Abstract
Objective:
To evaluate the safety of Fecal Microbial Transplant (FMT) in individuals with immune-mediated dry eye (DE).
Design:
Open-label, non-randomized clinical trial.
Study Population:
Ten individuals with DE symptoms and signs meeting criteria for Sjögren’s or positive early Sjögren’s markers.
Procedures:
Two FMTs from a single healthy donor were delivered via enema, one week apart.
Main Outcome Measures:
The primary outcome was safety. In addition, gut microbiome profiles, DE metrics, and T cell profiles in blood were examined at baseline before FMT, and at 1 week, 1 month, and 3 months after FMT.
Results:
Mean age of the population was 60.4 years; 30% were male, 50% were white, and 50% were Hispanic. At baseline, all subjects had significantly different gut microbiome profiles compared to the donor including higher mean diversity indices. Subjects had a decreased abundance of genera Faecalibacterium, Prevotella, and Ruminococcus and an increased abundance of genera Alistipes, Streptococcus, and Blautia compared to the donor. Effector and regulatory T cell profiles were positively correlated with each other and with DE symptom severity (Th1: r=0.76, p=0.01; Th17: r=0.83, p=0.003; CD25: r=0.66, p=0.04; FoxP3: r=0.68, p=0.03). No adverse events were noted with FMT. After FMT, gut microbiome profiles in 8 subjects moved closer to the donor’s profile. As a group, gut microbiome profiles at all follow-up time points were more similar to the original recipients’ than the donor’s microbiome, however certain phyla, classes, and genera OTU numbers remained closer to the donor versus recipients’ baseline profiles out to 3 months. Five individuals subjectively reported improved dry eye symptoms 3 months after FMT.
Conclusions:
FMT was safely performed in individuals with immune-mediated DE, with certain bacterial profiles resembling the donor out to 3 months after FMT. Half the subjects reported improved DE symptoms. The most effective FMT administration method is yet to be determined.
Keywords: microbiome, dry eye, fecal microbial transplant, autoimmune, T cell, gut
Introduction
Dry eye (DE) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film accompanied by ocular symptoms. Tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1 Many individuals with DE have associated systemic immune abnormalities, with Sjögren’s disease (Sjögren’s) being a common comorbidity.2 Chronic CD4+ T cell infiltration, autoreactive B cells, proinflammatory cytokines, and acinar cell apoptosis in the salivary and lacrimal glands have been found in individuals with Sjögren’s and are thought to drive DE and other disease manifestations.3,4 Interestingly, many of these pathophysiologic contributors are associated with gut microbiome dysbiosis, which has been described in Sjögren’s5,6 as well as other autoimmune diseases.7–9 Currently however, it is not understood whether these abnormalities are contributors or consequences of disease.
There is biologic plausibility that gut dysbiosis may contribute to immune-mediated DE and other autoimmune diseases. It is well-established that the gut microbiome impacts systemic inflammation and immunity through the generation of proinflammatory effector T cells (e.g. Th17) and anti-inflammatory regulatory T (Treg) cells.10,11 For example, specific Clostridia species have been shown to induce Th17 cells proliferation in intestinal and extra-intestinal sites, while other Clostridia species produce short chain fatty acids (SFCA) which support Treg development. Similarly, certain Bacteroides species express molecules that suppress Th17 inflammatory responses.12 An optimal balance between pro- and anti-inflammatory T cells is important locally to protect the mucosa from pathogenic microorganisms while also limiting excessive T cell responses. Various mediators have been implicated in maintaining this balance including transforming growth factor (TGF)-β, Interleukin (IL)-6, retinoic acid, and SCFAs.
Various autoimmune diseases have been associated with gut dysbiosis. For example, decreased gut microbial diversity has been found in individuals with rheumatoid arthritis (RA)13, with a lower abundance of common commensals such as Bifidobacteria and Bacteroides and an increase in Prevotella copri.14,15 Importantly, beyond its presence, P copri has been shown to have immune relevance in RA. Specifically, a 27-kd protein of P copri stimulated effector T cell responses in 17 of 40 individuals with RA.16 Additionally, 41 of 127 individuals with RA had IgG or IgA antibody reactivity with P copri, yet this was rarely found in individuals with other types of arthritis. These data suggest that gut dysbiosis may contribute to disease manifestations in several immune-mediated diseases, including DE. As such, microbiome manipulation by way of dietary changes, probiotics, or fecal microbial transplant (FMT) has been suggested as potential therapies for DE.17
In FMT, intestinal microbiota are transferred from a healthy donor to the patient, with the goal of replacing an abnormal gut microbiome with a stable and healthy one. FMT is remarkably effective in treating Clostridium difficile infections,18 leading to eradication of infection and symptoms in 90% of individuals after one treatment according to a meta-analysis.19 FMT has also been used as a treatment for autoimmune diseases of the gastrointestinal (GI) tract.20 In a randomized control trial of 75 patients, weekly FMT from healthy anonymous donors for 6 weeks induced a significantly larger ulcerative colitis (UC) remission rate (24%) at week 7 compared to placebo (5%).21 FMT has also been applied to immune diseases outside of the GI tract, including metabolic syndrome, multiple sclerosis, and idiopathic thrombocytopenic purpura (ITP).22–24 For example, nine individuals metabolic syndrome had increased insulin sensitivity six weeks after one dose of FMT delivered via gastroduodenal tube from lean donors.22 In another open-label study, 3 individuals with multiple sclerosis and constipation underwent FMT daily for 1–2 weeks and noted an improvement in neurological symptoms, including regaining the ability to walk, decreased paresthesias, and improved energy levels.23 Finally, an individual with UC and idiopathic ITP underwent one session of FMT via enema with regression of UC symptoms and normalization of platelet counts, effects that lasted for years after transplantation.25 Most relevant to DE, FMT has also been used in graft-versus-host disease (GVHD), a condition in which DE is an important component. In one open-label study, 4 individuals underwent FMT (one or two doses, one week apart, via enema from healthy spouses or relatives) with subsequent increased abundances of beneficial bacteria Lactobacillus, Bacteroides, Bifidobacterium, and Faecalibacterium at 4 weeks post-FMT. Concomitantly, GI symptoms such as defecation consistency and frequency also improved.26 The success of FMT in the above conditions suggests its potential clinical applications in other autoimmune diseases associated with gut dysbiosis.
We previously found that individuals with immune-mediated DE, including those with Sjögren’s, had gut microbiome alterations compared to healthy controls.6 Specifically, individuals with DE had a more diverse phyla and different genera compared to controls, including a decrease in Faecalibacterium and Viellonella, and increase in Megasphaera, Parabacteroides, and Prevotella.6 Based on our preliminary work and the use of FMT in GVHD, we hypothesized that gut microbiome modulation through FMT may be a potential therapeutic approach for immune-mediated DE.
