Abstract
Objective:
To evaluate race and ethnicity differences in cesarean birth and maternal morbidity in low-risk nulliparous people at term.
Methods:
We conducted a secondary analysis of a randomized trial of expectant management versus induction of labor in low-risk nulliparous people at term. The primary outcome was cesarean birth. Secondary outcome was maternal morbidity, defined as: transfusion of ≥ 4 units of red blood cells, any transfusion of other products, postpartum infection, ICU admission, hysterectomy, venous thromboembolism, or maternal death. Multivariable modified Poisson regression was used to evaluate associations between race and ethnicity, cesarean birth, and maternal morbidity. Indication for cesarean was assessed using multivariable multinomial logistic regression. A mediation model was used to estimate the portion of maternal morbidity attributable to cesarean by race and ethnicity.
Results:
Of 5759 included participants, 1158 (20.1%) underwent cesarean; 1404 (24.3%) identified as non-Hispanic Black, 1670 (29.0%) as Hispanic, and 2685 (46.6%) as non-Hispanic White. Adjusted models showed increased relative risk of cesarean among non-Hispanic Black (adjusted relative risk [aRR] 1.21, 95% CI 1.03-1.42) and Hispanic (aRR 1.26, 95%CI 1.08-1.46) people compared with non-Hispanic White people. Maternal morbidity affected 132 (2.3%) individuals, and was increased among non-Hispanic Black (aRR 2.05, 95%CI 1.21-3.47) and Hispanic (aRR 1.92, 95%CI 1.17-3.14) people compared with non-Hispanic White people. Cesarean birth accounted for an estimated 15.8% (95% CI 2.1%-48.7%) and 16.5% (95% CI 4.0% - 44.0%) of excess maternal morbidity among non-Hispanic Black and Hispanic people, respectively.
Conclusion:
Non-Hispanic Black and Hispanic nulliparous people who are low-risk at term undergo cesarean more frequently than low-risk non-Hispanic White nulliparous people. This difference accounts for a modest portion of excess maternal morbidity.
Précis:
Term, low-risk nulliparous Black and Hispanic people have higher rates of cesarean birth than White people, which accounts for a portion of excess maternal morbidity.
Introduction
Despite increasing attention to safely reducing cesarean birth rates among nulliparous people with singleton, vertex pregnancies at term, approximately 25% of deliveries in the U.S. occur via primary cesarean in this group.1,2 Cesarean birth increases short-term peripartum morbidity and risks of future pregnancy complications.3 As with other perinatal outcomes, racial and ethnic disparities in the rate of cesarean birth persist, with non-Hispanic Black people undergoing primary cesarean more commonly than their non-Hispanic White counterparts.1,4–11
Limited studies have focused on differences in primary cesarean birth rates among low-risk nulliparous people by race and ethnicity; in general, these studies are retrospective, using administrative data or single-institution chart reviews.4,6,9,10,12,13 Hypotheses accounting for the observed increased rates of cesarean among non-Hispanic Black people include increased rates of unmeasured co-morbidities that predispose patients to cesarean birth, institutional differences in labor management, physician or other health care professional behavior, and others.7,14–24
Given the association between cesarean birth and increased maternal morbidity, increased rates of cesarean birth among non-Hispanic Black people would intuitively be associated with increased maternal morbidity.3,23,25,26 Racial inequities in maternal morbidity have received increasing attention from the public and medical communities, spurring health care professionals, institutions, and policy makers to address them.27–29 However, the association between maternal morbidity, race, ethnicity, and primary cesarean among otherwise low-risk nulliparous laboring people is understudied.
The ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management) provides a unique opportunity to assess the relationships between cesarean birth, race, ethnicity, and maternal morbidity. The trial included only term low-risk nulliparous people, eliminating many of the confounding risk factors present in other studies of race, ethnicity, and cesarean birth. Though the distribution of cesarean by race and ethnicity was not different in ARRIVE as a result of study group assignment (i.e., induction did not change the distribution of cesarean by race or ethnicity), there was a higher frequency of cesarean birth among non-Hispanic Black and Hispanic individuals in the study overall. However, in a medically homogenous population, the frequency of primary cesarean birth should be equally distributed among racial and ethnic groups. Therefore, our objective was to assess whether racial and ethnic disparities in cesarean birth exist in this group of geographically and racially diverse nulliparous people who were low-risk at term. Secondarily, we sought to assess whether: 1) indication for cesarean birth differed by race and ethnicity, and 2) increased cesarean births among non-Hispanic Black or Hispanic people could explain racial or ethnic inequities in maternal morbidity.
Methods
This is a secondary analysis of ARRIVE, a multicenter randomized trial of induction of labor versus expectant management at term conducted at 41 centers across the United States.30 To briefly review, low-risk nulliparous people with a reliably dated singleton fetus in vertex presentation with no contraindications to a trial of labor were screened for eligibility between 34 0/7 - 38 6/7 weeks gestation from March 2014 to August 2017. Low-risk was defined as having no medical, obstetric, or fetal indications for delivery prior to 40 5/7 weeks gestation. Individuals consented earlier in gestation were re-assessed between 38 0/7 - 38 6/7 weeks to ensure they continued to meet low-risk criteria. At 38 0/7 - 38 6/7 weeks, eligible participants were randomized to either induction of labor between 39 0/7 - 39 4/7 weeks or expectant management (awaiting spontaneous onset of labor or a medical or obstetric indication for delivery, with delivery initiated no later than 42 2/7 weeks). The trial was conducted pragmatically, with no pre-specified protocols for induction or labor management. Trained research personnel abstracted all medical record data.
All participants in the primary trial with available outcome data were eligible for this analysis. The primary exposure was participant self-reported race and ethnicity. Due to small numbers, participants who identified as other than non-Hispanic Black, Hispanic, or non-Hispanic White, or whose race and ethnicity were missing, were excluded.
