Abstract
Objectives:
To analyze trends in the incidence and use of diagnostic modalities for GCA in a population-based cohort over the past seven decades. To explore survival trends in patients with GCA compared with the general population.
Methods:
A population-based cohort of patients diagnosed with GCA was extended with new incident cases from 2010-2019. Three time periods were compared: Period One (1950-1979), Period Two (1980-1999), and Period Three (2000-2019). Cases were classified as: Diagnostic Group One, temporal artery biopsy (TAB) positive; Diagnostic Group Two, TAB-negative or not done with positive large-vessel imaging; or Diagnostic Group Three, clinical diagnosis of GCA. Survival was evaluated by comparing Kaplan-Meier estimated mortality rates for cases of GCA against expected mortality rates from Minnesota life tables
Results:
Age- and sex-adjusted incident rates per 100,000 ≥ 50 years of age (95% CI) were 13.5 (10.1, 16.9) in Period One, 21.0 (17.1, 25.0) in Period Two, and 15.0 (12.4, 17.5) in Period Three. The percent of patients in Diagnostic Group One decreased over the three time periods (89%, 86%, and 72%) while the patients in Diagnostic Group Three increased (11%, 14%, and 17%). Standardized mortality ratios (95% CI) were 1.03 (0.79, 1.32), 1.11 (0.91, 1.34), and 0.82 (0.64, 1.04) across Periods 1-3, respectively.
Conclusions:
Incidence of GCA in females in the population declined, resulting in a decreasing overall incidence. More patients have been identified by large-vessel imaging and fewer by positive TABs. No significant difference in survival between patients with GCA and the general population was observed.
Keywords: giant cell arteritis, epidemiology, incidence, diagnosis, survival
1. INTRODUCTION
Giant cell arteritis (GCA) is a primary systemic vasculitis characterized by inflammation in the aorta and/or its medium and large arterial branches, generally occurring in adults 50 years of age or more.(1) The incidence of disease is higher in females and increases with age, reaching a peak in the eighth decade of life.(2) Estimates of the incidence of GCA vary among studies and populations but generally range from 18-29 cases per 100,000 persons ≥ 50 years of age in populations of Northern European ancestry.(3)
The historic gold standard diagnostic test for GCA has been temporal artery pathology revealing transmural inflammation.(4) However temporal artery inflammation, while common, is not universal and approximately 23% of patients with GCA may have negative temporal artery biopsy (TAB).(5) Therefore, the diagnosis ultimately remains clinical and an over-emphasis on a positive TAB for diagnosis runs the risk of under-inclusion. More recently, advanced imaging techniques of the large vessels have provided assistance in the identification of patients with large-vessel GCA in whom TABs were negative or not performed, and recent studies, particularly clinical trials, have included patients identified using such methods.(6-8)
In addition to variance in diagnostic modalities, the risk of mortality in patients with GCA has been debated with different findings observed. Prior studies have demonstrated no change in the overall mortality risk between those with GCA and population-based controls, except among the subset of patients with GCA diagnosed in a hospital setting or those with large-vessel complications of aortic aneurysm and/or dissection.(9-11) However, this survival parity has recently been called into question.(12-15)
The purpose of this study was three-fold: first, to identify trends in the incidence estimates of GCA in a well-established population-based cohort over seven decades; second, to determine if the use of diagnostic modalities for GCA has changed in recent decades; and third, to compare survival rates of patients with GCA to that of the general population..
2. METHODS
2.1. Approach
This was a retrospective, observational cohort study of Olmsted County, MN residents diagnosed and classified with GCA between 1950 and 2019. The incidence of disease was estimated, cases of GCA were characterized by diagnostic modality, and the survival of patients with GCA was compared to expected rates in the general population from the same geographic area.
2.2. The Population
Olmsted County, MN has been the site of many population-based epidemiologic studies. The residents of Olmsted County primarily receive healthcare services from the Mayo Clinic, Olmsted Medical Center (another group practice), and their affiliated hospitals. In 2010, the population was 144,248, 84.2% white, largely middle class, and approximately 94.5% of the adult population had graduated from high school. Apart from a higher proportion of the working population employed in the healthcare industry, the characteristics of the Olmsted County population are similar to those of US whites.
