Extended Summary
Introduction
Although enhanced recovery pathways (ERP) provide a safe and effective way to improve the recovery of children undergoing bladder reconstruction, ERPs have not been widely adopted in pediatric urology. We describe a quality improvement initiative and outcomes after implementing a 24-element ERP at a single, freestanding children’s hospital.
Study Design
Multiple stakeholder meetings were planned and executed, initially with pediatric practitioners with ERP experience to understand potential implementation barriers then with anesthesiologists, nurses, case managers, and other ancillary staff to draft our institution-specific ERP. A standardized order set was generated to improve ERP adherence. ERP adherence audits and cyclic performance evaluations held every 6–9 months facilitated continuous pathway refinement. Patient outcomes were compared with a pre-ERP historic cohort.
Results
Time from initial ERP planning to first implementation was 7 months. ERP was implemented in twenty consecutive patients undergoing bladder reconstruction (median age 11.3 years, range 4.1–21.1) who were compared to twenty consecutive pre-ERP patients (median age 11.4 years, range 7.7–25.1). Median post-operative length of stay (LOS) significantly decreased from 9 days (range 2–31) pre-ERP to 4 days (range 3–29) post-ERP (p<0.05). A median of 16 (range 12–19) of 24 institutional pathway elements were implemented for each patient. Balancing measures showed no significant increases in highest Clavien complication grade, readmission rate, or unplanned return to the operating room within 30 post-operative days.
Discussion
Implementation of ERP is feasible but requires commitment from multi-disciplinary stakeholders. While we were unable to consistently achieve 80% of the elements, we successfully implemented the pathway and improved our patients’ recovery processes (indirectly reflected by a decreased post-operative LOS) with adherence to a median of 67% of elements. Our implementation and effectiveness results are specific to our center and may not be generalizable. However, our experience may offer some insight for others interested in ERP implementation and encourage initiation of their own institutional pathways.
Conclusion
Successful ERP implementation at our hospital for children undergoing bladder reconstruction was facilitated by open communication, early stakeholder involvement, and monitoring ERP adherence. ERP implementation significantly decreased LOS without increasing post-operative complications and readmissions.
Keywords: Bladder Augmentation, Enhanced Recovery Pathways, Quality Improvement
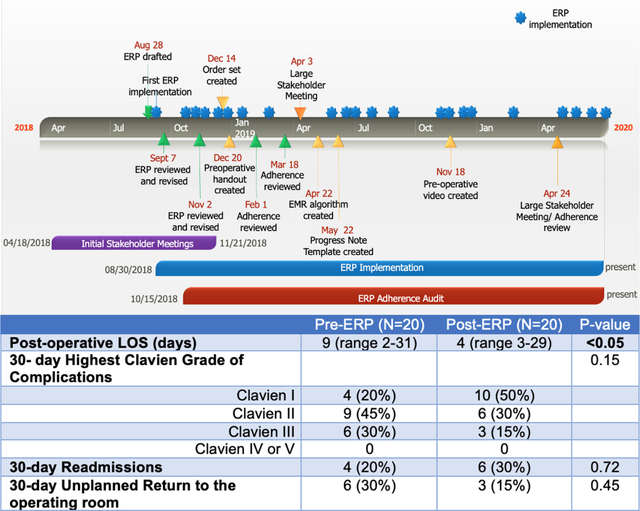
Summary Figure.
Pathway Development and Implementation Timeline and Clinical Outcomes
Introduction
Bladder reconstruction surgeries, such as bladder augmentation, bladder neck reconstruction, and creation of continent catheterizable channels, are performed in hundreds of children annually in the United States to improve their bladder capacity, protect their renal function, or facilitate social continence.[1, 2] Given the complexity of these procedures, post-operative recovery may be prolonged; average in-hospital length of stay (LOS) is 9 days, ranging from 8–12 days.[1] Recovery is often hindered by ileus leading to delayed feeding. Impaired mobility secondary to poor pain control and the presence of surgical drains further prolongs recoveries. Poor baseline nutritional status may increase risk of post-operative complications.[3] Collectively, these factors cause significant distress for patients and families.
We aimed to improve the recovery process for children undergoing bladder reconstruction at our institution. Implementation of an enhanced recovery pathway (ERP) in adults is associated with reduced LOS and complication rates.[4–7] Similar data are emerging for children undergoing bladder reconstruction.[8] The ERP promotes a multi-disciplinary approach to peri-operative care that focuses on improving post-operative mobility, peri-operative nutritional status, and reducing risk factors for post-operative ileus. We simultaneously enrolled our patients in an ongoing, multi-center trial (the Pediatric Urology Recovery after Surgery Endeavor (PURSUE)) evaluating the impact of ERPs on patients undergoing bladder reconstruction[9] and drew upon the experience of the PURSUE Study Group to implement an institutional ERP. This paper details our single-center experience with the implementation process, encountered barriers, and early outcomes.
