Abstract
High nut consumption is associated with reduced total and certain cause-specific mortality in general populations. However, its association with cancer outcomes among long-term breast cancer survivors remains unknown. We examined the associations of nut consumption (including peanuts and tree nuts), assessed at five-years post-diagnosis, with overall survival (OS) and disease-free survival (DFS) among 3,449 long-term breast cancer survivors from the Shanghai Breast Cancer Survival Study, applying Cox regression analysis. During a median follow-up of 8.27 years post dietary assessment, there were 374 deaths, including 252 breast cancer deaths. Among 3,274 survivors without previous recurrence at the dietary assessment, 209 developed breast cancer-specific events, i.e., recurrence, metastasis, or breast cancer mortality. At five-years post dietary assessment (i.e., 10-years post-diagnosis), regular nut consumers had higher OS (93.7% vs 89.0%) and DFS (94.1% vs 86.2%) rates. After multivariable adjustment, nut consumption was positively associated with OS (Ptrend=0.022) and DFS (Ptrend=0.003) following a dose-response pattern, with hazard ratios (HR, 95% CI) of 0.72 (0.52, 1.05) for OS and 0.48 (0.31, 0.73) for DFS, for participants with >median nut intake compared with non-consumers. The associations did not vary by nut type. Stratified analyses showed that the associations were more evident among participants with a higher total energy intake for OS (Pinteraction=0.02) and among participants with early-stage (I-II) breast cancers for DFS (Pinteraction=0.04). The nut-DFS associations were not modified by estrogen receptor/progesterone receptor status or other known prognostic factors. In conclusion, nut consumption was associated with better survival, particularly DFS, among long-term breast cancer survivors.
Keywords: nut consumption, breast cancer, long-term breast cancer survivors, overall survival, disease-free survival
Introduction
Breast cancer is the most frequently diagnosed cancer worldwide and the fourth and second leading cause of cancer death among women in China and the U.S., respectively.1,2 The breast cancer mortality rate increased in China from 2007 to 20113 but has declined in the U.S. since 1989, the latter being attributed to improvements in treatment and early detection.2,4,5 The five-year breast cancer survival rate in China was 82% between 2012–2015.6 The corresponding U.S. rate was 90% as of January 1, 2019,7 and there were over 3.8 million women with a breast cancer history living in the U.S.2 Despite an overall high survival rate, 20%−40% of breast cancer patients eventually develop recurrence.8 While most recurrence occurs within five years of the initial diagnosis, particularly for estrogen receptor (ER)-negative breast cancer, late recurrence (recurrence which occurs five or more years after diagnosis) may occur in 10%−41% of ER-positive breast cancer9 and remains a significant challenge for managing the disease and decreasing breast cancer mortality.8 Therefore, identification of modifiable factors is of great importance to improve long-term breast cancer survival.
Diet has long been linked with breast cancer risk10,11 and survival.12 A high quality diet consistent with prudent dietary patterns, and meeting dietary guidelines of the Chinese Food Pagoda (CHFP) and Dietary Approaches to Stop Hypertension, have been inversely associated with all-cause mortality among breast cancer survivors.13,14 As nutrient dense foods incorporated in healthy diets, nuts have been found in several cohort studies to be associated with reduced all-cause and cause-specific mortality, particularly mortality due to cardiovascular diseases.15–20 However, despite several studies that assessed nut consumption in association with breast cancer risk,21–24 with inconsistent results, nut intake has not been investigated for associations with survival outcomes among breast cancer patients.
To fill this knowledge gap, using data from a population-based cohort study, we investigated overall survival (OS) and disease-free survival (DFS) in association with consumption of total nuts and nut subtypes among long-term breast cancer survivors.
Materials and Methods
Study population
This study included participants of the Shanghai Breast Cancer Survival Study (SBCSS) who survived over five-years post cancer diagnosis. Details of the SBCSS have been described elsewhere.25 Briefly, the SBCSS is a longitudinal, population-based study of 5,042 women, aged 20–75 years, who were diagnosed with primary breast cancer between March 2002 and April 2006. Among them, 3,575 participants who survived five years or longer after cancer diagnosis and completed a detailed dietary assessment at the five-year post-diagnosis follow-up interview, between October 2007 and October 2011, were eligible for the current study.14 We further excluded participants who had in situ breast cancer (n=125) or had a consumption of any type of nuts exceeding 500 g/day (n=1), leaving 3,449 breast cancer survivors included in the final analysis.