2.3. Rochester Epidemiology Project (REP)
The REP is a medical record linkage system established in 1966 which combines healthcare data from different providers throughout Olmsted County, MN. This comprehensive medical record linkage system transcends specific sites and organizations of care to capture the richness and diversity of a patient’s medical history. The resulting dataset synthesizes the notes of a patient’s medical record with high fidelity. Analyses of participation have shown that over 90% of subjects have authorized REP research participation to all their health providers.(16)
2.4. The Existing GCA Cohort (1950-2009)
The Division of Rheumatology had already assembled a population-based incidence cohort of 248 subjects with GCA diagnosed between 1/1/1950 and 12/31/2009 in Olmsted County, MN. The details of the cohort during these years have previously been described.(17)
2.5. Cohort Expansion (2010-2019) and Inclusion Criteria
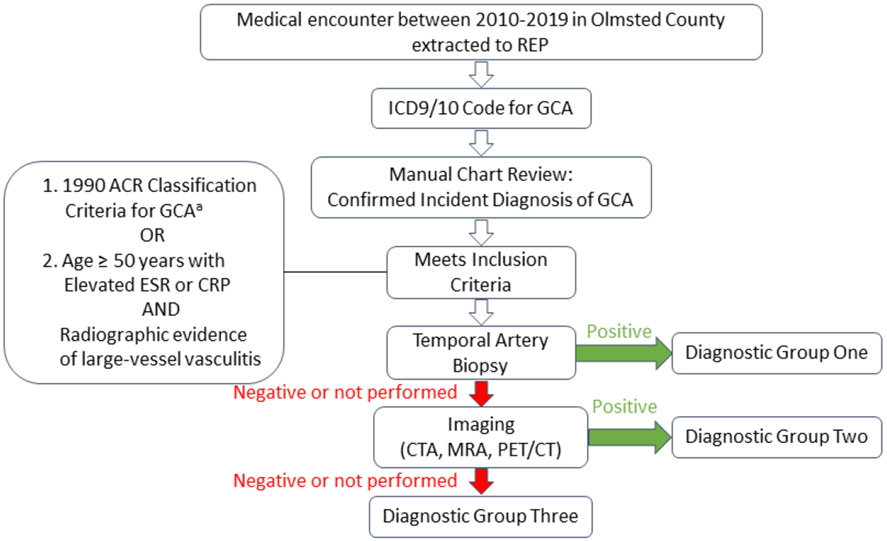
All patients with an ICD9/10 code of GCA between 1/1/2010 and 12/31/2019 in the records of the REP were identified. These cases with a clinical diagnosis of GCA were then reviewed to see if they met criteria for inclusion into the GCA cohort (Figure 1). Patients who moved to Olmsted County with previously diagnosed GCA (i.e., prevalent cases) were not included.
Figure 1. Assignment to Diagnostic Group based on Biopsy and Imaging results.
aElevated c-reactive protein ≥ 10 mg/L could be used for inclusion instead of elevated ESR
Between 1950 and 1999, patients were included in the cohort if they met the American College of Rheumatology (ACR) 1990 criteria for classification of GCA.(18) Between 2000 and 2019, patients were included either if they met the 1990 ACR criteria (with the addition of using either elevated C-reactive protein [CRP] or elevated erythrocyte sedimentation rate [ESR]) or if they met the following criteria: age ≥ 50 years with elevated inflammatory markers (i.e. ESR ≥ 50 mm/hour or CRP ≥ 10 mg/L) and radiographic evidence of large-vessel vasculitis. Studies providing radiographic evidence of large-vessel vasculitis included computed tomography angiography (CTA), magnetic resonance angiography (MRA), and positron emission tomography-computed tomography (PET-CT). Imaging consistent with large-vessel vasculitis included one or more of the following attributable to GCA without other identifiable cause: wall thickening ≥ 2 mm (CTA, MRA), mural wall edema (MRA), wall enhancement (CTA, MRA), arterial wall hypermetabolism (PET-CT), stenosis, aneurysm, and dissection. Patients who were diagnosed with GCA based only on clinical presentation (negative biopsy and imaging/not done) were reviewed by two of the authors (TDG and KJW) with further adjudication by a third investigator (MJK) when needed.
2.6. Case Review
The medical records of all potential GCA cases were reviewed by TDG with assistance from the coinvestigators. All GCA subjects’ medical records were followed until either death, migration from Olmsted County, or December 31st, 2020 (end of follow-up for the study).
Incident cases were organized by date of incident diagnosis into three time periods: Period One −1950-1979; Period Two - 1980-1999; and Period Three - 2000-2019. They were also categorized based on results of diagnostic testing. Patients in Diagnostic Group One had positive TAB results; patients in Diagnostic Group Two had large-vessel imaging consistent with vasculitis and TABs were either negative or not performed; and patients in Diagnostic Group Three had both TAB and large-vessel imaging that was either negative or not performed (Figure 1).
2.7. Statistical Analysis
Descriptive statistics (percentage, mean, etc.) were used to summarize the data. Comparisons of characteristics between time periods were performed using chi-square and rank-sum tests. Age- and sex-specific incidence rates were calculated for each time period using the number of incident cases as the numerator and population estimates from the REP census as the denominator. Overall incidence rates were age- and sex-adjusted to the 2010 white population of the US. To compute 95% confidence intervals (95% CI) for incidence rates it was assumed that the number of incident cases followed a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods. The annual incidence rates were graphically illustrated using a three-year, centered, moving average to reduce random fluctuations over time. Mortality rates were estimated using Kaplan-Meier methods, and were compared to the lifetable rates of the Minnesota white population. The standardized mortality ratio was estimated as the ratio of the observed and expected number of deaths. Ninety-five percent confidence intervals for the standardized mortality ratio were calculated assuming that the expected rates were fixed and the observed rates followed a Poisson distribution. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
2.8. Data Statement
Mayo Clinic Institutional Review Board (IRB) policy does not allow full access of patient information to be provided to a third party without prior approval from the IRB committee overseeing this study. However, access to the complete de-identified data can be made available following approval. Requests for additional study related data can be sent to Cynthia S. Crowson at crowson@mayo.edu.
