Abstract
Introduction
The Frequent Hemodialysis Network (FHN) Daily and Nocturnal trials aimed to compare the effects of hemodialysis (HD) given six versus three times per week. More frequent in-center HD significantly reduced left ventricular mass (LVM), with more pronounced effects in patients with low urine volumes. In this study, we aimed to explore another potential effect modifier: the predialysis serum sodium (SNa) and related proxies of plasma tonicity.
Methods
Using data from the FHN Daily and Nocturnal Trials, we compared the effects of frequent HD on LVM among patients stratified by SNa, dialysate-to-predialysis serum-sodium gradient (GNa), systolic and diastolic blood pressure, time-integrated sodium-adjusted fluid load (TIFL), and extracellular fluid volume (ECFV) estimated by bioelectrical impedance analysis.
Results
In 197 enrolled subjects in the FHN Daily Trial, the treatment effect of frequent HD on ΔLVM was modified by Sna. When the FHN Daily Trial participants are divided into lower and higher predialysis SNa groups (less and greater than 138 mEq/L), the LVM reduction in the lower group was substantially higher [−28.0 (95% CI −40.5 to −15.4) g], than in the higher predialysis SNa group [−2.0 (95% CI −15.5 to 11.5) g)]. Accounting for GNa, or TIFL also showed more pronounced effects among patients with higher GNa, or higher TIFL. Results in the Nocturnal Trial were similar in direction and magnitude but did not reach statistical significance.
Discussion/Conclusion
In the FHN Daily Trial, the favorable effects of frequent HD on LVH were more pronounced among patients with lower pre-dialysis SNa and higher GNa and TIFL. Whether these metrics can be used to identify patients most likely to benefit from frequent HD or other dialytic or non-dialytic interventions remains to be determined. Prospective, adequately powered studies studying the effect of GNa reduction on mortality and hospitalization are needed.
Keywords: Frequent hemodialysis, left ventricular mass, blood pressure, fluid overload, effect modification
Introduction
Cardiovascular risk is markedly increased in patients with reduced glomerular filtration rate [1] and the risk of adverse cardiovascular outcomes does not improve following commencement of standard renal replacement therapy [2]. Adverse cardiovascular outcomes result from increased vascular events, often in association with inflammation [1, 3], and an increased incidence of congestive heart failure and left ventricular hypertrophy [4]. The prevalence and severity of left ventricular hypertrophy is increased in patients with decreased residual diuresis [5] and with increased volume load [6-8]. Left ventricular hypertrophy is also associated with higher serum concentrations of fibroblast growth factor-23 (FGF-23) which is upregulated in response to hyperphosphatemia [9, 10].
Sodium management and control of excessive ECFV has proven effective in reducing blood pressure [4, 11, 12] and in decreasing the risk of left ventricular hypertrophy [6, 4, 12]. Beneficial effects on both blood pressure control and left ventricular hypertrophy have been reported with use of more frequent hemodialysis, a modification of the conventional thrice-weekly dialysis regimen, which, in addition to improving volume control, also facilitates control of hyperphosphatemia, and improves health-related quality of life [13].
In 2003 the National Institute of Health and the Centers for Medicare and Medicaid Services (CMS) initiated the Frequent Hemodialysis Network (FHN) Daily and Nocturnal Trials. The Trials were designed to establish the safety and efficacy of more frequent hemodialysis on two co-primary endpoints: death, or change in left ventricular mass (LVM) and death, or change in the Physical Health Composite of the RAND-36 instrument [14, 15]. Effect of frequent HD on the co-primary and a series of secondary outcomes have been previously reported [16-21, 12].
To quantify interdialytic volume overload, we computed a novel parameter designed to represent a Time-Integrated (“area-under the curve”) estimate of extracellular Fluid Load (TIFL), a concept which was reported to associate with changes in left ventricular mass in the trials [12]. During the development of the TIFL, which incorporates serum sodium and the dialysate to serum sodium gradient we noticed that the favorable treatment effect (i.e., a reduction in LVM) appeared to be more prominent among patients with lower predialysis serum sodium (SNa) values and those with higher dialysate to serum sodium gradient (GNa) values. The purposes of this study were to quantify this effect, and to explore a number of possible mechanisms that might explain the effect modification, especially those related to ECFV and inflammation.
Materials and Methods
Patient selection
Patients for the Daily Trial (ClinicalTrials.gov number: NCT00264758) were recruited by two consortia of in-center hemodialysis units, one organized by the Renal Research Institute in New York, and the other by dialysis units at university and free-standing hemodialysis centers in California and Texas. Patients for the separate Nocturnal Trial (ClinicalTrials.gov: NCT00271999) were recruited from home-dialysis training centers in the United States and Canada. All patients signed informed consent. Both trials were approved by local Institutional Review Boards (Beth Israel Medical Center in New York, NY; Stanford University School of Medicine in Stanford, CA; and Wake Forest School of Medicine, in Winston-Salem, NC) and conducted according to the Declaration of Helsinki.
