Abstract
Background
Findings from autopsies have provided evidence on systemic microvascular damage as one of the underlying mechanisms of Coronavirus disease 2019 (COVID-19). The aim of this study was to correlate autopsy-based cause of death in SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients with chest imaging and severity grade of pulmonary and systemic morphological vascular pathology.
Methods
Fifteen SARS-CoV-2 positive autopsies with clinically distinct presentations (age 22–89 years) were retrospectively analyzed with focus on vascular, thromboembolic, and ischemic changes in pulmonary and in extrapulmonary sites. Eight patients died due to COVID-19 associated respiratory failure with diffuse alveolar damage in various stages and/or multi-organ failure, whereas other reasons such as cardiac decompensation, complication of malignant tumors, or septic shock were the cause of death in 7 further patients. The severity of gross and histopathological changes was semi-quantitatively scored as 0 (absent), 1 (mild), and 3 (severe). Severity scores between the 2 groups were correlated with selected clinical parameters, initial chest imaging, autopsy-based cause of death, and compared using Pearson χ<sup>2</sup> and Mann-Whitney U tests.
Results
Severe pulmonary endotheliitis (p = 0.031, p = 0.029) and multi-organ involvement (p = 0.026, p = 0.006) correlated significantly with COVID-19 associated death. Pulmonary microthrombi showed limited statistical correlation, while tissue necrosis, gross pulmonary embolism, and bacterial superinfection did not differentiate the 2 study groups. Chest imaging at hospital admission did not differ either.
Conclusions
Extensive pulmonary endotheliitis and multi-organ involvement are characteristic autopsy features in fatal COVID-19 associated deaths. Thromboembolic and ischemic events and bacterial superinfections occur frequently in SARS-CoV-2 infection independently of outcome.
Keywords: Coronavirus disease-19, Respiratory failure, Endothelial dysfunction, Adult respiratory distress syndrome, Multi-organ failure
Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is a challenge for health-care systems and for affected patients worldwide [1]. Hypoxic respiratory failure is the leading cause of death in patients with severe COVID-19 disease as is also seen in other respiratory viral infections. The general histological correlate of acute respiratory distress syndrome (ARDS) is diffuse alveolar damage (DAD) characterized by intra-alveolar hyaline membrane formation, edema, and hemorrhage in the acute stage, followed by fibroblast proliferation in the organizing stage. DAD is a nonspecific finding with a long list of potential etiologies, including other viral diseases, like influenza and SARS-CoV [2, 3, 4]. However, severe COVID-19 is associated with early pronounced gas exchange disturbance and particularly severe hypoxemic respiratory failure compared to the degree of radiographic abnormalities and more severe multi-organ failure compared to, for example, influenza, particularly in patients with vascular risk factors [2, 4, 5, 6].
Among the distinctive attributes of COVID-19 are associated microvascular changes and thrombosis [2, 7]. Endothelial cells not only play an important role in the pathogenesis of ARDS but also in the pathogenesis of multi-organ failure. This is thought to be due to vascular inflammation (endotheliitis) and mediating inflammatory cell infiltration as well as apoptotic bodies found in different organ systems [7, 8]. This systemic endotheliitis with evidence of modulation of vessel barrier integrity, promoting a pro-coagulative state could thus explain the disproportional hypoxemia in relation to radiological changes and complications seen in different organs in patients with COVID-19 [9]. Quantitative RT-PCR results for SARS-CoV-2 also demonstrated that SARS-CoV-2 has organotropism beyond the respiratory tract, including the heart, the brain, skin, subcutaneous tissue, neuromuscular system, the kidneys, the intestine, and the liver [5, 6, 10, 11, 12, 13, 14, 15]. Recent terms such as cardio-COVID-19 define the increased risk for cardiac injury, such as arrhythmias, fulminant myocarditis, while neuromuscular-COVID-19 refers to a broad spectrum of associated neuromuscular disorders, hyposmia, anosmia, etc [10, 11, 12].
Risk factors for a severe disease course of COVID-19, such as old age, obesity, hypertension, and diabetes mellitus are all risk factors for pre-existing vascular dysfunction with altered endothelial metabolism, and therefore might explain the worse outcome in these patient groups [16]. Since the outbreak of the pandemics, several further parameters including tissue-based factors have been identified in association with severe disease course in COVID-19 [5, 6, 13, 17, 18, 19]. Complement activation and increased neutrophilic infiltration of pulmonary capillaries and extracellular spaces (so called NETosis) have been described in autopsy studies and have been associated with an explosive inflammatory response and a poor prognosis in COVID-19 [17, 19, 20]. The interaction between viral-induced endothelial necrosis, excessive cytokine production (“cytokine storm”), and a systemic viral vasculopathy syndrome have been described in several autopsy-based studies [5, 13, 18, 20]. A recent meta-analysis on published COVID-19 autopsies conclude that DAD is the most common pulmonary mortality cause and that a broad spectrum of ischemic necrosis, endotheliitis, and thromboembolism characterize extrapulmonary COVID-19 disease [6]. Different from other respiratory pandemics, such as in SARS-CoV-1, the extensive thromboembolic events and multifocal endotheliitis is more often seen in COVID-19 [6]. Damaged endothelial cell activation and increased plasma von Willebrand factor antigen have been described as the key drivers in severe COVID-19 disease along with associated coagulopathy [21, 22, 23]. Whether a sum of the aforementioned factors or any single parameter alone may contribute to poor prognosis or can predict lethal outcome in COVID-19 is a challenging question to address in individual patients.