Data collection
Approximately 6.5 months after cancer diagnosis, consented study participants were enrolled to the study via in-person interviews. A structured questionnaire was applied to collect information on demographic characteristics, reproductive history, disease history, medication use, selected lifestyle factors, soy food and cruciferous vegetable intake, use of complementary and alternative medicines, and quality of life. Clinical information collected included cancer stage, tumor ER and progesterone receptor (PR) status, and primary treatments (surgery/mastectomy, radiation therapy, chemotherapy, immunotherapy, and hormonal therapy such as tamoxifen). Inpatient medical charts were reviewed to verify clinical information. Cancer diagnoses were confirmed by a combination of medical record review and central review of pathological slides. A Charlson comorbidity index26 was created at baseline, based on a validated comorbidity scoring system and diagnostic codes from the International Classification of Disease (ICD-9).
In-person follow-up surveys were conducted at 18 months and 3, 5, and 10 years after diagnosis to collect or update information on cancer outcomes, including recurrence and metastasis, comorbidity, lifestyle, and medication use. At the five-year post-diagnosis survey, a comprehensive dietary survey was conducted using a validated food frequency questionnaire (FFQ)27 designed to measure the consumption of commonly consumed foods in Shanghai. Study participants were asked how frequently (in 5 categories: daily, weekly, monthly, yearly, or never) they consumed each food or food group, followed by a question on the amounts of foods consumed in liangs (1 liang=50 g) per unit of time during the previous 12 months. Amount of nut consumption, including peanuts, walnuts, and other nuts, was converted into g/week. Total nut consumption was calculated as the sum of intake from peanuts, walnuts, and other nuts. Participants with total nut consumption >0 g/week were defined as nut consumers, and those with no nut consumption were non-consumers. Consumption of total nuts was further categorized into three groups (0, 0-median [17.32 g/week], and >median). Soy food consumption was calculated as the dry weight of soy foods, including tofu, soy milk, fresh soybeans, and other soy products, as well as meat, fish, and cruciferous vegetables. Energy intakes were estimated based on FFQ data and nutrient contents from the Chinese Food Composition 2002.28 Diet quality score based on CHFP-2007 was calculated for each participant, as previously described.14
Statistical analysis
The primary study outcomes in this study were OS and DFS. Events for OS included death due to any cause; events for DFS included breast cancer recurrence, metastasis, and breast cancer-specific death. Participants who had a recurrent cancer or/and metastasis prior to the dietary assessment (n=175) were excluded in the DFS analyses. Information on DFS events was recorded in the ten-year follow-up survey. Vital status was further supplemented by regular record linkages with the Shanghai Vital Statistics Registry. Vital status was censored at the date of last in-person contact or the latest linkage date of December 31, 2017, whichever was more recent.
Descriptive characteristics between nut consumers and non-consumers were compared using chi-square tests for categorical variables and t-tests for continuous variables. Five-year survival rates post-dietary survey (approximate to the ten-year post-diagnosis) were evaluated using Kaplan-Meier curves, with the entry time of age at dietary assessment. The log-rank test was used to compare survival differences between survivors who consumed a particular type of nuts and those with no consumption of any nuts, with event-free observations censored at the end of the tenth year after diagnosis.
Multivariable Cox proportional hazards models were employed to evaluate the hazard ratios (HRs) for OS and DFS events associated with nut intake, using age as the time scale. Entry time was defined as the age at dietary assessment, and exit time was defined as the age at event or censoring. Models were adjusted for age at the initial diagnosis of breast cancer, disease (TNM) stage, ER status, PR status, menopause age, income and education assessed at baseline, weight change, total energy intake, physical activity, CHFP-2007 score, soy food intake, and body mass index (BMI) assessed at the five-year follow-up. Ptrend was estimated by assigning each category of total nut consumption an ordinal value (no-0, ≤median-1, and >median-2) and treating it as a continuous variable in the model. The associations between HRs and nut subtypes (i.e., peanuts, walnuts, and other nuts) were also evaluated. In these analyses, participants with no consumption of any nuts were considered as the fixed reference group, and those who only consumed nut subtypes other than the ones of interest were categorized as a group adjusted in the analyses. A sensitivity analysis using the entry time of age at breast cancer diagnosis was also performed.