Study design
The FHN Daily and Nocturnal trials were controlled and randomized. The co-primary endpoints were assessed after a follow-up period of 12 months [22]. Inclusion and exclusion criteria were nearly identical for both trials, except for residual kidney function at trial entry. In the Daily Trial, we excluded patients with residual urea clearance >3mL/min per 35 L of urea distribution volume, while in the Nocturnal Trial, where practical recruitment issues required a focus on incident patients, we set the residual kidney function exclusion considerably higher and accepated patients with average residual clearances of urea and creatinine up to 10mL/min per 1.73 m2 [22].
Measurements
We performed cardiac magnetic resonance imaging (cMRI) at baseline and at month 12. SNa was measured monthly by potentiometric methods in certified local laboratories. Dialysate Na (DNa) was not measured but retrieved from the dialysis provider medical record for each patient at each time point. The sodium gradient during hemodialysis (Gna) was calculated as the prescribed DNa minus the measured predialysis SNa. The interdialytic weight gains (IDWG) were assessed according to the dialysis facility routine. To quantify interdialytic volume overload, we computed a novel parameter designed to represent a Time-Integrated (“area-under the curve”) estimate of extracellular Fluid Load (TIFL). The TIFL curve was computed based on the interdialytic weight gain, which is affected by the residual urine volume, the GNa and the session length. Based on data collected in a previous study we calculated an estimated post-dialysis SNa as outlined in [12]. This estimated post-dialysis SNa was in turn used to estimate the volume of post-dialysis fluid intake required after dialysis to restore the pre-dialysis SNa level to its usual value. This sodium-gradient-adjusted modeled TIFL aims to separately account for both, the thirst-driven immediate post-dialytic fluid intake and the sodium and fluid intake during the remainder interdialytic period. The value for TIFL is calculated in liter x days, or, the number of liters of extracellular fluid overload times the number of days in the interdialytic interval that this overload exists. The value for TIFL is different from conventional time-integrated fluid overload measures in that it also takes into account the gradient between dialysate and predialysis SNa, making the assumptions that the postdialysis SNa will be very close to the dialysate value, and also that, soon after cessation of dialysis, a patient will drink until the SNa returns to its predialysis value. In a previous publication, we found that the calculated value for improvement in TIFL in liter×days with more frequent dialysis was associated with reduction of LVM, unless substantial residual renal function was present [12]. For details on the model see [12] and for simulations of TIFL for subjects with different gradients in the two study arms see Supp. Fig. 1.
Bioimpedance measurements
We used single frequency bioimpedance analysis (BIA) (RJL Systems, Clinton Township, NJ) to estimate body water compartments. As per protocol, we performed BIA prior to a mid-week hemodialysis treatment, however deviations in a minority of cases may be noted. Accuracy and precision of the BIA method that was used has been reported elsewhere [23, 24]. Extracellular fluid volume (ECFV) was calculated as the difference between BIA-estimates of total body water (TBW) [25] and intracellular fluid volume [26].
Statistical analyses
We evaluated the treatment effect of frequent versus conventional hemodialysis on LVM by employing linear mixed effects models adjusted for clinical center, age, diabetes mellitus and baseline value. We explored effect modification by inclusion of the interaction term between treatment effect and the respective parameter in the linear mixed effects model and subsequent analysis in strata of that parameter. Changes in LVM were further depicted as a box-whisker plot stratified into quintiles of SNa. P-values less than 0.05 were considered significant. We conducted all analyses using SAS version 9.2 (SAS Institute, Cary, NC) and R v3.6.0 (codename “Planting of a Tree”; [27]) using packages dplyr, reshape2, stringr, doBy, haven and stringr.
Results
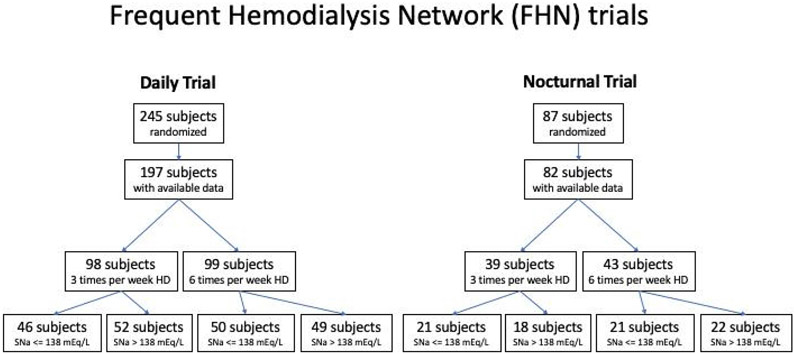
We included 197 patients enrolled in the Frequent Hemodialysis Network (FHN) Daily trial and 82 patients enrolled in the FHN Nocturnal Trial with available data for SNa at baseline (Fig. 1). Demographics for the overall population stratified as per the median predialysis SNa (138 mEq/L) are shown in Table 1.
Fig. 1:
Flowchart of patient inclusion.