Methods
Study Population:
The study population included 10 individuals with DE symptoms (Dry Eye Questionnaire ≥627) and signs (corneal staining score ≥2 on the National Eye Institute (NEI) scale28) that (1) met the 2016 American College of Rheumatology criteria for Sjögren’s, having a total weighted-score of ≥4 from the sum of the following: (a) anti-SSA/Ro antibody positivity and focal lymphocytic sialadenitis with a focus score of ≥1 foci/4mm2, each scoring 3; (b) an abnormal ocular staining score of ≥5, a Schirmer’s test result of ≤5 mm/5 minutes, and an unstimulated salivary flow rate of ≤0.1 ml/minute, each scoring 129 or (2) had one or more elevated early Sjögren’s markers (antibodies against salivary protein 1 (SP1), parotid secretory protein (PSP), and carbonic anhydrase 6 (CA6) at a level of 20 EU/ml or greater).30
Ethical approval:
The University of Miami Institutional Review Board (IRB) approved the prospective evaluation and treatment of patients. Informed consent was obtained from all subjects and the study was adherent with the principles of the Declaration of Helsinki. The trial was registered on clinicaltrials.gov (NCT03926286) and was performed under Investigational New Drug (IND) 17994 granted by the Food and Drug Administration (FDA).
Clinical metrics:
Demographic information for each participant was collected including age, gender, race, ethnicity, past ocular and medical history and current medications. A complete ophthalmic exam was performed. Patients filled out standardized questionnaires for dry eye, pain, gastrointestinal symptoms, and mental health (patient health questionnaire, PHQ-9).31
DE symptoms:
At each visit, participants completed two standardized DE symptom questionnaires: DEQ527 (score 0–22) and the Ocular Surface Disease Index (OSDI)32 (score 0–100).
DE signs:
Participants underwent a complete ocular surface exam of both eyes in the following order:
Ocular surface inflammation via matrix metalloproteinase (MMP) 9 levels (Inflammadry, Quantel, San Diego, CA)33 graded as absent or present based on the presence of a pink line (0 = no line, 1 = pink line present).
Tear breakup time (TBUT) using fluorescein stain measured three times in each eye and averaged.
Corneal staining using fluorescein graded to the National Eye Institute (NEI) scale which assesses 5 areas of the cornea on a 0–3 scale (total scale 0–15).28
Basal tear production after anesthesia placement (measured in mm at 5 minutes) using Schirmer’s strips.
Meibum quality evaluated after expression with intermediate pressure applied to the lower eyelid (0 = clear; 1 = cloudy; 2 = granular; 3 = toothpaste; 4 = no meibum extracted).
Immune profiles:
Blood was obtained at each visit and sent for flow cytometry (Duraclone IF, Beckman Coulter). T cell profiles obtained included percentages of effector T cells (Teff) (T helper 1 cells, Th1, defined by CD3+CD4+IFNγ+ and T helper 17 cells, Th17, defined by CD3+CD4+IL17+), and T regulatory cells (Treg) (CD25, defined by CD3+CD4+CD25hi and FoxP3, defined by CD3+CD4+FoxP3+).
Intervention:
Individuals received two FMT (OpenBiome, Boston, MA) via enema one week apart. All FMT came from the same healthy donor (33-year-old white female, body mass index (BMI) 20.3) with no known ocular or medical diagnoses. Three aliquots of product (90 cc total) were thawed at room temperature for at least 45 minutes prior to delivery and poured into a hanging enema bag with lubricated tip. Enemas were delivered in a private room by a registered nurse. Individuals were placed in a left lateral decubitus position with knees bent to receive the enema over a period of ~2 minutes and asked to hold the product in for one hour after delivery. Monitoring for adverse events occurred during that time. Vital signs were checked by a nurse pre- and post-delivery.
Time points of data collection:
Individuals underwent 4 clinical examinations, one prior to FMT (baseline), and then 1 week, 1 month, and 3 months after FMT. An at-home stool sample was collected at each of these time points (4 total).
Processing of stool specimens:
Stool samples were collected and placed in a glycerol suspension, homogenized, and sent to OpenBiome (Cambridge, MA). Specimens were then frozen at −80°C until analysis. Total DNA was isolated using Power-soil/fecal DNA isolation kit (Mo-Bio, Germantown, MD) as per manufacturer’s specifications. All samples were quantified using the Qubit® Quant-iT dsDNA Broad-Range Kit (Life Technologies, Grand Island, NY) to ensure that they met minimum concentration and mass of DNA. To enrich the sample for the bacterial 16S V4 rDNA region, DNA was amplified using fusion primers designed against the surrounding conserved regions that are tailed with sequences to incorporate flow cell adapters and indexing barcodes (Illumina, San Diego, CA). Each sample was PCR amplified with two differently barcoded V4-V5 fusion primers and were advanced for pooling and sequencing. For each sample, amplified products were concentrated using a solid-phase reversible immobilization method for the purification of PCR products and quantified by electrophoresis using an (Agilent, Santa Clara, CA) 2100 Bioanalyzer. The pooled 16S V4V5-enriched, amplified, barcoded samples were loaded into the MiSeq cartridge (Illumina Inc, San Diego, CA), and then onto the instrument along with the flow cell. After cluster formation on the MiSeq Instrument (Illumina, San Diego, CA), the amplicons were sequenced for 250 cycles with custom primers designed for paired-end sequencing.
In addition to patient samples, reagent controls were supplied in triplicate as background. Samples producing amplicons at later cycles compared to majority of samples were concentrated using Agencourt AMPureXP beads (Beckman Coulter, Indianapolis, IN). All samples (donor and subjects) were sequenced together after barcode-normalization subsequent to a preliminary sequencing run.
Sequences were quality filtered and de-multiplexed using exact matches to the supplied DNA barcodes and primers, using QIIME 2.0.34 Resulting sequences were clustered at 97% to obtain phylogenetic identities using SILVA database (v123). OTU tables were rarefied to the sample containing the lowest number of sequences in each analysis.
Statistical analysis:
Descriptive statistics were first used to summarize patient demographic and clinical information.
Comparison of diversity between donor and recipients:
α-diversity matrices were calculated using QIIME2.0 for the donor at baseline and the recipients’ baseline profiles, and at 1 week, 1 month, and 3 months post-FMT. Mean differences between the groups (donor vs recipient) were compared using an independent student t test and over time within recipients using paired t tests. Both Shannon index (which takes into account both the number of species present and the relative abundance of each species) and Chao 1 (which assumes that the number of organisms identified for a taxa has a Poisson distribution and corrects for variance) were calculated and compared. Chao 1 is especially useful for data sets skewed toward low-abundance calls, as is often the case with microbes. In both cases (Shannon and Chao1), an increase in value is correlated with increased richness and evenness of the species (OTUs). β-diversity was compared via UNIFrac Principal Coordinate Analysis (pCOA) using observation ID (OTU) level. The PERMANOVA test was utilized to find significant whole microbiome differences among discrete categorical or continuous variables with randomization/Monte Carlo permutation tests (with Bonferroni correction). The fraction of permutations with greater distinction among categories (larger cross-category differences) than that observed with the non-permuted data were reported as the p-value. False discovery rate (FDR) corrected p-value (q-value) <0.05 was considered significant across groups.
