Abstract
B cells play multiple roles in immune responses related to autoimmune diseases as well as different types of cancers. As such, strategies focused on B cell targeting attracted wide interest and developed intensively. There are several common mechanisms various B cell targeting therapies have relied on, including direct B cell depletion, modulation of B cell antigen receptor (BCR) signaling, targeting B cell survival factors, targeting the B cell and T cell costimulation, and immune checkpoint blockade. Nanocarriers, used as drug delivery vehicles, possess numerous advantages to low molecular weight drugs, reducing drug toxicity, enhancing blood circulation time, as well as augmenting targeting efficacy and improving therapeutic effect. Herein, we review the commonly used targets involved in B cell targeting approaches and the utilization of various nanocarriers as B cell-targeted delivery vehicles.
Keywords: Nanomedicines, B cell targeting, Autoimmunity, Immunotherapy
Graphical Abstract
1. INTRODUCTION
Nanocarrier-based drug delivery, a promising area in medicine development, is effective in diagnosis and treatment of disease. The physicochemical properties of nanocarriers such as size, charge, biocompatibility and absence of toxicity make them superior carriers for drug delivery. Manipulating the size of nanocarriers enables control of the intravascular half-life [1, 2]. Simultaneously, capture of nanocarriers by the mononuclear phagocyte system (MPS) can be minimized by adjusting their size, chemical composition and/or surface characteristics.
Nanocarriers can be broadly divided into two categories: inorganic nanocarriers and organic nanocarriers. The inorganic nanocarriers generally consist of an inorganic core such as gold [3], quantum dots [4], iron oxide [5] or silica nanoparticles [6] and a shell (usually synthetic polymers) to provide a linker for biomolecule conjugation or prevent core damage due to unwanted physicochemical interactions within the biological microenvironment. The organic nanocarriers include water soluble polymers [7–9], polymeric micelles [10, 11], liposomes [12], vesicular carriers [13–16], etc. Both inorganic and organic nanocarriers can be used as diagnostics and delivery vehicles by conjugation or entrapment with therapeutic agents such as peptides, proteins, siRNA, and drugs [17–21]. The large surface-to-volume ratio of nanocarriers enables them to possess a high-loading capacity to deliver therapeutic or imaging agent. Surface modification of nanocarriers with amphiphilic polymers such as polyethylene glycol (PEG) could reduce the uptake by the MPS, liver, and spleen [22, 23]. The utilization of nanocarriers elongates drug circulation time, minimizes toxicity, protects active drug against degradation and attains controlled drug release. The biophysical and biocompatible properties of tunable nanocarriers could be easily adjusted, which makes them versatile in application in biomedical use [24].
Passive targeting of nanomedicines in tumor tissue takes advantages of the enhanced permeation and retention (EPR) effect due to blood vessel leakage and inefficiency of lymphatic drainage in tumor tissues [25–28]. Active targeting nanocarriers contain specific structures (targeting moieties) that bind to specific surface structures on target cells, thus significantly enhancing therapeutic results and minimizing off-target effects. Multivalence is another notable advantage of nanocarriers as it allows better targeting, enhanced internalization and changes in subcellular trafficking, as well as augmented therapeutic efficacy [29, 30]. Various types of molecules have been conjugated to nanocarriers for active targeting including peptides [31, 32], antibodies [33], antibody fragments [34] aptamers [35], affibodies [36, 37], and small chemical molecules such as receptor ligands [38]. Although nanomedicines are extensively used, the limitations are not to be ignored such as toxicity/drug leak into the blood stream of liposomes, difficulty to scale up for polymeric nanoparticles, low drug-loading efficacy due to crystalline structure for solid lipid nanoparticles and toxicities for nanoemulsions and metallic nanoparticles [39]. Here we shall discuss the utilization of nanocarriers in B cell targeted therapy as B cells are essential in the development of adaptive immunity related to tumor microenvironment and chronic inflammation.
B cells initiate from hematopoietic stem cells (HSC) in mammalian bone marrow [40]. HSCs pass through sequential developmental stages such as pro-B cells, pre-B cells and grow into immature B cells in a stepwise manner, at which stage B cells are capable to recognize antigens [41]. The immature B cells subsequently migrate to secondary lymphoid tissues (such as spleen and nymph nodes) where they divide into mature naïve B cells [42]. Activation of mature naïve B cells happens when they bind with their respective antigen. Activated naïve B cells are selected and differentiated into plasmablasts depending on bound antigens followed by development of antibody producing plasma B cells [43]. More details of selected surface marker expression and disease association along with B cell development stages are summarized in Figure 1.
Figure 1.
