Abstract
Primary Objective
We hypothesized that, in patients with acute severe traumatic brain injury (TBI) who recover basic language function, speech-evoked blood-oxygen-level-dependent (BOLD) functional MRI (fMRI) responses within the canonical language network increase over the first six months post-injury.
Research Design
We conducted a prospective, longitudinal fMRI pilot study of adults with acute severe TBI admitted to the intensive care unit and age- and sex-matched healthy subjects.
Methods and Procedures
We evaluated BOLD signal in bilateral superior temporal gyrus (STG) and inferior frontal gyrus (IFG) regions of interest acutely and six months post-injury. Given evidence that regions outside the canonical language network contribute to language processing, we also performed exploratory whole-brain analyses.
Main Outcomes and Results
Of the 16 patients enrolled, eight returned for follow-up fMRI, all of whom recovered basic language function. We observed speech-evoked longitudinal BOLD increases in the left STG, but not in the right STG, right IFG, or left IFG. Whole-brain analysis revealed increases in the right supramarginal and middle temporal gyri but no differences between patients and healthy subjects (n=16).
Conclusion
This pilot study suggests that, in patients with severe TBI who recover language function, speech-evoked BOLD responses in bihemispheric language-processing cortex reemerge by six-months post-injury.
Keywords: traumatic brain injury, language, recovery, functional MRI, consciousness
Introduction
Reemergence of language function is a critical early milestone for patients with severe traumatic brain injury (TBI) and an indicator of the transition from unconsciousness to consciousness (1). Recovery of basic language function (e.g., command-following)_predicts better short-term and long-term outcomes for patients with severe TBI (2, 3), particularly with respect to social reintegration and return to work (4). Importantly, evidence of higher-level language behaviors, such as verbal fluency and comprehension of complex yes/no questions, also affects functional outcome after severe TBI (5). Despite the key role of language in recovery, the neurobiological mechanisms underlying reemergence of language function in patients with severe TBI are poorly understood. Consequently, there are no early biological markers to identify patients who are likely to recover language function and no interventions to promote this recovery.
It is well established that a bilateral language network (left-dominant in most right-handed individuals) supports the three core processes of intact language function: 1) perception (i.e., identifying sounds as phonetic features, syllables, and words); 2) comprehension (i.e., understanding the meaning of words and sentences); and 3) expression (i.e. using speech, writing, or gesture to express ideas) (6–11). The canonical language network is anchored by bilateral nodes in the superior temporal gyrus (STG) and inferior frontal gyrus (IFG) (6, 12–17). Additionally, according to current models of cortical speech processing, regions outside of the canonical network also support comprehension and expression via a bilateral ventral stream and a left-dominant dorsal stream, respectively (6, 10, 11). Understanding how language networks change after severe TBI may contribute to improved diagnostic and prognostic precision (18–20), as well as the development of early interventions targeted at repairing the nodes and connections critical for language perception, comprehension, and expression. However, the STG, IFG, and other language-related cortical regions have not been studied longitudinally in patients who recover language function after severe TBI.
We conducted a prospective, longitudinal study of functional MRI (fMRI) responses to speech versus rest in patients with disorders of consciousness (DoC) caused by acute severe TBI. We hypothesized that in patients who recover basic language function after severe TBI, fMRI responses to language stimuli increase over time in bilateral frontotemporal language regions (within the right and left superior temporal gyrus [STGR, STGL], and right and left inferior frontal gyrus [IFGR, IFGL]). Additionally, given the complex neuroanatomy of speech processing in the human brain, with known contributions from cortical regions across ventral and dorsal streams (6, 10, 11), we conducted an exploratory whole-brain analysis to identify regions outside of the canonical frontotemporal language network related to recovery of language. Finally, to examine whether longitudinal fMRI changes are associated with reorganization versus reemergence of language networks, we compared fMRI responses in patients who recovered basic language function to those in healthy control subjects.
Materials and Methods
Experimental design
We prospectively and consecutively screened all adult patients with TBI admitted to the intensive care unit (ICU) at an academic hospital during the 3-year pilot phase of an ongoing observational study (ClinicalTrials.gov NCT03504709) approved by the local Institutional Review Board. Inclusion and exclusion criteria have been previously reported (21). Briefly, inclusion criteria were: 1) age 18 to 65 years; and 2) head trauma with Glasgow Coma Scale (GCS) score of 3–8 with no eye opening for at least 24 hours. Exclusion criteria were: 1) life expectancy less than six months; 2) prior severe brain injury or neurodegenerative disease; 3) penetrating TBI with intracranial metal or other body metal precluding MRI; and 4) no fluency in English prior to the injury (because the fMRI paradigms and language assessments could only be administered in English). Surrogate decision-makers provided written informed consent. Acute fMRI was performed as soon as patients were stable for transport to the MRI scanner, as determined by treating clinicians. Follow-up fMRI and neurobehavioral assessments were performed approximately 6–12 months after injury. Sixteen age- and sex-matched healthy subjects, with no history of neurological, psychiatric, cardiovascular, pulmonary, renal or endocrinological disease, also completed the fMRI paradigm.
Behavioral language assessment
Prior to each fMRI, we assessed patients using the Coma Recovery Scale-Revised (CRS-R) (1), components of the Confusion Assessment Protocol (CAP) (22), and the California Verbal Learning Test–II (CVLT-II) (23). We defined behavioral recovery of basic language function (i.e. comprehension of simple sentences and expression at the word-level) as consistent command-following, intelligible speech, and functional communication on the CRS-R and the CAP, as well as the ability to validly complete the CVLT-II. The CRS-R, CAP, and CVLT-II assessments were performed by a neurologist (B.L.E.), who was blind to the fMRI results at the time of the assessments.
The CRS-R is a 23-item, 6-subscale assessment of auditory, visual, motor, verbal, and communication function as well as arousal in patients with DoC (1). Emergence of volitional cortically-mediated responses (e.g., visual pursuit, object recognition) on CRS-R assessment indicates transition from unconsciousness (i.e., coma or vegetative state [VS]) to a minimally conscious state (MCS). Return of functional communication or use of common objects, but ongoing disorientation coupled with other cognitive and clinical symptoms, is indicative of a post-traumatic confusional state (PTCS) (24). On the CRS-R, grossly intact language function is evidenced by the ability to follow commands, respond to questions, or speak intelligibly (3). The CAP is a composite measure of cognition, orientation, and clinical symptoms that measures severity of PTCS. The CAP includes several components that require language comprehension (e.g., a series of four semantically complex questions such as: do you put your shoes on before your socks?) and expression (e.g., verbal responses to prompts). The CVLT-II assesses verbal memory and learning and consists of five trials of word-list repetition (i.e., immediate recall) followed by assessment of short-delay free recall, short-delay cued recall, long-delay free recall, long-delay cued recall, and total recognition discrimination. We defined valid completion of the CVLT-II as intelligible, fluid, and appropriate (but not necessarily accurate) responses to all test questions, as this provides evidence for language perception, comprehension, and expression (23).
fMRI data acquisition
MRI data were acquired with a 32-channel head coil on a 3 Tesla Skyra MRI scanner (Siemens Medical Solutions) located in the Neurosciences ICU. Auditory stimuli were presented to all subjects via MRI-compatible earphones (Newmatic Medical) connected to the scanner’s sound system. The blood oxygen level-dependent (BOLD) fMRI sequence used the following parameters: echo time = 30ms, repetition time = 4000ms, in-plane resolution = 2.0×2.0mm, slice thickness = 2mm, interslice gap = 2.5mm, matrix = 94×94, field-of-view = 192×192mm2, 49 slices, 2x GRAPPA acceleration.