Descriptive examination of microbiome composition:
Cladogram qualitative analysis was used to examine relative compositions of bacteria with respect to phyla, genera, and class levels. We then created a heat map tree of the entire hierarchy from phylum to species to examine which aspects of the tree were increased or decreased compared to the donor. Data were analyzed for significance (p≤0.05) by 2-tailed student t- and Mann-Whitney U tests (GraphPad Prism 8).
Linear discriminant analysis:
A stricter measure, namely linear discriminant analysis (LDA) was next used on the dataset. This analysis integrates statistical significance with biological consistency (effect size) estimation. The statistics is based on non-parametric factorial Kruskal-Wallis (KW) rank-sum test to detect features with significant differential abundance, followed by LDA to estimate the effect size of each differentially abundant features.
Correlations between microbiome, clinical, and immune cells metrics:
Correlation coefficients were used to evaluate relationships between metrics of interest.
Change in the microbiome composition over time:
We examined change in OTU number over time in the phyla, class, and genera noted to be significantly different between recipients and donor at baseline. P values were calculated between the donor and recipient at various time points using 2-tailed independent student t-tests.
Results
Study population:
10 subjects were enrolled in the study (Table 1). The mean age of the population was 60.4 years (range 51–66, standard deviation (SD) 4.2), 3 (30%) were male, 5 (50%) were white, and 5 (50%) were Hispanic. Full Sjögren’s criteria was met by 5 (50%) subjects and early Sjögren’s criteria was met by the other 5 (50%) subjects by PSP (n=2) or SP1 (n=3) positivity. Comorbidities included hypertension (n=4), type 2 diabetes (n=2), and Grave’s disease (n=1). Five subjects had one or more comorbid autoimmune diseases (rheumatoid arthritis (n=4), ulcerative colitis (n=2), systemic lupus erythematosus (n=2)). Gastrointestinal symptoms at time of enrollment included nausea (n=4), heartburn (n=3), abdominal pain (n=3), constipation (n=5), and blood in stool (n=1). All individuals were on a stable combination of oral and topical medications, including numerous dry eye therapies.
Table 1:
Population demographics, comorbidities, and medications
| Demographics | Subjects (n=10) |
|---|---|
| Age, years, mean ± SD (range) | 60.4 ± 4.2 (51–66) |
| Gender, male, n (%) | 3 (30%) |
| Race, white, n (%) | 5 (50%) |
| Ethnicity, Hispanic, n (%) | 4 (40%) |
| Past smoker, n (%) | 2 (20%) |
| Current smoker, n (%) | 1 (10%) |
| Comorbidities | |
| Hypertension, n (%) | 4 (40%) |
| Diabetes, n (%) | 2 (20%) |
| Medications | |
| Anti-hypertensive | 5 (50%) |
| Lipid lowering | 1 (10%) |
| Glucose lowering | 1 (10%) |
| Analgesic | 9 (90%) |
| Anti-depressant | 2 (20%) |
| Anxiolytic | 2 (20%) |
| Anti-histamine | 2 (20%) |
| Oral Antibiotics | 1 (10%) |
| Topical Medications | |
| Artificial Tears | 10 (100%) |
| Topical cyclosporine 0.05% | 6 (60%) |
| Lifitegrast 5% | 3 (30%) |
| Autologous Serum Tears | 3 (30%) |
| Topical Corticosteroid | 2 (20%) |
| Topical Antibiotic | 1 (10%) |
| Punctal Plug | 4 (40%) |
SD=standard deviation, n=number in group
At baseline, DE symptoms scored in the severe range with a mean DEQ5 of 15.3 and a mean OSDI of 48 (Table 2). Furthermore, all individuals had notable ocular surface abnormalities with an Inflammadry positivity in 70% of patients and a mean corneal staining of 5.3.
Table 2:
Baseline ocular and non-ocular symptoms and dry eye signs
| Dry eye symptoms | Mean ± SD (range) |
|---|---|
| DEQ5 | 15.3 ± 3.3 (9–18) |
| OSDI | 48 ± 22 (15–83) |
| Dry eye signs * | |
| Inflammation via Inflammadry | 70% |
| Tear break up time, seconds | 6.2 ± 2.9 (2–11) |
| Corneal staining | 5.3 ± 2.7 (2–10) |
| Schirmer’s score, mm wetting 5 min | 11.3 ± 6.5 (3–23) |
| Meibum quality | 1 ± 0.9 (0–3) |
| Non-Ocular Symptoms | |
| Depression (via PHQ-9) | 10.6 ± 8.5 (0–25) |
| Average non-ocular pain (1 week recall) | 6.6 ± 2.0 (3–10) |
DEQ5=Dry Eye Questionnaire 5; OSDI=Ocular Surface Disease Index; PHQ=Patient Health Questionnaire; SD=standard deviation (range)
more severe value between the two eyes.
Baseline gut microbiome profiles of subjects as compared to the donor:
At baseline, all subjects had a gut microbiome profile that was significantly different from the donor. This was first examined by the α- diversity matrices Shannon and Chao. Recipients had higher mean Shannon and Chao indices compared to the donor (Figure 1A and B).
