcriptional Addiction in Cancer. Cell 168, 629–643. 10.1016/j.cell.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flavahan WA et al. (2016) Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114. 10.1038/nature16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Negre N et al. (2010) A comprehensive map of insulator elements for the Drosophila genome. PLoS genetics 6, e1000814. 10.1371/journal.pgen.1000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie X et al. (2007) Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites. Proc Natl Acad Sci U S A 104, 7145–7150. 10.1073/pnas.0701811104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kustatscher G et al. (2017) Pervasive coexpression of spatially proximal genes is buffered at the protein level. Mol Syst Biol 13, 937. 10.15252/msb.20177548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zinani OQH et al. (2021) Pairing of segmentation clock genes drives robust pattern formation. Nature 589, 431–436. 10.1038/s41586-020-03055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giudicelli F et al. (2007) Setting the Tempo in Development: An Investigation of the Zebrafish Somite Clock Mechanism. PLoS Biol 5, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ay A et al. (2013) Short-lived Her Proteins Drive Robust Synchronized Oscillations in the Zebrafish Segmentation Clock. Development 140, 3244–3253 [DOI] [PubMed] [Google Scholar]
- 62.Schroter C et al. (2012) Topology and dynamics of the zebrafish segmentation clock core circuit. PLoS Biol 10, e1001364. 10.1371/journal.pbio.1001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGary KL et al. (2013) Physical linkage of metabolic genes in fungi is an adaptation against the accumulation of toxic intermediate compounds. Proc Natl Acad Sci U S A 110, 11481–11486. 10.1073/pnas.1304461110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H et al. (2019) Synchronization of stochastic expressions drives the clustering of functionally related genes. Sci Adv 5, eaax6525. 10.1126/sciadv.aax6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagee D et al. (2020) Genomic clustering within functionally related gene families in Ascomycota fungi. Comput Struct Biotechnol J 18, 3267–3277. 10.1016/j.csbj.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]