Methods
Study Design
An institutional ERP was created and implemented as a quality improvement initiative to facilitate smoother post-operative recoveries for patients undergoing bladder reconstruction at our free-standing children’s hospital, an academic center with rotating residents/fellows and 11 pediatric urologists (four who regularly perform these procedures) where 10–15 eligible reconstructive procedures are performed annually. Patients undergoing creation of catheterizable channels with or without bowel anastomoses, bladder augmentations, bladder neck reconstructions were eligible. Patients undergoing initial urinary tract reconstruction for exstrophy-epispadias or cloaca were excluded. ERP elements addressed key drivers that prolong post-operative recuperation, including poor mobility, inadequate pain control, ileus, and differences in patient/provider expectations for recovery. We aimed to improve post-operative recovery and complications following bladder reconstruction by aiming to achieve at least 80% compliance of the ERP elements (Supplemental Figure 1).
Adherence and outcomes data were collected by retrospective chart review for patients who underwent surgery pre-ERP and for ERP patients who underwent surgery before the PURSUE study enrollment. Institutional Review Board approval was obtained, and the need for informed consent was waived for retrospective chart review (IRB 2020–3260). For patients enrolled in PURSUE, consent was obtained on day of surgery which allowed for prospective data collection (IRB 2019–2566). As PURSUE is still ongoing, this report is focused on the ERP implementation process at our single center; multi-center implementation efforts and outcomes will be reported in subsequent PURSUE Study Group manuscripts.
Multidisciplinary Team
A multidisciplinary team was assembled (Supplemental Table 1). Multiple stakeholder meetings were held from April to November 2018 (Figure 1). ERP champions from each care team, whose participation was supported by their respective administrators, were identified. These members communicated pathway changes with their colleagues, evaluated barriers to implementation, and iteratively refined the pathway. After pathway creation, core members of the team, including urology, anesthesia, and nursing, met every six to nine months to review pathway adherence and discuss further modifications. Large stakeholder meetings were held yearly. Our institutional patient safety and quality improvement department provided guidance on pathway implementation and sustainability.
Figure. 1.
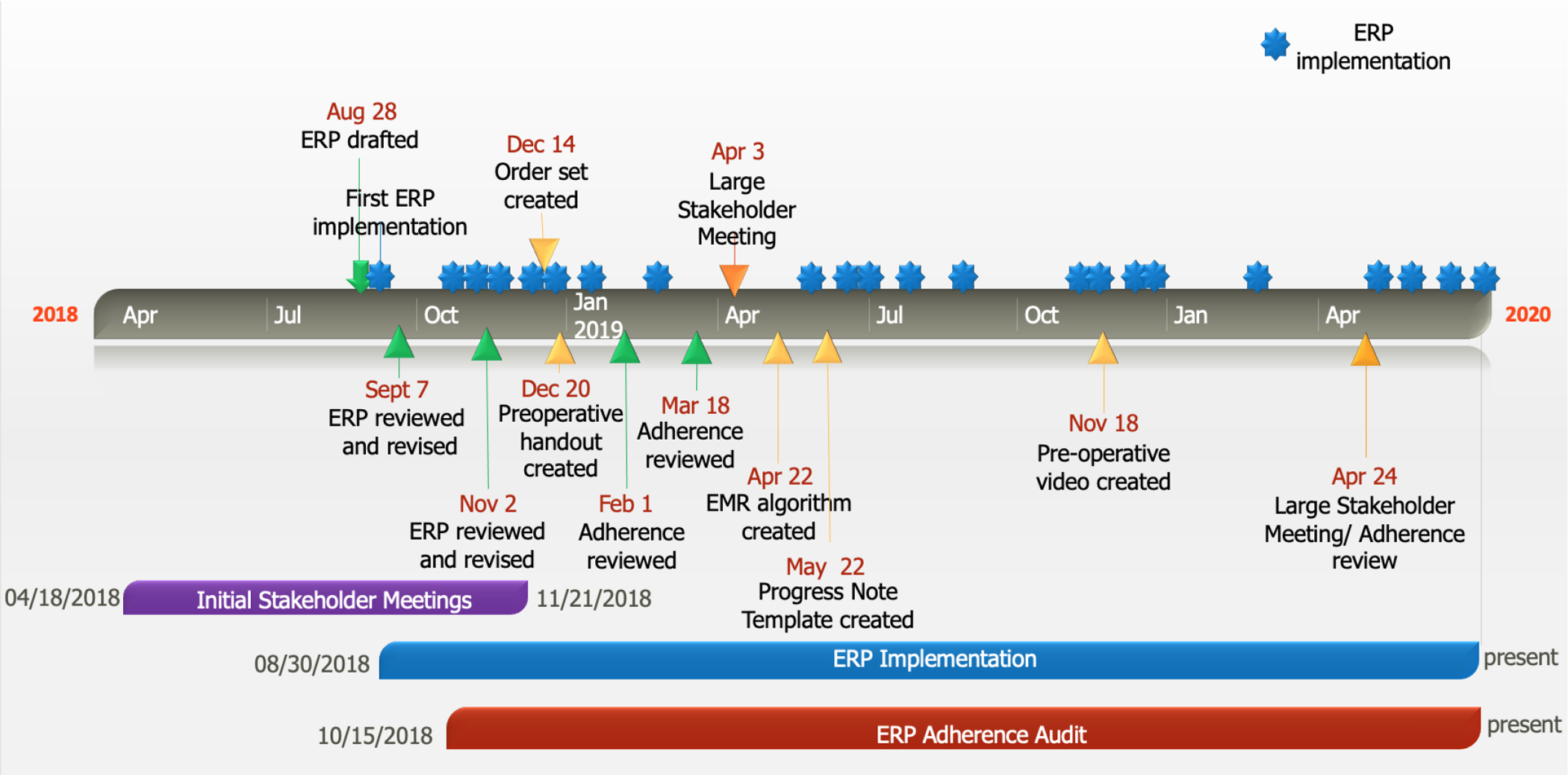
Pathway Development and Implementation Timeline
Pathway Development