The aim of this study was to correlate autopsy-based cause of death in SARS-CoV-2 positive autopsies with selected clinical parameters and with the severity grade of pulmonary and systemic morphological vascular pathology. We addressed the questions (a) whether SARS-CoV-2 infection has any distinct morphological vascular pattern depending on the autopsy-based cause of death, (b) if pulmonary thromboembolic events and bacterial superinfection have an impact on COVID-19-associated death, (c) how the involvement of extrapulmonary sites affects fatal outcome, and (d) if some clinical parameter may correlate with morphological findings or cause of death identified at autopsy. In order to answer these questions, we analyzed 15 SARS-CoV-2 positive autopsies with 2 distinct clinical late phase disease patterns: 8 patients deceased due to COVID-19 associated respiratory or multi-organ failure and 7 patients died due to other reasons based on autopsy results.
Material and Methods
Postmortem examinations on 15 SARS-CoV-2 positive deceased patients were performed at the Institute of Pathology and Molecular Pathology of the University Hospital Zurich, Switzerland, according to standard procedures in a biosafety level 3 isolation room.
The diagnosis of SARS-CoV-2 infection was confirmed by real-time reverse transcriptase-polymerase chain reaction testing (rRT-PCR) on nasopharyngeal swabs prior to and during hospitalization in 14 of 15 patients. In 1 patient (patient 2), postmortem formalin-fixed and paraffin-embedded (FFPE) lung tissue was positive for SARS-CoV-2 using rRT-PCR in the Reference Infectious Pathology of Switzerland in the Institute of Pathology Cantonal Hospital Liestal Switzerland using the methodology as described earlier [5]. In all patients, a complete autopsy was performed including macroscopic organ assessment and histological analysis of a predefined organ list. The organs of the central nervous systems were examined in the Institute of Neuropathology, University Hospital Zurich (in collaboration with KF).
For histological diagnosis, tissue sections from FFPE tissues (2 μm) from all organs (fixed in 4% paraformaldehyde) were stained with hematoxylin and eosin (H&E) according to standard techniques. The diagnoses and histopathological features in this study were carried out on gross examination and using routine H&E stains on tissue sections. Fibrin thrombi were confirmed with fibrin stains (modified Picro-Mallory stain). A large subset of cases underwent further immunohistochemistry analyzes and were characterized by a panel of lympho- and hematopoietic and further markers (such as CD3, CD4, CD45, CD68, and ACE2). Selected lung, kidney, and heart samples were tested for the presence of viral particles and viral-like particles using FFPE or frozen snap tissues for immunofluorescence (in collaboration with XZ) and glutaraldehyde fixed samples for electron microscopy (in collaboration with FS) as described earlier [5, 24, 25]. Postmortal swabs and FFPE samples of selected organs in a subset of autopsies were tested for SARS-CoV-2 by rRT-PCR as described above.
Patient Cohort
Altogether, 15 autopsies with SARS-CoV-2 infection were included in this study. The first autopsy was performed in March 2020 and the autopsy of the last included subject was performed in January 2021. Ten patients were male and 5 patients were female. Age at the time of the autopsy ranged between 22 and 89 years (median 72.9 years). All 15 patients were hospitalized (2–40 days, median 16.6 days). Five patients were not mechanically ventilated (do not intubate order) and 10 patients were intubated (intubation time ranged between 1 and 24 days [median 11.5 days]). Cardiovascular risk factors were present in all 15 patients: hypertension in 10 of 15 patients (67%), diabetes mellitus in 2 of 15 patients (13%), and smoking in 6 of 15 patients (40%), in 3 of 15 patients, there was no information on smoking from patient history. Obesity defined as body mass index >30 kg/m2 was present in 6 of 15 patients (40%). Comorbidities included COPD (2 of 15), pulmonary hypertension (2 of 15), chronic interstitial lung disease (3 of 15), asthma (1 of 15), coronary artery disease (4 of 15), hypertensive heart disease (2 of 15), atrial fibrillation (7 of 15), chronic renal failure (7 of 15), current malignant tumor (3 of 15), recurrence-free malignant tumor (3 of 15), solid organ transplantation (2 of 15), autoimmune disorder (1 of 15), hematological disease (2 of 15), and depression (3 of 15). Clinical data are shown Table 1.
Table 1.
