Abstract
Single-cell combinatorial indexing (sci) with transposase-based library construction increases the throughput of single-cell genomics assays but produces sparse coverage in terms of usable reads per cell. We develop symmetrical strand sci (‘s3’), a uracil-based adapter switching approach that improves the rate of conversion of source DNA into viable sequencing library fragments following tagmentation. We apply this chemistry to assay chromatin accessibility (s3-ATAC) in human cortical and mouse whole brain tissues, with mouse datasets demonstrating a 6-to-13-fold improvement in usable reads per cell compared with other available methods. Application of s3 to single-cell whole genome sequencing (s3-WGS) and to whole genome plus chromatin conformation (s3-GCC) yields 148 and 14.8 fold improvements, respectively, in usable reads per cell compared with sci-DNA-seq and sci-HiC. We show that s3-WGS and s3-GCC resolve subclonal genomic alterations in patient-derived pancreatic cancer cell lines. We expect that the s3 platform will be compatible with other transposase-based techniques, including sci-MET or CUT&Tag.
Introduction
Single-cell genomics assays have emerged as a dominant platform for interrogating complex biological systems. Methods to capture various properties at the single-cell level typically suffer a tradeoff between cell count and information content, which is defined by the number of unique and usable reads acquired per cell. We and others have described workflows that utilize single-cell combinatorial indexing (sci)1, leveraging transposase-based library construction2 to assess a variety of genomic properties in high throughput; however, these techniques often produce sparse coverage for the property of interest. Here, we describe an adaptor-switching strategy, ‘s3’, capable of producing one-to-two order-of-magnitude improvements in usable reads obtained per cell for chromatin accessibility (s3-ATAC), whole genome sequencing (s3-WGS), and whole genome plus chromatin conformation (s3-GCC), while retaining the same high-throughput capabilities of predecessor ‘sci’ technologies. We apply s3 to produce high-coverage single-cell ATAC-seq profiles of mouse brain and human cortex tissue; and whole genome and chromatin contact maps for two low-passage patient-derived cell lines from a primary pancreatic tumor.
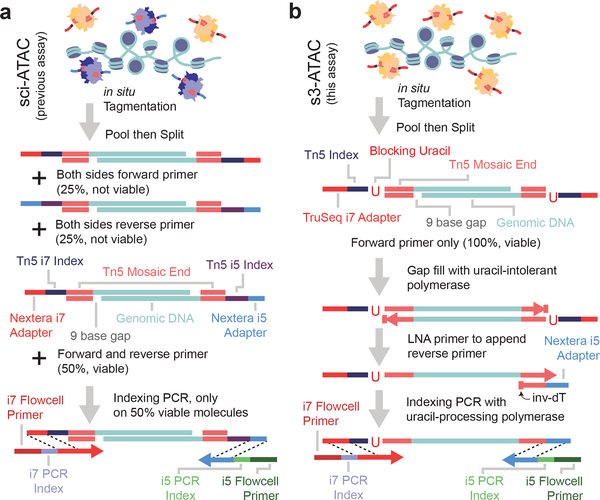
The core component of many sci- assays, as well as ATAC-seq, is the use of transposase-based library construction. While the transposition reaction itself (tagmentation) is highly efficient, viable sequencing library molecules are only produced when two different adaptors, in the form of forward or reverse primer sequences, are incorporated at each end of the molecule. This occurs only 50% of the time (Figure 1a). To combat this inefficiency, strategies including the use of larger complements of adaptor species3, incorporation of T7 promoters to enable amplification via in vitro transcription4–6, or reverse adaptor introduction through targeted7 or random priming8, or ligation9 have been developed; however, these methods are often complex and result in limited efficiency improvements. Here, we present a strategy of adapter replacement to produce library molecules tagged with both forward and reverse adaptors for top and bottom strands. In addition to overcoming the 50% yield limitation, the efficiency of opposite adapter incorporation is also improved when compared to standard tagmentation. This is due to the use of multiple rounds of extension as opposed to a single extension prior to PCR. This format permits the use of a DNA index sequence embedded within the transposase adaptor complex, enabling single-cell combinatorial indexing (sci) applications, where two rounds of indexing are performed — the first at the transposition stage, and second at the PCR stage1,8,10.
Figure 1 |. Symmetrical strand single-cell combinatorial indexing ATAC-seq (s3-ATAC) improves molecular capture rate.
(a) Schematic of standard sci-ATAC library construction. (b) Schematic of s3-ATAC library construction with intermediate steps of adapter switching leading to increased genomic molecule capture rate.
Our strategy, symmetrical strand sci (s3; Figure 1b) uses single-adapter transposition to incorporate the forward primer sequence, the Tn5 mosaic end sequence and a reaction-specific DNA barcode. As with standard tagmentation workflows, extension through the bottom strand is then performed to provide adaptor sequences on both ends of each molecule; however, the s3 transposome complexes contain a uracil base immediately following the mosaic end sequence. Use of a uracil-intolerant polymerase therefore prevents extension beyond the mosaic end into the DNA barcode and forward adaptor sequence. A second template oligo is then introduced that contains a 3’-blocked (inverted dT) locked nucleic acid (LNA) mosaic end reverse complement sequence with a reverse adaptor sequence 5’ overhang. This oligo favorably anneals to the copied mosaic end sequence, due to the higher melting temperature of LNA, and acts as a template for the library molecule to extend through and copy the reverse adaptor. This results in all library fragments having both a forward and reverse adaptor sequence. The LNA-templated extension is carried out over multiple rounds of thermocycling to ensure maximum efficiency of reverse adaptor incorporation, which provides an additional improvement over traditional tagmentation workflows where only a single pre-PCR extension is possible. Furthermore, adapter sequences are designed such that standard sequencing recipes can be used instead of the custom workflows and primers that are required for current indexed transposition technologies (Supplementary Tables 1–5)11,12, making use of the TruSeq read 2 and index read 1 sequencing primer and the standard Nextera read 1 and index read 2 primer.
Results
s3-ATAC creates high-content chromatin profiles
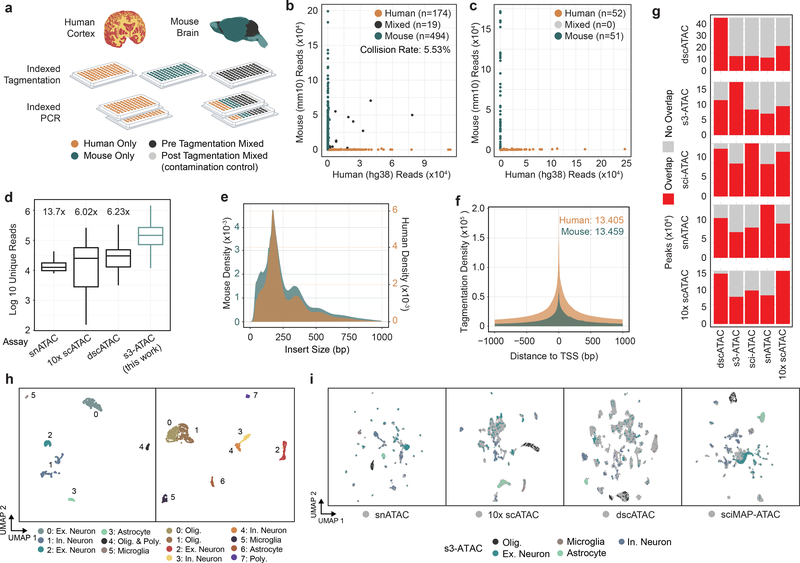
We first sought to establish the s3 technique to assess chromatin accessibility. In s3-ATAC, nuclei are isolated and tagmented using our single-ended, indexed transposomes and carried through the adaptor-switching s3 workflow (Figure 1b). To ensure we attain true single-cell libraries without contamination from other nuclei, and minimal barcode collisions, we performed a mixed-species experiment on primary frozen human cortical tissue from the middle frontal gyrus and frozen mouse whole brain tissue (Figure 2a). We elected to perform this test on primary tissue samples instead of an idealized cell line setting to more accurately capture the rates of cross-cell contamination. Levels of crosstalk were assessed at both points of possible introduction: the tagmentation and PCR stages; by mixing nuclei from the two samples before tagmentation as well as after. Additionally, pure species libraries were produced by leveraging the inherent sample multiplexing capabilities of sci workflows. In the experimental condition where nuclei were mixed prior to any processing, i.e. pre-tagmentation, we observed a total estimated collision rate of 5.53% (Figure 2b and c; 2 × 2.77% detected human-mouse collisions), comparable to existing methods and tunable based on the number of nuclei deposited into each PCR indexing reaction. Zero collisions were observed in the post-tagmentation experimental conditions, suggesting no molecular crosstalk during s3 adapter switching or PCR.
Figure 2 |. s3-ATAC on human cortex and mouse whole brain.
(a) Experimental flow through and plate layout for the mixed-species experiment, including tagmentation and PCR plate conditions per well. (b) Scatter plots of single-cell libraries with counts of unique reads aligned to mouse or human chromosomes in a chimeric reference genome. Points are colored to reflect species assignment (see Online Methods) in both pre-tagmentation mixing and (c) post-tagmentation mixing. (d) Comparison of unique read counts per cell, restricted to only properly-paired reads for s3-ATAC mouse whole-brain sampled cells to unique read counts produced for previously reported data sets (n=3,034, 4,117, 46,653, and 298 cells, for snATAC, 10x scATAC, dscATAC and ours, respectively). All comparisons to our data are significantly less (Welch’s two-sample t-test, p-values < 5.7×10−42, 3.1×10−40, 1.6×10−37, respectively). Fold improvement of our library complexity per method is listed above the method13–15. Box plot represents median and center quartiles with whiskers at 10th and 90th percentile. (e) Insert size distribution of human and mouse libraries reflect nucleosome banding. (f) Enrichment of reads at transcription start sites (“TSS”) for human and mouse libraries with enrichment calculation following ENCODE standard practices. (g) Stacked bar plot for comparison of peak overlaps across mouse whole brain data sets. Each row is for a different peak set, with each column showing peak overlap in red. (h) UMAP projection of mouse whole brain cell samples (n=837 cells) colored by cluster and cell type assignment (left). UMAP projection human cortex cell samples (n=2,175 cells; right). (i) Integration of s3-ATAC mouse data with other datasets. Points colored by cell type (respective of panel h) or external data set (gray).