The primary outcome was cesarean birth. Indication for cesarean was based on the primary indication identified during chart abstraction, and categorized as non-reassuring fetal status, labor dystocia (arrest of dilation or arrest of descent), or other (abnormal presentation, abruption, umbilical cord prolapse, suspected macrosomia, preeclampsia, and other/unspecified). We did not subcategorize dystocia due to sample size constraints.
Composite maternal morbidity included transfusion of ≥4 units of packed red blood cells, transfusion of any other blood products, postpartum infection (including postpartum endometritis, wound complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia of unknown source, or septic pelvic thrombophlebitis), intensive care unit (ICU) admission, hysterectomy, venous thromboembolism, or maternal death. This composite includes diagnoses available in our data that the Centers for Disease Control and Prevention’s (CDC) includes in their definition of severe maternal morbidity. However, we used a broader definition of infection-related morbidity (CDC definition includes only sepsis).31
Baseline sociodemographic and clinical variables assessed at randomization included maternal age, employment (full- or part-time vs none), insurance (private vs self-pay or public), marital status (married or partnered vs not partnered), body mass index (BMI), tobacco use (none vs any during pregnancy), and history of pregnancy loss (any loss < 20 weeks). Modified Bishop score (cervical dilation, fetal station, cervical effacement) was assessed on admission for delivery and categorized as < 5 or ≥ 5. Post-randomization obstetric and neonatal variables were obtained via chart abstraction and included preeclampsia or gestational hypertension (defined according to American College of Obstetrics and Gynecology guidelines32), clinical chorioamnionitis (clinical diagnosis any time between start of labor until delivery), abruption, small for gestational age (SGA) neonate (birthweight < 10th percentile for gestational age33), neonatal intermediate or intensive care unit (NICU) admission, and, among those with non-reassuring fetal status as the indication for cesarean, Apgar score ≥ 7 at 1 minute. NICU admission and Apgar score served as a crude safety signal for indicated cesarean delivery.
Bivariate analyses were conducted to compare baseline and outcome variables between race and ethnicity groups. We used Fisher’s exact or chi-square as appropriate for categorical variables, and Wilcoxon rank-sum test for continuous.
Multivariable modified Poisson regression was used to assess the association between cesarean birth and race and ethnicity. Any pre-delivery covariates that differed by race or ethnicity (p < 0.05) and were statistically related to cesarean birth with p <0.20 were included in the multivariable models. Covariates that did not statistically alter the models were removed in a reverse stepwise fashion. However, we retained study treatment group, relationship status, employment, and insurance irrespective of their statistical influence. An adjustment for site was considered, but sample size did not permit the required number of covariates.
We assessed indication for cesarean via multivariabe multinomial logistic regression, which permits simultaneous evaluation of multiple categories of the outcome variable. The categories of indication for cesarean (non-reassuring fetal status, labor dystocia, and other) were compared with vaginal birth (referent). We used the reverse stepwise process described above to identify covariates. Given fewer events in the “Other” indication category for cesarean birth, we performed a sensitivity analysis removing this indication from the models.
Multivariable modified Poisson regression was used for the maternal morbidity outcome. We next performed a mediation analysis to determine if and to what extent cesarean delivery may account for excess risk of maternal morbidity among non-Hispanic Black and Hispanic people. Because adjusted coefficients in logistic regression cannot be directly compared to assess for mediation,34 we used SAS procedure PROC CAUSALMED. This mediation analysis uses a counterfactual statistical approach to determine the percentage of the association between race and ethnicity and maternal morbidity that is accounted for by cesarean delivery. Bootstrapping was used to obtain 95% confidence intervals for these mediation estimates.
Institutional Review Board (IRB) approval was obtained for the primary study at all participating centers. All participants provided written informed consent at enrollment. This secondary analysis was considered exempt from review by the University of Utah IRB. A p-value < 0.05 was used to define statistical significance, and all tests were two-tailed. Imputation for missing data was not performed. All analyses were performed using SAS version 9.4 (SAS Institute, Cary NC). We followed STROBE reporting guidelines for cohort studies.
Results
Of the 6,106 people randomized in the parent trial, outcome data were available for 6,096. Of these, 5,759 (94.5%) were included in this analysis. One hundred ninety-two Asian people and 145 people who reported their race and ethnicity as any other race, multiple races, or who declined to report were excluded. Table 1 provides the distribution of sociodemographic, maternal, and obstetric factors by race and ethnicity, as well as missingness for covariates. Non-Hispanic White individuals were more likely than both non-Hispanic Black and Hispanic individuals to be older, married or partnered, and employed, have private insurance, lower BMI, and less likely to have a modified Bishop score < 5 at admission or a diagnosis of preeclampsia or gestational hypertension after randomization. Compared to non-Hispanic White people, non-Hispanic Black people were more likely to use tobacco or have prior pregnancy losses, while Hispanic individuals used tobacco less frequently and were more likely to develop clinical chorioamnionitis. Of note, randomization resulted in similar distribution of race and ethnicity within randomized study groups.
Table 1.
