Abstract
Background:
Genetic evaluation and testing for hereditary breast and ovarian cancer (HBOC) remain suboptimal. We evaluated the feasibility of using a screening tool at a breast imaging center to increase HBOC assessment referrals.
Methods:
We developed and added a brief questionnaire based on the National Comprehensive Cancer NetworkTM HBOC genetic counseling referral guidelines to the standard intake forms of patients undergoing mammography at a community breast imaging center from 2012 through 2015. Patients who met the criteria in the guidelines were referred for genetic counseling.
Results:
We screened 34,851 patients during the study period and found 1246 (4%) patients eligible for referral; 245 of these patients made a genetic counseling appointment, and 142 patients received genetic counseling. 40 (28%) had a personal history of breast cancer but were not previously tested. Following counseling, we tested 105 patients for BRCA1/2. Eight (8%) tested positive for a pathogenic mutation, and nine (9%) had a variant of unknown significance. Although they tested negative, many patients met criteria to add breast MRI to their screening due to greater than 20% lifetime breast cancer risk based on their family cancer history. Our study led to improved clinical risk management in 67% of the patients who underwent genetic counseling.
Conclusions:
Our study shows that large-scale screening of patients for HBOC syndromes at time of breast imaging is practical and highly feasible. The screening tool identified women with actionable BRCA1/2 mutations and mutation-negative but high-risk women, leading to significant changes in their risk management; these women would otherwise have been missed.
Keywords: BRCA, HBOC, Genetic Testing, Family History, Genetic referral, Breast cancer
Lay summary
Hereditary Breast and Ovarian Cancer (HBOC) caused by pathogenic mutations in breast cancer genes (BRCA1/ BRCA2) increase an individual’s lifetime risk of getting HBOC. Identifying these high-risk individuals and using proven preventive clinical risk management strategies can significantly reduce their lifetime risk of HBOC. Using an innovative family cancer history questionnaire, we screened 34,000 women at a community breast imaging center and provided genetic counseling and testing to eligible women from the screening. We identified several women at high risk for HBOC leading to positive clinical risk management changes. These women would have been missed if not for our intervention.
Precis
The study establishes a new methodology to screen women for Hereditary Breast and Ovarian Cancer. Through genetic counseling and BRCA gene testing, the study identifies women at-risk for HBOC and potentiates changes in their clinical risk management.
INTRODUCTION
Up to 10% of all breast cancers are attributed to inherited genetic mutations, most commonly in BRCA1 and BRCA21, 2. Patients with a germline pathogenic BRCA1/2 mutation have up to 70% lifetime risk of breast cancer and up to a 17– 45% lifetime risk of ovarian cancer, as well as increased lifetime risks for central gastrointestinal adenocarcinomas (e.g., pancreatic) and melanoma3, 4. Identifying cancer patients and their family members with pathogenic BRCA1/2 mutations can inform treatment options for patients, as well as risk management and prevention strategies. However, despite these known treatment and prevention benefits, studies have reported that less than 50% of individuals who would be appropriate potential candidates for BRCA1/2 genetic testing receive a referral for genetic counseling and testing5.
Screening for cancer family history is an essential and effective tool to identify women carrying BRCA1/2 mutations and is recommended as part of comprehensive patient care6. Evidenced-based recommendations from several organizations, including the US Preventive Services Task Force, suggest that women be referred for genetic counseling and evaluation if their family history indicates an increased risk for BRCA1/2 mutations7. The American College of Obstetrics and Gynecology, American College of Medical Genetics, and National Comprehensive Cancer Network (NCCN) have all endorsed similar recommendations. Yet many mutations go undetected, and compliance with recommendations has been suboptimal; only 29–34% of eligible individuals are referred for genetic counseling and testing8–10. Primary care settings are poised to play a significant role in individualized risk assessment, given their focus on disease prevention and screening.
Annual screening mammography appointments present an ideal opportunity for administering screening tools to improve genetic counseling referrals. In the United States, adherence to US Preventive Services Task Force mammography screening guidelines is high (between 76% and 81%)11. These data suggest that breast imaging centers (BICs) may serve as logical primary care entry points to screen for cancer family history and identify women at increased risk for breast cancer who would benefit from genetic counseling and possibly testing.
Tools for assessing family history in primary care should be comprehensive and guideline-driven, as well as easy to administer and interpret so that these tools optimally facilitate referral to genetic counseling12–14. In prior studies, various family history-based individualized risk assessment tools to facilitate genetic counseling referrals have been developed and evaluated in various healthcare settings15–18. Researchers in such studies have utilized existing risk models (e.g., Myriad, Tyrer-Cuzick, BRCAPro), modified existing tools (e.g., National Cancer Institute Breast Cancer Risk Assessment Tool), and developed new paper- or computer-based questionnaires. Typically, the implementation of such tools has resulted in marginal improvement in the use of genetic counseling services. Among patients who completed questionnaires using a wireless tablet prior to receiving a mammogram, 6.1% were found to be at high risk and could benefit from genetic counseling19. Although risk assessment questionnaires increased the rate of genetic testing in ovarian cancer by 24%20, in another study, only 10% of all eligible patients who received a mammogram completed genetic counseling for breast cancer9. Innovative models to identify eligible women and provide access to genetic services are needed to enable subsequent cancer prevention and early detection.
Our study aimed to develop a practical streamlined guideline-based screening tool to assess BRCA1/2 mutation risk and to evaluate the feasibility of implementing the tool in community BICs to identify women who should be referred for genetic counseling. We hypothesized that use of the guideline-based screening tool would improve identification of high-risk women eligible for genetic counseling, and subsequently improve their clinical risk management.
