Abstract
Introduction:
Acute traumatic coagulopathy (ATC) is an endogenous impairment in hemostasis that often contributes to early mortality after trauma. Endothelial glycocalyx damage is associated with trauma-induced coagulation abnormalities; however, the specific relationship between hyaluronan (HA), a key glycocalyx constituent, and ATC has not been evaluated.
Methods:
We performed a secondary analysis of prospectively collected data from a recent study in which trauma patients (>18 years) admitted to our Level I trauma center with an ABC Score≥2 were enrolled. Partial thromboplastin time (PTT), international normalized ratio (INR) and thromboelastography (TEG) parameters were recorded at arrival. Injury characteristics and clinical outcomes were obtained. Plasma HA levels were measured in healthy controls (HC) and in trauma subjects at arrival (t = 0h) and 12, 24, and 48h. ATC was defined as admission INR>1.2 or PTT≥36.5 sec. Comparisons of HA levels were assessed, and Spearman’s correlations were performed between 0h and 24h HA levels, coagulation measures and clinical outcomes. P-values <0.05 were considered significant.
Results:
Forty-eight trauma patients and 22 controls were enrolled for study. Sixteen trauma subjects were coagulopathic at admission. HA levels in subjects with ATC were higher than non-coagulopathic subjects at all time points and elevated above HC levels at 24 and 48h. At arrival, HA levels correlated with TEG R-time, PTT, and INR. HA levels at 24h correlated with increased transfusion requirements and intensive care unit and hospital lengths of stay.
Conclusion:
Shed HA is associated with early coagulation abnormalities in trauma patients, which may contribute to worse outcomes. These findings highlight the need for additional studies to evaluate the mechanistic role of HA in ATC.
Keywords: glycocalyx, hyaluronic acid, coagulation, trauma, hemorrhagic shock
1. INTRODUCTION
Trauma remains the leading cause of death for Americans ages 1 to 44 years old (1). Uncontrollable hemorrhage is the primary cause of trauma-related deaths occurring within the first several hours of injury (2), often attributable to injury-induced abnormalities in coagulation that prevent hemostasis. Acute traumatic coagulopathy (ATC) is defined as an endogenous impairment in hemostasis that occurs within minutes of severe injury prior to resuscitation-associated causes of coagulopathic bleeding (e.g., hemodilution, hypocalcemia) (3). ATC is present at hospital arrival in approximately 25% of trauma patients (4,5), and the presence of ATC is associated with a four-fold increase in early-stage death (5). Although, in some cases, implementation of balanced resuscitation strategies designed to mitigate ATC have decreased mortality rates associated with early exsanguination (6,7), the precise etiologic mechanisms driving coagulopathy and uncontrollable bleeding in the minutes to hours after injury remain elusive.
Physiologic regulation of hemostasis involves complex interactions between coagulation factors, anticoagulants, protease inhibitors, and localized cellular mechanisms. The vascular endothelial cell surface plays a critical role in localizing hemostasis to sites of injury by governing clot formation/propagation via interactions with platelets and mediators of coagulation (e.g., tissue factor, protein C, thrombomodulin). However, trauma-related tissue injury and hemorrhagic shock cause damage to the endothelial surface layer that can disrupt coagulation mechanisms. Specifically, damage to the endothelial glycocalyx layer, as evidenced by circulating syndecan-1, is associated with trauma-induced coagulopathy present at hospital arrival (8). Therefore, uncovering mechanisms by which glycocalyx injury promotes ATC may prove critical to identifying novel strategies that will ameliorate this life-threatening disease process.