3. RESULTS
3.1. Description of the Entire Patient Cohort
The study cohort is comprised of 304 patients diagnosed with GCA from 1950 until 2019. Between 2010 and 2019, 55 incident cases of GCA were identified and added to the previously published cohort. Clinical signs and symptoms and laboratory results are presented according to each period (Table 1)
Table 1:
Characteristics of the Cohort including Temporal Artery Biopsy Results and Diagnostic Grouping organized by Time Period
| Period One 1950-1979 (N=63) |
Period Two 1980-1999 (N=111) |
Period Three 2000-2019 (N=130) |
Total Cohort 1950-2019 (N=304) |
p-value | |
|---|---|---|---|---|---|
| Age: Mean (SD) | 73.6 (8.2) | 76.3 (8.0) | 77.5 (8.1) | 76.3 (8.2) | 0.005 |
| Female Sex: # (%) | 49 (78%) | 88 (79%) | 97 (75%) | 234 (77%) | 0.683 |
| Symptom onset to diagnosis (months): N | 62 | 111 | 130 | 304 | 0.138 |
| Median (IQR) | 0.9 (0.6, 2.0) | 1.0 (0.5, 1.7) | 1.1 (0.7, 2.3) | 1.1 (0.6, 2.1) | |
| Fever1: # (%) | 11/61 (18%) | 19/109 (17%) | 19/128 (15%) | 49/298 (16%) | 0.807 |
| Weight loss2: # (%) | 14/61 (23%) | 25/109 (23%) | 38/127 (30%) | 77/297 (26%) | 0.398 |
| Headache: # (%) | 45/61 (74%) | 80/109 (73%) | 92/129 (71%) | 217/299 (73%) | 0.913 |
| Jaw claudication: # (%) | 31/61 (51%) | 43/109 (39%) | 61/127 (48%) | 135/297 (45%) | 0.268 |
| Scalp tenderness: # (%) | 19/54 (35%) | 41/106 (39%) | 65/125 (52%) | 125/285 (44%) | 0.046 |
| Tender T.A.: # (%) | 5/38 (13%) | 39/100 (39%) | 36/122 (30%) | 80/260 (31%) | 0.012 |
| Blurred vision: # (%) | 8/61 (13%) | 13/109 (12%) | 32/127 (25%) | 53/297 (18%) | 0.016 |
| Transient vision loss: # (%) | 3/61 (5%) | 2/108 (2%) | 9/129 (7%) | 14/298 (5%) | 0.178 |
| Vision, permanent partial loss: # (%) | 8/61 (13%) | 5/109 (5%) | 6/128 (5%) | 19/298 (6%) | 0.054 |
| Vision, permanent complete loss: # (%) | 1/61 (2%) | 3/108 (3%) | 2/129 (2%) | 6/299 (2%) | 0.784 |
| Other visual symptoms: # (%) | 6/61 (10%) | 4/109 (4%) | 32/129 (25%) | 42/299 (14%) | <0.001 |
| Arm claudication: # (%) | 0/61 (0%) | 1/108 (1%) | 3/128 (2%) | 4/297 (1%) | 0.380 |
| Leg claudication: # (%) | 1/61 (2%) | 0/108 (0%) | 2/128 (2%) | 3/297 (1%) | 0.420 |
| Bruit: # (%) | 0/59 (0%) | 5/106 (5%) | 6/119 (5%) | 11/284 (4%) | 0.221 |
| Absent pulse: # (%) | 5/42 (12%) | 15/103 (15%) | 6/119 (5%) | 26/264 (10%) | 0.053 |
| PMR symptoms: # (%) | 14/61 (23%) | 35/109 (32%) | 38/129 (29%) | 87/299 (29%) | 0.448 |
| Other musculoskeletal pain: # (%) | 10/61 (16%) | 11/109 (10%) | 40/129 (31%) | 61/299 (20%) | <0.001 |
| Hemoglobin: N | 56 | 75 | 124 | 255 | 0.123 |
| Mean (SD) | 11.5 (1.3) | 11.9 (1.3) | 11.9 (1.3) | 11.8 (1.3) | |
| Sedimentation rate: N | 60 | 109 | 124 | 293 | <0.001 |
| Mean (SD) | 92.1 (19.6) | 73.8 (31.0) | 67.8 (31.2) | 75.0 (30.4) | |
| C-Reactive protein (mg/L): N | ND | ND | 104 | 104 | --- |
| Mean (SD) | 89.8 (83.0) | 89.8 (83.0) | |||
| Temporal Artery Biopsy Result | 0.002 | ||||
| Negative | 2 (3%) | 9 (8%) | 28 (22%) | 39 (13%) | |
| Positive | 56 (89%) | 96 (86%) | 93 (72%) | 245 (81%) | |
| Not performed | 5 (8%) | 6 (5%) | 9 (7%) | 20 (7%) | |
| Diagnostic Group | <0.001 | ||||
| 1 - Temporal artery biopsy positive | 56 (89%) | 96 (86%) | 93 (72%) | 245 (81%) | |
| 2 - Imaging positive with temporal artery biopsy negative or not performed | ND | ND | 15 (12%) | 15 (5%) | |
| 3 - Clinical diagnosis with both temporal artery biopsy and imaging negative or not performed | 7 (11%) | 15 (14%) | 22 (17%) | 44 (14%) |
Fever was defined as > 100 F
Weight loss was defined as > 5 pounds or > 10% of premorbid weight
Several significant changes occurred over the course of the seven-decade study period. The age at diagnosis of GCA has been slowly increasing in our cohort at a rate of 0.8 years of age each decade (p=0.005) (Figure 2). Scalp tenderness and tender temporal arteries at the time of diagnosis increased in frequency over time. Similarly, the frequency of patients reporting blurred vision and other visual symptoms (other than temporary or permanent loss) was higher in Period Three. Musculoskeletal pain (not consistent with polymyalgia rheumatica [PMR]) was reported more frequently in Period Three. The mean ESR was noted to be decreasing over the decades.