Figure 1:
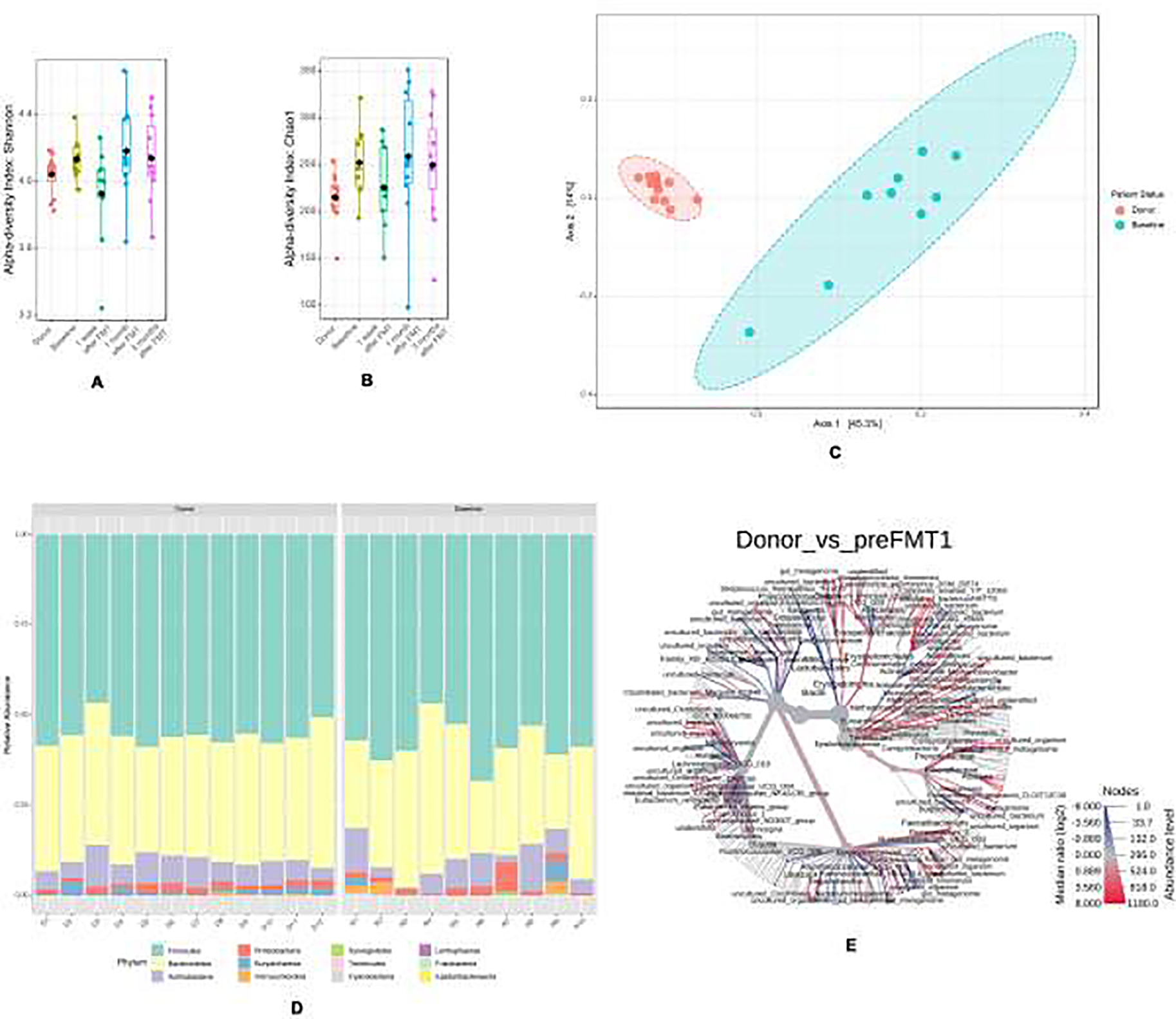
(a) Shannon alpha-diversity (b) Chao alpha-diversity (c) Principle co-ordinate analysis demonstrating beta-diversity (d) Descriptive analysis of the relative abundances of the phylum (e) Heat map tree of species.
Recipients were also significantly different compared to the donor with respect to β diversity, examined with pCoA. There was a clear separation between subjects (blue) and the donor (red), with a q-value of 0.0025 (p-value, 0.001) (Figure 1C). When examined descriptively, Firmicutes and Bacteroides were noted to be the dominant phyla in both the donor and recipients (Figure 1D). The following phylum were found to be increased in abundance in recipients compared to controls: Actinobacteria (1.3 fold), Bacteroidetes (1.2 fold), Cyanobacteria (163 fold), Firmicutes (1.3 fold), Proteobacteria (1.6 fold), and Verrucomicrobia (4.3 fold). The following phylum were found to be decreased in abundance in recipients compared to controls: Euryarchaeota (2.9 fold) and Fusobacteria (1.3 fold). The heat map tree (Figure 1E) demonstrates that certain species (e.g. Parabacteroides goldsteinii) were increased (red) compared to the donor and that certain species (e.g. Streptococcus thermophillus) were decreased (blue).
Compositional differences between the groups on a genus level were next examined by LDA (Figure 2). Overall, recipients had a decreased abundance of the genera Faecalibacterium, Prevotella, and Ruminococcus and an increased abundance of the genera Alistipes, Streptococcus and Blautia compared to the donor.
Figure 2:
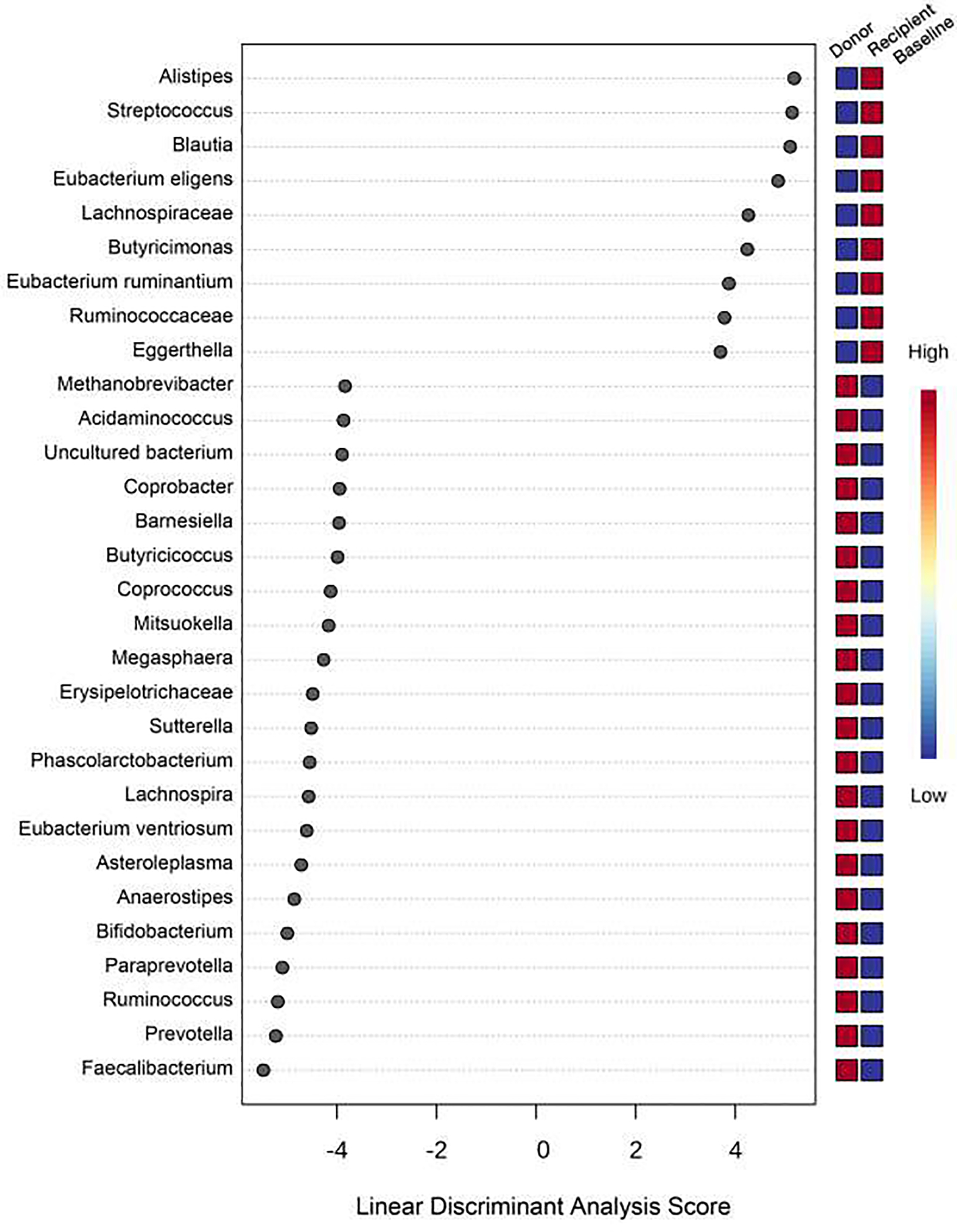
Linear discriminant analysis (LDA) showing the compositional differences between the groups on a genus level.