Hyaluronan (HA) is a major non-sulfated glycosaminoglycan of the endothelial glycocalyx layer that mediates barrier permeability and mechanotransduction signaling that is essential for nitric oxide production and vasoregulation (9,10). Its degradation and shedding from the endothelial surface alters the physical barrier of the glycocalyx and initiates a variety of signaling mechanisms that can influence leukocyte recruitment, endothelial cell proliferation and angiogenesis (10,11). Trauma-induced glycocalyx damage results in erosion of the endothelial glycocalyx, which entails HA cleavage/degradation and removal from the endothelial surface layer. As trauma patients with high plasma levels of HA demonstrate impaired ex vivo thrombin generation and reduced clot formation (12), this would suggest that HA may play a significant role in the pathogenesis of ATC. However, the relationship between plasma levels of HA and ATC has not been evaluated. Therefore, the goal of this study was to assess the associations between plasma HA levels, early coagulation abnormalities, and clinical outcomes after trauma.
2. METHODS
Study Design and Participants
We performed a secondary analysis of prospectively collected data from a recent clinical study performed between August 2018 and December 2019 (13). The original study included adult trauma patients (>18 years) presenting to our Level I Trauma Center with suspected hemorrhagic shock, as determined by an Assessment of Blood Consumption score ≥ 2. For controls, blood was collected from 22 healthy adult volunteers with no history of bleeding disorders or use of aspirin or non-steroidal anti-inflammatory drugs within 48 hours of sampling. The University of Alabama at Birmingham Institutional Review Board approved this study.
Clinical Data
Patient demographics, injury characteristics and clinical data were available from the original study. Base excess, activated partial thromboplastin time (aPTT), international normalized ratio (INR) and thromboelastography (TEG) parameters were measured upon hospital arrival. ATC was defined as admission INR > 1.2 or aPTT ≥ 36.5 sec. Prehospital crystalloid usage and units of packed red blood cells (pRBC), fresh frozen plasma (FFP) and platelets administered within 24 hours of hospitalization were recorded. Outcome measures included the development of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI), duration of mechanical ventilation, and intensive care unit (ICU) and hospital lengths of stay. Development of ALI or ARDS was defined according to the Berlin criteria (14), and AKI was defined using the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease criteria (15).
Plasma Collection and HA Measurements
Blood specimens were collected in sodium-citrated tubes, and platelet-poor plasma was collected, aliquoted and stored at −70°C. For the current study, plasma HA levels were measured using an enzyme-linked immunosorbent assay (R&D Systems Inc, Minneapolis, MN) in healthy controls (HC) and trauma subjects at hospital arrival (t = 0 hour) and 12, 24, and 48 hours after hospitalization.
Statistical Analyses
Analyses were performed using SPSS Version 25 (IBM Corp., Armonk, NY) and GraphPad Prism 9 (GraphPad Software, San Diego, CA). Categorical data are presented as number (proportion), and continuous data are presented as median (interquartile range) due to the nonparametric data distribution determined after Kolmogorov-Smirnov evaluation. Categorical data were compared using the chi-square test, and continuous data were analyzed using either the Mann-Whitney U test or a mixed-effects one-way analysis of variance followed by Dunn multiple comparisons tests as appropriate. Spearman’s rank correlations were performed between 0h and 24h HA levels, coagulation measures and clinical outcomes. Independent associations between HA levels and ATC were assessed using univariate and multivariate logistic regression analysis. All analyses were two-sided with an alpha level of 0.05. P values < 0.05 were considered significant.
3. RESULTS
Study Population
Forty-nine trauma subjects and 22 HC were enrolled in the original study (13). One trauma subject was excluded from the current analysis due to unavailable admission INR or aPTT data. Of 48 trauma subjects included for analysis, 16 (33.3%) subjects were coagulopathic at admission (Table 1). Injury severity score (ISS) and injury mechanism did not significantly differ between ATC groups. By definition, subjects with ATC had increased aPTT and INR at the time of hospitalization. These subjects also presented in a more severe state of hemorrhagic shock as evidenced by higher lactate levels and greater base deficits. Administration of prehospital fluids did not differ between groups. Coagulopathic subjects received more units of pRBCs during the initial 24 hours of hospitalization, but 24-hour requirements for other blood products did not differ. Subjects with ATC had increased ICU and hospital lengths of stay.
