Abstract
Background/Aim:
Adiposity trajectories reflect dynamic process of growth and may predict later life health better than individual measures. Prenatal phthalate exposures may program later childhood adiposity, but findings from studies examining these associations are conflicting. We investigated associations between phthalate biomarker concentrations during pregnancy with child adiposity trajectories.
Methods:
We followed 514 mother-child pairs from the Mexico City PROGRESS cohort from pregnancy through twelve years. We measured concentrations of nine phthalate biomarkers in 2nd and 3rd trimester maternal urine samples to create a pregnancy average using the geometric mean. We measured child BMI z-score, fat mass index (FMI), and waist-to-height ratio (WHtR) at three study visits between four and 12 years of age. We identified adiposity trajectories using multivariate latent class growth modeling, considering BMI z-score, FMI, and WHtR as joint indicators of latent adiposity. We estimated associations of phthalates biomarkers with class membership using multinomial logistic regression. We used quantile g-computation to estimate the potential effect of the total phthalate mixture and assessed effect modification by sex.
Results:
We identified three trajectories of child adiposity, a “low-stable”, a “low-high”, and a “high-high” group. A doubling of the sum of di (2-ethylhexyl) phthalate metabolites (ΣDEHP), was associated with 1.53 (1.08, 2.19) greater odds of being in the “high-high” trajectory in comparison to the “low-stable” group, whereas a doubling in di-isononyl phthalate metabolites (ΣDiNP) was associated with 1.43 (1.02, 2.02) greater odds of being in the “low-high” trajectory and mono (carboxy-isononyl) phthalate (MCNP) was associated with 0.66 (0.45, 97) lower odds of being in the “low-high” trajectory. No sex-specific associations or mixture associations were observed.
Conclusions:
Prenatal concentrations of urinary DEHP metabolites, DiNP metabolites, and MCNP, a di-isodecyl phthalate metabolite, were associated with trajectories of child adiposity. The total phthalate mixture was not associated with early life child adiposity.
Keywords: Phthalates, Children’s environmental health, Environmental epidemiology, Mixtures analysis
1. Introduction
Trajectories of childhood adiposity are predictors of later life cardiometabolic diseases (Barraclough et al., 2019; Norris Tom et al., 2021) and may have greater clinical implications than a single BMI measurement or overall BMI (Aris et al., 2019; Juonala et al., 2012; Owen et al., 2009). Early life exposures to environmental toxicants may lead to altered adiposity in early childhood that tracks through to adulthood via structural and functional changes in endocrine and adipocyte signaling. Longitudinal environmental health studies that do not consider adiposity trajectories may miss important trends that correlate with life stages at greater risk for later cardiometabolic risk.
Phthalates are prevalent chemicals with widespread use in consumer and industrial products (Wormuth et al., 2006). Although phthalates are rapidly metabolized and excreted, because of recurrent and frequent exposures, urinary phthalate biomarkers are ubiquitously detected in United States and Mexican populations, including pregnant women (Katsikantami et al., 2016; López-Carrillo et al., 2010; Wu et al., 2020). Research in experimental models suggests that phthalates may act as environmental “obesogens”(Hao et al., 2012; Lin et al., 2011; Schmidt et al., 2012), though human studies examining prenatal phthalate exposures and childhood adiposity present inconsistent or conflicting findings. Age differences may help to explain these associations, as the risk for obesity changes across childhood. For instance, the sum of urinary concentrations of prenatal di (2-ethylhexyl) phthalate metabolites (ΣDEHP) was negatively associated with fat mass from four to nine years in one study(Buckley et al., 2016b), while it was positively associated with multiple adiposity indicators from five to 12 years in another(Harley et al., 2017). Mono-3-carboxypropyl phthalate (MCPP) was positively associated with overweight status from four to seven years of age (Buckley et al., 2016a), while later work in the same cohort observed that monobenzyl phthalate (MBzP) was negatively associated with adiposity at age eight (Shoaff et al., 2017). In other cohorts, prenatal MBzP urinary concentrations were inversely associated with child’s BMI z-score from eight to 14 years (Yang et al., 2018).
Our objectives were to: 1) determine if prenatal urinary concentrations of individual phthalate biomarkers are associated with trajectories of child adiposity from four to 12 years and with general longitudinal childhood adiposity; 2) investigate associations of the overall phthalate mixture; and 3) characterize sex-specific effects.
2. Material and Methods
2.1. Study Participants
We used information from the Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) study, a prospective birth cohort that enrolled pregnant women in Mexico City, Mexico, from 2007 to 2011. Enrollment details are described elsewhere (Braun et al., 2014; Burris et al., 2013). Of the 948 women that delivered a live birth and agreed to follow-up, 786 had prenatal phthalate metabolites measurements. We excluded six women who reported smoking during pregnancy due to the adverse effects of prenatal tobacco smoke exposure on child development. Children were followed-up at a four-, six-, and eight-year visit with 573 children returning for the four-year follow-up visit and 514 with at least one other follow-up. All study protocols were approved by the institutional review boards of the participating institutions (Icahn School of Medicine human subject’s management #12-00751, Instituto Nacional de Salud Pública project #560, and Columbia University Human Research Protection Office protocol #AAAR0689). The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects’ research. Women provided written informed consent and children provided assent of study protocols.
2.2. Quantification of Maternal Urinary Phthalate Metabolites
We collected maternal spot urine samples during the 2nd and 3rd trimester visits. Samples were analyzed for 15 phthalate metabolites at the CDC by isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry as previously described (Silva et al., 2007). The limits of detection (LODs) ranged from 0.2 to 1.2 μg/L, with the detection frequency ranging from 100% to 81% (Supplemental Table S1). For concentrations reported to be below the LOD, we used the instrument reported value, as this may provide more information than a LOD based imputation (Schisterman et al., 2006), and has previously not altered associations between phthalates and other cardiometabolic risk factors in this population in comparison to an LOD/√2 imputation (Kupsco et al., 2021). To adjust for urinary dilution, we standardized biomarker concentrations by urine specific gravity (Duty et al., 2005), which was measured using a digital handheld refractometer (AR200, Reichert Technologies, Buffalo, NY). We replaced missing specific gravity values for 101 2nd trimester urine samples using the median value (1.016) (Wu et al., 2020).
2.3. Measurement of Child Adiposity
Children were followed-up at a four-year (N = 514; age range = 4.0 – 6.8; mean = 4.8), a six-year (N = 506; age range = 6.0 – 9.7; mean = 6.7), and an eight-year visit (N = 463; age range = 8.1 – 11.7; mean = 9.7) for adiposity assessments. Trained staff collected child weight and standing height twice using a professional digital scale (Health-o-meter, McCook, IL) and averaged the values. BMI z-scores were estimated using the World Health Organization guidelines (de Onis et al., 2007; de Onis and World Health Organization (WHO), 2006). We estimated fat mass using Tetrapolar bioelectrical impedance with the InBody 370 (Biospace Co, Ltd, Cerritos, CA) at the four-year visit and part of the six-year visit, then switched to the Inbody 230 in the six-year visit and the eight-year visit. As values on these two instruments differed systematically, we used a robust linear model fit on a calibration set of 36 children with concurrent measures to adjust the values (R2 = 0.99) (Renzetti et al., 2017). Fat mass index (FMI) was calculated as Fat Mass / Height (m)2. Waist circumference was measured above the iliac crests using a SECA measuring tape and waist to height ratio (WHtR) was calculated as the ratio between waist circumference and child height.
2.4. Covariates
We obtained prenatal covariate information from baseline questionnaires. An index of socioeconomic status (SES) was calculated based on the AMAI rule 13×6 that we collapsed into a relative three-level index of low, medium, and high (Carrasco, 2002). Covariates were selected based on directed acyclic graphs including: child sex, maternal age at enrollment, pre-pregnancy BMI (kg/m2) estimated from 2nd trimester weight as previously described (Soria-Contreras et al., 2020), parity (primiparous or multiparous), education (< high school, high school, or > high school) and SES at enrollment. We included date at first urine collection, which was associated with the exposures and outcomes, as a representation of long-term and seasonal trends. For longitudinal mixed-effects models of adiposity, we additionally included linear and quadratic terms for child age at each measurement to control for changes in outcomes with age. Models specified with only child age and sex were not meaningfully different from fully adjusted models, so we present fully adjusted models throughout.
2.5. Statistical Analyses
2.5.1. Phthalate Exposure Biomarkers
Phthalates metabolite data were treated as previously described (Kupsco et al., 2021; Wu et al., 2020). In brief, we obtained an estimate of pregnancy-wide exposures as the geometric mean of the 2nd and 3rd trimesters for each metabolite. To reduce dimensionality and account for high correlations among metabolites from the same parent compound, we calculated the molar sums for di (2-ethylhexyl) phthalate (ΣDEHP), di-iso-butyl phthalate (ΣDiBP), di-isononyl phthalate (ΣDiNP), and di-n-butyl phthalate (ΣDBP). The final nine phthalate biomarkers included in our analyses were: ΣDEHP, ΣDiBP, ΣDiNP, ΣDBP, MBzP, mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), mono (carboxy-isononyl) phthalate (MCNP), MCPP, and monoethyl phthalate (MEP), which were log2 transformed to reduce the influence of high values.
2.5.2. Multivariate Latent Class Growth Models
To empirically identify trajectories of childhood adiposity, we implemented multivariate latent class growth modeling (LCGM) with child BMI z-score, WHtR, and FMI as joint height-adjusted measures of underlying latent adiposity. First, we constructed trajectory models using non-centered BMI z-score, WHtR and FMI, that allowed for different starting adiposity values for each child. Next, we constructed trajectory models that centered BMI z-score, WHtR, and FMI at each child’s starting value. These approaches allow us to capture both trajectories of change irrespective of starting adiposity and those dependent on a starting adiposity value.
We used the R package lcmm to construct our trajectory models (Proust-Lima et al., 2017), including child-specific random intercepts in all analyses and age-specific random intercepts in non-centered models. We systematically evaluated varying model parameters to determine the best fitting models. We considered: 1) varying the number of classes from two to five; 2) modeling age as a linear or a quadratic term; 3) inclusion of outcome-specific random intercepts; and 5) multiple specifications of the link function for each adiposity outcome, including linear, quantile splines (2 to 3 knots), or equidistant splines (3 or 4 knots). To select the final models, we required a posterior probability > 0.7 and a class size greater than 10% of the population, then compared Bayesian information criterion to select the best fitting model. We confirmed model validity by verifying odds of correct classification > 5 and relative entropy values ~1 using LCTMtools (Lennon et al., 2018). We assigned each child to a trajectory class. To assess sex differences in adiposity trajectories, we fit new LCGM models considering sex and its interaction with the linear and quadratic terms of age for the fixed and mixture effects.
Final LCGM models for non-centered adiposity outcomes included: three classes, linear and quadratic fixed effects for child age at follow-up, and three quantile splines on each adiposity outcome. Final LCGM models for centered adiposity outcomes included: three classes, linear and quadratic terms for age centered at the first visit, and splines with four equidistant knots on BMI z-score and FMI, and a linear link function on WHtR. For the centered adiposity outcomes, the splines transformation entailed parameters at the boundary of the parameter space, which led to a lack of convergence. To correct this, the best positions from the original models for the quadratic I-splines with nodes at 4.2 for BMI z-score and at 10.8 for FMI were fixed at their starting values and models were refit without re-estimating those parameters. Model diagnostics are available in Supplemental Table S4.
2.5.3. Associations between Phthalate Biomarkers and Adiposity Trajectories and Longitudinal Adiposity Indicators.
To estimate associations between individual phthalate biomarkers and trajectories of adiposity, we ran covariate-adjusted, multinomial logistic regressions with class membership as the outcome using the stable low trajectory class as the reference category. Models included all log 2-transformed phthalate biomarkers in a single model. Results can be interpreted as the odds of being in a trajectory class per doubling of phthalate biomarker. To identify sex-specific associations, we used the sex-specific trajectories to determine class membership, then ran multinomial logistic regressions with the population stratified by child sex or including interactions between sex and each phthalate biomarker.
We used multivariable linear mixed-effects models with a random intercept for subject to estimate associations between the nine phthalate biomarkers and child BMI z-score, FMI, and WHtR, including all nine phthalate biomarkers in each model. We investigated child sex as an effect measure modifier by testing interaction terms of child sex and each phthalate biomarker and ran sex-stratified models to determine sex-specific effect estimates. Effect estimates are expressed as the change of the outcome per doubling in the exposure with the 95% confidence intervals (CI).
2.5.4. Mixture Analysis with Quantile G-Computation
We investigated the associations of the total biomarker mixture with trajectory class membership and longitudinal BMI z-score, FMI and WHtR in outcome-specific models, using quantile g-computation (Keil et al., 2020). Quantile g-computation calculates a weighted sum of all phthalate biomarkers based on their quantiles, then estimates the effect of increasing all exposures by one quantile simultaneously on the outcome, conditional on covariates, using a generalized linear mixed model implementation of g-computation with a random intercept for subject. We converted phthalate biomarkers into quartiles and ran 1000 bootstraps to estimate confidence bounds of the overall mixture effect. This can be interpreted as either the relative risk of membership in a trajectory class in comparison to the reference class per quartile increase in total phthalates or, for the continuous longitudinal outcomes, as the effect of increasing the total phthalates by one quartile on child BMI z-score, WHtR, or FMI.
2.5.5. Sensitivity Analyses
To determine if trimester-specific associations were driving our results, we repeated analyses with 2nd and 3rd trimester phthalate biomarkers. We also repeated multinomial logistic regression and linear mixed-effect models with each phthalate biomarker in separate models to determine the impact of co-exposure confounding on our results. We altered the number of trajectories from three to two or four to determine the robustness of our results under alternative specifications of the trajectories.
All statistical analysis were conducted using R software version 3.5.1 (R Core Team, 2018) and statistical significance was assessed at alpha = 0.05.
3. Results
3.1. Study Participant Characteristics
At enrollment, mothers were on average 27.7 years of age, of lower socioeconomic status, and with less than a high school education (Table 1). Of the 514 children included in the analysis 455 children attended all three visits and 59 children attended the four-year visit and either follow-up visit. Maternal characteristics at enrollment of children included in the analysis did not materially differ from those who were excluded (Supplemental Table S2). Average child BMI z-scores, fat mass, and FMI increased across follow-ups, while WHtR remained constant (Table 1). Adiposity outcomes were highly correlated within visits (Pearson’s r = 0.82 – 0.92) (Supplemental Figure S1). Intraclass correlation coefficients for BMI z-score, FMI and WHtR were 0.73, 0.51 and 0.64, respectively.
Table 1.
Characteristics of PROGRESS children included at each follow-up visit.
| Four year visit | Six year visit | Eight year visit | ||||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| Child Age (years) | 514 | 4.8 ± 0.57 | 506 | 6.7 ± 0.57 | 463 | 9.7 ± 0.67 |
| Height (cm) | 514 | 106 ± 5.4 | 506 | 118 ± 6.0 | 463 | 135 ± 7.5 |
| Waist Circumference (cm) | 514 | 52.6 ± 5.3 | 506 | 56.4 ± 7.7 | 463 | 67.3 ± 10.7 |
| Waist to Height Ratio | 514 | 0.5 ± 0.04 | 506 | 0.48 ± 0.05 | 463 | 0.5 ± 0.07 |
| BMI Z-score | 514 | 0.23 ± 1.1 | 506 | 0.4 ± 1.3 | 463 | 0.87 ± 1.3 |
| Fat Mass (kg) | 511 | 4.4 ± 1.9 | 505 | 6.0 ± 3.5 | 462 | 11.3 ± 6.0 |
| Fat Mass Index (kg/m2) | 511 | 3.9 ± 1.5 | 505 | 4.2 ± 2.1 | 462 | 6.0 ± 2.8 |
| Child Sex | ||||||
| Female | 258 | 50.2 | 256 | 50.6 | 234 | 50.5 |
| Male | 256 | 49.8 | 250 | 49.4 | 229 | 49.5 |
| Maternal Characteristics at Enrollment | Four year visit Population | Six year visit Population | Eight year visit Population | |||
| Age (years) | 514 | 27.7 ± 5.7 | 506 | 27.8 ± 5.7 | 463 | 27.7 ± 5.7 |
| Pre-Pregnancy BMI (kg/m2) | 514 | 26.4 ± 4.1 | 506 | 26.4 ± 4.1 | 463 | 26.5 ± 4.1 |
| Parity | ||||||
| Multi-parous | 320 | 62.3 | 314 | 62.1 | 290 | 62.6 |
| Primi-parous | 194 | 37.7 | 192 | 37.9 | 173 | 37.4 |
| Socioeconomic Status | ||||||
| Low | 267 | 51.9 | 261 | 51.6 | 243 | 52.5 |
| Medium | 195 | 37.9 | 193 | 38.1 | 176 | 38 |
| High | 52 | 10.1 | 52 | 10.3 | 44 | 9.5 |
| Education | ||||||
| Less than High School | 208 | 40.5 | 203 | 40.1 | 188 | 40.6 |
| High School | 180 | 35 | 178 | 35.2 | 165 | 35.6 |
| More than High School | 126 | 24.5 | 125 | 24.7 | 110 | 23.8 |
MEP and ΣDEHP had the highest concentrations in maternal urine during pregnancy (Supplemental Table S3). Phthalate biomarkers had low to moderate correlations (Pearson’s r = 0.12 – 0.75) (Supplemental Figure S2). The lowest correlations were observed between MEP and other biomarkers (r = 0.12 – 0.27) and the highest correlation was detected between MCPP and ΣDBP metabolites (r = 0.75). Women included in our analyses had lower urinary MCPP, ΣDBP, ΣDiBP and MBzP concentrations than women whose child missed the four-year follow-up (Supplemental Table S3).
3.2. Childhood adiposity trajectories from four to twelve years
We characterized three trajectories of childhood adiposity based on non-centered and centered outcomes. For non-centered outcomes, we identified a “low-stable” (N = 260), a “low-high” (N = 147), and a “high-high” (N = 107) class within our population (Figure 1A). The “low-stable” class was characterized by average starting BMI z-scores of approximately zero. BMI z-scores and FMI remained stable through 12 years, while WHtR decreased slightly over time. The “low-high” class was characterized by adiposity indicators that remained low through the six-year visit, then increased sharply from six years to eight years. Children in the “high-high” class started with the greatest values for each adiposity outcome. In the “high-high” class, BMI z-scores and WHtR increased early then stabilized, while FMI increased steadily.
Figure 1.
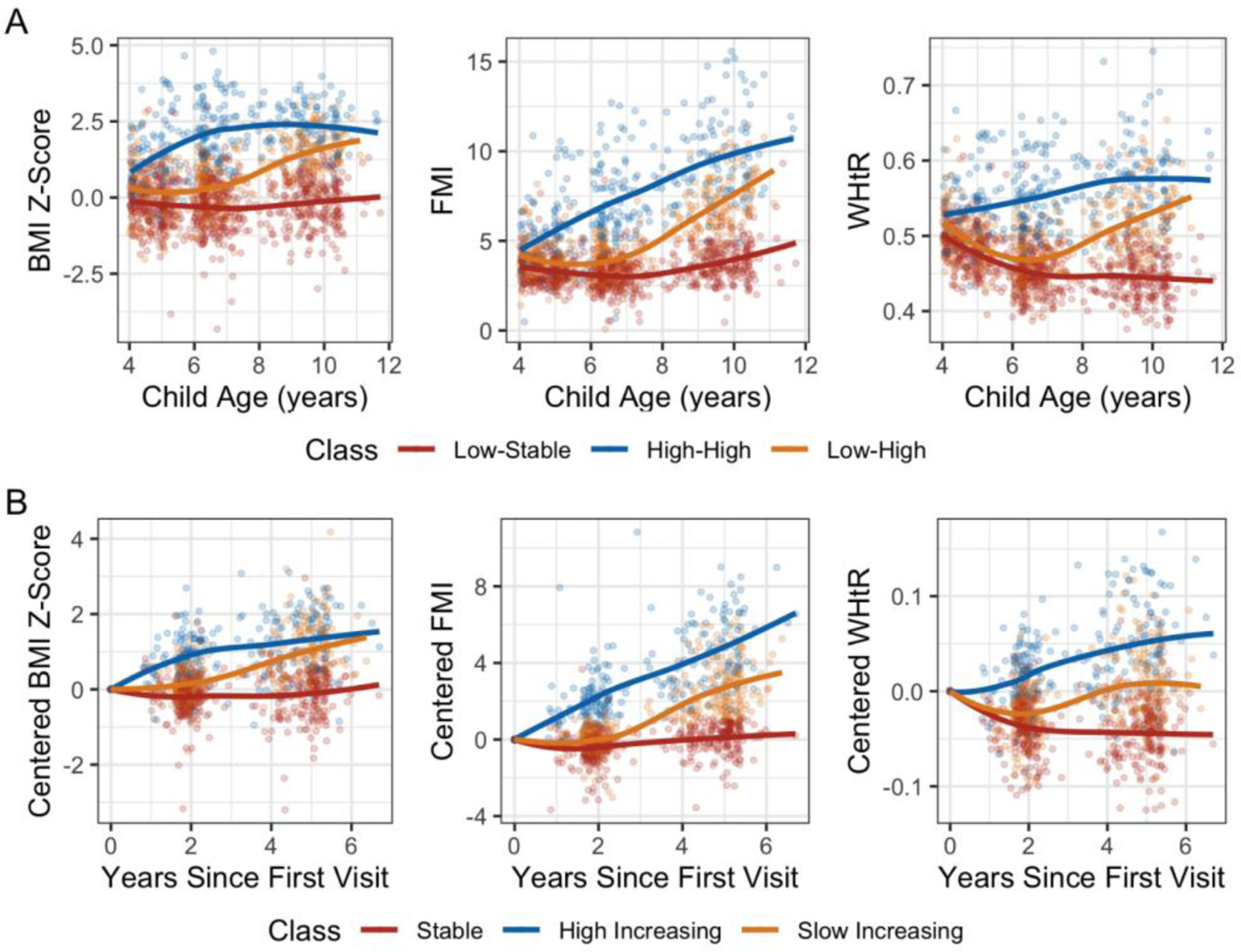
Adiposity trajectory classes identified by latent class growth modeling for A) non-centered BMI z-score, FMI and WHtR, and B) first visit centered BMI-zscore, FMI, and WHtR. Colors indicate class membership (red: “low-stable”/”stable”, blue: “high-high”/”high-increasing” and orange: “low-high”/”slow increasing”) and smooth loess curves describe the trend of each trajectory. LCGM models for non-centered adiposity outcomes included linear and quadratic fixed effects for child age at follow-up, random intercepts for age and subject and link functions of 3 quantile splines on each adiposity outcome. LCGM models for centered adiposity outcomes included: linear and quadratic terms for age centered at the 4-year visit, random intercepts for subject, 4 equidistant splines on BMI z-score and FMI, and a linear link function on WHtR.
Centered trajectories characterized a “stable” class (N = 221), a “slow increasing” (N = 175), and a “high increasing” (N = 118) class (Figure 1B). The “slow increasing” class remained stable until the six-year visit then increased, while the “high increasing” increased immediately after the first visit. While there was considerable overlap between the “stable” and “low-stable” trajectory classes, the increasing and higher adiposity classes were more dissimilar (Supplemental Table S5).
3.3. Phthalate-specific and overall mixture associations with childhood adiposity trajectories and overall adiposity
ΣDEHP was associated with increased odds of being in the “high-high” class (Adjusted Odds Ratio = 1.53 [1.08, 2.19]; p = 0.02), but with lower odds of being in the “low-high” class (0.73 [0.52, 1.00]; p = 0.05) in comparison to the “low-stable” group (Figure 2A). A doubling of ΣDiNP was also associated with 1.43 (1.02, 2.02) greater odds of being in the “low-high” class (p = 0.04). A doubling in MCNP resulted in 0.66 (0.45, 97) lower odds of being in the “low-high” trajectory (p = 0.03). For centered adiposity trajectories, MCNP was borderline associated with lower odds of being in the “slow increasing” class (0.70 [0.48, 1.01]; p = 0.06) (Figure 2B). When individual adiposity outcomes were examined longitudinally throughout childhood, prenatal urinary MCNP was also negatively associated with child BMI z-score (−0.18 [−0.36, 0.002]; p = 0.05) (Figure 2C). Total prenatal phthalate biomarkers were not associated with childhood adiposity (Table 2). The greatest positive weights were observed for ΣDEHP (0.72 – 0.51), whereas the greatest negative weights were observed for MCPP (−0.68 – −0.06) (Supplemental Figure S3).
Figure 2.
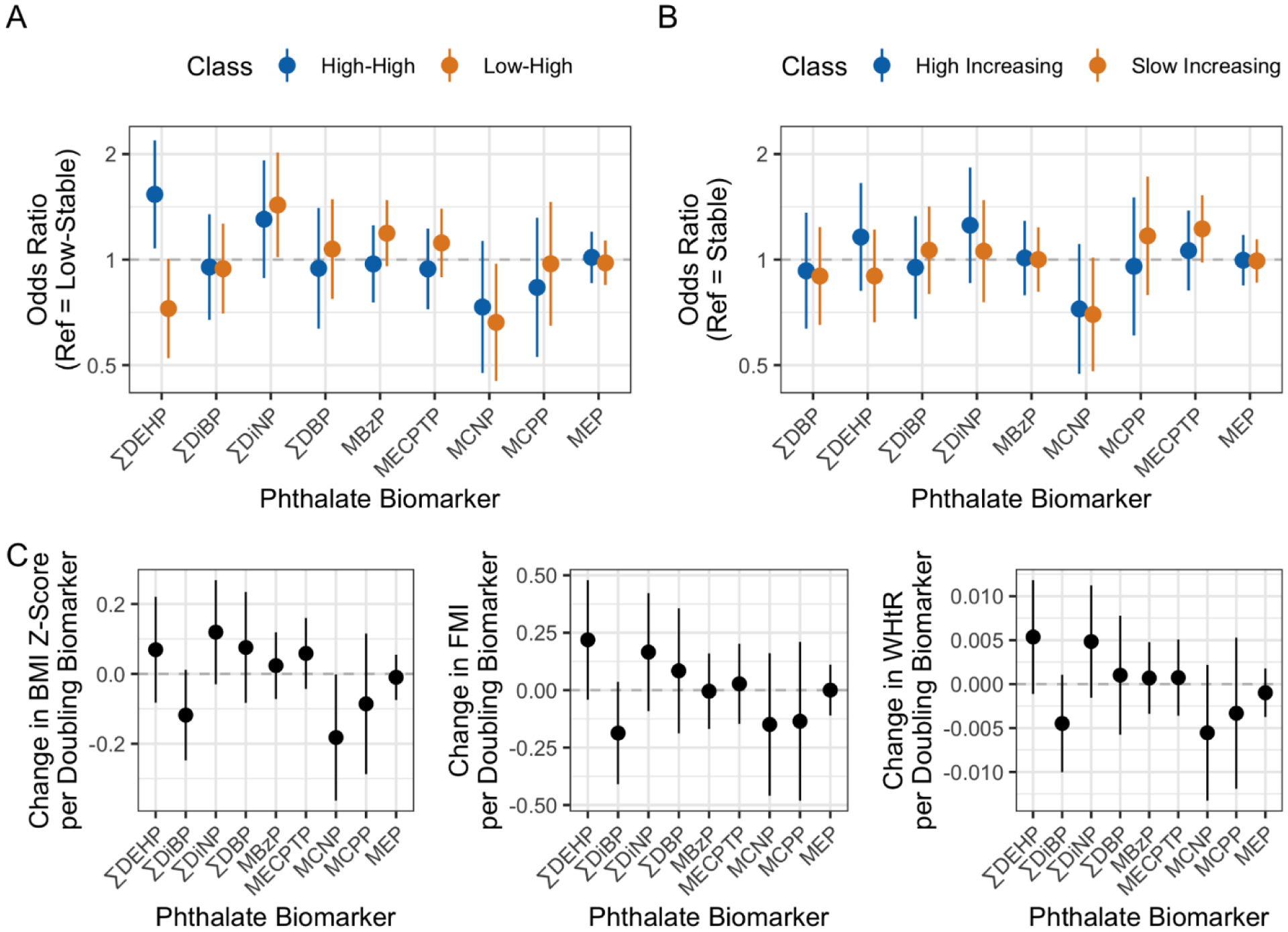
Associations of 2nd and 3rd trimester geometric mean urinary phthalate metabolites with A) non-centered adiposity trajectory classes, B) centered adiposity trajectory classes, and C) overall BMI z-score, fat mass index (FMI) and WHtR (waist-to-height ratio), using multinomial logistic regression with the “low-stable” and “stable” classes as the reference groups (A and B, respectively), or linear mixed-effects models (C). Logistic regression model estimates are presented as odds ratios per doubling of metabolite concentrations with 95% confidence intervals (CI’s). Colors represent the comparison classes (blue: “high-high” or “high-stable”; yellow: “low-high” or “high increasing”. Linear mixed-effects models are presented as the change in outcome per doubling of phthalate biomarker with 95% CI’s. All models included all 9 phthalate biomarkers and were adjusted for maternal age, maternal pre-pregnancy BMI, parity, socioeconomic status, education, child sex, and date and phthalate measurement. Mixed-effects models were additionally adjusted for linear and quadratic terms for child age at visit.
Table 2.
Associations of a quartile increase in the total phthalates mixture with overall adiposity and adiposity trajectories as measured by quantile g-computation (N = 514).
| Model Type | Outcome | Rate Ratio/ Effect Estimate (95% CI)* | P Value |
|---|---|---|---|
| Centered Trajectories | Stable (N = 221) | 1.00 (Ref) | |
| High Increasing (N = 118) | 1.12(0.94, 1.35) | 0.19 | |
| Slow Increasing (N = 175) | 0.99 (0.85, 1.14) | 0.90 | |
| Non-Centered Trajectories | Low Stable (N = 260) | 1.00 (Ref) | |
| Low-High (N = 147) | 1.01 (0.85, 1.19) | 0.93 | |
| High-High (N = 107) | 1.07 (0.87, 1.31) | 0.51 | |
| Overall Adiposity | BMI Z-score | 0.02 (−0.10, 0.14) | 0.73 |
| FMI | 0.08 (−0.13, 0.28) | 0.47 | |
| WHtR | 0 (0, 0) | 0.93 |
All models adjusted for maternal age, maternal pre-pregnancy BMI, parity, socioeconomic status, education, child sex, and date and phthalate measurement. Mixed-effects models were additionally adjusted for linear and quadratic terms for child age at visit.
3.4. Sex-Specific Associations
Adiposity trajectories for male and female children were highly similar (Supplemental Figure S4), with no meaningful differences between the number of males and females in each class (Supplemental Table S6). We observed no sex-specific associations between urinary phthalate biomarkers and adiposity trajectories (Supplemental Figure S5A–B). MCPP concentrations were negatively associated with FMI (−0.58 [−1.04, −0.12]; p = 0.02) and WHtR (−0.015 [−0.026, −0.003]; p = 0.02) in males but not in females (0.21 [−0.30,0.32] for FMI; and 0.007 [−0.006,0.019] for WHtR). Similar to the results in the full population, no mixture associations were observed (Supplemental Table S7 and Supplemental Figure S6).
3.5. Sensitivity Analyses
Correlations between phthalate metabolite concentrations measured in the 2nd and 3rd trimesters were low to moderate depending on the metabolite (Pearson’s R = 0.16 – 0.39; Supplemental Figure S7). Evaluating 2nd and 3rd trimester phthalate biomarkers separately revealed no meaningful trimester-specific differences (Supplemental Figure S8). Specifying separate models for each individual phthalate biomarker resulted in slightly attenuated effect estimates for associations with trajectory class membership, though the direction of effect was retained (Supplemental Figure S9A and S9B). The association between MNCP concentrations and longitudinal BMI z-score was attenuated (Supplemental Figure S9C). Specifying two to four adiposity trajectory classes produced similar groups as in the main analyses (Supplemental Figure S10–S13). The overall associations of phthalate biomarkers with trajectory classes remained consistent.
4. Discussion
4.1. Trajectories of Childhood Adiposity
Modeling strategies using centered and non-centered outcomes provide similar yet distinct insight into changes in child adiposity. Adiposity indicators that are not centered at the earliest visit capture children who may already have elevated adiposity at the start of follow-up, reflecting increases in adiposity prior to four years. We observe this phenomenon in the “high-high” class of children. In contrast, by examining centered adiposity outcomes, we are able to profile children who have the greatest changes in BMI independent of starting values.
Several studies have observed childhood adiposity trajectories similar to those presented here that are predictive of later life cardiometabolic health risk (Blond et al., 2020; Lycett et al., 2020; Norris Tom et al., 2021). Australian children in early and late rising BMI groups had greater atherogenic lipoprotein expression at age fourteen years in comparison to a normal BMI group, although those who were early risers had worse cardiometabolic profiles (Barraclough et al., 2019), suggesting that the trajectories identified here are relevant for later cardiometabolic risk. Others populations demonstrate that although all overweight trajectories associate with increased cardiometabolic risk in adulthood in comparison to normal classes, the trajectory classes that start normal then increase to obesity have worse cardiometabolic profiles than trajectories that started with a higher BMI (Norris Tom et al., 2021). Furthermore, membership in moderate and high BMI trajectories in childhood was associated with greater risk of hypertension, type 2 diabetes, and dyslipidemia (Yuan et al., 2020). Taken together, these studies suggest that we were able to identify trajectories that provide meaningful insight into future cardiometabolic risk.
4.2. Comparison of Prenatal Phthalates and Childhood Adiposity to Previous Literature
A growing number of studies have examined associations between prenatal phthalates exposures and childhood adiposity with inconsistent results, which may be due to differences in biomarker concentrations or developmental timing of exposures, the metabolites quantified, timing of follow-up, or other population characteristics. A recent review on the effects of early life DEHP exposures on rodent obesity found a significant positive effect on fat weight and a nonsignificant negative effect on body weight (Wassenaar and Legler, 2017). Population studies have reported positive (Harley et al., 2017), exposure timing-dependent (Shoaff et al., 2017), and nonlinear associations (Yang et al., 2018) with prenatal DEHP biomarker concentrations. Although we observed no differences by sex, studies have reported opposing associations between prenatal ΣDEHP and adiposity in boys and girls, however, results vary between studies (Buckley et al., 2016a; Vafeiadi et al., 2018). Furthermore, experimental research has found obesogenic effects of DEHP only in males (Fan et al., 2020) or only in females (Klöting et al., 2015; Neier et al., 2019b). That we did not observe any sex-specific associations may be due to our small sample size when stratifying the population by sex. Future studies on sex-specific associations of phthalates with adiposity trajectories would benefit from a larger population.
We also observed greater odds of being in the “low-high” adiposity class with increasing ΣDiNP concentrations. DiNP has been used as an industrial alternative for DEHP. Mice perinatally exposed to DiNP have greater body weights (Neier et al., 2019a), as well as greater abdominal circumference accompanied by sex-specific changes in fatty acid, lipid, and cholesterol composition (Huang et al., 2019; Yang et al., 2021). Human population studies investigating associations between prenatal concentrations of DiNP metabolites, such as MCOP, with childhood adiposity have reported either null (Berman et al., 2020; Harley et al., 2017) or weakly positive (Berger et al., 2021) associations. More research on DiNP is needed to determine its potential as an environmental obesogen.
Finally, we observed an association between prenatal MCNP, a metabolite of DiDP, and lower odds of being in the “slow-increasing” class and a negative association with BMI z-score. One study observed positive associations between prenatal MCNP and odds of being overweight/obese at five years of age (Berger et al., 2021), suggesting the importance of further research on DiDP to evaluate its potential impacts on child health.
We observed little evidence for an association of the total prenatal phthalate biomarker mixture with childhood adiposity, which could be explained opposing directions of effect by different phthalate biomarkers as shown by biomarker weights. To date, few studies have investigated mixture associations of phthalate metabolites. One study found that the sum of five high-molecular-weight phthalate metabolites was associated with lower BMI z-scores in boys and higher BMI z-scores in girls at 4 and 7 years of age (Valvi et al., 2015). We also previously observed negative associations between the prenatal phthalate biomarker mixture and child triglycerides and non-high density lipoprotein levels in the PROGRESS cohort (Kupsco et al., 2021).
4.3. Potential Mechanisms of Action
Exposure to environmental stressors during pregnancy can program future child health via effects on developing cells (La Merrill and Birnbaum, 2011). Obesogenic impacts of phthalates may arise from their role as peroxisome-proliferator-activated receptor (PPAR) agonists (Hao et al., 2012). Mono-2-ethylhexyl phthalate (MEHP), the hydrolytic monoester metabolite of DEHP, activates human and rodent PPARα and PPARγ receptors in vitro, leading to expression of key PPAR target genes and metabolites (Ellero-Simatos et al., 2011; Feige et al., 2007; Hurst and Waxman, 2003). Prenatal MEHP concentrations may also activate PPARγ responses in vivo (Hao et al., 2012). DEHP replacements, DiNP and DiDP, induced adipocyte differentiation and expression of adipocyte specific genes in vitro, potentially via binding of PPARγ (Pomatto et al., 2018; Zhang et al., 2019). In silico analyses of phthalate binding to human PPAR subtypes found that DiDP had high affinity for PPARγ (Josh et al., 2014). The effects of DiNP on rodent liver hepatocyte proliferation, palmitoyl-CoA oxidase activity, and enzymes involved in β- and ω-oxidation of fatty acids were also found to be PPARα dependent (Valles et al., 2003). Aberrant activation of the PPAR signaling pathways during early development may result in long term disruption of adipose tissue metabolism leading to altered childhood adiposity.
4.4. Strengths and Limitations
This study has several strengths, including implementation of a sophisticated trajectory modeling approach to provide new information on the impact of prenatal phthalates exposures on childhood adiposity and use of a novel mixture method to assess the overall effects. We characterized a mean measure of phthalate biomarker concentrations from two prenatal time points, which may be more reliable than measures from a single spot urine sample. Phthalate biomarker concentrations vary throughout pregnancy, from day to day, and within a 24-hour period (Johns et al., 2015; Wu et al., 2020). In the present analysis, the phthalate biomarker concentrations measured in the 2nd to 3rd trimester were only weakly correlated, indicating that individual spot measures are likely unreliable. However, while residual measurement error is likely present, our sensitivity analyses of 2nd and 3rd trimester phthalate biomarker concentrations did not reveal substantial differences in associations between trimesters.
Although we adjusted for a number of relevant confounders in our analyses, our associations may be impacted by residual or unmeasured confounding. Consumption of packaged and processed foods is a source of phthalates exposure (Buckley et al., 2019), and an unhealthy diet during pregnancy may contribute to childhood adiposity via maternal BMI or other cardiometabolic factors. Unfortunately, prenatal dietary information was unavailable in this cohort. Furthermore, we were unable to account for certain negative confounders, which may contribute to certain null findings. For instance, over the counter supplements can be an important source of phthalates exposures (Kelley et al., 2012); however, maternal supplement use may indicate a greater health consciousness that could result in lower childhood adiposity. Similarly, electronics have been demonstrated to also contain phthalates (Yang et al., 2020); however, high electronics use may also be indicative of a higher socioeconomic status that was not captured by our SES variable. Because our adiposity measures were collected beginning at four years, we were unable to examine infant peak adiposity or adiposity nadir, which are also relevant for cardiometabolic risk (Aris et al., 2019). Finally, our study population consisted of Mexican children and may not be generalizable to other populations.
5. Conclusions
In sum, prenatal urinary concentrations of biomarkers of three high molecular weight phthalates were associated with differing trajectories of mid-childhood adiposity, which may be relevant for adult cardiometabolic risk. However, we observed no evidence for an association of the phthalate biomarker mixture, nor any robust sex-specific effects suggesting that research in larger, diverse populations is necessary to fully understand the impacts of prenatal phthalate exposures on childhood adiposity. Future research should explore the contribution of childhood lifestyle factors, such as physical activity and diet, to these associations and how they may modify the impact of prenatal phthalates exposures on child adiposity. Furthermore, analysis of the pathways that may be mediating this relationship, such as birthweight or timing of pubertal onset, would provide greater insight into the factors contributing to our findings. Finally, additional analysis with growth trajectories from birth through adolescence would provide stronger evidence for the ability of the prenatal environment to shape adult cardiometabolic risk.
Supplementary Material
Acknowledgements
This work was supported by funding from the National Institute of Environmental Health Sciences [K99 ES030749 to AK, R00 ES023474 to ALD, R00 ES023450 to ACJ, R01 ES021357 and P30 ES009089 to AAB, R01 ES024381 to JMB, R01 ES028805 to MAK, and P30 ES023515; R01 ES014930; R01 ES013744 and R24 ES028522 to ROW].
Funding Information:
This work was supported by funding from the National Institute of Environmental Health Sciences [K99 ES030749 to AK, R00 ES023474 to ALD, R00 ES023450 to ACJ, R01 ES021357 and P30 ES009089 to AAB, R01 ES024381 to JMB, R01 ES028805 to MAK, and P30 ES023515; R01 ES014930; R01 ES013744 and R24 ES028522 to ROW].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement: The authors declare no conflict of Interest.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References Cited
- Aris IM, Rifas-Shiman SL, Li L-J, Kleinman KP, Coull BA, Gold DR, Hivert M-F, Kramer MS, Oken E, 2019. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. International Journal of Epidemiology 48, 157–167. 10.1093/ije/dyy286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough JY, Garden FL, Toelle BG, Marks GB, Baur LA, Ayer JG, Celermajer DS, 2019. Weight Gain Trajectories from Birth to Adolescence and Cardiometabolic Status in Adolescence. The Journal of Pediatrics 208, 89–95.e4. 10.1016/j.jpeds.2018.12.034 [DOI] [PubMed] [Google Scholar]
- Berger K, Hyland C, Ames JL, Mora AM, Huen K, Eskenazi B, Holland N, Harley KG, 2021. Prenatal Exposure to Mixtures of Phthalates, Parabens, and Other Phenols and Obesity in Five-Year-Olds in the CHAMACOS Cohort. Int J Environ Res Public Health 18. 10.3390/ijerph18041796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman YE, Doherty DA, Main KM, Frederiksen H, Keelan JA, Newnham JP, Hart RJ, 2020. The influence of prenatal exposure to phthalates on subsequent male growth and body composition in adolescence. Environmental Research 110313. 10.1016/j.envres.2020.110313 [DOI] [PubMed] [Google Scholar]
- Blond K, Aarestrup J, Vistisen D, Bjerregaard LG, Jensen GB, Petersen J, Nordestgaard BG, Jørgensen ME, Jensen BW, Baker JL, 2020. Associations between body mass index trajectories in childhood and cardiovascular risk factors in adulthood. Atherosclerosis 314, 10–17. 10.1016/j.atherosclerosis.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo Y Ortiz M, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM, 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13, 50. 10.1186/1476-069X-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, Richardson DB, Xu Y, Calafat AM, Wolff MS, Lanphear BP, Herring AH, Rundle AG, 2016a. Prenatal phthalate exposures and body mass index among 4 to 7 year old children: A pooled analysis. Epidemiology 27, 449–458. 10.1097/EDE.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, Wolff MS, Herring AH, 2016b. Prenatal Phthalate Exposures and Childhood Fat Mass in a New York City Cohort. Environ Health Perspect 124, 507–513. 10.1289/ehp.1509788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E, Rebholz CM, 2019. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int 131, 105057. 10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Braun JM, Byun H-M, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM, 2013. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 5, 271–281. 10.2217/epi.13.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco AV, 2002. The AMAI system of classifying households by socio-economic level: ESOMAR; 2002.
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J, 2007. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85, 660–667. 10.2471/blt.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, World Health Organization (WHO), 2006. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. WHO Multicentre Growth Reference Study Group, Geneva. [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R, 2005. Personal Care Product Use Predicts Urinary Concentrations of Some Phthalate Monoesters. Environ Health Perspect 113, 1530–1535. 10.1289/ehp.8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellero-Simatos S, Claus SP, Benelli C, Forest C, Letourneur F, Cagnard N, Beaune PH, de Waziers I, 2011. Combined Transcriptomic–1H NMR Metabonomic Study Reveals That Monoethylhexyl Phthalate Stimulates Adipogenesis and Glyceroneogenesis in Human Adipocytes. J Proteome Res 10, 5493–5502. 10.1021/pr200765v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Qin Y, Chen M, Li X, Wang R, Huang Z, Xu Q, Yu M, Zhang Y, Han X, Du G, Xia Y, Wang X, Lu C, 2020. Prenatal low-dose DEHP exposure induces metabolic adaptation and obesity: Role of hepatic thiamine metabolism. J Hazard Mater 385, 121534. 10.1016/j.jhazmat.2019.121534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B, 2007. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem 282, 19152–19166. 10.1074/jbc.M702724200 [DOI] [PubMed] [Google Scholar]
- Hao C, Cheng X, Xia H, Ma X, 2012. The endocrine disruptor mono-(2ethylhexyl)phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 32, 619–629. 10.1042/BSR20120042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Berger K, Rauch S, Kogut K, Henn BC, Calafat AM, Huen K, Eskenazi B, Holland N, 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatric Research 82, 405–415. 10.1038/pr.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Sun F, Tan H, Deng Y, Sun Z, Chen H, Li J, Chen D, 2019. DEHP and DINP Induce Tissue- and Gender-Specific Disturbances in Fatty Acid and Lipidomic Profiles in Neonatal Mice: A Comparative Study. Environ. Sci. Technol 53, 12812–12822. 10.1021/acs.est.9b04369 [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ, 2003. Activation of PPARα and PPARγ by Environmental Phthalate Monoesters. Toxicological Sciences 74, 297–308. 10.1093/toxsci/kfg145 [DOI] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD, 2015. Exposure Assessment Issues in Epidemiology Studies of Phthalates. Environ Int 85, 27–39. 10.1016/j.envint.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josh MKS, Pradeep S, Amma KSV, Balachandran S, Jaleel UCA, Doble M, Spener F, Benjamin S, 2014. Phthalates efficiently bind to human peroxisome proliferator activated receptor and retinoid X receptor α, β, γ subtypes: an in silico approach. Journal of Applied Toxicology 34, 754–765. 10.1002/jat.2902 [DOI] [PubMed] [Google Scholar]
- Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JSA, Dwyer T, Raitakari OT, 2012. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. Obstetrical & Gynecological Survey 67, 156–158. 10.1097/OGX.0b013e3182483780 [DOI] [PubMed] [Google Scholar]
- Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi O-I, Tsatsakis AM, Rizos AK, 2016. A global assessment of phthalates burden and related links to health effects. Environment International 97, 212–236. 10.1016/j.envint.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2020. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 128, 47004. 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KE, Hernández-Díaz S, Chaplin EL, Hauser R, Mitchell AA, 2012. Identification of Phthalates in Medications and Dietary Supplement Formulations in the United States and Canada. Environ Health Perspect 120, 379–384. 10.1289/ehp.1103998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöting N, Hesselbarth N, Gericke M, Kunath A, Biemann R, Chakaroun R, Kosacka J, Kovacs P, Kern M, Stumvoll M, Fischer B, Rolle-Kampczyk U, Feltens R, Otto W, Wissenbach DK, von Bergen M, Blüher M, 2015. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PLoS One 10. 10.1371/journal.pone.0143190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsco A, Wu H, Calafat AM, Kioumourtzoglou M-A, Tamayo-Ortiz M, Pantic I, Cantoral A, Tolentino M, Oken E, Braun JM, Deierlein AL, Wright RO, Téllez-Rojo MM, Baccarelli AA, Just AC, 2021. Prenatal maternal phthalate exposures and child lipid and adipokine levels at age six: A study from the PROGRESS cohort of Mexico City. Environmental Research 192, 110341. 10.1016/j.envres.2020.110341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Birnbaum LS, 2011. CHILDHOOD OBESITY AND ENVIRONMENTAL CHEMCALS. Mt Sinai J Med 78, 22–48. 10.1002/msj.20229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon H, Kelly S, Sperrin M, Buchan I, Cross AJ, Leitzmann M, Cook MB, Renehan AG, 2018. Framework to construct and interpret latent class trajectory modelling. BMJ Open 8, e020683. 10.1136/bmjopen-2017-020683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, Wei Z, Lv Z, Chen X, Xia W, Xu S, 2011. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. American Journal of Physiology-Endocrinology and Metabolism 301, E527–E538. 10.1152/ajpendo.00233.2011 [DOI] [PubMed] [Google Scholar]
- López-Carrillo L, Hernández-Ramírez RU, Calafat AM, Torres-Sánchez L, Galván-Portillo M, Needham LL, Ruiz-Ramos R, Cebrián ME, 2010. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ Health Perspect 118, 539–544. 10.1289/ehp.0901091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett K, Juonala M, Magnussen CG, Norrish D, Mensah FK, Liu R, Clifford SA, Carlin JB, Olds T, Saffery R, Kerr JA, Ranganathan S, Baur LA, Sabin MA, Cheung M, Dwyer T, Liu M, Burgner D, Wake M, 2020. Body Mass Index From Early to Late Childhood and Cardiometabolic Measurements at 11 to 12 Years. Pediatrics 146. 10.1542/peds.2019-3666 [DOI] [PubMed] [Google Scholar]
- Neier K, Cheatham D, Bedrosian LD, Dolinoy DC, 2019a. Perinatal exposures to phthalates and phthalate mixtures result in sex-specific effects on body weight, organ weights, and IAP DNA methylation in weanling mice. J Dev Orig Health Dis 10, 176–187. 10.1017/S2040174418000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neier K, Cheatham D, Bedrosian LD, Gregg BE, Song PXK, Dolinoy DC, 2019b. Longitudinal Metabolic Impacts of Perinatal Exposure to Phthalates and Phthalate Mixtures in Mice. Endocrinology 160, 1613–1630. 10.1210/en.2019-00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom Norris, Liina Mansukoski, Gilthorpe Mark S., Hamer Mark, Hardy Rebecca, Howe Laura D., Hughes Alun D., Li Leah, O’Donnell Emma, Ong Ken K., Ploubidis George B., Silverwood Richard J., Viner Russell M., Johnson William, 2021. Distinct Body Mass Index Trajectories to Young-Adulthood Obesity and Their Different Cardiometabolic Consequences. Arteriosclerosis, Thrombosis, and Vascular Biology 41, 1580–1593. 10.1161/ATVBAHA.120.315782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CG, Whincup PH, Orfei L, Chou Q-A, Rudnicka AR, Wathern AK, Kaye SJ, Eriksson JG, Osmond C, Cook DG, 2009. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 33, 866–877. 10.1038/ijo.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto V, Cottone E, Cocci P, Mozzicafreddo M, Mosconi G, Nelson ER, Palermo FA, Bovolin P, 2018. Plasticizers used in food-contact materials affect adipogenesis in 3T3-L1 cells. J Steroid Biochem Mol Biol 178, 322–332. 10.1016/j.jsbmb.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Liquet B, 2017. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm | Proust-Lima | Journal of Statistical Software. J. Stat. Softw 78, 1–56. 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Renzetti S, Just AC, Burris HH, Oken E, Amarasiriwardena C, Svensson K, Mercado-García A, Cantoral A, Schnaas L, Baccarelli AA, Wright RO, Téllez-Rojo MM, 2017. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environmental Research 152, 226–232. 10.1016/j.envres.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A, 2006. The limitations due to exposure detection limits for regression models. Am. J. Epidemiol 163, 374–383. 10.1093/aje/kwj039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J-S, Schaedlich K, Fiandanese N, Pocar P, Fischer B, 2012. Effects of Di(2-ethylhexyl) Phthalate (DEHP) on Female Fertility and Adipogenesis in C3H/N Mice. Environ Health Perspect 120, 1123–1129. 10.1289/ehp.1104016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, Yolton K, Braun JM, 2017. Early-Life Phthalate Exposure and Adiposity at 8 Years of Age. Environ Health Perspect 125. 10.1289/EHP1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM, 2007. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 860, 106–112. 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Soria-Contreras DC, Trejo-Valdivia B, Cantoral A, Pizano-Zárate ML, Baccarelli AA, Just AC, Colicino E, Deierlein AL, Wright RO, Oken E, Téllez-Rojo MM, López-Ridaura R, 2020. Patterns of Weight Change One Year after Delivery Are Associated with Cardiometabolic Risk Factors at Six Years Postpartum in Mexican Women. Nutrients 12, 170. 10.3390/nu12010170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, Venihaki M, Sarri K, Vassilaki M, Leventakou V, Stephanou EG, Kogevinas M, Chatzi L, 2018. Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Front Public Health 6. 10.3389/fpubh.2018.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles EG, Laughter AR, Dunn CS, Cannelle S, Swanson CL, Cattley RC, Corton JC, 2003. Role of the peroxisome proliferator-activated receptor α in responses to diisononyl phthalate. Toxicology 191, 211–225. 10.1016/S0300-483X(03)00260-9 [DOI] [PubMed] [Google Scholar]
- Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M, 2015. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect 123, 1022–1029. 10.1289/ehp.1408887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar PNH, Legler J, 2017. Systematic review and meta-analysis of early life exposure to di(2-ethylhexyl) phthalate and obesity related outcomes in rodents. Chemosphere 188, 174–181. 10.1016/j.chemosphere.2017.08.165 [DOI] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K, 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 26, 803–824. 10.1111/j.1539-6924.2006.00770.x [DOI] [PubMed] [Google Scholar]
- Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E, Braun JM, Mercado-Garcia A, Cantoral A, Téllez-Rojo MM, Wright RO, Baccarelli AA, 2020. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ. Sci. Technol 54, 1740–1749. 10.1021/acs.est.9b05836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Harris SA, Jantunen LM, Kvasnicka J, Nguyen LV, Diamond ML, 2020. Phthalates: Relationships between Air, Dust, Electronic Devices, and Hands with Implications for Exposure. Environ. Sci. Technol 54, 8186–8197. 10.1021/acs.est.0c00229 [DOI] [PubMed] [Google Scholar]
- Yang TC, Peterson KE, Meeker JD, Sánchez BN, Zhang Z, Cantoral A, Solano M, Tellez-Rojo MM, 2018. Exposure to Bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr Obes 13, 550–557. 10.1111/ijpo.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sun F, Chen H, Tan H, Yang L, Zhang L, Xie J, Sun J, Huang X, Huang Y, 2021. Postnatal exposure to DINP was associated with greater alterations of lipidomic markers for hepatic steatosis than DEHP in postweaning mice. Science of The Total Environment 758, 143631. 10.1016/j.scitotenv.2020.143631 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Chu C, Zheng W-L, Ma Q, Hu J-W, Wang Y, Yan Y, Liao Y-Y, Mu J-J, 2020. Body Mass Index Trajectories in Early Life Is Predictive of Cardiometabolic Risk. The Journal of Pediatrics 219, 31–37.e6. 10.1016/j.jpeds.2019.12.060 [DOI] [PubMed] [Google Scholar]
- Zhang L, Sun W, Duan X, Duan Y, Sun H, 2019. Promoting differentiation and lipid metabolism are the primary effects for DINP exposure on 3T3-L1 preadipocytes. Environmental Pollution 255, 113154. 10.1016/j.envpol.2019.113154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