Because guidelines for pediatric bladder reconstruction have not yet been established by the ERAS® society, an institutional pathway was developed with input from the PURSUE Study Group[9] and those with experience with ERP in other specialties at our institution. Members of the PURSUE Study Group shared their experiences with barriers encountered during their local implementations. Our colleagues with ERP experience in other specialties offered insight into available institutional resources that would help us overcome implementation barriers and better integrate ERP into our workflows. A final pathway consisting of 24 elements was generated (Table 1). Twenty of these elements were specified by the PURSUE Study Group. Additional institutional elements including pre-habilitation, perioperative gabapentinoids, nutritional optimization, and post-operative gum chewing were added given potential benefits cited in the literature.[10–14] Criteria for implementation of PURSUE ERP elements were defined by the PURSUE Study Group.
Table 1.
Institutional ERP Elements
| Criteria for implementation | |
|---|---|
| Pre-operative (clinic) | |
| Preoperative counseling on ERP |
|
| Carbohydrate load |
|
| Avoid prolonged fasting |
|
| Avoid bowel preparation |
|
| Prehabilitation |
|
| Pre-operative (Day of Surgery) | |
| Minimize opioids |
|
| Intra-operative | |
| Administer pre-op antibiotics within 60 minutes of incision |
|
| Thromboembolic prophylaxis |
|
| Regional anesthetic |
|
| Maintain normothermia |
|
| Maintain euvolemia |
|
| Minimize opioids |
|
| Minimize drains |
|
| Minimally invasive approach when able |
|
| Post-operative | |
| Avoid routine nasogastric tube (NGT) |
|
| Encourage sugar free gum chewing |
|
| Prevention of post-operative nausea/vomiting |
|
| Adjunct pain mediation |
|
| Minimize opioids |
|
| Early feeding |
|
| Early discontinuation of IVF |
|
| Early mobilization |
|
| Early drain removal |
|
| Peri-operative nutritional optimization |
|
Bolded elements are specified by PURSUE.
We concurrently implemented and refined the pathway as patients accrued. This approach benefited patients undergoing surgery during pathway development and enabled our team to better identify and overcome institution-specific barriers.
Key Pathway Components
Pre-operative
The pre-operative phase focused on patient counseling regarding perioperative expectations. The importance of patient and family engagement in achieving surgical milestones was emphasized for a diverse array of learners through verbal counseling in-person or by phone, written education packets, and an educational video (https://youtu.be/TpxbS5k7gwA).
Contrary to pre-ERP practices, prolonged fasting and pre-operative mechanical bowel preparation were avoided unless the patient underwent open cecostomy button placement as one of the surgical components. Patients were admitted after surgery as opposed to the day prior. Patients received an oral, liquid carbohydrate load 2–2.5 hours pre-operatively. This conflicted with our institution’s routine preoperative fasting guidance, which is more conservative than the American Society of Anesthesiologists fasting guidelines. Prescreening nurses who call patients before surgery to review instructions were engaged through an in-person in-service to ensure that ERP-specific fasting guidance was provided.
