Abstract
PURPOSE:
To examine the shape of the relationship between physical activity (PA) and total energy expenditure (TEE) and to explore the role of energy balance status (negative, stable, positive) in influencing this association.
METHODS:
Cross-sectional. Participants were 584 older adults (50–74 yrs.) participating in the Interactive Diet and Activity Tracking in AARP study. TEE was assessed by doubly labeled water and PA by accelerometer. The relationship between PA and TEE was assessed visually and using non-linear methods (restricted cubic splines). Percent weight change (>3%) over a six-month period was used as a proxy measurement of energy balance status.
RESULTS:
TEE generally increased with increasing deciles of PA averaging 2354 (SD=351) kcal/d in the bottom decile to 2693 (SD=480) kcal/d in the top decile. Cubic spline models showed an approximate linear association between PA and TEE (linear relation p<0.0001, curvature p=0.920). Results were similar in subgroup analyses for individuals classified as stable or positive energy balance. For those in negative energy balance, TEE was generally flat with increasing deciles of PA averaging 2428 (SD=285) kcal/d in the bottom decile to 2372 (SD=560) kcal/d in the top decile.
CONCLUSION:
Energy balance status appears to play an important role in the relationship between PA and TEE. When in a positive energy balance, the relationship between TEE and PA was consistent with an additive model, however, when energy balance was negative, TEE appears to be consistent with a constrained model. These findings support PA for weight gain prevention by increasing TEE; however, the effect of PA on TEE during periods of weight loss may be limited. An adequately powered, prospective study is warranted to confirm these exploratory findings.
Keywords: physical activity, doubly labeled water, accelerometer, energy balance, total energy expenditure
INTRODUCTION
Over the past three decades the prevalence of obesity has increased by more than two-fold (1) and has been recognized as a key threat to public health (2). It is well established that obesity increases the risk for many chronic diseases, such as heart disease, hypertension, diabetes, (3, 4) and certain types of cancer (5, 6). Current obesity prevention and treatment strategies promote increasing physical activity to achieve clinically meaningful weight loss (≥3–5%; (7)). It is assumed that increased physical activity will lead to associated increases in total energy expenditure (TEE); thereby promoting a negative energy balance and subsequent weight loss. It was previously believed that increases in physical activity would be tightly correlated with increases in TEE (8, 9); however, recent evidence would suggest that TEE is constrained at high levels of physical activity as the body adjusts to maintain TEE through both behavioral and metabolic adaptations (10).
The “Constrained Energy Expenditure Model” suggests that TEE increases linearly with lower amounts of physical activity but plateaus with higher amounts of physical activity (10) and this has been observed in both animals (11–14) and young adults (24–45yrs.; (15)). However, the mechanisms for energy constraint are unclear. One possible explanation is energy intake or energy balance status. Energy intake and expenditure are interdependent variables that are dynamically influenced by each other and body weight (16). Attempts to alter energy balance through diet or exercise are countered by physiological adaptations that resist weight loss (17). Thus, during a period of negative energy balance, it is plausible that the body conserves energy even during periods of increased activity. During a period of positive energy balance, typical of western populations, this energy conservation would be unnecessary in the presence of increased physical activity; thus, physical activity and TEE would be more congruent.
In the current study we leveraged data from the Interactive Diet and Activity Tracking in AARP (iDATA) study to examine the shape of the relationship between physical activity, TEE, and energy balance status (negative, stable, positive) in a large sample of free-living older adult men and women. We hypothesized that the constrained energy expenditure model would be observed in adults who were in negative energy balance but not adults in positive or stable energy balance.
METHODS
Participants and Study Design.
Participants were a convenience sample of AARP members from Pittsburgh, PA aged 50–74 years, who spoke English, had internet access, were not on a weight-loss diet, had a BMI 18.5–40 kg/m2, and were free of major medical conditions (e.g., history of renal failure, congestive heart failure, or other conditions involving disturbances in fluid balance) and mobility limitations. Initial contact was made via automated phone call and invitation letter directing those interested to contact study staff. Prior to any research procedures participants provided written informed consent. The study was approved by the National Cancer Institute Special Studies Institutional Review Board. Consented participants visited the study center up to three times over 12-months and completed several diet and physical activity measurements. Individuals who completed the full study received $450. The primary aim of iDATA study was to evaluate how well internet-based, self-report instruments measure food intake and physical activity levels and their relationship with disease (18).
Of the 1,082 individuals who enrolled in the study (see Supplemental Figure 1, Supplemental Digital Content, Appendix), 741 were scheduled to complete DLW assessments. Of these, we excluded those with invalid DLW values (n=54), participants without at least one valid day of accelerometer data (n=45), those missing anthropometric data (n=39), and those with extreme TEE or physical activity [vertical axis CPM/d and vector magnitude CPM/d] values (i.e., twice the interquartile range; n=19).
Participants were assigned to one of four groups and followed a specified 12-month assessment protocol. Groups 1 and 3 followed the same assessment schedule while Groups 2 and 4 followed an alternative schedule (Table 1).
Table 1.