Potential effect modifiers, including ER/PR status of breast cancer, BMI, physical activity, comorbidity, soy food intake, CHFP-2007 score, total energy intake, radiotherapy, and TNM stage were evaluated in stratified analyses to assess whether these factors modified the associations of nut intake with OS and DFS. The ordinal nut intake variable was treated as a continuous variable in the analyses. All statistical analyses were performed using SAS Enterprise Guide (Version 7.15 HF8), and all statistical tests were based on two-tailed probability with a significance level set at α<0.05.
Results
Among 3,449 participants included in the final analysis, 3,148 were nut consumers (Table 1). The majority of participants in both consumer and non-consumer groups were diagnosed with ER-positive or PR-positive cancers, though nut consumers were more likely than their counterparts to be diagnosed at stage I (38% vs 30%, P=0.04). In general, compared with non-consumers, nut consumers had a younger age at diagnosis, lower BMI, higher total energy intake, higher CHFP-2007 score, and higher soy food intake. In addition, nut consumers were more likely to have a higher education, higher personal income, higher physical activity level [≥7.5 metabolic equivalent of task (MET)-hour/week] and received immunotherapy.
Table 1.
Characteristics of long-term breast cancer survivors in Shanghai Breast Cancer Survival Study by nut consumption
| Any nut |
|||||
|---|---|---|---|---|---|
| No (n=301) | Yes (n=3148) | P-value | ≤ Mediana (n=1573) | > Mediana (n=1575) | |
|
| |||||
| Age at diagnosis (years) | 55.61 (10.75) | 53.36 (9.64) | <0.001 | 53.88 (9.91) | 52.83 (9.34) |
| Time interval (diagnosis to survey) | 5.320 (0.162) | 5.320 (0.170) | 0.945 | 5.316 (0.163) | 5.323 (0.176) |
| Total energy intake (Kcal/day) | 1339 (357) | 1459 (343) | <0.001 | 1401 (309) | 1518 (364) |
| Total energy intake > medianb | 40% | 51% | <0.001 | 43% | 59% |
| Education ≥ high school | 37% | 55% | <0.001 | 50% | 60% |
| Income >1,000 yuan/month | 25% | 44% | <0.001 | 40% | 48% |
| BMI (kg/m2) | 24.86 (4.05) | 24.28 (3.48) | 0.007 | 24.25 (3.53) | 24.31 (3.43) |
| BMI > 25kg/m2 | 42% | 38% | 0.173 | 38% | 38% |
| Weight change during follow-up (kg) | 0.32 (6.65) | 0.91 (5.11) | 0.06 | 0.87 (5.37) | 0.96 (4.84) |
| TNM stage | 0.041 | ||||
| I | 30% | 38% | 38% | 39% | |
| II | 57% | 50% | 51% | 50% | |
| III/IV | 8% | 6% | 6% | 7% | |
| Unknown | 5% | 5% | 5% | 5% | |
| ER | 0.978 | ||||
| Positive | 66% | 66% | 67% | 64% | |
| Negative | 33% | 33% | 32% | 35% | |
| Unknown | 1% | 1% | 1% | 1% | |
| PR | 0.512 | ||||
| Positive | 62% | 59% | 60% | 58% | |
| Negative | 37% | 40% | 38% | 41% | |
| Unknown | 1% | 1% | 2% | 1% | |
| Menopause ≤ 49.5 years | 53% | 47% | 0.072 | 49% | 46% |
| Physical activity ≥ 7.5 MET-hour/week | 43% | 57% | <0.001 | 54% | 61% |
| Comorbidity | 46% | 46% | 0.848 | 47% | 45% |
| CHFP-2007 score | 32.27 (5.79) | 35.47 (5.12) | <0.001 | 34.68 (5.22) | 36.27 (4.89) |
| Soy food intake in dry weight (g/day) | 17.65 (16.03) | 21.14 (21.70) | 0.007 | 19.20 (15.98) | 23.08 (26.05) |
| Chemotherapy | 91% | 92% | 0.289 | 91% | 94% |
| Radiotherapy | 27% | 31% | 0.188 | 28% | 33% |
| Immunotherapy | 9% | 16% | 0.004 | 14% | 17% |
| Tamoxifen use | 55% | 53% | 0.482 | 54% | 53% |
Values are means (SD) for continuous variables; percentages for categorical variables
Values of polytomous variables may not sum to 100% due to rounding
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; MET, metabolic equivalent of task; CHFP-2007, Chinese Food Pagoda 2007
Median of total nut intake=17.32 g/week
Median of total energy intake=1429.75 kcal/day.