Figure 2.
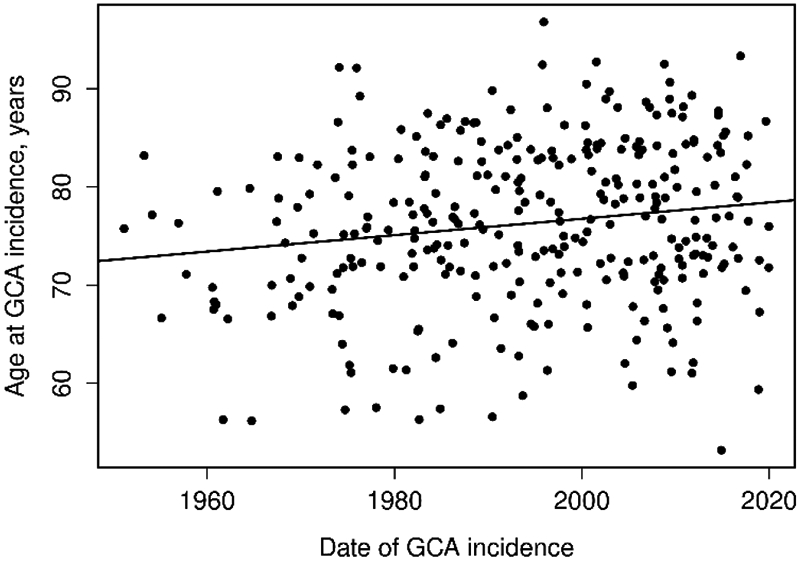
Plot of age at GCA incidence date by date of GCA incidence.
3.2. Changing Trends in Diagnostic Modalities
In the cohort there was a significant decrease over time in the percent of patients with positive TAB: 89% in Period One; 86% in Period Two; and 72% in Period Three (Table 1). This also corresponded to a significant decrease in the percent of patients in Diagnostic Group One over the three time periods with an accompanying increase in the percent of patients in Diagnostic Group Three. Diagnostic Group Two made up 12% of the patients in Period Three and 5% of the total cohort however comparisons across time periods for the group cannot be made because imaging was not performed or used as inclusion criteria to the cohort in Periods One and Two.
Within Period Three the frequencies of diagnostic modalities from the most recent decade (2010-2019) were compared to those of the first decade within that period (2000-2009). Of the 55 patients diagnosed between 2010-2019, there were 37 (67%) in Diagnostic Group One, 10 (18%) in Diagnostic Group Two, and 8 (15%) in Diagnostic Group Three. In contrast, of the 75 patients diagnosed between 2000-2009 there were 56 (75%) in Diagnostic Group One, 5 (7%) in Diagnostic Group Two, and 14 (19%) in Diagnostic Group Three. However, the difference in the Diagnostic Group composition did not reach statistical significance (p=0.12), despite an increased use of imaging and a decline in diagnosis by TAB in the most recent decade.
During the first decade of Time Period 3 (2000-2009) 11 of 56 (19.6%) in Diagnostic Group One, 5 of 5 (100%) in Diagnostic Group Two, and 6 of 14 (42.9%) in Diagnostic Group Three were evaluated with arterial imaging. In the most recent decade (2010-2019) 24 of 37 (64.9%), 10 of 10 (100%), and 6 of 8 (75%) underwent arterial imaging in the Diagnostic Groups One, Two, and Three, respectively. Among patients in the 2000-2009 portion of period 3, the most common imaging study was CTA (n=13), followed by MRA (n=8), PET (n=2), and conventional angiogram (n=1). Similarly, in the 2010-2019 portion, CTA was most often used (n=29), compared to MRA (n=8) and PET (n=8). Ultrasound was not employed as a screening modality during the listed study period. During the 2000-2009 portion of Time Period 3, eight patients were found to have evidence of large vessel vasculitis on imaging. Features observed included aortic/arterial wall thickening > 2 mm (n=6), non-atherosclerotic stenosis (n=2), aneurysm/dilatation (n=2). During the 2010-2019 portion of Time Period 3, 16 patients had evidence of large vessel vasculitis as demonstrated by wall thickening (n=12), non-atherosclerotic stenosis (n=6), aneurysm/dilatation (n=1), and arterial hypermetabolism (n=4). Distribution of the biopsy and imaging findings according to diagnostic group are outlined in Table 2.