Assessment Timeline
| Month-1 | Month-6 | Month-12 | |
|---|---|---|---|
| Recruitment Groups 1 and 3 | |||
| Anthropometrics | X* | X* | X |
| Physical Activity | |||
| Actigraph | X* | X | |
| Total Daily Energy Expenditure | |||
| Doubly Labeled Water | X* | ||
| Recruitment Groups 2 and 4 | |||
| Anthropometrics | X | X* | X* |
| Physical Activity | |||
| Actigraph | X* | X | |
| Total Daily Energy Expenditure | |||
| Doubly Labeled Water | X* |
Measures used in current investigation
TEE and Body Composition.
TEE was assessed via doubly labeled water over a 14-day period using methods previously described by Subar et. al (19). Baseline urine specimens were collected from each participant prior to oral dosing with a mixed solution of deuterium and oxygen-18 based on body weight. Three additional urine samples were collected on the dosing day and two final urine samples were collected 14 days after the dosing day. The deuterium is eliminated from the body as water and oxygen-18 as both water and carbon dioxide. The difference between the elimination rates provides a measure of carbon dioxide production which can be used to calculate TEE. Total body water, determined by stable isotope dilution (20, 21), was used to derive fat free mass [FFM(kg)=TBW/0.732)] (22) and subsequently fat mass [FM(kg)=body weight (kg)−FFM (kg)].
Physical activity.
Physical activity was assessed by ActiGraph GT3X tri-axial accelerometers (Actigraph LLC., Pensacola, FL, U.S.A.). Participants were asked to wear the ActiGraph GT3X monitors all day on an elastic belt on their waist, for seven consecutive days, and to remove the monitors when showering, bathing, and swimming and for bed at night. The data collection interval was set at one second epoch with a minimum of 10 hours of wear constituting a valid monitored day. One valid day was required to be included in the analysis (23). Monitor wear logs were used to confirm wear dates and define the waking day, and the Choi algorithm was used to estimate non-wear time during this period. The vertical axis total activity counts per day (TAC/d) variable was defined as the mean daily activity counts accumulated on valid monitoring days. Mean activity counts per minute (CPM/d) were also computed for the vertical axis and as vector magnitude. CPM/d has been shown to be a valid proxy for total physical activity volume (24). Total physical activity (minutes/day) was also calculated as mean acceleration counts per minutes >100 during wear time. A mean of approximately 6 (SD = 1.6) valid days (90.2% of sample with ≥4 valid days) of accelerometer data with 14 (SD = 1.3) hours per day of wear time were available.
Anthropometrics.
Anthropometric measures (weight and standing height) aligning with physical activity and TEE assessments and the subsequent 6-month follow-up measures were used. Up to three measurements were taken and the average of the two closest repeated readings at each visit were recorded. Body mass index (BMI) was calculated from height and weight (kg/m2).
STATISTICAL ANALYSIS
All analyses were performed using SAS Software, version 9.4 (SAS Institute Inc., Cary, NC) and figures were created using GraphPad Prism, version 8.4.3 (GraphPad Software Inc., San Diego, CA). Participant characteristics were summarized using means and standard deviations for continuous variables, and frequencies and percentages for categorical variables.
This statistical approach was designed to replicate the strategy used by Pontzer and colleagues (15). We first examined the relationship of wear time and recruitment group on accelerometer-measured activity counts using linear regression and found significant associations (data not shown). To control the effect of these covariates, residual adjusted activity counts were calculated for each subject by adding the residuals from a regression model to mean activity counts (TAC/d and CPM/d). Adjusted activity counts were used for all analyses. Accelerometer-derived TAC/d was classified into age- and sex-specific quartiles of US population-referenced TAC/d.; population-referenced TAC/d percentiles standardized by age and sex were published in 2015 (25). To determine how our sample’s physical activity level compared to US population younger age groups, TAC/d were matched to ages 20, 30, and 40 yr. old sex-specific quartiles of the US population-referenced TAC/d.
Similar to accelerometer data, linear regression was used to examine the relationship of fat mass, fat-free mass, height, age, sex, race/ethnicity, and recruitment group on TEE. To control for the effect of these covariates, residual adjusted TEE was calculated for each subject by adding the residuals from the regression model to mean TEE. Adjusted TEE (TEEADJ) was used for all analyses. Controlling for these variables is necessary in order to examine the effect of physical activity, measured via accelerometry as mean CPM/d, without spurious effects of covariates. Using resting energy expenditure estimated from the Mifflin St-Jeor equation (REEMifflin) (26), physical activity level (PAL) was calculated as TEEadj divided by REEMifflin.