Table 1:
Demographic information and injury characteristics at admission for trauma subjects with and without acute traumatic coagulopathy.
| −ATC (n = 32) |
+ATC (n = 16) |
p-value* | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (years) | 36 (25, 55) | 40 (28, 68) | 0.47 |
| Male Gender, n (%) | 24 (75) | 9 (56) | 0.19 |
| INJURY CHARACTERISTICS | |||
| Blunt Mechanism of Injury, n (%) | 9 (28) | 9 (56) | 0.06 |
| Injury Severity Score | 10 (6, 24) | 17 (9, 38) | 0.07 |
| Severe Head Injury (AIS ≥ 3), n (%) | 3 (9) | 2 (13) | 0.74 |
| aPTT (sec) | 26 (24, 27) | 29 (25, 35) | 0.01 |
| INR | 1.1 (1.0, 1.2) | 1.3 (1.3, 1.5) | <0.01 |
| Lactate (mmol/L) | 3.4 (2.0, 5.5) | 5.4 (2.6, 15.3) | 0.04 |
| Base Deficit (mmol/L) | 2.8 (1.6, 5.1) | 8.1 (3.3, 16.9) | <0.01 |
| PREHOSPITAL FLUID ADMINISTRATION | |||
| Crystalloids, n (%) | 12 (38) | 3 (19) | 0.19 |
| Blood Product, n (%) | 4 (13) | 3 (19) | 0.56 |
| 24-HOUR TRANSFUSION REQUIREMENTS | |||
| Packed Red Blood Cells (units) | 3 (0, 6) | 5 (3, 8) | 0.04 |
| Fresh Frozen Plasma (units) | 2 (0, 4) | 4 (2, 7) | 0.12 |
| Platelets (units)† | 0 (0, 1) | 1 (0, 2) | 0.13 |
| Cryoprecipitate (units) | 0 (0, 0) | 0 (0, 0) | 0.34 |
| OUTCOMES | |||
| AKI, n (%) | 5 (16) | 4 (25) | 0.43 |
| ALI/ARDS, n (%) | 0 | 0 | --- |
| Mechanical Ventilation Duration (days) | 2 (0, 4) | 3 (1, 13) | 0.15 |
| ICU Length of Stay (days) | 4 (2, 9) | 11 (5, 24) | 0.01 |
| Hospital Length of Stay (days) | 9 (4, 17) | 17 (7, 38) | 0.04 |
| Mortality, n (%) | 0 | 0 | --- |
Emboldened p-values signify statistically significant comparisons.
1 unit of apheresis platelets is equivalent to 4–6 units of whole blood-derived platelet concentrates.
AKI represents acute kidney injury; ALI/ARDS, acute lung injury/acute respiratory distress syndrome; aPTT, activated partial thromboplastin time; ATC, acute traumatic coagulopathy; ICU, intensive care unit; INR, international normalized ratio.
HA Characterization and Association with the ATC Phenotype
Plasma levels of HA rose following injury and were greatest 24 hours following hospital admission (Figure 1). Further, HA levels in ATC subjects were higher than those without ATC at all time points and were significantly higher than HC levels 24 and 48 hours after hospitalization. Plasma levels of HA in subjects without ATC were not different from HC levels at any time following admission.
Figure 1. Plasma hyaluronan (HA) levels in traumatically injured patients with and without acute traumatic coagulopathy (ATC) compared to healthy controls (HC).