Sociodemographic and obstetric factors by race and ethnicity*
| Non-Hispanic Black (n=1404) |
p-value† | Hispanic (n=1670) |
p-value† | Non-Hispanic White (n=2685) |
|
|---|---|---|---|---|---|
| Baseline demographic and clinical characteristics | |||||
| Maternal age (years) | 21.0 (20.0-24.0) | <.001 | 22.0 (20.0-26.0) | <.001 | 26.0 (22.0-30.0) |
| Private insurance | 238 (17.0) | <.001 | 278 (16.7) | <.001 | 2007 (74.8) |
| Employed full or part time | 523 (37.3) | <.001 | 516 (30.9) | <.001 | 1902 (71.2) |
| Married or living with partner | 361 (25.7) | <.001 | 835 (50.0) | <.001 | 2140 (79.7) |
| BMI (kg/m2) | 31.8 (27.8-37.2) | <.001 | 31.0 (28.0-35.1) | <.001 | 30.0 (27.1-34.2) |
| History of pregnancy loss‡ | 413 (29.4) | <.001 | 384 (23.0) | 0.70 | 604 (22.5) |
| Tobacco use | 169 (12.0) | 0.005 | 40 (2.4) | <.001 | 248 (9.2) |
| Modified Bishop score < 5 at admission | 529 (39.0) | <.001 | 555 (35.2) | <.001 | 648 (26.4) |
| Gestational age at delivery (weeks) | 39 (39-40) | 0.15 | 39 (39-40) | 0.63 | 39 (39-40) |
| Randomized to induction of labor | 706 (50.3) | 0.62 | 866 (51.9) | 0.12 | 1328 (49.5) |
| Clinical diagnoses after randomization | |||||
| Preeclampsia or gestational hypertension | 221 (15.7) | <.001 | 231 (13.8) | <.001 | 229 (8.5) |
| Chorioamnionitis (suspected or confirmed) | 173 (12.3) | 0.28 | 293 (17.5) | <.001 | 300 (11.2) |
| Abruption | 4 (0.3) | 0.50 | 6 (0.4) | 0.35 | 5 (0.2) |
Abbreviations: BMI – body mass index
Incomplete data: Missing Bishop score in 369, missing BMI in 28, missing employment in 17.
Data presented are n (%) or median (interquartile range).
Based on Chi-square or Fisher’s exact test for categorical variables or Wilcoxon rank-sum test for continuous variables. P-values are for pairwise comparisons of Non-Hispanic Black or Hispanic vs. Non-Hispanic White as the referent.
Any prior pregnancy delivered < 20 weeks’ gestation
Table 2 shows the frequency of primary and secondary outcomes, as well as descriptive neonatal outcomes, by race and ethnicity. Just under 18% of non-Hispanic White people underwent cesarean birth, compared to 22.8% (p<0.001) of non-Hispanic Black and 21.9% (p<0.001) of Hispanic people had cesarean births. Overall, 2.3% (n=132) of people had a maternal morbidity event. Among non-Hispanic Black and Hispanic individuals, maternal morbidity was present in 3.1% and 3.2% of deliveries respectively, compared with 1.3% of deliveries to non-Hispanic White people (p<0.001 for each pairwise comparison). Of those with morbidity, infection was the most common (n=110, 83.3%). There were no significant differences in SGA or NICU admission by race or ethnicity, nor in 1-minute Apgar > 7 among infants delivered via cesarean for non-reassuring fetal status.
Table 2.
Distribution of cesarean birth, indication for cesarean, maternal morbidity, and descriptive neonatal outcomes by race and ethnicity*
| Non-Hispanic Black (n=1404) |
p-value† | Hispanic (n=1670) |
p-value† | Non-Hispanic White (n=2685) |
|
|---|---|---|---|---|---|
| Cesarean birth (any indication)‡ | 320 (22.8) | <.001 | 366 (21.9) | <.001 | 472 (17.6) |
| Non-reassuring fetal status | 190 (13.5) | 185 (11.1) | 194 (7.2) | ||
| Labor dystocia | 110 (7.8) | 153 (9.2) | 239 (8.9) | ||
| Other | 20 (1.4) | 28 (1.7) | 39 (1.5) | ||
| Maternal morbidity composite§ | 43 (3.1) | <.001 | 54 (3.2) | <.001 | 35 (1.3) |
| Transfusion ≥ 4u pRBC | 4 (0.3) | 6 (0.4) | 4 (0.2) | ||
| Transfusion any other products | 6 (0.4) | 6 (0.4) | 4 (0.2) | ||
| Postpartum infection‖ | 38 (2.7) | 44 (2.6) | 28 (1.0) | ||
| ICU admission | 4 (0.3) | 3 (0.2) | 5 (0.2) | ||
| Hysterectomy | -- | 1 (0.1) | -- | ||
| Venous thromboembolism | 1 (0.1) | 2 (0.1) | -- | ||
| Neonatal outcomes | |||||
| Small for gestational age¶ | 113 (8.1) | 0.28 | 142 (8.5) | 0.54 | 243 (9.1) |
| NICU admission | 174 (12.4) | 0.63 | 199 (11.9) | 0.97 | 319 (11.9) |
| 1-minute Apgar ≥ 7# | 149 (78.4) | 0.54 | 153 (82.7) | 0.10 | 147 (75.8) |
Abbreviations: pRBC – packed red blood cells; ICU – intensive care unit; NICU – neonatal intermediate or intensive care unit
Data presented are n (%)
Chi-square test for categorical variables. P-values for pairwise comparisons of Non-Hispanic Black or Hispanic vs. referent Non-Hispanic White.
The proportions attributed to each indication for cesarean are relative to the total number of births, including vaginal births.
Distributions for each component of the composite are presented as column percentages because components are not mutually exclusive. There were no maternal deaths.
Postpartum infection defined as any postpartum endometritis, wound complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia of unknown source, or septic pelvic thrombosis
Defined as birthweight <10th percentile for gestational age
Apgar score evaluated only among those women undergoing cesarean for non-reassuring fetal status
Table 3 displays unadjusted and adjusted regression models predicting cesarean birth, indication for cesarean, and maternal morbidity. Model 2 is adjusted for obstetric risk factors significant in bivariate analyses, including maternal age, BMI, modified Bishop score on admission, and study intervention group. Model 3 additionally adjusts for socioeconomic factors including employment, insurance, and marital status. Of note, hypertensive disorders of pregnancy, smoking, and prior pregnancy loss did not change the results and were removed from the models. Including assigned study group (elective induction vs expectant management) did not meaningfully alter the results, indicating that the disparity in cesarean birth was present irrespective of study group assignment; because this is a secondary analysis of a randomized controlled trial, study group was retained in the models. Non-Hispanic Black people had significantly increased relative risk of cesarean birth in all models [Model 1 unadjusted RR 1.30 (95% CI 1.14-1.47); Model 2 aRR 1.28 (95% CI 1.11-1.47); Model 3 aRR 1.21 (95% CI 1.03-1.42)]. Similarly, we found a significantly increased relative risk of cesarean birth in all models for Hispanic people [Model 1 unadjusted RR 1.25 (95% CI 1.10-1.41); Model 2 aRR 1.29 (95%CI 1.13-1.47); Model 3 aRR 1.26 (95% CI 1.08-1.46)]. Figure 1 displays a forest plot of Model 3 results.