Clinical parameters in both groups with SARS-CoV-2 infection (patients 1–8 died from severe COVID-19, patients 9–15 died due other causes)
| Clinical characteristics | Patient |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | 5+ | 6+ | 7+ | 8+ | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Age, years | 70 | 77 | 58 | 79 | 56 | 61 | 79 | 72 | 22 | 69 | 67 | 87 | 58 | 89 | 78 |
| Sex | M | F | F | M | M | M | M | M | M | F | M | F | F | M | M |
| BMI, kg/m2 | 22.7 | 24.5 | 37 | 33.7 | 34.2 | 30.9 | 22.6 | 23.1 | 27.8 | 31.1 | 27.5 | 35 | 27.2 | 19.8 | 27.7 |
| Days in hospital until death | 8 | 9 | 18 | 7 | 19 | 20 | 18 | 40 | 1 | 32 | 11 | 5 | 23 | 2 | 28 |
| Days intubated | 8 | 0 | 17 | 0 | 15 | 1 | 0 | 20 | 1 | 0 | 10 | 4 | 15 | 0 | 24 |
| Cardiovascular risk factors | |||||||||||||||
| Hypertension | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Diabetes | No | No | Yes | No | No | No | No | No | No | No | No | No | Yes | No | No |
| Smoking (packs per year) | Yes* | 0 | N/A | Yes* | 0 | 0 | 60 | 7.5 | Yes* | 0 | N/A | N/A | 0 | 0 | Yes* |
| Obesity1 | No | No | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | No | No | No |
| Comorbidities | |||||||||||||||
| COPD | No | No | No | No | No | No | Yes | No | No | No | Yes | No | No | No | No |
| Pulmonary hypertension | No | No | No | Yes | No | No | No | No | No | No | No | Yes | No | No | No |
| Interstitial lung disease | No | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No | Yes |
| Asthma | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No |
| Coronary artery disease | Yes | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes | Yes |
| Hypertension cardiopathy | No | No | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No |
| Atrial fibrillation | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | No | No |
| Chronic renal failure | Yes | No | No | Yes | Yes | No | No | Yes | No | Yes | No | No | No | Yes | Yes |
| Malignant solid tumor (current) | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No | Yes | No |
| Malignant solid tumor (recurrence free) | No | Yes | No | No | Yes | No | Yes | No | No | No | No | No | No | No | No |
| Solid organ transplantation | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Autoimmune disease | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No |
| Hematological disorder | No | No | No | Yes | No | Yes | No | No | No | No | No | No | No | No | No |
| Depression | No | Yes | No | No | No | No | No | Yes | No | No | No | No | Yes | No | No |
| Severity of disease | |||||||||||||||
| SOFA (mean) | 12 | N/A | 12.3 | N/A | 11.5 | 10.5 | N/A | 10.9 | 17 | N/A | 12 | 11.5 | 7.4 | N/A | 8.75 |
| SOFA (max) | 14 | N/A | 19 | N/A | 16 | 15 | N/A | 18 | 17 | N/A | 12 | 12 | 11 | N/A | 11 |
| SOFA (delta)2 | 2 | N/A | 11 | N/A | 7 | 9 | N/A | 12 | 0 | N/A | 5 | 1 | −3 | N/A | 8 |
Patients 9–15 died due to other causes. N/A, not assessed; SOFA, Sequential Organ Failure Assessment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; BMI, body mass index.
Obesity was defined as BMI >30.
Difference of SOFA score upon admission and last registered score.
Patient of the first COVID-19 wave − death due to COVID-19.
Patient of second COVID-19 wave − death due to COVID-19.
Current/former smoker − pack years not assessed.
Statistical Analyses
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26.0. IBM Corp., Armonk, NY, USA. The Mann-Whitney U test and Pearson R test were used for comparison of morphological findings in autopsy tissue between groups. p value <0.05 was regarded as statistically significant. Parameters being statistically significant with both tests were weighted as biologically relevant. Graphics were plotted using GraphPadPrism (version 9.0).
Scoring of Autopsy Findings and Definition of Groups
Definition of Severity Scores
Macro- and histopathological scoring: Various parameters were scored according to the severity of histological appearance and/or gross findings. Score 0: absent (not apparent on H&E section and/or not evident with ancillary immunohistochemistry). Low intensity as score 1 (discrete and modest changes were pooled in 1 low intensity score 1): discrete (score 1) and modest (score 2) presence of macro -und histopathological feature only in small anatomical compartments and in focal (only 1 anatomical region) or only in scattered (in max. 2 anatomical compartments per organ) distribution. Ancillary tests were used if not apparent on H&E staining. Score 3: diffuse expression of the feature involving multiple (>2 foci per organ) anatomical compartments.
Multi-organ involvement and necrosis scoring: 1 score point was allocated to each organ showing histological signs of endotheliitis or necrosis, respectively. The sum of all affected organs was taken as the final score.
Definition of Biological Groups
To test the hypothesis “correlation of morphological autopsy findings with clinical course/severity/cause of death in SARS-Cov-2 positive deceased patients”, autopsy cases were assigned to 1 of 2 groups. Group 1: “Death due to COVID-19” were patients with fatal course of COVID-19 disease − hospitalized due to COVID-19 and autopsy-based cause of death was due to respiratory or cardio-respiratory failure with diffuse alveolar damage. Group 2: “Death due to other causes” were SARS-CoV-2 positive patients, with or without clinical symptoms of COVID-19 and death defined at autopsy due to other causes such as underlying disease, for example, malignancy or hematological disorder (Table 2).
Table 2.
Cause of death based on overall findings at autopsy
| Patient | Cause of death | Death due to COVID-19 |
|---|---|---|
| 1° | Respiratory failure with diffuse alveolar damage | Yes |
| 2° | Respiratory failure with diffuse alveolar damage | Yes |
| 3° | Respiratory failure with diffuse alveolar damage | Yes |
| 4° | Respiratory failure with diffuse alveolar damage | Yes |
| 5 | Respiratory failure with diffuse alveolar damage | Yes |
| 6 | Respiratory failure with diffuse alveolar damage | Yes |
| 7 | Respiratory failure with diffuse alveolar damage | Yes |
| 8 | Respiratory failure with diffuse alveolar damage | Yes |
| 9 | Acute myocardial infarction | No |
| 10 | Complication, metastatic colorectal cancer | No |
| 11 | Complication, metastatic non-small cell lung cancer | No |
| 12 | Cardiac decompensation and pulmonary hypertension | No |
| 13 | Cardio-respiratory insufficiency due to chronic interstitial lung disease | No |
| 14 | Complication, metastatic malignant melanoma | No |
| 15 | Septic shock and pulmonary aspergillosis | No |
COVID-19, coronavirus disease 2019. Patients of first COVID-19 wave.