Relationships between baseline gut microbiome profile and clinical metrics:
There were no significant relationships between diversity indices and DE metrics. However, DE symptoms (via DEQ5, OSDI) were negatively correlated to diversity indices indicating that higher symptoms severity was correlated with lower diversity (Shannon and DEQ5: r=−0.37, OSDI: r=−0.46, p>0.05).
Relationships between baseline immunes profile and clinical metrics:
The majority of individuals had T effector percentages that fell within the normal range (Table 3). With respect to Tregs, 2 individuals had CD25 percentages that fell above the normal range; 1 individual had a FoxP3+ percentage that fell above the normal range and 2 had FoxP3+ percentages that fell below the normal range. T cell profiles were related to each other. Specifically, positive correlations were noted between several cell types including T effector cells (Th1 and Th17: r=0.72, p=0.02), T regulatory cells (CD25 and FoxP3: r=0.63, p=0.05), and T effector and regulatory cells (Th17 and FoxP3: r=0.78, p=0.008). Negative correlations were noted between naïve T cells and all other T cell types (Th1: r=−0.6, p=0.06; FoxP3: r=−0.65, p=0.045).
Table 3:
Baseline T cell profiles
| T effector cells | Mean ± SD (range) | Normal range | Number in normal range |
|---|---|---|---|
| Th1 (CD3+CD4+IFNγ+) | 21.3% ± 6.5 (12.3–32.7) | 10.9%–48.7% | 10 |
| Th17 (CD3+CD4+IL17+) | 2.2% ± 1.5 (0.3–5.4) | 1.3%–6.9% | 9 |
| T regulatory cells | |||
| CD25 (CD3+CD4+CD25hi) | 6.4% ± 4.4 (1.5–17.2) | 1.0%–7.9% | 8 |
| FoxP3 (CD3+CD4+FoxP3+) | 6.1% ± 5.5 (0.5–16.9) | 2.0%–14.1% | 7 |
| Naïve T cell | |||
| CD3+CD4+CD45RA+ | 19.8% ± 19.2 (2.1–61.5) | 8.6%–58% | 4 |
SD=standard deviation
T cell profiles correlated with microbiome diversity indices, but relationships did not reach statistical significance in our small population. Both effector and regulatory T cells negatively correlated with diversity metrics (Shannon and Th1: r=−0.63, p=0.05; Th17: r=−0.50, p=0.14, CD25: r=−0.52, p=0.12; FoxP3: r=−0.52, p=0.05) indicating higher T cell populations in individuals with lower diversity.
T cell profiles were also related to clinical metrics of DE. Specifically, positive correlations were noted between symptom severity (OSDI) and effector T cells (Th1: r=0.76, p=0.01; Th17: r=0.83, p=0.003). Similar, but less robust, relationships were found with regards to T regulatory cells (CD25: r=0.66, p=0.04; FoxP3: r=0.68, p=0.03). This indicates higher symptom scores in individuals with higher effector and regulatory T cell populations. With regards to DE signs, positive relationships were noted between corneal staining and effector T cells (Th1: r=0.48, p=0.19; Th17: r=0.47, p=0.21) while negative relationships were noted between corneal staining and regulatory T cells (CD25: r=−0.66, p=0.06; FoxP3: r=−0.54, p=0.13), indicating higher effector T cell population but lower regulatory population in individuals with more severe corneal staining. The latter relationships, however, did not meet statistical significance in our small population.
Gut microbiome profile changes after FMT:
All individuals received two sessions of FMT delivered by enema 1 week apart. No adverse reactions to the FMT were reported. As examined by pCoA, the host microbiome migrated closer to the donor after FMT in all but 2 individuals (both of which were early marker positive), with the closest distance achieved at 1 week (n=2), 1 month (n=4) and 3 months (n=2) (Figure 3). In one individual, the host microbiome at 3 months was indistinguishable from that of the donor. However, at all time points, the recipient microbiome was significantly different than the donor microbiome (q-value <0.0025, p-value <0.001) and not significantly different than the baseline microbiome prior to FMT (p value>0.05 for all comparisons). When we examined phyla, class, and genera that were different between donor and recipient at baseline, some OTU counts remained closer to the donor even out to 3 months (Figure 4), including the phylum Euryarchaeota, the classes Lentisphaeria and Methanobacteria, and the genera Prevotella and Ruminiclostridium.