METHODS
Study design and participants
Study participants included a consecutive series of women who came to the Memorial Hermann Breast Imaging Center, a suburban, private practice community BIC for screening or diagnostic mammograms between August 1, 2012, and August 31, 2015. The study team consisted of a lead breast imaging radiologist, breast center manager, nurse navigator and hospital administrator. To explore the feasibility of adding a genetic counseling service line to the BIC, a personal and family cancer history questionnaire was added to other standard intake forms that all participants filled out. The nurse navigator kept a prospective Excel worksheet of all patients identified as high risk and referred for genetic counseling and made charts on all patients who went to genetic counseling. All initial paper forms were also kept in the nurse navigator’s office. The retrospective analysis of this data was approved by the Institutional Review Board with consent waiver at The University of Texas MD Anderson Cancer Center.
Procedure
The EPIS (Exploration, Preparation, Implementation, Sustainment) framework was selected and utilized as the guiding model for this study including the mapping of implementation strategies to EPIS stages, and selection, content, and timing of measurement protocols.
Development of a screening tool to assess BRCA1/2 risk
Pilot Study:
In the pilot phase, all patients who presented for a screening or diagnostic mammogram at BIC were asked to complete an 8 item “red flag” questionnaire recommended by Myriad Inc., the company that held a patent on genetic testing at the time (Supplemental Data, Form A). A total of 4,236 patients were screened, and 139 (3.2%) were identified to be eligible for genetic referral. Of the 139, 17 (12.2%) scheduled an appointment for genetic counseling, and 3 completed genetic counseling, representing a no-show rate of 82%. Using feedback from radiologists and nurse navigators several modifications were made to the study protocol including establishing weekly on-site genetic counseling services when patients were previously required to travel to an academic center approximately 10 miles away from BIC. The questionnaire was also adapted to improve administration, interpretation and increase patient comprehension.