Results
Postmortem Pulmonary Findings
Gross Findings
Macroscopic examination of the lungs was in line with full ARDS in 8 cases (Nr 1–8, 100% of the group) and showed a broad panel of gross lesions in 7 further cases. There were bilateral diffuse or focal, partially confluent large pale to yellowish hemorrhagic zones of consolidation and edema (shown in Fig. 1d, h). Macroscopic pulmonary infarction was evident in 6 of 15 cases (40%), macroscopic thromboembolism in 5 of 15 cases (30%).
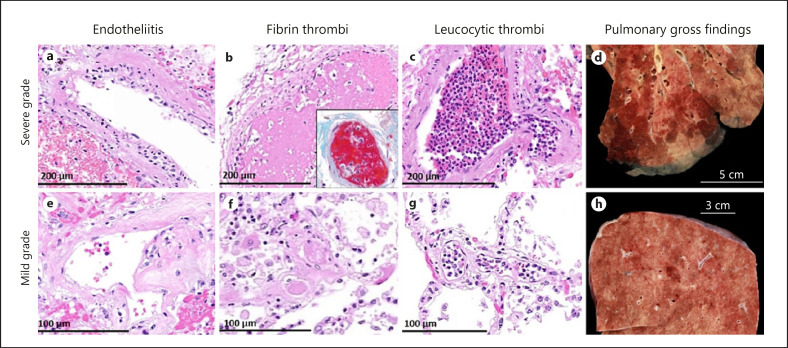
Fig. 1.
Histological vascular and gross pulmonary vascular findings in SARS-CoV-2 positive autopsies. Images demonstrate severe grade of vascular changes (larger involved areas and high number of foci) (a–d), images show mild grade morphological changes (smaller or only scattered areas involved) (e–h). Numerous (a) and only scattered (e) lymphocytes beneath the endothelium. Multiple middle- and small-sized (b and c) and only scattered small-sized peripheral (f, g) fibrin and leucocytic thrombi. Large multiple areas (d) and only small foci (h) of pulmonary hemorrhagic infarction and consolidation. a−g: H & E stain. b (inset): modified Picro-Mallory stain. H&E, hematoxylin and eosin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Histological Findings
Microscopic examination of the lungs revealed signs of DAD in different stages in 8 patients (53%) who died from COVID-19. Bronchopneumonia was identified in 8 of 15 cases (53%). Endotheliitis of small and medium-sized pulmonary vessels was seen in all but 1 patient (14 of 15, 93%) (Fig. 1a, e). Fibrin thrombi were evident in 7 of 15 cases (47%) (Fig. 1b, f) and leucocytic vessel occlusion (“leucocytic thrombi”) in 9 of 15 cases (60%) (Fig. 1c, g). Thrombotic vessel occlusions were noted within a few small pulmonary artery branches as often seen in DAD but also in small to medium-sized vessels and in areas not affected by DAD.
The extension of pulmonary endotheliitis significantly correlated with autopsy-based cause of death due to COVID-19 (R-test p = 0.031, U test p = 0.029) (Fig. 2a, 3a). Individual severity scores of macro and microscopic pathology findings are shown in Table 3.
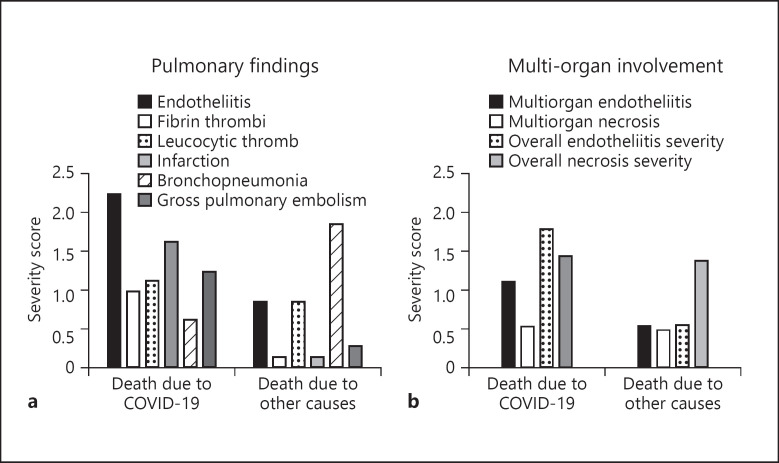
Fig. 2.
Correlation between severity scores of macroscopic and histopathological findings and autopsy-based cause of death in SARS-CoV-2 positive autopsies. Axe Y demonstrates mean score per gross or histopathological pulmonary findings (a) and of organ involvement (b). Axe X shows the distribution of autopsy findings in terms of cause of death identified at the autopsy. The extension of pulmonary endotheliitis and the evidence of multi-organ involvement correlated significantly with COVID-19 associated cause of death. Individual scores are shown in Table 3. Statistical correlation between scores and autopsy-based cause of death is shown in Table 4. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; CT, computed tomography.
Fig. 3.