Peri-operative and Intra-operative
On the day of surgery, a multimodal pain regimen was initiated pre-operatively, and a scopolamine patch was placed for nausea prevention. Elements of ERP were incorporated into the surgical time-out to ensure adherence. After induction of anesthesia, patients with spinal pathology underwent bilateral erector spinae plane catheter placement while patients without spinal pathology received thoracic epidural catheters. Decision on type of regional anesthetic was made in collaboration with our regional pain team. Intraoperative infusions of ketamine or dexmedetomidine and intravenous acetaminophen at closing were used to minimize opioid usage. To maintain euvolemia and normothermia, infusion pumps were used to precisely guide intravenous fluid administration, and operative room staff employed blankets, forced air warmers, and ambient temperature control to keep the temperature within a predetermined range of 36–38° Celsius.
Post-operative
Patients were admitted to a urology floor post-operatively where care was provided under the supervision of the attending pediatric urologist by nurses and an inpatient urology team familiar with the pathway. Early mobility and care of urinary catheters were emphasized in the post-operative period. Patients were mobilized with assistance on post-operative day (POD) 1. Mobility goals were set by the physical therapists (PT) and were tailored to the patient’s ambulatory status and home care needs. Catheter irrigation and teaching were initiated on POD 1. Clear liquid diet was initiated on POD 0. If this is tolerated without nausea/vomiting or significant abdominal distention, diet is advanced to a regular diet. Postoperative opioid-sparing analgesia was achieved with regional anesthesia catheters, scheduled acetaminophen, gabapentinoids, nonsteroidal anti-inflammatory drugs, and as-needed muscle relaxants. Patient-controlled analgesia with hydromorphone was used for breakthrough pain. Surgical milestones were presented in a pictorial diagram in the patient’s room. Discharge planning was initiated on POD 1. Patients were discharged after meeting surgical milestones and demonstrating comfort and proficiency with catheter care.
An algorithm detailing the daily pathway elements was generated and is easily accessible in the electronic medical record. A standardized order set and progress note with data-extractable fields were created to facilitate adherence and auditing. Educational materials were created for rotating surgical trainees and new nurses.
Pathway Induction
The process of pathway induction is illustrated in Supplemental Figure 2. After a bladder reconstruction is scheduled, all ERP stakeholders are informed a week pre-operatively via electronic mail. The anesthesia ERP champion reviews the pathway with the case’s anesthesia team. The post-operative ERP order set used generates an ERP banner in the patient’s chart with a link to the algorithm. These measures help maintain pathway adherence.
Outcomes
The primary outcome metric was patient LOS. Balance metrics included 30-day readmissions, unplanned returns to the operating room (OR), and post-operative complications by Clavien grade, and the process metric assessed was ERP element adherence.[15] Outcomes of the first 20 consecutive patients on ERP (“post-ERP”) were compared to 20 consecutive patients who underwent bladder reconstruction immediately prior to ERP implementation (“pre-ERP”).
Statistical Analysis
Descriptive statistics summarized demographic variables. Fisher’s exact tests and Kruskal-Wallis tests were used to compare distributions of categorical and continuous variables, respectively. Run charts depict post-operative LOS and ERP element implementation.
Results
Time to Implementation and Early Outcomes
Time from initial planning to first ERP implementation was 7 months (Figure 1). The first ERP implementation occurred in August 2018, and the last ERP implementation in this cohort occurred in April 2020. Details of meeting discussions and pathway modifications during this period are shown in Supplemental Table 2. Outcomes were compared to 20 consecutive pre-ERP patients who underwent surgery from March 2017-July 2018. ERP implementation decreased median post-operative LOS without significantly increasing 30-day complications, readmissions, or unplanned returns to the OR. Following ERP implementation, median post-operative LOS decreased from 9 days (range 2–31) to 4 days (range 3–29) (p<0.05) (Figure 2). Highest complication Clavien grade, readmissions, and unplanned returns to the OR within the first 30 post-surgical days were not statistically different between the two cohorts (Table 2).
Figure 2. Run Chart of Post-operative Length of Stay Pre- and Post-ERP Implementation.