In total, we generated 2,175 human and 837 mouse single-cell ATAC-seq profiles passing quality filters (Online Methods) across four PCR indexing plates (Figure 2a). We then assessed the total unique sequence reads obtained per cell as a function of the total aligned reads, i.e. the library complexity. One of our mixed species plates was sequenced to beyond 50% saturation (duplicate reads / total reads), to represent the sequencing depth obtained where diminishing returns of increased sequence depth become excessive10. For this plate, the mean sequencing saturation per cell was 63.6% and resulted in a median unique read count per cell of 178,069 (mean = 258,859; statistics on all plates can be found in Supplementary Table 6). The human cells reached a mean sequencing saturation of 56.6% with a median unique reads per cell of 99,882 (mean = 175,361). We additionally sequenced a plate that contained only human cells to a mean sequencing saturation of 70.4% which produced a median of 100,280 (mean = 146,937) unique reads per cell (Supplementary Table 6). When compared to other single-cell ATAC-seq datasets performed on mouse whole brain tissue, our mouse s3-ATAC libraries contain substantially greater properly paired, unique, nuclear reads per cell with 13.7x, 6.02x and 6.22x fold improvement compared to snATAC, 10X Genomics scATAC, and dscATAC, respectively (Figure 2d, Supplementary Table 7)13–15. Read count increases can be indicative of poor ATAC-seq library quality, with increased depth reflecting increased noise and loss of signal at open chromatin regions. To address this, we first assessed read pair insert sizes, revealing the characteristic nucleosome-size banding distribution of ATAC-seq (Figure 2e)16. We next calculated transcription start site (TSS) enrichment using the approach defined by the ENCODE project (Online Methods). This produced significant enrichment for both species at 13.4 for human, well above the ‘ideal’ standard (>7) and 13.5 for mouse, within the acceptable range and just below ideal (>15; Figure 2f). Similarly, the fraction of reads in pile-up genomic regions (“peaks”; FRiP) was comparable to other single-cell ATAC technologies at 31.95% and 29.15% as measured using 292,156 and 174,653 peaks for human and mouse cells respectively. However, FRiP is largely dependent on the number of peaks called, which influenced heavily by cell number and total sequence depth obtained. When expanding to a human cortex high-depth ATAC-seq peak set, a mean of 48.1% of reads were present in peaks, and mean of 78.2% of reads for mouse cells using a high-depth mouse brain ATAC-seq peak set (Supplementary File 1; Online Methods). We further compared peak overlap between the matched data sets to assess any systematic bias in s3-ATAC, with all assays performing comparably with respect to the proportion of overlapping reads for each other assay (Figure 2g).
s3-ATAC resolves cell types in the mammalian brain
With ample signal, we next sought to discern cell types present within the complex tissues. For each species, we used peaks called on aggregate data to construct a count matrix followed by dimensionality reduction using the topic-modeling tool cisTopic17 which we then visualized using UMAP18, performed graph-based clustering at the topic level, and processed via Signac19. Clear separation of cell types was observed using marker gene signal and differential accessibility profiles (Figure 1h, Supplementary File 2, Supplementary Figure 1a)15,20. Finally, we assessed any systematic bias that may affect biological interpretation by integrating our data sets with snATAC, 10X Genomics scATAC, dscATAC and sciMAP-ATAC (Figure 2i)13–15,21. We observed that our libraries readily integrate across platforms, maintaining cell type discrimination between clusters.
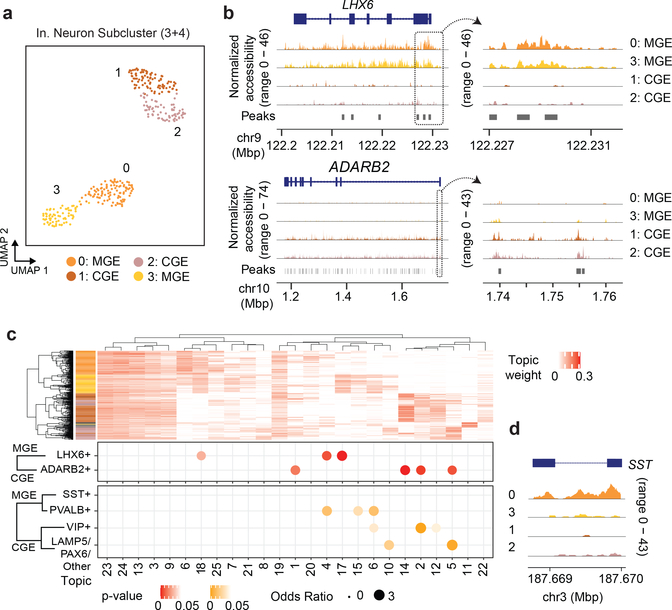
Notably, even with the modest cell count produced by this experiment, the quality improvements allow us to interrogate subclusters of inhibitory neurons previously difficult to distinguish in atlas-level datasets (Figure 3a)22. With our improved cell depth, we were able to discern caudal and medial ganglionic eminence inhibitory neurons by marker gene coverage plots across 342 GAD1+ cells (“CGE” and “MGE”, respectively). From these, we identified 157 GAD1+, ADARB2+ CGE cells and 168 GAD1+, LHX6+ MGE cells (Figure 3b). We identified 17 cells (subcluster 4) as putative doublets given the co-expression of both LHX6 and ADARB2 and excluded them from subsequent analyses. Aggregated genomic signal over our Topic-based dimensionality reduction was used to support our marker gene cell subtype discrimination and describe differentially accessible loci in human cortical inhibitory neurons (Supplementary File 2). We grouped topics based on cell embeddings (Figure 3c, top) through hierarchical clustering and observed topic-based enrichment of sites overlapping cell type specific marker genes previously defined through transcriptomics (Figure 3c, bottom)20. This analysis did not identify a specific topic (or topics) associated with MGE/SST+ cells; however, accessibility at SST was elevated specifically in cluster 0 (Figure 3c, right).
Figure 3 |. s3-ATAC on human cortex inhibitory neurons.
(a) Subclustering and UMAP projection of human cortical inhibitory neurons (clusters 3 and 4 from panel h., n=342) (b) Genome coverage track of human inhibitory neurons (n=342) aggregated over 4 subclusters for genomic locations overlapping MGE and CGE marker genes LHX6 and ADARB2, respectively, with a zoomed in view of the promoter region (on right). (c) Hierarchical clustering of topic weight per cell (top). Hypergeometric test of gene set analysis enrichment for human inhibitory neuron marker genes (bottom; Fisher’s exact test, see Online Methods), with a genome track of SST to delineate MGE cell types (right).
To further assess the impact of increased coverage in our s3-ATAC data, we performed a random read down sampling analysis followed by peak calling, topic modeling and cell type discrimination (Online Methods; Supplementary Figure 1b). As expected, the increased reads produced a greater number of called peaks (Supplementary Figure 1c), though peak calling is a function of the total reads, and can be improved by either greater cell number or greater coverage per cell. When examining the down sampled data performance on cell type discrimination, major cell types could be discerned with only 15 or 20% of reads utilized for human and mouse datasets, respectively (Supplementary Figure 1d). However, as reads increased, cell types separated more cleanly and produced additional granularity (e.g. inhibitory neurons). As a final examination of the advantages provided by improved library complexity, we assessed the impact on sequencing depth required to reach a comparable number of unique, passing reads per cell in our s3-ATAC library when compared to sci-ATAC, which produces libraries with a lower overall complexity (Supplementary Figure 1e). When targeting a total of 10,000 unique reads per cell, the s3-ATAC library resulted in the removal of only 1.2% of reads as PCR duplicates when compared to 27–38% for the lower-complexity preparations. This directly translates to a reduction in sequencing costs of roughly 1/3, enabling studies that want to profile larger cell numbers at a lower depth to do so more efficiently.
s3 for whole genome and chromatin conformation capture
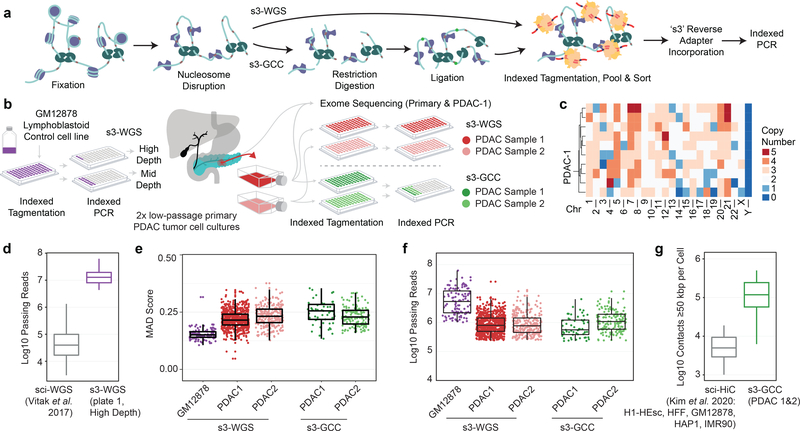
We then extend the improvements in data quality produced by s3-ATAC to other sci- workflows. This includes our previously-described sci-DNA-seq method10 that produces single-cell whole genome sequencing libraries (s3-WGS) and a strategy to incorporate the core components of HiC library preparation but without ligation junction enrichment to produce whole genome and chromatin conformation information (s3-GCC; Figure 4a). Both strategies disrupt nucleosomes to acquire sequence reads uniformly across the genome10, which we also improved for the s3 assay by optimizing fixation conditions. All experiments were performed using the same number of indexed tagmentation reactions and the same number of nuclei deposited into each PCR indexing well to achieve a comparable expected doublet rate as with the s3-ATAC experiments. We first tested s3-WGS by producing two small-scale libraries on the diploid lymphoblastoid cell line, GM12878. The first library comprised only four wells at the PCR stage for a target of 60 cells, allowing us to sequence the library to high depth (Figure 4b). This produced a median passing read count per cell of 12,789,812 (mean = 15,238,184), across 45 QC-passing cells (75% cell capture efficiency). With our sequenced library at 72.35% saturation; our complexity is notably higher than the predecessor sci-DNA-seq technology which produced a median of 43,367 reads per cell (mean = 103,138) at the same sequencing saturation (295 and 148 fold improvement in median and mean, respectively; Figure 4d)10. This improvement is based on a combination of the s3 workflow, optimization of the nucleosome disruption and the added benefit of thermocycling during s3 adapter switching which likely improves crosslink-reversal prior to PCR. The second preparation performed comparably, though sequenced to a lower total depth (15.98% saturation). We also confirmed that the coverage was uniform by assessing the median absolute deviation (MAD) across 500 kbp bins, which fell within 0.152 ± 0.025 (mean ± s.d.), comparable to other single-cell genome sequencing techniques (Figure 4e)10,23,24.