Development of improved screening tool to assess BRCA1/2 risk:
Using data from the pilot phase, we developed a one-page self-administered screening tool where the questions were mostly put into table format and were organized by the following categories to simplify interpretation by providers and to readily indicate the need for guideline-based referral to genetic counseling. (Figure 1) Personal cancer history was separated from family cancer history, and the age of diagnosis was added to each question where appropriate. Specific questions of Ashkenazi Jewish ancestry and personal and family history of BRCA1/2 genetic testing were added to the end of the form as they did not adapt to table format. A column for a simple “no” answer was also added after radiologist feedback. The forms were modified later to stratify family history by a first-degree relative, maternal, and paternal histories. In one form version, the question of would you like to speak with a genetic counselor was added, then removed from the tool as this did not find any more genetic counseling patients but did add work to the nurse navigator and genetic counselor from many “worried well” BIC patients. No new patients were identified with that question. The family history of prostate cancer was added to the family history table section when NCCN guidelines changed during this study period21
Figure 1.
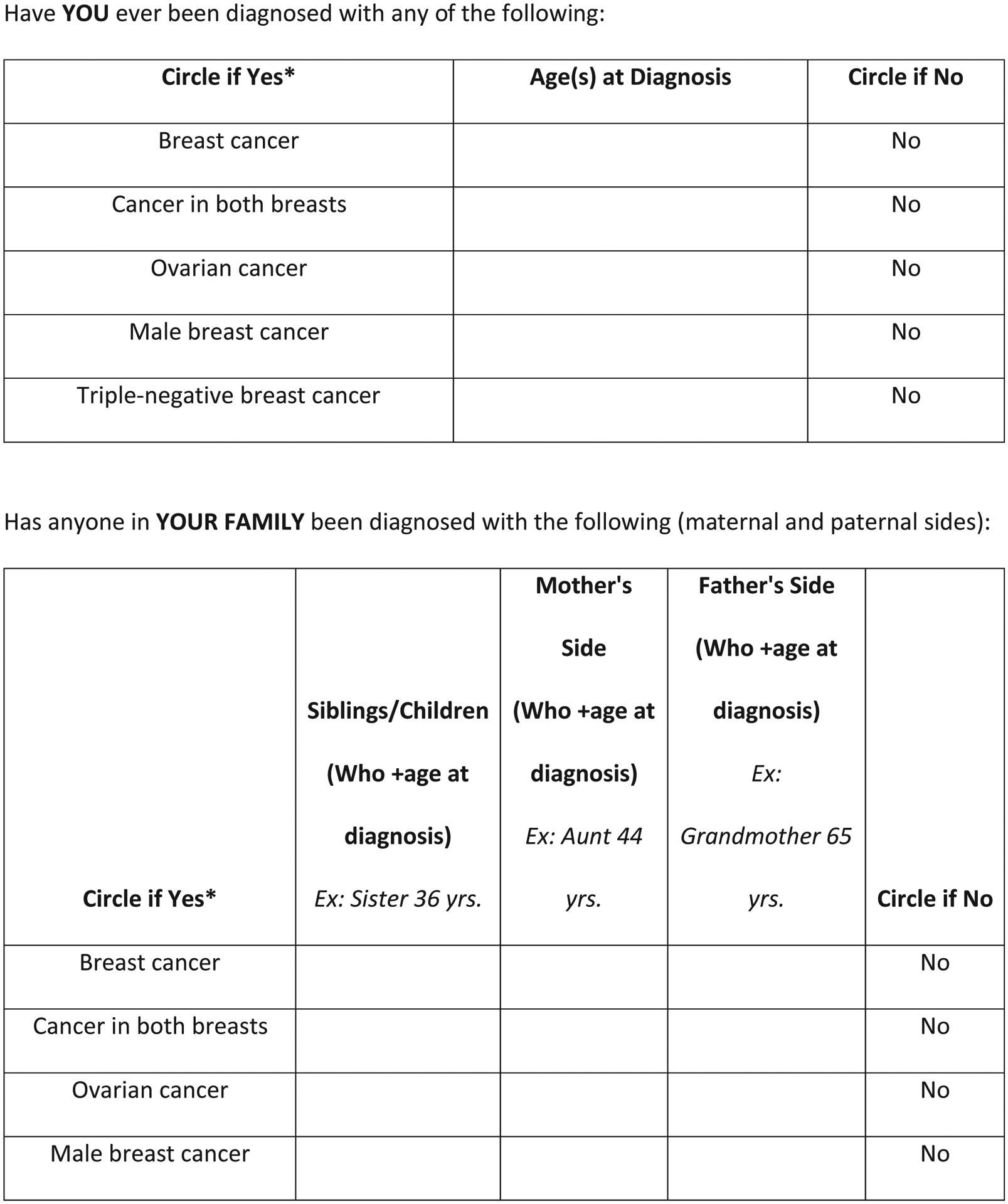
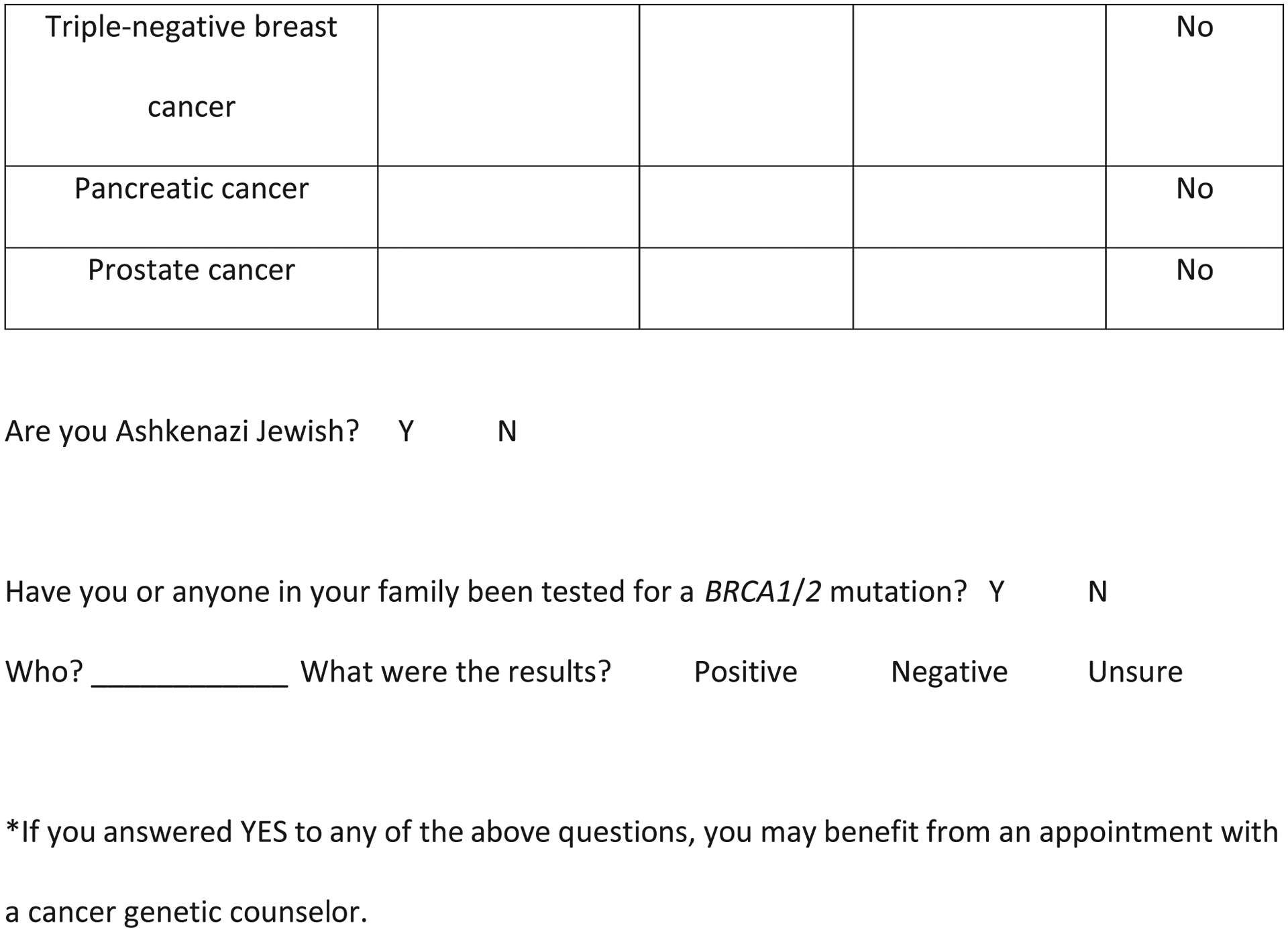
Screening tool used at community breast imaging centers in our study.
Formal evaluation of organizational capacity:
Prior to implementation, a process mapping of the workflow, including the screening tool, was completed to optimize efficiency, identify personnel and training needs, and determine patient contact and notification points.