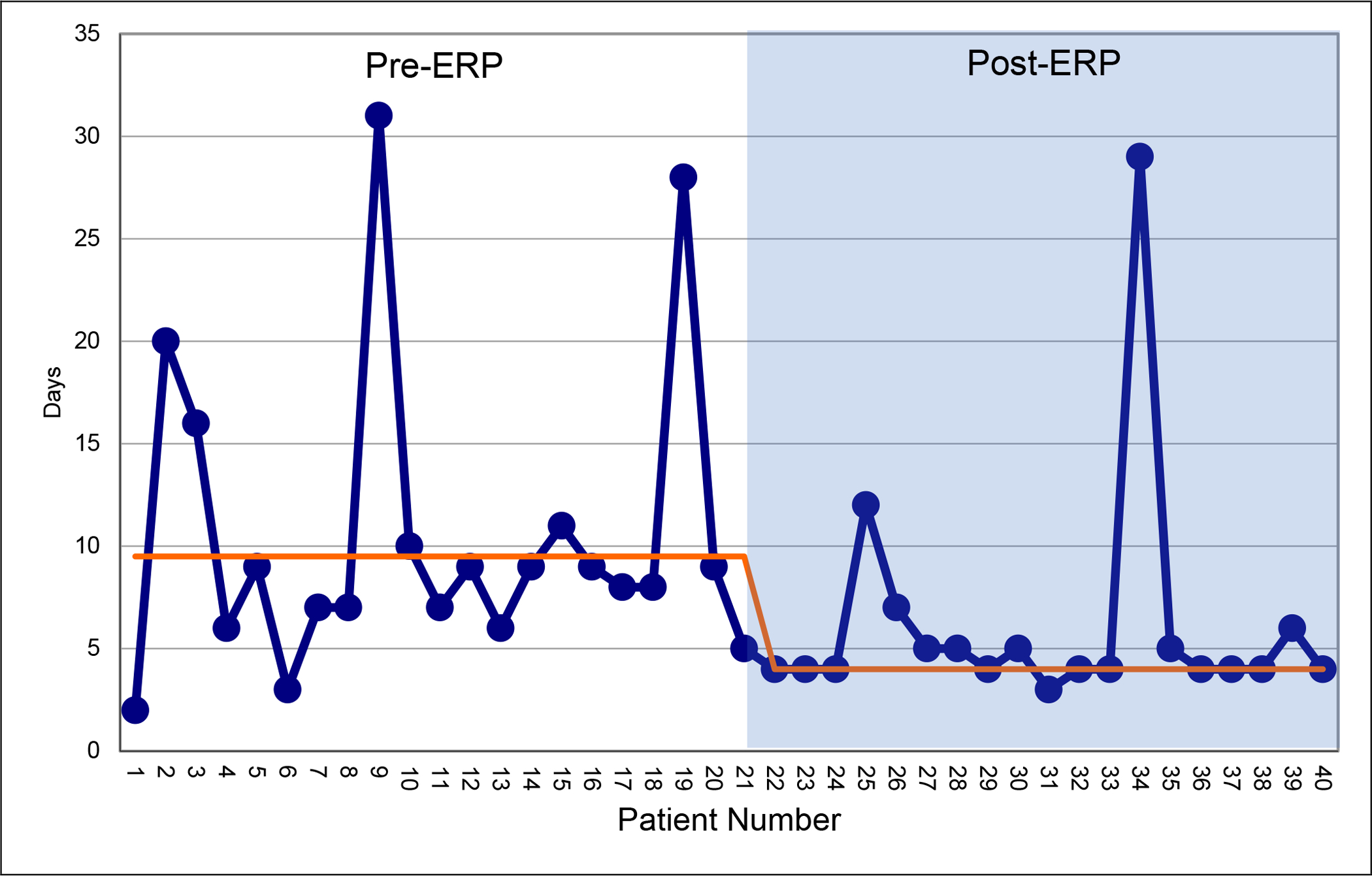
Median length of stay (LOS) is depicted by the orange line.
Table 2.
Patient Demographics and Early Outcomes
| P-value | |||
|---|---|---|---|
| Median Age at Surgery | 11.4 years (7.7–25.1) | 11.3 years (4.1–21.4) | 0.61 |
| Sex | 0.52 | ||
| Male | 7 (35%) | 10 (50%) | |
| Female | 13 (65%) | 10 (50%) | |
| Presence of Ventriculoperitoneal Shunt | 7 (35%) | 8 (40%) | 0.99 |
| Procedure * | |||
| Ileocystoplasty | 13 (65%) | 18 (90%) | 0.13 |
| Continent Catheterizable Channel | 14 (70%) | 10 (50%) | 0.33 |
| Bladder neck reconstruction, bladder neck sling, or bladder neck closure | 7 (35%) | 3 (15%) | 0.27 |
| Ureteral reimplant | 2 (10%) | 0 | 0.49 |
| Malone Antegrade Continence Enema (MACE) | 5 (25%) | 4 (20%) | 0.99 |
| Post-operative Length of Stay (days) | 9 (range 2–31) | 4 (range 3–29) | <0.05 |
| 30- day Highest Clavien Grade of Complications | 0.15 | ||
| Clavien I | 4 (20%) | 10 (50%) | |
| Clavien II | 9 (45%) | 6 (30%) | |
| Clavien III | 6 (30%) | 3 (15%) | |
| Clavien IV or V | 0 | 0 | |
| 30-day Readmissions | 4 (20%) | 6 (30%) | 0.72 |
| 30-day Unplanned Return to the Operating Room | 6 (30%) | 3 (15%) | 0.45 |
Patients may have more than one procedure
Pathway Adherence
A median of 16 of 24 (range 12–19) elements were implemented in the ERP cohort (Supplemental Figure 3). Rates of adherence to individual ERP elements are shown in Supplemental Figure 4.