During a median follow-up of 8.27 years post dietary assessment, there were 374 deaths from any cause, including 252 deaths from breast cancer. At ten-years post diagnosis, nut consumers had higher OS (93.7% vs 89.0%, P=0.003) and DFS (94.1% vs 86.2%, P<0.001) rates compared with non-consumers. Similar survival differences were observed for consumption of peanuts, walnuts, and other nuts (Table 2).
Table 2.
Overall and disease-free survival rate among long-term breast cancer survivors in Shanghai Breast Cancer Survival Study by nut consumption
| Overall (N=3449) |
Disease-freea (N=3274) |
|||||
|---|---|---|---|---|---|---|
| N/total deaths | 5-year survival rateb (%) | P-value | N/eventsc | 5-year survival rateb (%) | P-value | |
|
| ||||||
| None (reference) | 301/52 | 89.0 | 285/38 | 86.2 | ||
| Any nuts | 3148/322 | 93.7 | <0.001 | 2989/171 | 94.1 | <0.001 |
| Peanuts | 2501/270 | 93.9 | 0.006 | 2365/134 | 94.2 | 0.004 |
| Walnuts | 2476/221 | 94.9 | <0.001 | 2361/120 | 94.8 | <0.001 |
| Other nuts | 2295/200 | 94.6 | <0.001 | 2178/118 | 94.2 | 0.002 |
175 subjects whose events occurred before the dietary assessment were excluded for disease-free analyses
The entry time was age at the dietary survey; i.e., approximate 5-year post breast cancer diagnosis; the 5-year survival rate is approximate to the 10-year pot-diagnosis survival rate among long-term breast cancer survivors.
Events include breast cancer recurrence, metastasis, and death.
After adjustment for age at diagnosis, total energy intake, income, education, disease stage, ER status, PR status, menopause age, physical activity, CHFP-2007 score, soy food intake, BMI, and weight change, ever nut consumption was associated with significantly better DFS (HR=0.52, 95% CI: 0.35, 0.75), but a non-significantly improved OS (HR=0.90, 95% CI: 0.66, 1.23) (Table 3). Analyses by amount of nut intake showed a dose-response relationship for both OS (Ptrend=0.022) and DFS (Ptrend=0.003). Compared with non-consumers, those with nut consumption amounts greater than the median (17.32 g/week) and equal to or less than the median had a respective 52% (HR=0.48, 95% CI: 0.31, 0.73) and 45% (HR=0.55 95% CI: 0.37, 0.81) lower risk of cancer recurrence, metastasis, or breast cancer-specific death, with a linear dose-response relationship. Point estimates for OS, however, did not reach statistical significance. Similar association patterns were observed for consumption of peanuts, walnuts, and other nuts. The sensitivity analysis using age at diagnosis as the entry time showed very similar results (Table 3).
Table 3.
Associations of overall and disease-free survival with nut consumption among long-term breast cancer survivors
| Overall |
Disease-freea |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of participants | No. of events | HR (95% CI)b | HR (95% CI)c | No. of participants | No. of events | HR (95% CI)b | HR (95% CI)c | |
|
| ||||||||
| Nuts | ||||||||
| No | 301 | 52 | 1.00 (ref) | 1.00 (ref) | 285 | 38 | 1.00 (ref) | 1.00 (ref) |
| Yes | 3148 | 322 | 0.90 (0.66, 1.23) | 0.90 (0.66, 1.23) | 2989 | 171 | 0.52 (0.35, 0.75) | 0.52 (0.36, 0.76) |
| ≤ Mediand | 1573 | 193 | 1.00 (0.73, 1.38) | 1.00 (0.73, 1.38) | 1486 | 91 | 0.55 (0.37, 0.81) | 0.55 (0.37, 0.82) |
| > Median | 1575 | 129 | 0.74 (0.52, 1.05) | 0.74 (0.53, 1.06) | 1503 | 80 | 0.48 (0.31, 0.73) | 0.49 (0.32, 0.74) |
| P trend e | 0.022 | 0.022 | 0.003 | 0.004 | ||||
| Peanuts | 2501 | 250 | 0.85 (0.63, 1.20) | 0.87 (0.63, 1.20) | 2365 | 134 | 0.50 (0.34, 0.74) | 0.51 (0.35, 0.75) |
| Walnuts | 2476 | 221 | 0.82 (0.59, 1.13) | 0.82 (0.59, 1.14) | 2361 | 120 | 0.46 (0.31, 0.69) | 0.47 (0.32, 0.70) |
| Other nuts | 2295 | 200 | 0.82 (0.59, 1.14) | 0.82 (0.59, 1.14) | 2178 | 118 | 0.51 (0.34, 0.76) | 0.52 (0.35, 0.77) |
CI, confidence interval
Models were adjusted for age at diagnosis, total energy intake, income, education, TNM stage, estrogen receptor status, progesterone receptor status, menopause age, physical activity, Chinese Food Pagoda 2007 score, soy food intake, body mass index, and weight change during first 5-year follow-up
175 subjects whose events occurred before the dietary assessment were excluded for disease-free analyses; events include breast cancer recurrence, metastasis, and death
The entry time was age at dietary assessment
The entry time was age at breast cancer diagnosis
Median of total nut intake=17.32 g/week
Ptrend was estimated by treating categories of total nut consumption as ordered values (no-0, ≤median-1, and >median-2).