Initially, the linearity of the relation between physical activity and TEE was visually examined by boxplots indicating medians and quartiles of TEEADJ for each decile of CPM/d. To assess the shape of the relationship between physical activity and TEEADJ, we fit three different regression models to a scatterplot of TEEADJ against CPM/d. First, a linear regression model was fit to test the relationship between TEEADJ and physical activity. Second, restricted cubic spline curves were fit to test for non-linearity of these associations using the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms, SAS macro “glmcurv9” (27). Splines ranging from 3 to 10 knots were examined. Visual inspection was used to ensure results were robust, regardless of the number of knots employed. Analyses with knots >5 produced high levels noise, therefore as recommended Durrleman et. al (28), the results from 5 knots (5th 25th 50th 75th 95th percentiles) models are presented. Third, we evaluated the effect of CPM/d on TEEADJ via a series of linear regression analysis for all subjects with CPM/d values above a threshold CPM/d = i and iterated this analysis over the range of CPM/d thresholds i = (1, 2, 3….i). This simply means that we conducted several linear regressions with subsamples above a CPM/d threshold of our original full sample. The resulting set of β and SE values for each subsample regression were plotted by CPM thresholds. Subgroup analysis was conducted to assess the role of energy balance status on the shape of the relationship between physical activity and TEEADJ. Percent weight change over the six-month period following the doubly labeled water assessment was calculated as a proxy measurement of energy balance status and individuals were classified using AHA/ACC/TOS Guidelines for clinically meaningful weight change into positive energy balance (> +3%), stable energy balance (±3%), or negative energy balance (< −3%) categories and relationship of physical activity to TEEADJ was assessed for each subgroup as previously described (29).
RESULTS
Descriptive characteristics of the 584 participants with valid data that were included in this analysis are presented in Table 2. Compared to the full eligible sample, the analytic sample was similar on reported demographic characteristics (see Supplemental Table 1, Supplemental Digital Content, Appendix). The analytic sample was 63.2 ± 5.9 years old, 51 percent female, and 93 percent non-Hispanic white. Seventy-three percent of the sample were classified with overweight or obesity (BMI ≥ 25.0 kg/m2) with 16%, 68%, 16% classified as positive, stable, or negative energy balance status; respectively. Calculated PAL (TEEadj – REE as calculated using Mifflin et al.) ranged from 1.0 to 2.9 times resting energy expenditure predicted. Participants were more likely to be physically active compared to the general US population, with ~36% in the highest US population-reference TAC/d quartiles for activity (i.e., ≥ 75th percentile) and 11% in the lowest quartile (i.e., ≤ 25th percentile). Even when compared to 20 yr. old US population-reference TAC/d quartiles for activity, ~12% of participants remained in the highest quartile (see Supplemental Figure 2, Supplemental Digital Content, Appendix).
Table 2.
Sample Characteristics
| Analytic Sample (n=584) |
||
|---|---|---|
| N | % | |
| Female (%) | 297 | 50.9 |
| Non-Hispanic White (%) | 545 | 93.3 |
| Mean | SD | |
| Age (yrs.) | 63.2 | 5.9 |
| BMI (kg/m2) | 28.1 | 4.7 |
| Height (cm) | ||
| Weight Change (%) | −0.2 | 3.9 |
| Fat Mass (kg) | 30.6 | 9.6 |
| Fat Free Mass (kg) | 50.5 | 10.7 |
| TEE (kcal/d) | 2446 | 479 |
| PAL (TEEadj/REEmifflin) | 1.7 | 0.3 |
| Vertical Axis (TAC/d) | 254200 | 93186 |
| Vertical Axis (CPM/d) | 300.0 | 110.2 |
| Vector Magnitude (CPM/d) | 751.0 | 216.8 |
Note: SD = standard deviations; yrs. = years; cm = centimeters; BMI = body mass index (kg/m2); kg = kilogram; m= meter; TEE = total energy expenditure; adj = adjusted; kcal = kilocalories; d = day; PAL = physical activity level; REEmifflin = resting energy expenditure estimated by Mifflin St-Jeor equation; TAC = total activity counts; CPM = counts per minute
To be consistent with previously published literature regarding constrained TEE, the main analysis presented throughout uses vertical axis CPM/d. Results were similar to those of the main analysis when assessing physical activity using vector magnitude CPM/d and total physical activity minutes/day (see Supplemental Tables 2–3 and Supplemental Figures 3–4, Supplemental Digital Content, Appendix).
Anthropometric measurements explained over 60% of the variation in TEE (df = 577, adjusted r2 [adj r2] = 0.64, p <0.0001; Table 3, model 1), with fat-free mass the strongest single factor. Adding recruitment group to account for variation in assessment schedules did not improve the fit (df = 574, adj r2 = 0.64, p <0.0001; Table 2, model 2). Measures of physical activity (vertical axis CPM/d) accounted for an additional 4% of the variation in TEE (df = 573, adj r2 = 0.68, p <0.0001; Table 3, model 3). To examine the effects of physical activity on TEE, we calculated adjusted TEE (TEEADJ) from the residuals of model 2 in Table 1, thereby controlling for the effects of fat-free mass, fat mass, age, height, sex, and recruitment group on TEE. Physical activity (vertical axis CPM/d) explained 8% of the variation in TEEADJ (df = 582, adj r2 = 0.08, p <0.0001; Table 3, model 4).
Table 3.