Table 1:
Baseline characteristics of FHN Trial participants who had baseline sodium concentration values
| Characteristic | N (available data for respective parameter ) |
3X Daily Pts (N=98) |
3X Daily Pts with SNa=<138 mEq/L (N=46) |
3X Daily Pts with SNa>138 mEq/L (N=52) |
N (available data for respective parameter ) |
6X Daily Pts (N=99) |
6X Daily Pts with SNa=<138 mEq/L (N=50) |
6X Daily Pts with SNa>138 mEq/L (N=49) |
|---|---|---|---|---|---|---|---|---|
| Age (yrs.) | 98 | 52.1 ± 12.7 | 52.9 ± 12.8 | 51.3 ± 12.7 | 99 | 48.7 ± 12.7 | 48.2 ± 12.6 | 49.1 ± 12.8 |
| Female (%) | 98 | 38 (39%) | 21 (40%) | 17 (37%) | 99 | 38 (38%) | 23 (47%) | 15 (30%) |
| Race | 98 | 99 | ||||||
| Black (%) | 44 (45%) | 18 (35%) | 26 (57%) | 40 (40%) | 18 (37%) | 22 (44%) | ||
| White (%) | 34 (35%) | 22 (42%) | 12 (26%) | 32 (32%) | 16 (33%) | 16 (32%) | ||
| Native American, Aboriginal Canadian, Alaskan | 4 (4%) | 3 (6%) | 1 (2%) | 4 (4%) | 1 (2%) | 3 (6%) | ||
| Native (%) | 5 (5%) | 3 (6%) | 2 (4%) | 9 (9%) | 4 (8%) | 5 (10%) | ||
| Asian (%) | 3 (3%) | 3 (6%) | 0 | 1 (1%) | 0 | 1 (2%) | ||
| Native Hawaiian or other Pacific Islander (%) | 8 (8%) | 3 (6%) | 5 (11%) | 13 (13%) | 10 (20%) | 3 (6%) | ||
| Other/mixed/unknown (%) | ||||||||
| Ave. Weekly Predialysis Systolic BP (mmHg) | 98 | 146 ± 18 | 146 ± 20 | 145 ± 17 | 99 | 148 ± 17 | 149 ± 17 | 147 ± 18 |
| Ave. Weekly Predialysis Diastolic BP (mmHg) | 98 | 78.1 ± 11.0 | 77.6 ± 10.5 | 78.7 ± 11.7 | 99 | 81.7 ± 11.4 | 82.4 ± 9.1 | 80.9 ± 13.3 |
| Ave. Weekly Postdialysis Systolic BP (mmHg) | 98 | 133 ± 18 | 133 ± 18 | 133 ± 17 | 99 | 135 ± 19 | 135 ± 18 | 135 ± 20 |
| Ave. Weekly Postdialysis Diastolic BP (mmHg) | 98 | 71.6 ± 10.3 | 70.4 ± 9.3 | 72.9 ± 11.3 | 99 | 73.7 ± 11.6 | 74.4 ± 10.7 | 73.0 ± 12.5 |
| Left Ventricular Mass (g) | 98 | 145 ± 52 | 147 ± 55 | 143 ± 49 | 99 | 146 ± 58 | 139 ± 50 | 152 ± 64 |
| Left Ventricular End-diastolic Vol. (ml) | 98 | 184 ± 70 | 188 ± 60 | 179 ± 80 | 99 | 179 ± 57 | 177 ± 48 | 181 ± 65 |
| Extra-cellular fluid volume (L) | 97 | 22.9 ± 4.7 | 23.0 ± 4.6 | 22.9 ± 5.0 | 94 | 22.7 ± 4.2 | 22.7 ± 4.1 | 22.7 ± 4.4 |
| Extra-cellular fluid volume / Total Body fluid volume (L) [BIA] | 97 | 0.528 ± 0.063 | 0.530 ± 0.066 | 0.525 ± 0.060 | 94 | 0.517 ± 0.073 | 0.530 ± 0.069 | 0.505 ± 0.075 |
| Extra-cellular fluid volume / Ave. Weekly Postdialysis Wt. | 97 | 0.298 ± 0.040 | 0.301 ± 0.043 | 0.294 ± 0.036 | 94 | 0.298 ± 0.045 | 0.301 ± 0.046 | 0.294 ± 0.045 |
| IDWG (% post HD body weight) | 88 | 4.21 ± 1.30 | 4.64 ± 1.38 | 3.75 ± 1.03 | 85 | 4.25 ± 1.36 | 4.43 ± 1.46 | 4.09 ± 1.27 |
| IDWG (% of ECV) | 88 | 14.2 ± 4.0 | 15.5 ± 4.1 | 12.8 ± 3.4 | 82 | 14.5 ± 4.8 | 14.9 ± 4.4 | 14.1 ± 5.2 |
| time-integrated fluid load (TIFL; units liter × day) | 88 | 4.74 ± 2.06 | 5.71 ± 2.05 | 3.69 ± 1.50 | 82 | 4.72 ± 2.33 | 5.57 ± 2.54 | 3.99 ± 1.88 |
| Body Mass Index (kg/m2) | 97 | 28.8 ± 7.2 | 28.7 ± 6.7 | 28.8 ± 7.7 | 94 | 28.9 ± 6.5 | 28.7 ± 7.0 | 29.0 ± 6.1 |
| Predialysis Bicarbonate (mEqL) | 98 | 23.7 ± 4.2 | 22.4 ± 4.5 | 25.2 ± 3.5 | 99 | 23.9 ± 3.3 | 23.7 ± 3.5 | 24.1 ± 3.0 |
| Diabetes (%) | 98 | 40 (41%) | 26 (50%) | 14 (30%) | 99 | 39 (39%) | 23 (47%) | 16 (32%) |
| Left Ventricular Ejection Fraction (%) | 98 | 56.2 ± 12.4 | 55.4 ± 12.7 | 57.0 ± 12.2 | 99 | 57.8 ± 10.0 | 57.7 ± 10.3 | 57.9 ± 9.8 |
Plus-minus values are means ±SD. Bracketed values are medians with interquartile ranges.