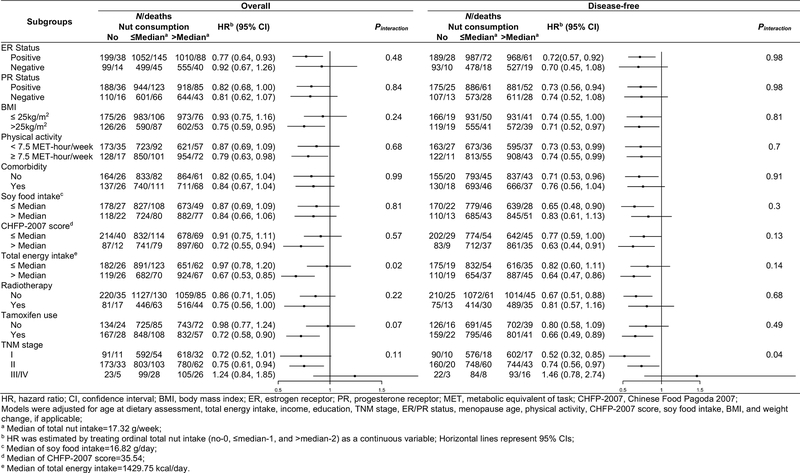
Stratified analyses showed that higher nut consumption was associated with significantly better OS among several subgroups of breast cancer patients, including those who had ER-positive or PR-positive breast cancer, higher BMI, higher physical activity, higher CHFP-2007 score, higher total energy intake, radiotherapy, tamoxifen use, and those diagnosed with stage I-II breast cancers, although the test for multiplicative interaction was only significant for energy intake (Figure 1). Higher nut consumption was associated with better OS (HR=0.67, 95% CI: 0.53, 0.85) among participants who had total energy intakes greater than the median (1429.75 kcal/day). However, no significant association with OS was found among those with lower total energy intakes (HR=0.97, 95% CI: 0.78, 1.20, Pinteraction=0.02). The association between total nut consumption and DFS was consistently observed across almost all the subgroups of breast cancer patients. An effect modification by TNM stage was observed for DFS. The nut-DFS association was only significant among participants who were diagnosed at stage I (HR=0.52, 95% CI: 0.32, 0.85) and stage II (HR=0.74, 95% CI: 0.56, 0.97, Pinteraction=0.04).
Figure 1.
Overall and disease-free survival associated with nut consumption by selected prognostic factors
Discussion
In this population-based study, we found that nut consumption was associated with improved DFS and OS among long-term breast cancer survivors, following a dose-response pattern. Such associations were observed for all types of nuts under study. While the nut-OS association was only observed in some subgroups of patients and modified by total energy intake, the nut-DFS association was consistently seen across subgroups of patients and was modified by TNM stage.
The health benefits of nut consumption have been well-documented, though no previous research investigated the association among long-term breast cancer survivors. In several large cohort studies of general populations, nut consumption has been associated with 8%−47% lower total mortality, with a dose-response relationship.15–20 In our study, we observed a trend of lower total mortality associated with higher nut consumption overall and among several subgroups of patients, although point estimates for overall analysis failed to reach statistical significance. The latter may be attributable to a much smaller sample size, and thus, lower statistical power that our study has compared with that of previous cohort studies.15–20 The levels of nut consumption were relatively low in our study population (median=17.32 g/week) compared with the 42.5 g/week recommended by the American Heart Association.29 Therefore, it is also possible that the levels of nut consumption among breast cancer survivors in our study did not reach a threshold of health benefits for OS.