Model parameters for multivariate analyses of TEE and TEEADJ
| TEE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 Adj R2 0.64 |
Model 2a Adj R2 0.64 |
Model 3 Adj R2 0.68 |
|||||||
| Variable | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| (intercept) | 1014.8 | 301.9 | <0.001 | 1083.3 | 305.5 | <0.001 | 506.2 | 295.3 | 0.087 |
| FFM (kg) | 39.8 | 2.4 | <0.0001 | 40.1 | 2.5 | <.0001 | 42.2 | 2.3 | <.0001 |
| Fat Mass (kg) | −2.7 | 1.6 | 0.0849 | −2.8 | 1.6 | 0.083 | −1.2 | 1.5 | 0.442 |
| Height (cm) | −1.6 | 1.7 | 0.3418 | −1.8 | 1.7 | 0.289 | −2.0 | 1.6 | 0.212 |
| Age (yrs.) | −5.5 | 2.1 | 0.0093 | −5.7 | 2.1 | 0.008 | −2.4 | 2.0 | 0.249 |
| Sex (1 = M, 0 = F) | −59.5 | 48.5 | 0.2205 | −57.9 | 48.7 | 0.234 | −110.3 | 46.2 | 0.017 |
| Race (1 = Non-Hispanic White, 0 = other) | 171.2 | 48.7 | <0.001 | 167.9 | 48.8 | <0.001 | 163.9 | 45.9 | <0.001 |
| Recruitment group: 1 | - | - | - | - | - | - | |||
| Recruitment group: 2 | −32.5 | 39.8 | 0.415 | −39.9 | 37.5 | 0.288 | |||
| Recruitment group: 3 | −54.0 | 36.7 | 0.142 | −59.6 | 34.6 | 0.085 | |||
| Recruitment group: 4 | −53.2 | 36.0 | 0.140 | −51.2 | 33.9 | 0.132 | |||
| Vertical Axis (CPM/d) | 0.9 | 0.1 | <.0001 | ||||||
| TEEADJ | |||||||||
| Model 4 Adj R2 0.08 |
|||||||||
| Variable | β | SE | p-value | ||||||
| (intercept) | 2136.0 | 46.8 | <.0001 | ||||||
| Vertical Axis (CPM/d) | 1.0 | 0.1 | <.0001 | ||||||
residuals from Model 2 used to calculated TEEADJ.
TEE = total energy expenditure; adj = adjusted; SE = standard error; FFM = fat free mass; kg = kilogram; cm = centimeters; yrs. = years; M = male; F = female; CPM = counts per minute; d = day
TEEADJ was shown to generally increase with increasing deciles of physical activity (CPM/d) averaging 2,354 (SD = 351) kcal/d in the bottom decile to 2,693 (SD = 480) kcal/d in the top decile (Figure 1A). Yet, the variability of TEEADJ within each decile of CPM/d was high. The range of variation within any decile of CPM/d far exceeded the difference in median TEEADJ across the range of CPM/d (see Supplemental Table 3, Supplemental Digital Content, Appendix). The mean coefficient of variation within CPM/d deciles (16% [SD = 0.9%]) was equivalent to the difference in mean TEEADJ between the 1st and 10th deciles (16%).
Figure 1.

The Relationship between TEE and Physical Activity. (A) TEEADJ (kcal/d) and physical activity (CPM/d). Boxplots indicate medians, quartiles, and range of TEEADJ for each decile of CPM/d and are centered on the median CPM/d value for each decile. Restricted cubic splines (dash) and linear (solid) regression lines are shown. (B) Series of linear regression analyses for the association of CPM/d with TEEADJ for subjects above increasing CPM/d thresholds. Black dots show the β value for CPM/d for subjects above a given CPM/d threshold; grey bars represent ±SE.
Restricted cubic spline analysis showed a linear association between physical activity and TEEADJ for the entire analytic sample (p for linear relation <0.0001, p for curvature = 0.920; Figure 1A). Figure 1B shows the effect (β) of CPM/d on TEEADJ at increasing CPM/d thresholds. These results were consistent with the restricted cubic spline analysis, showing a significant association between CPM/d and TEE across increasing CPM/d thresholds (all p < 0.05) with an upward inflection in TEE at higher activity levels. Based on statistical methods and visual inspection, results were similar to the entire analytic sample for two energy balance subgroups: stable energy balance (p for linear relation <0.0001, p for curvature = 0.950; Figure 2B). and positive energy balance (p for linear relation <0.001, p for curvature = 0.542; Figure 2C). TEEADJ was shown to generally increase with increasing deciles of physical activity (CPM/d) averaging 2,346 (SD = 346) kcal/d (stable energy balance) and 2,175 (SD = 408) kcal/d (positive energy balance) in the bottom decile to 2,776 (SD = 376) kcal/d (stable energy balance) and 2,880 (SD = 511) kcal/d (positive energy balance) in the top decile (Figure 2B–C). However, in the negative energy balance subgroup, physical activity (CPM/d) was not associated with TEEADJ (p for linear relation = 0.851, p for curvature = 0.724; Figure 2A). In this subgroup, TEEADJ was shown to be generally flat with increasing deciles of physical activity (CPM/d) averaging 2,428 (SD = 285) kcal/d in the bottom decile to 2,372 (SD = 560) kcal/d in the top decile (Figure 2A).
Figure 2.