Table 3.
Crude and adjusted relative risks for cesarean birth and maternal morbidity, and crude and adjusted odds of indication for cesarean, by race and ethnicity*
| Non-Hispanic Black* RR (95% CI) |
Hispanic* RR (95% CI) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome |
Model
1 Unadjusted |
Model 2 Adjusted for clinical factors† |
Model 3 Adjusted for clinical and SES factors‡ |
Model
1 Unadjusted |
Model 2 Adjusted for clinical factors† |
Model 3 Adjusted for clinical and SES factors‡ |
| Cesarean birth | 1.30 (1.14-1.47) | 1.28 (1.11-1.47) | 1.21 (1.03-1.42) | 1.25 (1.10-1.41) | 1.29 (1.13-1.47) | 1.26 (1.08-1.46) |
| Cesarean indication§ | ||||||
| Non-reassuring fetal status | 2.00 (1.62-2.47) | 1.97 (1.55-2.51) | 1.74 (1.32-2.29) | 1.62 (1.31-2.00) | 1.67 (1.33-2.11) | 1.51 (1.16-1.97) |
| Labor Dystocia | 0.94 (0.74-1.19) | 0.93 (0.71-1.21) | 0.90 (0.66-1.21) | 1.09 (0.88-1.35) | 1.16 (0.92-1.48) | 1.21 (0.92-1.59) |
| Other | 1.05 (0.61-1.80) | 1.21 (0.55-2.66) | 1.29 (0.53-3.12) | 1.22 (0.75-1.99) | 1.63 (0.85-3.15) | 1.66 (0.77-3.58) |
| Maternal morbidity composite‖ | 2.35 (1.51-3.65) | 2.22 (1.41-3.48) | 2.05 (1.21-3.47) | 2.48 (1.63-3.78) | 2.21 (1.44-3.37) | 1.92 (1.17-3.14) |
| Adjusted for cesarean birth¶ | --- | --- | 1.84 (1.11-3.05) | --- | --- | 1.76 (1.10-2.83) |
Abbreviations: RR – relative risk; CI – confidence interval
All models, including the multinomial model for cesarean indication, use vaginal birth as the outcome referent, and non-Hispanic White as the race and ethnicity referent.
Clinical factors include: maternal age, maternal body mass index (BMI) at admission, modified Bishop score, and treatment group.
SES factors include: employment status, insurance status, and marital status
Multinomial models for cesarean indication produce crude and adjusted odds ratios
Maternal morbidity composite defined as in Table 2/text. Maternal morbidity models are additionally adjusted for chorioamnionitis during labor and exclude modified Bishop score as a covariate.
Maternal morbidity Model 3 additionally adjusted for cesarean birth
Figure 1:
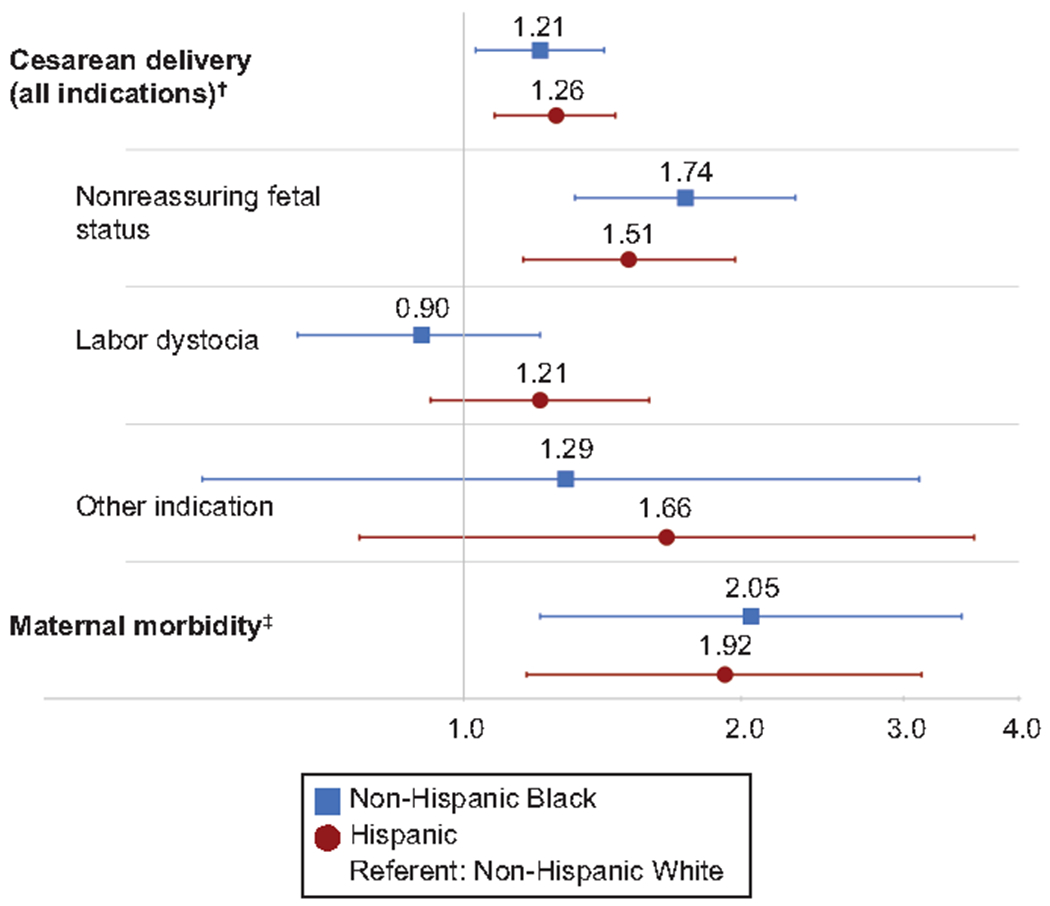
Adjusted* relative risks (aRRs) for cesarean birth and maternal morbidity, and adjusted* odds of cesarean indications, by race and ethnicity. Blue squares with blue lines indicate aRR or adjusted odds ratio (aOR), with error bars for 95% CIs for non-Hispanic Black individuals vs non-Hispanic White individuals. Red circles with red lines indicate aRR or aOR, with error bars for 95% CIs for Hispanic individuals vs non-Hispanic White individuals. Vertical grey line is an indicator for a relative risk or odds of 1.0 (line of unity). *Adjusted for clinical factors (maternal age, body mass index, modified Bishop score at admission, study group) and socioeconomic factors (employment status, insurance status, and marital status). †Vaginal delivery is the outcome referent for all models in which cesarean birth is the primary outcome, including multinomial model of indication for cesarean. ‡Composite includes transfusion of 4 or more units of packed red blood cells, transfusion of any other blood products, postpartum infection (postpartum endometritis, wound complication, cellulitis requiring antibiotics, pneumonia, pyelonephritis, bacteremia of unknown source, or septic pelvic thrombosis), intensive care unit admission, hysterectomy, venous thromboembolism, or maternal death. Maternal morbidity models are additionally adjusted for chorioamnionitis and excluded the covariate of modified Bishop score.
In adjusted multinomial regression models evaluating indications for cesarean, only non-reassuring fetal status differed significantly for non-Hispanic Black and Hispanic people compared with their non-Hispanic White counterparts (Table 3, Figure 1). Among non-Hispanic Black people, the adjusted odds of having a cesarean for non-reassuring fetal status (relative to vaginal birth) was 74% higher than for non-Hispanic White people (aOR 1.74, 95% CI 1.32-2.29). Among Hispanic people, the adjusted odds of cesarean for non-reassuring fetal status was 51% higher (aOR 1.51, 95% CI 1.16-1.97). Sensitivity analyses removing “Other” as an indication for cesarean birth did not alter the results (analyses not shown).
For both non-Hispanic Black (aRR 2.05, 95% CI 1.21-3.47) and Hispanic (aRR 1.92, 95% CI 1.17-3.14) people, the adjusted relative risk of maternal morbidity was twice that of non-Hispanic White people (Table 3). Since the majority of morbidity in this cohort stemmed from infection-related causes, adjusted maternal morbidity models included clinical chorioamnionitis as a covariate. The proportion of the relative risk of maternal morbidity accounted for by cesarean birth among non-Hispanic Black people was 15.8% (95% CI 2.1%-48.7%). For Hispanic people, cesarean accounted for 16.5% (95% CI 4.0%-44.0%) of the excess maternal morbidity.