According to the breast cancer survivors report from 2018, Diet, Nutrition, Physical Activity and Breast Cancer Survivors,30 there has been no strong evidence to support individual food items in favor of breast cancer survival. Our study is among the first to show that nut consumption, overall or by subtype, was associated with up to ~50% reduced risk of breast cancer recurrence, metastasis, or mortality. This association persisted after adjustment for the CHFP-2007 score, a surrogate of overall dietary quality, indicating an independent beneficial effect of nut consumption on DFS. Nuts are rich in several pro-health nutrients, including unsaturated fatty acids, protein, fiber, vitamins, minerals, and other bioactive constituents such as phytosterols and phenolic compounds.31 Previous in vitro studies have identified several potential molecular mechanisms indicative of growth inhibitory effects of nut components (e.g., ellagic acid,32 alpha-linolenic acid,33 and β-sitosterol33,34) on human breast cancer MCF-7 cells, which may prevent breast cancer recurrence. Other impacts of nut consumption on some intermediate markers, such as lowering cholesterol levels,35 inhibiting oxidation,36–38 and regulating endothelial dysfunction,39 may also contribute to better DFS and OS among breast cancer survivors. It is worth mentioning that we found an interaction between nut and energy intake on OS, and between nut intake and breast cancer stage on DFS. One explanation is that patients with higher energy intake or those with early stage of breast cancer may be, in general, healthier than their counterparts. Indeed, in our study, breast cancer survivors with higher energy intake were younger and had higher education, higher income, higher BMI, higher physical activity, higher CHFP-2007 score, and lower comorbidity (Supplemental Table 1) than their counterparts. Thus, they are less likely to be affected by reserve causation. However, chance findings cannot be ruled out. More studies are needed to confirm our findings and to investigate the biological mechanism(s) underlying these interactions.
One of the strengths of this study is the inclusion of a large number of long-term breast cancer survivors. Prior studies have suggested that breast cancer patients might modify their diets in both the short-term and long-term.40,41 The validated semiquantitative FFQ,27 designed to capture long-term dietary habits, was implemented at the five-year follow-up, when survivors had completed cancer treatments and established a new life normalcy. This minimized the influences of disease- and treatment-related dietary changes. The comprehensive information gathered from the FFQ, baseline and follow-up surveys enabled us to investigate the associations of major types of nuts and perform an in-depth evaluation of effect modifications and adjustments for confounders of cancer stage, characteristics, treatments, and a wide range of post-diagnosis lifestyle factors.
There are also some limitations to this study. First, the recurrence and metastasis statuses were self-reported. Misclassification, particularly regarding the event date, is likely. Second, despite the large number of long-term breast cancer survivors included in the study, the number of events was relatively small for subgroups of patients, resulting in a limited statistical power for stratified analyses. Third, nut consumption information was collected only once, at the five-year follow-up survey. Information on change of nut consumption was not captured. Bias due to reverse causality is possible because individuals with chronic diseases and poor health statuses may change their dietary habits. However, we excluded from DFS analyses all women who had a recurrence at dietary assessment. Our stratified analysis showed no evidence of differential associations between individuals with and without comorbidities. Furthermore, our findings of a general non-significant association with OS, but a significant association with DFS, also argue against reverse causation as the sole explanation. Further studies that track dietary changes during the course of survivorship, or randomized clinical trials, are needed to draw a firm conclusion.
In summary, in this large cohort study including 3,449 long-term breast cancer survivors, we found that nut consumption was associated with better survival. Nuts are important components of healthy diets. Promoting this modifiable lifestyle factor should be emphasized in breast cancer survivor guidelines.
Supplementary Material
Novelty and Impact:
The association of nut consumption with recurrence and mortality among long-term breast cancer survivors is unknown. Our cohort study found that nut consumption among long-term breast cancer survivors was associated with a 52% reduced risk of recurrence or breast cancer mortality following a dose-response pattern. The association was stronger for survivors with stage I-II breast cancer than those with stage III-IV cancer. This study provides evidence for promoting nut consumption among breast cancer survivors.
Acknowledgments
We would like to thank the participants and research staff members of the Shanghai Breast Cancer Survival Study, without whom this study would not have been possible. We also thank Dr. Mary Shannon Byers for her help in editing this manuscript.