Three-dimensional T1-weighted multi-echo magnetization prepared gradient echo (MEMPRAGE) anatomical images were acquired for registration purposes (25): field of view = 256×256mm2, acquisition matrix = 256×256, 176 sagittal slices, 3x GRAPPA acceleration, echo time = 1.69, 3.55, 5.41, and 7.27 ms, repetition time = 2530ms, inversion time = 1200–1300ms, 1.0 mm3 isotropic resolution, flip angle = 7°. Patients received sedation before or during the acute MRI at the discretion of the treating clinicians.
The block design fMRI language paradigm consisted of two runs. Each run included three 24-second rest blocks and two 24-second stimulation blocks. The first stimulation block was a clip from John F. Kennedy’s (JFK) Inaugural Address played forward, while the second block was the same clip played backward. The backward language condition was not included in this study.
fMRI preprocessing and first-level analysis
First-level analysis used the FMRI Expert Analysis Tool (FEAT) (26, 27) version 6.00 in FSL 5.0.7 (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl) (28). Structural and functional volumes were normalized into Montreal Neurological Institute (MNI) space. We applied motion correction using MCFLIRT (29), brain extraction using BET (30), and spatial smoothing using a 10 mm FWHM Gaussian kernel. To further minimize motion-related confounding, we supplemented standard MCFLIRT motion correction with extraction of rotational and translational motion outliers for each dataset using the “fsl_motion_outliers” command. We then included these motion outliers as additional confounder covariates in the general linear model. Absolute motion parameters as well as the number of timepoints excluded from analysis due to excessive motion are presented in Supplementary Table S1. We contrasted forward language with rest. The resulting Z-statistic images were cluster thresholded (Z ≥ 3.1 and P ≤ 0.05) (31).
Functional MRI region-of-interest (ROI) analysis
We selected frontotemporal ROIs (STGL, STGR, IFGL, IFGR; Supplemental Materials; Figure S1) based on prior fMRI studies of language (6, 21, 32–35) and created ROIs using the Harvard-Oxford Cortical atlas with a probability threshold of 5%. The threshold of at least 5% probability that a given voxel is within STG or IFG was chosen to account for variability in functional responses to language after severe TBI, the effects of lesions on the spatial distribution of the BOLD signal, and potential neural reorganization within the canonical language network. We extracted the mean Z-score for the voxels within each ROI using FEATquery in the FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fsl). In this context, the Z-score represents the average BOLD signal intensity in each ROI in response to the language stimulus relative to the mean BOLD signal intensity of all voxels in the brain. We tested for differences between mean Z-scores in acute and follow-up scans using a Wilcoxon signed-rank test with Bonferroni correction (corrected P significance threshold = 0.0125). An increased response to the fMRI stimulus was defined as a statistically significant difference between mean Z-scores of suprathreshold voxels in acute and follow-up fMRI scans within each ROI. We performed statistical analyses with GraphPad Prism 7 (GraphPad; LaJolla, CA).
Functional MRI whole-brain analysis
We conducted whole-brain analyses using the fixed-effects model within FSL (FMRIB’s Local Analysis of Mixed Effects; FLAME) and generated individual and group-level cluster-thresholded Z-statistic maps (Z ≥ 3.1 and P ≤ 0.05). We also compared fMRI responses to language stimuli between patients and healthy subjects at thresholds of Z ≥ 3.1. Given the low statistical power of our study, we also performed the comparison at the less conservative threshold of Z ≥ 2.3, which was the threshold commonly used in BOLD fMRI studies prior to 2016 (31).
Data Sharing
All anonymized fMRI data are released at:: openneuro.org/datasets/ds002675. Data processing scripts can be found at: github.com/ComaRecoveryLab/LongitudinalLanguagefMRI. No part of the study procedures or analysis was pre-registered prior to the research being conducted.
Results
Patient demographics, clinical characteristics, and in-scanner motion
Injury characteristics and demographics are reported in Table 1. We enrolled 16 patients with acute severe TBI and a DoC. Two patients died in the ICU after withdrawal of life-sustaining therapy. Of the 14 patients who survived, 8 returned for follow-up MRI. The remaining 6 patients were unable or unwilling to return to the hospital for MRI assessment. Six patients received sedative, anxiolytic, or analgesic medications before or during the acute fMRI (see Supplementary Table S5 for specific medications and doses administered). Sedation was not administered to any patient for the follow-up fMRI. On average, prior to filtering out individual timepoints with excessive motion, patients moved in the scanner more than healthy subjects (healthy versus acute patients p < 0.02; healthy versus follow-up patients p < 0.05). The common, though arbitrary, threshold for average “high” levels of motion (i.e., 0.5 mm displacement when comparing the middle volume to all other volumes) was exceeded by two patients acutely (patients 5 and 8) (29). Nevertheless, motion parameters did not differ between patients scanned acutely versus at follow-up. Notably, the number of timepoints excluded in the analysis due to excessive motion did not differ between healthy subjects and patients (see Supplementary Table 1).
Table 1.
Patient Demographics
| Patient ID | Age (yrs) | Sex | TBI mechanism | iGCS | Handedness | Day of acute fMRI | CRS-R subscale scores at acute fMRI | LoC at acute fMRI | Day of follow-up fMRI | Follow-up CVLT-II trial 1 total correct words, (standard score)c | Focal lesions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | M | MVA | 5T | R | 16 | A4V5M6O3C2Ar3 | PTCS* | 206 | 4 (−1.5) | R frontal, temporal, and parietal contusions; R frontal SDH |
| 2 | 21 | M | Ped vs. car | 4–8T | R | 1 | A0V0M3O1C0Ar0 | MCS | 174 | 11 (2.0) | L frontal evidence of prior EVD tract |
| 3 | 19 | F | MVA | 5T | R | 3 | A0V0M1O0C0Ar0 | Coma* | 371 | 7 (−0.5) | None |
| 4 | 19 | M | Fall | 3–7T | Amb | 17 | A4V5M6O3C2Ar3 | PTCS | 576 | 9 (1.0) | Bifrontal and R temporal contusions |
| 5 | 28 | F | MVA | 3 | L | 7 | A0V1M2O1C0Ar2 | VS* | 656 | 7 (−0.5) | L frontal contusion |
| 6 | 22 | F | Ped vs. car | 6T | R | 14 | A4V5M6O3C1Ar3 | PTCS* | 187 | 7 (−0.5) | L temporo-parietal contusion; R thalamic hemorrhage |
| 7 | 29 | M | Ped vs. car | 4–7 | R | 7 | A0V0M3O0C0Ar0 | MCS* | 235 | 8 (0.5) | L insular contusion; R frontal evidence of prior EVD tract |
| 8 | 33 | M | Fall | 3–4 | L | 3 | A4V2M5O0C0Ar1 | MCS* | 191 | 5 (−1) | None |
| Patients (8) | 24.5 [20–28.5]a | 5M | 5R, 2L, 1 Amb | 7 [3–15]a |
1 Coma, 1 VS, 3 MCS, 3 PTCS | 220.5 [189–473.5]a |
7.25 (2.2)b |
||||
| Controls (16) | 27 [22–32]a |
12M | 13R, 3L |
The initial Glasgow Coma Scale (iGCS) range is defined by the best (i.e. highest) and worst (i.e. lowest) post-resuscitation GCS scores assessed by a qualified clinician who performed a reliable examination (not confounded by sedation and/or paralytics) prior to intensive care unit admission. Level of consciousness (LoC) is assessed via behavioral evaluation with the Coma Recovery Scale-Revised (CRS-R) as coma, vegetative state (VS), minimally conscious state (MCS), or post-traumatic confusional state (PTCS; emerged from MCS but disoriented). The subscales for the CRS-R are Auditory Function (A), Visual Function (V), Motor Function (M), Oromotor Function (O), Communication (C), and Arousal (Ar); bolded subscales are those that rely on language function. Additional abbreviations: Amb = Ambidextrous; CVLT-II = California Verbal Learning Test-II; EVD = external ventricular drain; F = female; M = male; MVA = motor vehicle accident; Ped = pedestrian; SDH = subdural hemorrhage; T = intubated; TBI = traumatic brain injury.
