Abstract
Introduction
While SARS-CoV-2's main transmission route is through respiratory droplets, research has found that viral RNA could be detected in blood samples, causing concerns over the safety of blood donations and blood products. This paper therefore aims to systematically search for studies that have addressed their country's lack of donations and analyse the risk of blood transfusion-transmission. As such, it will answer the question “should blood services focus more on donation vigilance or worry more about the risks of transmission through blood products?”
Methods
38 articles were identified through a systematic review adopting the PRISMA and STROBE guidelines. Meta-analysis was conducted using OpenMeta software.
Results
The average decrease in blood donations was found to be 38%, with some regions showing up to 67% decrease. To assess the risk of actual blood transfusion-transmission, three datasets were analysed. Firstly, the viral load in COVID-19 patients was studied and found to have less than 1% detection rate (ARD = −0.831, 95% −0.963, −0.699). Secondly, the prevalence of finding viral RNA in a pool of donations was nearly −1.503 (ARD = −1.538, −1.468). Lastly, recipients who were given blood products of positive donors were found to be −0.911 (ARD 95% = −1.247, −0.575).
Discussion/Conclusion
Blood centres should focus more on launching initiatives and policies that would increase their countries' blood supply as the virus has no direct threat to blood safety.
Keywords: COVID-19, Blood transfusion, Donations, RNAaemia
Introduction
Blood transfusion is considered an integral part of medicine that treats thousands of patients every year, making blood products' management, safety, and storage fundamental in every country's national healthcare policy [1, 2]. Maintaining an adequate supply of blood products is no easy feat considering blood components have a short shelf life ranging from 5 to 42 days, and the fact that blood collection relies entirely on generous donations from the public [3]. However, not everyone can or is willing to donate; hence, constant encouragement from blood centres is therefore a necessary prosocial behaviour so that blood can be collected from all ethnicities and blood types. Due to its reliance on donations, the supplies can easily be diminished when disaster strikes especially in the cases of pandemics [4, 5].
Since late 2019, the world was plagued by a new disease that originated from Wuhan, China, infecting over 28 million people and causing at least 900,000 deaths across 188 countries [4]. Healthcares were suddenly hit with critical shortages of PPEs, ventilators, and hospital beds, causing governments around the world to act in an unprecedented manner to mitigate the exponential rise of infected cases [6, 7, 8]. Social distancing was implemented, as well as closing down public venues, schools, universities, and any non-essential work [7, 8]. This lockdown and the fear of virus transmission has not only affected the public's health and a decline in economy, it also caused a significant drop in the number of blood donations across the world, creating shortages at various blood banks and diminishing nationwide blood supply.
The Rise of SARS-COV-2
Scientists identified the virus and found that it was 88% homologous to a bat SARS-like CoV genome and named it Coronavirus Disease 2019 (COVID-19) − later renamed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by WHO [9, 10]. They found that this new virus is distinctly different yet fairly similar to the two previous coronavirus infections, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), that occurred in 2002 and 2012, respectively [9]. All three diseases are caused by a genus of zoonotic β-coronavirus which consists of positive single-stranded RNA viruses that can infect the human lower respiratory tracts and induce mild to severe complications [9, 10, 11]. So far, the main transmission of the virus was through direct contact between people through respiratory droplets produced when the infected sneezes or coughs and or indirect contact from contaminated surfaces [12, 13].
The clinical presentation of SARS-CoV-2 can range from asymptomatic to life-threatening and death. Analyses show that roughly 80% of confirmed cases exhibit none to mild symptoms that included fever, dry cough, fatigue, loss of smell, myalgia, dyspnea, and other flu-like symptoms that appear within 14 days of exposure [14, 15]. The other 20% experienced more severe complications that ranged from pneumonia, respiratory failure, pulmonary edema, and acute respiratory distress syndrome (ARDS) that could place the patient in the ICU with mechanical ventilations [11, 13, 15]. However, studies have shown evidence the haematopoietic system is also affected [11, 16]. They found that one of the main pathogenic mechanisms of the virus is inducing a cytokine storm that leads to inflammatory reactions and thus causing organ failures, cardiomyopathy, and thromboembolisms [15, 17]. Early case reports have described coagulopathies in severely affected patients, including an increase in procoagulant factors, D-dimers, and prolonged PT times which contributed to a high mortality rate [11, 13].
In terms of pathogenesis, SARS-CoV-2 genome encodes spike proteins that recognise and bind to a host cell's angiotensin converting enzyme 2 (ACE2) receptor, widely expressed in tissue cells such as in heart, liver, testes, brain, kidney, placenta, and endothelial cells [14]. Hence even though the CoV family are mainly respiratory diseases, scientists believe that because ACE2 is found everywhere, transmission is not limited to the respiratory system. In fact, reports have found viral RNA in other human body fluids such as the urine, tears, and blood [11, 18].
The Impact of SARS-CoV-2 on Blood Collection
On top of lockdown, social distancing, and fear of blood virus transmission, more challenges surfaced for the blood transfusion community. The older population, who contributes the most to donations, are amongst the most vulnerable to the virus, which led to decreased donations on their part [19]. Furthermore, asymptomatic cases also soared, making it more difficult to detect and thus indirectly posed a risk in blood collection safety [20, 21]. In response to the decreased donations, hospitals were forced to preserve their supplies by cancelling non-urgent surgeries and organ transplants or reducing transfusion volumes just to avoid blood shortages [22, 23]. Though reducing the demand for blood worked for a while, an increase in blood component wastage was also observed due to the decrease of demand coinciding with the blood products' short life span [23]. The idea of transporting blood products across various blood collection centres was also not feasible. During the first SARS epidemic, China had tried to do so but faced temperature and packaging problems that led to increases in errors, costs, and wastes [24, 25].
Emergency plans have always been in place in all transfusion services in the past years, but due to the uncertainty, lack of vaccine, and increasing infection rates, blood shortages became a primary concern across the world [4, 14]. WHO noted this decrease and released guidelines including pre-donation screening procedures to help promote staff and donors' safety [26]. These measures included basic proper hygiene, use of masks, increased spaces between donors, and forbidding anyone who had recently travelled to donate. It also included constantly deep cleaning all donation stations and any surfaces at risk of potential contamination. Studies also emerged from various countries that took actions to increase donations. By adapting and developing their own strategies to adapt to the current situation, an increase in blood products and donations was observed in those regions [17].
