Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by diffuse inflammation of the colorectal mucosa. Unlike Crohn’s disease, which has long been characterized as a progressive disease which results in bowel damage, the similarly progressive nature of UC has only more recently been considered and characterized by the extent of colonic involvement, the development of neoplasia, altered colonic permeability, and dysmotility/anorectal dysfunction.1 Indirect support for these observations are found in the pivotal trials of therapies for UC, which demonstrate discrepancies between clinical remission and measures of mucosal healing.2,3. We therefore postulated that a potential explanation for these findings is a type of progressive damage from chronic inflammation in UC that results in diminished rectal compliance.
We performed a prospective controlled study of compliance of the rectum in adult patients with UC and non-IBD controls. We recruited patients who were scheduled for routine sigmoidoscopies or colonoscopies and at the conclusion of the endoscopic examination we performed a barostat examination, in which a rectal balloon was incrementally insufflated to an initial pressure of 5 mm Hg and up to a maximal pressure of 60 mmHg, and the corresponding rectal balloon volumes (mL) were measured by the barostat (see Supplemental Methods). Clinical and demographic data were collected from electronic medical records, and a Simplified Clinical Colitis Activity Index (SCCAI), Mayo endoscopic subscores, and biopsies of the rectum were obtained.
We assessed static rectal compliance using a validated power exponential model4 in which the volume (Vol) at any given pressure (P) is defined as
where Vol is the rectal volume, relative pressure , and Vmax is the maximal rectal volume in the compliance assessment. The parameter β reflects the overall shape of the curve, and k is the change in volume as a function of at any given point. ϵ is a static error correction value. We compared static rectal compliance between all patients with UC and controls and assessed the effect of disease-related factors on the compliance parameters.
We recruited 93 people, 73 patients with UC and 20 non-IBD controls. Patients with UC had a mean disease duration of 16.9y (SD 11.9), 51% were women and 71% had extensive disease. The control and UC groups were similar across all demographics characteristics apart from age with a mean age of 61.1y (SD 11.3) in the control group and 51.5y (SD 14.4) in the UC group (p=0.007). (Supplementary Table 1).
Static rectal compliance was lower in patients with UC compared with the control group (Vmax= 265.7 mL vs Vmax= 311.1 mL, p=0.047), which remained significant after adjusting for the age differences between the groups (p=0.043). (Figure 1A.) Patients with UC who had an SCCAI scores of ≥ 5 compared with those with a score <5, had lower compliance (Vmax= 226.7 mL vs Vmax= 271.7 mL, p = 0.0379). (Figure 1B.) Stool frequency (Vmax= −11.5 mL, p = 0.034; k 1.01, p = 0.02) and the Mayo endoscopic subscore (β= −0.07, p = 0.02) had a negative effect on compliance. History of biologic therapy was associated with reduced compliance (Vmax= 244.07mL vs Vmax= 290.64mL, p=0.043). (Figure 1C.) Disease duration, disease extent, age, and sex were not associated with differences in rectal compliance.
Figure 1:
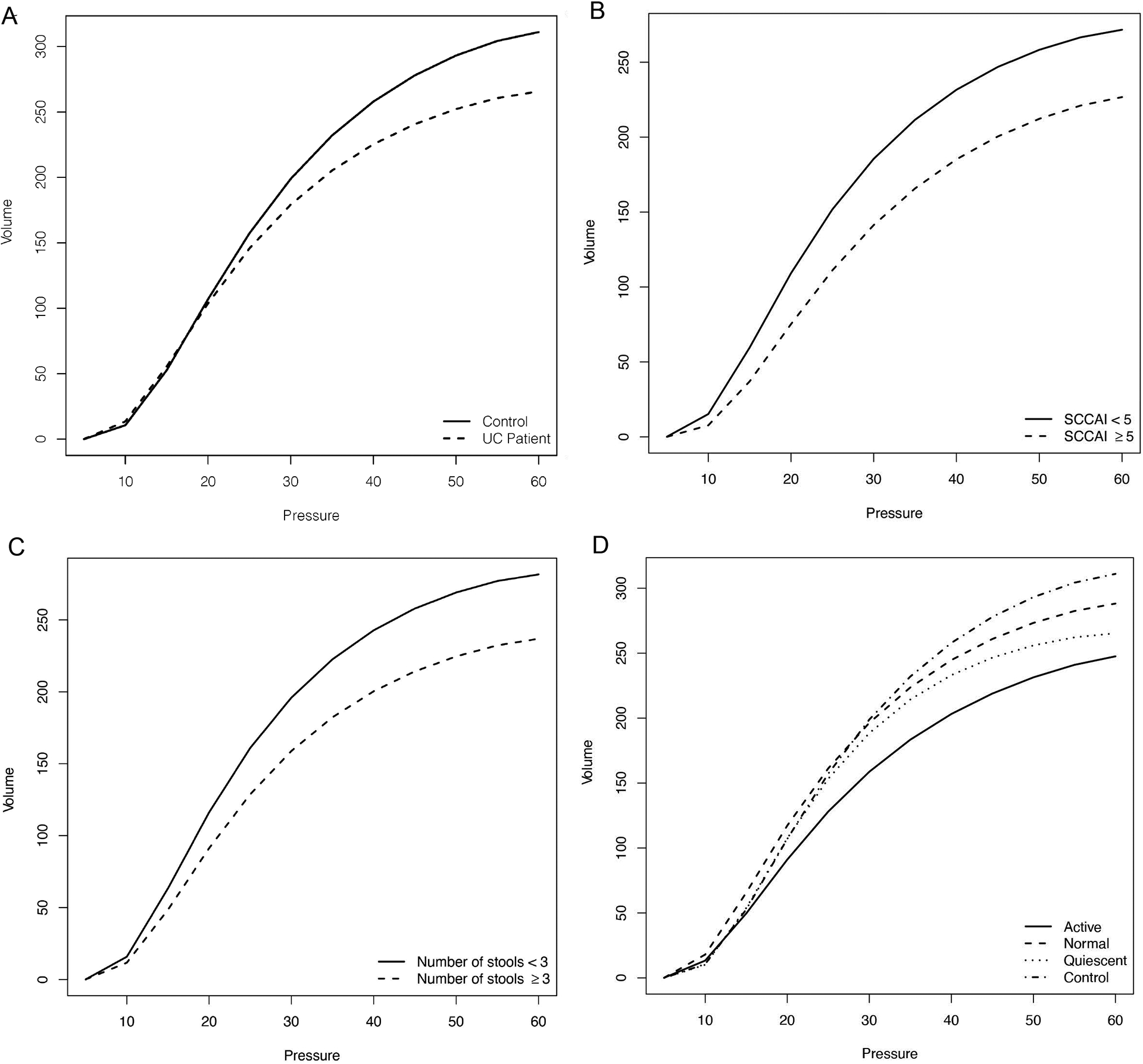
Static rectal compliance curves. A) Ulcerative colitis (UC) and non-IBD control patients B) Simple Clinical Colitis Activity Index (SCCAI) in UC patients. C) Stool frequency scores in UC patients D) Histology scores: Active=acute active inflammation in UC patients; Normal=normalized histology in UC patients; Quiescent=quiescent histology in UC patients; Control=non-IBD normal histology
In the analysis of histologic disease, 28 patients had active inflammation, 22 quiescent disease and 23 patients normalized their histology. Rectal compliance was lower in patients with active histologic disease (Vmax=247.6mL, SE=16.9) compared to the healthy controls (Vmax = 311.1mL, SE = 20.0; p = 0.01) and compared to quiescent histologic disease (Vmax= 265.3mL, SE = 19.1; p=0.03). The histologically quiescent group had lower rectal compliance (Vmax = 265.32 mL, SE=19.08) compared to the control group (Vmax= 311.1 mL, SE=20.0; p = 0.015). The group who normalized their histology had similar rectal compliance (Vmax = 288.21 mL, SE = 18.67) when compared with the controls (Vmax = 311.06 mL, SE=20.02; p = 0.287). When comparing the controls with all UC patients (active, quiescent and normalized histology) there was a significant difference in rectal compliance between the two groups (Vmax= 311.07mL, SE = 20.25 vs Vmax= 265.7mL, SE = 10.6, p=0.045). (Figure 1D.)