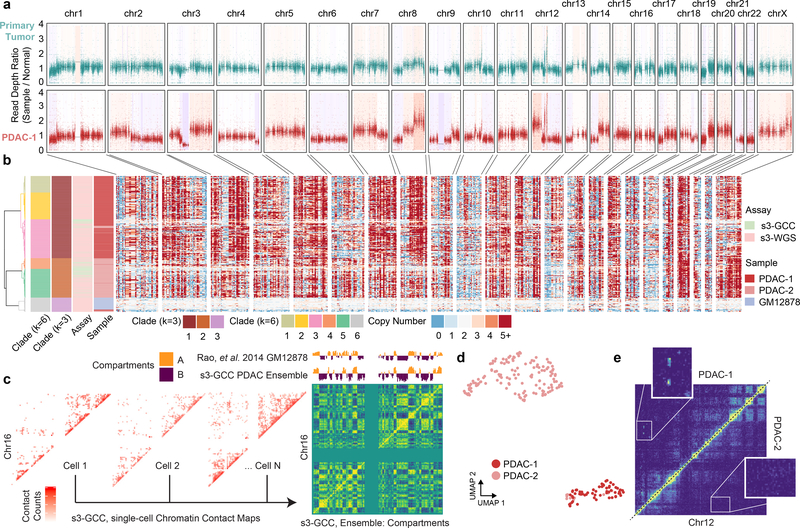
Figure 4 |. s3 whole genome sequencing (s3-WGS) and genome conformation capture (s3-GCC).
(a) Schematic of sci-WGS and sci-GCC library construction. (b) Experimental flow through and plate layout for control GM12878 diploid line (left) and PDAC cell lines (right). (c) Heatmap summary of chromosome count per cell in PDAC-1 SKY data. Example karyotype of PDAC-1 cell (bottom). (d) Boxplot of unique read count per cell for matched GM12878 cell line (n=3,576, 45 cells for sci-WGS and s3-WGS, respectively)10. (e) Boxplot of MAD score per cell per sample and assay (n=111, 698, 257, 57, and 145 cells, listed left to right). (f) Boxplot of reads passing filter per cell. Cell count same as panel e. (g) Comparison boxplot of s3-GCC and sci-HiC distal contacts (≥50kbp) per cell (n=2,312, 202 cells for sci-HiC and s3-GCC, respectively)31. Boxplots depicts median and center quartiles with 10th and 90th percentile whiskers.
We performed s3-WGS and s3-GCC on two cell lines derived from a single primary pancreatic ductal adenocarcinoma (PDAC) tumor (Figure 4b). PDAC is a highly-aggressive cancer that typically presents at an advanced stage, making early detection and study of tumor progression key25. PDAC studies suffer from a low cancer cell fraction in biopsied samples, thus we used patient derived cell lines (PDCLs) maintained at fewer than 10 passages. This method allows for multiple modalities of characterization and perturbation, while maintaining a large portion of the heterogeneity present in the tumor sample26. We targeted two PDCLs (referred to as PDAC-1 and PDAC-2) derived from the same parent tumor which had a driver mutation in the oncogene KRAS (p.G12D) and profound genomic instability, as indicated by karyotyping (Figure 4c). For our s3-WGS preparations, we produced 773 and 256 single-cell libraries with a mean passing read counts of 1,181,128 and 1,299,949 for PDAC-1 and 2 (at a combined median of 28.46% saturation), respectively. The s3-GCC libraries contained 57 and 145 cells produced a mean passing read count of 973,397 and 1,588,926 (combined median 73.25% sequencing saturation) for PDAC-1 and 2, respectively (Figure 4f). MAD scores for the two lines were greater than that of the diploid karyotype of GM12878, 0.219 ± 0.041 (mean ± s.d.); however, this is expected given the widespread copy number alterations present in the samples. In addition to the WGS component, the s3-GCC libraries also contained reads that were identified as chimeric ligation junctions that provide HiC-like chromatin conformation signal, with a distal contact distribution comparable to bulk HiC datasets (Supplementary Figure 3a). Across both samples, we identified a mean of 118,048 reads per cell that capture genomic contacts at least 50 kbp apart from one another, a 14.8-fold improvement over the previous high-throughput single-cell combinatorial indexing technique, sci-HiC27 (Figure 4g; Supplementary File 3), and comparable to low-throughput scHiC methods that process cells individually28, with the exception of Dip-C, which can achieve exceeding 1×106 contact counts3. Read pairs spanning ≥50 kbp accounted for a median of 15.6% and 17.0% of the total reads obtained per cell, which equates to an enrichment of 361- and 402-fold over that of the s3-WGS libraries for PDAC-1 and 2, respectively (Supplementary Table 8).
s3-WGS and s3-GCC resolve subclonal alterations in PDCLs
We first focused our analysis on the s3-WGS and the WGS component of the s3-GCC libraries to examine the copy number alterations present in the lines. To get a sense of the genomic landscape, we first performed copy number calling on whole exome sequencing (WES) libraries that were generated using primary tumor tissue and on the PDCL line PDAC-1, derived from the tumor (Figure 5a). This revealed a profile of copy number aberrations at finer resolution, with a more pronounced profile in the PDCL sample, likely due in part to less euploid stromal cell contamination. We then processed all single-cell libraries using SCOPE23 which revealed a highly altered genomic landscape within each of the two samples. In line with paired karyotyping and bulk exome data, we see a similar pattern per cell of multi-megabasepair copy number aberrations when performing breakpoint analysis on 500 kbp windows, with a median depth per window of 81 reads. Using the inferred copy number profile within genomic windows for the three samples, GM12878 and two PDAC lines, we performed hierarchical and K-means clustering on the Jaccard distance between cell breakpoint copy numbers at two different centroid counts. For our optimal centroid value, we found a relatively clean separation between cell lines (k=3), for subclonal analysis we used a higher centroid count at local optima (k=6). s3-WGS and s3-GCC cells cluster dependent on cell line, reflecting our ability to capture genome-wide copy number data in our s3-GCC libraries (Figure 5b). We generated pseudo-bulk clades from the single-cell read count bins, with an average of 211.3 cells per clade and an average read count of 3,750 per 50 kbp bin. This revealed multiple fixed and subclonal genomic arrangements (Supplementary Figure 2a,b). In PDAC-1 and PDAC-2 we see shared copy number loss of tumor supressor genes CDKN2A, SMAD4 and BRCA225,29. In PDAC-2 we observed a subclonal amplification of PRSS1, a mutation that was fixed within our sampling for PDAC-1 and is associated with tumor size, tumor node metastasis rate30. This suggests that while the lines have the same origin, each culture captured different subsets of tumor clonal populations.
Figure 5 |. s3-WGS for copy number calling and s3-GCC for genome conformation changes.
(a) Whole exome sequencing of primary tumor biopsy and PDAC-1 cell line. Scatterplot of reads per bin with a shading of called copy number variation. (b) Single-cell whole genome copy number calling on 500 kbp bins genome-wide. Cells (rows) are hierarchically clustered and annotated by assay, sample, and assigned clade (left). (c) Representative single-cell contact maps (raw counts) at 1 Mbp resolution for chromosome 16 and ensemble contact map profile at 500 kbp resolution with compartment calling. Compartment eigenvectors are plotted above and compared to GM12878 high-depth bulk HiC data from Rao et al. 2014. (d) UMAP projection of scHiC topic modeling dimensionality reduction and clustering of single-cell distal contact profiles. (e) Putative subclonal translocation on chr12 specific to PDAC-1 (top left) when compared to PDAC-2 (bottom right).
Duplications and deletions are not the sole form of genomic rearrangement that may induce a competitive advantage in cancer cell growth. Genomic inversions are difficult to assess through standard karyotyping and chromosome painting methods, whereas chromosomal translocations are difficult to uncover in whole-genome amplification methods, since only reads capturing the breakpoint would provide supportive evidence. To address both of these limitations, we utilized the HiC-like component of our s3-GCC libraries. Using read pairs spanning ≥50 kbp, we produced chromatin contact maps that produced clear chromatin compartmentalization signal (Figure 5c)27. Single cells were separated by their distal contact information via scHiC topic modeling and observed distinct clusters by PDCLs31. Notably, even at this relatively low sequencing depth, we were able to reliably tell PDCL line sparse contact profiles apart (Figure 5d, Supplementary Figure 3b,c). Differences between the aggregated contact maps between clusters were then used to assess unique translocation and inversion events across the sampled cells. This identified a putative intrachromosomal translocation between the 8.5–9.5 Mbp and 88.5–91.0 Mbp regions of chromosome 12 (Figure 5e), which exhibited contact signal comparable to proximal regions in sequence space (Supplementary Figure 3d). The putative translocation contained ATP2B1, which is commonly overexpressed in PDAC32 and the tumor suppressor gene DUSP633 and is only present in PDAC-1.