Criteria for referral to genetic counseling
On the basis of self-reported information provided on the screening tool (Figure 1), patients were considered eligible for a genetic counseling referral if they met any of the following guideline-based criteria: 1) personal breast cancer diagnosis at age 45 years or younger; 2) personal diagnosis at any age of bilateral, triple-negative, or male breast cancer or ovarian cancer; 3) personal diagnosis of breast cancer at any age and any relative diagnosed with breast cancer when younger than 50 years or any relative diagnosed with ovarian, pancreatic, male breast, bilateral breast, or triple-negative breast cancer at any age; 4) family history of ovarian or male breast cancer, or female breast cancer diagnosed at age 45 years or younger; 5) more than one case of female or male breast cancer, triple-negative or bilateral breast cancer, ovarian cancer, or pancreatic cancer diagnosed on the maternal or paternal side of the family at any age; 6) self-reported as Ashkenazi Jewish and met any other criteria on the form; 7) self-reported a BRCA1/2 mutation in the family. A history of prostate cancer was used only if diagnosed at a young age as a substitute for a high Gleason score. Prostate cancer history was used more as a contributing factor. It did not weigh as high as the other factors in this section.
Selection of measures and ways to monitor:
Patients who answered “yes” to any question in the personal history section of the form, or yes to two items on the family history section of the form, or a personal history of breast cancer at any age as well as a positive family history, were selected for further analysis. Patients of Ashkenazi Jewish ancestry with any positive responses as well as any patient who reported a known genetic mutation in their family also qualified for referral. This tool which was laminated and placed at every BIC radiologist’s reading station along with current NCCN HBOC guidelines.
Implementation of the tool and study procedure:
Initial training on the implementation and interpretation of the screening tool was facilitated by the study lead radiologist and the BIC genetic counselor in routine radiology meetings and a mandatory CME training meeting. This training was supplemented with tutorials and emails to update radiologists on changes in NCCN™ genetic testing guidelines and practices. NCCN™ guidelines for hereditary breast and ovarian cancer genetic counseling and testing were placed adjacent to all radiology workstations during the study.
All patients receiving mammographic imaging at the BIC completed the screening tool along with standard intake forms at registration. A Spanish translated form was also available. Mammography technologists reviewed completed tools with patients during their imaging examination for accuracy and legibility. The screening tools were evaluated by radiologists upon the interpretation of the radiology study, and the radiologists identified patients who met the criteria for genetic counseling referral. For patients who met the criteria, the form was flagged, and the recommendation for a genetic counseling referral was added to the radiology report. The form was scanned into the electronic health record along with the other paperwork used by the hospital system during the study period. Although the nurse navigator was able to discuss genetic counseling with some patients while they were still in the BIC, the majority of patients that had left the BIC before their imaging was interpreted and their HBOC risk identified. In those cases, the nurse navigator and/or genetic counselor made at least one and up to three attempts to contact patients by telephone. All patients identified for genetic counseling referrals received a letter with their mammogram results indicating that they may be at increased risk for inherited cancer and genetic counseling should be considered. A copy of the letter was sent along with the radiology results to the referring physician as well.
The nurse navigator or genetic counselor verified all information with the patient prior to scheduling a 60 minute genetic counseling appointment. During the study period, a genetic counselor was on site one day per week to see patients referred for counseling and risk assessment. Very few patients received same day genetic counseling as the counselors’ schedule was usually full and booked in advance. Patients were charged a flat fee of $50 for all counseling services, which was determined by the hospital administrators to cover overhead costs for this new service line. No insurance was billed for counseling services as we thought this would decrease barriers for patients and simplify the administrative costs of this program.
The genetic counselor performed a detailed family pedigree and ran the Gail, BRCAPro, and Tyrer-Cuzick risk models if applicable22–24. The counselor discussed the likelihood of identifying a genetic mutation with the patient. Patients underwent genetic testing if appropriate criteria were satisfied, the patient provided informed consent, and the patient’s insurance covered genetic testing. The counselor discussed whether the patient qualified for chemoprevention or high-risk screening magnetic resonance imaging (MRI) as indicated by Gail or Tyrer-Cuzick results, respectively.
If the results were negative, the genetic counselor discussed the results over the phone. If the results were positive or showed a variant of unknown significance, a follow-up in-person appointment was made.
Statistical analysis
Descriptive statistics were used to summarize the patient samples at each step. No statistical tests were run.
RESULTS
Patient population
A total of 34,851 consecutive patients were screened for genetic counseling referral during the study period. Of these patients, 64% were insured by managed care, 30% by Medicare, 3% by Medicaid, 1.5% by self-pay, and 1.5% by others. The breakdown of patients’ ages by the decade was as follows: 7% <39 years, 23% 40–49 years, 29% 50–59 years, 26% 60–60 years, and 15% >70 years.
Identification of patients eligible for genetic evaluation
A flowchart describing the screening and referral identification process is shown in Figure 2. Of the 34,851 patients, 1246 patients (4%) were identified as appropriate candidates for genetic evaluation; only 189 (15%) of these had had prior genetic testing done by their physicians. The remaining 1057 patients (3% of the study population) received a letter and a phone call referring them for genetic counseling.
Figure 2.