Serum sodium modifies the effect of frequent HD on left ventricular mass
Irrespective of the treatment allocation, sNa, dNa, and the resulting GNa, remained consistent in magnitude for the studied population over the entire period of the study (Supp. Fig. 2a-c). In both, the Daily and the Nocturnal Trial, SNa at baseline was not significantly different in patients with congestive heart failure, myocardial infarction or those with an open prescription of antihypertensive medication at baseline in both trials.
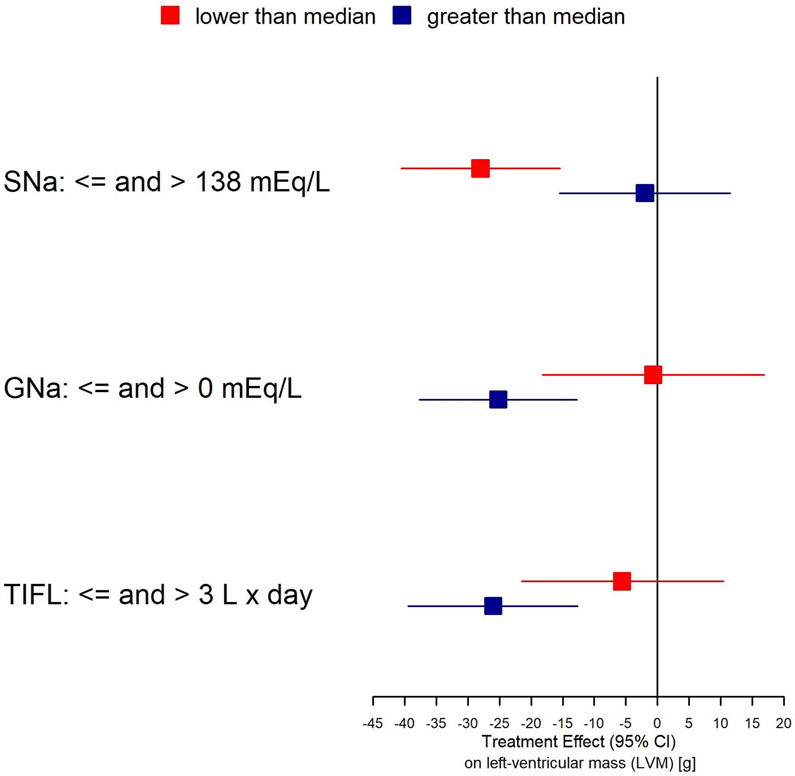
As shown in Supplementary Table 1 and Fig. 2, baseline predialysis sNa significantly modified the effect of frequent HD on LVM (p=0.027); the interaction of treatment x baseline GNa was of marginal significance (p=0.06). Stratified by median values, the change in LVM was −28.0 (95% CI −40.5 to −15.4) g in patients with baseline sNa <=138 mmol/L and −2.0 (95% CI −15.5 to 11.5) g in patients with baseline sNa >138 mmol/L; Supplementary Table 1]. Repeating the analyses stratifying by quintiles of SNa showed a comparable trend (Fig. 3). For GNa, the interaction of treatment x baseline GNa was only of borderline significance (p=0.06). Reductions in LVM associate with more frequent hemodialysis for patients with a positive gradient (GNa>0 mEq/L) were −25.2 (95% CI −37.6 to −12.7) g and −0.7 (95% CI −18.2 to 16.8) g; Supplementary Table 1]. In the Nocturnal Trial where the power to detect interactions was considerably lower, the findings regarding SNa, GNa and TIFL were of similar direction but none were statistically significant (Supplementary Table 2 and Supplementary Table 3).
Fig. 2:
Forest plot depicting the magnitude of treatment effect of more frequent hemodialysis on left ventricular mass reduction in subgroups of parameters tested for interaction.
