Abstract
Background:
Hispanic patients have a higher incidence of gastric cancer when compared to non-Hispanics. Outlining clinicodemographic characteristics and assessing the impact of ethnicity on stage-specific survival may identify opportunities to improve gastric cancer care for this population.
Methods:
Patients with gastric cancer in the US Safety Net Collaborative (2012-2014) were retrospectively reviewed. Demographics, clinicopathologic characteristics, operative details, and outcomes were compared between Hispanic and non-Hispanic patients. Early onset gastric cancer was defined as age <50 years. Kaplan-Meier and Cox proportional-hazards models were used to identify the impact of ethnicity on disease-specific survival (DSS).
Results:
Seven hundred and ninety-seven patients were included, of which 219 (28%) were Hispanic. Hispanic patients were more likely to seek care at safety-net hospitals (66 vs 39%) and be uninsured (36 vs 17%), and less likely to have a primary care provider (PCP) (46 vs 75%; all P <0.05). Hispanic patients were twice as likely to present with early onset gastric cancer (28 vs 15%) and were more frequently diagnosed in the emergency room (54 vs 37%) with both abdominal pain and weight loss (44 vs 31%; all P <0.05). Treatment paradigms, operative outcomes, and DSS were similar between Hispanic and non-Hispanic patients when accounting for cancer stage. Cancer stage, pathologically positive nodes, and negative surgical margins were independently associated with DSS.
Conclusions:
A diagnosis of gastric cancer must be considered in previously healthy Hispanic patients who present to the emergency room with both abdominal pain and weight loss. Fewer than 50% of Hispanic patients have a PCP, indicating poor outpatient support. Efforts to improve outpatient support and screening may improve gastric cancer outcomes in this vulnerable population.
Keywords: Gastric cancer, Hispanic, Safety-net, Survival
Introduction
In 2017, nearly 25,000 people were diagnosed with gastric cancer in the United States (U.S.), 4,000 of which were Hispanic1. Though the overall incidence for gastric cancer is decreasing, Hispanics have a higher risk of early onset gastric cancer (age <50 years) and are 50%-60% more likely to develop gastric cancer than non-Hispanic whites2, 3. The presence of environmental and genetic risk factors for gastric cancer, like Helicobacter Pylori and CDH mutations, have also been shown to disproportionately affect low socioeconomic status and Hispanic individuals, respectively3, 4. Together, these findings make gastric cancer an important public health diagnosis in Hispanic patients in the U.S.
Safety-net hospitals (SNHs) provide a significant proportion of health-related services to the U.S. Hispanic population5. In the setting of emergent care, Hispanic patients are also more likely to present to SNHs when compared to non-Hispanic white patients living in the same zip code6. As a result, SNHs provide a significant proportion of gastric cancer care in the Hispanic population. Identifying clinical presentation patterns associated with gastric cancer in Hispanic patients may help SNHs mitigate the already apparent cancer health disparities observed in ethnic minorities for other cancers7.
In this study, we use a large mutli-institutional database to compare presentation patterns, treatment, and survival outcomes of Hispanic patients with gastric cancer at both SNHs and their affiliated academic medical centers (AMCs). We hypothesize that there will be important differences in clinical disease presentation among Hispanic patients which may lead to actionable targets to improve disease-specific survival.
Methods
Cohort and variables
Data were collected from the U.S. Safety Net Collaborative (USSNC) gastric cancer database, which represents 5 major academic medical centers and their affiliated safety net hospitals in the U.S: The University of Texas Southwestern/Parkland Memorial Hospital; Emory University/Grady Memorial Hospital; New York University /Bellevue Hospital; the University of Miami/Jackson Memorial Hospital; and University of Illinois at Chicago/John H. Stroger Jr. Hospital of Cook County. Individual IRB approvals and waivers of informed consent for retrospective chart review were obtained at each participating institution prior to inclusion in analysis.
Clinicodemographic and pathologic features were collected for each patient and include age, race, ethnicity, insurance status, hospital designation, medical history, clinical symptoms, cancer stage at diagnosis, diagnostic workup, treatment approach, therapy type, perioperative data, surgical pathology, post-operative complications, and follow up data including disease-specific survival (DSS). Gastric cancer staging is based on the American Joint Committee on Cancer (AJCC) 8th edition. Patients diagnosed with primary gastric cancer between January 1, 2012 and December 31, 2014 were included. Patients were grouped by self-reported ethnicity (Hispanic or non-Hispanic), and clinicodemographic, treatment, and survival data were compared. Subgroup analysis was performed to look specifically at Hispanic patients with early onset gastric cancer (defined as age <50 years).