In this prospective controlled study using a barostat and advanced statistical analysis, we identified that rectal compliance is significantly lower in patients with UC compared with non-UC controls. A prior small study in patients with actively inflamed UC demonstrated reduced compliance and increased rectal hypersensitivity5 as a physiologic correlate to the active symptoms patients experience when the rectum is inflamed. Our controlled study confirms that there is also diminished compliance in the quiescent phase of UC and contributes to the possibility that other pathophysiologic factors that are in addition to or beyond mucosal inflammation may lead to diminished compliance of the rectum. These factors may include anatomical variables such as changes to the bowel wall constituents such as muscularis mucosal hypertrophy, submucosal fibrosis, as well as changes to the presacral spaces, all of which have been demonstrated in UC6,7.
One of the most remarkable findings of our study was the identification that patients with UC who had normalized their histology had improved rectal compliance compared with those with histologic quiescence, and that these patients had compliance similar to that of healthy controls. This finding suggests that the unique endpoint of histologic normalization may also be associated with clinically relevant functional result and that reduced rectal compliance may be preventable or reversible with sufficient disease control. Such muscle “remodeling” has been described in other diseases8.
In conclusion, we demonstrate that patients with active or quiescent UC have diminished rectal compliance compared with healthy controls, and provide further evidence that UC is a progressive condition. We propose rectal compliance as a functional outcome and a marker of increased disease severity and quality of life for patients with UC.
Supplementary Material
Acknowledgements:
The authors wish to acknowledge Jingzhou Wang, MD, Christopher R. Weber, MD, PhD, and Dezheng Huo, PhD for their assistance with this study.
Disclosures:
NKC is a consultant for Takeda and Arena pharmaceuticals.
VR, KEJ, MCG, and SSR have no relevant disclosures.
DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Arena Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Genentech/Roche, Gilead Sciences, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Pfizer, Prometheus Laboratories, Reistone, Takeda, and Techlab Inc.
Grant support:
funded in part by the Gastro-intestinal Research Foundation of Chicago, the Scholtz Family Foundation, and the Digestive Disease Research Core Center of the University of Chicago (DK42086).
References
- 1.Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18(7):1356–1363. doi: 10.1002/ibd.22839 [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. New England Journal of Medicine. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516 [DOI] [PubMed] [Google Scholar]
- 3.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. New England Journal of Medicine. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Ford MJ. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment Pharmacol Ther. 1998;12(4):287–302. doi: 10.1046/j.1365-2036.1998.00305.x [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, Read NW, Davison PA, Bannister JJ, Holdsworth CD. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1987;93(6):1270–1275. doi: 10.1016/0016-5085(87)90255-1 [DOI] [PubMed] [Google Scholar]
- 6.Alp MH, Sage MR, Grant AK. The significance of widening of the presacral space at contrast radiography in inflammatory bowel disease. Aust N Z J Surg. 1978;48(2):175–177. doi: 10.1111/j.1445-2197.1978.tb07298.x [DOI] [PubMed] [Google Scholar]
- 7.Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47(7):922–939. doi: 10.1111/apt.14526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87(3):755–763. doi: 10.1161/01.ci.87.3.755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