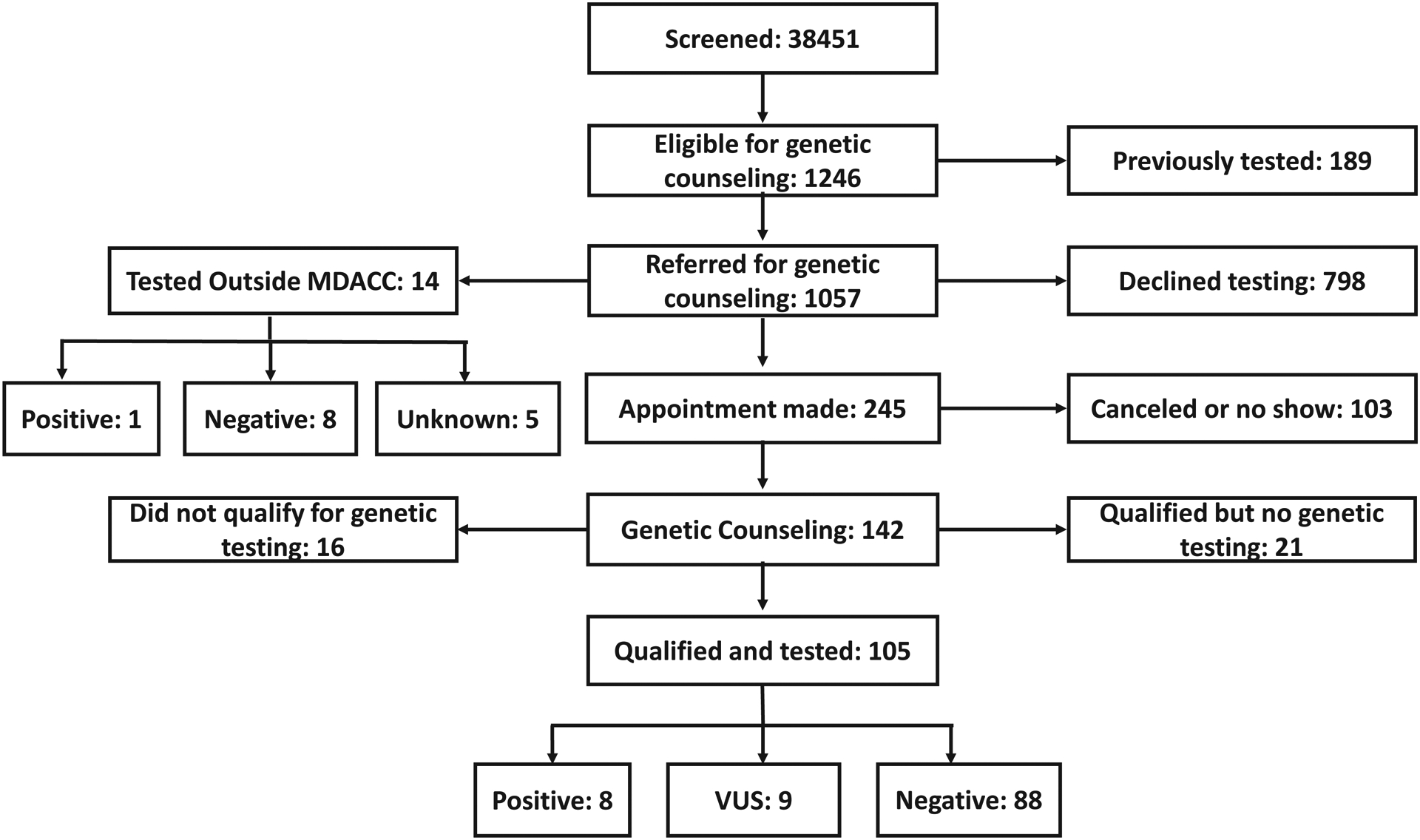
Flow chart of patients who were screened for genetic counseling and testing in our study. VUS indicates variant of unknown significance.
Genetic counseling and genetic testing
Of the 1057 patients eligible for a genetic counseling referral, 798 declined the referral or could not be reached by phone. A total of 245 made an appointment with our genetic counselor; 142 kept the appointment, and the remaining 103 either canceled or did not show for the appointment.
Among those who kept the appointment 14 patients received genetic testing by their outside physician after our contact (1 patient was found to have a pathogenic mutation, 8 were negative, and 5 had unknown results), Among the 142 patients who kept their appointment and were seen by our genetic counselor, 126 met clinical criteria for genetic testing and 105 underwent testing: 8 patients had a pathogenic germline BRCA1/2 mutation (3 BRCA1 and 5 BRCA2; Table 2), 9 had a variant of unknown significance, and 88 were mutation-negative.
Table 2.
Personal and family history of cancer in the 8 patients who were genetically positive for hereditary breast and ovarian cancer
| Patient no. | Age, years | History of breast cancer/age at diagnosis | History of ovarian cancer | Known gene mutation in the family | Family history of breast cancer | Family history of ovarian cancer | Gail | Tyrer-Cusick | Gene | Details |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | no | no | yes | yes | no | n/a* | n/a | BRCA1 | |
| 2 | 58 | yes/32 years | no | no | yes | yes | n/a | n/a | BRCA1 | |
| 3 | 61 | yes/40 and 50 years | no | no | yes | no | n/a | n/a | BRCA1 | Bilateral breast cancer, father had breast cancer |
| 4 | 51 | yes/51 years | no | no | yes | no | n/a | n/a | BRCA2 | Family history of bilateral and male breast cancer |
| 5 | 49 | yes/44 and 49 years | no | no | no | no | n/a | n/a | BRCA2 | Did not get flagged until second cancer diagnosis |
| 6 | 54 | no | no | yes | no | yes | n/a | n/a | BRCA2 | |
| 7 | 58 | no | no | yes | no | yes | >1.6 | <20% | BRCA2 | |
| 8 | 55 | no | no | no | no | yes | <1.6 | <20% | BRCA2 | Insurance denied in 2013, diagnosed with breast cancer 2016 and tested positive for BRCA mutation |
n/a indicates not applicable.