Discussion
With a concerted, multidisciplinary effort, an institutional ERP was developed, implemented, and sustained for children undergoing bladder reconstruction to improve their recovery. Similar to Rove et al., ERP implementation resulted in a significant decrease in post-operative LOS[8], which may be an indirect reflection of smoother recovery processes facilitated by a streamlined and standardized post-operative care pathway. However, a change in post-operative complications was not detected, which could be secondary to our small, unmatched cohort sizes. Our initial experiences with implementation and outcomes were encouraging.
Our complications and readmissions rates are in-line with rates reported in the literature.[1, 2] While the total number of complications was equal between the pre- and post-ERP groups, 75% of patients in the pre-ERP group had Clavien II or III complications compared to 45% in the post-ERP group. Review of reasons for readmissions and return to the OR did not reveal any set pattern to suggest specific contributing intrinsic or extrinsic factors. Given the small number of outcome events, any such findings would be exploratory. Multiple factors influenced decisions to readmit for observation, including the patients’ underlying medical comorbidities and complex social situations. We have a low threshold to readmit as a brief period of observation can help us better identify areas of need to enhance home care and long-term outcomes.
During pathway development and implementation, challenges similar to what Vacek et al. noted in their study of barriers to ERP implementation in pediatric surgery were encountered.[16] The authors reported lack of buy-in from surgeons and anesthesiologists as a common barrier. While 20 of the 24 institutional ERP pathway elements were pre-determined by the PURSUE Study Group, multiple discussions across specialties were held at our center to find acceptable implementation approaches (Figure 1). This ensured practitioner comfort and acceptance of the pathway as ERP does require significant changes in perioperative practices. Identification of invested stakeholders who actively participated in pathway development and facilitated buy-in from their colleagues was critical. It is important to note that some of our urologists had stopped routine pre-operative bowel preparations prior to ERP implementation. This may have eased the transition as many pediatric urologists have reservations with avoiding pre-operative bowel preparations in these cases.[17]
Vacek et al. also noted lack of resources for implementation, data collection, and analysis as additional barriers.[16] Pathway development and sustainability requires significant time commitment which is challenging for many active clinicians. To address this, we solicited the expertise of our institutional quality improvement team who facilitated pathway integration into the electronic medical record. Continuous auditing of pathway adherence and outcomes was also important in pathway refinement and addressing barriers. During initial development, the surgical team monitored adherence to enact immediate changes. After pathway development, our research team performed continuous auditing with regular reviews by stakeholders. Although ERP elements were not changed in the process, approaches to implementation were modified to improve adherence and workflow (Supplemental Table 2).
The focus of the group shifted towards pathway sustainability after pathway development. Performance monitoring and pathway educational activities improve pathway sustainability.[18, 19] Ease in implementation was also critical. Use of a standard order set and involvement of nursing educators and our inpatient urology physician assistant help reduce implementation variations as new trainees and nurses rotate through. Since pathway implementation, median number of elements implemented has remained at 16 of 24 (Supplemental Figure 3). As patients vary in their underlying comorbidities and required surgical interventions, inter-individual differences in implementable number of ERP elements are expected.
Adherence was 50% or less for nine elements. Preoperatively, these included ERP counseling and prehabilitation. It is important to note that the ERP educational handout was still under development during initial implementation. While 95% (N=19) of patients received verbal counseling, six did not receive the written handout and therefore did not qualify as having received full ERP counseling. Patient willingness, insurance coverage, and transportation issues remain challenges for prehabilitation.
Intraoperative elements with 50% or less adherence included maintenance of normothermia, minimally invasive (MIS) approach, and drain minimization. Normothermia was a challenging element to achieve, especially if patients became hypothermic prior to incision. This often occurred during regional anesthesia placement so warm blankets and the Bair Hugger™ were used to keep the patient warm. As MIS approaches to reconstruction are not favored at our institution for a majority of eligible patients, this ERP element has low adherence. Pelvic drains were avoided in uncomplicated ileocystoplasties or isolated catheterizable channels.