Table 2:
Diagnostic group and distribution of patients undergoing arterial imaging
| Biopsy and Imaging Distribution; N (%) | 2000-2009 (N=75) |
2010-2019 (N=55) |
|
|---|---|---|---|
| Diagnostic Group One | TAB(+) / Imaging (+) | 3 (4) | 6 (11) |
| TAB(+) / Imaging (−) | 8 (10.7) | 18 (32.7) | |
| TAB (+) / Imaging N.D. | 45 (60) | 13 (23.6) | |
| Diagnostic Group Two | TAB(−) or N.D. / Imaging (+) | 5 (6.7) | 10 (18.2) |
| Diagnostic Group Three | TAB(−) or N.D. / Imaging (−) | 6 (8) | 6 (10.9) |
| TAB (−) or N.D. / No imaging | 8 (10.7) | 2 (3.6) | |
N.D., not done; TAB, temporal artery biopsy
3.3. Declining Incidence
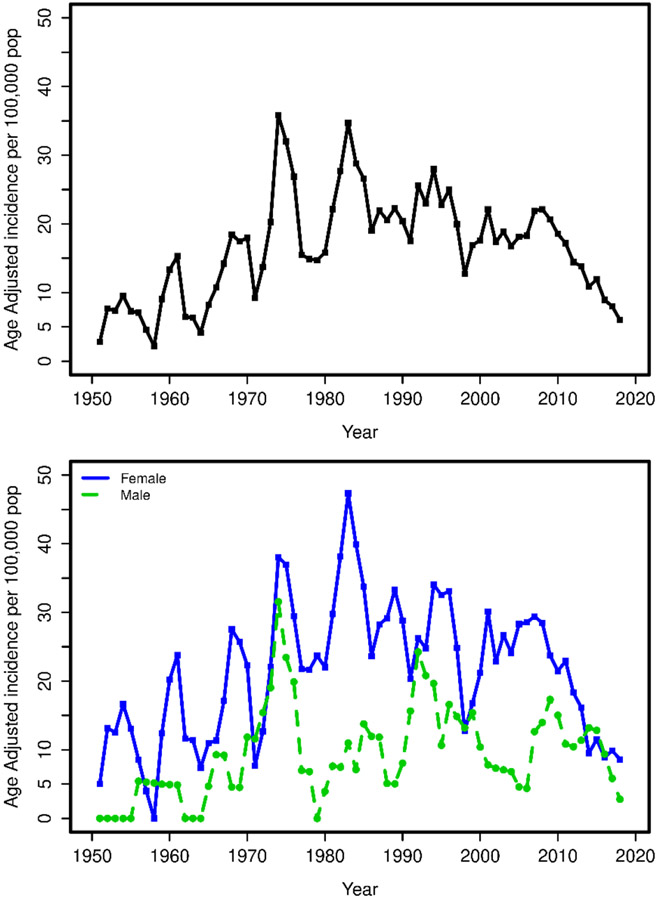
The age- and sex-adjusted incident rates (95% CI) per 100,000 for females, males, and the total population were 18.2 (13.0, 23.3), 7.8 (3.6, 12.1), and 13.5 (10.1, 16.9) in Period One; 27.8 (21.9, 33.8), 12.4 (7.2, 17.6), and 21.0 (17.1, 25.0) in Period Two; and 19.4 (15.5, 23.2), 9.3 (6.1, 12.5), and 15.0 (12.4, 17.5) in Period Three (Table 3)(Figure 3).
Table 3.
Age- and Sex-adjusted Incidence Rates by period for the cohort
| Time Period |
N cases Female |
N cases male |
N cases total |
Female Age-adjusted rate per 100,000 |
Male Age-adjusted rate per 100,000 |
Total age- and sex- adjusted rate per 100,000 |
|---|---|---|---|---|---|---|
| 1: 1950-79 | 49 | 14 | 63 | 18.2 (13.0, 23.3) | 7.8 (3.6, 12.1) | 13.5 (10.1, 16.9) |
| 2: 1980-99 | 88 | 23 | 111 | 27.8 (21.9, 33.8) | 12.4 (7.2, 17.6) | 21.0 (17.1, 25.0) |
| 3: 2000-19 | 97 | 33 | 130 | 19.4 (15.5, 23.2) | 9.3 (6.1, 12.5) | 15.0 (12.4, 17.5) |
Figure 3.
Trends in Incidence of GCA in Olmsted County (1950-2019): Top Panel – Incidence in the total population; Bottom Panel – Incidence by sex
Fifty-five new incident cases were diagnosed between 2010-2019; 37 females (67%) and 18 males (33%). The incidence rate of GCA in the most recent decade (2010-2019) was compared to that in the first decade of period Three (2000-2009). The age and sex adjusted incidence rates (95% CI) per 100,000 between 2010-2019 for females, males, and the total population were 13.0 (8.8, 17.3), 8.6 (4.6, 12.7), and 10.8 (8.0, 13.7), respectively. The corresponding incidence rates from 2000-2009 were 28.0 (21.0, 35.1), 10.2 (5.0, 15.5), and 20.5 (15.9, 25.1), respectively. This represents a significant decline in the incidence rates in females (p<0.001) and the total group (p<0.001) between the 2000–2009 and 2010-2019 cohorts but no change in males (p=0.64).
3.4. Survival Trends
There was no difference in survival between the GCA cohort and the general population across the entire cohort or individual Periods. The standardized mortality ratios (95% CI) were 1.03 (0.79, 1.32), 1.11 (0.91, 1.34), and 0.82 (0.64, 1.04) across Periods One, Two, and Three respectively (Table 4). The 10-year survival percentage in the 3 periods were 57.0 (45.8, 70.7), 53.2 (44.6, 63.3), and 58.1 (49.2, 68.7) respectively. The survival is plotted in Figure 4.