median [interquartile range];
mean (standard deviation);
the standard score is derived based on a normative sample provided by the CVLT-II Comprehensive Scoring System (San Antonio, TX). The average CVLT-II score for the patient group is within the mean (SD) Trial 1 CVLT-II score of healthy subjects (mean=6.0–7.0 (2.0)) reported in prior studies (23, 36).
patient received sedative, anxiolytic, or analgesic medication at before or during acute fMRI (see Supplemental Material, Table S5 for specific medications and doses
Standardized behavioral language assessments
All patients who returned for follow-up fMRI recovered basic language function, as demonstrated by the following standardized assessments: 1) CRS-R total score of 23 (the maximum possible score), 2) correct responses to all semantically complex questions on the CAP and 3) valid completion of the CVLT-II.
On the first trial of the CVLT-II immediate recall test, the mean (SD) total words recalled was 7 (2.2), which is within one SD of the group-level mean score (i.e., mean = 6.0–7.0 (2.0)) reported in prior studies of healthy subjects (23, 36). Moreover, all individual patient CVLT-II scores were no more than 1.5 standard deviations away from the healthy normative data provided by the CVLT-II Comprehensive Scoring System (San Antonio, TX), suggesting intact basic language comprehension and expression. CVLT-II scores for the remainder of the assessment are not reported as they are indicators of verbal memory and learning rather than language function.
ROI-based fMRI responses
Acute fMRI responses observed in patients with severe TBI in the ICU, as well as in healthy controls, were reported previously (21). Longitudinally, we observed an increase in language-evoked fMRI responses in the STGL (p=0.0117, significant after multiple-comparison correction), but not in the STGR, IFGL, or IFGR (p=0.0296, p=0.0193, and p=0.4355, respectively; Figure 1).
Fig. 1 – Region-of-interest analysis shows significant longitudinal increase in left STG responses.
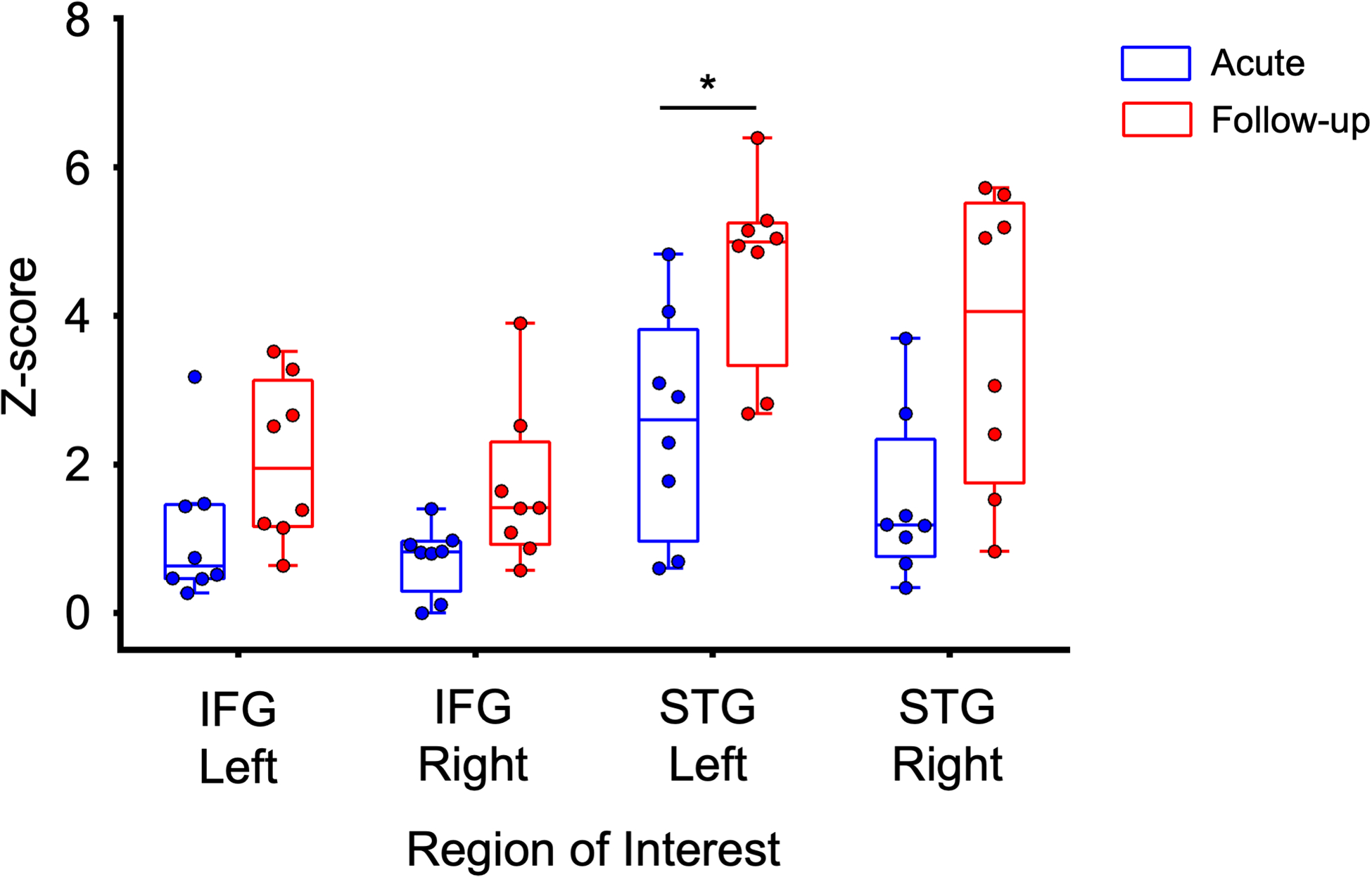
The mean Z-score of suprathreshold activation in response to the language stimulus is shown stratified by region of interest in acute (blue) and follow-up (red) scans. Box plots illustrate median and interquartile ranges across subjects. There is a significant increase in activation between acute and follow-up scans in the left superior temporal gyrus (STG), but not right STG, left inferior frontal gyrus (IFG) and right IFG. We used a Wilcoxon signed-rank test for statistical comparison, Bonferroni corrected for multiple comparisons. * corrected P significance threshold = 0.0125
Whole-brain fMRI responses
In healthy subjects, whole-brain fMRI responses to spoken language compared to rest were observed in the bilateral STG and IFG, as well as other cortical and subcortical regions underlying language processing (Figure 2). Group-level acute patient fMRI responses were limited to language-related regions within the left hemisphere, including the planum temporale in the posterior STG. At follow-up, patients showed responses within the bilateral STG, as well as the right supramarginal, angular and middle temporal gyri. When comparing follow-up to acute patient responses, there were significant longitudinal increases within the right supramarginal and middle temporal gyri (Figure 2), including peaks within the STGR ROI. MNI coordinates of group-level cluster peaks and local maxima are provided in Supplementary Table S2.