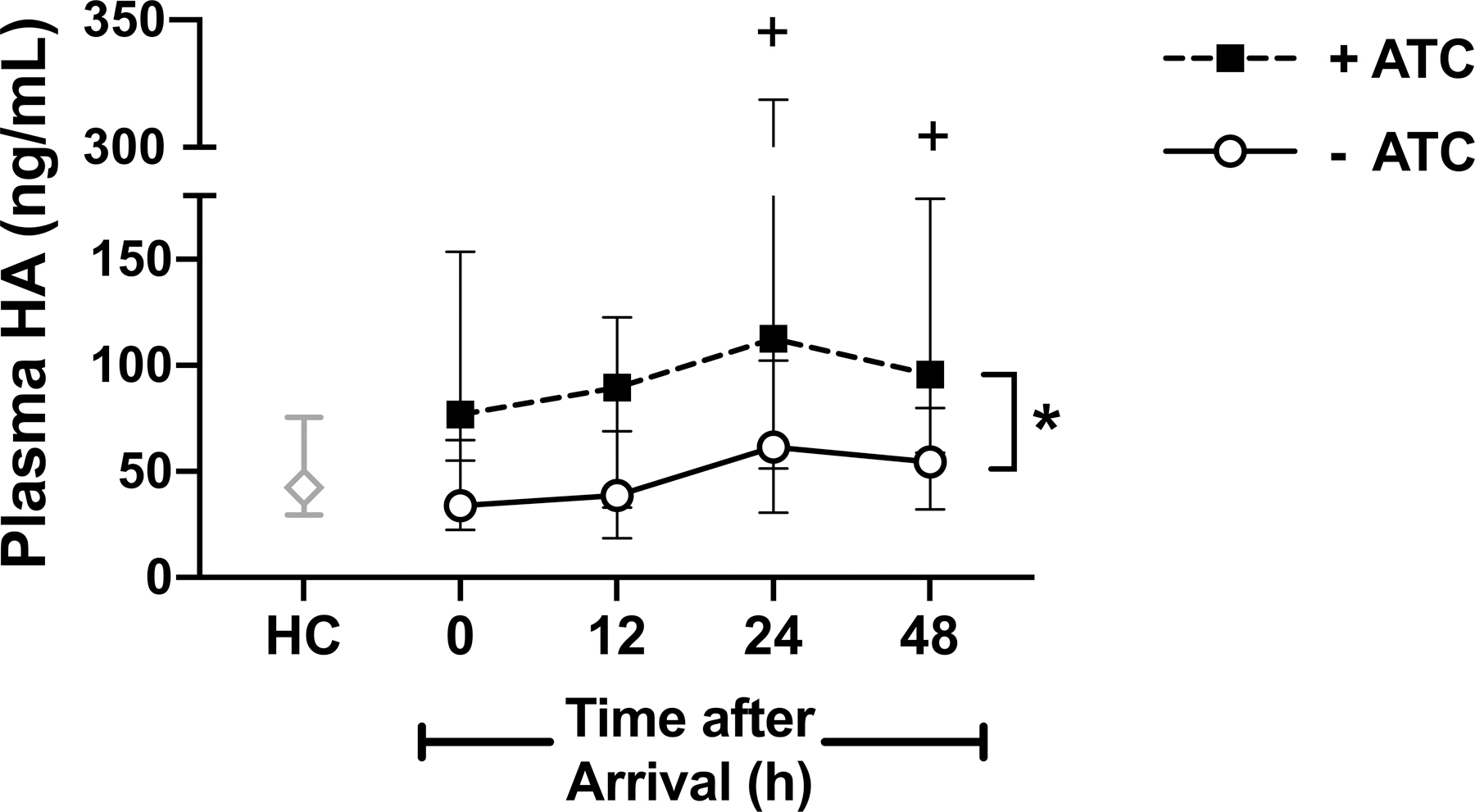
Data are reported as median with interquartile range. *p<0.05 for +ATC vs −ATC at all time points; +p<0.05 for +ATC vs HC.
We then performed a multivariate logistic regression analysis to evaluate the relationship between glycocalyx HA shedding and presence of the ATC phenotype, adjusting for injury severity and degree of shock. In the univariate analysis, ISS was not significantly associated with the development of ATC, whereas admission base deficit and HA levels were significantly associated with an ATC phenotype (Table 2). After adjusting for ISS and base deficit, HA levels remained significantly associated with ATC.
Table 2:
Univariate and multivariate logistic regression analysis with presence of ATC as endpoint.