Table 4.
Survival Rates for Olmsted County residents by Period
| Measure | Period One (1950-1979) |
Period Two (1980-1999) |
Period Three (2000-2019) |
Total Cohort (1950-2019) |
|---|---|---|---|---|
| Number of patients | 63 | 111 | 130 | 304 |
| Number of deaths | 62 | 107 | 68 | 237 |
| Expected number of deaths | 60.0 | 96.8 | 82.9 | 239.8 |
| Standardized mortality ratio (95% CI) | 1.03 (0.79, 1.32) | 1.11 (0.91, 1.34) | 0.82 (0.64, 1.04) | 0.99 (0.87, 1.12) |
| 1-sample log rank test p-value | 0.80 | 0.30 | 0.10 | 0.86 |
| 10-year survival, % (95% CI) | 57.0 (45.8, 70.7) | 53.2 (44.6, 63.3) | 58.1 (49.2, 68.7) | 56.4 (50.8, 62.5) |
Figure 4.
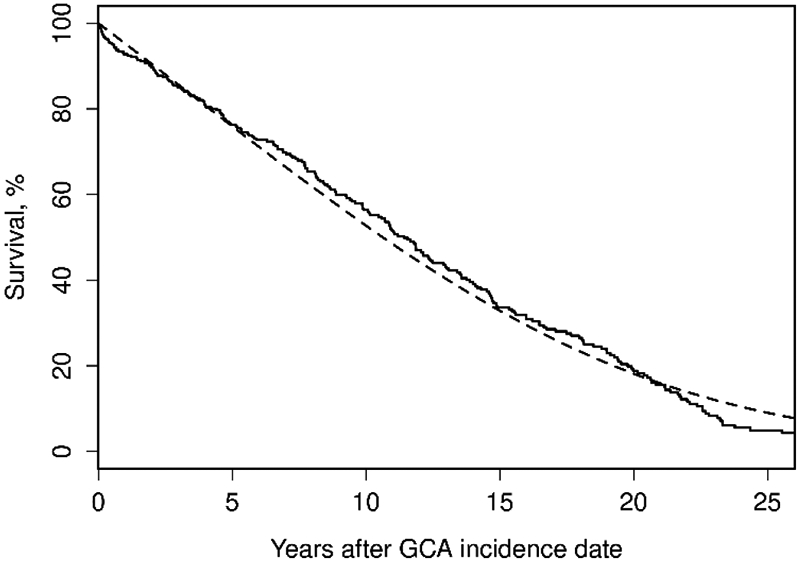
Overall survival of Olmsted county residents with incident GCA in 1950-2019 compared to expected rates from Minnesota lifetables (observed: solid line; expected dashed line) to last follow-up (no truncation – followed through 12/31/2020).
Among patients who both were diagnosed in 2000-2019 and underwent arterial imaging, survival in those with (N=24) and without (N=38) radiographic evidence of large-vessel vasculitis was compared to the general population. Survival did not differ between those with large-vessel vasculitis [standardized mortality ratio (95% CI): 0.88 (0.40, 1.67); p=0.70] or without large-vessel vasculitis [standardized mortality ratio (95% CI): 0.65 (0.34, 1.14); p=0.13] when compared to the general Minnesota population life tables. Age and sex-adjusted Cox model comparing large-vessel vasculitis to those without large-vessel vasculitis similarly did not show a difference in survival between these two groups [hazard ratio (95% CI): 1.98 (0.73-5.37); p=0.18].
4. DISCUSSION
This population-based cohort of patients with GCA is the first to report incidence and mortality trends over a 70-year period. In addition, this is the first North American study to describe trends in GCA diagnostic methodology through the inclusion of patients in whom large-vessel imaging was positive, but TAB pathology was negative or not performed.
The incidence of GCA decreased between Periods Two and Three but this decrease did not reach significance. When analyzing Period Three in greater detail a significant decline in the annual incidence of GCA between 2000-2009 and 2010-2019 was observed. This decline was driven predominantly by a decrease in the incidence of GCA among women. This phenomenon does not appear to be isolated. A similar reduction in the incidence of GCA was observed recently in Northern Italy.(19) There, the annual age- and sex-adjusted incidence rates increased significantly by 15.9% every three years until 2000, after which the incidence fell by an average of −4.8% per three years from 2001-2012. This reduction was also predominantly due to a decrease in incident GCA in women. The overall annual incidence rates have been declining in both Southern Sweden and Southern Norway as well.(12, 20) The cause of these declines is unknown.
This study highlights the complementary but sometimes different roles that pathology, imaging, and clinical evaluation can play in accurate diagnosis of GCA. Over the 70-year study period the percentage of positive TABs decreased with an accompanying rise in patients diagnosed clinically or by advanced imaging studies. Involvement of the large vessels in GCA has been well-known and described since early autopsy studies; however, an understanding of the differential expression of GCA phenotypes contrasting cranial-GCA and large-vessel GCA was not proposed until the late 1990s.(21, 22) An increase in the availability and utilization of non-invasive large-vessel imaging has further informed the heterogenous clinical spectrum of GCA.(23) Consequently experts have suggested an expansion of classification criteria to include non-invasive arterial studies, including MRI, CT, PET, and ultrasound.(24) When revised classification criteria for GCA include these modalities they have been noted to improve specificity compared to the prior 1990 ACR classification criteria.(25) They also appear to have better sensitivity for cases with more constitutional rather than cranial symptoms. Further investigations will help better define and individualize diagnostic strategies based on presentation to maximize study yield and minimize testing-associated morbidity.