Funding
This work was supported by the U.S. Department of Defense Breast Cancer Research Program [grant number DAMD 17-02-1-0607 to X.O.S.]; National Cancer Institute at the National Institutes of Health [grant number R01 CA118229 to X.O.S.]; and National Natural Science Foundation of China [grant number 81402734 to P.B.].
Abbreviations:
- BMI
body mass index
- CHFP
Chinese Food Pagoda
- CI
confidence interval
- DFS
disease-free survival
- ER
estrogen receptor
- FFQ
food frequency questionnaire
- HR
hazard ratio
- ICD
International Classification of Disease
- MET
metabolic equivalent of task
- OS
overall survival
- PR
progesterone receptor
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest.
Ethics Statement
This study was approved by the institutional review board and ethic committee of Vanderbilt University and Shanghai Municipal Center for Disease Control and Prevention. The study was carried out in accordance with the relevant guidelines and regulations. Written consents were obtained from all study participants.
Data Availability Statement
Data are available from the corresponding author on request.
References
- 1.Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Communications. 2019;39(1):22. doi: 10.1186/s40880-019-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Sauer AG, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69(6):438–51. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JDF, Feuer EJ. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. New England Journal of Medicine. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 5.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, Berry DA, Burnside ES, Chang Y, Chisholm G, de Koning HJ, Ali Ergun M, Heijnsdijk EAM, Huang H, Stout NK, Sprague BL, Trentham-Dietz A, Mandelblatt JS, Plevritis SK. Effects of Screening and Systemic Adjuvant Therapy on ER-Specific US Breast Cancer Mortality. J Natl Cancer Inst. 2014;106(11). doi: 10.1093/jnci/dju289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. The Lancet Global Health. 2018;6(5):e555–67. doi: 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 8.Takeshita T, Yan L, Asaoka M, Rashid O, Takabe K. Late recurrence of breast cancer is associated with pro-cancerous immune microenvironment in the primary tumor. Sci Rep. 2019;9. doi: 10.1038/s41598-019-53482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. New England Journal of Medicine. 2017;377(19):1836–46. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, Rosner B, Willett WC. Association of Dietary Intake of Fat and Fatty Acids With Risk of Breast Cancer. JAMA. 1999;281(10):914–20. doi: 10.1001/jama.281.10.914 [DOI] [PubMed] [Google Scholar]
- 11.Hirayama T Epidemiology of breast cancer with special reference to the role of diet. Preventive Medicine. 1978;7(2):173–95. doi: 10.1016/0091-7435(78)90244-X [DOI] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Trudeau ME, Hood N. Diet and Breast Cancer: Evidence That Extremes in Diet Are Associated With Poor Survival. JCO. 2003;21(13):2500–7. doi: 10.1200/JCO.2003.06.121 [DOI] [PubMed] [Google Scholar]
- 13.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary Patterns and Breast Cancer Recurrence and Survival Among Women With Early-Stage Breast Cancer. J Clin Oncol. 2009;27(6):919–26. doi: 10.1200/JCO.2008.19.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Cai H, Gu K, Shi L, Yu D, Zhang M, Zheng W, Zheng Y, Bao P, Shu X-O. Adherence to Dietary Recommendations among Long-Term Breast Cancer Survivors and Cancer Outcome Associations. Cancer Epidemiol Biomarkers Prev. 2020;29(2):386–95. doi: 10.1158/1055-9965.EPI-19-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luu HN, Blot WJ, Xiang Y-B, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao Y-T, Zheng W, Shu X-O. Prospective Evaluation of the Association of Nut/Peanut Consumption With Total and Cause-Specific Mortality. JAMA Intern Med. 2015;175(5):755–66. doi: 10.1001/jamainternmed.2014.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaccio M, Castelnuovo AD, Curtis AD, Costanzo S, Bracone F, Persichillo M, Donati MB, de Gaetano G, Iacoviello L. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli-sani study. British Journal of Nutrition. 2015;114(5):804–11. doi: 10.1017/S0007114515002378 [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of Nut Consumption with Total and Cause-Specific Mortality. New England Journal of Medicine. 2013;369(21):2001–11. doi: 10.1056/NEJMoa1307352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hshieh TT, Petrone AB, Gaziano JM, Djoussé L. Nut consumption and risk of mortality in the Physicians’ Health Study. Am J Clin Nutr. 2015;101(2):407–12. doi: 10.3945/ajcn.114.099846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guasch-Ferré M, Bulló M, Martínez-González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M, Lapetra J, Vinyoles E, Lamuela-Raventós RM, Serra-Majem L, Pintó X, Ruiz-Gutiérrez V, Basora J, Salas-Salvadó J. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Medicine. 2013;11(1):164. doi: 10.1186/1741-7015-11-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: a cohort study and meta-analysis. Int J Epidemiol. 2015;44(3):1038–49. doi: 10.1093/ije/dyv039 [DOI] [PubMed] [Google Scholar]