Study Aim
Even without pandemics, blood collection in itself is an ongoing challenge. When the COVID-19 hit this year and without signs of it slowing down, it is imperative for blood services all over the world to come up with strategies and policies that will maintain their country's blood supply. In Australia itself, Lifeblood Chief Shelly Park has issued statements of an urgent need for more donations as more than 900 donor cancellations occur almost every week around the country [27]. This paper therefore aims to systematically search for studies that have addressed their country's lack of donations and to also analyse the risk of blood transfusion-transmission. As such, it will answer the question “should blood services focus more on donation vigilance or worry about the risks of transmission through blood products?”
Methods and Materials
Study Design
A systematic review and meta-analysis were performed using the guidelines and procedures outlined the PRISMA to identify the articles needed to evaluate the impact of COVID-19 to blood transfusion services. Furthermore, qualitative analysis of each paper was assessed through the STROBE (strengthening the reporting of observational studies in epidemiology) checklist.
Search Strategy
Three databases were thoroughly searched: PubMed/Medline, SCOPUS, and ProQuest for eligible articles. Considering that this paper focused only on COVID-19, the time frame was limited to 2020. Language was selected for only English-based papers, though there were several papers that were translated directly from Chinese. Keywords included “COVID-19” searched alongside “impact on blood centres,” “blood transfusion,” “blood donors,” “blood donations,” “blood products,” “viremia,” “blood biochemical characteristics.” Additional papers were obtained from the references mentioned in various papers.
Inclusion and Exclusion Criteria
For data pertaining to COVID-19's impact on blood centres, articles included were mostly retrospective studies from countries that conducted an actual research on how their respective blood services were faring. These research articles took physical numbers of donations made before the pandemic started and compared it to donations during the peak of their pandemic period. Articles were excluded if only the percentage decrease or reports of critical levels were noted in their paper.
For data related to evaluating the amount of viral RNA found in blood, articles were obtained from research conducting various biochemical properties of the virus. These articles contained any research that was done to obtain qRT-PCR values from blood, serum, or plasma of patients who were admitted to the hospital, along with any articles that screened for RNAaemia in pools of blood donors. Excluded were letters, editorials, and literature reviews, though their references were double checked and added. It's important to note that the term “viremia” was not used in these articles as it implies COVID-19 can cause infections through blood. Hence the term “RNAaemia” was used instead to indicate presence of the virus in the blood.
Data Extraction
Data was extracted and tabulated into tables containing the primary author, year of publication, and their specific location if possible. Tables also had information on time frame of blood donations, number of blood donations before and during pandemic, number of total cases, and population of region. The other tables contained type and timing of blood collection, number of positive RNAaemia with total number of patients, CT values, detection techniques, RNAaemia in a pool of random blood donors, and number of recipients of positive COVID-19 blood products transfused.
Data Synthesis and Statistical Analysis
Synthesis of data and meta-analysis were conducted using the OpenMeta[Analyst] software, version 12.11.14 from the Brown University website. The data were grouped according to the specific type of outcomes measured in this paper and measured with a maximum likelihood binary effects model. For comparing donation rates between two time periods, a two-arm proportion analysis was implemented. The difference in arcsines transformed proportions was conducted for the rest of the data. Heterogeneity, p values, and 95% confidence intervals were calculated by OpenMeta and plotted into forest plots.
Results
Overall Study Characteristics
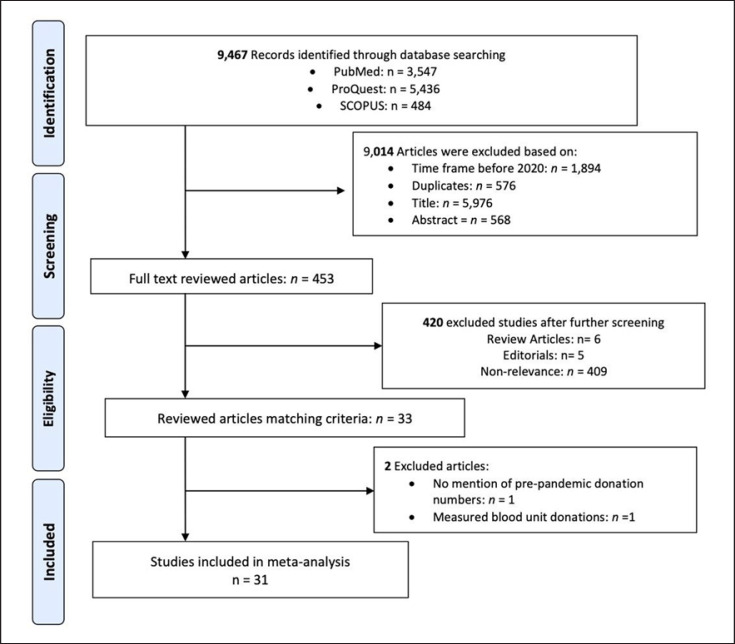
A PRISMA flowchart was made to display how potential articles were pooled and screened (shown in Fig. 1). From the three databases, 9,467 articles were initially obtained. Following removal of 576 duplicates, 1,894 articles that were not from 2020, and 6,633 non-relevant titles and abstracts, 453 full text articles were then analysed. A thorough examination of the 453 articles showed that the majority of them were letters, editorials, and articles that included hematological disorders as part of COVID-19's impact on all blood-related things. After these removals, only 34 papers were left. Another thorough screening revealed that only 32 studies were included in meta-analysis as 2 studies did not contain suitable metadata.
Fig. 1.
Flowchart showing screening methods of potential articles using the PRISMA guidelines.
The majority of these articles came from China; hence, the combined data also included which regions and hospitals the papers conducted their study on to make sure no crossover studies were added; however, it cannot be certain if there were no crossovers of patients.
STROBE Analysis
To analyse each paper qualitatively, the STROBE guidelines were adapted. For articles relating to donation trends, hospitals or blood centres where research was conducted needed to be included, along with period of collection date. Articles relating to RNAaemia also had to provide location, time, and type of blood samples. Only three articles did not meet this criterion. One was the article by Mohammadi et al. [28] in which only the mean donation rate was recorded during the pandemic, not before. The second was that by Gniadek et al. [29], where whole blood units were recorded rather than donation numbers. The other was by Yu et al. [30] as there were no specifications of where the positive RNAaemia samples were collected from. Yu et al. [30] had reported 323 different biochemical samples collected from 76 patients, but whether these samples were from the same patient or different patients is unknown.
Study Characteristics of Donation Trends
Of the 33 articles reviewed, 8 related to the number of blood donations received before and during pandemic period (Table 1). Of those 8 articles, only 5 were included in the meta-analysis (Table 2). Those 3 articles were highly relevant to the topic in question but were unfortunately excluded as one reported whole blood unit collections, while the others did not provide numbers of donation before the pre-pandemic period. All donation numbers refer to any blood products received. The number of active COVID-19 positive cases during the time of research were also manually searched and added to provide a comparison of donation decrease to the infected population.