Statistical analysis
Univariate analyses were performed to identify any differences in patient characteristics, gastric cancer symptoms, treatment approach, and survival outcomes by AJCC stage in Hispanic patients. Nearly all variables included in analysis had minimal missing data (<10%), with the exception of primary care provider (PCP) status (11% missing), H. Pylori status (24% missing), the presence of peptic ulcer disease (14% missing), metastatic disease identified on laparoscopy (85% missing), and initial stage at diagnosis (19% missing). Independent two-sample Student’s t-tests were used for continuous variables, while Chi-Squared tests (or Fisher Exact when appropriate with cell counts < 5 events) were used for categorical variables. Continuous data were evaluated for distribution normality by histogram and Shapiro-Wilk test (P <0.05 indicating non-normal distribution). Non-normally distributed continuous variables were then analyzed using Mann-Whitney U-tests for significance, when indicated. A variable had to be significant on univariable analysis between ethnic groups with P <0.05, or known to impact patient survival (i.e., gastric cancer stage) in order to be included in Cox-proportional hazards modeling. Kaplan-Meier analyses were performed to generate survival graphics, with log-rank tests utilized to assess for significance. Cox-Proportional hazards models with time-dependent covariates were used to confirm the finding that ethnicity and patient age were not associated with DSS. A P <0.05 was considered significant. All statistical analysis was performed using SPSS v21.0.0.0 (IBM Inc. Armonk, NY).
Results
Hispanic versus non-Hispanic gastric cancer
Seven hundred and ninety-seven patients were included, 578 (72.5%) of which were non-Hispanic and 219 (27.5%) who were Hispanic (Table 1). Significant demographic differences were observed between the two ethnic groups, with Hispanic patients more frequently seeking care at SNHs (66 vs 39%), more likely to be uninsured (32 vs 17%), and less likely to have U.S. citizenship (41 vs 66%; all P <0.05). Hispanic patients were also less likely to have a primary care provider (PCP) (46 vs 75%; P < 0.05), and among the entire cohort, patients without a PCP were more likely to be diagnosed in the ED (65 vs 29%, P <0.001), more likely to present to the ED with abdominal symptoms within 6 months prior to diagnosis (32 vs 19%, P <0.001), and more likely to be diagnosed with metastatic disease on presentation (62 vs. 44%, P <0.001). Notably, Hispanic patients were twice as likely to present with early onset gastric cancer (28% vs 15%), and were more frequently diagnosed in the emergency room (54% vs 37%) with both abdominal pain and weight loss (44% vs 31%; all P <0.05). When comparing SNHs versus AMCs, similar disparities were seen. SNH patients were more likely to be diagnosed in the ED with gastric cancer (61% vs 25%; P <0.001) and more likely to be uninsured (41% vs. 4%; P <0.001).
Table 1 –
Clinicodemographic characteristics of patients with gastric cancer, by ethnicity.