Discussion
In a geographically and racially diverse cohort enrolled in a randomized trial limited to nulliparous people who were verified to be low-risk in the 38th week of gestation, non-Hispanic Black and Hispanic people had higher relative risk of cesarean birth compared with non-Hispanic White people. For both non-Hispanic Black and Hispanic individuals, these inequities were driven by an increased relative risk of cesarean birth for non-reassuring fetal status. Furthermore, cesarean birth may account for approximately 16% of the excess maternal morbidity among non-Hispanic Black and Hispanic people in this cohort.
Cesarean birth for non-reassuring fetal status was primarily responsible for the association between maternal Black race or Hispanic ethnicity and cesarean birth, a finding that comports with previous retrospective analyses.10,12 Though assigning a “primary” indication for cesarean birth can obscure a more complex clinical picture, these data do not permit us to ascertain details of the medical decision making. It is worth noting that despite attempts at standardization, the interpretation of fetal heart rate tracings remains somewhat subjective – particularly among the wide range of Category II tracings – and correlation with fetal outcomes is weak.35,36 Indeed, despite increased relative risk of cesarean for non-reassuring fetal status among Black and Hispanic individuals, we found no differences by race in indicators of neonatal compromise at delivery such as NICU admission or Apgar score. Therefore, the finding that a relatively subjective indication for cesarean birth accounts for the difference in cesarean rates for low-risk non-Hispanic Black and Hispanic people raises several possible interpretations.
First, the higher rate of cesarean due to fetal tracing among non-Hispanic Black and Hispanic people may stem from the inequitable use of a subjective indication for cesarean due to clinician bias, whether conscious or unconscious.37–39 This cohort of low-risk nulliparous PEOPLE lacks the most common risk factors (e.g., chronic hypertension, diabetes, fetal growth restriction) for placental insufficiency and accordingly greater risk of fetal intolerance of labor. Consequently, one would expect that abnormal fetal tracings leading to cesarean should be randomly distributed across racial and ethnic groups. We hypothesize that various potential biases (and likely a combination) during intrapartum management could explain this phenomenon. For instance, clinical obstetric teaching about the relationship between placental insufficiency and race may create an educational bias40 that leads clinicians to act prematurely on the fetal tracing of a non-Hispanic Black person that they might manage expectantly for a White person. Communication bias, in which clinicians word their concerns about fetal status differently depending upon the mother’s race or ethnicity, may lead a birthing person to more readily agree to a cesarean or feel more pressured to do so. Power dynamics and patients’ sense of agency may also play a role; people of color have reported lower birth satisfaction, increased stress in labor, and higher rates of mistreatment intrapartum.41
There may also be differences by race or ethnicity in antenatal management or access to care after randomization that could contribute to the risk for cesarean delivery, including cesarean due to non-reassuring fetal status. For instance, structural racism results in differences in access to prenatal care by race.42–44 Limited access to care at term among non-Hispanic Black or Hispanic people could create disparities in the timely diagnosis of post-randomization indications for delivery, and it is possible that delay in some diagnoses could increase risk of cesarean birth. Similarly, implicit and explicit bias have been associated with inappropriate dismissal of patient concerns and poor communication,41,45,46 which may also lead to delays that could increase the risk for cesarean. Notably, adjusting for hypertensive disorders of pregnancy did not significantly alter the association between race or ethnicity and cesarean birth, but we cannot exclude that other post-randomization diagnoses may have contributed to differential risk of cesarean. Even if that were so, however, it does not alter the fundamental finding: even among individuals who remain low risk just days before full term, there are still disparities in cesarean delivery by race and ethnicity that account for some of the variation in severe maternal morbidity.
Finally, one retrospective study found differences by race or ethnicity in selected fetal tracing parameters on computerized cardiotocography, a type of monitoring not routinely used in the US.47 The study did not evaluate whether physicians or midwives providing obstetric care can detect these differences in clinical practice, nor if they contribute to clinical decision making such as cesarean delivery. Furthermore, as race is a social construct, these differences, if present, would not be inherent to race itself. Given this, it seems unlikely that such differences contribute to the findings we observed in a US cohort.
The positive association between maternal morbidity and race was consistent with prior work.3 Although cesarean birth may account for only a modest proportion of excess morbidity among non-Hispanic Black or Hispanic people, if applied to the population, even small changes in primary cesarean may have broad ramifications for maternal morbidity. Approximately 800,000 of the >3 million births in the US each year are to low-risk nulliparous people at term, of whom approximately 12-13% are non-Hispanic Black and 20% Hispanic.2,48 Demonstrating this modest association between primary low-risk cesarean birth and excess maternal morbidity among non-Hispanic Black and Hispanic individuals contributes urgency to attempts to safely reduce primary cesarean birth, and should prompt future studies to further evaluate the relationship.
Our study does have limitations. As with any randomized trial, those who chose to enroll in ARRIVE may differ from the general population of term, low-risk nulliparous PEOPLE,49 but this limitation to generalizability would not be expected to explain racial or ethnic differences in cesarean birth. Additionally, as a secondary analysis, this study primarily serves as a hypothesis generating exercise. Of note, while the overall sample size is large, the sample sizes for some outcomes (e.g. maternal morbidity) are lower, resulting in relatively wide confidence intervals. Therefore, point estimates for these outcomes should be interpreted with caution. However, observational studies provide the bulk of the evidence regarding inequities in perinatal outcomes by self-reported race or ethnicity. Consistency of these findings across contexts, institutions, and settings provides confidence in the existence of a true relationship.6,8,10
Our study has several strengths. Previous studies used retrospective review of either medical records or administrative data,6,8,10 but individuals’ participation in ARRIVE depended upon a standardized, prospective assessment of eligibility. Ensuring that all included individuals were equally low-risk in the 38th week of pregnancy provides confidence in the diagnosis of “low-risk at term.” Data collection by trained personnel offers reliability of collected covariates and the indication for cesarean. The multi-site nature of the parent study improves generalizability compared with existing single-institution or single-state studies by increasing institutional, geographic, racial, and ethnic diversity in the cohort. Finally, this study provides an estimate of the relationship between primary cesarean birth and excess maternal morbidity among non-Hispanic Black and Hispanic women in a low-risk cohort, a novel contribution to the literature.
Future studies should seek to understand the factors that lead to increased primary cesarean birth among non-Hispanic Black and Hispanic people. Our findings suggest that the safe reduction of the primary cesarean rate among Black and Hispanic people may not only produce equity in surgical outcomes, but may partially address inequities in maternal morbidity. Reduction of primary cesarean birth among low-risk nulliparous people of color will likely require a multi-pronged approach to address institutional, structural, and clinician biases in addition to high-quality, safe intrapartum obstetric care overall.
Supplementary Material
Acknowledgements:
The authors thank Torri D. Metz, MD, MS for her contributions to the design of this secondary analysis and review and editing of the manuscript; Gail Mallett, R.N., M.S., C.C.R.C. and Kim Hill, R.N., B.S.N. for protocol development and coordination between clinical research centers; Lindsay Doherty, M.S. for protocol and data management; and Elizabeth A. Thom, Ph.D., Madeline M. Rice, Ph.D., and Robert M. Silver, M.D. for protocol development and oversight.
Financial Support:
Supported by grants (HD40512, U10 HD36801, HD27869, HD34208, HD68268, HD40485, HD40500, HD53097, HD40560, HD40545, HD27915, HD40544, HD34116, HD68282, HD87192, HD68258, HD87230) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MPD receives salary support from the March of Dimes and American Board of Obstetrics and Gynecology as part of the Reproductive Scientist Development Program. MMC receives salary support from the NICHD.
Footnotes
A list of other members of the NICHD MFMU Network is available in Appendix 1, available online at http://links.lww.com/AOG/C505.
Financial Disclosure
Geeta K. Swamy reports money was paid to them from GlaxoSmithKline, Pfizer, Moderna, and UpToDate (contributor). Money was paid to their institution from the CDC. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Dr. Rouse, Associate Editor (Obstetrics) of Obstetrics & Gynecology, was not involved in the review or decision to publish this article.
This work was accepted for presentation at the 67th Annual Meeting of the Society for Reproductive Investigation, Vancouver, British Columbia, Canada, March 10-14, 2020 [conference canceled secondary to COVID-19 pandemic].
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final Data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47. [PubMed] [Google Scholar]
- 2.Boyle A, Reddy UM, Landy HJ, Huang C-C, Driggers RW, Laughon SK. Primary Cesarean Delivery in the United States. Obstet Gynecol. 2013;122(1):33–40. doi: 10.1097/AOG.0b013e3182952242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver RM. Implications of the First Cesarean: Perinatal and Future Reproductive Health and Subsequent Cesareans, Placentation Issues, Uterine Rupture Risk, Morbidity, and Mortality. Seminars in Perinatology. 2012;36(5):315–323. doi: 10.1053/j.semperi.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 4.Edmonds JK, Yehezkel R, Liao X, Simas TAM. Racial and ethnic differences in primary, unscheduled cesarean deliveries among low-risk primiparous women at an academic medical center: a retrospective cohort study. BMC Pregnancy and Childbirth. 2013;13(1):168. doi: 10.1186/1471-2393-13-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDorman MF, Menacker F, Declercq E. Cesarean Birth in the United States: Epidemiology, Trends, and Outcomes. Clinics in Perinatology. 2008;35(2):293–307. doi: 10.1016/j.clp.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 6.Getahun D, Strickland D, Lawrence JM, Fassett MJ, Koebnick C, Jacobsen SJ. Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. American Journal of Obstetrics and Gynecology. 2009;201(4):422.e1–422.e7. doi: 10.1016/j.ajog.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 7.Janevic T, Loftfield E, Savitz DA, Bradley E, Illuzzi J, Lipkind H. Disparities in Cesarean Delivery by Ethnicity and Nativity in New York City. Matern Child Health J. 2014;18(1):250–257. doi: 10.1007/s10995-013-1261-6 [DOI] [PubMed] [Google Scholar]
- 8.Min CJ, Ehrenthal DB, Strobino DM. Investigating racial differences in risk factors for primary cesarean delivery. American Journal of Obstetrics and Gynecology. 2015;212(6):814.e1–814.e14. doi: 10.1016/j.ajog.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 9.Salahuddin M, Mandell D, Lakey D, Patel D. Maternal Risk Factor Index and Primary Cesarean Delivery among Low-Risk Women in Texas in 2015 [19E]. Obstetrics & Gynecology. 2018;131:57S. doi: 10.1097/01.AOG.0000533038.04320.a3 [DOI] [Google Scholar]
- 10.Washington S, Caughey AB, Cheng YW, Bryant AS. Racial and Ethnic Differences in Indication for Primary Cesarean Delivery at Term: Experience at One U.S. Institution. Birth. 2012;39(2):128–134. doi: 10.1111/j.1523-536X.2012.00530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yee LM, Costantine MM, Rice MM, et al. Racial and Ethnic Differences in Utilization of Labor Management Strategies Intended to Reduce Cesarean Delivery Rates. Obstetrics & Gynecology. 2017;130(6):1285. doi: 10.1097/AOG.0000000000002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris T, Meredith O, Schulman M, Morton CH. Race, Insurance Status, and Nulliparous, Term, Singleton, Vertex Cesarean Indication: A Case Study of a New England Tertiary Hospital. Women’s Health Issues. 2016;26(3):329–335. doi: 10.1016/j.whi.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Sebastiao Y. Racial and Ethnic Differences in Low-Risk Cesarean Deliveries in Florida. Graduate Theses and Dissertations. Published online October 21, 2016. https://scholarcommons.usf.edu/etd/6583 [Google Scholar]
- 14.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. American Journal of Obstetrics and Gynecology. 2010;202(4):335–343. doi: 10.1016/j.ajog.2009.10.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson NS, Corwin EJ, Hernandez TL, Holt E, Lowe NK, Hurt KJ. Association between provider type and cesarean birth in healthy nulliparous laboring women: A retrospective cohort study. Birth. 2018;45(2):159–168. doi: 10.1111/birt.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffey R, Andrews R, Moy E. Racial, Ethnic, and Socioeconomic Disparities in Estimates of AHRQ Patient Safety Indicators. Medical Care. 2005;43(3). Accessed January 6, 2020. insights.ovid.com [DOI] [PubMed] [Google Scholar]
- 17.Creanga AA, Bateman BT, Mhyre JM, Kuklina E, Shilkrut A, Callaghan WM. Performance of racial and ethnic minority-serving hospitals on delivery-related indicators. American Journal of Obstetrics and Gynecology. 2014;211(6):647.e1–647.e16. doi: 10.1016/j.ajog.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Declercq E, MacDorman M, Osterman M, Belanoff C, Iverson R. Prepregnancy Obesity and Primary Cesareans among Otherwise Low-Risk Mothers in 38 U.S. States in 2012. Birth. 2015;42(4):309–318. doi: 10.1111/birt.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grobman WA, Bailit JL, Rice MM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX Study. Am J Obstet Gynecol. 2014;211(2):147.e1–147.e16. doi: 10.1016/j.ajog.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henke RM, Wier LM, Marder WD, Friedman BS, Wong HS. Geographic variation in cesarean delivery in the United States by payer. BMC Pregnancy Childbirth. 2014;14. doi: 10.1186/s12884-014-0387-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huesch M, Doctor JN. Factors Associated With Increased Cesarean Risk Among African American Women: Evidence From California, 2010. Am J Public Health. 2015;105(5):956–962. doi: 10.2105/AJPH.2014.302381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozhimannil KB, Arcaya MC, Subramanian SV. Maternal clinical diagnoses and hospital variation in the risk of cesarean delivery: analyses of a National US Hospital Discharge Database. PLoS Med. 2014;11(10):e1001745. doi: 10.1371/journal.pmed.1001745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tangel V, White RS, Nachamie AS, Pick JS. Racial and Ethnic Disparities in Maternal Outcomes and the Disadvantage of Peripartum Black Women: A Multistate Analysis, 2007–2014. Am J Perinatol. 2019;36(8):835–848. doi: 10.1055/s-0038-1675207 [DOI] [PubMed] [Google Scholar]