Fig. 2 – Whole-brain analysis of longitudinal functional MRI responses to language stimuli.
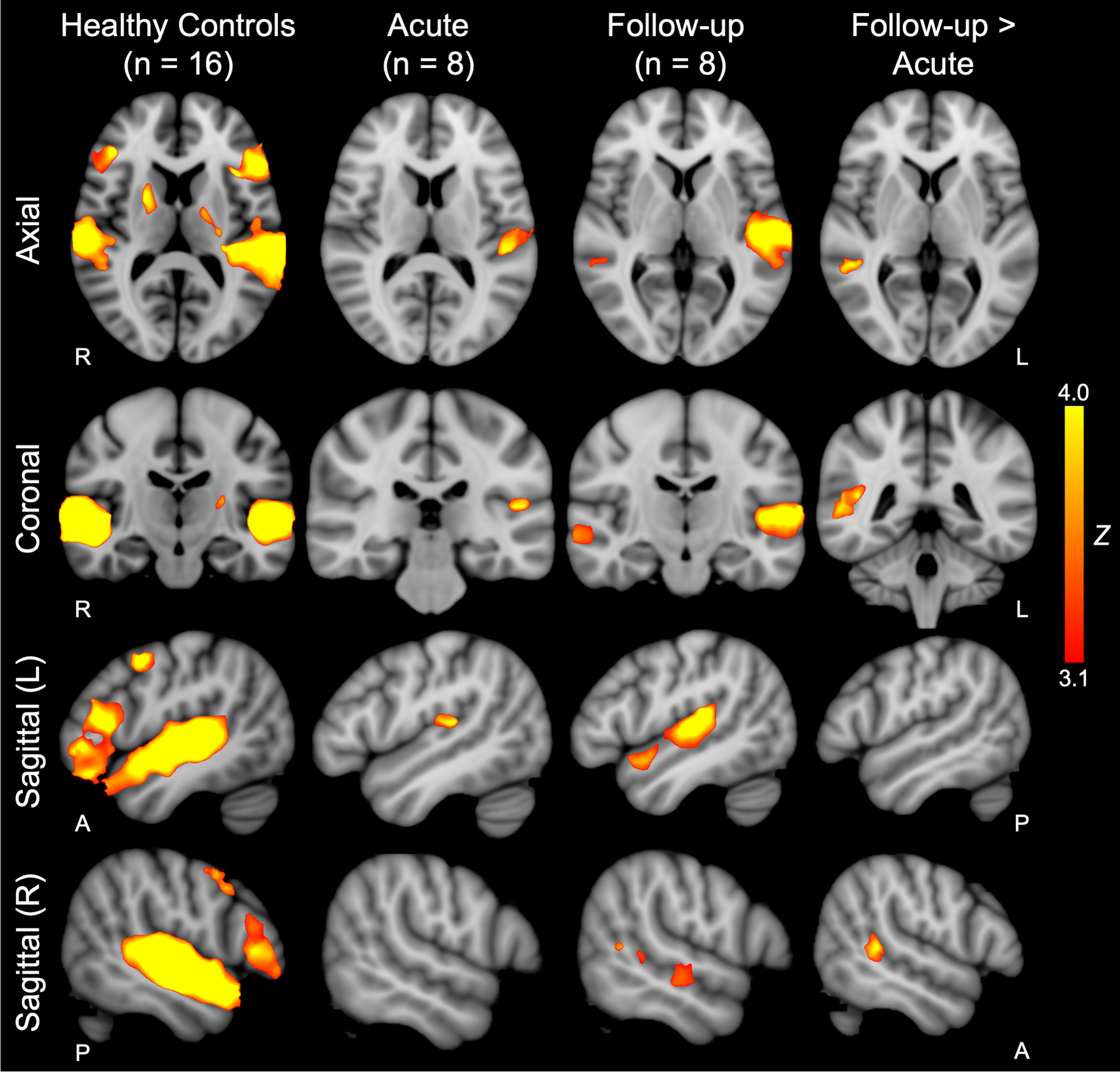
Whole-brain group-level functional MRI (fMRI) responses to language stimuli in 16 healthy age- and sex-matched controls are shown in the left column. Acute and follow-up whole-brain group-level patient responses to language stimuli are shown in the second and third columns from the left, respectively. Whole-brain group-level longitudinal comparison of follow-up > acute fMRI responses to language stimuli are shown in the right column. Z-statistic maps are cluster-thresholded (Z ≥ 3.1 and P ≤ 0.05). See Supplemental Material; Table S2 for local maxima coordinates and Harvard-Oxford Cortical Atlas labels. Z = Z-score.
Individual Subject Results
Individual subject results are presented in Table 2, Figure 3 (healthy control subjects) and Figure 4 (patients). Supplementary Table S3 presents the single-subject statistical analysis of each of the four language ROIs. BOLD responses in STGL, STGR, or both were observed in all 16 healthy subjects. BOLD responses in in IFGL, IFGR, or both were observed in 14 healthy subjects. Three patients (Patient 3, 4, and 6) did not have STGL activation acutely, but recovered STGL activation by follow-up assessment. Of the six patients (Patients 1, 3, 4, 5, 6, and 8) who did not have STGR activation acutely, five recovered STGR activation by the follow-up MRI. The one patient (Patient 1) who did not show STGR activation at follow-up had significant encephalomalacia in the right temporal lobe due to a large contusion. Five patients (Patient 3, 4, 5, 6, and 7) did not have IFGL activation acutely, and three recovered IFGL activation at follow-up (Patient 4, 6 and 7). Four patients (Patients 3, 4, 7, 8) did not have IFGR activation acutely and two (Patients 4, and 8) recovered IFGR activation at follow-up.
Table 2:
Individual subject results of fMRI BOLD responses to speech in the canonical language network
| ROI | |||||
|---|---|---|---|---|---|
| IFG Left | IFG Right | STG Left | STG Right | ||
| Healthy Subjects | |||||
| 1 | + | + | + | + | |
| 2 | + | + | + | + | |
| 3 | − | − | + | − | |
| 4 | + | + | + | + | |
| 5 | + | − | + | + | |
| 6 | + | − | + | + | |
| 7 | + | + | + | + | |
| 8 | + | + | + | + | |
| 9 | + | + | + | + | |
| 10 | + | + | + | + | |
| 11 | + | + | + | + | |
| 12 | − | − | − | + | |
| 13 | − | + | + | + | |
| 14 | + | + | + | + | |
| 15 | + | + | + | + | |
| 16 | + | + | + | + | |
| Patients | |||||
| 1 | Acute* | + | + | + | − |
| Follow-up | + | − | + | − | |
| 2 | Acute | + | + | + | + |
| Follow-up | + | + | + | + | |
| 3 | Acute* | − | − | − | − |
| Follow-up | − | − | + | + | |
| 4 | Acute | − | − | − | − |
| Follow-up | + | + | + | + | |
| 5 | Acute* | − | + | + | − |
| Follow-up | − | − | + | + | |
| 6 | Acute* | − | + | − | − |
| Follow-up | + | + | + | + | |
| 7 | Acute* | − | − | + | + |
| Follow-up | + | − | + | + | |
| 8 | Acute* | + | − | + | − |
| Follow-up | + | + | + | + | |
For each subject and each ROI, a “+” indicates that an fMRI BOLD response to speech was detected while a “−“ indicates that an fMRI BOLD response to speech was not detected. In all healthy subjects, responses were detected in right, left or bilateral STG. In 14 healthy subjects, responses were detected in right, left or bilateral IFG. No IFG response were detected in 2 healthy subjects. In 5 patients, responses were detected in right, left or bilateral STG acutely and in all 8 patients, responses were detected in right, left or bilateral STG at follow-up. Similarly, in 5 patients, responses were detected in right, left or bilateral IFG acutely. In 6 patients, responses were detected in right, left or bilateral IFG at follow-up
patient received sedative, anxiolytic, or analgesic medication before or during the acute functional MRI (see Supplemental Material, Table S5 for specific medications and doses).