Discussion
Our s3 workflow provides marked improvements over the predecessor sci platform with respect to passing reads obtained per cell without sacrificing signal enrichment in the case of s3-ATAC, or coverage uniformity for s3-WGS. We also introduce another variant of combinatorial indexing workflows, s3-GCC to obtain both genome sequencing and chromatin conformation, with improved chromatin contacts obtained per cell when compared to sci-HiC. We demonstrate the utility of these approaches by assessing two patient-derived tumor cell lines with genomic instability. Our analysis reveals patterns of focal amplification for disease-relevant genes, and uncover wide-scale heterogeneity at a throughput not attainable with standard karyotyping. Additionally, we highlight the joint analysis of our protocols for uncovering the chromatin compartment disrupting effect of copy number aberrations. Furthermore, the s3 workflow has the same inherent throughput potential of standard single-cell combinatorial indexing, with the ability to readily scale into the tens and hundreds of thousands of cells by expanding the set of transposome and PCR indexes. We also expect that this platform will be compatible with other transposase-based techniques, including sci-MET8, or CUT&Tag34. Lastly, unlike sci workflows, the s3 platform does not require custom sequencing primers or custom sequencing recipes, removing one of the major hurdles that groups may face while implementing these technologies.
Online Methods
PDCL propagation
Low-passage, patient-derived cell lines (PDCLs) were propagated from rapidly dissociated PDAC tumors and cultured for continuous propagation in culture medium containing ROCK inhibitor (Y-276320)35. Briefly, approximately 50,000 viable, disaggregated tumor cells were plated to a 35mm diameter, collagen-coated well (Gibco, A11428–02) and passaged 1:3 while subconfluent until reaching 85% confluence on a 10cm diameter dish. From a fraction of these cells, DNA was extracted to validate the presence of KRAS-G12 mutations by ddPCR (Bio-Rad, 1863506) and to validate an STR profile that matches normal leukocyte DNA from the same patient (Genetica). PDCLs exhibited morphologies consistent with epithelial tumor cells and abundant KRT expression was detected by immunocytofluorescence using the monoclonal antibodies: AE1/AE3, C-11, and Cam5.2. To ensure that only tumor cells were cultured, G-banded karyotyping was performed by the Knight Diagnostic Research Cytogenetics Lab at OHSU. Chromosome spreads from more than 20 cells were observed to ensure that the culture contained 100% tumor cells.
Whole Exome Sequencing and Analysis
Whole exome sequencing libraries for the patient blood sample, tumor biopsy, and PDCL were carried out by the Knight Diagnostic Research Cytogenetics Lab at OHSU. Libraries were prepared using 500 ng of fragmented gDNA using KAPA Hyper-Prep Kit (KAPA Biosystems) with Agilent SureSelect XT Target Enrichment System and Human All Exon V5 capture baits (Agilent Technologies), following manufacturer’s protocols. Sequencing was carried out using the Illumina HiSeq 2500 platform by the OHSU Massively Parallel Sequencing Shared Resource (MPSSR). Paired-end reads were aligned with bwa mem (v0.7.15-r1140) to GRCh38 (“hg38”,Genome Reference Consortium Human Reference 38 (GCA_000001405.2))36. The data was processed following the best practices workflow for the GATK pipeline (v4.1.9.0)37. Exome regions annotated as “protein-coding” were extracted from GenCode (v35)38 and used as the intervals for processing. The following commands were then used for WES data normalization and segmentation with additional options were specified: PreprocessInvertals, CollectReadCounts, AnnotateIntervals, FilterIntervals, CreateRedCountPanelOfNormals (using the matched blood sample as the normal, with minimum-interval-median-percentile set to 5.0), and finally PlotDenoisedCopyRatios. The output was then plotted with ggplot2 (v3.3.2) in R (v4.0.0). The geom_rect function was used to shade the genomic region based on the relative copy number with segmentation interval, and geom_point was used to plot normalized bin reads (Figure 5a).
s3-ATAC Library Generation
A formatted stepwise protocol for s3-ATAC is available for review at:
https://dx.doi.org/10.17504/protocols.io.bd6wi9fe
Prior to sample handling, 96 uniquely indexed transposome complexes were assembled using previously-described methods11. Complexes were diluted to 2.5uM in a protein storage buffer composed of 50% (v/v) glycerol (Sigma G5516), 100 mM NaCl (Fisher Scientific S271–3), 50 mM Tris pH 7.5 (Life technologies AM9855), 0.1 mM EDTA (Fisher Scientific AM9260G), 1 mM DTT (VWR 97061–340), and stored at −20°C (Supplementary Table 2). At the time of nuclei dissociation, 50mL of nuclei isolation buffer (NIB-HEPES) was freshly prepared with final concentrations of 10 mM HEPES-KOH (Fisher Scientific, BP310–500 and Sigma Aldrich 1050121000, respectively), pH 7.2, 10 mM NaCl, 3mM MgCl2 (Fisher Scientific AC223210010), 0.1 % (v/v) IGEPAL CA-630 (Sigma Aldrich I3021), 0.1 % (v/v) Tween (Sigma-Aldrich P-7949) and diluted in PCR-grade Ultrapure distilled water (Thermo Fisher Scientific 10977015). After dilution, two tablets of Pierce™ Protease Inhibitor Mini Tablets, EDTA-free (Thermo Fisher A32955) were dissolved and suspended to prevent protease degradation during nuclei isolation.
For s3-ATAC tissue handling, primary samples of C57/B6 mouse whole brain were extracted and flash frozen in a liquid nitrogen bath, before being stored at −80°C. Human cortex samples from the middle frontal gyrus were sourced from the Oregon Brain Bank from a 50-year-old female of normal health status. Tissue was collected at 21 hours post-mortem and then placed in a −80°C freezer for storage. An at-bench dissection stage was set up prior to nuclei extraction. A petri dish was placed over dry ice, with fresh sterile razors pre-chilled by dry-ice embedding. 7mL capacity dounce homogenizers were filled with 2mL of NIB-HEPES buffer and held on wet ice. Dounce homogenizer pestles were held in in ice cold 70% (v/v) ethanol (Decon Laboratories Inc 2701) in 15mL tubes on ice to chill. Immediately prior to use, pestles were rinsed with chilled distilled water. For tissue dissociation, mouse and human brain samples were treated similarly. The still frozen block of tissue was placed on the clean pre-chilled petri dish and roughly minced with the razors. Razors were then used to transport roughly 1 mg the minced tissue into the chilled NIB-HEPES buffer within a dounce homogenizer. Suspended samples were given 5 minutes to equilibrate to the change in salt concentration prior to douncing. Tissues were then homogenized with 5 strokes of a loose (A) pestle, another 5 minute incubation, and 5–10 strokes of a tight (B) pestle. Samples were then filtered through a 35 μm cell strainer (Corning 352235) during transfer to a 15mL conical tube, and nuclei were held on ice until ready to proceed. Nuclei were pelleted with a 400 rcf centrifugation at 4°C in a centrifuge for 10 minutes. Supernatant was removed and pellets were resuspended in 1mL of NIB-HEPES buffer. This step was repeated for a second wash, and nuclei were once again held on ice until ready to proceed. A 10uL aliquot of suspended nuclei was diluted in 90uL NIB-HEPES (1:10 dilution) and quantified on either a Hemocytometer or with a BioRad TC-20 Automated cell counter following manufacturer’s recommended protocols. The stock nuclei suspension was then diluted to a concentration of 1400 nuclei/uL.
Tagmentation plates were prepared by the combination of 420 uL of 1400 nuclei/uL solution with 540 uL 2X TD Buffer (Nextera XT Kit, Illumina Inc. FC-131–1024). From this mixture, 8uL (~5000 nuclei total) was pipetted into each well of a 96 well plate dependent on well schema (Figure 1b). 1uL of 2.5uM uniquely indexed transposase was then pipetted into each well. Tagmentation was performed at 55°C for 10 minutes on a 300 rcf Eppendorf ThermoMixer. Following this incubation, plate temperature was brought down with a brief incubation on ice to stop the reaction. Dependent on experimental schema pools of tagmented nuclei were combined and 2uL 5mg/mL DAPI (Thermo Fisher Scientific D1306) was added.
Nuclei were then flow sorted via a Sony SH800 to remove debris and attain an accurate count per well prior to PCR. A receptacle 96 well plate was prepared with 9uL 1X TD buffer (Nextera XT Kit, Illumina Inc. FC-131–1024,diluted with ultrapure water), and held in a sample chamber kept at 4°C. Fluorescent nuclei were then flow sorted gating by size, internal complexity and DAPI fluorescence for single nuclei following the same gating strategy as previously described39. Immediately following sorting completion, the plate was sealed and spun down for 5 minutes at 500 rcf and 4°C to ensure nuclei were within the buffer.
Nucleosomes and remaining transposases were then denatured with the addition 1uL of 0.1% SDS (~0.01% f.c.) per well. 4uL of NPM (Nextera XT Kit, Illumina Inc) per well was subsequently added to perform gap-fill on tagmented genomic DNA, with an incubation at 72°C for 10 minutes. 1.5 uL of 1uM A14-LNA-ME oligo was then added to supply the template for adapter switching (Supplementary Table 3). The polymerase based adapter switching was then performed with the following conditions: initial denaturation at 98°C for 30 seconds, 10 cycles of 98°C for 10 seconds, 59°C for 20 seconds and 72°C for 10 seconds. The plate was then held at 10°C. After adapter switching 1% (v/v) Triton-X 100 in ultrapure H2O (Sigma 93426) was added to quench persisting SDS. At this point, some plates were stored at −20°C for several weeks while others were immediately processed.