Figure 3:
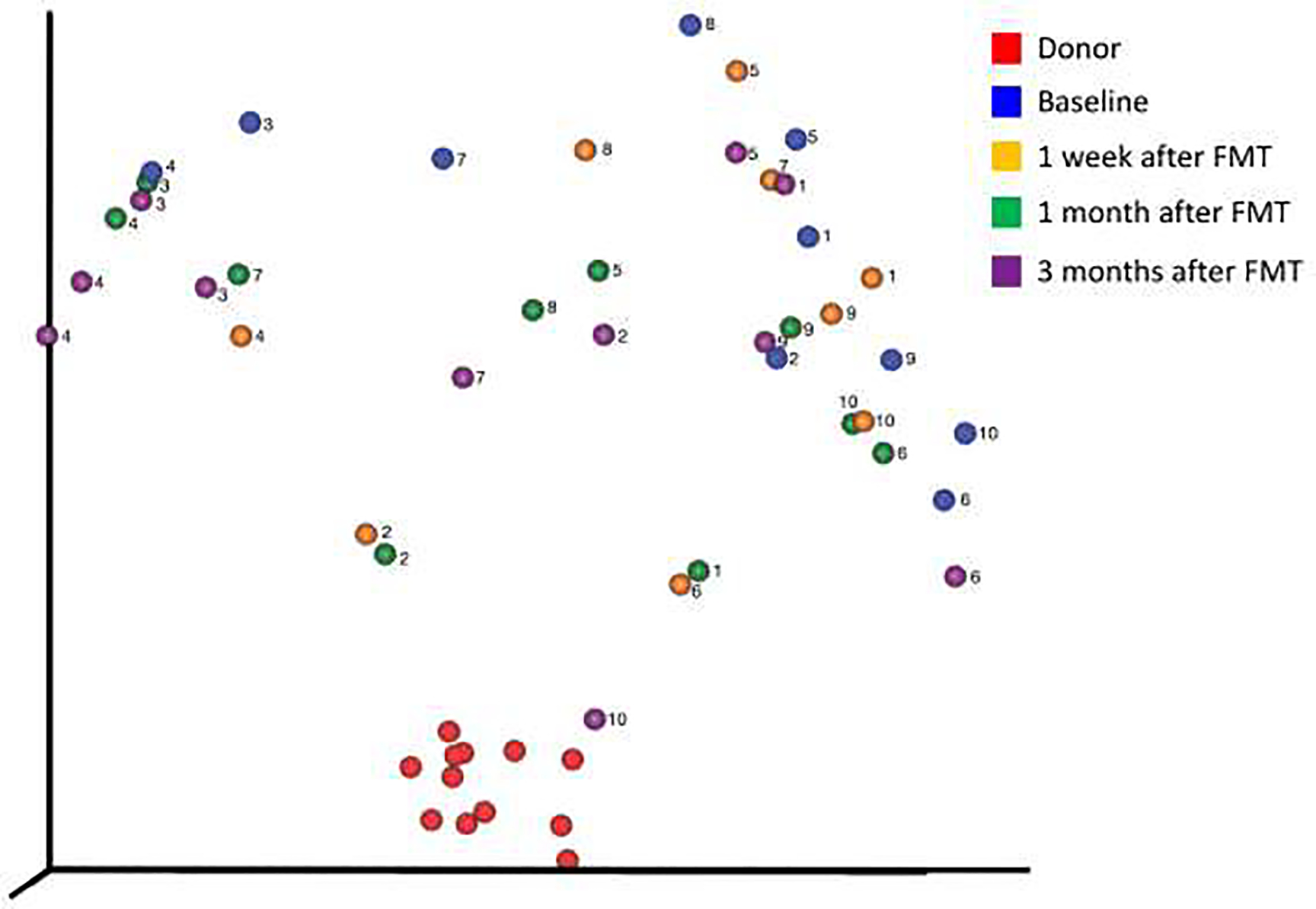
Principle co-ordinate analysis demonstrating the migration of the host microbiome to or from the donor microbiome at all time points.
Figure 4:
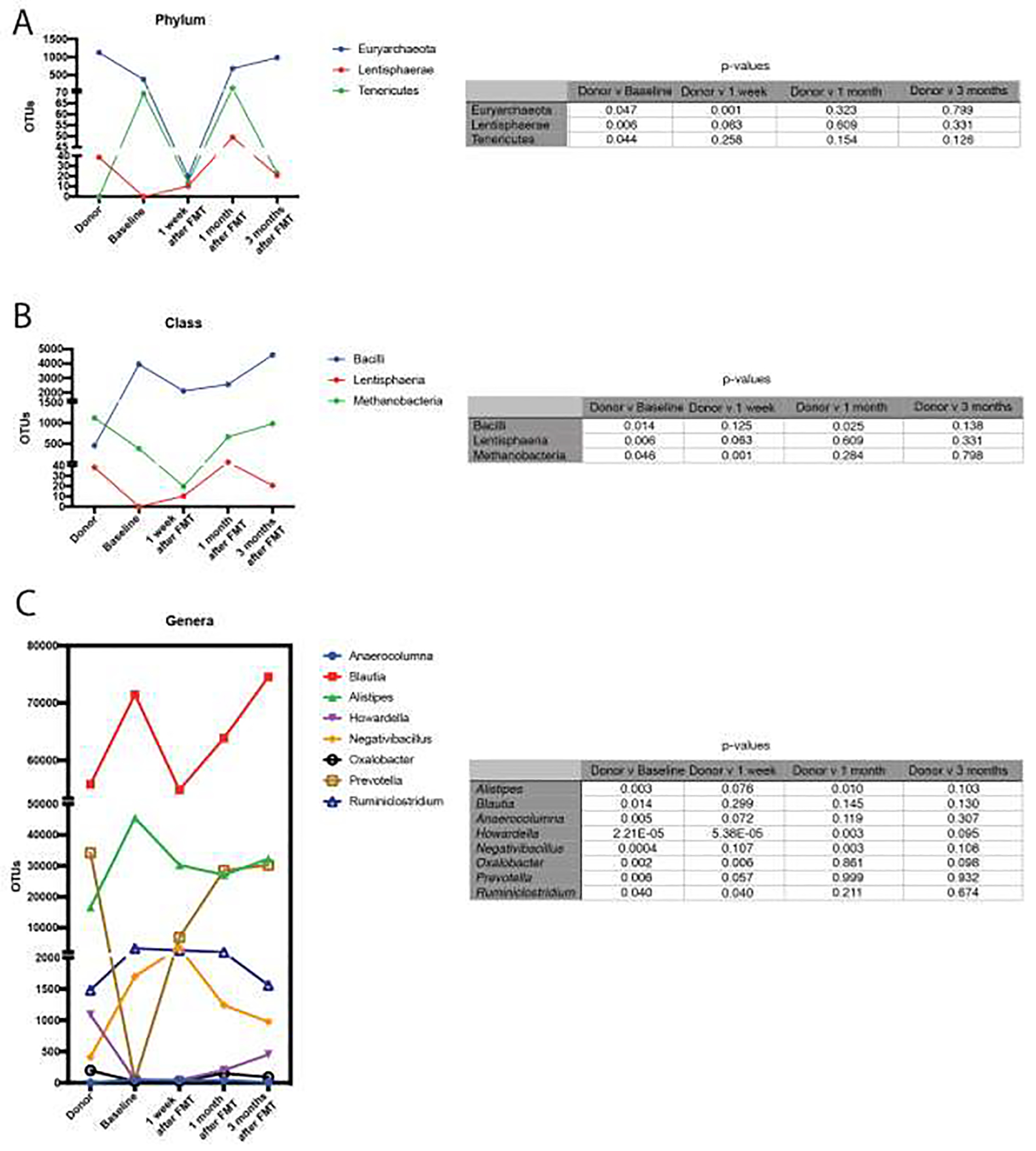
Comparison of the relative abundance of phyla, class, and genera that were different in the recipient compared to donor at baseline and their change over all time points.