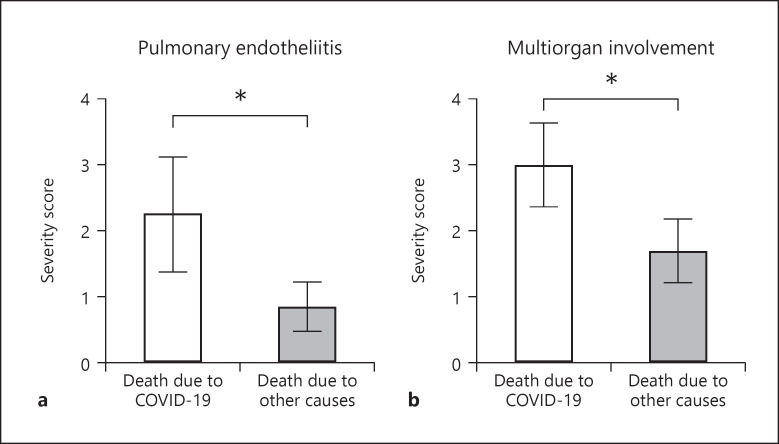
a The extension of pulmonary endotheliitis (R-test p = 0.031, U test p = 0.029) and b) the evidence of multi-organ involvement (R-test p = 0.026, U test p = 0.006) correlated significantly with COVID-19 associated cause of death (Pearson χ2 and Mann-Whitney tests). COVID-19, coronavirus disease 2019.
Table 3.
Scores of histological and gross macroscopic findings at autopsy
| Histological scoring | Patients |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | 5+ | 6+ | 7+ | 8+ | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Lung1 | |||||||||||||||
| Endotheliitis2 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Fibrin thrombi2 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Leucocytic thrombi2 | 3 | 1 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 0 |
| Infarction | 1 | 0 | 0 | 0 | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Bronchopneumonia2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 3 | 0 | 3 | 0 | 1 | 3 | 3 |
| Macroscopic thrombi | 0 | 0 | 0 | 0 | 1 | 3 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Heart1 | |||||||||||||||
| Endotheliitis2 | 1 | 1 | 1 | 1 | 3 | 0 | 3 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Fibrin thrombi2 | 1 | 0 | 1 | 1 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leucocytic thrombi2 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| All organ systems | |||||||||||||||
| Involved organs | 5 | 2 | 4 | 3 | 2 | 3 | 3 | 4 | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| Endotheliitis in different organs2, 3 | 9 | 2 | 8 | 5 | 6 | 3 | 9 | 3 | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| Necrosis in different organs | 3 | 0 | 2 | 1 | 3 | 1 | 4 | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 1 |
Patients 9–15 died due to other causes. COVID-19, coronavirus disease 2019.
Pooled low intensity score 1 versus high intensity score 3.
Microscopic evaluation only.
Sum score of all affected organs.
Patients of the first COVID-19 wave, death due to COVID-19.
Patients of second COVID-19 wave, death due to COVID-19.
Viral RNA was evident in 30–80% of selected organs in 10 of 15 autopsies, being the highest in lung samples and showing a lower but similar distribution in the extrapulmonary organs (see online suppl. Fig. 1; see www.karger.com/doi/10.1159/000518914 for all online suppl. material). There was no statistically significant difference in RNA positive samples between the 2 study groups (χ2 test, p = 0.991, not significant at p < 0.05).
Chest CT Morphology
Chest computed tomography (CT) at initial hospital admission showed a different extent of bilateral pulmonary ground-glass opacities and consolidations in both autopsy groups. Severe or mild vascular damage at autopsy did not correlate with the extent of pulmonary ground-glass opacities, which was evidenced in patients who died due to COVID-19 and those who succumbed to other underlying causes (Fig. 4a-d).
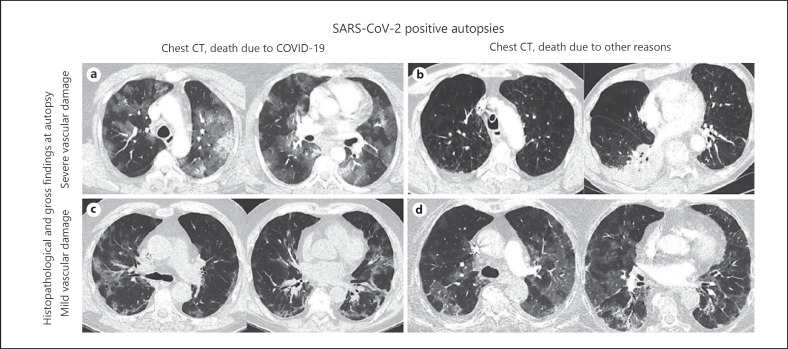
Fig. 4.
Chest CT morphology in SARS-CoV-2 infected and deceased patients at initial hospital admission. CT images show no correlation to different extent of histopathological and gross vascular changes at the autopsy and are not associated with clinical course. Images (a, c) show diffuse bilateral pulmonary consolidations (with severe and mild histopathological changes, respectively). Image (b) displays focal consolidations at severe histopathological findings at autopsy. Image (d) shows diffuse bilateral pulmonary consolidations at only mild histopathological changes at autopsy. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; CT, computed tomography.
Vascular Postmortem Findings in Further Organ Involvement
Heart
Endotheliitis was a frequent finding in the small intramyocardial and subepicardial vessels present in all but 2 cases (13/15). Fibrin thrombi were less frequent (5 of 15 cases, 30%) and leucocytic thrombi in 7 of 15 cases (47%).