Table 1.
Characteristics of the 33 articles included in the systematic review, following the STROBE guidelines
| First author [Ref.] | Detailed description of settings and methods of collection | Provided specific criteria for participants/ donations | Clearly defined all variables and bias | Clear description of statistical analysis | Provided clear results and conclusion |
|---|---|---|---|---|---|
| Grandone [31] | Y | Y | N | N | Y |
| Gupta [33] | Y | Y | Y | Y | Y |
| Ou-Yang [25] | Y | Y | Y | Y | Y |
| Raturi [26] | Y | Y | Y | y | Y |
| Wang [32] | Y | Y | Y | Y | Y |
| Yahia [34] | Y | Y | Y | Y | Y |
| Mohammadi [28]* | N | N | N | Y | N |
| Gniadek [29]* | Y | Y | N | Y | Y |
| Chen [49] | Y | Y | Y | Y | Y |
| Chan [46] | Y | Y | Y | Y | Y |
| Lescure [50] | Y | Y | Y | Y | Y |
| Wang [51] | Y | Y | Y | Y | Y |
| Chan [52] | Y | Y | Y | Y | Y |
| Zhou [47] | Y | Y | Y | Y | Y |
| Huang [5] | Y | Y | Y | Y | Y |
| Kim [53] | Y | Y | Y | Y | Y |
| Peng [54] | Y | Y | Y | Y | Y |
| Zhang [55] | Y | Y | Y | Y | Y |
| Yu [30] | N | Y | Y | Y | Y |
| Huang [56] | Y | Y | Y | Y | Y |
| Chen [57] | Y | Y | Y | Y | Y |
| Wu [58] | Y | Y | Y | Y | Y |
| Zheng [35] | Y | Y | Y | Y | Y |
| Chang [17] | Y | Y | Y | Y | Y |
| Chang [59] | Y | Y | Y | Y | Y |
| Xu [38] | Y | Y | Y | Y | Y |
| Cappy [36] | Y | Y | Y | Y | Y |
| Corman [60] | Y | Y | Y | Y | Y |
| Kwon [40] | Y | Y | Y | Y | Y |
| Percivalle [37] | Y | Y | Y | Y | Y |
| Waheed [39] | Y | N | Y | Y | Y |
| Anurathapan [42] | Y | N | Y | N | Y |
| Lázaro [41] | Y | N | Y | N | Y |
Y, yes, the article met the criteria; N, article was missing part or all of the criteria.
Articles that were not included in meta-analysis due to incompatible metadata.
Table 2.
Donation numbers before and during the pandemic period of different countries
| First author [Ref.] | Location | Time frame (2020) | Before pandemic | During pandemic | Number of total cases | Population of region |
|---|---|---|---|---|---|---|
| Grandone [31] | Apulia, Italy | March ‘19/March ‘20 | 18,100 | 16,200 | 2,077 (Apulia) | 4,008,296 |
|
| ||||||
| Wang [32]a | Zhejiang, China | Feb ‘19/Feb ‘20 | 15,609 | 5,253 | 1,205 (Feb 26) | 1,935,000 |
|
| ||||||
| Ou-Yang [25]b | Guangdong province | Jan to Mar ‘19/Jan to Mar ‘20 | 56,100 (26,400 same time frame before measures) | 48,800 (17,800 before measures) | 1,501 (March 31, Guangdong province) | 13,302,000 (Guangdong) |
|
| ||||||
| Raturi [26]c | Dehradun, Uttarkhand, India | Jan–early Feb Feb-March | 1,343 | 600 | 7/1,559 (Uttarkhand/ India) | 11,701,989 (Uttarkhand) |
|
| ||||||
| Gupta [33]d | India (1 unknown centre) | March ‘20 April ‘20 | 877 | 613 | 724 (March 27) | 30,291,000 (Delhi) |
|
| ||||||
| Yahia [34] | Bisha, Saudi Arabia | Sept-Dec.'19/ Jan-May ‘20 | 1,360 | 1,009 | 22,752 (May 1) | 34,813,871 |
Numbers obtained from 38 blood centres.
Actions were made after January 30 2020, thereby increasing donations.
Research separated their data between two phases. First phase was between October 2019 to middle half of February 2020. The second phase was between late half of February to June. Only the numbers added in this table were from January to first half of February 2020, and first half of February to March.
Lockdown started after March 25.
As for the 5 articles, results are based on reports of each blood bank service of their respective regions, not reflecting the entire country's donations. All the articles were retrospective studies with no controls as it was hard to define one for what blood donation numbers should be. Each article had a different way of comparing their pre- and during-pandemic periods as countries were hit by the virus in varying months. In the case of three articles, Grandone et al. [31], Wang et al. [32], and Ou-Yang et al. [25], their comparison focused on their donation numbers between the same months in 2020 and in 2019. As for the others, Raturi and Kusum [22], Gupta et al. [33], and Yahia [34], manual counts were done to calculate the relevant data needed for the meta-analysis as they each reported their results differently. All three articles measured their donations within spans of months before 2020 and during 2020. Only Raturi and Kusum's [22] study was reported.