The following was then combined per well for PCR: 16.5 ul sample, 2.5uL indexed i7 primer at 10 uM, 2.5uL indexed i5 primer at 10 uM, 3 uL of ultrapure H2O, and 25 uL of NEBNext Q5U 2X Master mix (New England Biolabs M0597S), and 0.5uL 100X SYBR Green I (Thermo Scientific S7563) for a 50 uL reaction per well (Supplementary Table 4–5). A real time PCR was performed on a BioRad CFX with the following conditions, measuring SYBR fluorescence every cycle: 98°C for 30 seconds; 16–18 cycles of 98°C for 10 seconds, 55°C for 20 seconds, 72°C for 30 seconds, fluorescent reading, 72°C for 10 seconds. After fluorescence passes an exponential growth and begins to inflect, the samples were held at 72°C for another 30 seconds then stored at 4°C.
Amplified libraries were then cleaned by pooling 25 uL per well into a 15 mL conical tube and cleaned via a Qiaquick PCR purification column following manufacturer’s protocol (Qiagen 28106). The pooled sample was eluted in 50 uL 10 mM Tris-HCl, pH 8.0. Library molecules then went through a size selection via SPRI selection beads (Mag-Bind® TotalPure NGS Omega Biotek M1378–01). 50 uL of vortexed and fully suspended room temperature SPRI beads was combined with the 50 uL library (1X clean up) and incubated at room temperature for 5 minutes. The reaction was then placed on a magnetic rack and once cleared, supernatant was removed. The remaining pellet was rinsed twice with 100 uL fresh 80% ethanol. After ethanol was pipetted out, the tube was spun down and placed back on the magnetic rack to remove any lingering ethanol. 31 uL of 10 mM Tris-HCl, pH 8.0 was then used to resuspend the beads off the magnetic rack and allowed to incubate for 5 minutes at room temperature. The tube was again placed on the magnetic rack and once cleared, the full volume of supernatant was moved to a clean tube. DNA was then quantified by Qubit dsDNA High-sensitivity assay following manufacturer’s instructions (Thermo Fisher Q32851). Libraries were then diluted to 2ng/uL and run on an Agilent Tapestation 4150 D5000 tape (Agilent 5067–5592). Library molecule concentration within the range of 100–1000bp was then used for final dilution of the library to 1 nM. Diluted libraries were then sequenced on High or Mid capacity 150 bp sequencing kits on the Nextseq 500 system following manufacturer’s recommendations (Illumina Inc. 20024907, 20024904). For greater sequencing effort, select libraries were also sequenced on a NovaSeq S2 flowcell, again following manufacturer’s recommendations (Illumina Inc. 20028315). For both machines, libraries were sequenced as paired-end libraries with 10 cycle index reads and 85 cycles for read 1 and read 2.
s3-WGS Library Generation
A formatted stepwise protocol for s3-WGS is available for review at:
https://dx.doi.org/10.17504/protocols.io.beb3jaqn
Prior to processing the following buffers were prepared: 50mL of NIB HEPES buffer as described above, as well as 50mL of a Tris-based NIB (NIB Tris) variant with final concentrations of 10 mM Tris HCl pH 7.4, 10 mM NaCl, 3mM MgCl2, 0.1 % (v/v) IGEPAL CA-630, 0.1 % (v/v) Tween and diluted in PCR-grade Ultrapure distilled water. After dilution, two tablets of Pierce™ Protease Inhibitor Mini Tablets, EDTA-free were dissolved and suspended to prevent protease degradation during nuclei isolation.
s3-WGS library preparation was performed on cell lines as follows. For patient derived PDCL cell lines, cells were plated at a density of 1×106 on a T25 flask the day prior to processing. At harvest, cells were washed twice with ice cold 1X PBS (VWR 75800–986) and then trypsinized with 5mL 1X TrypLE (Thermo Fisher 12604039) for 15 minutes at 37°C. Suspended cells were then collected and pelleted at 300 rcf at 4°C for 5 minutes. For suspension-growth cell lines (GM12878), cells were pipetted from growth media and pelleted at 300 rcf at 4°C for 5 minutes.
Following the initial pellet, cells were washed with ice cold 1mL NIB HEPES twice. After the second wash, pellets were then resuspended in 300 uL NIB HEPES. Nuclei were aliquoted and quantified as described above, then aliquots of 1 million nuclei were generated based on the quantification. The aliquots were pelleted by a 300 rcf centrifugation at 4°C for 5 minutes and resuspended in 5 mL NIB HEPES. 246 uL 16% (w/v) formaldehyde (Thermo Fisher 28906) was then added to nuclear suspensions (f.c. 0.75% formaldehyde) to lightly fix nuclei. Nuclei were fixed via incubation in formaldehyde solution for 10 minutes on an orbital shaker set to 50 rpm. Suspensions were then pelleted at 500 rcf for 4 minutes at 4°C and supernatant was aspirated. Pellet was then resuspended in 1 mL of NIB Tris Buffer to quench remaining formaldehyde. Nuclei were again pelleted at 500 rcf for 4 minutes at 4°C and supernatant was aspirated. The pellet was washed once with 500uL 1X NEBuffer 2.1 (NEB B7202S) and then resuspended with 760 uL 1X NEBuffer 2.1. 40 uL 1% SDS (v/v) was added and sample was incubated on a ThermoMixer at 300 rcf set to 37°C for 20 minutes. Nucleosome depleted nuclei were then pelleted at 500 rcf at 4°C for 5 minutes and then resuspended in 50 uL NIB Tris. A 5 uL aliquot of nuclei was taken and diluted 1:10 in NIB Tris then quantified as described above. Nuclei were diluted to 500 nuclei/uL with addition of NIB Tris, based on the quantification. Dependent on experimental setup, the 420 uL of nuclei at 500 nuclei/uL were then combined with 540 uL 2X TD buffer. Following this, nuclei were tagmented, stained and flow sorted, genomic DNA was gap-filled and adapter switching was performed as described for the s3-ATAC protocol. Library amplification was performed by PCR as described above with fewer total cycles (13–15) likely due to more initial capture events per library. Libraries were then cleaned, size selected, quantified and sequenced as described previously.
s3-GCC Library Generation
A formatted stepwise protocol for s3-WGS is available for review at:
https://dx.doi.org/10.17504/protocols.io.beb4jaqw
The same cultured cell line samples were harvested as described for s3-WGS library generation, and processed from the same pool of fixed, nucleosome depleted nuclei. Following quantification of nuclei, the full remaining nuclear suspensions (~2–3 million nuclei per sample) were pooled respective of sample. Nuclei were pelleted at 500 rcf at 4°C for 5 minutes and resuspended in 90 uL 1X Cutsmart Buffer (NEB B7204S). 10 uL of 10U/uL AluI restriction enzyme (NEB R0137S) was added to each sample. Samples were then digested for 2 hours at 37°C at 300 rpm on a ThermoMixer. Following digestion, nuclear fragments then underwent proximity ligation. Nuclei were pelleted at 500 rcf at 4°C for 5 minutes and resuspended in 100uL ligation reaction buffer. Ligation buffer is a mixture with final concentrations of 1X T4 DNA Ligase Buffer + ATP (NEB M0202S), 0.01 % TritonX-100, 0.5mM DTT (Sigma D0632), 200 U of T4 DNA Ligase, diluted in ultrapure H2O. Ligation took place at 16°C for 14 hours (overnight). Following this incubation, nuclei were pelleted at 500 rcf at 4°C for 5 minutes and resuspended in 100 uL NIB HEPES buffer. An aliquot of nuclei were quantified as described previously, and were then diluted, aliquoted, tagmented, pooled, DAPI stained, flow sorted, genomic DNA was gap-filled and adapter switching was performed as described for the s3-ATAC protocol. Library amplification occurred at the same rate as the s3-WGS libraries (13–15 cycles) and libraries were subsequently pooled, cleaned, quantified and sequenced as described above.
Computational Analysis
Preprocessing
The initial processing of all library types was the same. After sequencing, data was converted from bcl format to FastQ format using bcl2fastq (v 2.19.0, Illumina Inc.) with the following options with-failed-reads, no-lane-splitting, fastq-compression-level=9, create-fastq-for-index-reads. Data were then demultiplexed, aligned, de-duplicated using the in-house scitools pipeline (ref 39). Briefly, FastQ reads were assigned to their expected primer index sequence allowing for sequencing error (Hamming distance ≤2) and indexes were concatenated to form a “cellID”. Reads that could be assigned unambiguously to a cellID were then aligned to reference genomes. For s3-WGS and s3-GCC libraries, paired reads were aligned with bwa mem (v0.7.15-r1140) to hg3836. For s3-ATAC libraries, reads were first aligned to a concatenated hybrid genome of hg38 and GRCm38 (“mm10”, Genome Reference Consortium Mouse Build 38 (GCA_000001635.2)). Reads were then de-duplicated to remove PCR and optical duplicates by a perl (v5.16.3) script aware of cellID, chromosome and read start, read end and strand. From there putative single-cells were distinguished from debris and error-generated cellIDs by both unique reads and percentage of unique reads.
s3-ATAC Analysis
Barnyard Analysis
With single-cell libraries distinguished, we next quantified contamination between nuclei during library generation. We calculated the read count of unique reads per cellID aligning to either human reference or mouse reference chromosomes (Figure 1C). CellIDs with ≥90% of reads aligning to a single reference genome were considered bona fide single-cells. Those not passing this filter (2.7%,19/687 cells for pre-tagmentation barnyard) were considered collisions. Collision rate was estimated to account for cryptic collisions (mouse cell-mouse cell or human cell-human-cell) by multiplying by two (final collision rate of 5.5%, Figure 2b). Bona fide single-cell cellIDs were then split from the original FastQ files to be aligned to the proper hg38 or mm10 genomes with bwa mem as described above. Human and mouse assigned cellIDs were then processed in parallel for the rest of the analysis. After alignment, reads were again de-duplicated to obtain proper estimates of library complexity (Supplementary Table 6).