Fig. 3:
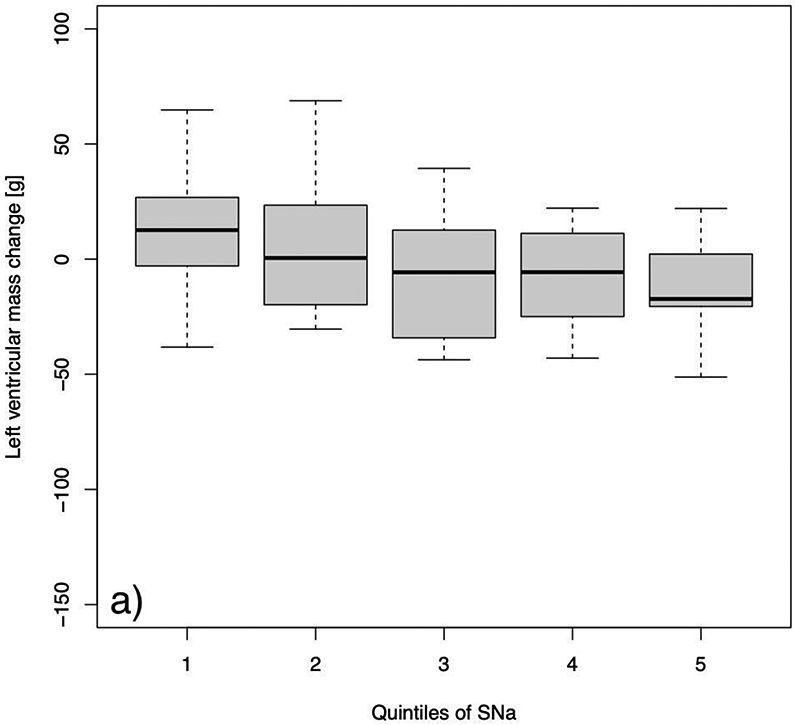
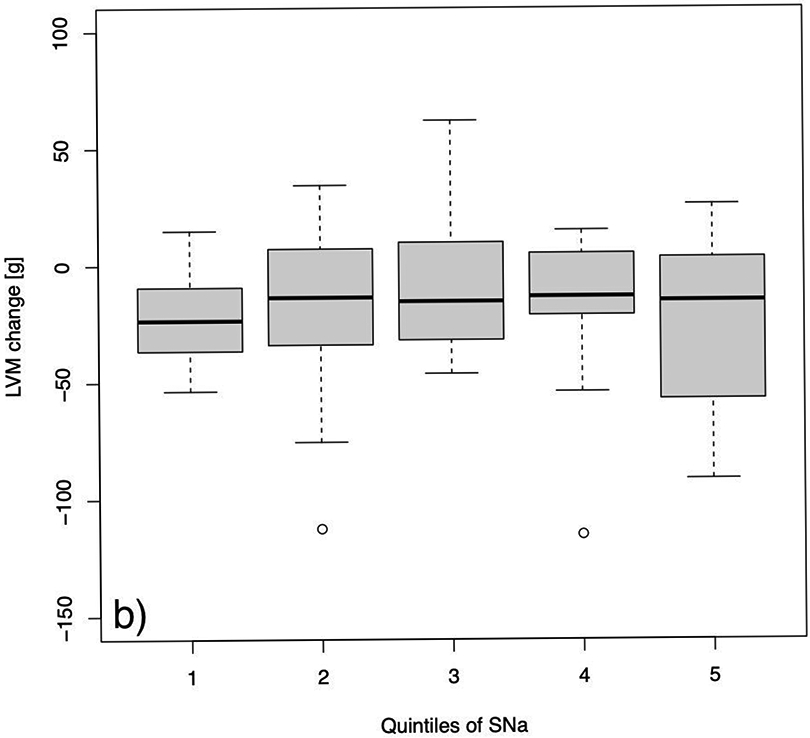
Changes in left ventricular mass from baseline to follow-up after a 12 months study period in a) the control group and b) the more frequent hemodialysis group. Quintiles were defined as follows: Q1: 129 to 135; Q2: 136 to 137; Q3: 137 to 139; Q4: 139 to 141; and Q5: 141 to 145 mEq/L.
TIFL as an effect modifier
Formal testing of the treatment x TIFL interaction was not significant (p=0.22). However, when stratified by TIFL <=3.0 L x days and TIFL >3 Lxdays, we observed a more pronounced effect in the latter group [−26.0 (95% CI −39.43 to −12.59) g] for those with greater TIFL (Supplementary Table 1 and Fig. 2). In the Nocturnal Trial the treatment effects were smaller and of comparable magnitude in terms of direction and subgroup differences, but not statistically significant (Supplementary Table 3).
Discussion/Conclusion
Our analysis quantified and documented our observation, that in patients with lower predialysis SNa values, the use of frequent hemodialysis was associated with a more marked reduction in LVH compared to those with higher predialysis SNa values. When the FHN Daily Trial participants are divided into lower and higher predialysis SNa groups, the LVM reduction in the lower group was substantially higher [−28.0 (95% CI −40.5 to −15.4) g], than in the higher predialysis SNa group [−2.0 (95% CI −15.5 to 11.5) g); see Supplementary Table 1 and Fig. 2]. This interaction was statistically significant and remained significant when the predialysis SNa level was examined as a continuous interaction variable. Notably, assignment to more frequent dialysis had no effect on the predialysis SNa value.