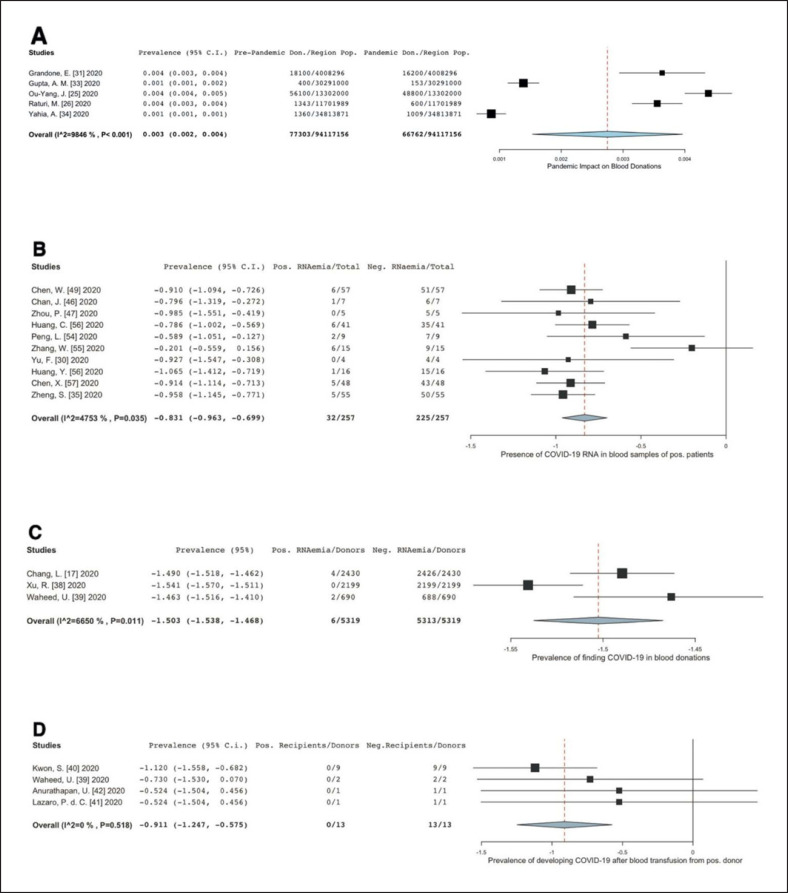
The donation trend was analysed in a forest plot (shown in Fig. 2A). The largest presented decreased rate was from the report by Wang et al. [32], whereby the region of Zhejiang, China, experienced a 66% decrease in donation when compared to the numbers in February 2019. It is important to note that the high number of donations in comparison to the rest of the data came from 38 blood centres scattered across different regions in Zhejiang. Meanwhile, the 400 donations made in the research from India by Gupta et al. [33]were obtained from one donation centre in a span of 2 months of comparison, nevertheless still showing a 62% decrease.
Fig. 2.
Forest plot analyses on the four data sets to show impact of COVID-19 on donation trend and its prevalence of blood. transfusion-transmission. A Impact on donation trends in various countries. The numbers depicted in the graph are obtained by subtracting the donations during pandemic from pre-pandemic period numbers per person as a fraction of the underlying regional population to demonstrate the number of decreased donations. Several articles actually recorded the numbers via trend lines; hence data extraction was manually done by estimating the numbers. Efforts were made to be as accurate as possible. B Prevalence of the presence of COVID-19 viral RNA in the blood samples of positive patients and asymptomatic blood donors. One study, Zhenget al. [35], had two results added into the meta-analysis as they tested blood samples in two different onset of symptoms and added it to their conclusion. C Prevalence of finding RNAaemia in a pool of random donations. Line of null effect is not shown by the data. D Prevalence of COVID-19 blood transfusion-transmission.
Furthermore, the 13% decrease from research by Ou-Yang et al. [25] in Guangdong province does not represent the entire percentage decrease. This is due to the fact that the number 48,800 of donations is the total between January to March 2020, during which after the month of February, policies were issued out by the government and blood centres to encourage more blood donations as their January period reached critically low levels of blood supply.
The statistical method used for the forest plot was a two-arm proportion analysis using the donation numbers prior to the pandemic as controls. The arcsine risk difference (ARD) was calculated to be 0.003 (95% 0.002, 0.004), while the heterogeneity (I2) was found to be 98.5%.
Study Characteristics of Viral Load in COVID-19-Positive Patients and Asymptomatic Donors
Of the 22 articles in Table 3, 18 articles were identified that performed viral nucleic acid detection by RT-PCR in the blood samples of patients and a pool of asymptomatic donors. The majority of the reports mentioned that their CT values were above 30, which is considered quite high and nearly hits the limit of detection.
Table 3.
Tabulated data on the prevalence of viral load in case-positive COVID-19 patients, asymptomatic donors, or in a random pool of blood donations [5, 17, 30, 35, 36, 37, 38, 39, 46, 47, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60]
| First author [Ref.] | Location | Type and timing of blood collection | Positive for RNA | CT value | Notes |
|---|---|---|---|---|---|
| Chen [49]* | Guangzhou Eighth People's hospital | Serum; positives collected 6-12 days after onset | 6/57 | 24-39 | 6/6 progressed to severe stage 12/57 who were not blood positive were severe cases |
|
| |||||
| Chan [46] | Hong Kong | Plasma; 4–13 days after onset | 10/47 | NS | Used RdRp-P2 assay; did not state clinical symptoms of patients |
|
| |||||
| Lescure [50] | France: Paris + Bordeaux | Plasma/WB − collected day 7–9, and 12 after onset of symptoms | 1/5 | 37.1 | Patient was old and died after 24 days |
|
| |||||
| Wang [51] | China: Hubei, Shandong, Beijing | Collected throughout the illness | 3/307 | 34.6 | 205 patients, 307 blood specimen samples |
|
| |||||
| Chan [52]* | China: Shenzen | Serum sample; positive sample came 6 days after onset | 1/7 | 40 | Single RNAaemia patient had the most severe disease |
|
| |||||
| Zhou [47]* | China: Wuhan (Jin Yin Tan Hospital) | Blood collected 18–29 days after onset | 0/5 | NS | All patients had severe pneumonia; only 5 serum samples obtained from all 7 patients |
|
| |||||
| Huang [5]* | China: Wuhan (Jin Yin Tan Hospital) | Plasma | 6/41 | 35.1 | All patients had pneumonia. 2/6 were placed in ICU care |
|
| |||||
| Kim [53] | Korea | Serum detected 3–5 days after onset | 6/74 | NA | Measured in copies/µL (127) |
|
| |||||
| Peng [54]* | China: Guangzhou (Sun Yat-sen Hospital) | Blood − 3 days from onset | 2/9 | NA | Measured qRT-PCR, copies/mL (8.04 and 9.1) |
|
| |||||
| Zhang [55]* | China: Wuhan Pulmonary Hospital | Whole blood | 6/15 | 30.3–32.1 | None of the blood positves were oral/anal swabs positive; 2/6 had severe diseases |
|
| |||||
| Yu [30]* | China: Beijing Ditan Hospital | Plasma; unclear timing | 0/4 | Cutoff 38 | Unclear whether from 4 samples of 4 different patients |
|
| |||||
| Huang [56]* | China: Guangzhou Medical University | Blood; 10–12 days after onset | 1/16 | 30.10–37.57 | All 16 patients admitted in ICU |
|
| |||||
| Chen [57]* | China: General Hospital of Central Theatre Command, Wuhan | Serum | 5/48 | 34.58–39.01 | The number of patients tested positive for N gene was 3, and 5 for the ORF1 antibody; all 5 were from critically ill group, 2 of which died; serum samples from moderate or severe group tested negative |
|
| |||||
| Wu [58] | China: Renmin Hospital, Wuhan | Blood; NS timing | 4/132 | NS | Did not specify clinical symptoms of these 4 patients |
|
| |||||
| Zheng [35]** | China: Zhejiang University | Serum: after admission | 39/95 | Cutoff <38 | 6/22 were from mild patients; 33/75 were from severe patients |
|
| |||||
| Serum 4 weeks after admission | 5/55 | All 5 remaining samples that tested positive were from severe patients | |||
|
| |||||
| Corman [60] | Germany | Plasma (7/8 blood samples from that 1 patient tested negative) | 1/18 | NS | Patient had ARDS; low detection, yielding 179 copies/mL |
|
| |||||
| Cappy [36] | France: Paris | Blood samples from asymptomatic donors | 3/311 | CT >37 | These 311 are all positive patients that reported sickness. Donor 1's products were transfused with no transmission recorded; unknown statuses of all 311 patients |
|
| |||||
| Percivalle [37] | Italy: Lodi Red Zone (10 cities) | Blood samples from asymptomatic donors | 20/390 | NS | 8 reported no symptoms, while the rest developed mild symptoms −390/2,272 total blood donors |
|
| |||||
| Chang [17] | China: Hubei (12 blood centres) | Whole blood and platelet products from a random pool of donations | 0/98,342 | NS | Unknown how many of those 98,342 donors are actually COVID-19 positive |
|
| |||||
| Change [59]# | China: Wuhan Blood Centre | Whole blood and platelet products from a random pool of donors | 4/2,420 | NS | All 4 donors were asymptomatic during time of donation; 2 remained asymptomatic, while the other 2 developed fever |
|
| |||||
| Xu [38]# | China: Guangzhou | Whole blood products from a random pool of donors | 0/2,199 | NS | 7 donors were reactive for SARS-CoV-2 antibodies, but ultimately all tested negative for viral RNA |
|
| |||||
| Waheed [39]# | Pakistan: Kashmir | FFP from a random pool of donors | 2/690 | NS | Both donors remained asymptomatic |
Articles that were pooled for meta-analysis whereby the study was conducted in China and came from one local origin.