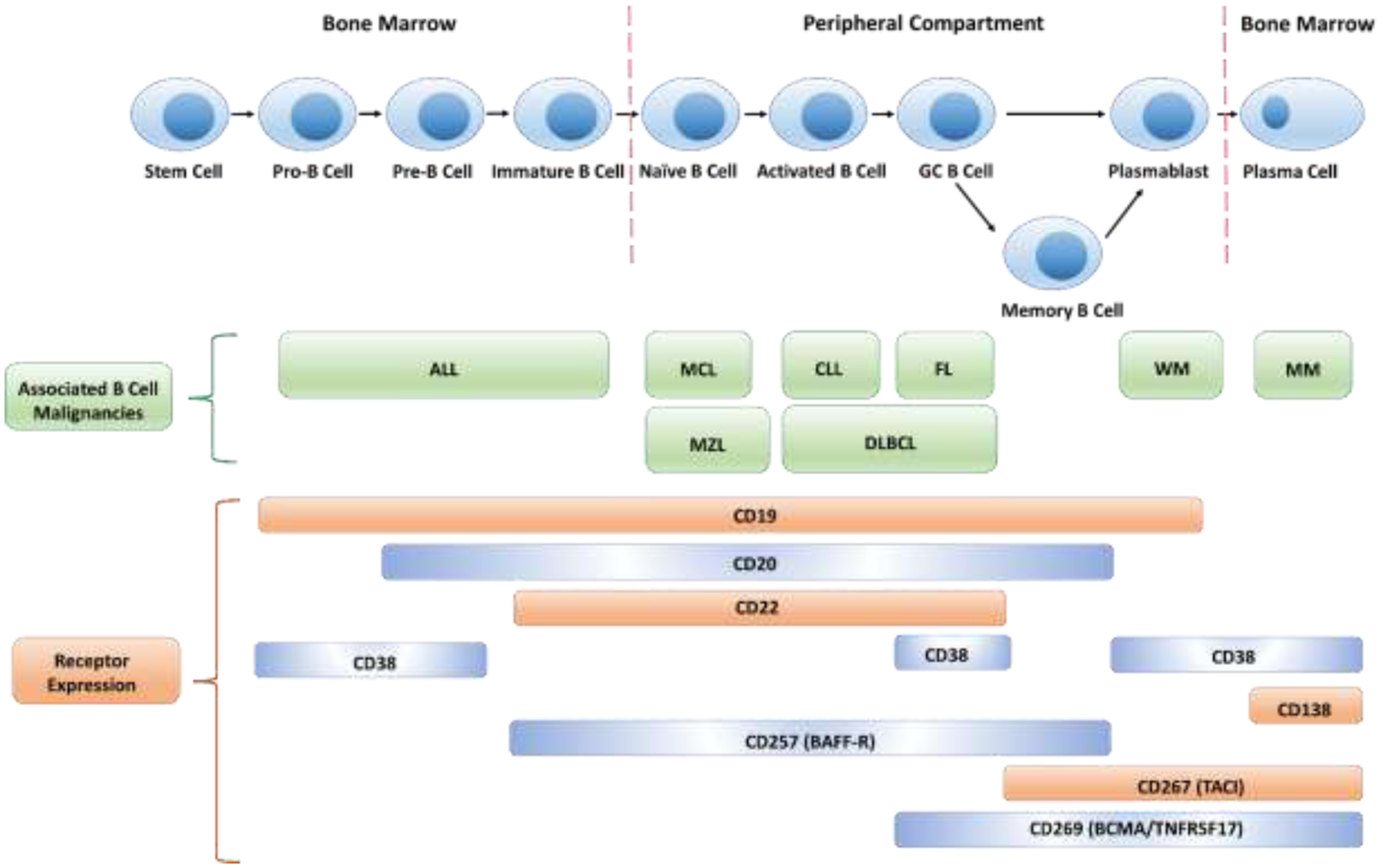
B cell lineage development along with corresponding biomarker expression and associated B cell malignances. GC: germinal center; ALL: acute lymphocytic leukemia; MCL: mantle cell lymphoma; CLL: chronic lymphocytic leukemia; FL: follicular lymphoma; MZL; marginal zone lymphoma; DLBCL: diffuse large B-cell lymphoma; WM: Waldenstrom macroglobulinemia; MM: multiple myeloma.
B cells contribute to immunity through antibody-independent functions and antibody-dependent functions.
There are two major antibody-independent functions B cells perform to affect T cell activity and other immune cells. Antigen-activated B cells could act as antigen-presenting cells (APCs), participating in the generation or regulation of immune responses by activating or tolerating T cells at even low antigen concentration [44]. There are three ways for APCs to internalize antigen: phagocytosis, pinocytosis and receptor-mediated endocytosis. Other APCs like macrophages and dendritic cells internalize antigens via phagocytosis and pinocytosis while B cells internalize antigens through B cell antigen receptor (BCR)-mediated biorecognition. Thus, B cells serve as APCs with high sensitivity and specificity in normal immunity and autoimmune diseases [45, 46].