Kidney
Acute tubular necrosis was a frequent finding seen in 13 of 15 patients (87%). In contrast to the lungs and the heart, the kidneys demonstrated only very mild endotheliitis with few infiltrating cells in 2 of 15 patients (13%).
Liver
Patchy centrilobular hepatocyte necrosis and confluent necrosis associated with fibrin thrombi in the respective vessels were found in those patients with multi-organ failure (in 8 of 15 cases [53%]).
Small Intestine
Small intestine necrosis was present in 4 of 15 patients (27%), leading in the setting of multi-organ failure to death in all 4 patients. Identical histomorphological changes were seen in all cases, with microangiopathic chances of the submucosal vessels consisting of fibrin thrombi, endotheliitis, and apoptotic endothelial cells. Individual severity scores of pathology findings are shown in Table 3.
Association between cause of death based on autopsy findings and vascular manifestations of COVID-19
Severe pulmonary endotheliitis (R-test p = 0.031, U test p = 0.029) and multi-organ involvement of COVID-19 (R-test p = 0.026, U test p = 0.006) correlated significantly with COVID-19 associated death (Fig. 2, 3; Table 4). Histological evidence of small fibrin thrombi was more frequent in COVID-19 associated death (U test p = 0.040), while tissue necrosis, gross thromboemboli, and bacterial superinfection did not differentiate the 2 study groups. Chest imaging at hospital admission did not differ either and lacked correlation to any pathological factors analyzed. We did not find any statistically relevant interaction between clinical SOFA scores, the use of ventilation and morphological parameters at the autopsy.
Table 4.
Summary of statistical comparison between cause of death (2 groups) and autopsy findings as well as SOFA score in SARS-CoV-2 positive autopsies
| Clinical course of disease | ||||||
|---|---|---|---|---|---|---|
| SOFA (mean) | SOFA (max) | SOFA (delta) | ||||
| Pearson χ2 | 0.540 | 0.189 | 0.358 | |||
| Mann-Whitney | 0.841 | 0.056 | 0.247 | |||
| Pulmonary autopsy findings | ||||||
|---|---|---|---|---|---|---|
| pulmonary endotheliitis | pulmonary fibrin thrombi | pulmonary leucocytic thrombi | pulmonary infarction | broncho-pneumonia | gross pulmonary embolism | |
| Pearson χ2 | 0.031 | 0.060 | 0.875 | 0.084 | 0.186 | 0.182 |
| Mann-Whitney | 0.029 | 0.040 | 0.779 | 0.072 | 0.152 | 0.336 |
| Multi-organ autopsy findings | ||||||
|---|---|---|---|---|---|---|
| multi-organ endotheliitis | overall endotheliitis severity | multi-organ necrosis | overall necrosis severity | |||
| Pearson χ2 | 0.026 | 0.113 | 0.138 | 0.374 | ||
| Mann-Whitney | 0.006 | 0.001 | 0.955 | 1.000 | ||
p values <0.05 are considered as statistically significant. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment.
Discussion
In this study, we retrospectively analyzed the correlation between morphological vascular findings, autopsy-based cause of death and clinical parameters (such as SOFA score) and chest imaging in 15 COVID-19 autopsies with a different clinical course. We could show in our cohort that severe grade pulmonary endotheliitis, multi-organ involvement of endotheliitis, and tissue necrosis significantly correlate with COVID-19 associated death. Vascular changes were less prevalent in COVID-19 positive patients dying from other causes. Pulmonary infarction, bronchopneumonia, grossly visible pulmonary embolism, and frequent neutrophilic thrombi were common pathological findings in the autopsies. However, disease severity and cause of death did not correlate with these additional findings. Neither the initial chest CT imaging nor clinical disease severity parameters such as SOFA score or duration of ventilation correlated with autopsy findings or morphological cause of death. According to our knowledge, this autopsy-based study is the first to address the relationship between the magnitude and site involvement of morphological vascular changes, clinical parameters, and autopsy-based cause of death in COVID-19 autopsies.
The correlation between morphological pathological autopsy findings and imaging modalities especially chest CT at initial presentation or hospitalization have been the subject of several previous studies [8, 22, 26, 27, 28, 29]. In a previous autopsy-based study, the typical ground-glass opacities on imaging harbored a high correlation to a broad spectrum of pulmonary autopsy findings; however, all these patients died due to COVID-19 so a correlation to the cause of death in SARS-CoV-2 positive autopsies was not possible [27]. In our autopsy series, 7 SARS-CoV-2 positive patients died due to other causes than COVID-19 although a direct comparison between initial CT images and the magnitude of histopathological and gross autopsy findings or RNA viral evidence in selected organs did not show any association. These findings were seen in all patients, independent of the use of mechanical ventilation in our cohort, and are similar to those described in COVID-19 autopsy series from other centers as well as in influenza pneumonia and findings during the 2002–2003 SARS (SARS-CoV-1) pandemic [4, 27, 30, 31, 32].
The correlation between clinical parameters (such as SOFA scores or neutrophilic activation) to disease course has been observed in previous studies indicating the possibility to monitor disease severity [17, 19]. In our series, the relationship between dynamic SOFA scores and the degree of histopathological and gross vascular autopsy findings did not prove to be significant and we could not identify any morphological factors in our autopsy series harboring a link to clinical SOFA scores. Interestingly, none of the SOFA scores (mean, maximal, and delta) could be correlated to COVID-19 associated cause of death in our series, possibly pointing to the biological role and relevance of global immunological SARS-CoV-2 induced host response independently of morphological disease extension. However, the extent of endotheliitis has been described to be associated with critical illness and fatal COVID-19 disease, which further strengthens our data [21].