Zheng had two data reported but only one data was used.
Articles that were pooled in a separate meta-analysis forest plot as they were data showcasing single origin blood centres performing RT-PCRs, on donated blood products.
Of the 18 articles, 16 conducted their research on patients that were already admitted in hospitals, with many from the ICU. Several observations from the table could be made. Firstly, out of a total 935 plasma, serum, or whole blood samples that were tested, only 119 samples tested positive for detectable viral RNA. Of those positive RNAaemia, the viral load was still quite low. Secondly, detection of RNAaemia highly varied in the courses of detection, some being detected just 3 days after symptoms, while others could still be detected 4 weeks after onset of symptoms. However, all agreed that the duration of RNAaemia lasts only a few days and could show up at different times of symptom onset.
Such was in the case of Zheng's group, where they noticed that the positivity rate increased from the first week after onset of symptoms and gradually decreased by the third week [35]. Thirdly, all 16 articles found an association between the severity of the disease with positive RNAaemia. Many of the patients had ended up in the ICU, with varying symptoms including ARDS, severe pneumonia, and sometimes death.
As for the other 2 articles, Cappy et al. [36] and Percivalle et al. [37], a total of 701 donated blood products from asymptomatic donors were screened, and 23 positives for RNAaemia were found. These donors were ones that have called their blood centres to report symptoms related to COVID-19 or had tested positive for the virus within 15 days of their donation. While the rest developed COVID-19 like symptoms, several were mild, while 10 were completely asymptomatic. However, Cappy did not follow up the disease progression in their 311 donors, so no association with severity of disease can be made in their paper.
In the meta-analysis conducted, only 10 articles were actually pooled in an odds ratio forest plot analysis − those that were from China and from a single local blood centre. The ARD was found to be −0.831 (95% −0.963, −0.699), with a p value of 0.035, indicating a significantly low prevalence of RNAaemia. Only one article touched the line of no null effect. Heterogeneity of the data, however, reached 47.5%.
Study Characteristics of RNAaemia within a Random Pool of Blood Donations
Of the 22 articles shown in Table 3, four research papers were found to have screened for viral RNA in donated blood products. To date, only two countries, China and Pakistan, have published data on screening for viral RNA during the peak of their pandemic period, mostly between January and April 2020. A total of 103,661 blood products were screened by RT-PCR, and only 6 tested positive for RNAaemia. It is important to note, however, that this does not mean that the 103,661 donors were not infected or asymptomatic. No follow-ups were made to them unlike the previous data in Table 3. Of the 6 donors, 2 developed mild symptoms, while 4 remained asymptomatic. Interestingly, Xu et al. [38] found that 7 out of 2,199 donated blood products had COVID-19 antibodies in their blood samples, though none of them tested positive for viral RNAaemia.
A forest plot analysis of this dataset is shown in Figure 2C, with the study of Chang et al. [17] being excluded as the data came from multiple centres unlike the other three which had a single origin. The ARD was −1.503 (95% −1.538, −1.468), with a p value of 0.011. The average was so low that the line of null effect was not even shown. Heterogeneity, however, was 66.50%.
Study Characteristics of COVID-19 Blood Transmission
Due to ethical reasons, no actual research on the transmissibility of positive COVID-19 blood products has been conducted. However, four articles have been found to report cases whereby blood products of COVID-19-positive donors had been transfused to recipients. As shown in Table 4, all 13 recipients who received those donors' blood products did not develop the disease nor was their blood found to have viral RNA.
Table 4.
Tabulated results of COVID-19 blood transfusion transmission
| First author [Ref.] | Country | Time frame | Donation type | Recipient developing COVID-19/ total recipients | Notes |
|---|---|---|---|---|---|
| Kwon [40] | Korea | Feb. 2020 | 6 plasma 3 whole blood | 0/9 | The 6 COVID-19 donors were asymptomatic at time of donation and had negative RNAaemia |
|
| |||||
| Waheed [39] | Pakistan: Kashmir | Mar.–Apr. 2020 | RCCs | 0/2 | The 2 donors were RNA positive and developed no symptoms after donation |
|
| |||||
| Anurathapan [42] | Thailand: Bangkok | Apr. 2020 | Bone marrow stem cells | 0/1 | Donor (a child) turned out to be nasal-swab positive with no RNAaemia; donor remained asymptomatic |
|
| |||||
| Làzaro [41] | Spain | Mar. 2020 | Donor HCT | 0/1 | Unknown status of donor after donation, but she was positive during donation |
In screening for viral RNA in a random pool of blood donations as mentioned in the previous dataset, Waheed et al. [39] investigated the two donors who tested positive for RNAaemia. Their red blood cell products had already been transfused to two recipients by the time of the study. Follow-ups were made for both donors and recipients. None of the recipients tested positive from nasopharyngeal swabs, while the two donors remained completely asymptomatic. In the paper by Kwon et al. [40], seven donors were identified as COVID-19 positive through donor follow-ups. By the time of notification, their blood products (6 platelet units and 3 red blood cell units) had already been transfused to 9 patients. Upon notification, the donors' repository samples were tested for viral RNA and found to be negative.