The current cohort is the first North American population-based incidence study to compare diagnostic methods over time. Only one other study has included non-invasive arterial imaging, in addition to or in lieu of TAB, in the diagnosis of GCA for a population-based incidence analysis.(20) Andersen et al. reported on 206 patients with GCA in Southern Norway diagnosed between 2000-2013 in which 96% of patients met 1990 ACR Criteria, 74% were biopsy-proven, and only 7% of the total cohort was included based on arterial imaging confirmation without biopsy. While the percentage of biopsy-proven GCA cases in our cohort for a similar time period (2000-2019) was similar (72%), we observed a higher percentage of patient included by imaging diagnosis (12%). The decreasing percentage of biopsy-proven cases across time periods in our study reflects a shift in the diagnostic modalities utilized in evaluating patients with GCA. This has also been reported in a recent meta-analysis by Rubenstein et al.(5)
Patients with a predominantly large-vessel GCA presentation less often have a positive TAB and less frequently satisfy the 1990 ACR classification criteria which has raised concern that these patients may be underrecognized in population-based estimates.(26) It was the expectation of the authors that expansion of diagnosis to include patients identified by non-invasive arterial imaging would capture additional patients with GCA and commensurately increase the incidence of GCA. However, despite the inclusion of such patients, the incidence in the current study declined over the most recent decade. Though the findings were contrary to our expectations, a similar lower annual incidence rate was noted by Andersen and colleagues over their study period, which included imaging, in comparison to historical incidence rates from the same population.(20) Future studies must include patients diagnosed by imaging without a biopsy or in whom biopsy was performed and negative in order to further understand the impact of these patients on the overall epidemiology of GCA.
Differences in clinical characteristics of patients with GCA were observed across the time periods in this study. Classical features of headache, jaw claudication, PMR, and constitutional symptoms were stable; however, scalp tenderness, non-ischemic visual symptoms, and non-PMR musculoskeletal pain were higher in the most recent periods. Given the retrospective nature of this study, these differences may have been affected by reporting bias. Notable variances observed in this study that would not be considered subject to such bias, however, include the significantly increasing age and lower ESR at GCA diagnosis. The increase in mean age at GCA diagnosis has been reported by our group previously but this trend is further confirmed in the most recent study period.(27)
In this population-based cohort of patients with GCA diagnosed over a 70-year period, the mortality of patients diagnosed in recent years was similar to that of the general population. In light of the higher risk seen in patients with complications of large-vessel vasculitis we were curious if the increased use of advanced imaging for cohort inclusion might have a measurable effect on the mortality risk of the entire group. Such an effect however was not seen in our study. These findings are in keeping with a recent population-based study and a recent meta-analysis concluding that long-term mortality in GCA is not increased.(10, 19) Notably, the results from other recent literature have been mixed. One systematic review showed improved mortality over time with rates decreasing by 0.14 per 1000 people per year (p=0.00076) while other recent studies have shown increased mortality.(28) In the United Kingdom, the first year after diagnosis has been noted to be a time of increased mortality and this effect is even more pronounced in those diagnosed at a young age (less than 65 years of age).(29) Ben Shabat and colleagues observed mortality was increased in patients with younger ages at time of diagnosis, less than 2 years before diagnosis, and more than 10 years after diagnosis.(30) Reduced survival in patients who were younger at diagnosis was also recently reported from Ontario, Canada.(15) This group also noted worse survival in males and an overall trend toward reduced survival across the population. Nevertheless, our study comprising seven decades of comparison between patients with GCA and the general population is the longest epidemiology study on the disease.
This study has many strengths. The REP allows capture of population-based data and has an excellent record in epidemiologic research. Each potential case identified in screening was reviewed and validated by a rheumatologist. The study must also be evaluated in the context of its limitations. Cases might have been missed in screening if they never received an ICD9/10 code for GCA during the course of their disease. Secondly, the unavailability of large-vessel imaging during Period One (1950-1979) and Period Two (1980-1999) limits our ability to compare the relative frequencies of diagnostic groups over time. Third, and inherent to all retrospective studies, the information available for abstraction was limited to that which was documented by the treating providers.
5. CONCLUSIONS
In a well-established population-based cohort of patients with GCA, incidence estimates have decreased over the most recent decade. Fewer patients are being diagnosed with positive temporal artery pathology and more patients are being diagnosed clinically or with abnormal large-vessel imaging. Mortality of GCA in Olmsted Country, Minnesota is similar to that of the general population. Further research is needed to understand the epidemiologic impact of advanced arterial imaging on the identification of patients with this disease.
Highlights.
The incidence of giant cell arteritis in Olmsted County, Minnesota, USA is decreasing over the most recent two decades due to a reduction in incidence among women.
Increasing numbers of patients are being diagnosed by clinical means or large-vessel imaging compared to temporal artery biopsy
Mortality among patients with GCA in this population is similar to the general population.