Fig. 3 -. Whole-brain functional MRI responses to language stimuli in individual healthy control subjects.
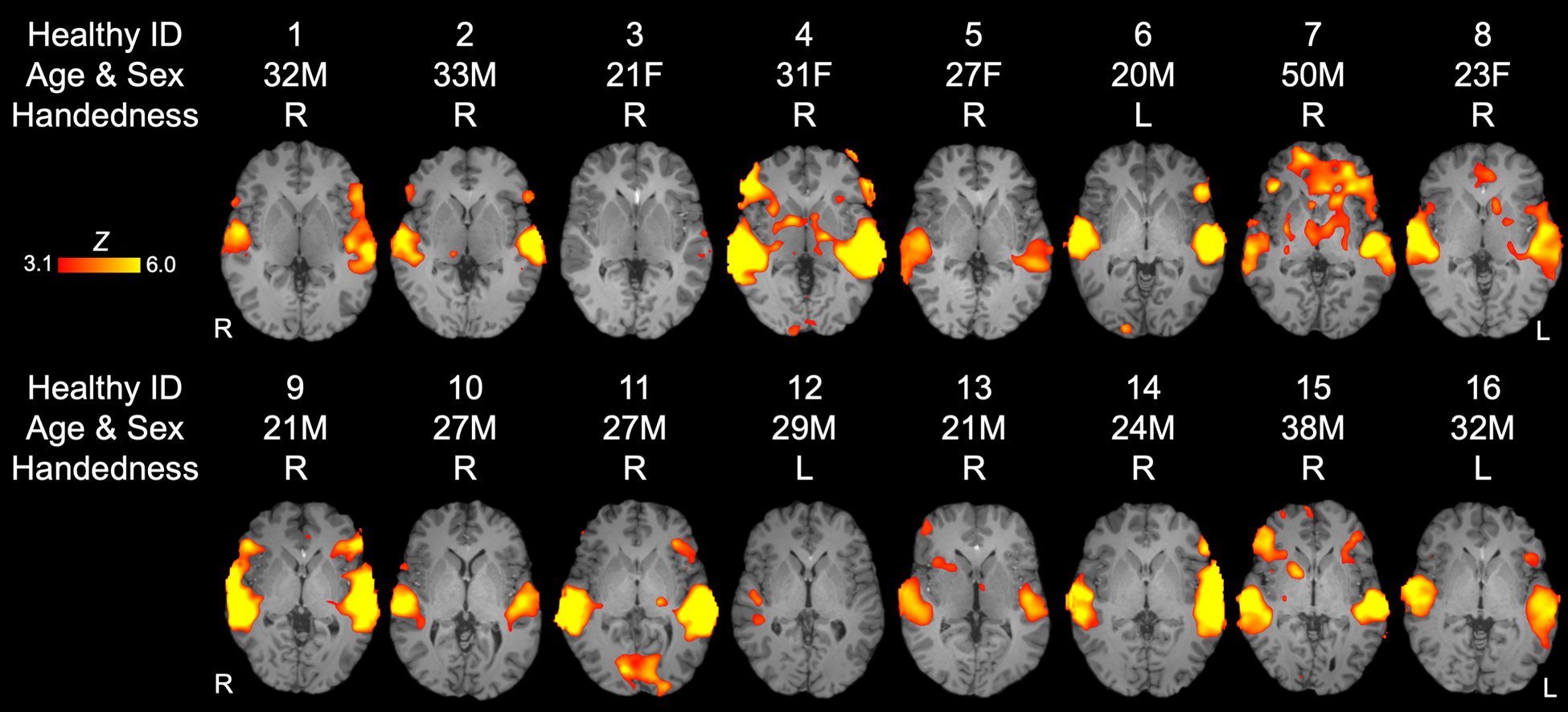
Z-statistic maps are cluster-thresholded (Z ≥ 3.1 and P ≤ 0.05). See Supplemental Material; Table S3 for qualitative data on individual fMRI responses to language stimuli within each language-related related region of interest. Abbreviations: Amb = ambidextrous; F = female; L = left; M = male; R = right.
Fig. 4 -. Whole-brain functional MRI responses to language stimuli in individual patients at acute and follow-up time points.
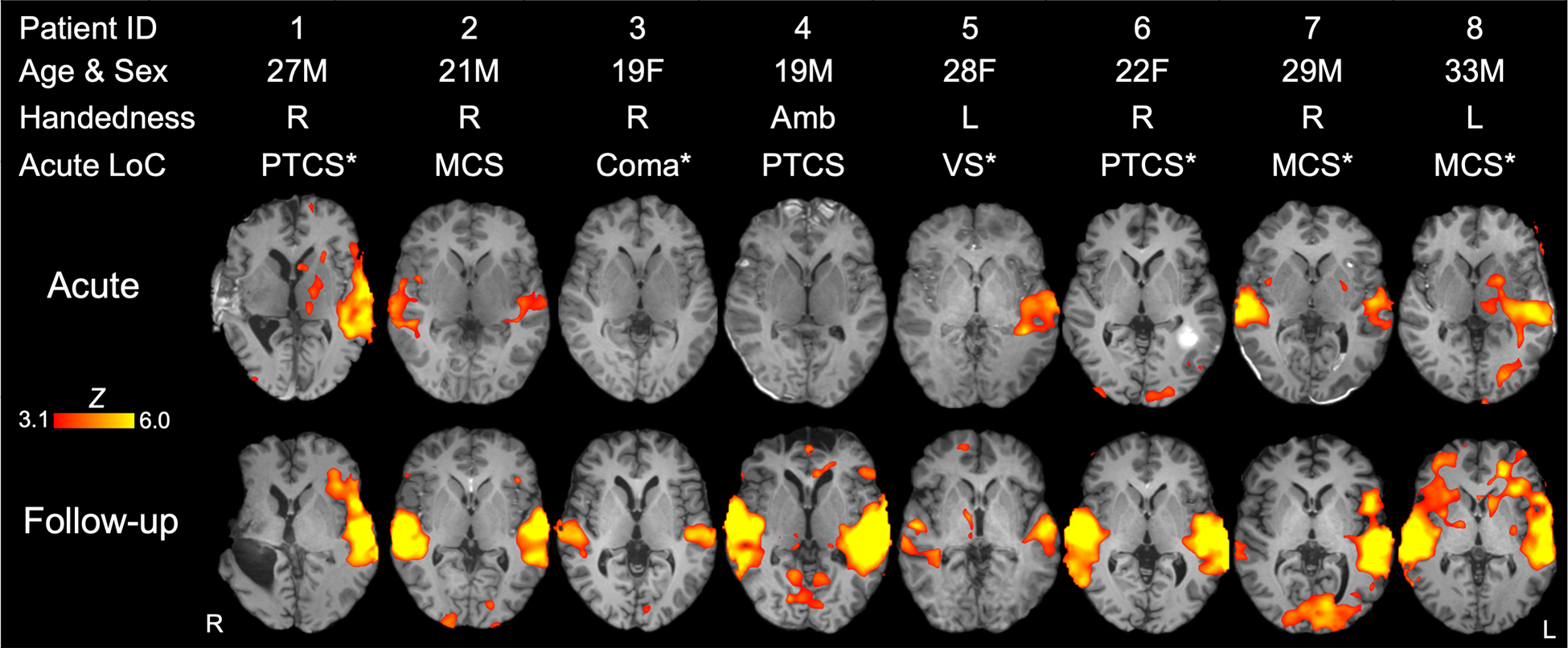
Z-statistic maps are cluster-thresholded (Z ≥ 3.1 and P ≤ 0.05). See Supplemental Material; Table S3 for qualitative data on individual fMRI responses to language stimuli within each language-related related region of interest. Abbreviations: Amb = ambidextrous; F = female; L = left; LoC = level of consciousness; M = male; MCS = minimally conscious state; PTCS = post-traumatic confusional state; R = right; VS = vegetative state; * = patient received sedative, anxiolytic, or analgesic medication before or during the acute functional MRI (see Supplemental Material, Table S5 for specific medications and doses).