Tagmentation Insert Quantification
To assess tagmentation insert size, samtools isize (v. 1.10) was performed and plotted with ggplot2 (v3.3.2) in R (v4.0.0) using the geom_density function (default parameters, Fig 2e). To assess library quality further, we generated tagmentation site density plots centered around transcription start sites (TSSs). We used the alignment position (chromosome and start site) for each read to generate a bed file that was then piped into the BEDOPS closest-feature command mapped the distance between all read start sites and transcription start sites (v 2.4.36)40. From this, we collapsed binned distances (100bp increments) into a counts table and generated percentage of read start site distances within each counts table. We plotted these data using R and ggplot2 geom_density function (default parameters) subset to 2000 base pairs around the start site to visualize enrichment. TSS enrichment values were calculated for each experimental condition using the method established by the ENCODE project (https://www.encodeproject.org/data-standards/terms/enrichment), whereby the aggregate distribution of reads ±1,000 bp centered on the set of TSSs is then used to generate 100 bp windows at the flanks of the distribution as the background and then through the distribution, where the maximum window centered on the TSS is used to calculate the fold enrichment over the outer flanking windows (Figure 2f).
Library Complexity Analysis
To project library complexity through sequencing effort, pre-de-duplicated cellID read sets were used to build a projection as follows8. Reads were randomly subsampled starting at 1% of the total reads with 5% of data added in increasing increments to build a simple saturation curve per cellID. A summarized saturation curve per species was generated and plotted in ggplot2 using the geom_smooth function, descripting the curves mean, median and standard error. For comparison to publicly available data sets of a matched tissue type, we focused our analysis on the mouse brain libraries. We plotted our PCR plate sequenced to 36.4% ± 17.4% unique reads/total reads for comparison to three other single-cell ATAC-seq methods which have been applied to post-natal mouse whole brain (n=3,034; 5,336; 46,653, for snATAC, 10X Genomics scATAC, and dscATAC, respectively)13–15. Our data was filtered to just unique reads which were properly paired via the samtools view “–f 2” argument to allow for proper read count comparison across data sets which report read pairs or “fragments”. Data passing self-reported filters were used for comparison and plotted with ggplot geom_boxplot function (Figure 2d). Welch’s two-sample T test comparisons between unique reads per cell were calculated with the t.test function in base R for a one-sided alternative hypothesis. For peak overlap comparison, we added an additional sci-ATAC low sequencing effort data set21 performed on adult mouse flash frozen brain tissue. We then counted unique peak overlaps between data sets and plotted as stacked bar plots via ggplot geom_bar function (Figure 2g).
For assessment on sequencing effort necessary to reach a median unique reads/cell threshold, we compared our s3ATAC mouse data to the publically available sci-ATAC matched sample data21. We randomly subsampled the bam files pre-deduplication and calculated per cellID library complexity, as described above10,21. The resulting model was plotted with geom_line. We then calculated amount of PCR duplicate reads at that threshold for sequencing effort comparison (Supplementary Figure 1e).
Dimensionality Reduction
Pseudo-bulked data (agnostic of cellID) was then used to call read pile-ups or “peaks” via macs2 (v.2.2.7.1) with option –keep-dup all41. Narrowpeak bed files were then merged by overlap and extended to a minimum of 500bp for a total of 292,156 peaks for human and 174,653 peaks for mouse. A scitools perl script was then used to generate a sparse matrix of peaks × cellID to count occurrence of reads within peak regions per cell. FRiP was calculated as the number of unique, usable reads per cell that are present within the peaks out of the total number of unique, usable reads for that cell for each peak bed file. Cells with less than 20% of reads within peaks were then filtered out. Tabix formatted files were generated using samtools and tabix (v1.7). The counts matrix and tabix files were then input into a SeuratObject for Signac (v1.0.0) processing19,42.We performed LDA-based dimensionality reduction via cisTopic (v0.3.0)with 27 topics for mouse cells and 24 topics for human cells17. The number of topics were selected after generating 25 separate models per species with topic counts of 5,10,20–30,40,50,55,60–70 and selecting the topic count using selectModel based on the second derivative of model perplexity. Cell clustering was performed with Signac FindNeighbors and FindClusters functions on the topic weight × cellID data frame. For FindClusters function call, resolution was set to 0.3 and 0.2 for human and mouse samples, respectively. The respective topic weight × cellID was then projected into two dimensional space via a uniform manifold approximation and projection (“UMAP”) by the function umap in the uwot package (v0.1.8, Figure 2h)43. Cis-coaccessibility networks (CCANs) were generated through the Signac wrapper of cicero (v1.3.4.10)44. Genome track plots with CCAN linkages were generated through Signac function CoveragePlot for marker genes previously described (Supplementary Figure 1a)19. Differential accessibility between clusters in one by one, and one by rest comparisons were generated using Signac function FindMarkers using options: test.use = ‘LR’, and only.pos=T, with latent.vars = ‘nCount_peaks’, to account for read depth. Cell type per cluster was assigned based on genome track plots and differentially accessible sites (Supplementary Tables 6–7, Supplementary File 2).
Subsampling
For subsampling analysis, the processed, deduplicated bam files were split by cellID into single-cell bam files. Each bam file was then subsampled randomly using samtools view –s argument for 0.5, 1, 2, 5, 10, 15, 20, 40, 50, 60, 80%, respectively. Following this single-cell subsampled bams were collated respective of downsampling percentage and processed through peak calling, dimensionality reduction and projection as described above with the following exception. Topic model generation was limited to 10,20–30 topics. Number of peaks callable per downsampled data set were plotted via geom_bar. The final projections were plotted via geom_point with the color of the cell type assignment in the full data set (Supplementary Figure 1b–d).
Cross-Platform Integration
Data used in library complexity and peak overlap comparisons was integrated using the Signac package as follows45. Counts matrices were generated using the platform-defined peak regions and formatted as Seurat objects. For each integration our s3-ATAC mouse data, we generated a new counts matrix was generated from the platform-defined peaks. Following this, counts matrices were merged and latent semantic indexing (“lsi”) was used for reduction. Harmony was used to integrate, and a UMAP projection was generated using dimensions 2–30 for sci-ATAC, scATAC, and snATAC data sets and 2–40 for dscATAC46. Integrated plots were generated using our defined cell types (Figure 2i).
Subclustering
After gross cell type assignment of mouse and human cell lines, human inhibitory neurons (GAD1+) clusters 3 and 4 were subset from the SeuratObject. Those 342 cells were then iteratively clustered by performing the same cisTopic, UMAP, and Signac processing with the following changes19,43,47. CisTopic was performed on the full set of human peaks (292,156) with those 342 subset cells. 12 Topic models were constructed (5, 10, 20–30 topics) and the 25 topic model was chosen on the second derivate of the model perplexity. A resolution of 0.5 was used in the Signac FindClusters on the topic weight × cellID call to attain 5 subclusters. One cluster was removed based on putative doublets (Figure 3a). Coverage plots were generated as reported above for ADARB2 and LHX6 (Figure 3b). Peaks were then assigned to topics using the cisTopic binarizecisTopics function with argument thrP=0.975 (mean count per topic: 2429 peaks). We then performed a simple gene set enrichment analysis on human cortical inhibitory neurons and subtypes based on RNA-identified marker genes defined previously20.We used a Fisher’s Exact test with the function fisher.test with function alternative.hypothesis=”greater” to look for enrichment of topic-assigned peaks in marker gene bodies for inhibitory neuron subclasses relative to all topic-assigned peaks. We filtered results to those with nominal enrichment (p value ≤ 0.05) and used ggplot geom_point with color reflecting the reported p-value and size proportional to odds ratio to generate a bubble plot (Figure 3c).
s3-WGS and s3-GCC Analysis
Quality Control
s3-WGS and s3-GCC cellIDs were initially filtered to samples with either ≥1×105 or ≥1×106 unique reads (PDCL and GM12878 samples, respectively). CellIDs were split after de-duplication into single-cell bam files. They were then processed via the pipeline in the package SCOPE (v1.1)23. The genome was split into 500 kbp bins with each bin being assigned a GC content and mappability score (generated through CODEX2)48. Reads with a mapping quality of Q ≥ 10 were counted in bins per cellID. Bins with a mappability score < 0.9 or GC content ≤ 20% or ≥ 80% were removed (5449 bins passing filter). Additionally, cellIDs with low coverage were removed (1268 samples passing filter). Median absolute deviation (MAD) scores were calculated per cell on 500kb bins of cells passing filter as previously described23. Briefly, let Yi,j be the raw read count for the ith cellID of the jth bin (from 1.. n bins). Let Ni be a cell-specific scaling factor (total read depth) and Bj be a bin-specific normalization, output as beta.hat from the function normalize_codex2_ns_noK. Such that
MAD scores were then plotted using the ggplot geom_jitter and geom_boxplot functions (Figure 4e).
Copy Number Calling
SCOPE assumes diploid cells within the sample for normalization steps. To this end we used GM12878 lymphoblastoid cell line as our normal diploid samples and used an a priori estimate of 2.6N based on averaged PDCL karyotyping results (Figure 4c). We then used the SCOPE function normalize_scope_foreach with the following options: K=5, T=1:6 to normalize read distributions per cell. We segmented the genome into breakpoints per chromosome and inferred copy number per breakpoint per cell by segment_CBScs allowing for a simple nested structure of copy number changes (max.ns=1). To plot inferred copy number per cell, we used the R library ComplexHeatmap (v2.5.5) by function Heatmap49. Pairwise distance between cells was generated by Jaccard distance through the R library philentropy (v0.4.0)50 on windows categorized as “neutral” (2N), “amplified” (>2N) or “deleted” (<2N). Cells then underwent hierarchical clustering by the “ward.D2” argument in the function hclust. The resultant dendrogram was then cut into both 3 and 6 clades based on the two independent optimal k value searches using the find_k function in the R library dendextend (v1.14.0) given a range of 2 to 10 and 5 to 10 clusters, respectively (Figure 5b)51. Cells with shared clade membership were then combined into “pseudobulk” clades for higher resolution copy number calling. After combining counts data across 50 kbp bins (and filtered as described above), we had 6 clades with 154, 250, 363, 100, 268 and 133 cells, with mean reads per bin of 1207, 2442, 4662, 2071, 2700, and 9416, respectively. These pseudobulk sampled were then normalized as described above with clade 6, containing 83.45% GM12878 cells (111/133 cells) as the normal diploid sample. The genome per sample was then segmented as described above and normalized reads per bin as well as segmentation calls were plotted with ggplot2 geom_point and geom_rect functions (Supplementary Figure 2a). Select genomic locations25 of recurrently mutated genes were visualized and plotted using IGV with 5 bins (250kbp) up and downstream from the transcription start sites. (Supplementary Figure 2b)52.