- 21.van den Brandt PA, Nieuwenhuis L. Tree nut, peanut, and peanut butter intake and risk of postmenopausal breast cancer: The Netherlands Cohort Study. Cancer Causes Control. 2018;29(1):63–75. doi: 10.1007/s10552-017-0979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348. doi: 10.1136/bmj.g3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Colditz GA, Cotterchio M, Boucher BA, Kreiger N. Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res Treat. 2014;145(2):461–70. doi: 10.1007/s10549-014-2953-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, Wirfält E. Plant foods and oestrogen receptor α- and β-defined breast cancer: observations from the Malmö Diet and Cancer cohort. Carcinogenesis. 2008;29(11):2203–9. doi: 10.1093/carcin/bgn196 [DOI] [PubMed] [Google Scholar]
- 25.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–43. doi: 10.1001/jama.2009.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27.Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao Y-T, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738 [DOI] [PubMed] [Google Scholar]
- 28.CHANG YYXWGYPX. China Food Composition Table. Beijing Medical University Press; 2000. [Google Scholar]
- 29.Go Nuts (But just a little!) www.heart.org. Accessed October 14, 2020. https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/go-nuts-but-just-a-little
- 30.Diet, Nutrition, Physical Activity and Breast Cancer Survivors. World Cancer Research Fund/Amercan Institute for Cancer Research; 2018. Accessed November 15, 2020. https://www.wcrf.org/sites/default/files/Breast-cancer-survivors-report.pdf [Google Scholar]
- 31.Ros E Health Benefits of Nut Consumption. Nutrients. 2010;2(7):652–82. doi: 10.3390/nu2070652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H-S, Bai M-H, Zhang T, Li G-D, Liu M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. International Journal of Oncology. 2015;46(4):1730–8. doi: 10.3892/ijo.2015.2870 [DOI] [PubMed] [Google Scholar]
- 33.Heuvel JPV, Belda BJ, Hannon DB, Kris-Etherton PM, Grieger JA, Zhang J, Thompson JT. Mechanistic Examination of Walnuts in Prevention of Breast Cancer. Nutrition and Cancer. 2012;64(7):1078–86. doi: 10.1080/01635581.2012.717679 [DOI] [PubMed] [Google Scholar]
- 34.Awad AB, Chan KC, Downie AC, Fink CS. Peanuts as a source of beta-sitosterol, a sterol with anticancer properties. Nutr Cancer. 2000;36(2):238–41. doi: 10.1207/S15327914NC3602_14 [DOI] [PubMed] [Google Scholar]
- 35.Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102(6):1347–56. doi: 10.3945/ajcn.115.110965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins DJA, Kendall CWC, Josse AR, Salvatore S, Brighenti F, Augustin LSA, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136(12):2987–92. [DOI] [PubMed] [Google Scholar]
- 37.Torabian S, Haddad E, Rajaram S, Banta J, Sabate J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet. 2009;22(1):64–71. doi: 10.1111/j.1365-277X.2008.00923.x [DOI] [PubMed] [Google Scholar]
- 38.Stockler-Pinto MB, Mafra D, Moraes C, Lobo J, Boaventura GT, Farage NE, Silva WS, Cozzolino SF, Malm O. Brazil Nut (Bertholletia excelsa, H.B.K.) Improves Oxidative Stress and Inflammation Biomarkers in Hemodialysis Patients. Biol Trace Elem Res. 2014;158(1):105–12. doi: 10.1007/s12011-014-9904-z [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of Walnut Consumption on Endothelial Function in Type 2 Diabetic Subjects - A randomized controlled crossover trial. Diabetes Care. 2010;33(2):227–32. doi: 10.2337/dc09-1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabin C, Pinto B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-Oncology. 2006;15(8):701–12. doi: 10.1002/pon.1000 [DOI] [PubMed] [Google Scholar]
- 41.Alfano CM, Day JM, Katz ML, Herndon JE, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psycho-Oncology. 2009;18(2):128–33. doi: 10.1002/pon.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on request.