Healthy Control Subjects Compared with Patients
There were no significant differences in fMRI responses to spoken language between healthy control subjects and patients acutely or at follow-up at the statistical threshold of Z ≥ 3.1. However, when the threshold was decreased to Z ≥ 2.3, patients assessed acutely had reduced activation relative to healthy subjects in right hemispheric regions including the STGR and IFGR (Figure 5 and Supplementary Table S4), while patients at follow-up remained indistinguishable from healthy subjects.
Fig. 5 -. Regions for which fMRI responses to language stimuli are greater in healthy control subjects than patients acutely and at follow-up.
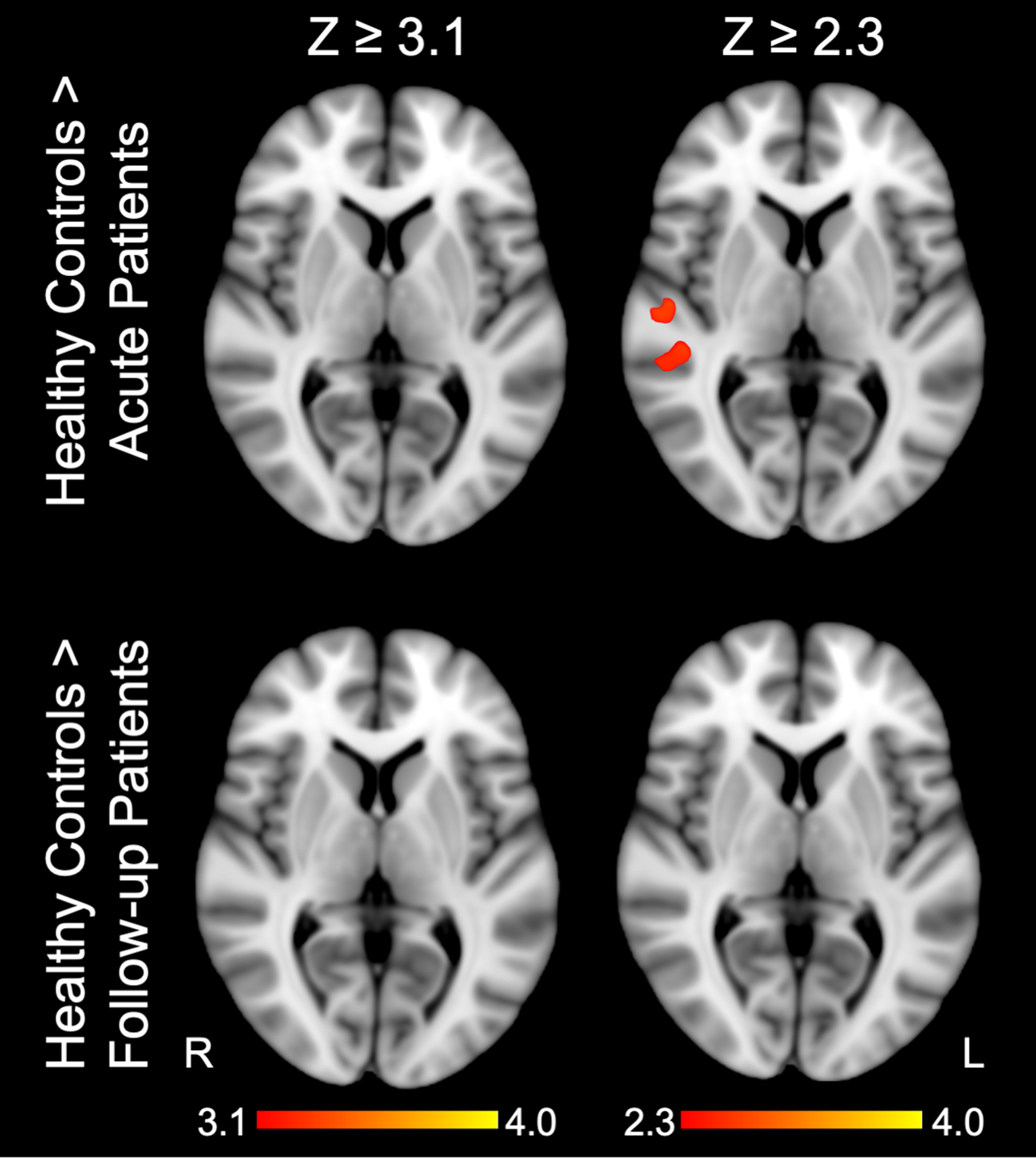
Z-statistic maps cluster-thresholded at Z ≥ 3.1 and Z ≥ 2.3. See Supplemental Materials; Table S4 for local maxima coordinates and Harvard-Oxford Cortical Atlas labels. Z = Z-score.
Discussion
In this pilot study of patients with severe TBI who recovered basic language function, we observed longitudinal increases in fMRI responses to spoken language within the STGL, right supramarginal gyrus, and right middle temporal gyrus. Although longitudinal increases in fMRI responses to speech were observed across all a priori selected ROIs, these changes did not reach statistical significance in the STGR, IFGR, and IFGL after correction for multiple comparisons. Due to the small sample size and multiple limitations detailed below, the findings in this study are preliminary. Nevertheless, the results suggest that, during recovery from severe TBI, changes within cortical nodes of the canonical language network are nonuniform and that reemergence of the entire language network may not be necessary to support recovery of language function. While the mechanistic contributions of the right supramarginal and right middle temporal gyri to language recovery are not fully understood, the supramarginal gyrus is connected to both the IFG and STG by branches of the arcuate fasciculus (37) and is believed to integrate the auditory representation of spoken words with their meaning (38). Evidence from fMRI and transcranial magnetic stimulation studies indicate that the middle temporal gyrus also contributes to comprehension of spoken and written words (39–41). Collectively, these findings provide early evidence that recovery of language after severe TBI is associated with reemergence of responses within and outside the canonical language network.