Generation of GCC Contact Profiles
s3-GCC contact profile raw counts were generated for cellIDs passing the read count and SCOPE filters (215 cells) as follows. For initial plotting of single-cell profiles, paired-end read bam files were filtered for an insert length of ≥50kbp via pysam53 and output as upper-triangle triple-sparse format at 1mbp bin sizes. Raw contact matrices were then plotted with R and ComplexHeatmap (Fig 5c, left). Merged ensemble plots were generated by summing single-cell contact matrices generated as described above for 500 kbp bins. Following this, we performed dimensionality reduction and clustering analyses using a topic modeling approach. We treated the GCC portion of single-cell sequencing fragments (read pairs separated by a genomic distance higher than 20kb) as traditional distal interactions. We analyzed these cells using our previously established topic model for analysis and characterization of single-cell Hi-C data31. In the topic modeling framework, each cell is treated as a mixture of “topics” where each topic corresponds to a set of distal interactions. The model is trained in an unsupervised manner to find the optimum number of topics that best describe the data and associates each distal interaction with a probabilistic mixture of topics.
We trained a topic model using the GCC data with the default parameters in Kim et al. However, we altered one parameter, which is the range of distal interactions that are input into the model. Due to high coverage of s3-GCC assays, we opted for distal interactions that are separated by a genomic distance of 20Mb or less, as opposed to original parameter where we used interactions that are separated by distances lower than 10Mb. After training, we found that the number of topics that best describe the data is 15. We visualized cells using UMAP and found that the majority of cells from two lines cluster separately (Figure 5c). Overall, these results validate the Hi-C like characteristics of GCC data and further show that we can capture the subtle differences in chromatin organization of the two lines.
Compartment Calling
We called compartments from pseudobulk Hi-C contact matrices and calculated contact probabilities as described in the original Hi-C paper by Lieberman-Aiden et al54. We briefly describe these methods here. To obtain compartment calls, we first ICE normalized and removed the distance effect in Hi-C matrices for each chromosome at 500kb resolution. For each normalized Hi-C contact matrix, we calculated the Spearman Correlation Coefficient matrix from the normalized Hi-C matrix, which yields a matrix with clear plaid pattern. Resulting matrices were reduced to one dimensional representation by performing eigendecomposition; the first eigenvector typically yields the compartment calls, which closely tracks the two dimensional plaid pattern in one dimension. For comparison we used compartment calls from Rao et al.55 on the GM12878 cell line (Figure 5c, right).
Calculation of contact probabilities
To calculate intrachromosomal contact probabilities for each genomic distance, we first calculated the mean Hi-C signal at a given genomic distance for a given resolution. For a Hi-C matrix binned at 500kb resolution, the mean Hi-C signal for distances less than 500kb is the mean of the diagonal, the mean Hi-C signal for distances between 500kb and 1000kb is the mean of the first off-diagonal, and so forth. After calculating the mean Hi-C signal vector for the every intrachromosomal matrix, we obtain a mean Hi-C signal vector at 500kb resolution that contains 500 elements, ranging from 0 to ~250Mb, since the largest chromosome (Chr1) is approximately 250Mb long. To convert this vector into probabilities, we simply divide the vector by the sum. When plotting contact probabilities, we typically omit the visualizing the contact probabilities for distances larger than 200Mb, as the mean Hi-C signal at such long distances is both sparse and noisy. External bulk Hi-C datasets have been downloaded from the ENCODE consortium’s data portal, https://www.encodeproject.org/ via accession codes ENCSR194SRI, ENCSR346DCU, ENCSR444WCZ, ENCSR079VIJ (Supplementary Figure 3a).
Data Availability
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE174226 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE174226). External single-cell ATAC data sets were downloaded from GEO sample accession number GSM2668124 for snATAC (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSM2668124), and external sites for dscATAC (https://github.com/buenrostrolab/dscATAC_analysis_code/blob/master/mousebrain/data/mousebrain-master_dataframe.rds), and 10X Genomics scATAC (https://cf.10xgenomics.com/samples/cell-atac/1.1.0/atac_v1_adult_brain_fresh_5k). External single-cell WGS dataset was downloaded from NCBI BioProject PRJNA326698 (https://www.ncbi.nlm.nih.gov/sra/SRX2005587). Single-cell Hi-C data sets were downloaded from the 4D Nucleosome project (https://data.4dnucleome.org/publications/048d4558–2cac-41d2-ac6e-ff2ac3f007c4/#expsets-table). External bulk Hi-C datasets have been downloaded from the ENCODE consortium’s data portal, https://www.encodeproject.org/ via accession codes ENCSR194SRI, ENCSR346DCU, ENCSR444WCZ, ENCSR079VIJ.
Code Availability
Code and custom scripts used in this study are available at https://github.com/adeylab/scitools, and https://mulqueenr.github.io/.
Reporting Summary –
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Supplementary Figure 1 | Marker sets for gross cell type discrimination in human cortex and mouse whole brain data sets and down sampling analysis. (a) Genome coverage plots of marker genes for mouse (top) and human (bot.) cell types. Cells are separated into tracks based on clustering and signal aggregated. (b) Table of down sampling analysis percentages (percentage of reads per cell) and mean unique reads per cell. (c) Number of called peaks for down sampled data for mouse (top) and human (bot.). (d) UMAPs of cells colored by full data set cluster identity for down sampled data on mouse (top) and human (bot.). Percentage of reads used per cell is listed in the top corner of each subpanel. (e) Log10 unique, passing reads as a function of library saturation for s3-ATAC and sci-ATAC datasets. Dashed line represents 10,000 unique passing reads, with the percentage of total reads discarded for each method to achieve that total.
Supplementary Figure 2 | Pseudo-bulk whole genome copy number calling. (a) Pseudo-bulk genome-wide relative copy number calling. Scatter plot of reads per bin (50 kbp) separated by clade and chromosome. Clade assignment shown on left and relate to Figure 5b (k=6). Recurrently mutated gene locations in PDAC25 highlighted along the read count bins for clade 6. Shading of plot colored by relative copy number in genomic locus. (b) Select genes with known copy number aberrations in PDAC bar plot for relative copy number per clade. Middle line per row is copy number neutral, amplifications in red and deletions in blue. Red boxes on chromosome ideograms at the top of each window indicate the position of the gene.
Supplementary Figure 3 | Clustering of s3GCC profiles. (a) Contact probability as function of genomic distance for s3-GCC data compared to bulk HiC datasets. (b) Unique contacts ≥50 kbp displayed on UMAP dimensions for quality control purposes. (c) Topic contribution scores plotted over UMAP projection of s3-GCC cells. (d) Normalized HiC contacts for PDAC-1 and PDAC-2 cell lines for chromosome 12, windows within the translocation specific to PDAC-1 exhibit elevated HiC contacts comparable to much shorter distances (<20 Mbp).
Supplementary File 1 | Mouse brain ATAC-seq peaks.
Supplementary File 2 | s3-ATAC differential accessibility and cell type classification.
Supplementary Table 1–5 | s3 oligonucleotide and primer design.
Supplementary Table 6 | s3-ATAC metadata.
Supplementary Table 7 | s3-ATAC public data comparisons between mouse whole brain preparations. Statistics for comparison between s3-ATAC data with public data sets using a Welch’s two-sided test. No adjustments made for multiple comparisons.
Supplementary Table 8 | s3-WGS and s3-GCC metadata.
Supplementary File 3 | s3-GCC A/B compartment eigenvector plots.
Acknowledgements
We would like to thank helpful suggestions and feedback from other members of the Adey Lab as well as Jay Shendure and Cole Trapnell. This work was funded by R01DA047237 (NIH/NIDA) and R35GM124704 (NIH/NIGMS) to A.C.A; and R01MH113926 (NIH/NIMH) to B.J.O. We would also like to thank the Oregon Brain Bank for the donated biological sample used in this study.
Competing Interests
D.P., F.Z., and F.J.S. are employees of Scale Bio. R.M.M., D.P., F.Z., F.J.S., and A.C.A. are authors on licensed patents that cover components of the technologies described in this manuscript. This potential conflict of interest for A.C.A. and R.M.M. has been reviewed and managed by OHSU.
References
- 1.Cusanovich DA et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science (80-.). 348, 910–914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adey A et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 11, R119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L, Xing D, Chang CH, Li H & Xie XS Three-dimensional genome structures of single diploid human cells. Science (80-.). 361, 924–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sos BC et al. Characterization of chromatin accessibility with a transposome hypersensitive sites sequencing (THS-seq) assay. Genome Biol. 17, 20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y et al. High-Throughput Single-Cell Sequencing with Linear Amplification. Mol. Cell 76, 676–690.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C et al. Single-cell whole-genome analyses by Linear Amplification via Transposon Insertion (LIANTI). Science (80-.). 356, 189–194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adey A & Shendure J Ultra-low-input, tagmentation-based whole-genome bisulfite sequencing. Genome Res. 22, 1139–1143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulqueen RM et al. Highly scalable generation of DNA methylation profiles in single cells. Nat. Biotechnol. 36, 428–431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang O et al. Efficient and unique cobarcoding of second-generation sequencing reads from long DNA molecules enabling cost-effective and accurate sequencing, haplotyping, and de novo assembly. Genome Res. 29, 798–808 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitak SA et al. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat. Methods 14, 302–308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amini S et al. Haplotype-resolved whole-genome sequencing by contiguity-preserving transposition and combinatorial indexing. Nat. Genet. 46, 1343–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adey A et al. In vitro, long-range sequence information for de novo genome assembly via transposase contiguity. Genome Res. 24, 2041–2049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preissl S et al. Single-nucleus analysis of accessible chromatin in developing mouse forebrain reveals cell-type-specific transcriptional regulation. Nature Neuroscience 1–8 (2018) doi: 10.1038/s41593-018-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datasets -Single Cell ATAC -Official 10x Genomics Support.