The other two reports were of haematopoietic stem cell products. Lázaro del Campo et al. [41] reported of a cancer patient who was transfused with cells from a relative who tested positive just days after her donation. Anurathapathan et al. [42] reported a child with β-thalassaemia who was transfused with stem cells from a relative known to be positive for COVID-19. The urgency of the transfusion along with the fact that the donor remained completely asymptomatic led the doctors to go ahead with the procedure. Both authors thoroughly examined the patients' nasopharyngeal and blood samples for viral RNA which came up negative many times.
An arcsine risk difference statistical analysis was still performed despite the zero outcomes of the recipients, as shown in Figure 2D. The ARD was found to be −0.911 (95% 0.-1.247, −0.575) with a p value of less than 0.001. Heterogeneity was found to be 0.
Discussion
Impact of COVID-19 on Donation Trend
There are many variabilities that need to be taken into account. Firstly, there were no controls mentioned in all the articles, as it is impossible to get such comparisons considering that the number of donations vary highly between countries. Simply put, the more advanced the country is, the higher their blood donations tend to be, as in the case of China. India and Saudi Arabia, however, already had trouble with obtaining sufficient donations even before the pandemic started. Secondly, the articles each had their own way of measuring their data − some took numbers from as many as 38 blood centres scattered across a big region, while others only took numbers from one centre and/or hospital. Their population sizes and sample sizes vastly differ from one another. Thirdly, each country and region had differing periods of lockdowns as they were hit by the peak of the pandemic at different times. China was hit earlier on between December to February, while the rest of the countries were hit between March and June. All these contributed to having a relatively high heterogeneity of 98%. In fact, one of the limitations of this study is that no statistical analysis was made to correlate infection rates to the total population of that region. This truly limited how associations between the pandemic and active donations could be made. Logic would say that the higher the infection rates of a region, the lesser the donations would be, which is what various articles have reported.
Prevalence of Viral Load in COVID-19-Positive Individuals
Viral load measurements from tissue samples are a way to study active viral replications and monitor diseases [35]. As such, the fact that COVID-19 RNA could be detected in tissue samples other than respiratory samples made scientists concerned over the transmissibility of the disease by any routes other than direct contact of respiratory droplets. Systematic and literature reviews have already been made on the viral load of COVID-19 in the blood samples of patients, with the majority concluding that viral load in blood samples are very low and even so, nearly undetectable as shown by their high CT values [43, 44, 45]. In fact, Zheng et al. [35] compared the first SARS and SARS-CoV-2 detection rate of viral RNA, finding that up to 79% of blood samples contained the former, while the latter was only 41%. Despite several limitations, this paper supports these conclusions and found the rate to be even lower.
One of the reasons for the relatively high heterogeneity of 47.5% could be that sample sizes included in this report were varied − the lowest reported 5 patients, while the highest was 390. Another reason could be the different targets of the nucleic acid tests that each article did, which may have affected detectability of viral load. Chan et al. [46] noticed that an RdRp-P2 assay was less sensitive than an RdRp/Hel assay, where 10 samples were negative in the former but positive in the latter. Of the pooled 18 articles, some targeted N genes and ORF1ab genes, others had targeted S genes. An early report by Zhou et al. [47] recommended using RdRp or E genes for routine detection, though it may have been discarded. However, no statistical analysis was made on this correlation but should be considered in terms of finding the best molecular assay to do viral blood screening.
Initial data from a literature review showed that RNAaemia can be correlated with severity of disease as most of the individuals had ended up in the ICU [43]. Zheng et al. [35]even showed that viral load was higher in patients with severe progression of disease compared to milder patients. However, this is rebutted by the fact that asymptomatic donors also had RNAaemia, so no correlations can be made between progression of disease and RNAaemia for this paper. Another thing to note is that the virus's detectability in the blood highly varies in terms of symptom onset (Table 3).
Prevalence of Finding RNAaemia in a Pool of Donors
The rising numbers of asymptomatic cases gave rise to concerns over transfusing positive donors' blood products to unsuspecting recipients. As such, China and Pakistan conducted real-time screening of SARS-CoV-2 in the blood products stored in various blood centres.
This dataset has limitations. Firstly, the sample size is low − only three of the four are included in the meta-analysis, two of which came from just China. There was not a lot of research made yet that conducted such findings as logically speaking, the higher the infection rate of a population in the region, the higher the chances of finding asymptomatic donors. Additionally, individuals who experience severe symptoms, which RNAaemia tends to be found with, will not be allowed nor will they have the ability to actually donate blood.
The articles from China agreed that as of now, there seems to be no direct threat to transmission via blood supplies. All four articles noted and agreed that the general decrease in blood supply around the world was of a higher concern.
Prevalence of Recipients Developing COVID-19 from Blood Products of Positive Donors
WHO has stated that the coronavirus family are not known to get transmitted via blood and blood products [48]. Hundreds of case reports have shown that recipients who were given blood products from positive donors did not develop the disease. This paper supports this fact.
Meta-analysis showed an arcsine risk difference of −0.911, and heterogeneity was found to be 0. An ARD result is not as always straightforward. Another form of data analysis could have been better suited for this paper. Furthermore, sample size again was low. There were only 13 patients analysed for this data set, but in reality, there are many other case reports regarding the entire coronavirus family. SARS-CoV-2 is still a fairly new virus, and at the time of publication, there are very little data regarding to transfusion-transmission. Additionally, some countries may not have done screening for viral RNAaemia in blood products so there's no way to gather that much data.
Impact of Results and Future Directions
More than half a year later, the COVID-19 pandemic has not ended. Concerns over blood transmission still hang over the heads of blood banks − but the data showing the decrease in donations, and thus the blood supply, was more significant as shown in this paper. As shown by the meta-analyses, only Figure 2A showed a positive ARD outcome, whereas the rest showed negative ARD outcomes. In terms of the actual risk of blood transfusion-transmission, this systematic search found that recipients who did indeed receive blood from positive donors did not develop the disease. Furthermore, the prevalence of finding RNAaemia in a pool of random donations was nearly zero, and even if products came from asymptomatic donors, SARS-CoV-2's RNA is nearly undetectable. It is also important to note that the data presented skims the surface on donation trends worldwide. Although only 8 papers were included in this study, there are more news articles, blogs, and editorials reporting how low their country's blood supplies have been due to COVID-19.