Current theories of speech processing involve dual cortical streams, a ventral stream (including STG and IFG) that is preferentially involved in decoding the meaning of spoken language and a dorsal stream (including pars opercularis, premotor areas, and posterior regions in the supramarginal gyrus) underlying articulation of spoken output. Based on this conceptualization, our fMRI paradigm, which involves passive listening to JFK’s inaugural address, should primarily target the ventral stream. However, our findings in healthy subjects and patients with severe TBI suggest additional language processing by the dorsal stream, which may be implicated in articulation of speech (42, 43). Dorsal stream processing may also suggest familiarity with the presented speech stimulus, although our subjects were likely only familiar with the famous “ask not what your country can do for you – ask what you can do for your country” quote rather than the entire inaugural address. Alternatively, studies supporting the dual stream model of language processing may not generalize to our sample because they: 1) rely largely on data from non-human primates, healthy humans, or patients with stroke (11), 2) utilize non-standardized fMRI paradigms, or 3) apply structural lesion mapping to infer function (10). Finally, if TBI disrupts the dual stream processing of language, it is possible that longitudinal reorganization of the language network reduces the functional dissociation between the ventral and dorsal streams.
Acutely, we anticipated decreased fMRI responses in the language versus rest condition in patients compared to healthy subjects. Although we did not find this difference at a stringent statistical threshold (31), at a lower threshold of Z ≥ 2.3, patients acutely had decreased right hemispheric cortical responses, including in STGR and IFGR. A larger sample size may have revealed this difference at the more stringent threshold. In the absence of pre-injury fMRI data, we cannot definitively differentiate between reorganization (i.e., neuroplasticity) and reemergence of cortical functions underlying language processing. However, fMRI activation maps of patients at follow-up were indistinguishable from healthy control subjects at Z ≥ 2.3, suggesting that recovery was primarily driven by reemergence, not reorganization, of language-processing regions.
Our results, generated from a small but unique longitudinal sample, should be considered in the context of multiple challenges associated with conducting imaging studies in critically ill patients. First, transporting patients from the ICU to the MRI scanner requires a travel ventilator, administration of multiple continuous intravenous infusions, and care by nurses, physicians, respiratory therapists and MRI technologists to ensure that lines and tubes do not become dislodged. Second, lying supine in an MRI scanner may exacerbate intracranial hypertension, delaying or precluding data acquisition during the acute phase of recovery. For patients who survive their ICU hospitalization, returning for follow-up fMRI studies is also difficult due to ongoing medical issues, complex transportation needs, and psychosocial factors.
Because TBI pathology is heterogenous and recovery is dynamic over the first six months after injury, we cannot determine whether fMRI responses are affected by specific neuroanatomic distributions of lesions or the total volume of injured brain tissue. In our study, both age and structural lesion site varied across patients, potentially influencing our results. It is for this reason, and to ensure that we were capturing the full variability in the location of language processing regions, that we chose a liberal ROI of voxels that were in the STG or IFG with at least 5% probability. This approach, which has been used in prior studies (21), decreases the false negative rate (i.e. missing voxels within an individual’s canonical language network) but increases the potential for false positive fMRI responses if voxels included in the ROI are outside the canonical language network. Furthermore, because patients moved in the scanner more than healthy subjects and two patients exceeded the threshold for “high” motion, it is possible that the decreased activations in patients compared to healthy subjects may be attributed to motion (44). Given the limitations in this pilot study, multi-center collaborations will be required to conduct large, rigorous trials that further elucidate the neurobiological processes underlying recovery of language after severe TBI.
The frequent need for patients to be sedated in the ICU, when the information provided by fMRI may be most useful, is especially problematic for studies of acute severe TBI as sedation may confound fMRI results. The relationship between sedation and fMRI responses is complex and difficult to measure because multiple patient-specific factors, including body mass, tolerance, metabolism, and clearance, may alter the effect of a sedative on the BOLD response. However, cortical function may be altered by severe brain injury more than by sedation, as evidenced by studies reporting cortical responses to a variety of stimuli even under varying levels of sedation (21, 32, 45, 46). Indeed, four of the six patients requiring sedation in the ICU demonstrated acute language-evoked responses in regions underlying language function. Nevertheless, given that the role of sedating medications on cortical responsiveness is not fully understood, the longitudinal findings in this study may be influenced by acute administration of these medications. In the absence of sedation, the two patients without acute fMRI responses may have responded to the language stimuli and the four patients with acute fMRI responses may have demonstrated stronger fMRI signals, ultimately leading to different longitudinal fMRI changes. The contribution of sedation to fMRI responses in patients with severe brain injuries requires further study, especially as the field moves towards clinical implications of these technologies (19, 47).
Notably, two patients in PTCS did not have suprathreshold language-evoked fMRI responses in the bilateral STG or IFG acutely (Patients 4 and 6; Figure 4). There are three potential explanations for this unexpected finding. First, it is possible that recovery of consciousness and recovery of language function were dissociable in these patients. Second, a lack of fMRI responses in the bilateral STG or IFG may be due to normal variability in response to the spoken language stimulus. Consistent with prior studies, our results suggest that even healthy individuals show variable responses to spoken language stimuli (48). Finally, the lack of a response in Patient 6 may have been attributable to the effects of lorazepam and haloperidol (though no medications were given to Patient 4). It is also notable that, because patients were critically ill at the time of enrollment, our fMRI paradigm was relatively brief. A longer paradigm may have increased the signal-to-noise ratio of the fMRI data, thereby reducing intra- and inter-subject variance.
Because our study did not include patients with persistently altered basic language function (i.e., aphasia), we are unable to determine whether our fMRI findings are specific to patients who recover language or are generalizable to all patients with acute DoC after severe TBI. Furthermore, although our analyses revealed fMRI changes specific to language cortex, we cannot exclude the possibility that the changes we observed may reflect recovery of consciousness, which co-occurred with recovery of language function. We also did not directly test other aspects of language function, such as confrontation naming, responsive naming, or verbal fluency. The mechanisms underlying language recovery after severe TBI therefore require further study in a cohort of subjects with varying levels of language function and the incorporation of more varied and robust measures of language function. Notably, our findings are consistent with prior studies suggesting that cortical responses to language stimuli may predict subsequent behavioral recovery (7, 18, 20, 33). Moreover, recent guidelines endorsed by multiple professional societies recommend fMRI as part of a multimodal approach to diagnosis and prognosis in the clinical management of patients with DoC (19, 47). These recommendations highlight the clinical relevance of stimulus-based functional neuroimaging for patients with severe brain injury. Studies involving serial fMRI and comprehensive language function assessment are needed to determine the temporal relationship between reemergence of fMRI responses in language cortices and recovery of language function.
Conclusion
In summary, we provide initial evidence that fMRI responses reemerge in multiple bihemispheric nodes within the language network in patients who recover basic language function after severe TBI. Furthermore, rather than reorganizing or integrating new nodes, our findings suggest that the language network can be restored following severe TBI. These results provide a foundation for further testing of stimulus-based fMRI as a potential clinical biomarker of language recovery, which could be leveraged to develop pharmacologic and rehabilitative interventions.