Table 1 presents the personal and family history reported in the screening tools by patients who received genetic counseling. Of the 142 patients who had genetic counseling, 40 (28%) reported a personal history of breast cancer and 3 (2%) reported a personal history of ovarian cancer but had not undergone genetic testing previously, 116 (82%) reported a family history of breast cancer, and 44 (31%) reported a family history of ovarian cancer. Of the 40 patients reporting a personal history of breast cancer, 26 also reported a family history of breast or ovarian cancer. Over two-thirds of our genetic counseling referrals (100 patients; 70%) were based on family history alone, thus demonstrating the importance of collecting a complete family cancer history.
Table 1.
Patient Characteristics and Screening results for patients who received genetic counseling in our study (n = 142)
| Patient Characteristics | N | (%) | |
|---|---|---|---|
| All | 142 | (100%) | |
| Age at Genetic Counseling Appointment | 20–29 | 3 | (2%) |
| 30–39 | 24 | (17%) | |
| 40–49 | 38 | (27%) | |
| 50–59 | 40 | (28%) | |
| 60–69 | 29 | (20%) | |
| 70–99 | 8 | (6%) | |
| Ashkenazi Jewish Descent | Yes | 8 | (6%) |
| No | 129 | (91%) | |
| Unknown | 5 | (4%) | |
| Personal History of Breast Cancer* | 40 | (28%) | |
| Age at Diagnosis - median (min, max) | 46.0 | (32.0, 72.0) | |
| Bilateral Breast Cancer | 5 | (4%) | |
| Triple Negative Breast Cancer | 4 | (3%) | |
| Male Breast Cancer | 0 | (0%) | |
| Personal History of Ovarian Cancer* | 3 | (2%) | |
| Family History of Breast Cancer | 116 | (82%) | |
| Number of relatives with History of Breast Cancer | |||
| 1 | 54 | (38%) | |
| 2 | 37 | (26%) | |
| 3 | 17 | (12%) | |
| >3 | 8 | (6%) | |
| Age of Youngest Relative with Breast Cancer - median (min, max) | N=111 | 45.0 | (21.0, 81.0) |
| Family history of Bilateral Breast Cancer | Yes | 34 | (24%) |
| No | 74 | (52%) | |
| Unknown | 8 | (6%) | |
| Family History of Male Breast Cancer | Yes | 3 | (2%) |
| No | 107 | (75%) | |
| Unknown | 6 | (4%) | |
| Family history of Triple Negative Breast Cancer | Yes | 2 | (1%) |
| No | 103 | (73%) | |
| Unknown | 11 | (8%) | |
| Family History of Ovarian Cancer | Yes | 44 | (31%) |
| Age of Youngest Relative with Ovarian Cancer - median (min, max) | N=33 | 54.0 | (25.0, 81.0) |
| Family History of Pancreatic Cancer | Yes | 13 | (9%) |
| No | 120 | (85%) | |
| Unknown | 9 | (6%) | |
| Number of Relatives with History of Pancreatic Cancer | 1 | 11 | (8%) |
| 2 | 1 | (1%) | |
| 3 | 1 | (1%) | |
| Family History of Prostate Cancer | Yes | 16 | (11%) |
| No | 117 | (82%) | |
| Unknown | 9 | (6%) | |
| Prostate Cancer Before Age 60 | Yes | 8 | (6%) |
| No | 7 | (5%) | |
| Unknown | 1 | (1%) | |
| Number of relatives with History of Prostate Cancer | 1 | 12 | (8%) |
| 2 | 3 | (2%) | |
| >3 | 1 | (1%) |
One patient reported a personal history of both breast and ovarian cancer.
During the genetic counseling session, breast cancer risk estimates, using the Tyrer-Cuzick and Gail risk models, were calculated for patients without a personal history of breast cancer. Lifetime breast cancer risk estimates from the Tyrer-Cuzick model were calculated for 91 of these patients. Forty-nine met NCCN criteria for annual MRI screening because of a ≥20% lifetime risk of breast cancer 23. A Gail score was calculated for 88 patients, and 46 met criteria for chemoprevention because of a 5-year Gail score ≥1.6%. Seventeen patients (18%) met criteria for both chemoprevention and MRI screening. The genetic counseling appointment risk assessments provided clinically actionable risk and screening information in 67% of patients without a personal history of breast or ovarian cancer (67/100).
DISCUSSION
Our findings showed that administering a streamlined screening tool to determine eligibility for genetic counseling at the time of breast imaging in a BIC is feasible and practical, and yields clinically actionable outcomes. Our implementation strategy identified 1057 women undergoing routine mammography screening who met NCCN criteria for referral to genetic counseling to evaluate hereditary breast cancer risk. Not all adhered to the referral recommendation; nonetheless, 126 underwent genetic counseling, resulting in 8 women with pathogenic BRCA1/2 variants, who otherwise may not have been identified in a timely manner. In addition, we identified women (67% ) who were advised to enhance their breast cancer risk management practices with the addition of MRI and/or chemoprevention. We also demonstrated the feasibility of offering genetic counseling on-site at a BIC; compared to our initial pilot implementation project, uptake of genetic counseling improved. Critical to the success of this project was the development and implementation of a streamlined, self-administered screening tool that was highly feasible for patients to complete and for providers to readily interpret, and that was designed to be adaptable to changes in NCCN guidelines and recommendations. Without our intervention, these women would not have been identified and would not have qualified for improved risk management strategies, thus showing the urgent need for such interventions among the general population.
Despite available guidelines for risk management and the therapeutic implications for BRCA1/2 mutation carriers, most individuals potentially at risk of carrying the mutation are not referred for genetic counseling and testing. US Preventive Services Task Force guidelines7 were recently released recommending screening for BRCA1/2 mutations not only in oncology but also in primary care. In women who test positive, undergoing risk-reductive surgeries could reduce breast and ovarian cancer risk by more than 90%. Furthermore, identification of a BRCA1/2 gene mutation would lead to predictive (cascade) testing of family members, which would further identify high-risk individuals who would be eligible for screening and risk-reductive interventions. This would result in the reduction of breast and ovarian cancer incidence in these families25.