China realised these problems and have already implemented initiatives to make sure their donation numbers are up once more. The observational research by Wang et al. [32] in Zhejiang, China, which saw a 67% drop in their province, saw 81% of their donor respondents' main concern was acquiring COVID-19 during the donation process. As such, 38 blood centres launched several initiatives. These included monitoring temperature of blood donors, increasing spaces, using air disinfectant machines that sterilises with UV irradiation systems, and providing online appointment through popular social media sites. They have also started questioning donor's travel history, following up on their donors' health statuses, and called out pleas to donate. Incredibly, the success rate was up to 3.2%, in which 5,253 donations of a total 163,791 was because of the recruitment policies. Additionally, they did not issue a quarantine system for donated blood which led to a more effective blood supply and demand. Other countries such as Pakistan, Saudi Arabia, Korea, and Italy followed suit.
Conclusion
In conclusion, blood centres should focus more on launching initiatives and policies that would increase their countries' blood supply. Research has shown that while concerns over blood transfusion-transmission risk is legitimate, SARS-CoV-2 itself has no direct threat to blood safety. Viral RNA in asymptomatic donors, who provide the majority of donations, is extremely low even when detected. However, the decrease in donations has become a serious issue. Simply decreasing blood demand will not help the problem in the long run as there will always be people in need of blood products.
Statement of Ethics
The paper is exempt from ethical committee approval because there was no need for one as this is a systematic review, not an original clinical study.
Conflict of Interest Statement
Authors declare no conflicts of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
C.C. performed and wrote the systematic review and meta-analysis, T.N., K. A., H. H. and J.H.H. provided intellectual input, review of the paper, D.J. directed the systemic review and meta-analysis and edited the manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Acknowledgements
I would like to express my deepest appreciation to my professor, Denise Jackson, for always being there to help and give great suggestions. I would also like to thank the rest of my group members Khader Alghamdi, Thao Nguyen, and Joon Hee Han for helping me get on the right track to performing a meta-analysis and giving me statistical advice. I would also like to thank BTS for giving me company and strength to finish the paper.
References
- 1.Chassé M, McIntyre L, English SW, Tinmouth A, Knoll G, Wolfe D, et al. Effect of Blood Donor Characteristics on Transfusion Outcomes: A Systematic Review and Meta- Analysis. Transfusion Medicine Reviews. 2016;30((2)):69–80. doi: 10.1016/j.tmrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47((3)):385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger R. Deutsches Aerzteblatt Online. 2020. Correspondence. [Google Scholar]
- 4.WHO WHO. Blood safety and availability: WHO.int. World Health Organization. 2020. [Available from: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and- availability]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano MB, Hess JR, Tsang HC, Staley E, Gernsheimer T, Sen N, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington State. Transfusion. 2020;60((5)):908–11. doi: 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Ren M, Chen F, Li L, Lei H, Wang X. Blood transfusion during the COVID-19 outbreak. Blood Transfus. 2020;18((2)):79–82. doi: 10.2450/2020.0076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, et al. Essential Role of Patient Blood Management in a Pandemic: A Call for Action. Anesth Analg. 2020;131((1)):74–85. doi: 10.1213/ANE.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahu KK, Raturi M, Siddiqui AD, Cerny J. “Because Every Drop Counts”: Blood donation during the COVID-19 Pandemic. Transfus Clin Biol. 2020;27((3)):105–8. doi: 10.1016/j.tracli.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang L, Yan Y, Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus Med Rev. 2020;34((2)):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Z, Chen D, Chen X, Wei Y. Estimation of the number of blood donors during the COVID-19 incubation period across China and analysis of prevention and control measures for blood transfusion transmission. Transfusion. 2020;60((8)):1778–84. doi: 10.1111/trf.15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debuc B, Smadja DM. Is COVID-19 a New Hematologic Disease? Stem Cell Rev Rep. 2020:1–5. doi: 10.1007/s12015-020-09987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhury N, Mathur A, Smit Sibinga CT, Aatm O. COVID‐19 Pandemic - blood supply challenges and approaches in AATM member countries. Voxs. 2020;15((4)):353–61. [Google Scholar]
- 14.Deng X, Liu B, Li J, Zhang J, Zhao Y, Xu K. Blood biochemical characteristics of patients with coronavirus disease 2019 (COVID-19): a systemic review and meta-analysis. Clin Chem Lab Med. 2020;58((8)):1172–81. doi: 10.1515/cclm-2020-0338. [DOI] [PubMed] [Google Scholar]
- 15.Fang B, Meng QH. The laboratory's role in combating COVID-19. Crit Rev Clin Lab Sci. 2020;57((6)):400–14. doi: 10.1080/10408363.2020.1776675. [DOI] [PubMed] [Google Scholar]
- 16.Ngo A, Masel D, Cahill C, Blumberg N, Refaai MA. Blood Banking and Transfusion Medicine Challenges During the COVID-19 Pandemic. Clin Lab Med. 2020;40((4)):587. doi: 10.1016/j.cll.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Yan Y, Zhao L, Hu G, Deng L, Su D, et al. No evidence of SARS-CoV-2 RNA among blood donors: A multicenter study in Hubei, China. Transfusion. 2020;60((9)):2038–46. doi: 10.1111/trf.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayedahmed AMS, Ali KAM, Ali SBS, Ahmed HSM, Shrif FSM, Ali NAA. Coronavirus disease (COVID-19) and decrease in blood donation: A cross sectional study from Sudan. ISBT Science Series. 2020;15((4)):381–5. [Google Scholar]
- 19.Mohseni AH, Taghinezhad-S S, Xu Z, Fu X. Body fluids may contribute to human-to-human transmission of severe acute respiratory syndrome coronavirus 2: evidence and practical experience. Chinese Med. 2020;15((58)):1–4. doi: 10.1186/s13020-020-00337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323((13)):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Arcot PJ, Kumar K, Mukhopadhyay T, Subramanian A. Potential challenges faced by blood bank services during COVID-19 pandemic and their mitigative measures: The Indian scenario. Transfus Apher Sci. 2020;59:102877. doi: 10.1016/j.transci.2020.102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raturi M, Kusum A. The blood supply management amid the COVID-19 outbreak. Transfus Clin Biol. 2020;27((3)):147–51. doi: 10.1016/j.tracli.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhiman Y, Patidar GK, Arora S. Covid-19 pandemic- response to challenges by blood transfusion services in India: a review report. ISBT Sci Ser. 2020;15((4)):365–73. [Google Scholar]