When examining potential mechanisms for the treatment effect of predialysis serum Na on LVM, we assessed whether the predialysis serum Na value was related to the predialysis systolic blood pressure, or to the baseline value of LVM, because in previous analyses we had found that the treatment effect of more frequent dialysis on LVM was strongly associated with baseline LVM, also there was a trend for a larger reduction in LVM to occur with more frequent dialysis in patients with the highest predialysis systolic BP values at baseline [16]. In the present analysis, baseline predialysis SNa level was not related to either the systolic or the diastolic predialysis BP (Table 1), nor to the baseline measure of LVM. Similarly, baseline ejection fractions were similar in the lower and higher predialysis SNa groups.
In previous analyses, we found that there appeared to be an interaction between the presence of diabetes and response of LVM to frequent dialysis in the Daily Trial [16], though the relationship in the current data the modifying effect of diabetes was in the opposite direction. The reduction in LVM with more frequent dialysis was greater in those patients without diabetes. This particular interaction was of borderline significance in all models tested. We did find that presence of diabetes was increased in the half of patients with lower predialysis SNa values (Table 1) and it is well known that high predialysis glucose level can lower the measured predialysis SNa [28-30]. Unfortunately serum glucose was not included in the routine measurements in the FHN trial, so it is not possible to calculate whether or not the presence of hyperglycemia alone could account for the lower measured Sna values, nor to what extent poorly controlled diabetes, and the associated hyperglycemia which we were unable to account for, might have been responsible for a lower predialysis SNa. In our current analyses, the modifying effect of predialysis SNa on the LVM-frequent dialysis interaction was in the opposite direction to the modifying effect of diabetes (Table 1). The modifying effect of low predialysis SNa on the LVM-frequent dialysis interaction may in fact have been attenuated due to more patients having diabetes in the lower SNa group compared to those in the higher SNa group.
One popular explanation for the beneficial effects of more frequent dialysis on left ventricular hypertrophy is better control of excess fluid volume, and specifically, extracellular fluid (ECF) volume. In those patients with lower predialysis SNa values at baseline, the ECF volume did not seem to be increased: Left ventricular end-diastolic volumes were similar in the lower and higher predialysis SNa groups, and bioimpedance-measured ECF volumes were not significantly different between the groups presenting with lower and higher SNa (Table 1).
One volume-related measure that was higher in the patients with lower SNa values was the interdialytic weight gains, whether factored by postdialysis body weight or by postdialysis impedance-calculated ECFV (Table 1). In previous publications, we have proposed a new metric to quantify extracellular fluid “load” in dialysis patients, which we have termed TIFL, or time integrated fluid load [12].
In the present analysis, in addition to dividing patients up based on predialysis SNa values, we also divided them by dialysate-serum Na gradient. In 62 out of 197 patients so-analyzed, the sodium gradient was < 0 (dialysate Na less than serum Na), and in this subset the mean treatment effect of more frequent dialysis in the Daily Trial was −0.7 (95% CI −18.2 to 16.8) g, or essentially null, while in 111 out of 197 patients in whom the dialysate Na was greater than predialysis serum Na, the reduction in LVM associated with more frequent HD was −25.2 (95% CI −37.6 to −12.7) g. Similarly when we calculated the value for TIFL, when the value for TIFL was < 3L×days, the reduction in LVM was −5.6 (95% CI –−21.5 to 10.4 g), whereas when TIFL was >= 3L×days, the reduction was much larger, −26.0 (95% CI −39.43 to −12.59) g (Supplementary Table 1 and Fig. 2). However, the examination of the interaction between the treatment arm allocation and a) the baseline predialysis serum Na, b) the sodium gradient, and c) TIFL as a continuous variable, showed a significant interaction only for the predialysis SNa. This suggests a non-linear relationship between treatment effect and these potential effect-modifying parameters and requires further investigation and analyses.
Our primary analysis was performed on patients in the FHN Daily Trial. We also divided the patients in the FHN nocturnal trial into two groups based on baseline predialysis SNa values, to explore whether or not any trends or differences found in the Daily Trial Trial might be present in the Nocturnal Trial patients. Such trends were present, but none were statistically significant, as documented in the Supplementary Table 2 and Supplementary Table 3.
Our post-hoc analysis has a number or limitations, among them are a reduced sample size and the lack of available data to explore all possible mechanisms that might potentially explain the modifying effect of baseline predialysis SNa on the reduction of LVM with more frequent dialysis. We were not able to measure or estimate the non-osmotically stored sodium in storage sites such as skin and muscle over the course of the study. These relatively newly described storages are not yet well enough understood to generate hypotheses as to how frequent hemodialysis might have affected them; however, one recently described association between non-osmotically stored sodium and LVM [31] suggests that this topic definitely merits further research. Further, it will be important to validate the model allowing the quantification of TIFL and the pathophysiological correlates in cohorts other than the studied. However, in regard of the biological context, the underlying physiology and the adequate power of our analysis, we believe the found dynamics to be describing a generally applicable phenomenon. Prospective, adequately powered, studies aiming to reduce the GNa and then to study the effects of the reduction on hard outcomes such as LVM, mortality, hospitalization; but also soft outcomes such as skin and muscle sodium storage are needed to fully understand the complex interrelationship found in our data.