| Variable | Non-Hispanic Ethnicity (n = 578) | Hispanic Ethnicity (n = 219) |
P-value |
|---|---|---|---|
| Safety net hospital care (n,%) | 226 (39) | 145 (66) | 0.001 |
| Age group (years, SD) | 0.001 | ||
| Up to 49 | 77 (15) | 61 (28) | |
| 50 and older | 451 (85) | 154 (72) | |
| Male gender (n,%) | 364 (63) | 122 (56) | 0.199 |
| US citizenship (n,%) | 379 (66) | 89 (41) | 0.001 |
| BMI ≥ 30 (n,%) | 108 (19) | 47 (22) | 0.740 |
| Insurance type (n,%) | 0.001 | ||
| Private | 206 (36) | 41 (19) | |
| Government | 241 (42) | 97 (44) | |
| Hospital card | 32 (6) | 11 (5) | |
| Uninsured | 95 (17) | 70 (32) | |
| Primary care provider (n,%) | 376 (75) | 96 (46) | 0.001 |
| Hypertension (n,%) | 297 (52) | 86 (39) | 0.004 |
| Alcohol abuse (n,%) | 72 (13) | 19 (9) | 0.228 |
| Smoking history (n,%) | 185 (33) | 57 (26) | 0.191 |
| H. Pylori (n,%) | 98 (23) | 29 (15) | 0.049 |
| Peptic ulcer disease (n,%) | 137 (28) | 46 (23) | 0.166 |
| Location diagnosed (n,%) | 0.007 | ||
| Emergency room | 210 (37) | 117 (54) | |
| Primary care/office | 334 (59) | 93 (43) | |
| Incidentally found | 19 (3) | 8 (4) | |
| Any symptoms (n,%) | |||
| Abdominal pain | 331 (58) | 153 (71) | 0.001 |
| Weight loss | 251 (44) | 119 (55) | 0.012 |
| Bleeding | 167 (29) | 76 (35) | 0.102 |
| Obstruction | 300 (53) | 125 (58) | 0.456 |
| Abdominal pain and weight loss (n,%) | 0.002 | ||
| Neither | 162 (29) | 40 (19) | |
| Abdominal pain only | 154 (27) | 57 (26) | |
| Weight loss only | 74 (13) | 23 (11) | |
| Both abdominal pain and weight loss | 177 (31) | 96 (44) |
Some values may not add up to 100% secondary to missing values and rounding
In terms of radiographic tumor characteristics, Hispanic and non-Hispanic patients had similar tumor sizes, lymph node involvement, and evidence of metastases (Table 2). However, Hispanic patients were significantly more likely to present with antral/body tumors (71 vs 60%). Rates of staging laparoscopy were similar, but low (17 vs. 15%, P=0.433); however, when performed, Hispanic patients more frequently had metastatic disease identified (64 vs 43%; P=0.039). As a result, on initial clinical assessment, Hispanic patients were more frequently defined as metastatic (58 vs 45%), and had a trend toward more advanced AJCC stage at diagnosis.
Table 2 –
Clinicopathologic features of gastric cancer patients, by ethnicity.
| Variable | Non-Hispanic Ethnicity (n = 578) | Hispanic Ethnicity (n = 219) | P-value |
|---|---|---|---|
| Radiographic tumor size (cm) (median, IQR) | 4.0 (3.8) | 4.2 (5.8) | #0.702 |
| Radiographic lymph node involvement (n,%) | 310 (57) | 138 (66) | 0.061 |
| Radiographic metastatic disease (n,%) | 228 (41) | 102 (49) | 0.142 |
| Tumor Location (n,%) | 0.01 | ||
| Proximal (GE Junction/cardia) | 211 (40) | 60 (29) | |
| Distal (Body/antrum) | 318 (60) | 149 (71) | |
| EGD performed (n,%) | 521 (92) | 203 (96) | 0.18 |
| EUS performed (n,%) | 165 (30) | 41 (20) | 0.011 |
| Staging laparoscopy performed (n,%) | 82 (15) | 36 (17) | 0.433 |
| Metastatic disease on staging laparoscopy (n,%) | 35 (43) | 23 (64) | 0.039 |
| Initial clinical assessment (n,%) | 0.025 | ||
| Early | 101 (19) | 26 (13) | |
| Locally advanced | 196 (37) | 62 (30) | |
| Metastatic | 238 (45) | 119 (58) | |
| Stage at diagnosis* (n,%) | 0.133 | ||
| I | 67 (15) | 24 (13) | |
| II | 78 (17) | 20 (11) | |
| III | 91 (20) | 32 (17) | |
| IV | 224 (49) | 113 (60) |
Some values may not add up to 100% secondary to missing values and rounding
Stage refers to AJCC 8th Edition
Indicates Mann-Whitney U-Test
Treatment characteristics by AJCC stage and ethnicity were similar between the two groups (Table 3). The majority of stage I gastric cancer patients were treated with surgery alone in both Hispanics and non-Hispanics (50 and 61%, P=0.147). Similarly, both Hispanic and non-Hispanic Stage II and Stage III patients were frequently treated with both surgery and chemotherapy (80 vs 56% for Stage II; 56 vs 53% for Stage III), while Stage IV patients were often treated with systemic chemotherapy (59 vs 58%; P=0.996). When comparing SNHs versus AMCs, treatment characteristics for stage I-III gastric cancer were again similar, with the exception of stage IV patients, who were slightly more likely to undergo systemic therapy alone at SNHs (40% vs. 37%; P=0.029).