Our streamlined screening tool identified 4% of patients at a BIC as possible candidates for hereditary breast and ovarian cancer risk assessment. Other studies have reported identification rates ranging from 1.9% to 9% using various settings, cohorts, and study methods9, 15, 19. Two studies used either retrospective chart review or identification of the cohort from a mammogram database20, 26. In several studies, tablets were given to patients to fill out, and the risk was then calculated using various models, including Tyrer-Cuzick, Myriad, and BRCAPro17, 27
Our study prospectively evaluated patients who were flagged by the screening tool and correlated these findings with the genetic counseling results. We found that only 15% of patients we identified as high-risk had received prior genetic counseling or testing. This is consistent with the recent results by Childers et al.8, who found in pooled data from United States national databases that only 15.1% of patients with a history of ovarian cancer had discussed genetic testing with their physician, and only 10.5% underwent testing. For breast cancer, 35.6% met one or more eligibility criteria, but of those, only 29% discussed testing with their physician, and only 15.3% underwent testing. They estimated that 1.2 to 1.3 million women in the United States qualified for testing but did not receive it. This estimate does not include the number of patients who qualify for testing based on family history alone.
Of the 142 patients in our cohort who received counseling, 126 (89%) qualified for genetic testing. These results are similar to those reported by Ricker et al. (62.5%) and Woodson et al. (87%); both of these studies used genetic counseling clinic results from primarily oncology referrals, indicating that the interest and perceived value of testing in this population are similar28, 29. Genetic counseling results in our study showed that 8% of tested patients were positive for BRCA1/2 and 9% had a variant of unknown significance. Woodson et al.29 reported that 8.4% of patients referred from oncology clinics at a community hospital tested positive for BRCA mutations Whitworth et al30 reported a deleterious mutation rate of 3.8%. Three of our eight patients who tested positive for BRCA1/2 had a known mutation in their family. This is somewhat surprising, indicating that risk model assessments that do not ask about gene mutations in the family will miss many extremely high-risk patients. It is unclear why these patients with a known BRCA1/2-positive relative did not undergo testing prior to the BIC screening. This may be due to a lack of education, counseling barriers, cost barriers, or a combination of these factors.
We also found that our program’s genetic counseling component changed clinical risk and screening management recommendations in more than two-thirds of the patients who underwent genetic counseling; these patients qualified for breast MRI screening (in addition to mammograms) and/or chemoprevention. This clearly shows that our family history questionnaire tool is not limited to identifying individuals with a hereditary mutation but also has a broader application amongst the general population in identifying individuals at an increased risk for breast cancer and leading to better risk management of these individuals.
There are limitations to our study; we had limited demographic data and the community based private practice setting did not have an infrastructure to track MRI screening in patients that were identified as high risk via this tool; this study was not designed and therefore does not have reasons on why non-adherent patients did not schedule or attend genetic counseling sessions; we don’t know what the non-adherence rate would have been without the patient navigator and in uninsured or Medicaid populations. Another important limitation is that women age less than 40 years will not be identified with this tool as they are not evaluated by mammogram routinely.
Our findings also raise important questions beyond the current study’s scope, primarily relating to alternative service delivery models that may help improve efficiency and patient follow-up, such as telegenetics. Only 23% of eligible patients in our study made genetic counseling appointments. Amongst them, there was a 42% no-show rate, which may be related to several real and perceived barriers to care that requires further evaluation of barriers to genetic counseling.
In summary, our study demonstrated that it is feasible to implement a simple NCCN-based tool to screen a large number of patients to identify women eligible for genetic counseling and potential BRCA1/2 testing. In our screening cohort, 4% of women were found to be eligible for genetic counseling who would otherwise have been missed, and 67% of women without a personal history of breast or ovarian cancer who underwent genetic counseling received improved clinical risk management. Therefore, we propose that this tool be used in community BICs to successfully identify individuals eligible for genetic counseling and testing. The availability and increasing use of telegenetics would make this approach even more practical and implementable at other primary care centers, which currently lack genetic counseling resources. Further studies could evaluate this approach in other clinical care settings, such as primary care clinics, OB/GYN offices, and/or internal medicine practices.