Temporal artery histology and large-vessel imaging provide complementary information in the diagnosis of giant cell arteritis raising the possibility for individualization of the diagnostic approach to GCA based on patient presentation
FUNDING:
This study was made possible by through use of the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- ACR
American College of Rheumatology
- CRP
C-reactive protein
- ESR
Erythrocyte Sedimentation Rate
- GCA
Giant Cell Arteritis
- IRB
Institutional Review Board
- PMR
Polymyalgia Rheumatica
- REP
Rochester Epidemiology Project
- TAB
Temporal Artery Biopsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author). Dr Warrington receives clinical trial support from Eli Lilly and Kiniksa, but funding for this study was not provided. The other authors declare no competing interests.
REFERENCES
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61(10):1454–61. [DOI] [PubMed] [Google Scholar]
- 3.Dejaco C, Brouwer E, Mason JC, Buttgereit F, Matteson EL, Dasgupta B. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat Rev Rheumatol. 2017;13(10):578–92. [DOI] [PubMed] [Google Scholar]
- 4.Cavazza A, Muratore F, Boiardi L, Restuccia G, Pipitone N, Pazzola G, et al. Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol. 2014;38(10):1360–70. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein E, Maldini C, Gonzalez-Chiappe S, Chevret S, Mahr A. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59(5):1011–20. [DOI] [PubMed] [Google Scholar]
- 6.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. [DOI] [PubMed] [Google Scholar]
- 7.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis Rheumatol. 2017;69(4):837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med. 2017;377(4):317–28. [DOI] [PubMed] [Google Scholar]
- 9.Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51(2):264–8. [DOI] [PubMed] [Google Scholar]
- 10.Hill CL, Black RJ, Nossent JC, Ruediger C, Nguyen L, Ninan JV, et al. Risk of mortality in patients with giant cell arteritis: A systematic review and meta-analysis. Semin Arthritis Rheum. 2017;46(4):513–9. [DOI] [PubMed] [Google Scholar]
- 11.Kermani TA, Warrington KJ, Crowson CS, Ytterberg SR, Hunder GG, Gabriel SE, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2013;72(12):1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad AJ, Nilsson JA, Jacobsson LT, Merkel PA, Turesson C. Incidence and mortality rates of biopsy-proven giant cell arteritis in southern Sweden. Ann Rheum Dis. 2015;74(6):993–7. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Shabat N, Tiosano S, Shovman O, Comaneshter D, Shoenfeld Y, Cohen AD, et al. Mortality among patients with giant-cell arteritis: A large-scale population-based cohort study. J Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Baslund B, Helleberg M, Faurschou M, Obel N. Mortality in patients with giant cell arteritis. Rheumatology (Oxford). 2015;54(1):139–43. [DOI] [PubMed] [Google Scholar]
- 15.Barra L, Pope JE, Pequeno P, Gatley JM, Widdifield J. Increased mortality for individuals with Giant Cell Arteritis: a population-based study. Arthritis Care Res (Hoboken). 2021. [DOI] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandran AK, Udayakumar PD, Crowson CS, Warrington KJ, Matteson EL. The incidence of giant cell arteritis in Olmsted County, Minnesota, over a 60-year period 1950-2009. Scand J Rheumatol. 2015;44(3):215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. [DOI] [PubMed] [Google Scholar]
- 19.Catanoso M, Macchioni P, Boiardi L, Muratore F, Restuccia G, Cavazza A, et al. Incidence, Prevalence, and Survival of Biopsy-Proven Giant Cell Arteritis in Northern Italy During a 26-Year Period. Arthritis Care Res (Hoboken). 2017;69(3):430–8. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JB, Myklebust G, Haugeberg G, Pripp AH, Diamantopoulos AP. Incidence Trends and Mortality of Giant Cell Arteritis in Southern Norway. Arthritis Care Res (Hoboken). 2021;73(3):409–14. [DOI] [PubMed] [Google Scholar]
- 21.Cooke WT, Cloake PC, et al. Temporal arteritis; a generalized vascular disease. Q J Med. 1946;15:47–75. [DOI] [PubMed] [Google Scholar]
- 22.Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42(2):311–7. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Gay MA, Ortego-Jurado M, Ercole L, Ortego-Centeno N. Giant cell arteritis: is the clinical spectrum of the disease changing? BMC Geriatr. 2019;19(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56(4):506–15. [DOI] [PubMed] [Google Scholar]
- 25.Wiberg F, Naderi N, Mohammad AJ, Turesson C. Evaluation of revised classification criteria for giant cell arteritis and its clinical phenotypes. Rheumatology (Oxford). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford). 2015;54(3):463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kermani TA, Schafer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2010;69(4):780–1. [DOI] [PubMed] [Google Scholar]
- 28.Li KJ, Semenov D, Turk M, Pope J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Neogi T, Jick S. Mortality in Patients With Giant Cell Arteritis: A Cohort Study in UK Primary Care. Arthritis Care Res (Hoboken). 2018;70(8):1251–6. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shabat N, Tiosano S, Shovman O, Comaneshter D, Shoenfeld Y, Cohen AD, et al. Mortality among Patients with Giant Cell Arteritis: A Large-scale Population-based Cohort Study. J Rheumatol. 2020;47(9):1385–91. [DOI] [PubMed] [Google Scholar]