Opioid minimization, use of adjunct pain medications, early feeding, and early discontinuation of intravenous fluids also had 50% or less adherence. Per PURSUE guidelines, opioid minimization is defined as <=0.15 mg/kg/day of IV morphine equivalent. As patients had varying degrees of sensation, a patient-controlled analgesia delivering on-demand opioids was utilized to ensure patient comfort and cooperation with PT. The authors accepted that not all patients would satisfy the opioid minimization element but hoped that they would mobilize on POD 1. To satisfy criteria for adjunct pain medications, patients must receive ketorolac and intravenous acetaminophen within 4 hours post-operatively. Although all patients received acetaminophen within this timeframe, ketorolac administration was delayed due to transient rises in serum creatinine on post-operative labs (N=2) or low post-operative urine output (N=2). In cases of baseline chronic kidney disease (N=3) or solitary kidney (N=2), ketorolac was not given. However, these patients were still receiving additional non-opioid analgesics. Although not stringently monitored, no patients required cessation of gabapentinoids due to medication side effects. Early feeding is defined as ordering a clear liquid diet on POD 0 and regular diet on POD 1. As nausea or abdominal distention remained complaints on POD 1, we had reservations with advancing to regular diets until patients tolerated clears. This typically occurred by POD 2 or 3, which is earlier compared to historical practices. Similarly, intravenous fluids were continued until patients demonstrated adequate oral tolerance.
Our study has certain limitations. We emphasize that our implementation and effectiveness results are specific to our center. Although our ERP stems mostly from the PURSUE Study protocol, implementation processes depend on institutional context and may not be generalizable.[20] The cohort sizes are relatively small, reflect the heterogenous nature of patients needing bladder reconstruction, and are unmatched, so biases may be present. Some patients underwent Mitrofanoff placements without need for bowel anastomoses, resulting in shorter LOS than those who underwent more complex reconstructions. However, more patients underwent bladder augmentation (90%) post-ERP compared to the pre-ERP group (65%) and still had a lower median LOS. Differences in baseline medical complexity and care needs between patients may have affected adherence. As ERPs are complex and contain multiple elements, it is difficult to decipher which elements are most critical in reducing LOS and influencing post-operative outcomes. Our paper is primarily focused on the implementation process and is not intended to evaluate the full effects of ERP on patient outcomes. This is being evaluated by the PURSUE trial and is beyond the scope of this paper.[9] While we were unable to consistently achieve 80% of the elements as stated in our aims statement, we successfully implemented the pathway and improved our patients’ recovery processes (indirectly reflected by a decreased post-operative LOS) with adherence to a median of 67% of elements. Our preliminary results demonstrate the feasibility and sustainability of ERP in children undergoing bladder reconstruction.
Future Directions
Since pathway implementation, additional endeavors have been undertaken to further improve the care of these patients. As prehabilitation is challenging to facilitate given insurance and traveling constraints, we collaborated with our physical therapists to increase implementation. Our PT team also created post-discharge milestones and guidelines to encourage mobility at home. Areas for enhancing patient education have been identified by our urology and nursing stakeholders, leading to further collaboration. An educational video instructing patients and families on catheter care and irrigation is in development. In the interim, parents are filmed irrigating catheters to use as a point of reference after discharge.
Conclusion
ERP implementation in children undergoing complex bladder reconstruction is feasible through a concerted multidisciplinary effort. Identification of invested stakeholders is critical in pathway development and sustainability. We have outlined our institutional experience with hopes that it will provide insight for others also interested in implementing ERP. ERP implementation has significantly reduced LOS for our patients without increasing perioperative morbidity. Continuous adherence auditing and feedback will enable further pathway refinement.
Supplementary Material
Acknowledgements:
We would like to acknowledge the following individuals for their significant contribution to pathway development and implementation: Nicoletta Alavanja MS RD LDN, Diana K. Bowen MD, Mary Cheng PT DPT, Kelly Conforti, Vicki Grabowski MSN RN, Sarah Harlan PT, David Krodel MD, Ryan Marcelino MD, Maureen McCarthy-Kowols MSN RN CCRN, Nisha Pinto MD, Jamie Portnoy RD, Kris Razma MS OTR/L, Kalee Ryan MSN RN, Doreen Stein BSN RN CPN, Kristina Stein OTR/L, Jeanine Talsky RN, Lisa Tieman BSN RN NPD-BC CPN, Jennifer Wolf BSN RN CPN, Christina Wilbur MSN RN CPN, Lurie Children’s Creative and Marketing team, members of the Lurie Children’s prescreening nursing team, and members of the PURSUE Study group.