Supplementary Material
Funding disclosure:
Supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Footnotes
Conflict of Interest disclosure: None declared
REFERENCES
- 1.Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. November 2000;83(10):1301–8. doi: 10.1054/bjoc.2000.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. December 6 2006;98(23):1694–706. doi: 10.1093/jnci/djj465 [DOI] [PubMed] [Google Scholar]
- 3.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. June 20 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 4.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. May 2003;72(5):1117–30. doi: 10.1086/375033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood ME, Kadlubek P, Pham TH, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. March 10 2014;32(8):824–9. doi: 10.1200/JCO.2013.51.4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke W, Culver J, Pinsky L, et al. Genetic assessment of breast cancer risk in primary care practice. Am J Med Genet A. March 2009;149A(3):349–56. doi: 10.1002/ajmg.a.32643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Force USPST, Owens DK, Davidson KW, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. August 20 2019;322(7):652–665. doi: 10.1001/jama.2019.10987 [DOI] [PubMed] [Google Scholar]
- 8.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J Clin Oncol. December 1 2017;35(34):3800–3806. doi: 10.1200/JCO.2017.73.6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morman NA, Byrne L, Collins C, Reynolds K, Bell JG. Breast Cancer Risk Assessment at the Time of Screening Mammography: Perceptions and Clinical Management Outcomes for Women at High Risk. J Genet Couns. August 2017;26(4):776–784. doi: 10.1007/s10897-016-0050-y [DOI] [PubMed] [Google Scholar]
- 10.Stuckey A, Febbraro T, Laprise J, Wilbur JS, Lopes V, Robison K. Adherence Patterns to National Comprehensive Cancer Network Guidelines for Referral of Women With Breast Cancer to Genetics Professionals. Am J Clin Oncol. August 2016;39(4):363–7. doi: 10.1097/COC.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 11.Narayan A, Fischer A, Zhang Z, Woods R, Morris E, Harvey S. Nationwide cross-sectional adherence to mammography screening guidelines: national behavioral risk factor surveillance system survey results. Breast Cancer Res Treat. August 2017;164(3):719–725. doi: 10.1007/s10549-017-4286-5 [DOI] [PubMed] [Google Scholar]
- 12.Sabatino SA, McCarthy EP, Phillips RS, Burns RB. Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev. 2007;31(5):375–83. doi: 10.1016/j.cdp.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Sweet KM, Bradley TL, Westman JA. Identification and referral of families at high risk for cancer susceptibility. J Clin Oncol. January 15 2002;20(2):528–37. doi: 10.1200/JCO.2002.20.2.528 [DOI] [PubMed] [Google Scholar]
- 14.Tyler CV Jr., Snyder CW. Cancer risk assessment: examining the family physician’s role. J Am Board Fam Med. Sep-Oct 2006;19(5):468–77. doi: 10.3122/jabfm.19.5.468 [DOI] [PubMed] [Google Scholar]
- 15.Bellcross CA, Lemke AA, Pape LS, Tess AL, Meisner LT. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. November 2009;11(11):783–9. doi: 10.1097/GIM.0b013e3181b9b04a [DOI] [PubMed] [Google Scholar]
- 16.Brinton JT, Barke LD, Freivogel ME, Jackson S, O’Donnell CI, Glueck DH. Breast cancer risk assessment in 64,659 women at a single high-volume mammography clinic. Acad Radiol. January 2012;19(1):95–9. doi: 10.1016/j.acra.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozanne EM, Loberg A, Hughes S, et al. Identification and management of women at high risk for hereditary breast/ovarian cancer syndrome. Breast J. Mar-Apr 2009;15(2):155–62. doi: 10.1111/j.1524-4741.2009.00690.x [DOI] [PubMed] [Google Scholar]
- 18.Dominguez FJ, Jones JL, Zabicki K, et al. Prevalence of hereditary breast/ovarian carcinoma risk in patients with a personal history of breast or ovarian carcinoma in a mammography population. Cancer. November 1 2005;104(9):1849–53. doi: 10.1002/cncr.21393 [DOI] [PubMed] [Google Scholar]
- 19.Ray D, Grumet S, Lagmay-Fuentes P, et al. Short-term outcomes of the implementation of a computer-based breast cancer risk assessment program during screening mammography. J Community Support Oncol. June 2014;12(6):209–11. doi: 10.12788/jcso.0050 [DOI] [PubMed] [Google Scholar]
- 20.Drescher CW, Beatty JD, Resta R, et al. The effect of referral for genetic counseling on genetic testing and surgical prevention in women at high risk for ovarian cancer: Results from a randomized controlled trial. Cancer. November 15 2016;122(22):3509–3518. doi: 10.1002/cncr.30190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCN. National Comprehensive Cancer Network: Genetic/familial high-risk assessment: Breast and Ovarian. . 2017;Version2.2012-1.2017 [Google Scholar]
- 22.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. December 20 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879 [DOI] [PubMed] [Google Scholar]
- 23.Berry DA, Iversen ES Jr., Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. June 1 2002;20(11):2701–12. doi: 10.1200/JCO.2002.05.121 [DOI] [PubMed] [Google Scholar]
- 24.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. April 15 2004;23(7):1111–30. doi: 10.1002/sim.1668 [DOI] [PubMed] [Google Scholar]
- 25.Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol. September 20 2010;28(27):4214–20. doi: 10.1200/JCO.2010.28.0719 [DOI] [PubMed] [Google Scholar]
- 26.Jones JL, Hughes KS, Kopans DB, et al. Evaluation of hereditary risk in a mammography population. Clin Breast Cancer. April 2005;6(1):38–44. doi: 10.3816/CBC.2005.n.007 [DOI] [PubMed] [Google Scholar]
- 27.McDonnell CH, Seidenwurm DJ, McDonnell DE, Bobolis KA. Self administered screening for hereditary cancers in conjunction with mammography and ultrasound. Fam Cancer. December 2013;12(4):651–6. doi: 10.1007/s10689-013-9641-z [DOI] [PubMed] [Google Scholar]
- 28.Ricker C, Lagos V, Feldman N, et al. If we build it … will they come?--establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns. December 2006;15(6):505–14. doi: 10.1007/s10897-006-9052-5 [DOI] [PubMed] [Google Scholar]
- 29.Woodson AH, Profato JL, Park M, et al. Service Delivery Model and Experiences in a Cancer Genetics Clinic for an Underserved Population. J Health Care Poor Underserved. August 2015;26(3):784–91. doi: 10.1353/hpu.2015.0090 [DOI] [PubMed] [Google Scholar]
- 30.Whitworth P, Beitsch P, Arnell C, et al. Impact of Payer Constraints on Access to Genetic Testing. J Oncol Pract. January 2017;13(1):e47–e56. doi: 10.1200/JOP.2016.013581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