- 15.Lareau CA et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. (2019) doi: 10.1038/s41587-019-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buenrostro JD, Giresi PG, Zaba LC, Chang HY & Greenleaf WJ Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravo González-Blas C et al. cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. Nat. Methods 16, 397–400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becht E et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Stuart T, Srivastava A, Lareau C & Satija R Multimodal single-cell chromatin analysis with Signac. bioRxiv 2020.11.09.373613 (2020) doi: 10.1101/2020.11.09.373613. [DOI] [Google Scholar]
- 20.Hodge RD et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton CA et al. Spatially mapped single-cell chromatin accessibility. Nat. Commun. 12, 1274 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domcke S et al. A human cell atlas of fetal chromatin accessibility. Science 370, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Lin DY & Jiang Y SCOPE: A Normalization and Copy-Number Estimation Method for Single-Cell DNA Sequencing. Cell Syst. 10, 445–452.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laks E et al. Clonal Decomposition and DNA Replication States Defined by Scaled Single-Cell Genome Sequencing. Cell 179, 1207–1221.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raphael BJ et al. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32, 185–203.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenburger K et al. AB024. S024. Drug responses of patient-derived cell lines in vitro that match drug responses of patient PDAc tumors in situ. Ann. Pancreat. Cancer 1, AB024–AB024 (2018). [Google Scholar]
- 27.Ramani V et al. Massively multiplex single-cell Hi-C. Nat. Methods 14, 263–266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagano T et al. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed S, Bradshaw A-D, Gera S, Dewan M & Xu R The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J. Clin. Med. 6, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H et al. PRSS1 genotype is associated with prognosis in patients with pancreatic ductal adenocarcinoma. Oncol. Lett. 19, 121–126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H-J et al. Capturing cell type-specific chromatin compartment patterns by applying topic modeling to single-cell Hi-C data. PLOS Comput. Biol. (2020) doi: 10.1371/journal.pcbi.1008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sritangos P et al. Plasma membrane Ca2+ atpase isoform 4 (PMCA4) has an important role in numerous hallmarks of pancreatic cancer. Cancers (Basel). 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad MK, Abdollah NA, Shafie NH, Yusof NM & Razak SRA Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer. Cancer Biology and Medicine vol. 15 14–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaya-Okur HS et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 12, 439–451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Online Methods References
- 36.Li H & Durbin R Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poplin R et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 201178 (2017) doi: 10.1101/201178. [DOI] [Google Scholar]
- 38.Frankish A et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinnamon JR et al. The accessible chromatin landscape of the murine hippocampus at single-cell resolution. Genome Res. 29, 857–869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neph S et al. BEDOPS: high-performance genomic feature operations. Bioinformatics 28, 1919–1920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler A, Hoffman P, Smibert P, Papalexi E & Satija R Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mcinnes L, Healy J, Saul N & Großberger L UMAP: Uniform Manifold Approximation and Projection Software • Review • Repository • Archive. (2018) doi: 10.21105/joss.00861. [DOI] [Google Scholar]
- 44.Pliner HA et al. Cicero Predicts cis-Regulatory DNA Interactions from Single-Cell Chromatin Accessibility Data. Mol. Cell (2018) doi: 10.1016/j.molcel.2018.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart T et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korsunsky I et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Blas CB et al. Cis-topic modelling of single cell epigenomes. bioRxiv 370346 (2018) doi: 10.1101/370346. [DOI] [Google Scholar]
- 48.Jiang Y et al. CODEX2: Full-spectrum copy number variation detection by high-throughput DNA sequencing. Genome Biol. 19, 202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Z, Eils R & Schlesner M Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (2016). [DOI] [PubMed] [Google Scholar]
- 50.Drost H-G Philentropy: Information Theory and Distance Quantification with R. J. Open Source Softw. 3, 765 (2018). [Google Scholar]
- 51.Galili T dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31, 3718–3720 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson JT et al. Integrative genomics viewer. Nature Biotechnology vol. 29 24–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.pysam-developers/pysam.
- 54.Lieberman-aiden E et al. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science (80-.). 326, 289–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao SSP et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 12, 439–451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H & Durbin R Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poplin R et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 201178 (2017) doi: 10.1101/201178. [DOI] [Google Scholar]
- 39.Frankish A et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinnamon JR et al. The accessible chromatin landscape of the murine hippocampus at single-cell resolution. Genome Res. 29, 857–869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neph S et al. BEDOPS: high-performance genomic feature operations. Bioinformatics 28, 1919–1920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler A, Hoffman P, Smibert P, Papalexi E & Satija R Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mcinnes L, Healy J, Saul N & Großberger L UMAP: Uniform Manifold Approximation and Projection Software • Review • Repository • Archive. (2018) doi: 10.21105/joss.00861. [DOI] [Google Scholar]
- 45.Pliner HA et al. Cicero Predicts cis-Regulatory DNA Interactions from Single-Cell Chromatin Accessibility Data. Mol. Cell 71, 858–871.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuart T et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korsunsky I et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Blas CB et al. Cis-topic modelling of single cell epigenomes. bioRxiv 370346 (2018) doi: 10.1101/370346. [DOI] [Google Scholar]
- 49.Jiang Y et al. CODEX2: Full-spectrum copy number variation detection by high-throughput DNA sequencing. Genome Biol. 19, 202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu Z, Eils R & Schlesner M Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (2016). [DOI] [PubMed] [Google Scholar]
- 51.Drost H-G Philentropy: Information Theory and Distance Quantification with R. J. Open Source Softw. 3, 765 (2018). [Google Scholar]
- 52.Galili T dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31, 3718–3720 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson JT et al. Integrative genomics viewer. Nature Biotechnology vol. 29 24–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.pysam-developers/pysam. https://github.com/pysam-developers/pysam.
- 55.Lieberman-Aiden E et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science (80-.). 326, 289–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao SSP et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 | Marker sets for gross cell type discrimination in human cortex and mouse whole brain data sets and down sampling analysis. (a) Genome coverage plots of marker genes for mouse (top) and human (bot.) cell types. Cells are separated into tracks based on clustering and signal aggregated. (b) Table of down sampling analysis percentages (percentage of reads per cell) and mean unique reads per cell. (c) Number of called peaks for down sampled data for mouse (top) and human (bot.). (d) UMAPs of cells colored by full data set cluster identity for down sampled data on mouse (top) and human (bot.). Percentage of reads used per cell is listed in the top corner of each subpanel. (e) Log10 unique, passing reads as a function of library saturation for s3-ATAC and sci-ATAC datasets. Dashed line represents 10,000 unique passing reads, with the percentage of total reads discarded for each method to achieve that total.
Supplementary Figure 2 | Pseudo-bulk whole genome copy number calling. (a) Pseudo-bulk genome-wide relative copy number calling. Scatter plot of reads per bin (50 kbp) separated by clade and chromosome. Clade assignment shown on left and relate to Figure 5b (k=6). Recurrently mutated gene locations in PDAC25 highlighted along the read count bins for clade 6. Shading of plot colored by relative copy number in genomic locus. (b) Select genes with known copy number aberrations in PDAC bar plot for relative copy number per clade. Middle line per row is copy number neutral, amplifications in red and deletions in blue. Red boxes on chromosome ideograms at the top of each window indicate the position of the gene.
Supplementary Figure 3 | Clustering of s3GCC profiles. (a) Contact probability as function of genomic distance for s3-GCC data compared to bulk HiC datasets. (b) Unique contacts ≥50 kbp displayed on UMAP dimensions for quality control purposes. (c) Topic contribution scores plotted over UMAP projection of s3-GCC cells. (d) Normalized HiC contacts for PDAC-1 and PDAC-2 cell lines for chromosome 12, windows within the translocation specific to PDAC-1 exhibit elevated HiC contacts comparable to much shorter distances (<20 Mbp).
Supplementary File 1 | Mouse brain ATAC-seq peaks.
Supplementary File 2 | s3-ATAC differential accessibility and cell type classification.
Supplementary Table 1–5 | s3 oligonucleotide and primer design.
Supplementary Table 6 | s3-ATAC metadata.
Supplementary Table 7 | s3-ATAC public data comparisons between mouse whole brain preparations. Statistics for comparison between s3-ATAC data with public data sets using a Welch’s two-sided test. No adjustments made for multiple comparisons.
Supplementary Table 8 | s3-WGS and s3-GCC metadata.
Supplementary File 3 | s3-GCC A/B compartment eigenvector plots.
Data Availability Statement
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE174226 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE174226). External single-cell ATAC data sets were downloaded from GEO sample accession number GSM2668124 for snATAC (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSM2668124), and external sites for dscATAC (https://github.com/buenrostrolab/dscATAC_analysis_code/blob/master/mousebrain/data/mousebrain-master_dataframe.rds), and 10X Genomics scATAC (https://cf.10xgenomics.com/samples/cell-atac/1.1.0/atac_v1_adult_brain_fresh_5k). External single-cell WGS dataset was downloaded from NCBI BioProject PRJNA326698 (https://www.ncbi.nlm.nih.gov/sra/SRX2005587). Single-cell Hi-C data sets were downloaded from the 4D Nucleosome project (https://data.4dnucleome.org/publications/048d4558–2cac-41d2-ac6e-ff2ac3f007c4/#expsets-table). External bulk Hi-C datasets have been downloaded from the ENCODE consortium’s data portal, https://www.encodeproject.org/ via accession codes ENCSR194SRI, ENCSR346DCU, ENCSR444WCZ, ENCSR079VIJ.