- 24.Witt WP, Wisk LE, Cheng ER, et al. Determinants of Cesarean Delivery in the U.S.: A Lifecourse Approach. Matern Child Health J. 2015;19(1):84–93. doi: 10.1007/s10995-014-1498-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe Obstetric Morbidity in the United States: 1998–2005. Obstetrics & Gynecology. 2009;113(2):293–299. doi: 10.1097/AOG.0b013e3181954e5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrows LJ, Meyn LA, Weber AM. Maternal Morbidity Associated With Vaginal Versus Cesarean Delivery. Obstetrics & Gynecology. 2004;103(5):907–912. doi: 10.1097/01.AOG.0000124568.71597.ce [DOI] [PubMed] [Google Scholar]
- 27.Jain JA, Temming LA, D’Alton ME, et al. SMFM Special Report: Putting the “M” back in MFM: Reducing racial and ethnic disparities in maternal morbidity and mortality: A call to action. American Journal of Obstetrics and Gynecology. 2018;218(2):B9–B17. doi: 10.1016/j.ajog.2017.11.591 [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services. Healthy Women, Healthy Pregnancies, Healty Futures: Action Plan to Improve Maternal Health in America.; 2020:171. https://aspe.hhs.gov/system/files/aspe-files/264076/healthy-women-healthy-pregnancies-healthy-future-action-plan_0.pdf
- 29.Office of the Surgeon General. The Surgeon General’s Call to Action to Improve Maternal Health. Office of the Surgeon General; 2020:71. https://www.hhs.gov/sites/default/files/call-to-action-maternal-health.pdf [Google Scholar]
- 30.Grobman WA, Rice MM, Reddy UM, et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. New England Journal of Medicine. 2018;379(6):513–523. doi: 10.1056/NEJMoa1800566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–1036. doi:http://10.1097/AOG.0b013e31826d60c5 [DOI] [PubMed] [Google Scholar]
- 32.ACOG Practice Bulletin No. 222: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2020;135(6):e237–e260. doi: 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 33.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–231. doi: 10.1023/a:1022381506823 [DOI] [PubMed] [Google Scholar]
- 34.MacKinnon DP. Introduction to Statistical Mediation Analysis. Routledge; 2008. doi: 10.4324/9780203809556 [DOI] [Google Scholar]
- 35.ACOG Practice Bulletin No. 106: Intrapartum Fetal Heart Rate Monitoring: Nomenclature, Interpretation, and General Management Principles. Obstetrics & Gynecology. 2009;114(1):192–202. doi: 10.1097/AOG.0b013e3181aef106 [DOI] [PubMed] [Google Scholar]
- 36.Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of Fetal Heart Rate Categories and Short-Term Neonatal Outcome. Obstetrics & Gynecology. 2011;118(4):803–808. doi: 10.1097/AOG.0b013e31822f1b50 [DOI] [PubMed] [Google Scholar]
- 37.Chapman EN, Kaatz A, Carnes M. Physicians and Implicit Bias: How Doctors May Unwittingly Perpetuate Health Care Disparities. J Gen Intern Med. 2013;28(11):1504–1510. doi: 10.1007/s11606-013-2441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehon E, Weiss N, Jones J, Faulconer W, Hinton E, Sterling S. A Systematic Review of the Impact of Physician Implicit Racial Bias on Clinical Decision Making. Academic Emergency Medicine. 2017;24(8):895–904. doi: 10.1111/acem.13214 [DOI] [PubMed] [Google Scholar]
- 39.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18. doi: 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai J, Ucik L, Baldwin N, Hasslinger C, George P. Race Matters? Examining and Rethinking Race Portrayal in Preclinical Medical Education. Academic Medicine. 2016;91(7):916–920. doi: 10.1097/ACM.0000000000001232 [DOI] [PubMed] [Google Scholar]
- 41.Vedam S, Stoll K, Taiwo TK, et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod Health. 2019;16. doi: 10.1186/s12978-019-0729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green TL. Unpacking Racial/Ethnic Disparities in Prenatal Care Use: The Role of Individual-, Household-, and Area-Level Characteristics. Journal of Women’s Health. 2018;27(9):1124–1134. doi: 10.1089/jwh.2017.6807 [DOI] [PubMed] [Google Scholar]
- 43.Andaya E Race-ing Time: Clinical Temporalities and Inequality in Public Prenatal Care. Med Anthropol. 2019;38(8):651–663. doi: 10.1080/01459740.2019.1590826 [DOI] [PubMed] [Google Scholar]
- 44.Salm Ward TC, Mazul M, Ngui EM, Bridgewater FD, Harley AE. “You learn to go last”: perceptions of prenatal care experiences among African-American women with limited incomes. Matern Child Health J. 2013;17(10):1753–1759. doi: 10.1007/s10995-012-1194-5 [DOI] [PubMed] [Google Scholar]
- 45.Hall WJ, Chapman MV, Lee KM, et al. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am J Public Health. 2015;105(12):e60–e76. doi: 10.2105/AJPH.2015.302903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saluja B, Bryant Z. How Implicit Bias Contributes to Racial Disparities in Maternal Morbidity and Mortality in the United States. Journal of Women’s Health. 2020;30(2):270–273. doi: 10.1089/jwh.2020.8874 [DOI] [PubMed] [Google Scholar]
- 47.Tagliaferri S, Esposito FG, Fagioli R, et al. Ethnic analogies and differences in fetal heart rate variability signal: A retrospective study. Journal of Obstetrics and Gynaecology Research. 2017;43(2):281–290. doi: 10.1111/jog.13213 [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Troendle J, Reddy UM, et al. Contemporary Cesarean Delivery Practice in the United States. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10. doi: 10.1016/j.ajog.2010.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmichael SL, Snowden JM. The ARRIVE Trial: Interpretation from an Epidemiologic Perspective. Journal of Midwifery & Women’s Health. 2019;64(5):657–663. doi: 10.1111/jmwh.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