The extent of endothelial damage described in this study is most prominent in the lung, while extrapulmonary manifestations are less evident. Endothelial cell injury at the blood-gas barrier may explain the observed high alveolo-arterial gradient with pronounced hypoxemia both in spontaneous breathing patients with less severe dyspnea than expected and in the early phase of mechanical ventilation with severe hypoxemia but initially only mildly reduced lung compliance than ARDS of other origins [1, 8, 17, 19, 22, 23, 33, 34]. Corroborating with these clinical, tissue, and autopsy-based previous observations, our study data support the preferential role of the magnitude in pathological vascular alterations especially in pulmonary vascular involvement and the role of morphological multi-organ involvement in COVID-19 [17, 19, 21, 22].
The vascular pattern described in this study may explain the adverse outcome of COVID-19 in patients with pre-existing endothelial dysfunction, and thus less capacity to restore the injured vasculature and inhibit microthrombosis. In line with the observed association of the extent of systemic microvascular damage with clinical signs of organ failure in this study, circulating markers of endotheliopathy were associated with mortality in a plasma profiling study in a small sample of COVID-19 patients.
Endothelial tropism and endothelial cell apoptosis have been observed in case of infection with other respiratory viruses, for example, influenza A infection or SARS-CoV-1 have been suggested as underlying mechanism of, for example, encephalopathy in influenza infection [35, 36]. In an autopsy-based meta-analysis, SARS-CoV-1, Influenza A, and SARS-CoV-2 infections share the high prevalence of DAD as the main pulmonary pathology; however, extrapulmonary findings are different especially in terms of multifocal pulmonary and extrapulmonary thrombosis and multifocal endotheliitis pointing to higher frequency of multi-organ involvement at COVID-19 [2, 5, 6]. It is however at current time not possible to answer the question whether the multi-organ involvement triggers the endotheliitis or contrariwise or whether both factors represent 2 biologically distinct mechanisms in COVID-19. If the endothelium of the blood-gas barrier is damaged or dysfunctional, both diffusion impairment and ventilation-perfusion mismatch will further increase and aggravate hypoxemia [8, 23, 26, 28]. Therefore, treatment strategies that reduce ventilation-perfusion mismatch might be particularly important (e.g., prone positioning). The effect of substances active on the pulmonary endothelium needs to be systematically studied. Secondary preventive measures in patients with pre-existing endothelial dysfunction who seem to be prone to a severe course of COVID-19 might be of epidemiological interest [17, 19, 22, 33]. Therapeutic implications of the described vascular damage, such as anticoagulation are established, however, which other interventions might be helpful against the procoagulant state in case of microvascular damage are still subject to debate [17, 19, 22]. Whether survivors of severe COVID-19 will suffer from long-term vascular damage (e.g., pulmonary hypertension or COVID-19-promoted atherosclerosis) and other consequences of microvascular damage-associated multi-organ failure should be systematically studied during a long-term follow-up.
Limitations of the Study
The small sample size (n = 15 autopsies) and the variable clinical presentations (such as diverging time-frame between first diagnosis and death, different postmortem time to autopsy and individual pre- and in-hospital treatment) likely explain why morphological vascular findings and viral RNA evidence in available selected tissues are discordant in this study. Discrepant results between virus detection and clinical outcome have been described in previous studies and were linked to numerous co-factors such as the presence of viral load in vivo, the disease phase and treatment modalities [5, 13, 18, 37]. Higher viral load resulting from direct viral attack is consequently mostly measured in pulmonary vessels, while extrapulmonary sites (e.g., kidney, skin, brain, heart, etc.) harbor rather a low viral load due to docking pseudovirions (spikes and neurocapsid SARS-CoV-2 elements [5, 14, 18, 30, 37]).
Conclusions
In conclusion, our autopsy-based data show that a distinct histopathological pattern in terms of extensive pulmonary endotheliitis and manifestation of systemic multi-organ vascular and tissue damage correlates with COVID-19 associated death and differs in SARS-CoV-2 positive patients dying due to other causes. Pulmonary infarction, bronchopneumonia, grossly visible pulmonary embolism and increased neutrophilic thrombi were common pathological findings in autopsies of SARS-CoV-2 positive patients independent of disease severity or the cause of death. Clinical parameters such as initial chest CT imaging, SOFA score, duration of ventilation, or tissue-based viral RNA evidence did not correlate with autopsy findings or with morphological cause of death. The vascular pattern in fatal COVID-19 described here is another piece of the puzzle to support treatment strategies focusing on the endothelium, inflammatory response, and on avoidance of thrombi.
Statement of Ethics
Consent to perform the autopsy was given in all cases and the Institutional Review Board (Department of Pathology and Molecular Pathology of the University Hospital Zurich, Switzerland) approved the study (PN626). Ethical aspects in research on autopsy tissue of deceased patients, postmortem diagnostic and molecular analyses were completed in accordance with the Swiss Federal Research Regulations (BASEC2020-1316).
Conflict of Interest Statement
The authors have no financial or other conflict of interests to reveal.
Funding Sources
No external funding was necessary for this study.