In summary, of all the potential mechanisms examined, it appears most likely that the association between baseline predialysis SNa and LVM-response to more frequent dialysis is related to a greater impact on control of ECFV with more frequent dialysis in the slightly hyponatremic patients, in whom the time-averaged sodium-adjusted ECFV load TIFL may be magnified due to a higher sodium gradient. However, the data and mechanistic explanations remain preliminary, and it is not clear to what extent more frequent dialysis should be recommended for slightly hyponatremic patients.
Supplementary Material
Acknowledgement
The authors wish to acknowledge and thank the entire Frequent Hemodialysis Network (FHN) Trials Group. Part of the data presented in this manuscript was presented at the Renal Week 2014 of the American Society of Nephrology in Philadelphia, PA, USA and submitted to the Renal Week 2018 of the American Society of Nephrology in San Diego, CA, USA.
Statement of Ethics
All recruited patients signed informed consent. Both trial trial protocols were approved by local Institutional Review Boards (Beth Israel Medical Center in New York, NY; Stanford University School of Medicine in Stanford, CA; and Wake Forest School of Medicine, in Winston-Salem, NC) and conducted according to the Declaration of Helsinki. Both protocols were registered (ClinicalTrials.gov number NCT00264758 for the Daily Trial and NCT00271999 for the Nocturnal Trial) and approved and funded by the National Instiute of Health (U.S. NIH Grant/Contract number 5U01DK066597).
Funding Sources
Supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), the Center for Medicare and Medical Services and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter, and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics, Fresenius Medical Care, Renal Advantage, Renal Research Institute, and Satellite Healthcare.
Appendix
FHN Trial Group
Chair, Steering Committee: Kliger A; NIDDK: Eggers P, Briggs J, Hostetter T, Narva A, Star R; Centers for Medicare and Medical Services: Augustine B, Mohr P; Data Coordinating Center – Cleveland Clinic: Beck G (PI), Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, MacKrell J, Wiggins K, Sherer S, Weiss B; MRI Core – Ohio State University and Mt. Sinai Medical Center: Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M; Tran T, West J; Central Quality of Life Core – University of Pittsburgh: Unruh M; Keene R, Schlarb J; Central Holter Core– Toronto General Hospital: Chan C; McGrath-Chong M; Biospecimen Repository – Fisher BioServices: Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C; Data Safety and Monitoring Board: Eknoyan G (Chair), Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E;
Daily Trial Clinical Sites – University of California San Francisco (UCSF)/Stanford Consortium: Chertow G (PI); UCSF and San Francisco Bay Area: James S, Chertow G, Tamura M, Hall Y, McCulloch C, Painter P, Gorodetskaya I, Tichy M, Humphreys M, Luan J, Escalada R, Rodriquez R; UC Davis and Sacramento Area: Depner T, Kaysen G, Suter M, Sonico J, Anderson S; El Camino Hospital and Satellite Health Care: Ting G, Schiller B, Coplon N, Doss S, Rogers J, Dominguez A, Atwal J, Lemus D; UCLA and Los Angeles Area: Rastogi A, Nissenson A, Goodman W, Salusky I, Schweitzer S, Rivas M, Smith M, Gayda P, Hernandez A, Rashid M; UCSD and San Diego Area: Mehta R, Pepas J, Bharti B, Nabali A, Manaster R, Mathew R, Shah S, Sanz G, Wei J; University of Texas, San Antonio: Ayus J, Achinger S, Gutierrez M; Renal Research Institute (RRI) New York Consortium: Levin N (PI); Bay W, Carter M, Geronemus R, Kuhlmann M, Handelman G, Gotch F, Finkelstein F, Kimmel P, Lacson E, Ornt D, Greenwood R, Vassalotti J, Burrowes J; RRI New York City: Levin N, Kotanko P, Kaufman A, Winchester J, Meisels I, Radbill B, Chang J, Fofie Y, Ramos R, Sergeyeva O, Callegari J, Arthur B, Tarallo M, Ulloa D, Apruzzese R; University of Western Ontario: Lindsay R, Suri R, Garg A, Bullas
Nocturnal Trial Clinical Sites – Wake Forest University School of Medicine Consortium: Rocco M (PI); Barnes-Jewish/Washington University: Miller B, Riley J, Schuessler R; Lynchburg Nephrology: Lockridge R, Pipkin M, Peterson C; Rubin Dialysis: Hoy C, Fensterer A, Steigerwald D; University of Iowa: Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E; University of Toronto: Chan C, McGrath-Chong M; University of British Columbia: Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D; University of Western Ontario: Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E; Wake Forest University School of Medicine: Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T; Humber River Regional Hospital: Pierratos A, Chan W, Regozo K, Kwok S.
Footnotes
Conflict of Interest Statement
Peter Kotanko holds stock in Fresenius Medical Care North America. Nathan W. Levin is a scientific advisor to Aethlon and Affymax, received funding from the US National Institute of Health and owns stock in Fresenius Medical Care North America. Robert M. Lindsay has unrestricted research support from Fresenius Medical Care Canada. Glenn M. Chertow serves on the Board of Directors of Satellite Healthcare. Jochen Raimann and Peter Kotanko are employees of the Renal Research Institute. All other authors have no relevant conflicts of interest.