Table 3 –
Treatment approach, by stage and ethnicity.
| Variable | Non-Hispanic Ethnicity | Hispanic Ethnicity | P-value |
|---|---|---|---|
| Stage I * | n = 67 | n = 24 | 0.147 |
| No therapy (n,%) | 6 (9) | 0 (0) | |
| Systemic therapy alone (n,%) | 4 (6) | 1 (4.2) | |
| Surgery alone (n,%) | 41 (61) | 12 (50) | |
| Surgery and chemotherapy** (n,%) | 16 (24) | 11 (46) | |
| Stage II | n = 78 | n = 20 | 0.199 |
| No therapy (n,%) | 11 (14) | 0 (0) | |
| Systemic therapy alone (n,%) | 8 (10) | 1 (5) | |
| Surgery alone (n,%) | 15 (19) | 3 (15) | |
| Surgery and chemotherapy** (n,%) | 44 (56) | 16 (80) | |
| Stage III | n = 91 | n = 32 | 0.474 |
| No therapy (n,%) | 7 (8) | 2 (6) | |
| Systemic therapy alone (n,%) | 29 (32) | 12 (38) | |
| Surgery alone (n,%) | 7 (8) | 0 (0) | |
| Surgery and chemotherapy** (n,%) | 48 (53) | 18 (56) | |
| Stage IV | n = 224 | n = 113 | 0.996 |
| No therapy (n,%) | 61 (27) | 31 (27) | |
| Systemic therapy alone (n,%) | 130 (58) | 67 (59) | |
| Surgery alone (n,%) | 7 (3) | 3 (3) | |
| Surgery and chemotherapy** (n,%) | 26 (12) | 12 (11) |
Some values may not add up to 100% secondary to missing values and rounding
P-values represent Fisher Exact tests comparing the distribution of treatment types within each AJCC Stage.
Stage refers to AJCC 8th Edition
Chemotherapy refers to neoadjuvant and/or adjuvant
Among patients treated with surgical intervention, Hispanic patients exhibited similar rates of curative intent surgery (88 vs 92%), partial gastrectomy as the operation of choice (45 vs 45%), and D2 lymphadenectomy (75 vs 83%) (Table 4; all P>0.05). On pathology, the rates of nodal involvement (59 vs 55%), perineural invasion (38 vs 47%), signet cell histology (55 vs 46%), advanced T stage (54 vs 48%), poorly differentiated histology (76 vs 64%), and histologically negative (R0) margin (83 vs 87%) were similar (all P>0.05). The only significant differences observed were in non-Hispanic patients, who were more likely to undergo surgery alone without systemic therapy (36 vs 22%) and receive a blood transfusion during surgery (12 vs 5%; both P<0.05).
Table 4 –
Treatment features of surgical patients, by ethnicity.
| Variable | Non-Hispanic Ethnicity (n = 273) | Hispanic Ethnicity (n = 96) | P-value |
|---|---|---|---|
| Treatment approach | 0.045 | ||
| Surgery alone (n,%) | 99 (36) | 21 (22) | |
| Neoadjuvant only (n,%) | 42 (15) | 23 (24) | |
| Adjuvant only (n,%) | 78 (29) | 32 (33) | |
| Neoadjuvant and adjuvant (n,%) | 54 (20) | 20 (21) | |
| Curative intent (n,%) | 246 (92) | 85 (88) | 0.341 |
| Subtotal/partial gastrectomy (n,%) | 121 (45) | 43 (45) | 0.401 |
| Estimated blood loss (mL) (median, IQR) | 200 (225) | 200 (213) | #0.481 |
| Intraoperative RBC transfusion (n,%) | 29 (12) | 4 (5) | 0.049 |
| D2 Lymphadenectomy (n,%) | 166 (83) | 61 (75) | 0.277 |
| Positive nodes (n,%) | 138 (55) | 51 (59) | 0.806 |
| Perineural invasion (n,%) | 102 (47) | 32 (38) | 0.173 |
| Signet cell (n,%) | 116 (46) | 46 (55) | 0.146 |
| T3/T4 stage on pathology (n,%) | 123 (48) | 49 (54) | 0.58 |
| Poorly differentiated histology (n,%) | 161 (64) | 68 (76) | 0.078 |
| R0 margin status (n,%) | 211 (87) | 70 (83) | 0.497 |
Stage refers to AJCC 8th Edition
Indicates Mann-Whitney U-Test
Early onset Hispanic gastric cancer
Sixty-one (28%) Hispanic patients presented with early onset gastric cancer, which was twice as prevalent as early gastric cancer in non-Hispanic patients. Table 5 shows the clinicopathologic features of Hispanic patients stratified by age. Patients in the early onset gastric cancer group had a higher proportion of signet cell histology (62 vs 40%; P=0.013) and were found to have metastatic disease at the time of diagnostic laparoscopy (100% vs 56%; P=0.02) and pathologically positive nodes at the time of curative surgery (75 vs 52%; P=0.002) than those presenting at a later age. Early onset Hispanic gastric cancer patients were more likely to receive care at SNHs (79 vs 61%), more likely to be uninsured (43 vs 27%), but significantly less likely to have a dependent functional status or diagnosed medical comorbidities such as hypertension, diabetes, prior cardiac event, alcohol abuse, or smoking than their older counterparts (Table 5).
Table 5 –
Clinicopathologic features of Hispanic gastric cancer patients, by age.
| Variable | Hispanic under 50 (n = 61) | Hispanic 50 years and over (n = 154) | P-value |
|---|---|---|---|
| Male (n,%) | 33 (54) | 88 (57) | 0.685 |
| Safety net hospital care (n,%) | 48 (79) | 94 (61) | 0.014 |
| Insurance type (n,%) | 0.133 | ||
| Private | 11 (18) | 30 (20) | |
| Government | 21 (34) | 75 (49) | |
| Hospital card | 3 (5) | 8 (5) | |
| Uninsured | 26 (43) | 41 (27) | |
| Primary care provider (n,%) | 20 (33) | 74 (48) | 0.074 |
| US citizenship (n,%) | 20 (33) | 66 (43) | 0.063 |
| Partially dependent functional status (n,%) | 0 (0) | 23 (15) | 0.001 |
| Hypertension (n,%) | 4 (7) | 80 (52) | 0.001 |
| Diabetes (n,%) | 5 (8) | 41 (27) | 0.011 |
| Prior Cardiac Event (n,%) | 0 (0) | 17 (11) | 0.003 |
| Alcohol abuse (n,%) | 2 (3) | 17 (11) | 0.056 |
| Smoking (n,%) | 10 (17) | 47 (31) | 0.037 |
| Location diagnosed (n,%) | 0.039 | ||
| Emergency room | 41 (67) | 74 (48) | |
| Primary care/office | 18 (30) | 74 (48) | |
| Incidentally found | 2 (3) | 5 (3) | |
| Radiographic tumor size (cm) (median, IQR) | 4.3 (3.9) | 4 (6.5) | #0.921 |
| Radiographic lymph node involvement (n,%) | 42 (72) | 95 (65) | 0.314 |
| Tumor Location (n,%) | 0.667 | ||
| Proximal (GE Junction/cardia) | 16 (27) | 43 (30) | |
| Distal (Body/antrum) | 44 (73) | 102 (70) | |
| Staging laparoscopy performed (n,%) | 8 (14) | 27 (18) | 0.444 |
| Metastatic disease on staging laparoscopy (n,%) | 8 (100) | 15 (56) | 0.02 |
| Signet cell (n,%) | 28 (62) | 40 (40) | 0.013 |
| Stage at diagnosis (n,%) | 0.064 | ||
| I | 4 (7) | 20 (15) | |
| II | 9 (17) | 11 (8) | |
| III | 5 (9) | 26 (20) | |
| IV | 36 (67) | 75 (57) | |
| Curative intent (n,%) | 21 (84) | 62 (95) | 0.261 |
| Subtotal/partial gastrectomy (n,%) | 10 (40) | 32 (47) | 0.75 |
| D2 Lymphadenectomy (n,%) | 14 (70) | 46 (77) | 0.236 |
| Pathologically positive nodes (n,%) | 15 (75) | 33 (52) | 0.074 |
| Peritoneal disease (n,%) | 7 (29) | 2 (3) | 0.002 |
| T3/T4 stage on pathology (n,%) | 13 (57) | 35 (53) | 0.86 |
| Poorly differentiated histology (n,%) | 20 (93) | 47 (75) | 0.353 |
| R0 margin status (n,%) | 17 (85) | 52 (84) | 0.706 |
Some values may not add up to 100% secondary to missing values and rounding
Indicates Mann-Whitney U-Test
Survival
DSS was similar by AJCC stage in both Hispanic and non-Hispanic patients (Fig. 1). The median survival for Stage I gastric cancer in both ethnic groups was not reached at 5 years, while the median survival for Stage II was not reached in non-Hispanics but 51 months (mo.) in Hispanics (P=0.997). Similarly, the median survival for Stage III gastric cancer was 29 mo. in non-Hispanics and 18 mo. in Hispanics (P=0.773), while the median survival for Stage IV was 11.5 mo. in non-Hispanics versus 8.5 mo. in Hispanics (P=0.1). On multivariable Cox Regression, advanced AJCC stage (HR 2.97 [1.34-6.61]), nodal positivity (HR 2.25 [1.22-4.15]), and obtaining an R0 margin (3.22 [0.41-25.08]) remained independent predictors or DSS (Table 6), while the impact of hospital type and chemotherapy administration lost significance when controlling for stage (HR 0.51 [0.22-1.19]). There were no observed differences in the DSS by AJCC stage between early onset Hispanic gastric cancer patients and Hispanic patients over 50 years of age (Fig. 2A-D).
Fig. 1 –
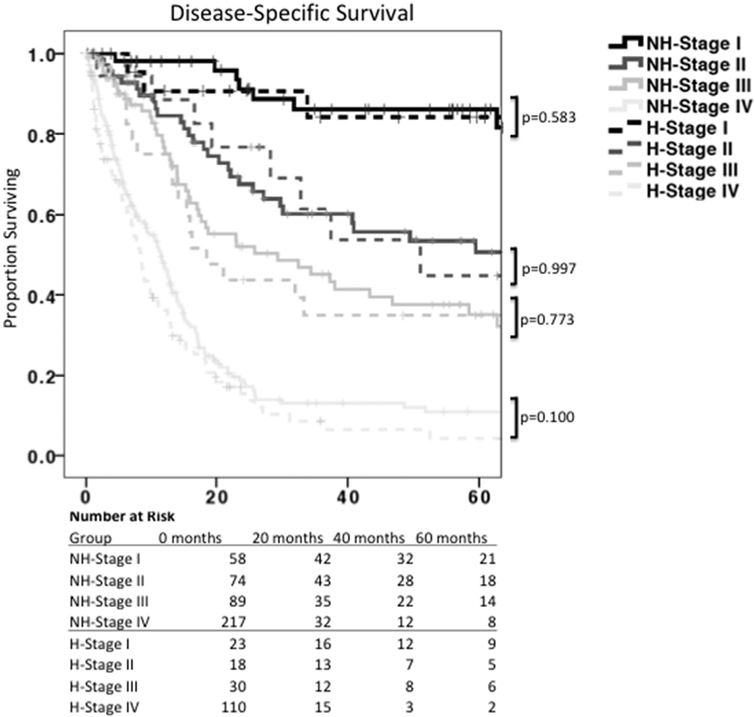
Disease-Specific Survival, by ethnicity and stage. Kaplan-Meier survival analysis showing disease-specific survival by ethnicity and AJCC stage. While AJCC staging adequately prognosticates survival, there was no difference in disease-specific survival between Hispanics and non-Hispanics. NH = non-Hispanic. H = Hispanic.
Table 6 –
Disease-specific survival model.
| Disease-specific survival |
||||
|---|---|---|---|---|
| Univariable |
Multivariable |
|||
| Variable | HR [95% CI] | P-value | HR [95% CI] | P-value |
| Hispanic ethnicity | 0.78 [0.64-0.96] | 0.02 | 0.76 [0.44-1.33] | 0.343 |
| Age under 50 | 1.18 [0.92-1.51] | 0.196 | n/a | n/a |
| Safety net hospital care | 0.70 [0.58-0.85] | 0.001 | 0.96 [0.51-1.91] | 0.959 |
| Private insurance type | 0.56 [0.42-0.74] | 0.001 | 0.66 [0.28-1.55] | 0.335 |
| Treatment approach | ||||
| Surgery alone | Reference | n/a | Reference | n/a |
| Neoadjuvant chemotherapy | 3.59 [2.14-6.02] | 0.001 | 1.36 [0.57-3.29] | 0.489 |
| Adjuvant chemotherapy | 1.57 [0.95-2.60] | 0.078 | 0.82 [0.39-1.75] | 0.611 |
| Neoadjuvant + adjuvant chemotherapy | 1.18 [0.67-2.07] | 0.567 | 0.51 [0.22-1.19] | 0.118 |
| Antrum/body tumor location | 0.93 [0.76-1.15] | 0.496 | n/a | n/a |
| Stage at diagnosis* | ||||
| Stage I | Reference | n/a | Reference | n/a |
| Stage II | 2.86 [1.52-5.39] | 0.001 | 1.76 [0.81-3.81] | 0.154 |
| Stage III | 4.74 [2.60-8.65] | 0.001 | 2.97 [1.34-6.61] | 0.008 |
| Stage IV | 12.70 [7.23-22.33] | 0.001 | 2.62 [1.04-6.61] | 0.042 |
| Pathologically positive nodes | 2.50 [1.64-3.82] | 0.001 | 2.25 [1.22-4.15] | 0.009 |
| R0 margin | 0.14 [0.07-0.29] | 0.001 | 0.30 [0.12-0.73] | 0.008 |
| Poorly differentiated histology | 4.70 [1.50-14.70] | 0.008 | 3.22 [0.41-25.08] | 0.265 |
| Signet cell type | 1.03 [0.79-1.35] | 0.83 | n/a | n/a |
Stage refers to AJCC eighth Edition
Fig. 2 –
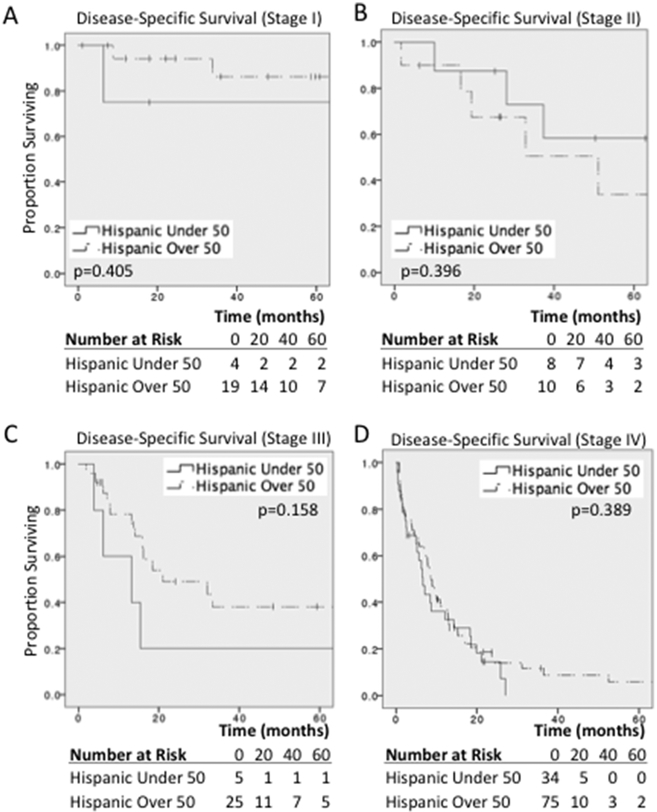
Disease-Specific Survival in early onset Hispanic gastric cancer. Stage I (A), Stage II (B), Stage III (C) and Stage IV (D) Kaplan-Meier survival analysis comparing early onset gastric cancer in Hispanic patients to Hispanic patients over 50 years of age. NH = non-Hispanic. H = Hispanic.
Discussion
The incidence of gastric cancer in the Hispanic population is rising, especially among young Hispanic men8. Since a large percentage of Hispanic patients receive medical care at SNHs5, SNHs are in the unique position to significantly improve gastric cancer outcomes in the U.S. Hispanic population by not only identifying at-risk patients early, but also defining prognostic features. In this study, we show that when compared to non-Hispanic patients, Hispanic patients more frequently present with early onset gastric cancer and more often exhibit symptoms (abdominal pain and weight loss). Furthermore, we show that despite being healthy without medical comorbidities, young Hispanic patients often present to SNHs emergency rooms with advanced or metastatic gastric cancer. Among the entire cohort, Hispanic patients were less likely to have a PCP than non-Hispanic patients, and patients without a PCP more frequently presented to the ED with abdominal symptoms and were more likely to be diagnosed with metastatic disease. These results suggest that for any Hispanic patient presenting to the emergency room with abdominal pain and weight loss, gastric cancer should be considered on the differential even in the presence of a healthy medical profile. It also argues that improved access to PCPs may help identify early stage gastric cancer in an at-risk Hispanic population.
The prevalence of advanced or metastatic gastric cancer in the Hispanic population in this study is concerning, yet the etiology is still poorly understood. Other groups have shown that age, sex, socioeconomic status, diet, and country of origin are all associated with gastric cancer location, histology, and incidence, and therefore should be considered during diagnosis and management9,10. Unfortunately, very few of the patients in our database had documented testing for CDH mutations. However, CDH mutations have recently been shown to be more prevalent in the Hispanic population and may contribute to the aggressive phenotype2. While this has important implications for surgical management, patient and family screening guidelines are not yet well established. Finally, H. Pylori infection is another well-defined risk factor for gastric cancer, though in this cohort H. Pylori was less prevalent in Hispanic patients. Consequently, a thorough workup of Hispanic patients with gastric cancer should include consideration of all of these factors (age, sex, socioeconomic status, diet, country of origin, H. Pylori, and CDH mutation status).
The present study shows that when controlling for AJCC Stage, Hispanic patients have similar DSS when compared to non-Hispanic patients. In this cohort, hospital designation and insurance status are also not associated with DSS when controlling for cancer stage. These results are encouraging, as it shows that SNHs are achieving equitable outcomes when compared to academic medical centers. Furthermore, since ethnicity does not independently impact outcomes, efforts focused on outpatient screening and early-stage diagnosis may directly improve gastric cancer outcomes in both Hispanic and non-Hispanic patients. Though there were only a small number of patients, our data also show that Hispanic patients with early-stage gastric cancer have similar DSS to older Hispanic patients with gastric cancer when controlling for stage. While this should be interpreted with caution given the small number of patients, this further suggests that diagnosis at an earlier stage (and a higher index of suspicion in young Hispanic patients) has the potential to improve survival outcomes.
There are several limitations of our study that should be considered. First, this was a multi-institutional study, and we could not control for institutional differences in SNH resources. In fact, each of the SNH in the US SNC is closely affiliated with an academic medical center, and many faculty hold dual appointments. Admittedly, this may reduce the generalizability of our findings to other public hospital systems or community hospitals. Second, the results indicating a high rate of metastases on staging laparoscopy in Hispanics with early-onset gastric cancer must be interpreted with caution, as overall rates of staging laparoscopy were low. Additional information regarding criteria and selection for staging laparoscopy is needed to draw a definitive conclusion in these age-dependent cohorts. Third, given the retrospective design of this study, associations but not causal relationships can be defined. Finally, although our study included nearly 800 patients, only a small number of patients with early onset Hispanic gastric cancer could be evaluated, limiting the number of patients included in this important subgroup.
Conclusion
A high index of suspicion for gastric cancer is needed in previously healthy, young Hispanic patients who present to the emergency room with abdominal pain and weight loss. While Hispanic patients more frequently present with early-onset gastric cancer and more advanced disease, disease-specific survival by stage is similar to non-Hispanics. Efforts to improve outpatient support and screening in high-risk Hispanic individuals may improve outcomes in this population.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclosure
The authors have declared that no conflicts of interest exist.
REFERENCES
- 1.Group UCSW. US Cancer Statistics Data Visualizations Tool, based on 2019 submission data (1999-2017). 2020. [Google Scholar]
- 2.Sanjeevaiah A, Cheedella N, Hester C, et al. Gastric cancer: recent molecular classification advances, racial disparity, and management implications. J Oncol Pract. 2018;14:217–224. [DOI] [PubMed] [Google Scholar]
- 3.Society, A.C. Cancer Facts & Figures for Hispanic/Latinos 2018-2020. American Cancer Society, Inc; 2018. [Google Scholar]
- 4.Wang SC, Yeu Y, Hammer STG, et al. Hispanic/Latino patients with gastric adenocarcinoma have distinct molecular profiles including a high rate of germline CDH1 variants. Cancer Res. 2020;80:2114–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Medicine (US) Committee on the Changing Market, M.C., and the Future Viability of Safety Net Providers. (2000) America’s health care safety net: intact but endangered., National Academies Press (US). [PubMed] [Google Scholar]
- 6.Hanchate AD, Paasche-Orlow MK, Baker WE, et al. Association of race/ethnicity with emergency department destination of emergency medical services transport. JAMA Netw Open. 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124:315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant SJ, Kim J, Choi AH, et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer. 2017;20:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang ET, Gomez SL, Fish K, et al. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila). 2012;5:150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]