Supplementary Material
Acknowledgements
We thank the nursing staff of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. We also thank the Massachusetts General Hospital MRI technologists for assistance with data acquisition. We are grateful to the patients and families involved in this study for their participation and support.
Funding:
This work was supported by the NIH Director’s Office (DP2HD101400), NIH National Institute of Neurological Disorders and Stroke (K23NS094538, R21NS109627, RF1NS115268), James S. McDonnell Foundation, Rappaport Foundation, and Tiny Blue Dot Foundation.
Role of the funding sources:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Footnotes
Declarations of interest: none.
References
- 1.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–9. Epub 2004/12/18. [DOI] [PubMed] [Google Scholar]
- 2.Whyte J, Cifu D, Dikmen S, Temkin N. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil. 2001;82(10):1355–9. Epub 2001/10/06. doi: 10.1053/apmr.2001.26091. [DOI] [PubMed] [Google Scholar]
- 3.Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol. 2019. Epub 2019/11/28. doi: 10.1007/s00415-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 4.Douglas JM, Bracy CA, Snow PC. Return to Work and Social Communication Ability Following Severe Traumatic Brain Injury. J Speech Lang Hear Res. 2016;59(3):511–20. Epub 2016/04/29. doi: 10.1044/2015_JSLHR-L-15-0025. [DOI] [PubMed] [Google Scholar]
- 5.Demir SO, Altinok N, Aydin G, Koseoglu F. Functional and cognitive progress in aphasic patients with traumatic brain injury during post-acute phase. Brain Inj. 2006;20(13–14):1383–90. Epub 2007/03/24. doi: 10.1080/02699050601081844. [DOI] [PubMed] [Google Scholar]
- 6.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–47. Epub 2012/05/16. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman MR, Rodd JM, Davis MH, Johnsrude IS, Menon DK, Pickard JD, et al. Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain. 2007;130(Pt 10):2494–507. Epub 2007/09/11. doi: 10.1093/brain/awm170. [DOI] [PubMed] [Google Scholar]
- 8.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6(2):78–84. Epub 2005/05/04. [DOI] [PubMed] [Google Scholar]
- 9.Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs right handers. Neuroimage. 1998;8(1):1–16. Epub 1998/08/12. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- 10.Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci U S A. 2016;113(52):15108–13. Epub 2016/12/14. doi: 10.1073/pnas.1614038114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. Epub 2007/04/14. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 12.Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115 (Pt 6):1753–68. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 13.Scott S The neural processing of phonetic information: the role of the superior temporal gyrus. In: Mody M, editor. Neural Mechanisms of Language. New York: Springer; 2000. p. 11–25. [Google Scholar]
- 14.Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114 (Pt 4):1803–17. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam MM. From sensation to cognition. Brain. 1998;121 (Pt 6):1013–52. Epub 1998/07/02. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 16.Jung-Beeman M Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9(11):512–8. Epub 2005/10/11. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Vigneau M, Beaucousin V, Herve PY, Jobard G, Petit L, Crivello F, et al. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage. 2011;54(1):577–93. Epub 2010/07/27. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Coleman MR, Davis MH, Rodd JM, Robson T, Ali A, Owen AM, et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain. 2009;132(Pt 9):2541–52. Epub 2009/08/28. doi: 10.1093/brain/awp183. [DOI] [PubMed] [Google Scholar]
- 19.Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil. 2018;99(9):1699–709. Epub 2018/08/14. doi: 10.1016/j.apmr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Di H, Hu X, Jing S, Thibaut A, Di Perri C, et al. Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Med. 2015;13:83. Epub 2015/04/17. doi: 10.1186/s12916-015-0330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edlow BL, Chatelle C, Spencer CA, Chu CJ, Bodien YG, O’Connor KL, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–414. Epub 2017/10/21. doi: 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehabil. 2005;86(5):896–904. Epub 2005/05/17. doi: 10.1016/j.apmr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–20. Epub 2006/07/18. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Sherer M, Katz DI, Bodien YG, Arciniegas DB, Block C, Blum S, et al. Post-traumatic Confusional State: A Case Definition and Diagnostic Criteria. Arch Phys Med Rehabil. 2020;101(11):2041–50. Epub 2020/08/02. doi: 10.1016/j.apmr.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 25.van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–69. Epub 2008/02/05. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–19. Epub 2004/10/27. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. Epub 2002/10/16. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–5. Epub 2016/07/01. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104(41):16032–7. Epub 2007/10/17. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di HB, Yu SM, Weng XC, Laureys S, Yu D, Li JQ, et al. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology. 2007;68(12):895–9. Epub 2007/03/21. doi: 10.1212/01.wnl.0000258544.79024.d0. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33(10):2487–98. Epub 2011/09/21. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adapa RM, Davis MH, Stamatakis EA, Absalom AR, Menon DK. Neural correlates of successful semantic processing during propofol sedation. Hum Brain Mapp. 2014;35(7):2935–49. Epub 2013/10/22. doi: 10.1002/hbm.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman MA, Evans JD, Miller WS, Heaton RK. Demographically corrected norms for the California Verbal Learning Test. J Clin Exp Neuropsychol. 2000;22(1):80–94. Epub 2000/01/29. doi: 10.1076/1380-3395(200002)22:1;1-8;FT080. [DOI] [PubMed] [Google Scholar]
- 37.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–61. Epub 2008/07/11. doi: S0010–9452(08)00111–1 [pii] 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR. Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci U S A. 2010;107(38):16494–9. Epub 2010/09/03. doi: 10.1073/pnas.1008121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acheson DJ, Hagoort P. Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci. 2013;25(10):1664–77. Epub 2013/06/19. doi: 10.1162/jocn_a_00430. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J Cogn Neurosci. 2012;24(8):1766–78. Epub 2012/05/25. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- 41.Yue Q, Zhang L, Xu G, Shu H, Li P. Task-modulated activation and functional connectivity of the temporal and frontal areas during speech comprehension. Neuroscience. 2013;237:87–95. Epub 2013/01/30. doi: 10.1016/j.neuroscience.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 42.Murakami T, Kell CA, Restle J, Ugawa Y, Ziemann U. Left dorsal speech stream components and their contribution to phonological processing. J Neurosci. 2015;35(4):1411–22. Epub 2015/01/30. doi: 10.1523/JNEUROSCI.0246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sammler D, Grosbras MH, Anwander A, Bestelmeyer PE, Belin P. Dorsal and Ventral Pathways for Prosody. Curr Biol. 2015;25(23):3079–85. Epub 2015/11/10. doi: 10.1016/j.cub.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. Epub 2012/08/29. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839–47. Epub 2008/01/26. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirsch M, Guldenmund P, Ali Bahri M, Demertzi A, Baquero K, Heine L, et al. Sedation of Patients With Disorders of Consciousness During Neuroimaging: Effects on Resting State Functional Brain Connectivity. Anesth Analg. 2017;124(2):588–98. Epub 2016/12/13. doi: 10.1213/ANE.0000000000001721. [DOI] [PubMed] [Google Scholar]
- 47.Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741–56. Epub 2020/02/25. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 48.Otzenberger H, Gounot D, Marrer C, Namer IJ, Metz-Lutz MN. Reliability of individual functional MRI brain mapping of language. Neuropsychology. 2005;19(4):484–93. Epub 2005/08/03. doi: 10.1037/0894-4105.19.4.484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