Clinical changes over time:
Five individuals subjectively reported improved symptoms (gastrointestinal and dry eye) 3 months after FMT while 5 individuals noted no differences. However, when examining T cell profiles and DE metrics over time, no significant trends were noted between baseline and 3 months in the 10 individuals (Supplementary Figures 1 and 2). Furthermore, no differences were noted between the 2 individuals whose microbiome did not trend toward the donor and the 8 individuals who saw at least a temporary shift in their microbiome.
Discussion
In this study we evaluated the safety of FMT in 10 individuals with immune-mediated dry eye, 5 of which met full Sjögren’s criteria. At baseline, all subjects had a different gut microbiome profile compared to the donor. Specifically, we found that subjects had a higher α-diversity compared to the donor, with a decreased abundance of the genera Faecalibacterium, Prevotella, and Ruminococcus and an increased abundance of the genera Alistipes, Streptococcus and Blautia. Interestingly, significant positive correlations were found between effector and regulatory T cell populations, which may reflect compensatory mechanisms.35 Both T cell populations were positively associated with DE symptom severity whereas corneal staining positively correlated with effector T cells and negatively with regulatory T cells. After the two FMT sessions, the host microbiome migrated towards the donor microbiome in all but two individuals but regressed back to the host microbiome in most individuals by 3 months. However, some phyla, classes, and genera OTU numbers remained closer to the donor versus recipients’ baseline profiles out to 3 months. Overall, our study did not find any adverse effects with 2 FMT doses delivered by enema in our patient population, although it is likely that this delivery schema was not sufficient to permanently alter the host microbiome.
Previous studies in mice have likewise found associations between gut microbiome metrics, DE parameters, and T cell profiles. In mice, antibiotic-induced intestinal dysbiosis combined with desiccating stress (DS) induced a more severe dry eye phenotype than DS alone. This included more severe corneal staining and increased numbers of effector T cells on the ocular surface.36 In a similar manner, germ-free mice compared to conventionally housed mice spontaneously developed more severe features of DE, including conjunctival goblet cell loss, corneal barrier dysfunction, lymphocytic (CD4+ and CD8+ T cells) infiltration into the lacrimal gland, and reduction of epidermal growth factor measured in tears.37 FMT has been shown to reverse some of these changes. When germ-free mice underwent one session of FMT from conventionally housed mice, the aforementioned DE measures were significantly improved 1 month later, including decreased corneal barrier disruption, improved goblet cell density, and decreased lymphocytic infiltration in the lacrimal gland.37 FMT has also reversed the severity of DE in a Sjögren’s model using CD25 knock-out (KO) mice. Corneal barrier dysfunction and goblet cell density improved while lymphocytic infiltration, CD4+IFN-γ+ cell number, and expression of interferon (IFN)-γ and IL-12 decreased after one FMT session from conventionally housed mice.38 Furthermore, to test the pathogenicity of CD4+ T cells, CD4+ T cells from either germ-free CD25KO mice, fecal-transplanted germ-free CD25KO, or conventionally housed CD25KO were adoptive transferred into immunodeficient RAG1KO recipient mice. RAG1KO transplanted with CD4+ T cells from fecal-transplanted germ-free CD25KO and conventionally housed CD25KO mice had less severe DE (less corneal barrier disruption, lower lacrimal gland infiltration, greater goblet cell density, and a decreased frequency of pathogenic CD4+IFN-γ+ cells) compared to mice transplanted from germ-free CD25KO mice.38 These studies suggest that a healthy microbiome helps maintain a healthy ocular surface, even in mice with a predisposition to develop dry eye.
Prior studies have also described gut microbiome difference in DE and Sjögren’s vs control populations.5,6 In a previous study by our group, the gut microbiome of 13 individuals with Sjögren’s, 8 individuals with immune-mediated DE who did not meet full Sjögren’s criteria, and 21 healthy controls were examined. Similar to the current study, increased phylogenetic diversity and a decreased abundance of Faecalibacterium were noted in DE compared to controls. On the other hand, an increased abundance of Prevotella was noted in the prior study, as compared to a decreased abundance in the current study in DE compared to controls.6 Another study examined stool from 10 individuals with Sjögren’s and compared it to 45 healthy controls from the Human Microbiome Project. Again, similarities included greater abundances of Blautia and Streptococcus and reduced abundances of Faecalibacterium, and Prevotella in individuals with DE compared to controls. Differences included a stronger negative relationship between severity of ocular and systemic disease and microbial diversity (r=−.72, p=.01)5 in the prior study which was not seen in our current study. In another study, 10 individuals with Sjögren’s dry eye and 14 individuals with non-Sjögren’s dry eye were compared to 12 healthy controls. Similar to the current study, Alistipes was associated with increased dry eye severity. Differences included decreased abundance of Blautia and increased abundance of Prevotella in individuals with Sjogren’s in the previous study.39 Differences between study results may be due to several factors, including population-based differences, varying processing and analytic techniques to examine the microbiome, or unaccounted confounders such as dietary or comorbid systemic or autoimmune diseases.
Relationships between T cell profiles and DE clinical signs have also been previously studied. Specifically, CD4+ T cell subsets, including Th1 and Th17 cells, have been found to be the major effector cells in DE in both mice and humans. For example, DS to the ocular surface of mice led to an increase in CD4+ T cells and the production of Th-17-associated genes in the conjunctival epithelium, including MMP-9, IL-1β, tumor necrosis factor (TNF)-α, IL-17, and IFN-γ.40 A similar study demonstrated increased numbers of Th1 cells and IFN-γ expression in draining lymph nodes after DS.41 Impaired Treg function has also been tied to DE. In one study, impaired Treg function was identified in DE mice through their reduced ability to suppress the proliferation of both naïve-T and primed-T cells, with a concomitant rise in pathogenic Th17 cells. The complex inter-relationship between T cell types was apparent when blockade of IL-17 significantly reduced the severity of DE (reduced corneal staining) and prevented the loss of Treg function.42 In humans, one study demonstrated higher levels of Th-17-downstream inflammatory mediators including MMP-9, IL-1β, TNF-α, IL-17, and IFN-γ in tears of 17 individuals with newly diagnosed DE (further details not specified).40 The novel contribution of our study is an examination of relationships between T cell profiles in blood and DE metrics in humans. Similar to animal models which studied T cells in the cornea, conjunctivae, and draining lymph nodes38,40,42, we found that corneal staining positively associated with effector T cells and negatively with regulatory T cells. Interestingly, both T cell categories had positive relationships to DE symptoms, a metric that is challenging to capture in animal models.