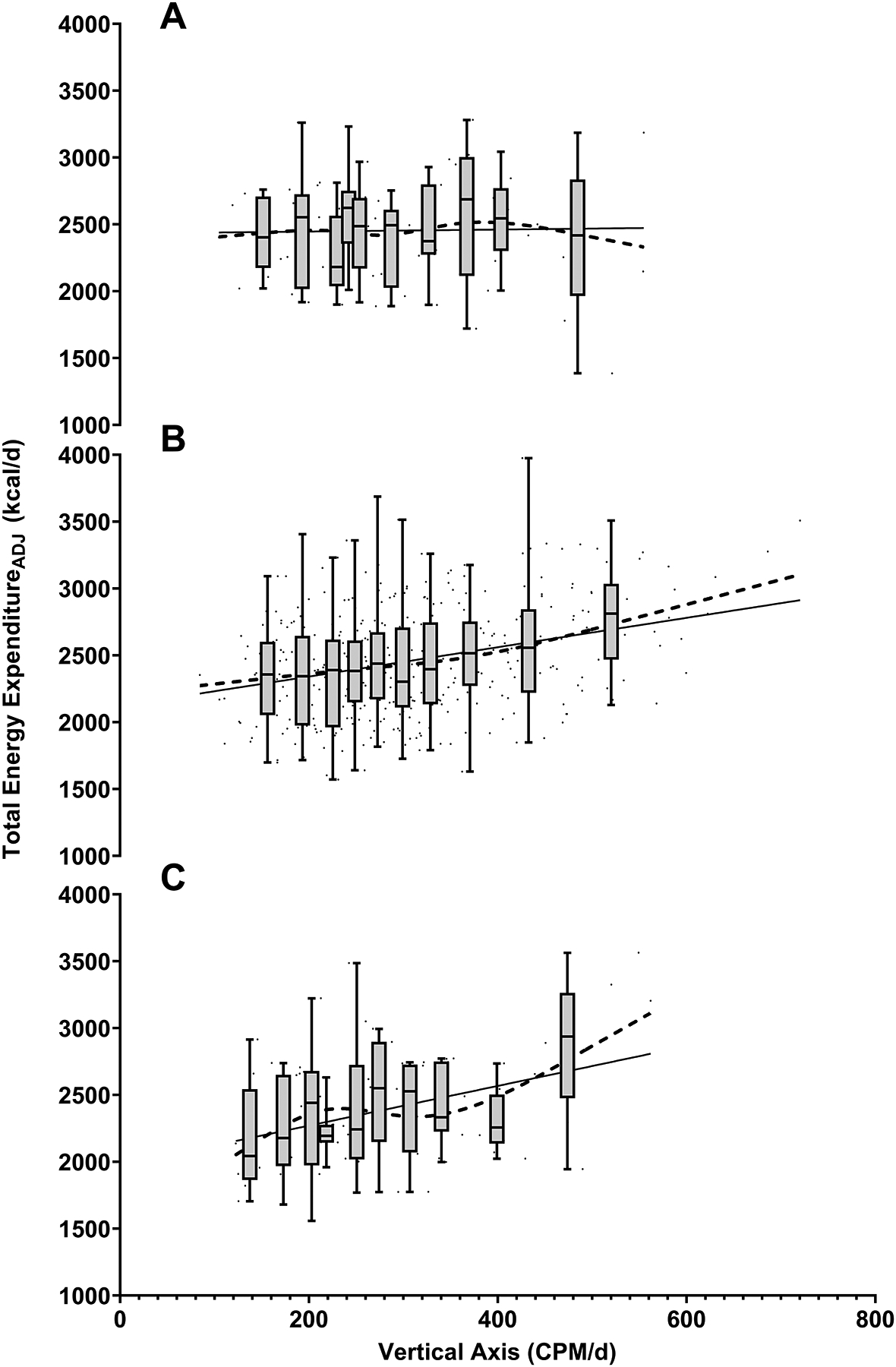
The Relationship between TEE and Physical Activity by energy balance status (A = negative, B = stable, C = positive). TEEADJ (kcal/d) and physical activity (CPM/d). Boxplots indicate medians, quartiles, and range of TEEADJ for each decile of CPM/d and are centered on the median CPM/d value for each decile. Restricted cubic splines (dash) and linear (solid) regression lines are shown.
DISCUSSION
The acute metabolic cost of physical activity is unquestionable; however, the impact of habitual levels of physical activity on TEE is less clear. The consensus view that increased physical activity leads to concomitant additive increases in TEE has been challenged (15, 30). The Constrained Energy Expenditure model has been proposed as an alternative explanation to the previous Additive model. The Constrained model suggests that physical activity and TEE display a linear relationship at lower levels of physical activity; however, as physical activity increases above a certain threshold, TEE plateaus, thereby conserving energy. The mechanisms for the Constrained model remain unclear; however, several hypotheses have been postulated including behavioral adaptations (e.g., increased sitting behavior and reduced fidgeting), and physiologic adaptations (increased muscular efficiency and decreased metabolic rates of non-muscular tissues). The present study contributes to this growing area of research by testing both Additive and Constrained models in a large western population. In addition, this study sought to determine the impact of energy balance status on these models. The primary findings of this study are that in a cohort of older adults, with a broad range of physical activity levels, it appears that the relationship between physical activity and TEE is additive. However, energy balance status appears to be an important factor in this relationship, with individuals in positive and stable energy balance aligning with the Additive model and individuals in negative energy balance exhibiting evidence of constrained TEE.