- 24.Gehrie E, Tormey CA, Sanford KW. Transfusion Service Response to the COVID-19 Pandemic. Am J Clin Pathol. 2020;154((3)):280–5. doi: 10.1093/ajcp/aqaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou-Yang J, Li SJ, Bei CH, He B, Chen JY, Liang HQ, et al. Blood Donor Recruitment in Guangzhou, China, during the 2019 Novel Coronavirus (COVID-19) Epidemic. Transfusion. 2020;60((11)):2597–610. doi: 10.1111/trf.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raturi M, Kusum A. The blood supply management amid the COVID-19 outbreak. Transfus Clin Biol. 2020 Aug;27((3)):147–51. doi: 10.1016/j.tracli.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7((10)):e756–e64. doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadi S, Tabatabaei Yazdi SM, Eshghi P, Norooznezhad AH. Coronavirus disease 2019 (COVID-19) and decrease in blood donation: experience of Iranian Blood Transfusion Organization (IBTO) Vox Sang. 2020;115((7)):595–6. doi: 10.1111/vox.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gniadek TJ, Mallek J, Wright G, Saporito C, AbiMansour N, Tangazi W, et al. Expansion of hospital-based blood collections in the face of COVID-19 associated national blood shortage. Transfusion. 2020;60((7)):1470–5. doi: 10.1111/trf.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020;71((15)):793–8. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grandone E, Mastroianno M, Caroli A, Ostuni A. Blood supply and transfusion support in southern Italy: findings during the first four weeks of the SARS-CoV-2 pandemic. Blood Transfus. 2020;18((3)):230–2. doi: 10.2450/2020.0107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Han W, Pan L, Wang C, Liu Y, Hu W, et al. Impact of COVID-19 on blood centres in Zhejiang province China. Vox Sang. 2020;115((6)):502–6. doi: 10.1111/vox.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AM, Ojha S, Poojary M, SHS, S H S, Dhokle R. Organization of the outdoor blood donation drives amid novel coronavirus pandemic and national lockdown: An experience from a tertiary care oncology institution in India. Transfus Apher Sci. 2020;59:102878. doi: 10.1016/j.transci.2020.102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahia AIO. Management of blood supply and demand during the COVID-19 pandemic in King Abdullah Hospital, Bisha, Saudi Arabia. Transfus Apher Sci. 2020;59:102836. doi: 10.1016/j.transci.2020.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappy P, Candotti D, Sauvage V, Lucas Q, Boizeau L, Gomez J, et al. No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood. 2020;136((16)):1888–91. doi: 10.1182/blood.2020008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25((24)) doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu R, Huang J, Duan C, Liao Q, Shan Z, Wang M, et al. Low prevalence of antibodies against SARS-CoV-2 among voluntary blood donors in Guangzhou, China. J Med Virol. 2020;93((3)):1743–7. doi: 10.1002/jmv.26445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waheed U, Wazeer A, Saba N, Qasim Z. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 RNA in Blood Donations. J Lab Physicians. 2020;12((2)):163–4. doi: 10.1055/s-0040-1716663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon SY, Kim EJ, Jung YS, Jang JS, Cho NS. Post-donation COVID-19 identification in blood donors. Vox Sang. 2020;115((8)):601–2. doi: 10.1111/vox.12925. [DOI] [PubMed] [Google Scholar]
- 41.Lázaro Del Campo P, de Paz Arias R, Ramírez López A, de la Cruz Benito B, Humala Barbier K, Sánchez Vadillo I, et al. No transmission of SARS-CoV-2 in a patient undergoing allogeneic hematopoietic cell transplantation from a matched-related donor with unknown COVID-19. Transfus Apher Sci. 2020:102921. doi: 10.1016/j.transci.2020.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anurathapan U, Apiwattanakul N, Pakakasama S, Pongphitcha P, Thitithanyanont A, Pasomsub E, et al. Hematopoietic stem cell transplantation from an infected SARS-CoV2 donor sibling. Bone Marrow Transplant. 2020;55:2359–60. doi: 10.1038/s41409-020-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leblanc JF, Germain M, Delage G, O'Brien S, Drews SJ, Lewin A. Risk of severe acute respiratory syndrome coronavirus 2 by transfusion: A literature review. Transfusion. 2020;60((12)):3046–54. doi: 10.1111/trf.16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bwire GM, Majigo MV, Njiro BJ, Mawazo A. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis. J Med Virol. 2020;93((2)):719–25. doi: 10.1002/jmv.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson M, Arancibia - Carcamo, Carolina V, Auckland K, Baillie JK, Barnes E, et al. SARS-CoV-2 RNA detected in blood samples from patients with COVID-19 is not associated with infectious virus. medRxiv. 2020 doi: 10.12688/wellcomeopenres.16002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated in vitro and with Clinical Specimens. J Clin Microbiol. 2020;58((5)):1–10. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579((7798)):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Organization WHO . Guidance on maintaining a safe and adequate blood supply during the coronavirus disease 2019 (COVID-19) pandemic and on the collection of COVID-19 convalescent plasma. World Health Organization; 2020. [Google Scholar]
- 49.Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9((1)):469–73. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20((6)):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323((18)):1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395((10223)):514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JM, Kim HM, Lee EJ, Jo HJ, Yoon Y, Lee NJ, et al. Detection and Isolation of SARS-CoV-2 in Serum, Urine, and Stool Specimens of COVID-19 Patients from the Republic of Korea. Osong Public Health Res Perspect. 2020;11((3)):112–7. doi: 10.24171/j.phrp.2020.11.3.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng L, Liu J, Xu W, Luo Q, Chen D, Lei Z, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020;92((9)):1676–80. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9((1)):386–9. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 Viral Load in Clinical Samples from Critically Ill Patients. Am J Respir Crit Care Med. 2020;201((11)):1435–8. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients Clinical Infectious Diseases. 2020. p. p. ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J, Liu J, Li S, Peng Z, Xiao Z, Wang X, et al. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis. 2020;37:p. 101673. doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang L, Zhao L, Gong H, Wang L, Wang L. Severe Acute Respiratory Syndrome Coronavirus 2 RNA Detected in Blood Donations. Emerg Infect Dis. 2020;26((7)):1631–3. doi: 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corman VM, Rabenau HF, Adams O, Oberle D, Funk MB, Keller-Stanislawski B, et al. SARS-CoV-2 asymptomatic and symptomatic patients and risk for transfusion transmission. Transfusion. 2020;60((6)):1119–22. doi: 10.1111/trf.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.