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
|
||||
| β (95% CI) | p-value* | β (95% CI) | p-value* | |
| Injury Severity Score, per unit | 1.05 (1.00–1.10) | 0.07 | 1.08 (1.01–1.16) | 0.02 |
| Base Excess, per mmol/L | 0.86 (0.76–0.96) | <0.01 | 0.81 (0.70–0.95) | <0.01 |
| Admission HA, per ng/mL | 1.02 (1.00–1.03) | 0.02 | 1.02 (1.00–1.04) | 0.02 |
Emboldened p-values signify statistically significant comparisons.
HA represents hyaluronan.
Correlation with Coagulation Measures
In trauma subjects, HA levels at admission correlated with admission TEG R-time, aPTT, and INR (Table 3). However, there was no correlation between HA and severity of hemorrhagic shock as assessed by base excess and lactate. Given the peak in HA levels 24 hours following hospitalization, we also evaluated the correlation between 24-hour plasma levels of HA and coagulation measures at 24 hours. Only a weak correlation between HA levels and INR was observed at 24 hours (rho = 0.317, p = 0.034).
Table 3:
Spearman’s rank correlations between admission plasma levels of hyaluronan and clinical measures of coagulopathy and hemorrhagic shock.
| rho | p-value* | |
|---|---|---|
| TEG R-Time | 0.395 | 0.02 |
| aPTT | 0.405 | <0.01 |
| INR | 0.420 | <0.01 |
| Base Excess | −0.043 | 0.77 |
| Lactate | 0.172 | 0.24 |
Emboldened p-values signify statistically significant comparisons.
aPTT represents activated partial thromboplastin time; INR, international normalized ratio; TEG, thromboelastograph.
Associations Between Plasma HA Levels and Clinical Outcomes
No subjects developed ALI or ARDS, suggesting that duration of mechanical ventilation was influenced by non-pulmonary clinical determinants (e.g., presence of shock or ongoing resuscitation, need for operative interventions, altered mental status or traumatic brain injury). Plasma HA levels were generally higher in subjects who developed AKI, though differences did not reach statistical significance (Supplemental Figure 1). Only 24-hour HA levels demonstrated significant correlations with clinical outcomes, specifically 24h total transfusion requirements, ICU length of stay, and hospital length of stay (Table 4).
Table 4:
Spearman’s rank correlations between 24-hour plasma levels of hyaluronan and clinical outcomes
| rho | p-value* | |
|---|---|---|
| 24h Total Blood Product Transfusion Requirement | 0.342 | 0.02 |
| Mechanical Ventilation duration | 0.106 | 0.48 |
| ICU Length of Stay | 0.325 | 0.03 |
| Hospital Length of Stay | 0.345 | 0.02 |
Emboldened p-values signify statistically significant comparisons.
ICU represents intensive care unit.
4. DISCUSSION
HA is an integral component of the endothelial glycocalyx, mediating barrier permeability and mechanosignaling that is essential for a diverse repertoire of endothelial cell functions (10,11). Glycocalyx shedding occurs after trauma, and while a relationship between syndecan-1 and early coagulopathy has been demonstrated (8), the association of ATC with plasma levels of HA is not known. In this study, we observed novel correlations between HA and measures of early coagulopathy that may provide new insight into the underlying pathophysiology of ATC. Understanding the types of mechanisms that regulate ATC are critical for the future development of targeted interventions that mitigate death by exsanguination.
In our study, HA levels in trauma patients with early coagulopathy were higher than HA levels in non-coagulopathic patients and healthy controls. Shed HA levels were independently associated with the ATC phenotype and significantly correlated with clinical measures of hypocoagulability but not hemorrhagic shock itself. These findings support a potential linkage specifically between HA shedding and coagulation abnormalities early after trauma. Our evaluation of the relationship between HA and coagulopathy were limited to routine clinical measures of coagulation as levels of clotting factors and other mediators of coagulation (e.g., fibrin, thrombin) were not measured. This limitation prevented us from evaluating whether HA may have a direct impact on intrinsic/extrinsic coagulation pathways. However, we did observe that ATC subjects developed lower platelet counts at 24 hours compared to non-ATC subjects (data not shown). It is likely that the lower platelet counts represent ongoing consumption of circulating platelets due to ongoing coagulation and endothelial adherence. Therefore, the inverse correlation between 24-hour platelet counts and 24-hour HA levels, may suggest a role of HA shedding from the vascular endothelial glycocalyx in ongoing coagulation abnormalities following trauma. Further, as plasma HA levels did not correlate with the degree of shock as represented by base deficit and lactate, our data also support the notion that HA shedding may be more substantially linked to coagulation abnormalities following trauma rather than the degree of shock.
At 24 hours, HA levels also correlated with transfusion requirements and lengths of ICU and hospital stay, indicating that continuous or persistent HA shedding is associated with worse clinical outcomes. Overall, subjects with ATC required greater transfusion volumes than subjects without ATC, although only RBC volumes were significantly different. Informed by the Pragmatic, Randomized Optimal Platelet and Plasma Ratios Phase III Trial (6), our institution generally follows a balanced resuscitation strategy of transfusing platelets, FFP and RBCs in a 1:1:1 ratio. Further, our institution uses apheresis platelet units, which is equivalent to transfusing 4 to 6 units of whole blood-derived platelet concentrates. However, due to the small sample size of our study groups, the apparent non-significant difference in FFP and platelet transfusion volumes between ATC and non-ATC subjects most likely represents a type II (beta) error. Ultimately, higher HA levels are believed to correspond with coagulation impairments that clinically warrant increased blood product transfusion.
Our study also demonstrates that elevated HA levels at 24 hours may be associated with organ dysfunction such as AKI. Others have demonstrated that HA excretion is largely attributed to an extrarenal process in the liver, which may mean that the association between HA levels and AKI is a reflection of endotheliopathy rather than post-trauma HA levels rising due to a defect in renal tubular absorption and clearance (16). Other groups have also identified associations between shed glycocalyx components (e.g., syndecan-1), endotheliopathy and AKI after trauma (17), which support our conclusion that the relationship between high levels of shed HA and organ injury is reflective of endothelial dysfunction.
The close interplay between endothelial glycocalyx damage, specifically syndecan-1 shedding, and coagulopathy triggered by trauma has also been documented (8). However, the relationship between HA and coagulation is less characterized. On one hand, the interaction between soluble HA and fibrinogen is proposed to accelerate clot formation and increase clot stability (18). Conversely, HA coatings on biomaterials act as anti-coagulants to prevent thrombus formation (19). Furthermore, in the case of hemorrhagic shock and injury, HA levels are elevated in patients with reduced colloid osmotic pressure (COP) compared to those with normal COP, which is associated with impaired thrombin generation (12). These findings suggest that HA glycocalyx fragments may play a role in impaired thrombus formation and delayed hemostasis. HA shedding may also impact the binding of pro- and/or anti-coagulant proteins to the exposed endothelial cell surface, which could have important implications on the regulation of clot formation and/or stability. Together, our data, along with other studies examining other glycocalyx components such as syndecan-1 and heparan sulfate, suggest glycocalyx components do have association with coagulopathy. Further mechanistic evaluations are needed, but clarification of the role of HA fragments in coagulation could have tremendous therapeutic benefit. Importantly, future studies should also consider the molecular weight of shed HA fragments, as low and high-molecular weight HA have been shown to have divergent effects on biological activities (20).
Several limitations exist in our study. As stated, the use of a small, single-center population limits the external validity of our findings and increases the risk of a type II error. As with all observational studies, the determined associations may not be causal, and further studies are needed to confirm the mechanistic contribution of HA shedding to endogenous coagulation abnormalities following trauma. Finally, the use of INR > 1.2 to define ATC identifies patients with relatively mild coagulation abnormalities; however, this cut-off does identify patients at risk for worse outcomes (21). Larger studies capable of evaluating HA levels in trauma subjects with greater coagulation derangements are needed to validate the current findings.
5. CONCLUSIONS
Coagulopathy begins early after trauma—often prior to hospital arrival. Mechanisms of ATC are not clearly understood, although our findings suggest a potential association between plasma HA levels, development of ATC, and clinical outcomes in trauma patients. Further elucidation of ATC pathophysiology is crucial in developing targeted resuscitation techniques to be used in the acute management of trauma patients.
Supplementary Material
Source of Funding:
Jillian R. Richter received funding from the National Institutes of Health (5R35GM137958–02).
Footnotes
Conflicts of Interest: The authors declare no disclosures.
REFERENCES
- 1.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, and Friese RS: Increasing trauma deaths in the United States. Ann Surg 260(1):13–21, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Callcut RA, Kornblith LZ, Conroy AS, Robles AJ, Meizoso JP, Namias N, Meyer DE, Haymaker A, Truitt MS, Agrawal V, et al. : The why and how our trauma patients die: A prospective Multicenter Western Trauma Association study. J Trauma Acute Care Surg 86(5):864–70, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushimoto S, Kudo D, and Kawazoe Y: Acute traumatic coagulopathy and trauma-induced coagulopathy: an overview. J Intensive Care 5(6):1–7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brohi K, Singh J, Heron M, and Coats T: Acute traumatic coagulopathy. J Trauma 54(6):1127–30, 2003. [DOI] [PubMed] [Google Scholar]
- 5.MacLeod JB, Lynn M, McKenney MG, Cohn SM, and Murtha M: Early coagulopathy predicts mortality in trauma. J Trauma 55(1):39–44, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. : Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 313(5):471–82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. : Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med 379(4):315–26, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Johansson PI, Stensballe J, Rasmussen LS, and Ostrowski SR: A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg 254(2):194–200, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, and Kajiya F: Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285(2):H722–6, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Dogne S and Flamion B: Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am J Pathol 190(4):768–80, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Queisser KA, Mellema RA, and Petrey AC: Hyaluronan and Its Receptors as Regulatory Molecules of the Endothelial Interface. J Histochem Cytochem 69(1):25–34, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, Ostrowski SR, Johansson PI, Holcomb JB, and Wade CE: Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 13:117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlich RM, Richter RP, Hu PJ, Kirkman AA, Ashtekar AR, Zheng L, Walker SC, Reynolds LM, Griffin RL, Jansen JO, et al. : Temporal Dysregulation of the Angiopoietin-2/−1 Ratio After Trauma and Associations With Injury Characteristics and Outcomes. Shock 54(6):703–9, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, and Slutsky AS: Acute respiratory distress syndrome: the Berlin Definition. JAMA 307(23):2526–33, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, and Acute Dialysis Quality Initiative w: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8(4):R204–12, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser JR, Laurent TC, Pertoft H, and Baxter E: Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J 200(2):415–24, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatton GE, Isbell KD, Henriksen HH, Stensballe J, Brummerstedt M, Johansson PI, Kao LS, and Wade CE: Endothelial Dysfunction is Associated With Increased Incidence, Worsened Severity, and Prolonged Duration of Acute Kidney Injury After Severe Trauma. Shock 55(3):311–15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeBoeuf RD, Raja RH, Fuller GM, and Weigel PH: Human fibrinogen specifically binds hyaluronic acid. J Biol Chem 261(27):12586–92, 1986. [PubMed] [Google Scholar]
- 19.Verheye S, Markou CP, Salame MY, Wan B, King SB 3rd, Robinson KA, Chronos NA, and Hanson SR: Reduced thrombus formation by hyaluronic acid coating of endovascular devices. Arterioscler Thromb Vasc Biol 20(4):1168–72, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Cyphert JM, Trempus CS, and Garantziotis S: Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int J Cell Biol 2015:563818, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, Johansson PI, Stanworth S, Thiemermann C, and Brohi K: Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost 8(9):1919–25, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