In 2015, Pontzer et al. proposed that the relationship between physical activity and TEE is constrained (31). This model was supported by both animal data and a pooled sample of adults living in five populations of African descent (Ghana, South Africa, Seychelles, Jamaica, and United States; (15)). In that study of adult humans, data demonstrated a breakpoint at 230 Actical-based CPM/d (~70th percentile), where higher physical activity below this breakpoint was associated with proportionally higher TEE, but physical activity levels above this breakpoint were not associated with further elevated TEE. Consistent with that study, we found that accelerometer-measured physical activity was weakly associated with TEE, and that the mean TEE difference between the lowest and highest physical activity deciles (~340 kcal/d) was substantially less than the variance in TEE within each decile (Figure 1). However, we failed to identify a similar physical activity threshold whereby the relationship between physical activity and TEE was nullified. In fact, in the full cohort analysis, there was an upward inflection at ~400 CPM/d suggesting that at higher levels of physical activity, TEE was associated more strongly than at lower levels of physical activity. While the additive effect of physical activity on TEE appears to be small compared to the other factors examined, the relationship appears to be relatively linear in this population of older US adults.
Differences in local environments, age of individuals recruited, and differences in devices employed makes direct comparison between studies difficult. It is possible that the US based older adult population of the current report, as a whole, are less active than the younger populations of African descent observed by Pontzer and colleagues. If this is the case, physical activity levels presented here may not have been at a high enough level to elicit a constrained response. Differences in devices used to estimate physical activity counts (Actical vs Actigraph) impedes direct comparison in distribution of physical activity levels between the two studies. However, when comparing our sample to national estimates we found that, on average, older adults participating in the iDATA study were more physically active compared to age and sex-matched general population and this distribution of activity held when compared to younger US populations. Additionally, there was a large amount of variability in PAL using a predicted REE (TEEadj/REEmifflin), with levels ranging from 1.0 (extremely inactive) to 2.9 (extremely active) times estimated resting energy expenditure (32). Therefore, the wide spectrum of physical activity observed in the current investigation should have been sufficient to detect a plateau in TEE.
One plausible mechanism for TEE constraint is energy balance status or energy intake. During periods of negative energy balance, it is possible that the relationship between physical activity and TEE would be constrained, and during periods of positive energy balance the relationship would be additive. This hypothesis aligns with previous weight loss maintenance data, suggesting that individuals maintaining significant weight loss have a high amount of energy flux (high amounts of energy intake and energy expenditure; (33, 34)). Schoeller et al. (34) found that threshold of physical activity energy expenditure corresponding to 80 min/day of moderate activity or 35 min/d of vigorous activity was required for successful weight maintenance. Similarly, Ostendorf et al. (35) found that successful weight loss maintainers, defined as maintaining a weight loss of >30 lbs. for >1 year, engaged in high amounts of moderate-to-vigorous physical activity (~270 min/wk.). This resulted in high amounts of TEE, which were similar to their pre-weight loss TEE, suggesting that the higher amounts of physical activity associated with weight loss maintenance helps to increase TEE to promote energy balance without chronic restriction of caloric intake (36). This study also supports the notion that during times of positive and stable energy balance (84% of the current study sample) TEE is not constrained.
Our data does suggest that the relationship between physical activity and TEE during periods of negative energy balance may be constrained. These data align with the findings from rodent (14) and human studies (15, 37). Human studies reporting energy constraint have typically compared expenditures across populations with disparate lifestyles and diets (15, 37–40), and thus diet and energy balance status could play an important role in the energy constraint response. Indeed, Pontzer et al. speculated that energy balance may be an important determinant for TEE. In their analysis, individuals with higher amounts of percent body fat had higher adjusted TEE (15). From an evolutionary perspective, limiting energy expenditure during a period of negative energy balance, is an adaptive response to protect against starvation [24].
While the relationship between physical activity and TEE appears to be linear, the strength of association may be less than expected. Interestingly, there was a 70% difference in median physical activity CPM/d between the 1st and 10th deciles that equated to only a 17% difference in median TEEADJ. This observation of minimal increases in TEE can be explained if REE does display compensation for increased physical activity energy expenditure and is consistent with previous studies showing that only about half of the energy expended during exercise is added to TEE (41, 42). However, the suggestion that a negative energy balance reduces the metabolic response to increased physical activity conflicts with previous studies that have shown that exercise alone can produce clinically significant weight loss (≥3–5%) without purposeful energy intake restriction from pre-exercise conditions (43–46). If chronic negative energy balance constrains TEE, then weight loss from exercise should be minimal. Studies increasing physical activity modestly (<2000 kcal/wk.) have indeed found that weight loss is less than expected, in part due to lower energy expenditure than predicted (43). High amounts of physical activity (2000–5000 kcal/wk.) also result in less weight loss than predicted; however, mean weight loss is clinically meaningful (>5%) (44). It is possible that at very high levels of physical activity, energy expenditure eclipses the drive to maintain energy balance, resulting in weight loss. In fact, TEE can increase >5 fold basal levels during periods of high activity (e.g. marathon running, ultra-endurance racing, endurance bike racing), however, this level of energy expenditure is not sustainable for long periods of time (>100 days; (47)).