Data Availability Statement
The data used for this analysis can be requested from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. September 23;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 2.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;65(6 Suppl 1):A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. The American journal of clinical nutrition. 2004. August;80(2):324–32. [DOI] [PubMed] [Google Scholar]
- 4.Charra B, Chazot C. Volume control, blood pressure and cardiovascular function. Lessons from hemodialysis treatment. Nephron Physiology. 2003;93(4):p94–101. [DOI] [PubMed] [Google Scholar]
- 5.Araujo S, Lemes HP, Cunha DA, Queiroz VS, Nascimento DD, Ferreira Filho SR. Cardiac morphology and function in patients with and without residual diuresis on hemodialysis. Jornal brasileiro de nefrologia : 'orgao oficial de Sociedades Brasileira e Latino-Americana de Nefrologia. 2011. March;33(1):74–81. [PubMed] [Google Scholar]
- 6.Ozkahya M, Ok E, Cirit M, Aydin S, Akcicek F, Basci A, et al. Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol Dial Transplant. 1998. June;13(6):1489–93. [DOI] [PubMed] [Google Scholar]
- 7.Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of Fluid Management Guided by Bioimpedance Spectroscopy on Cardiovascular Parameters in Hemodialysis Patients: A Randomized Controlled Trial. Am J Kidney Dis. 2013. February 14. [DOI] [PubMed] [Google Scholar]
- 8.Ok E, Levin NW, Asci G, Chazot C, Toz H, Ozkahya M. Interplay of volume, blood pressure, organ ischemia, residual renal function, and diet: certainties and uncertainties with dialytic management. Semin Dial. 2017. September;30(5):420–29. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal I, Ide N, Ix JH, Kestenbaum B, Lanske B, Schiller NB, et al. Fibroblast growth factor-23 and cardiac structure and function. Journal of the American Heart Association. 2014. February 13;3(1):e000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka S, Fujita S, Kizawa S, Morita H, Ishizaka N. Association between FGF23, alpha-Klotho, and Cardiac Abnormalities among Patients with Various Chronic Kidney Disease Stages. PloS one. 2016;11(7):e0156860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin NW, Raimann JG, Rocco MV. Should the knowledge gained from the Frequent Hemodialysis Network (FHN) trials change dialysis practice? Curr Opin Nephrol Hypertens. 2011. November;20(6):577–82. [DOI] [PubMed] [Google Scholar]
- 12.Raimann JG, Chan CT, Daugirdas JT, Depner T, Gotch FA, Greene T, et al. The Effect of Increased Frequency of Hemodialysis on Volume-Related Outcomes: A Secondary Analysis of the Frequent Hemodialysis Network Trials. Blood Purif. 2016;41(4):277–86. [DOI] [PubMed] [Google Scholar]
- 13.Raimann JG, Thijssen S, Ramos R, Levin NW. More frequent hemodialysis: what do we know? Where do we stand? Contrib Nephrol. 2011;171:10–6. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010. December 9;363(24):2287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocco MV, Lockridge RS Jr., Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011. November;80(10):1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging. 2012. March;5(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012. April;23(4):727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan CT, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, Larive B, et al. Effects of daily hemodialysis on heart rate variability: results from the frequent hemodialysis network (FHN) daily trial. Nephrol Dial Transplant. 2013. September 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Effects of Frequent Hemodialysis on Ventricular Volumes and Left Ventricular Remodeling. Clin J Am Soc Nephrol. 2013. August 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013. January 23;83(5):949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis. 2013. May;61(5):748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007. February;71(4):349–59. [DOI] [PubMed] [Google Scholar]
- 23.Raimann JG, Abbas SR, Liu L, Zhu F, Larive B, Kotanko P, et al. Agreement of Single- and Multi-Frequency Bioimpedance Measurements in Hemodialysis Patients: An Ancillary Study of the Frequent Hemodialysis Network Daily Trial. Nephron Clinical practice. 2014. November 7;128(1-2):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raimann JG, Zhu F, Wang J, Thijssen S, Kuhlmann MK, Kotanko P, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014. April;85(4):898–908. [DOI] [PubMed] [Google Scholar]
- 25.Kotler DP, Burastero S, Wang J, Pierson RN Jr. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996. September;64(3 Suppl):489S–97S. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, St-Onge MP, Lecumberri B, Pi-Sunyer FX, Heshka S, Wang J, et al. Body cell mass: model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004. January;286(1):E123–8. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 28.Katz MA. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med. 1973. October 18;289(16):843–4. [DOI] [PubMed] [Google Scholar]
- 29.Robin AP, Ing TS, Lancaster GA, Soung LS, Sparagana M, Geis WP, et al. Hyperglycemia-induced hyponatremia: a fresh look. Clin Chem. 1979. March;25(3):496–7. [PubMed] [Google Scholar]
- 30.Penne EL, Raimann JG, Usvyat LA, Thijssen S, Levin NW, Kotanko P. Alignment of the dialysate sodium concentration with the serum sodium: role of plasma water fraction and Donna effects. J Am Soc Nephrol. 2010;21(Abstract Supplement):434A. [Google Scholar]
- 31.Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J Am Soc Nephrol. 2017. June;28(6):1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this analysis can be requested from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK).