Abstract
It has been hypothesized that solar and geomagnetic activity can affect the function of the autonomic nervous system (ANS) and melatonin secretion, which both may influence immune response. We investigated the association between solar geomagnetic activity and white blood cell counts in the Normative Aging Study (NAS) Cohort between 2000 and 2013. Linear mixed effects models with moving day averages ranging from 0 to 28 days were used to evaluate the effects of solar activity measures, Interplanetary Magnetic Field (IMF), and Sunspot Number (SSN), and a measure of geomagnetic activity, Kp Index (Kp), on total white blood cell (WBC), neutrophil, monocytes, lymphocyte, eosinophil, and basophil concentrations. Even after adjusting for demographic and health related factors, there were consistent significant associations between IMF, SSN, and Kp index, with reduced total WBC, neutrophils, and basophil counts that were stronger with longer moving averages. The associations were similar after adjusting for ambient air particulate pollution. Our findings suggest that periods of increased solar and geomagnetic activity result in lower WBC, neutrophil and basophil counts that may contribute to slight immune suppression.
Keywords: Solar activity, Geomagnetic activity, Leukocytes, Immune, White Blood Cell, Air Pollution
1. Introduction
Solar activity encompasses the quasi-periodic oscillation of all electromagnetic radiation emitted from the Sun to the space environment and Earth (Mendoza & Sánchez de la Peña, 2010). Alterations in solar intensity, magnetic field fluctuation, and solar winds modulate disturbances of the Earth’s magnetic field, influencing biological and physiological responses of terrestrial life (Anderson, 1992; Barkhatov et al., 2008). Studies suggest variations in solar activity and Earth’s magnetic field disturbances (or geomagnetic disturbances) may influence the function of the autonomic nervous system (ANS) through the disruption of the circadian rhythms (Breus et al., 1995; McCraty et al., 2017). The ANS is tied to immune signaling as neurotransmitters released by the sympathetic and parasympathetic systems induce peripheral immune modulatory responses through receptors on immune cells (Kenney & Ganta, 2014). ANS dysregulation also plays a role in peripheral white blood cell count changes suggesting that solar and geomagnetic activity may influence circulating white blood cell and differential count (Scheiermann et al., 2013).
Circulating leukocytes include neutrophils, lymphocytes, monocytes, eosinophils, and basophils that are derived from hematopoietic stem cells found in bone marrow. Depending on cell type, peripheral leukocytes may reflect bone marrow and systemic response to infection or response to an allergen or medication. Hematopoietic malignancies, pre-malignant conditions, and viral infections can disrupt bone marrow function that may be reflected in circulating white blood cell and differential counts (Chandran et al., 2015). Few studies have investigated associations between solar or geomagnetic activity and immune response. Stoupel et al. (1995) compared immunoglobulin (IgA, IgG and IgM) levels during solar minimum and maximum, noting a decrease in all three immunoglobulin levels as solar maximum was reached. A follow up study showed variations in autoimmune disease biomarkers including anticardiolipin and lupus anticoagulant with changes in geomagnetic activity, showing possible clinical implications related to geomagnetic storms (Stoupel et al., 2006). Interactions with solar and geomagnetic activity also occur beyond the boundaries of Earth’s atmosphere. Astronauts experience higher exposures to solar activity from space weather and the responses observed provide insight for potential exposure relationships. Stowe et al. (1999) explored the relationship between short-term spaceflight and changes in neutrophil and lymphocyte counts. Leukocyte subset changes attributable to sympathetic nervous system effects were also observed among astronauts, even after adjusting for length of spaceflight (Stowe et al., 2003).
In this study we investigated the effects of solar [interplanetary magnetic field (IMF), sunspot number (SSN)] and geomagnetic activity [Kp Index] on immune dysregulation by evaluating changes in peripheral leukocytes and differential counts in a cohort of elderly healthy males living in the Greater Boston Area, Massachusetts, USA. We also assessed these effects after adjustment for fine particulate matter, particle number, black carbon, and particle radioactivity (i.e. radioactivity associated with ambient particles). To the best of our knowledge, this is the first epidemiologic investigation to provide a comprehensive assessment of the association between solar activity and peripheral white blood cell and differential count in an aging cohort.
2. Methods
2.1. Study Populations
Study participants were members of the Normative Aging Study (NAS) cohort, a closed cohort established by the U.S. Department of Veterans Affairs in 1963 (Bell et al., 1966). The NAS longitudinal study recruited men from the greater Boston area. At the beginning of the study, the participants’ mean age was 42 years (range 21–81 years). All participants were free of chronic conditions at baseline and attended clinical physical examinations every 3 to 5 years. The study was approved by the Institutional Review Boards of Harvard School of Public Health and the VA Boston Healthcare System. All cohort members provided written informed consent prior to the start of the study. Participants underwent clinical examinations under conditions of abstaining from smoking and an overnight fast. At each visit occurring between 2000 and 2013, patients had a full physical and laboratory analysis. Participants with an ever diagnosis or cause of death from any hematologic cancers (ICD-9 Codes 200 to 208) were excluded from the study, as these cancer diagnosis or treatment methodologies may effect WBC differential values (de Jonge et al., 2011). In the final analysis, 728 subjects with 2,048 total visits were eligible for inclusion.
2.2. Questionnaire assessment
Standardized questionnaires were administered to NAS cohort members at each visit to collect information about their health and behaviors. The comprehensive questionnaire assessed the participant’s complete medical history, family medical history, social behaviors (smoking and alcohol consumption), medications, food consumption frequency and quantitative health evaluation measures (height and weight). A full list of parameters has been previously described elsewhere (Bell et al., 1966; Hu et al., 1996)
2.3. Blood measurements
Following an overnight fasting period, venous blood samples were collected from all participants attending clinical visits. Differential and total WBC counts were analyzed using a Sysmex XN-10™ system in the VA Boston hematology laboratory (De Labry et al., 1990). High sensitivity CRP was determined using an immunoturbidimetric assay on the Roche Cobas 6000 system (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from Roche. This high-sensitivity assay has a limit of detection of 0.03 mg/L.
2.4. Solar activity exposures
Measures of SSN, IMF, and Kp are proxy measures to quantify solar activity and geomagnetic activity. IMF captures the intensity of the sun’s magnetic field, a component of solar activity. SSN provides insights on the timepoint in the solar cycle, another aspect of solar activity. Kp represents disturbances is Earth’s magnetic field and geomagnetic activity. The Earth’s and Sun’s magnetic fields merge at a point called the magnetopause, where under specific conditions the IMF can affect the Earth’s magnetic field potentially generating geomagnetic disturbances (Lugaz, 2019). These three variables together constitute a larger picture of the interactions between the Sun and the Earth, providing additional data to quantify human health impacts.
SSN shown on the National Aeronautics and Space Administration (NASA) Goddard Space Flight Center’s OMNIWeb2 website was obtained from the Belgium SILSO Center. SSN is a parameter used as a proxy for solar activity (Hathaway, 2015). Sunspots are dark areas with strong magnetic fields on the Sun’s surface and have a diameter ranging from 3,500 to 6,000 km (Solanki, 2003). Over ~11-year solar cycle periods, SSN rapidly increases and slowly falls throughout the solar cycle (Hathaway, 2015). The solar maximum is defined as the point of highest sunspot activity.
IMF is a direct measure of the portion of the Sun’s magnetic field that is carried by solar wind into interplanetary space. IMF measures presented on the NASA Goddard Space Flight Center’s OMNIWeb website were obtained using numerous spacecraft measurements. Total IMF intensity [expressed in nanotesla (nT)] measures the combined IMF strength for all directions.
Kp index is a parameter used to quantify geomagnetic activity. High fluctuations in geomagnetic activity are called geomagnetic disturbances (GMD), which mostly result from increases in solar activity. Kp index is measured on a 0 to 9 scale, where zero is interpreted as very little activity and 9 is extreme geomagnetic storms. Kp index was calculated as the average of minute-by-minute data samples taken over a three-hour period. Kp index values presented on the NASA Goddard Space Flight Center’s OMNIWeb website were obtained from the German Research Centre for Geosciences. The Kp index was measured using NASA’s mapping of standard daily Kp index.
2.5. Air pollution data
Daily concentrations of PM2.5 in μg/m3 (particulate matter ≤2.5 μm in diameter) were measured using a Harvard impactor (Blomberg et al., 2020; Vieira et al., 2020). Continuous measurements of Black Carbon (BC) in μg/m3 were analyzed using an Aethalometer (Magee Scientific Company, model AE-16, Berkeley, CA). Continuous measurements of particle number (PN) concentrations were analyzed using a condensation particle counter (CPC, TSI Inc. Model 3022a, Shoreview, MN). All measurements were taken at the Harvard Supersite, located on the roof of the Harvard Medical School’s Countway Library of Medicine. This site is approximately three miles from downtown Boston and approximately 1 km from the Hospital were NAS cohort members were examined.
2.6. Particle Radioactivity
Particle gross β-activity levels were obtained from the U.S. EPA RadNet monitoring network. The network includes 135 sampling stations, which are equipped with high-volume air samplers. Total Suspended Particles (TSP) were collected on filters (10-cm-diameter synthetic fiber). Particle gross β activity was measured 5 hours after the end of sampling to allow for decay of short-lived radon progeny. Moving averages of samples over multiple days were calculated. Particle gross β-activity exposures (mBq/m3) were estimated using data from the Boston, MA, RadNet site. These values were then imputed to generate a daily Log(β) activity variable (Blomberg et al., 2020; Nyhan et al., 2018).
2.7. Weather parameters
Daily local weather data, including ambient temperature (°C) and relative humidity (%) were obtained from the National Oceanic Atmospheric Administration’s, National Climatic Data Center (NOAA).
2.8. Exposure Windows
Short- and medium-term exposure windows were calculated for SSN, IMF, and Kp index for using moving averages for day 0 (the day of visit), and up to 28 days after the visit. As solar activity occurs far from the Earth’s surface there is an expected lag between the solar event occurrence and the exposure metrics. The exposure window was limited to one month to eliminate potential confounding by seasonal changes in solar exposures or additional exposures.
2.9. Statistical Analysis
The effects of solar activity parameters and immune outcomes were estimated using mixed-effects linear models with a random intercept for each participant to account for longitudinal correlation and multiple measurements for each participant at different visits. All models were adjusted for the following variables: day of year (DOY), day of the week (DOW), ambient temperature (° C), relative humidity (%), and seasonality3. We also included the following individual predictors: age at time of sample, estimated glomerular filtration rate (eGFR) based on the chronic kidney disease epidemiology collaboration equation (CKD-EPI)4, and pack years of smoking cigarettes. Categorial variables included in the model consisted of: race (white or non-white), lung diseases5 (yes versus no), diabetes mellitus (yes versus no), history of any chronic heart disease (yes versus no), smoking history (categorical: never, current and former), and two or more alcoholic drinks per day (yes versus no) (Levey et al., 2009). A sensitivity analysis was performed excluding samples with CRP levels greater than 10 mg/L and samples where a CRP measurement was not performed to assess if observed associations remained consistent. An additional sensitivity analysis was performed excluding any participant visits that took place outside of Massachusetts. To ensure consistency of results an additional sensitivity analysis was performed using two categorical solar variable assignments. The first category consisted of solar exposures below the median for each moving day average and the second category consisted of solar exposures above the median for each moving day average. Each of the 28 moving day averages had a different median values so categorical assignments were conducted based on moving day averages for each of the solar variables
In post-hoc analysis, we additionally adjusted our primary model for air pollution and radioactivity variables, one at a time to understand the potential effects of these environmental exposures on the solar activity and immune response relationship. This was determined by first running a mixed linear model separately for PM2.5, BC, PN, or Log(β) estimating effects on immune cell counts using up to 28-day moving averages without including solar radiation variables. This was done to determine the most significant moving day average for each air pollution variable, that would be adjusted for in the final models. The moving day average with the lowest p-value for each air pollution variable was selected for each individual immune outcome. The air pollutant moving day averages, effect estimates, and associated p-values are shown in table S1.
The generic structure of the post-hoc model can be represented as:
Where for each individual i and visit j; B0 is the model intercept, b0i is the random intercept for each subject, B1 is the coefficient for each solar activity parameter included one at a time (lagged up to 28-days moving average), B2 is a the coefficient for air pollution and radioactivity variables included one at a time (each with a selected moving average lag), B3 and B4 are coefficients for temperature and relative humidity, B5 to B7 are coefficients for seasonal variables, B8 to 17 are vectors of coefficients for 9 individual-level factors, and eij is the residual error.
The results are presented as cell count per interquartile range (IQR) increase in solar activity variables. The residuals of models were examined for potential deviations from linearity. We chose compound-symmetry variance-covariance structure to handle potential of unbalanced longitudinal data. All analyses were performed using R software version 3.6.3.
3. Results
3.1. Descriptive Results
The descriptive statistics of the NAS Cohort study subjects for all visits and for the first visit are shown in Table 1. There were 728 individual subjects, with 2,048 visits between 2000 and 2013. Of the 728 subjects, 728 presented for one visit, 537 presented for 2 visits, 413 presented for 3 visits, 286 presented for 4 visits, 87 presented for 5 visits, and 2 presented for 6 visits. At baseline, the mean age was 75.8 (±6.9) years and 98% of participants were white. The study population had a mean body mass index of 27.9 (±4.1) kg/m3. Of the 728 individuals 222 (30.5%) never smoked, and 590 (81.0%) had less than two drinks per day. Over all visits the study population had a mean total WBC count of 6,347 (±1,854) thousands/mm3, a mean absolute neutrophil count of 3,986 (±1,312) thousands/mm3, a mean absolute monocyte count of 542 (±169) thousands/mm3, a mean absolute lymphocyte count of 1,575 (±1,042) thousands/mm3, a mean absolute eosinophil count of 214 (±154) thousands/mm3, and a mean absolute basophil count of 38.0 (±35.2) thousands/mm3. These values for absolute peripheral leukocytes counts are within the normal range and do not suggest a baseline hyper or hypo-inflammatory state.
Table 1:
Characteristics of the study population in the Normative Aging Study and weather (2000–2013)
| All Visits | Visit 1 | |
|---|---|---|
| N | N | |
| Total Unique Observations | 2,048 | 728 |
| Total Unique IDs | 728 | 728 |
| Mean ± SD | Mean ± SD | |
| Age (y) | 75.8 ± 6.9 | 73.0 ± 6.9 |
| BMI (kg/m3) | 27.9 ± 4.1 | 28.2 ± 4.0 |
| Cigarette Pack years (y) | 20.3 ± 24.0 | 21.6 ± 25.6 |
| Estimated Glomerular Filtration Rate (mL/min/1.73m2) | 68.1 ± 16.1 | 71.3 ± 15.6 |
| Relative Humidity (%) | 68.0. ± 17.4 | 69.6 ± 17.4 |
| Ambient Temperature (°C) | 12.8 ± 8.8 | 12.8 ± 8.4 |
| White Blood Cells (cells/mm3) | 6,347 ± 1,854 | 6,313 ± 2,109 |
| Neutrophils (cells/mm3) | 3,986 ± 1,312 | 3,934 ± 1,294 |
| Monocytes (cells/mm3) | 542 ± 169 | 535 ± 163 |
| Lymphocytes (cells/mm3) | 1,575 ± 1,042 | 1,604 ± 1,423 |
| Eosinophils (cells/mm3) | 214 ± 154 | 209 ± 141 |
| Basophils (cells/mm3) | 38.0 ± 35.2 | 33.9 ± 32.5 |
| n(%) | n(%) | |
| Race | ||
| White | 1986 (98.0) | 707 (98.1) |
| Nonwhite | 42 (2.0) | 14 (1.9) |
| Coronary Heart Disease | 717 (35.0) | 218 (29.9) |
| Diabetes | 223 (10.9) | 65 (8.9) |
| <Two Drinks/Day | 1,674 (81.7) | 590 (81.0) |
| Smoking Status | ||
| Current | 87 (4.3) | 35 (4.8) |
| Former | 1,318 (64.4) | 471 (64.7) |
| Never | 643 (31.4) | 222 (30.5) |
| Lung Function | ||
| Diagnosis of chronic bronchitis, emphysema or asthma | 370 (18.1) | 133 (18.3) |
| No diagnosis of chronic bronchitis, emphysema or asthma | 1,678 (81.9) | 595 (81.7) |
The descriptive statistics of the solar activity measures (IMF and SSN) and geomagnetic disturbances (Kp) are shown in Table 2. During the dates of analysis, the mean IMF was 5.9 (±3.0) nT, the mean Kp Index was 19.1 (±12.2), and the mean SSN were 85.88 (±74.6). Over the course of the study period solar activity and geomagnetic disturbances vary due to both the 11-year solar cycle and the location of Earth. During the years 2000 to 2013 maximum IMF of 29.2 nT and the KP maximum of 80.9 occurred on October 10, 2003. The maximum SSN of 351.5 occurred on July 19, 2000. Variations in solar activity and geomagnetic disturbances are shown in Figure 1.
Table 2:
Distribution of solar activity measures, particulate air pollution variables, and β activity (2000–2013)
| IQR | ||||
|---|---|---|---|---|
| IMF (nT) | 5.9 ± 3.0 | 1.8 | 29.2 | 3.0 |
| Kp Index* | 19.1 ± 12.2 | 0.0 | 80.9 | 16.2 |
| SSN | 85.8 ± 74.6 | 0.0 | 351.5 | 117 |
| PM2.5 (μg/m3) | 9.9 ± 6.6 | 1.1 | 58.4 | 6.6 |
| Black Carbon (μg/m3) | 0.8 ± 0.4 | 0.2 | 2.5 | 0.5 |
| Particle Number (#/cm3) | 21,978 ± 12,163 | 3,447 | 92,400 | 16137.9 |
| Log β Activity* | −5.9 ± 0.3 | −5.9 | −4.1 | 0.4 |
Dimensionless
Figure 1:

Variations in solar variables over the 13 year study period
3.2. Associations of Solar and Geomagnetic Activity Variables and Immune Outcomes
There was a significant inverse association between solar and geomagnetic activity variables (IMF, SSN, and Kp index) and absolute total WBC, NEUT, and BASO counts. With greater measures of solar and geomagnetic activity there were lower concentrations of total WBC, NEUT, and BASO counts. The largest magnitude of effect was observed in models with IMF as the exposure. While still significant, SSN and Kp index had smaller effect estimates per IQR of exposure for total WBC, absolute NEUT, and absolute BASO counts. Trends showed a greater magnitude of effect over time with greater effects for longer moving day averages. All observed trends and associations remained consistent after adjusting for ambient air pollution, based on the most significant air pollution moving day average. A full listing of leukocyte results is shown in Tables 3, 4, and 5. The sensitivity analysis excluding CRP >10 and no CRP, as well as visits taking place outside of Massachusetts did not change the magnitude or direction of association.
Table 3:
Changes in WBC (cells/mm3) associated with IQR increases in exposure variables (95% CI) for 2048 clinical visits (n=728)
| Exposure | Moving Average | No Air Pollution Adjustment | PM2.5 | BC | PN | Log β |
|---|---|---|---|---|---|---|
| IMF(nt) | 0 | −72.472(−198.576 , 53.633) | −63.263(−189.528 , 63.862) | −77.461(−233.921 , 48.999) | −29.656(−177.776 , 118.464) | −63.468(−183.502 , 56.566) |
| 1 | −83.801(−234.613 , 66.415) | −75.383(−225.742 , 74.976) | −82.494(−233.432 , 68.343) | −45.085(−211.924 , 119.854) | −79.537(−222.461 , 63.388) | |
| 7 | −200.066(−439.661 , 39.528) | −188.557(−428.955 , 51.798) | −177.945(−418.479 , 62.578) | −102.601(−368.196 , 162.994) | −131.081(−362.323 , 151.161) | |
| 14 | −165.750(−446.462 , 114.962) | −154.732(−437.422 , 127.958) | −124.189(−487.649 , 159.229) | −33.307(−357.486 , 283.874) | −114.357(−387.669 , 158.894) | |
| 21 | −201.607(−542.916 , 99.691) | −184.116(−487.824 , 119.592) | −145.379(−449.976 , 159.219) | −86.020(−427.971 , 255.932) | −180.231(−473.972 , 113.568) | |
| 28 | −206.012(−515.592 , 193.067) | −186.701(−497.896 , 124.495) | −141.134(−454.376 , 172.147) | −105.385(−459.485 , 248.715) | −181.142(−483.638 , 121.355) | |
| Kp | 0 | −1.569(−4.642 , 5.952) | −1.367(−3.854 , 1.121) | −1.523(−3.991 , 5.944) | −0.976(−3.591 , 1.639) | −1.316(−3.665 , 1.233) |
| 1 | −1.769(−4.528 , 4.989) | −1.619(−4.393 , 1.156) | −1.657(−4.457 , 1.892) | −1.144(−3.998 , 1.712) | −1.391(−4.117 , 1.228) | |
| 7 | −1.479(−5.699 , 2.742) | −1.330(−5.568 , 2.948) | −1.058(−5.261 , 3.144) | 0.107(−4.286 , 4.501) | −0.766(−4.798 , 3.265) | |
| 14 | −0.744(−5.688 , 4.199) | −0.594(−5.555 , 4.366) | −0.338(−5.263 , 4.587) | 0.048(−5.163 , 5.258) | −0.420(−5.181 , 4.345) | |
| 21 | −2.784(−8.576 , 2.536) | −2.741(−8.984 , 2.649) | −2.130(−7.418 , 3.149) | −1.939(−7.465 , 3.587) | −2.524(−7.648 , 2.599) | |
| 28 | −2.811(−8.326 , 2.753) | −2.779(−8.363 , 2.854) | −2.076(−7.582 , 3.431) | −1.710(−7.462 , 4.242) | −2.167(−7.512 , 3.179) | |
| SSN | 0 | −0.439(−7.855 , −0.682) | −0.421(−4.783 , −6.568) | −0.342(−8.715 , 1.739) | −0.570(−8.986 , −0.156) | −0.447(−5.791 , −9.194) |
| 1 | −0.432(−1.794 , −1.577) | −0.412(−1.776 , −7.349) | −0.334(−7.779 , 7.446) | −0.554(−9.971 , −9.139) | −0.440(−5.786 , −5.395) | |
| 7 | −0.414(−3.793 , −1.736) | −0.397(−5.777 , −5.118) | −0.310(−6.792 , 9.988) | −0.498(−1.932 , −8.464) | −0.433(−3.795 , −5.472) | |
| 14 | −0.405(−0.798 , −8.812) | −0.382(−9.777 , 1.211) | −0.298(−0.736 , 8.118) | −0.483(−2.938 , −5.528) | −0.416(−4.793 , −7.447) | |
| 21 | −0.445(−6.851 , −1.546) | −0.412(−1.819 , −3.255) | −0.325(−3.747 , 0.097) | −0.544(−1.319 , −1.374) | −0.455(−2.844 , −3.566) | |
| 28 | −0.428(−6.841 , −7.116) | −0.390(−0.854 , 4.824) | −0.296(−1.726 , 5.134) | −0.525(−1.009 , −0.841) | −0.435(−0.831 , −7.545) |
All models were adjusted for seasonality based on day of year (DOY), DOW, ambient temperature, relative humidity, age, race, eGFR, lung diseases, diabetes mellitus, history of any chronic heart disease, smoking history, two or more alcoholic drinks per day, pack years of smoking cigarettes, ambient air pollutant exposures (no air pollution, PM2.5, BC, PN, or Log β) and solar variable (IMF(nt), Kp or SSN)
Table 4:
Changes in NEUT (cells/mm3) associated with IQR increases in exposure variables (95% CI) for 2048 clinical visits (n=728)
| Exposure | Moving Average | No Air Pollution Adjustment | PM2.5 | BC | PN | Log β |
|---|---|---|---|---|---|---|
| IMF(nt) | 0 | −90.568(−188.841 , 7.773) | −83.192(−181.644 , 15.261) | −90.870(−189.559 , 7.819) | −57.382(−158.789 , 44.125) | −69.220(−168.361 , 29.925) |
| 1 | −100.429(−217.335 , 16.477) | −92.680(−269.773 , 24.411) | −95.471(−212.948 , 22.316) | −71.436(−192.986 , 54.113) | −74.015(−191.956 , 43.926) | |
| 7 | −194.350(−378.577 , −18.125) | −178.754(−364.116 , 6.521) | −168.813(−354.749 , 17.124) | −129.618(−336.863 , 71.626) | −136.604(−325.571 , 51.792) | |
| 14 | −207.210(−421.576 , 7.584) | −184.863(−431.518 , 31.791) | −163.683(−381.964 , 54.598) | −117.792(−367.877 , 125.292) | −126.808(−347.938 , 94.321) | |
| 21 | −246.131(−475.341 , −16.921) | −220.306(−452.398 , 11.786) | −191.817(−425.836 , 42.222) | −169.096(−433.136 , 94.945) | −158.682(−395.437 , 78.273) | |
| 28 | −262.875(−497.562 , −28.199) | −233.778(−471.292 , 3.734) | −201.956(−442.488 , 38.576) | −196.781(−469.627 , 76.745) | −171.720(−415.664 , 71.623) | |
| Kp | 0 | −1.560(−3.499 , 2.378) | −1.453(−3.464 , 4.497) | −1.483(−3.424 , 2.458) | −1.197(−3.192 , 8.796) | −1.175(−3.129 , 2.779) |
| 1 | −1.635(−3.798 , 4.527) | −1.558(−3.733 , 0.617) | −1.482(−3.644 , 5.679) | −1.396(−3.638 , 1.837) | −1.282(−3.461 , 8.896) | |
| 7 | −2.261(−5.544 , 1.022) | −2.087(−5.388 , 1.214) | −1.786(−5.669 , 1.496) | −1.272(−4.713 , 2.159) | −1.626(−4.954 , 1.712) | |
| 14 | −2.823(−6.659 , 1.911) | −2.517(−6.376 , 1.349) | −2.251(−6.393 , 1.596) | −2.012(−6.044 , 2.228) | −1.724(−5.644 , 2.195) | |
| 21 | −4.431(−8.525 , −1.343) | −4.201(−8.338 , −3.564) | −3.693(−7.798 , 5.411) | −3.922(−8.243 , 8.398) | −3.225(−7.429 , 9.977) | |
| 28 | −4.454(−8.732 , −7.207) | −4.261(−8.568 , 5.745) | −3.653(−7.922 , 4.615) | −3.891(−8.413 , 8.621) | −3.124(−7.492 , 1.243) | |
| SSN | 0 | −0.328(−7.655 , −6.252) | −0.296(−8.576 , −4.018) | −0.244(−9.534 , 0.244) | −0.330(−5.665 , −4.001) | −0.260(−4.548 , 3.919) |
| 1 | −0.316(−8.595 , −1.738) | −0.283(−8.564 , −8.923) | −0.231(−2.522 , 9.962) | −0.318(−2.649 , 5.013) | −0.247(−9.529 , 4.333) | |
| 7 | −0.302(−4.593 , −8.512) | −0.268(−4.561 , 5.224) | −0.210(−1.514 , 9.193) | −0.276(−9.629 , 7.375) | −0.225(−8.525 , 6.768) | |
| 14 | −0.291(−1.593 , 7.411) | −0.252(−6.557 , 8.352) | −0.193(−5.511 , 3.123) | −0.256(−7.629 , 2.115) | −0.204(−3.511 , 0.142) | |
| 21 | −0.308(−6.629 , 1.373) | −0.263(−5.577 , 2.051) | −0.198(−9.527 , 6.129) | −0.295(−7.685 , 1.693) | −0.220(−4.537 , 3.696) | |
| 28 | −0.301(−5.618 , 3.915) | −0.252(−5.572 , 4.267) | −0.182(−1.516 , 1.152) | −0.292(−5.698 , 6.145) | −0.211(−9.533 , 3.179) |
All models were adjusted for seasonality based on day of year (DOY), DOW, ambient temperature, relative humidity, age, race, eGFR, lung diseases, diabetes mellitus, history of any chronic heart disease, smoking history, two or more alcoholic drinks per day, pack years of smoking cigarettes, ambient air pollutant exposures (no air pollution, PM2.5, BC, PN, or Log β) and solar variable (IMF(nt), Kp or SSN)
Table 5:
Changes in BASO (cells/mm3) associated with IQR increases in exposure variables (95% CI) for 2048 clinical visits (n=728)
| Exposure | Moving Average | No Air Pollution Adjustment | PM2.5 | BC | PN | Log β |
|---|---|---|---|---|---|---|
| IMF(nt) | 0 | −5.459(−8.729 , −2.189) | −5.235(−8.699 , −1.771) | −5.293(−8.594 , −1.997) | −4.075(−7.478 , −8.674) | −5.782(−9.699 , −2.466) |
| 1 | −8.348(−12.225 , −4.471) | −8.193(−12.157 , −4.228) | −8.151(−12.963 , −4.241) | −6.477(−13.594 , −2.361) | −8.830(−12.767 , −4.894) | |
| 7 | −13.504(−19.524 , −7.483) | −11.756(−17.913 , −5.642) | −12.190(−18.336 , −6.551) | −9.311(−16.137 , −2.485) | −14.593(−23.813 , −8.384) | |
| 14 | −16.010(−22.947 , −9.173) | −13.474(−22.656 , −6.293) | −14.251(−21.369 , −7.133) | −10.840(−19.051 , −2.629) | −17.675(−24.997 , −14.445) | |
| 21 | −17.605(−24.994 , −11.216) | −14.875(−22.586 , −7.163) | −15.949(−23.536 , −8.361) | −11.729(−23.657 , −2.862) | −19.653(−27.367 , −11.946) | |
| 28 | −17.503(−25.761 , −9.947) | −14.622(−22.521 , −6.724) | −15.741(−23.494 , −7.987) | −11.085(−21.295 , −1.885) | −19.823(−27.748 , −11.899) | |
| Kp | 0 | −0.132(−8.198 , −8.668) | −0.127(−8.195 , −3.861) | −0.127(−0.192 , −8.962) | −0.115(−7.183 , −3.249) | −0.135(−0.201 , −3.969) |
| 1 | −0.154(−5.227 , −7.282) | −0.147(−1.221 , −6.173) | −0.150(−2.223 , −4.878) | −0.128(−6.244 , −1.653) | −0.157(−7.231 , −5.585) | |
| 7 | −0.235(−7.344 , −4.127) | −0.197(−7.311 , −1.785) | −0.219(−0.329 , −3.139) | −0.179(−0.294 , −4.164) | −0.240(−5.351 , −5.131) | |
| 14 | −0.263(−1.389 , −0.137) | −0.217(−0.349 , −1.485) | −0.243(−3.371 , −7.116) | −0.194(−5.329 , −3.767) | −0.279(−0.439 , −6.149) | |
| 21 | −0.291(−0.425 , −5.157) | −0.245(−5.387 , −0.134) | −0.269(−4.435 , −3.134) | −0.207(−1.351 , −1.163) | −0.318(−4.458 , −4.179) | |
| 28 | −0.304(−9.444 , −1.166) | −0.259(−1.446 , −6.112) | −0.282(−7.423 , −3.142) | −0.209(−6.368 , −1.564) | −0.332(−5.477 , −1.188) | |
| SSN | 0 | −0.016(−2.125 , −0.807) | −0.015(−0.024 , −4.506) | −0.013(−9.123 , −7.475) | −0.007(−0.618 , 6.234) | −0.017(−1.126 , −2.398) |
| 1 | −0.016(−2.425 , −3.357) | −0.015(−0.024 , −8.956) | −0.013(−8.323 , −0.614) | −0.006(−9.018 , 6.604) | −0.017(−1.526 , −3.098) | |
| 7 | −0.015(−9.325 , −5.556) | −0.014(−7.124 , −1.765) | −0.013(−2.323 , −6.353) | −0.005(−3.517 , 7.387) | −0.017(−0.927 , −3.817) | |
| 14 | −0.017(−2.427 , −5.307) | −0.015(−7.926 , −6.396) | −0.014(−4.225 , −8.984) | −0.005(−9.519 , 8.157) | −0.018(−5.529 , −1.818) | |
| 21 | −0.018(−7.329 , −5.909) | −0.016(−9.927 , −1.127) | −0.015(−8.326 , −2.285) | −0.007(−3.521 , 6.156) | −0.020(−1.731 , −7.617) | |
| 28 | −0.019(−1.129 , −0.019) | −0.017(−3.228 , −0.697) | −0.016(−0.927 , −7.955) | −0.007(−5.821 , 4.736) | −0.020(−6.131 , −8.510) |
All models were adjusted for seasonality based on day of year (DOY), DOW, ambient temperature, relative humidity, age, race, eGFR, lung diseases, diabetes mellitus, history of any chronic heart disease, smoking history, two or more alcoholic drinks per day, pack years of smoking cigarettes, ambient air pollutant exposures (no air pollution, PM2.5, BC, PN, or Log β) and solar variable (IMF(nt), Kp or SSN)
Mixed relationships were observed for other non-significant blood cell outcomes (MONO, LYMPH, and EOSIN). Inverse associations were observed between IMF, Kp and MONO counts, while a positive associated was seen between SSN and MONO. Positive associations were observed between IMF and Kp and LYMPH counts, while an inverse association was seen between SSN and LYMPH. A positive association was also seen between IMF and EOSIN, as well as with Kp and EOSIN, while an inverse association was seen between SSN and EOSIN. Full results for MONO, LYMPH, and EOSIN are shown in the supplementary materials tables S2, S3, and S4.
The results of the sensitivity analyses of the categorical solar variables are similar in direction and significance to those of the main linear mixed-effects model analysis, but offer a stronger magnitude of effect estimates. The full results of the categorical sensitivity analysis are shown in Tables S5 to.
3.3. White Blood Cells
The associations for WBC followed a downward trend across the 0–28 day moving averages with the largest magnitude of effects coinciding with 28th moving day average. WBC counts decreased by 0.429 cells/mm3 (95% CI: −0.841, −0.016) (p=0.041) for every IQR increase in SSN on the 28th moving day average (Figure 2). While no significant associations were observed between IMF and WBC or KP and WBC similar trends were seen with the largest magnitude of WBC count decreases occurring on the 28th moving day average. IMF and KP both induced a larger magnitude of decrease in total number of WBCs; however, these changes only resulted in significant changes of neutrophils and basophils and not significant changes in the overall total WBC count.
Figure 2:
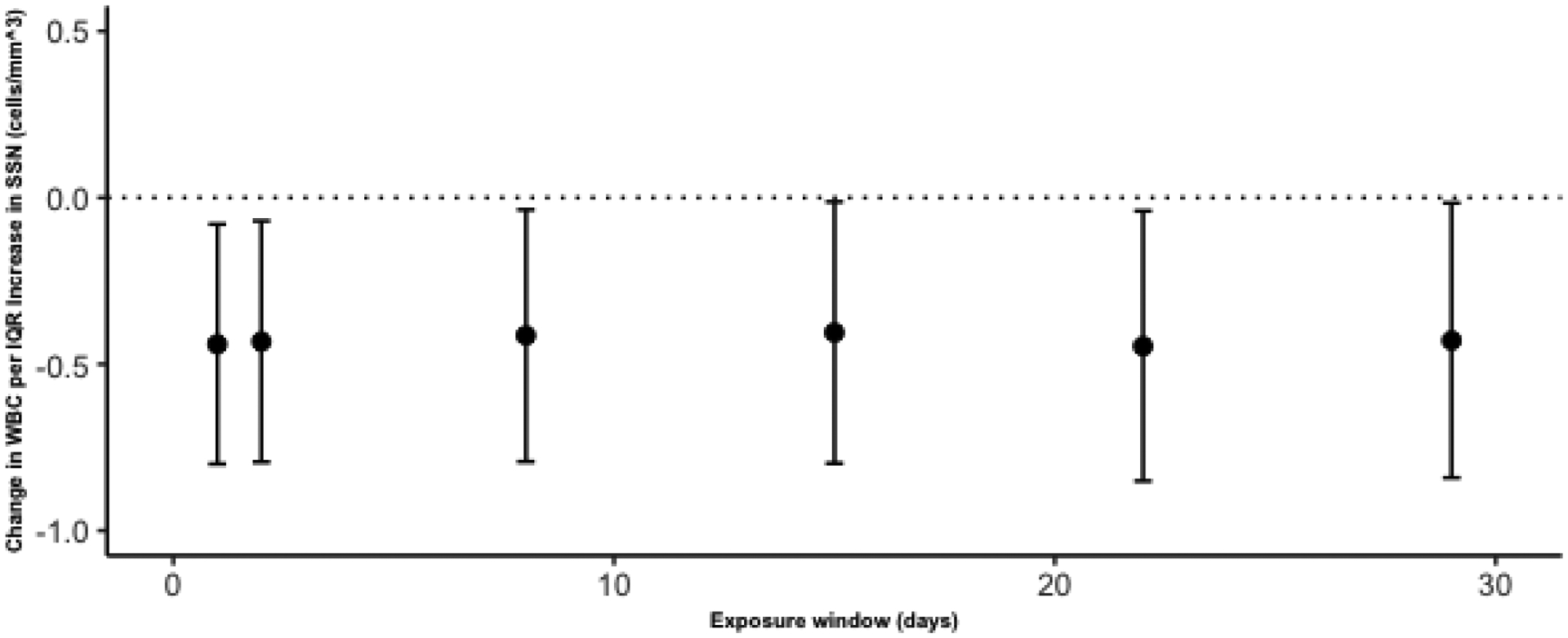
The change in White Blood Cells (WBC) per IQR increases in SSN over moving day average exposure windows of 0, 1, 7, 14, 21 and 28 days
3.4. Granulocytes
The associations for NEUT and BASO followed similar downward trend across the 0–28 day moving averages with the largest magnitude of effects coinciding with 28th moving day average NEUT counts decreased by 262.88 cells/mm3 (95% CI: −497.56, −16.92) (p=0.028) for every IQR increase in IMF on the 28th moving day average. Significant associations were also observed between KP and NEUT concentrations with a 4.45 cells/mm3 (95% CI: −8.70, −0.21) (p=0.039) decrease in NEUT counts per IQR increase in KP on the 28th moving day average (Figure 3).
Figure 3:

The change in Neutrophils (NEUT) per IQR increases in Solar Variables over moving day average exposure windows of 0, 1, 7, 14, 21 and 28 days (y-axis scales vary based on solar variable)
BASO counts decreased by 17.50 cells/mm3 (95% CI: −25.06, −9.95) (p<0.001) for every IQR increase in IMF on the 28th moving day average. Significant associations were also observed between SSN and BASO concentrations with a 0.019 cells/mm3 (95% CI:−0.029, −0.009) (p<0.001) decrease in BASO counts per IQR increase in SSN on the 28th moving day average. For every IQR increase in Kp Index there was a 0.30 cells/mm3 (95% CI: −0.44, −0.17) (p<0.001) change in BASO counts on the 28th moving day average (Figure 4).
Figure 4:

The change in Basophils (BASO) per IQR increases in Solar Variables over moving day average exposure windows of 0, 1, 7, 14, 21 and 28 days (y-axis scales vary based on solar variable)
4. Discussion
This study demonstrates an association between greater exposure to solar and geomagnetic activity and lower peripheral leukocytes counts, with effects on granulocyte cells types (NEUT, BASO) The largest magnitudes of effect (and lowest standard errors) were observed with greater cumulative exposures as suggested by greater effects seen with longer moving day averages. The findings are consistent with suppression of bone marrow granulocyte precursor cells that ultimately differentiates into neutrophils, basophils, and eosinophils. The decrease in these cell types are consistent with bone marrow effects caused by other exogenous exposures, such as some types of medications (i.e chemotherapy) or radiation exposures (Banfi et al., 2001; Kamiya et al., 2015). Bone marrow effects are also observed in several hematologic conditions, such as myelodysplastic syndrome or multiple myeloma, a diseases more commonly observed in older individuals (Friberg & Karlsson, 2003). Effects of myelosuppression include an increased risk of infection and bleeding complications (Carey, 2003). The association with neutrophils and other granulocytes, which are short-lived cells in comparison to lymphocytes are consistent with the week to month-s long exposure windows we are examining as compared to longer exposure periods. Biological systems and associated immune system responses are known to be influenced by solar and geophysical cycles through the mechanism of melatonin production, circadian rhythm disruption, and autonomic nervous system signaling.
One possible explanation for the effects of solar activity on peripheral absolute leukocyte counts could be due circadian rhythm disruption and ANS dysregulation (Wyse et al., 2020). The suprachiasmatic nucleus (SCN), part of the ANS situated at the base of the hypothalamus, is the principal circadian pacemaker in the human brain. As such, it regulates circadian rhythms during rest and activity, core body temperature, neuroendocrine function, autonomic function, memory, psychomotor performance, and a host of other behavioral and physiological processes. It is thus conceivable that SCN signaling may also have an impact on immune system regulation (Swaab, 2003).
The ANS and immune system communicate through an integrative interface, both the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS). (Elenkov et al., 2000). Activation of the ANS triggers pro-inflammatory responses, while increases in cytokines also results in increased vagus nerve activity (Ek et al., 1998; Saper et al., 2012). Multiple neural axes bridge the relationship between cytokine production and ANS activation, which in turn modulates the peripheral immune response. Neurotransmitters released by ANS nerve endings bind to receptors on immune cells, initiating an immune response (Kenney & Ganta, 2014). The SNS directly innervates lymphatic organs in signaling an immune response (Friedman & Irwin, 1997). This direct link between the nervous system and immune organs modulates immune response through the binding of norepinephrine, epinephrine, and dopamine to lymphocyte receptors, released by the SNS (Irwin, 1993). Environmental factors modulating changes in either the immune system or the nervous system, can have implications for long-term health including stress, depression, and overall immune function (Haskó & Szabó, 1998). Immune system effects also contribute to overall health via the ANS and SNS alterations resulting in hypertension, heart failure and fever (Ferrario & Strawn, 2006; Francis et al., 2004; Kang et al., 2009; Shi et al., 2010).
The pineal gland, responsible for melatonin secretion, is modulated by, and connected to, the SCN (Borjigin et al., 2012; Swaab, 2003). Melatonin is a biochemical factor modulating control of human circadian rhythms. The primary role of melatonin is synchronizing eukaryotic systems with associated photo periods (Currier et al., 2000). Dysregulation or removal of the pineal gland has a different effect on immune function, leading to an increase in total WBC count (Ana B. Rodriguez & Lea, 1994). Circadian fluctuations imposed by the SCN trigger fluctuations in melatonin secretion, often having direct implications for immune signaling or recruitment of inflammatory markers (A. B. Rodriguez et al., 1999; Scheiermann et al., 2013).
Variations of the geomagnetic field have been documented to induce changes in the biological response of animals and humans (Krylov et al., 2014; Touitou & Selmaoui, 2012). In addition, solar activity and geomagnetic field disturbances may reduce melatonin levels and impair the immune inflammatory responses to oxidative stress (Burch et al., 1999). Previous studies suggest an association between changes in geomagnetic activity and electromagnetic fields, decreased melatonin secretion and adverse physiological responses (Griefahn et al., 2002; Halgamuge, 2013; Weydahl et al., 2001).
Melatonin is a direct actor in immune system response through the stimulation of T-Lymphocytes, interleukin-2 and -6, and natural killer cells (Cherry, 2002; Peña et al., 2007; Szczepanik, 2007). The role of melatonin levels on immune system outcomes is thought to be modulated by cytokines. Melatonin receptors in T-Cells are a part of the melatonin-cytokine cascade, which induces the release of hematopoietic growth factors in bone marrow stimulated by interleukin production (Currier et al., 2000; García-Mauriño et al., 1999; Maestroni, 1995). Melatonin secretion, occurring primarily at night, has been linked both to inflammation and phagocytosis in animal studies (Hriscu, 2005; Terrón et al., 2004). Factors including alcohol, consumption, BMI, and gender differences have also been shown to alter nocturnal melatonin secretion (Cocco et al., 2005). Evidence also suggests that melatonin may delay leukocyte apoptosis and counteract the effects of age related oxidative stress in cells (Espino et al., 2010, 2011). Multiple links between melatonin and innate immune system responses, specifically responses in neutrophils and eosinophils have been investigated, thereby impacting total WBC counts (Calvo et al., 2013).
Neutrophils are a key component of the innate immune system, making up the largest percentage of the total white blood cell count (Kobayashi et al., 2005; Nauseef, 2007). In individuals taking melatonin supplements, an increased neutrophil response was observed along with increased intracellular chemokine expression; however, this response was not observed in monocytes or lymphocytes (Peña et al., 2007). In vitro studies have suggested a potential pathway by which melatonin contributes to the dysregulation of neutrophils, by inhibiting enzymes needed by L-selectin cleavage (Recchioni et al., 1998). L-selectin is a glycoprotein and adhesion molecule expressed on leukocytes, contributing to adhesion, migration, and signal transduction. Interestingly, L-selectin is expressed by all leukocytes except by activated, memory lymphocytes and thus may by an explanation for this observation (Ivetic et al., 2019).
Multiple additional pathways may influence the relationship between solar activity and peripheral leukocyte counts. Electromagnetic fields (EMF) have been shown to alter the action of cell membrane gates and flow through cation channels (Balcavage et al., 1996). Low frequency EMF has been shown to influence the interactions between leukocytes and the vascular endothelium (Ushiyama & Ohkubo, 2004). In mice exposed to low frequency EMF significant decreases in total leukocyte counts were observed (Bonhomme-Faivre et al., 1998). Alterations in membrane gate action coupled with direct EMF influences on leukocytes may also influence the relationship between solar activity and leukocytes leading to the observed effects.
Individual health susceptibility to solar radiation may vary temporally (daily or seasonal) and be influenced by solar activity periods. Furthermore, solar activity also appears to react synergistically with other environmental risk factors, such as ambient air pollution (Grandi & D’Ovidio, 2019). Solar activity is thought to play a major role in the atmospheric photochemical reactions, which affects the formation and concentration of aerosols. This reaction could intensify existing air pollutant concentration, specifically, quantitative measures of ultrafine particles (Ma & Birmili, 2015). Increased solar activity has been associated with increases in PN, with demonstrated seasonal fluxes (Vieira et al., 2020). Ambient air pollution also induces effects on peripheral leukocyte count (Steenhof et al., 2014). However, our results showed consistent pattern despite controlling for air pollutants.
Strengths & Limitations:
Solar activity measures do not account for indoor, medical, or anthropogenic sources of radiation exposure, which may contribute to the noted effects in immune response. This study was also limited to the analysis of one solar cycle, whereas a more robust exposure-outcome pathway would consider the variability in radiation measures between different solar cycles and control for this potential source of bias using data from two solar cycles, over a 22-year time window. In using the established NAS cohort, the results are limited to those observed in white men with an average age of 75.8 years and may not be generalizable to the entire population; however, the use of this limited population also prevents the introduction of additional confounding variables. This paper is among the first to investigate the epidemiological associations between solar activity, geomagnetic activity and peripheral leukocytes in an aging cohort. This research provides a basis for future research on human health effects of continuous environmental exposures.
5. Conclusions
Overall, we found that different parameters representing solar and geomagnetic activity were associated with a significant decrease in absolute white blood cell counts, absolute neutrophil counts, and absolute basophil counts thus alluding to the idea that increased solar and geomagnetic activity may have a generalized immune-suppressive effect. The results of this study represent a novel approach to understanding the implication of solar activity on the immune system. Multiple exposure metrics of solar activity were used to evaluate how variations in activity may impact immune outcomes after controlling for health and lifestyle confounders. Further research into the mechanisms of immune system disruption from solar activity by expanding the analysis to include additional measures of immune response, such as antibodies and interferon. The analysis should also be expanded across a larger population to generalize results.. The immune effects presented here are critical in understanding the totality of effects and potential interactions of solar activity and geomagnetic field fluctuations on human health
Supplementary Material
Acknowledgement
This publication was supported by USEPA grant RD-83479801 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. This study was also supported by NIH NIEHS R01 ES019853 and by resources and the use of facilities at the VA Boston Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
List of Abbreviations
- BMI
Body mass index
- eGFR
Estimated Glomular Filtration Rate
- DOW
Day of Week
- DOY
Day of Year
- PM2.5
Particulate matter with aerodynamic diameter less than or equal to 2.5 μm
- BC
Black carbon
- PN
Particle Number
- lbeta
Log β activity
- IMF
Interplanetary Magnetic Field
- Kp
Kp Index
- SSN
Sunspot Number
- EMF
Electromagnetic Field
- WBC
White blood cells
- MONO
Monocytes
- LYMPH
Lymphocytes
- EOSIN
Eosinophils
- BASO
Basophils
- ANS
Autonomic Nervous System
- SNS
Sympathetic Nervous System
- SCN
Suprachiasmatic Nucleus
- HPA
Hypothalamic-Pituitary-Adrena
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
OMNIWeb Data: https://omniweb.gsfc.nasa.gov/form/dx1.html
Calculated as Sin(2πDOY/365) + Cos(2πDOY/365)
Calculated as: eGFR=141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black]
Lung diseases include ever diagnosis of chronic bronchitis, emphysema or asthma
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anderson RY (1992). Possible connection between surface winds, solar activity and the Earth’s magnetic field. Nature, 358(6381), 51–53. 10.1038/358051a0 [DOI] [Google Scholar]
- Balcavage WX, Alvager T, Swez J, Goff CW, Fox MT, Abdullyava S, & King MW (1996). A Mechanism for Action of Extremely Low Frequency Electromagnetic Fields on Biological Systems. Biochemical and Biophysical Research Communications, 222(2), 374–378. 10.1006/bbrc.1996.0751 [DOI] [PubMed] [Google Scholar]
- Banfi A, Podestà M, Fazzuoli L, Sertoli MR, Venturini M, Santini G, Cancedda R, & Quarto R (2001). High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: A mechanism for post-bone marrow transplantation osteopenia. Cancer, 92(9), 2419–2428. [DOI] [PubMed] [Google Scholar]
- Barkhatov NA, Gromova LI, Levitin AE, & Revunov SE (2008). The relation between solar activity and orientation of the solar wind electric field relative to the Earth’s magnetic moment. Geomagnetism and Aeronomy, 48(6), 713–718. 10.1134/S0016793208060030 [DOI] [Google Scholar]
- Bell B, Rose CL, & Damon A (1966). The Veterans Administration longitudinal study of healthy aging. The Gerontologist, 6(4), 179–184. 10.1093/geront/6.4.179 [DOI] [PubMed] [Google Scholar]
- Blomberg AJ, Nyhan MM, Bind M-A, Vokonas P, Coull BA, Schwartz J, & Koutrakis P (2020). The role of ambient particle radioactivity in inflammation and endothelial function in an elderly cohort. Epidemiology, Publish Ahead of Print. 10.1097/EDE.0000000000001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme-Faivre L, Macé A, Bezie Y, Marion S, Bindoula G, Szekely AM, Frénois N, Auclair H, Orbach-Arbouys S, & Bizi E (1998). Alterations of biological parameters in mice chronically exposed to low-frequency (50 HZ) electromagnetic fields. Life Sciences, 62(14), 1271–1280. 10.1016/S0024-3205(98)00057-5 [DOI] [PubMed] [Google Scholar]
- Borjigin J, Zhang LS, & Calinescu A-A (2012). Circadian Regulation of Pineal Gland Rhythmicity. Molecular and Cellular Endocrinology, 349(1), 13–19. 10.1016/j.mce.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breus TK, Cornélissen G, Halberg F, & Levitin AE (1995). Temporal associations of life with solar and geophysical activity. Annales Geophysicae, 13(11), 1211–1222. 10.1007/s00585-995-1211-8 [DOI] [Google Scholar]
- Burch JB, Reif JS, & Yost MG (1999). Geomagnetic disturbances are associated with reduced nocturnal excretion of a melatonin metabolite in humans. Neuroscience Letters, 266(3), 209–212. 10.1016/S0304-3940(99)00308-0 [DOI] [PubMed] [Google Scholar]
- Calvo JR, González-Yanes C, & Maldonado MD (2013). The role of melatonin in the cells of the innate immunity: A review. Journal of Pineal Research, 55(2), 103–120. 10.1111/jpi.12075 [DOI] [PubMed] [Google Scholar]
- Carey PJ (2003). Drug-Induced Myelosuppression: Diagnosis and Management. Drug Safety, 26(10), 691–706. 10.2165/00002018-200326100-00003 [DOI] [PubMed] [Google Scholar]
- Chandran P, Le Y, Li Y, Sabloff M, Mehic J, Rosu-Myles M, & Allan DS (2015). Mesenchymal stromal cells from patients with acute myeloid leukemia have altered capacity to expand differentiated hematopoietic progenitors. Leukemia Research, 39(4), 486–493. 10.1016/j.leukres.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Cherry N (2002). Schumann Resonances, a plausible biophysical mechanism for the human health effects of Solar. Natural Hazards, 26(3), 279–331. 10.1023/A:1015637127504 [DOI] [Google Scholar]
- Cocco P, Cocco ME, Paghi L, Avataneo G, Salis A, Meloni M, Atzeri S, Broccia G, Ennas MG, Erren TC, & Reiter RJ (2005). Urinary 6-sulfatoxymelatonin excretion in humans during domestic exposure to 50 hertz electromagnetic fields. Neuro Endocrinology Letters, 26(2), 136–142. [PubMed] [Google Scholar]
- Currier NL, Sun LZ-Y, & Miller SC (2000). Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. Journal of Neuroimmunology, 104(2), 101–108. 10.1016/S0165-5728(99)00271-4 [DOI] [PubMed] [Google Scholar]
- de Jonge HJM, Valk PJM, de Bont ESJM, Schuringa JJ, Ossenkoppele G, Vellenga E, & Huls G (2011). Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: Relevance of mutated NPM1 and FLT3-ITD. Haematologica, 96(9), 1310–1317. 10.3324/haematol.2011.040592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Labry LO, Campion EW, Glynn RJ, & Vokonas PS (1990). White blood cell count as a predictor of mortality: Results over 18 years from the normative aging study. Journal of Clinical Epidemiology, 43(2), 153–157. 10.1016/0895-4356(90)90178-R [DOI] [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, & Ericsson A (1998). Activation of Vagal Afferents after Intravenous Injection of Interleukin-1β: Role of Endogenous Prostaglandins. The Journal of Neuroscience, 18(22), 9471–9479. 10.1523/JNEUROSCI.18-22-09471.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, & Vizi ES (2000). The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacological Reviews, 52(4), 595–638. [PubMed] [Google Scholar]
- Espino J, Bejarano I, Paredes SD, Barriga C, Reiter RJ, Pariente JA, & Rodríguez AB (2011). Melatonin is able to delay endoplasmic reticulum stress-induced apoptosis in leukocytes from elderly humans. Age (Dordrecht, Netherlands), 33(4), 497–507. 10.1007/s11357-010-9194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino J, Bejarano I, Redondo PC, Rosado JA, Barriga C, Reiter RJ, Pariente JA, & Rodríguez AB (2010). Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. The Journal of Membrane Biology, 233(1–3), 105–118. 10.1007/s00232-010-9230-0 [DOI] [PubMed] [Google Scholar]
- Ferrario CM, & Strawn WB (2006). Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. The American Journal of Cardiology, 98(1), 121–128. 10.1016/j.amjcard.2006.01.059 [DOI] [PubMed] [Google Scholar]
- Francis J, Chu Y, Johnson AK, Weiss RM, & Felder RB (2004). Acute myocardial infarction induces hypothalamic cytokine synthesis. American Journal of Physiology-Heart and Circulatory Physiology, 286(6), H2264–H2271. 10.1152/ajpheart.01072.2003 [DOI] [PubMed] [Google Scholar]
- Friberg L, & Karlsson M (2003). Mechanistic models for myelosuppression. Investigational New Drugs, 21(2), 183–194. 10.1023/A:1023573429626 [DOI] [PubMed] [Google Scholar]
- Friedman EM, & Irwin MR (1997). Modulation of immune cell function by the autonomic nervous system. Pharmacology & Therapeutics, 74(1), 27–38. 10.1016/s0163-7258(96)00200-8 [DOI] [PubMed] [Google Scholar]
- García-Mauriño S, Pozo D, Carrillo-Vico A, Calvo JR, & Guerrero JM (1999). Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sciences, 65(20), 2143–2150. 10.1016/S0024-3205(99)00479-8 [DOI] [PubMed] [Google Scholar]
- Grandi C, & D’Ovidio MC (2019). The Interplay Between Solar Radiation, Climate Change and Immunotoxicants in Relation to Immune Response Modulation: A Concern for Outdoor Workers’ Health. American Journal of Health Research, 6(6), 138. 10.11648/j.ajhr.20180606.13 [DOI] [Google Scholar]
- Griefahn B, Künemund C, Blaszkewicz M, Golka K, & Degen G (2002). Experiments on effects of an intermittent 16.7-Hz magnetic field on salivary melatonin concentrations, rectal temperature, and heart rate in humans. International Archives of Occupational and Environmental Health, 75(3), 171–178. 10.1007/s00420-001-0292-2 [DOI] [PubMed] [Google Scholar]
- Halgamuge MN (2013). Pineal melatonin level disruption in humans due to electromagnetic fields and ICNIRP limits. Radiation Protection Dosimetry, 154(4), 405–416. 10.1093/rpd/ncs255 [DOI] [PubMed] [Google Scholar]
- Haskó G, & Szabó C (1998). Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochemical Pharmacology, 56(9), 1079–1087. 10.1016/s0006-2952(98)00153-1 [DOI] [PubMed] [Google Scholar]
- Hathaway DH (2015). The Solar Cycle. Living Reviews in Solar Physics, 12(1), 4. 10.1007/lrsp-2015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hriscu ML (2005). Modulatory Factors of Circadian Phagocytic Activity. Annals of the New York Academy of Sciences, 1057(1), 403–430. 10.1196/annals.1356.032 [DOI] [PubMed] [Google Scholar]
- Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, & Rotnitzky A (1996). The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA, 275(15), 1171–1176. [PubMed] [Google Scholar]
- Irwin M (1993). Stress-induced immune suppression. Role of the autonomic nervous system. Annals of the New York Academy of Sciences, 697, 203–218. 10.1111/j.1749-6632.1993.tb49933.x [DOI] [PubMed] [Google Scholar]