This study does not come without limitations. First, this was a secondary analysis of a convenience sample of generally healthy older adults living in Eastern US who enrolled in a diet and physical activity measurement study. Therefore, the study was not designed or powered to examine the relationship between physical activity and TEE and the results of this study may not be generalizable to other populations. Furthermore, doubly labeled water assessment was only collected at a single time point. Multiple measures over time are needed to better understand the temporal relationship between TEE and physical activity. In addition, this study did not have device measured resting metabolic rate; thus, it was impossible to examine individual components of TEE. This study also used 6-month weight change data subsequent to physical activity and TEE assessments to determine energy balance status. This weight status may not reflect what was happening prior to or during the TEE and physical activity assessments. Additionally, it is unclear whether weight gain/loss was intentional in this population; it is possible that unintentional weight change is indicative of underlying health conditions that may influence energy expenditure. Finally, participants were only required to have one valid day of physical activity data and the TEE assessment was over 14 days; it is possible that this misalignment and intra-individual variation in both measures weakened the observable associations. Despite these limitations, this study was performed in a large sample and utilized robust assessments of physical activity using vertical and vector magnitude-based activity counts, minutes per day, and TEE from DLW; thus, these findings offer a unique and significant contribution to the literature on Additive vs. Constrained energy expenditure models.
There remain several unanswered questions and priorities for future research. Foremost would be prospective studies to test the constrained TEE model. These studies would require careful repeated measurements of all components of TEE using DLW and indirect calorimetry, along with device measured physical activity. Additionally, these studies should include repeated measures of weight to assess the effect of energy balance status on metabolic responses to physical activity and collect biomarkers related to reproductive, somatic, and immunologic function (8). Furthermore, studies should also examine how the allocation of energy contributes to the high degree of inter-individual variability in the magnitude and direction of weight change observed in weight loss studies evaluating exercise alone. In addition, other mechanisms that may influence the relationship between physical activity, TEE, and weight change such as timing of exercise within the 24 hour day (48), appetite regulation (49), or substrate utilization (50) need to be explored.
In summary, this study observed that TEE was positively associated with physical activity across a range of physical activity levels in a cohort of older US adults, providing evidence consistent with an Additive model. In subgroup analyses, among individuals who were in a negative energy balance, TEE appeared to be relatively flat around 2500 kcal/d with no further increase at higher levels of physical activity. This finding supports a constrained TEE model or compensation effects in response to a period of negative energy balance in the context of western diets. However, this study has highlighted several limitations to the current evidence base and an adequately powered, prospective trial is necessary to extend this prior work.
Supplementary Material
ACKNOWLEDGMENTS
The authors’ responsibilities were as follows—EW, CM, PSM, HP: conceptualized the research; EW: analyzed data; EW and SC: drafted the manuscript; EW, SC, PSM, SK, HP, DS, RT, CM: interpreted the results and revised the manuscript; EW: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
GRANT SUPPORT:
Z99 CA999999/Intramural NIH HHS/United States
Footnotes
The authors have no financial conflicts of interest regarding the results of this research. Results of the present study does not constitute endorsement by ACSM. Results are presented clearly (as possible), honestly, and without fabrication/falsification or with overt data manipulation.
SUPPLEMENTAL DIGITAL CONTENT (SDC)
Appendix.
File name: Online Supporting Material.docx
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama-J Am Med Assoc. 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutangadura GB. World Health Report 2002: Reducing Risks, Promoting Healthy Life: World Health Organization, Geneva, 2002, 250 pages, US $13.50, ISBN 9-2415-6207-2 In: No longer published by Elsevier; 2004. [Google Scholar]
- 3.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. Journal of obesity. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Fox CS et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–12. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New Engl J Med. 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly JE, Blair SN, Jakicic JM et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. [DOI] [PubMed] [Google Scholar]
- 8.Melanson E. The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obesity Reviews. 2017;18:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FAO/WHO/UNU. Human energy requirements, in Food and Nutrition Technical Report Series 1. 2001:17–24.