Funding:
Dr. Chu is supported by K23 DK125670 from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIH and NIDDK had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the official view of the NIH nor NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None declared
References
- [1].Schlomer BJ, Saperston K, Baskin L. National trends in augmentation cystoplasty in the 2000s and factors associated with patient outcomes. J Urol. 2013;190:1352–7. [DOI] [PubMed] [Google Scholar]
- [2].Maldonado N, Michel J, Barnes K. Thirty-day hospital readmissions after augmentation cystoplasty: A Nationwide readmissions database analysis. J Pediatr Urol. 2018;14:533 e1–e9. [DOI] [PubMed] [Google Scholar]
- [3].Ladd MR, Garcia AV, Leeds IL, Haney C, Oliva-Hemker MM, Alaish S, et al. Malnutrition increases the risk of 30-day complications after surgery in pediatric patients with Crohn disease. J Pediatr Surg. 2018;53:2336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heathcote S Sr., Duggan K, Rosbrugh J, Hill B, Shaker R, Hope WW, et al. Enhanced Recovery after Surgery (ERAS) Protocols Expanded over Multiple Service Lines Improves Patient Care and Hospital Cost. Am Surg. 2019;85:1044–50. [PubMed] [Google Scholar]
- [5].Palumbo V, Giannarini G, Crestani A, Rossanese M, Calandriello M, Ficarra V. Enhanced Recovery After Surgery Pathway in Patients Undergoing Open Radical Cystectomy Is Safe and Accelerates Bowel Function Recovery. Urology. 2018;115:125–32. [DOI] [PubMed] [Google Scholar]
- [6].Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J Surg. 2016;40:1741–7. [DOI] [PubMed] [Google Scholar]
- [7].Witcher A, Axley J, Novak Z, Laygo-Prickett M, Guthrie M, Xhaja A, et al. Implementation of an enhanced recovery program for lower extremity bypass. J Vasc Surg. 2020. [DOI] [PubMed] [Google Scholar]
- [8].Rove KO, Brockel MA, Saltzman AF, Donmez MI, Brodie KE, Chalmers DJ, et al. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol. 2018;14:252 e1–e9. [DOI] [PubMed] [Google Scholar]
- [9].Rove KO, Strine AC, Wilcox DT, Vricella GJ, Welch TP, VanderBrink B, et al. Design and development of the Pediatric Urology Recovery After Surgery Endeavor (PURSUE) multicentre pilot and exploratory study. BMJ Open. 2020;10:e039035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Byrne CM, Zahid A, Young JM, Solomon MJ, Young CJ. Gum chewing aids bowel function return and analgesic requirements after bowel surgery: a randomized controlled trial. Colorectal Dis. 2018;20:438–48. [DOI] [PubMed] [Google Scholar]
- [11].Mayo NE, Feldman L, Scott S, Zavorsky G, Kim DJ, Charlebois P, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–14. [DOI] [PubMed] [Google Scholar]
- [12].Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017;152:691–7. [DOI] [PubMed] [Google Scholar]
- [13].Baxter KJ, Hafling J, Sterner J, Patel AU, Giannopoulos H, Heiss KF, et al. Effectiveness of gabapentin as a postoperative analgesic in children undergoing appendectomy. Pediatr Surg Int. 2018;34:769–74. [DOI] [PubMed] [Google Scholar]
- [14].Anderson DE, Duletzke NT, Pedigo EB, Halsey MF. Multimodal pain control in adolescent posterior spinal fusion patients: a double-blind, randomized controlled trial to validate the effect of gabapentin on postoperative pain control, opioid use, and patient satisfaction. Spine Deform. 2020;8:177–85. [DOI] [PubMed] [Google Scholar]
- [15].Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [16].Vacek J, Davis T, Many BT, Close S, Blake S, Hu YY, et al. A baseline assessment of enhanced recovery protocol implementation at pediatric surgery practices performing inflammatory bowel disease operations. J Pediatr Surg. 2020;55:1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chan YY, Rosoklija I, Meade P, Burjek NE, Raval MV, Yerkes EB, et al. Utilization of and Barriers to Enhanced Recovery Pathway Implementation in Pediatric Urology. J Pediatr Urol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Doyle C, Howe C, Woodcock T, Myron R, Phekoo K, McNicholas C, et al. Making change last: applying the NHS institute for innovation and improvement sustainability model to healthcare improvement. Implement Sci. 2013;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ament SM, Gillissen F, Moser A, Maessen JM, Dirksen CD, von Meyenfeldt MF, et al. Identification of promising strategies to sustain improvements in hospital practice: a qualitative case study. BMC Health Serv Res. 2014;14:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