- Ivetic A, Hoskins Green HL, & Hart SJ (2019). L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Frontiers in Immunology, 10. 10.3389/fimmu.2019.01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, Zaharieva EK, Kimura Y, & Wakeford R (2015). Long-term effects of radiation exposure on health. The Lancet, 386(9992), 469–478. 10.1016/S0140-6736(15)61167-9 [DOI] [PubMed] [Google Scholar]
- Kang Y-M, Ma Y, Zheng J-P, Elks C, Sriramula S, Yang Z-M, & Francis J (2009). Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovascular Research, 82(3), 503–512. 10.1093/cvr/cvp073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, & Ganta CK (2014). Autonomic Nervous System and Immune System Interactions. In Terjung R (Ed.), Comprehensive Physiology (pp. 1177–1200). John Wiley & Sons, Inc. 10.1002/cphy.c130051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Voyich JM, Burlak C, & DeLeo FR (2005). Neutrophils in the innate immune response. Archivum Immunologiae Et Therapiae Experimentalis, 53(6), 505–517. [PubMed] [Google Scholar]
- Krylov VV, Zotov OD, Klain BI, Ushakova NV, Kantserova NP, Znobisheva AV, Izyumov YG, Kuz’mina VV, Morozov AA, Lysenko LA, Nemova NN, & Osipova EA (2014). An experimental study of the biological effects of geomagnetic disturbances: The impact of a typical geomagnetic storm and its constituents on plants and animals. Journal of Atmospheric and Solar-Terrestrial Physics, 110–111, 28–36. 10.1016/j.jastp.2014.01.020 [DOI] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, & CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150(9), 604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaz N (2019). Space Weather at Earth and in Our Solar System. In The Sun as a Guide to Stellar Physics (pp. 335–361). Elsevier. 10.1016/B978-0-12-814334-6.00012-1 [DOI] [Google Scholar]
- Ma N, & Birmili W (2015). Estimating the contribution of photochemical particle formation to ultrafine particle number averages in an urban atmosphere. Science of The Total Environment, 512–513, 154–166. 10.1016/j.scitotenv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Maestroni GJM (1995). T-Helper-2 lymphocytes as a peripheral target of melatonin. Journal of Pineal Research, 18(2), 84–89. 10.1111/j.1600-079X.1995.tb00144.x [DOI] [PubMed] [Google Scholar]
- McCraty R, Atkinson M, Stolc V, Alabdulgader A, Vainoras A, & Ragulskis M (2017). Synchronization of Human Autonomic Nervous System Rhythms with Geomagnetic Activity in Human Subjects. International Journal of Environmental Research and Public Health, 14(7), 770. 10.3390/ijerph14070770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza B, & Sánchez de la Peña S (2010). Solar activity and human health at middle and low geomagnetic latitudes in Central America. Advances in Space Research, 46(4), 449–459. 10.1016/j.asr.2009.06.021 [DOI] [Google Scholar]
- Nauseef WM (2007). How human neutrophils kill and degrade microbes: An integrated view. Immunological Reviews, 219(1), 88–102. 10.1111/j.1600-065X.2007.00550.x [DOI] [PubMed] [Google Scholar]
- Nyhan MM, Coull BA, Blomberg AJ, Vieira CLZ, Garshick E, Aba A, Vokonas P, Gold DR, Schwartz J, & Koutrakis P (2018). Associations Between Ambient Particle Radioactivity and Blood Pressure: The NAS (Normative Aging Study). Journal of the American Heart Association, 7(6). 10.1161/JAHA.117.008245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña C, Rincon J, Pedreanez A, Viera N, & Mosquera J (2007). Chemotactic effect of melatonin on leukocytes. Journal of Pineal Research, 43(3), 263–269. 10.1111/j.1600-079X.2007.00471.x [DOI] [PubMed] [Google Scholar]
- Recchioni R, Marcheselli F, Moroni F, Gáspár R, Damjanovich S, & Pieri C (1998). Melatonin increases the intensity of respiratory burst and prevents L-selectin shedding in human neutrophils in vitro. Biochemical and Biophysical Research Communications, 252(1), 20–24. 10.1006/bbrc.1998.9582 [DOI] [PubMed] [Google Scholar]
- Rodriguez AB, Marchena JM, Nogales G, Durán J, & Barriga C (1999). Correlation between the circadian rhythm of melatonin, phagocytosis, and superoxide anion levels in ring dove heterophils. Journal of Pineal Research, 26(1), 35–42. 10.1111/j.1600-079X.1999.tb00564.x [DOI] [PubMed] [Google Scholar]
- Rodriguez Ana B., & Lea RW (1994). Effect of pinealectomy upon the nonspecific immune response of the ring-dove (Streptopelia risoria). Journal of Pineal Research, 16(3), 159–166. 10.1111/j.1600-079X.1994.tb00096.x [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, & Scammell TE (2012). Neural circuitry engaged by prostaglandins during the sickness syndrome. Nature Neuroscience, 15(8), 1088–1095. 10.1038/nn.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, & Frenette PS (2013). Circadian control of the immune system. Nature Reviews Immunology, 13(3), 190–198. 10.1038/nri3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, & Raizada MK (2010). Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension, 56(2), 297–303. 10.1161/HYPERTENSIONAHA.110.150409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki SK (2003). Sunspots: An overview. Astronomy and Astrophysics Review, 11(2–3), 153–286. 10.1007/s00159-003-0018-4 [DOI] [Google Scholar]
- Steenhof M, Janssen NAH, Strak M, Hoek G, Gosens I, Mudway IS, Kelly FJ, Harrison RM, Pieters RHH, Cassee FR, & Brunekreef B (2014). Air pollution exposure affects circulating white blood cell counts in healthy subjects: The role of particle composition, oxidative potential and gaseous pollutants – the RAPTES project. Inhalation Toxicology, 26(3), 141–165. 10.3109/08958378.2013.861884 [DOI] [PubMed] [Google Scholar]
- Stoupel EG, Abramson E, Gabbay U, & Pick AI (1995). Relationship between immunoglobulin levels and extremes of solar activity. International Journal of Biometeorology, 38(2), 89–91. 10.1007/BF01270665 [DOI] [PubMed] [Google Scholar]
- Stoupel E, Monselise Y, & Lahav J (2006). Changes in Autoimmune Markers of the AntiCardiolipin Syndrome on Days of Extreme Geomamagnetic Activity. Journal of Basic and Clinical Physiology and Pharmacology, 17(4). 10.1515/JBCPP.2006.17.4.269 [DOI] [PubMed] [Google Scholar]
- Stowe RP, Sams CF, Mehta SK, Kaur I, Jones ML, Feeback DL, & Pierson DL (1999). Leukocyte subsets and neutrophil function after short-term spaceflight. Journal of Leukocyte Biology, 65(2), 179–186. 10.1002/jlb.65.2.179 [DOI] [PubMed] [Google Scholar]
- Stowe RP, Sams CF, & Pierson DL (2003). Effects of mission duration on neuroimmune responses in astronauts. Aviation, Space, and Environmental Medicine, 74(12), 1281–1284. [PubMed] [Google Scholar]
- Swaab DF (Ed.). (2003). Chapter 4 Suprachiasmatic nucleus (SCN) and pineal gland (Fig. 4A). In Handbook of Clinical Neurology (Vol. 79, pp. 63–125). Elsevier. 10.1016/S0072-9752(03)80011-8 [DOI] [PubMed] [Google Scholar]
- Szczepanik M (2007). Melatonin and its influence on immune system. Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society, 58 Suppl 6, 115–124. [PubMed] [Google Scholar]
- Terrón MP, Paredes SD, Barriga C, Ortega E, & Rodríguez AB (2004). Comparative study of the heterophil phagocytic function in young and old ring doves (Streptopelia risoria) and its relationship with melatonin levels. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology, 174(5), 421–427. 10.1007/s00360-004-0429-1 [DOI] [PubMed] [Google Scholar]
- Touitou Y, & Selmaoui B (2012). The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues in Clinical Neuroscience, 14(4), 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama A, & Ohkubo C (2004). Acute effects of low-frequency electromagnetic fields on leukocyte-endothelial interactions in vivo. In Vivo (Athens, Greece), 18(2), 125–132. [PubMed] [Google Scholar]
- Vieira CLZ, Garshick E, Alvares D, Schwartz J, Huang S, Vokonas P, Gold DR, & Koutrakis P (2020). Association between ambient beta particle radioactivity and lower hemoglobin concentrations in a cohort of elderly men. Environment International, 139, 105735. 10.1016/j.envint.2020.105735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydahl A, Sothern RB, Cornélissen G, & Wetterberg L (2001). Geomagnetic activity influences the melatonin secretion at latitude 70 degrees N. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 55 Suppl 1, 57s–62s. 10.1016/s0753-3322(01)90006-x [DOI] [PubMed] [Google Scholar]
- Wyse C, O’Malley G, Coogan AN, & Smith DJ (2020). Seasonal and Daytime Variation in Multiple Immune Parameters in Humans: Evidence from 329,261 Participants of the UK Biobank Cohort. MedRxiv, 2020.10.23.20218305. 10.1101/2020.10.23.20218305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