- 10.Pontzer H Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exerc Sport Sci Rev. 2015;43(3):110–6. [DOI] [PubMed] [Google Scholar]
- 11.Perrigo G, Bronson FH. Foraging effort, food intake, fat deposition and puberty in female mice. Biol Reprod. 1983;29(2):455–63. [DOI] [PubMed] [Google Scholar]
- 12.Wiersma P, Verhulst S. Effects of intake rate on energy expenditure, somatic repair and reproduction of zebra finches. J Exp Biol. 2005;208(Pt 21):4091–8. [DOI] [PubMed] [Google Scholar]
- 13.Breeding Perrigo G. and feeding strategies in deer mice and house mice when females are challenged to work for their food. Animal Behaviour. 1987;35(5):1298–316. [Google Scholar]
- 14.O’Neal TJ, Friend DM, Guo J, Hall KD, Kravitz AV. Increases in Physical Activity Result in Diminishing Increments in Daily Energy Expenditure in Mice. Curr Biol. 2017;27(3):423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontzer H, Durazo-Arvizu R, Dugas LR et al. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr Biol. 2016;26(3):410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95(4):989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochner CN, Tsai AG, Kushner RF, Wadden TA. Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations. The Lancet Diabetes & Endocrinology. 2015;3(4):232–4. [DOI] [PubMed] [Google Scholar]
- 18.Matthews CE, Keadle SK, Moore SC et al. Measurement of Active and Sedentary Behavior in Context of Large Epidemiologic Studies. Medicine and science in sports and exercise. 2018; 50(2):266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subar AF, Kipnis V, Troiano RP et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. American journal of epidemiology. 2003;158(1):1–13. [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ, Coward WA. Precision and accuracy of doubly labeled water energy expenditure by multipoint and two-point methods. Am J Physiol. 1992;263(5 Pt 1):E965–73. [DOI] [PubMed] [Google Scholar]
- 21.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267(4 Pt 1):E585–90. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr. 1999;69(5):833–41. [DOI] [PubMed] [Google Scholar]
- 23.Wolff-Hughes DL, McClain JJ, Dodd KW, Berrigan D, Troiano RP. Number of accelerometer monitoring days needed for stable group-level estimates of activity. Physiological measurement. 2016;37(9):1447. [DOI] [PubMed] [Google Scholar]
- 24.Chomistek AK, Yuan C, Matthews CE et al. Physical activity assessment with the ActiGraph GT3X and doubly labeled water. Medicine and science in sports and exercise. 2017;49(9):1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff-Hughes DL, Fitzhugh EC, Bassett DR, Churilla JR. Waist-worn actigraphy: population-referenced percentiles for total activity counts in US adults. Journal of Physical Activity and Health. 2015;12(4):447–53. [DOI] [PubMed] [Google Scholar]
- 26.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 27.Hertzmark E, Li R, Hong B, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory. 2012. [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MD, Ryan DH, Apovian CM et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American college of cardiology. 2014;63(25 Part B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 30.Westerterp KR. Exercise, energy expenditure and energy balance, as measured with doubly labelled water. Proceedings of the Nutrition Society. 2018;77(1):4–10. [DOI] [PubMed] [Google Scholar]
- 31.Pontzer H Constrained total energy expenditure and the evolutionary biology of energy balance. Exercise and sport sciences reviews. 2015;43(3):110–6. [DOI] [PubMed] [Google Scholar]
- 32.University UN, Organization WH. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17–24 October 2001. Food & Agriculture Org.; 2004. [Google Scholar]
- 33.Melby CL, Paris HL, Sayer RD, Bell C, Hill JO. Increasing Energy Flux to Maintain Diet-Induced Weight Loss. Nutrients. 2019;11(10):2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? The American journal of clinical nutrition. 1997;66(3):551–6. [DOI] [PubMed] [Google Scholar]
- 35.Ostendorf DM, Lyden K, Pan Z et al. Objectively Measured Physical Activity and Sedentary Behavior in Successful Weight Loss Maintainers. Obesity (Silver Spring). 2018;26(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostendorf DM, Caldwell AE, Creasy SA et al. Physical Activity Energy Expenditure and Total Daily Energy Expenditure in Successful Weight Loss Maintainers. Obesity (Silver Spring). 2019;27(3):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urlacher SS, Snodgrass JJ, Dugas LR et al. Constraint and trade-offs regulate energy expenditure during childhood. Sci Adv. 2019;5(12):eaax1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pontzer H, Raichlen DA, Wood BM, Mabulla AZ, Racette SB, Marlowe FW. Hunter-gatherer energetics and human obesity. PLoS One. 2012;7(7):e40503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebersole KE, Dugas LR, Durazo-Arvizut RA et al. Energy expenditure and adiposity in Nigerian and African-American women. Obesity (Silver Spring). 2008;16(9):2148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dugas LR, Harders R, Merrill S et al. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. Am J Clin Nutr. 2011;93(2):427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews CE, Keadle SK, Saint-Maurice PF et al. Use of time and energy on exercise, prolonged TV viewing, and work days. American journal of preventive medicine. 2018;55(3):e61–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willis EA, Herrmann SD, Honas JJ, Lee J, Donnelly JE, Washburn RA. Nonexercise energy expenditure and physical activity in the Midwest Exercise Trial 2. Med Sci Sports Exerc. 2014;46(12):2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly JE, Hill JO, Jacobsen DJ et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163(11):1343–50. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly JE, Honas JJ, Smith BK et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity (Silver Spring). 2013;21(3):E219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond). 2008;32(1):177–84. [DOI] [PubMed] [Google Scholar]
- 46.Nordby P, Auerbach PL, Rosenkilde M et al. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity (Silver Spring). 2012;20(11):2202–12. [DOI] [PubMed] [Google Scholar]
- 47.Thurber C, Dugas LR, Ocobock C, Carlson B, Speakman JR, Pontzer H. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci Adv. 2019;5(6):eaaw0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willis EA, Creasy SA, Honas JJ, Melanson EL, Donnelly JE. The effects of exercise session timing on weight loss and components of energy balance: midwest exercise trial 2. Int J Obes (Lond). 2020;44(1):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fillon A, Mathieu M, Boirie Y, Thivel D. Appetite control and exercise: Does the timing of exercise play a role? Physiology & Behavior. 2020;218:112733. [DOI] [PubMed] [Google Scholar]
- 50.Vieira AF, Costa RR, Macedo RC, Coconcelli L, Kruel LF. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016;116(7):1153–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