Several limitations should be considered when interpreting the results of this study. First, the optimal FMT dosing strategy for DE is not known. In UC studies where positive clinical results were noted, a higher number of FMT sessions (3–6) were performed.21,43 We modeled our protocol based on a previous GVHD study where 1–2 doses of FMT from healthy spouses or relatives were delivered via enema.26 Perhaps an alternative delivery method (oral pills or duodenal delivery by colonoscopy) or more frequent enemas would have led to more robust changes in the microbiome and clinical parameters in our study. Second, as is often the case in DE, our population was heterogenous with respect to DE signs and comorbidities. However, in our small study, no apparent differences in response were noted by baseline demographics or comorbidities. Third, we do not know the optimal donor composition to treat immune-associated DE and thus do not know if the donor microbiome composition was ideal. Fourth, 16s rRNA sequencing method has limited genera coverage, which limits a detailed study of the microbiome. Fifth, our study did not measure metabolic products of bacteria, such as butyrate, as this testing required in clinic stool sample collection. While this was originally planned, our population could not to produce stool on demand making this testing unfeasible. Sixth, with 2 markers, we acknowledge that we cannot definitively categorize T cells into pro- vs anti-inflammatory phenotypes. Finally, unrecorded confounders including diet, genetic factors, and environmental exposures may have influenced our results.
Despite these limitations, our study highlights that FMT can be safely delivered to individuals with immune-mediated DE, but the most effective route and frequency of administration are yet to be determined. Furthermore, our study demonstrates the complex interaction between the immune system and DE in humans, mirroring many of the findings in animal models. Despite the fact that symptoms and signs of DE were mostly unchanged after FMT in our population, gut microbiome manipulation remains a potential future therapy for immune-mediated DE. Its role in treating non-immune related DE is not as clear. However, given the complexity of FMT, gut microbiome manipulation via alternative methods such as prebiotics and probiotics should be explored with specific deficits in the DE microbiome targeted. Future studies are needed to focus on these lines of investigation.
Supplementary Material
Financial Support:
Supported by the Sjögren’s Foundation, the University of Miami Interdisciplinary Team Science Award (UM SIP 2018-2R), the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
Footnotes
Conflict of Interest:
No conflicting relationship exists for any author.
Trial Registration: The trial was registered on clinicaltrials.gov (NCT03926286) and was performed under Investigational New Drug (IND) 17994 granted by the Food and Drug Administration (FDA).
Supplemental Material available at AJO.com.
Disclaimer: This article was prepared while Santanu Banerjee, PhD was employed by University of Miami. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276–283. [DOI] [PubMed] [Google Scholar]
- 2.Ciurtin C, Ostas A, Cojocaru VM, Walsh SB, Isenberg DA. Advances in the treatment of ocular dryness associated with Sjogrens syndrome. Semin Arthritis Rheum. 2015;45(3):321–327. [DOI] [PubMed] [Google Scholar]
- 3.Both T, Dalm VA, van Hagen PM, van Daele PL. Reviewing primary Sjogren’s syndrome: beyond the dryness - From pathophysiology to diagnosis and treatment. Int J Med Sci. 2017;14(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. Ocul Surf. 2009;7(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Paiva CS, Jones DB, Stern ME, et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjogren Syndrome. Sci Rep. 2016;6:23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez R, Watane A, Farhangi M, et al. Gut microbial dysbiosis in individuals with Sjogren’s syndrome. Microb Cell Fact. 2020;19(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher JU, Littman DR, Abramson SB. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016;68(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Shao T, Li H, Xie Z, Wen C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs JP, Braun J. Immune and genetic gardening of the intestinal microbiome. FEBS Lett. 2014;588(22):4102–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHardy IH, Goudarzi M, Tong M, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015;6:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Wright K, Davis JM, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaahtovuo J, Munukka E, Korkeamaki M, Luukkainen R, Toivanen P. Fecal microbiota in early rheumatoid arthritis. J Rheumatol. 2008;35(8):1500–1505. [PubMed] [Google Scholar]
- 15.Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pianta A, Arvikar S, Strle K, et al. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017;69(5):964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watane A, Cavuoto KM, Banerjee S, Galor A. The Microbiome and Ocular Surface Disease. Current Ophthalmology Reports. 2019;7(3):196–203. [Google Scholar]
- 18.Dodin M, Katz DE. Faecal microbiota transplantation for Clostridium difficile infection. Int J Clin Pract. 2014;68(3):363–368. [DOI] [PubMed] [Google Scholar]
- 19.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–508. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(6):503–516. [DOI] [PubMed] [Google Scholar]
- 21.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149(1):102–109 e106. [DOI] [PubMed] [Google Scholar]
- 22.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916 e917. [DOI] [PubMed] [Google Scholar]
- 23.Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal Microbiota Transplantation (FMT) in Multiple Sclerosis (MS). Am J Gastroenterol. 2011;106:S352–S352. [Google Scholar]
- 24.Borody T, Campbell J, Torres M, Nowak A, Leis S. Reversal of Idiopathic Thrombocytopenic Purpura [ITP] with Fecal Microbiota Transplantation [FMT]. Am J Gastroenterol. 2011;106:S352–S352. [Google Scholar]
- 25.Borody T, Campbell J, Torres M, Nowak A, Leis S. Reversal of Idiopathic Thrombocytopenic Purpura [ITP] with Fecal Microbiota Transplantation [FMT]: 941. Official journal of the American College of Gastroenterology | ACG. 2011;106. [Google Scholar]
- 26.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60. [DOI] [PubMed] [Google Scholar]
- 28.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539–574. [DOI] [PubMed] [Google Scholar]
- 29.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16. [DOI] [PubMed] [Google Scholar]
- 30.Hubschman S, Rojas M, Kalavar M, Kloosterboer A, Sabater AL, Galor A. Association Between Early Sjogren Markers and Symptoms and Signs of Dry Eye. Cornea. 2020;39(3):311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidebottom AC, Harrison PA, Godecker A, Kim H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch Womens Ment Health. 2012;15(5):367–374. [DOI] [PubMed] [Google Scholar]
- 32.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. [DOI] [PubMed] [Google Scholar]
- 33.Lanza NL, Valenzuela F, Perez VL, Galor A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul Surf. 2016;14(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Choi SH, Kim YJ, et al. Clinical Effect of IRT-5 Probiotics on Immune Modulation of Autoimmunity or Alloimmunity in the Eye. Nutrients. 2017;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trujillo-Vargas CM, Schaefer L, Alam J, Pflugfelder SC, Britton RA, de Paiva CS. The gut-eye-lacrimal gland-microbiome axis in Sjogren Syndrome. Ocul Surf. 2020;18(2):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Zaheer M, Bian F, et al. Sjogren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaheer M, Wang C, Bian F, et al. Protective role of commensal bacteria in Sjogren Syndrome. J Autoimmun. 2018;93:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon J, Choi SH, Yoon CH, Kim MK. Gut dysbiosis is prevailing in Sjogren’s syndrome and is related to dry eye severity. PLoS One. 2020;15(2):e0229029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50(8):3802–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao HL, Chen SZ, Xu HM, et al. Efficacy and safety of fecal microbiota transplantation for treating patients with ulcerative colitis: A systematic review and meta-analysis. J Dig Dis. 2020;21(10):534–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