Author Contributions
M.H.: designed the study, performed several autopsies, collected samples, analyzed immunohistochemical and morphological data, and drafted the manuscript. E.I.S.: designed the manuscript, interpreted, collected clinical information and correlation, and drafted the manuscript P.S.: interpreted clinical information and correlation and critically revised the manuscript. K.F.: performed central nervous system autopsies, interpreted data on cerebral pathology, and critically revised the manuscript. F.S.: interpreted ultrastructural findings in routine autopsy service and critically revised the manuscript. X.Z.: provided antibodies for fluorescence labeled immunohistochemistry, interpreted results on immunofluorescence in routine autopsy service, and critically revised the manuscript. S.H.: coordinated necessary additional stains and immunostains in autopsy diagnostic procedure and critically revised the manuscript. H.M.: designed the study, interpreted clinical and pathological data, and critically revised the manuscript. Z.V.: designed the study, supervised the autopsies, analyzed all gross and histopathological autopsy findings, interpreted clinical and pathological data, and drafted the manuscript.
Data Availability Statement
Individual participant data that underlie the results reported in this article will only be available after de-identification upon justified request addressed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Burkhard-Koren NM, Haberecker M, Maccio U, Ruschitzka F, Schuepbach RA, Zinkernagel AS, et al. Higher prevalence of pulmonary macrothrombi in SARS-CoV-2 than in influenza A: autopsy results from “Spanish flu” 1918–1919 in Switzerland to coronavirus disease 2019. J Pathol Clin Res. 2021;7((2)):135–43. doi: 10.1002/cjp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S, Philip AT, Stoppacher R. Pathologic findings in novel influenza A (H1N1) virus (“Swine Flu”) infection: contrasting clinical manifestations and lung pathology in two fatal cases. Am J Clin Pathol. 2010;133:380–7. doi: 10.1309/AJCPXY17SULQKSWK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu A, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57:260–5. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, et al. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, et al. Postmortem findings associated with SARS-CoV-2: systematic review and meta-analysis. Am J Surg Pathol. 2021 doi: 10.1097/PAS.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 9.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–91. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo RB, Botelho BG, Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei SSML, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35((1)):4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020;94:959–69. doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi M, Padovani A, McArthur JC. Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol. 2020;267:1573–6. doi: 10.1007/s00415-020-09896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro CM, Mulvey J, Kubiak J, Mikhail S, Suster D, Crowson AN, et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50:151645. doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24((2)):168–75. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 15.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–2. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skok K, Stelzl E, Trauner M, Kessler HH, Lax SF. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Arch. 2021;478((2)):343–53. doi: 10.1007/s00428-020-02903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolai L, Leunig A, Brambs S, Kaiser R, Joppich M, Hoffknecht ML, et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemost. 2021;19((2)):574–81. doi: 10.1111/jth.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nienhold R, Ciani Y, Koelzer VH, Tzankov A, Haslbauer JD, Menter T, et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun. 2020;11:5086. doi: 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–7. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–82. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SE, Curley GF, Lavin M, Fogarty H, Karampini E, McEvoy NL, et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192((4)):714–9. doi: 10.1111/bjh.17273. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Babka AM, Kearney BJ, Radoshitzky SR, Kuhn JH, Zeng X. Molecular detection of SARS-CoV-2 in formalin-fixed, paraffin-embedded specimens. JCI Insight. 2020;5((12)):e139042. doi: 10.1172/jci.insight.139042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel A, et al. Electron microscopy of SARS-CoV-2: a challenging task: authors' reply. Lancet. 2020;395:e100. doi: 10.1016/S0140-6736(20)31185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–96. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henkel M, Weikert T, Marston K, Schwab N, Sommer G, Haslbauer J, et al. Lethal COVID-19: radiologic-pathologic correlation of the lungs. Radiol Cardiothorac Imaging. 2020;2((6)):e200406. doi: 10.1148/ryct.2020200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meizlish ML, Pine AB, Goshua G, Chang CH, Zhang H, Bishai J, et al. Circulating markers of angiogenesis and endotheliopathy in COVID-19. MedRxiv. 2020 doi: 10.1177/2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang-Hua Y, Le-Min W, Ai-Bin L, Zhu G, Riquan L, Xu-You Z, et al. Severe acute respiratory syndrome and venous thromboembolism in multiple organs. Am J Respir Crit Care Med. 2010;182:436–7. doi: 10.1164/ajrccm.182.3.436. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477:359–72. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–67. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan R, Rosoman NP, Henshaw DJE, Noble EP, Georgius P, Sommerfeld N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med Hypotheses. 2020;144:110024. doi: 10.1016/j.mehy.2020.110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, et al. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One. 2012;7:e47323. doi: 10.1371/journal.pone.0047323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumikoshi M, Hashimoto K, Kawasaki Y, Sakuma H, Suzutani T, Suzuki H, et al. Human influenza virus infection and apoptosis induction in human vascular endothelial cells. J Med Virol. 2008;80:1072–8. doi: 10.1002/jmv.21185. [DOI] [PubMed] [Google Scholar]
- 37.Massoth LR, Desai N, Szabolcs A, Harris CK, Neyaz A, Crotty R, et al. Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS-CoV-2 in human tissues. Am J Surg Pathol. 2021;45:14–24. doi: 10.1097/PAS.0000000000001563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
Individual participant data that underlie the results reported in this article will only be available after de-identification upon justified request addressed to the corresponding author.