As most antigens need to be processed before being presented to T cells, B cells - like other APCs - perform antigen processing and presentation with facilitation from major histocompatibility complex class II (MHCII) molecules [47]. Antigens internalized via BCR signaling are degraded into smaller fragments in the lysosomes. The MHCII molecule binds to desired antigenic peptide fragment and translocates it to the cell surface to present the antigen to T cells [48]. Human leukocyte histocompatibility complex DO (HLA-DO) is expressed on human B cell surface and serves as chaperone in this process [49]. It is also reported that B cells are related to the generation of ectopic germinal center (GC) in inflammatory tissues. The activation of B cells results in the activation of CD4+ T cells while non-B cell APCs cannot maintain T cell stimulation. Activated T and B cells promote development of ectopic GCs [50].
Another antibody-independent mechanism B cells are involved in relates to proinflammatory cytokine secretion. Activated B cells can produce chemokines CCL22 and CCL17, which are able to recruit CD4+ Th2 type T cells [51]. These T cells, in turn, initiate B cell differentiation towards cytokine secreting B cells. Cytokines secreted by B cells include TNF-α, lymphotoxin and IL-6; this enables B cells to modulate and amplify immune response [52]. On the other side, production of pro-tumorigenic cytokines from B cells can also support neoplastic cells as well as suppress cytotoxic T cell proliferation. Regulatory B cells suppress effector T cells and NK cells by expressing immune checkpoints, such as programmed death ligand PD-L1 [53, 54].
In the antibody-dependent mechanism, plasma cells differentiated from B cells secret autoantibodies which are present in the peripheral circulation. Autoantibody-mediated antigen uptake has been proved to be a pathogenic mechanism of T cell-mediated autoimmune diseases [55, 56]. Secreted autoantibodies could also activate or inhibit receptor functions. Autoantibodies to thyroid stimulating hormone (TSH) receptor can either inhibit the TSH receptor signaling pathway or activate different signaling cascades [57, 58].
In short, B cells execute anti-tumor effect through recognition of tumor-specific antigens, performance of antigen-activated B cells as APC and antibody production (IgG) by plasma cells. Secretion of effector cytokines by activated B cell promotes T cell responses in anti-tumor functions and B cells may mediate direct toxicity in cancer [59, 60]. Meanwhile, B cells could also have pro-tumor activities by involvement in solid tumorigenesis by multiple tumor-promoting mechanisms and activation of myeloid-derived suppressor cells (MDSCs). In addition, B cells also play important roles in progression of autoimmune diseases. Besides autoantibody secretion, production of inflammatory cytokines and APC function, more detailed mechanisms of B cells involved in autoimmune disease development and corresponding B cell targeting approaches have been summarized in [61].
Moreover, B cell targeting strategies show promise in the treatment of many other diseases such as cardiovascular diseases. As atherosclerosis is lipid-driven, plasma cholesterol level is a cornerstone treatment modality. However, even with optimal lipid-lowering, there is still a considerable risk of cardiovascular disease, which may be attributed to inflammation [62]. Conventionally, B cells play an overall protective role in atherogenesis [63]. However, there are increased data indicating depletion of B cells is beneficial for preventing atherosclerosis development/progression in mouse models [64, 65]. Improvement of lipid profiles was noticed in these patients treated with B cell depletion therapy [63]. B cell activation may enhance atherosclerosis by producing autoantibody and inflammatory cytokines as described above, depending on the B cell subsets [65, 66]. Thus, selective B cell depletion strategies could possibly be advantageous to atherosclerosis management.
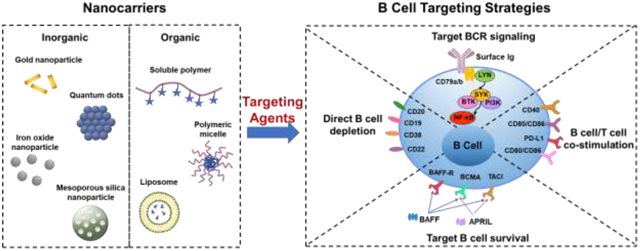
As critical players in B cell malignancies, immunity in tumor microenvironment and development of autoimmune diseases, B cells have been progressively recognized as essential participants in therapies of cancer and autoimmune diseases [53, 67]. Based on the roles B cells play in pathogenic pathways, therapeutic strategies for targeting B cells can be generally categorized into direct B cell depletion [68], modulation of BCR signaling [69], targeting B cell survival [70], targeting B cell/T cell costimulation [71], and immune checkpoint blockade [72]. In this review, we summarize the main strategies employed in targeting B cells and corresponding applications of diverse nanocarriers (Figure 2 & Table 1).
Figure 2.
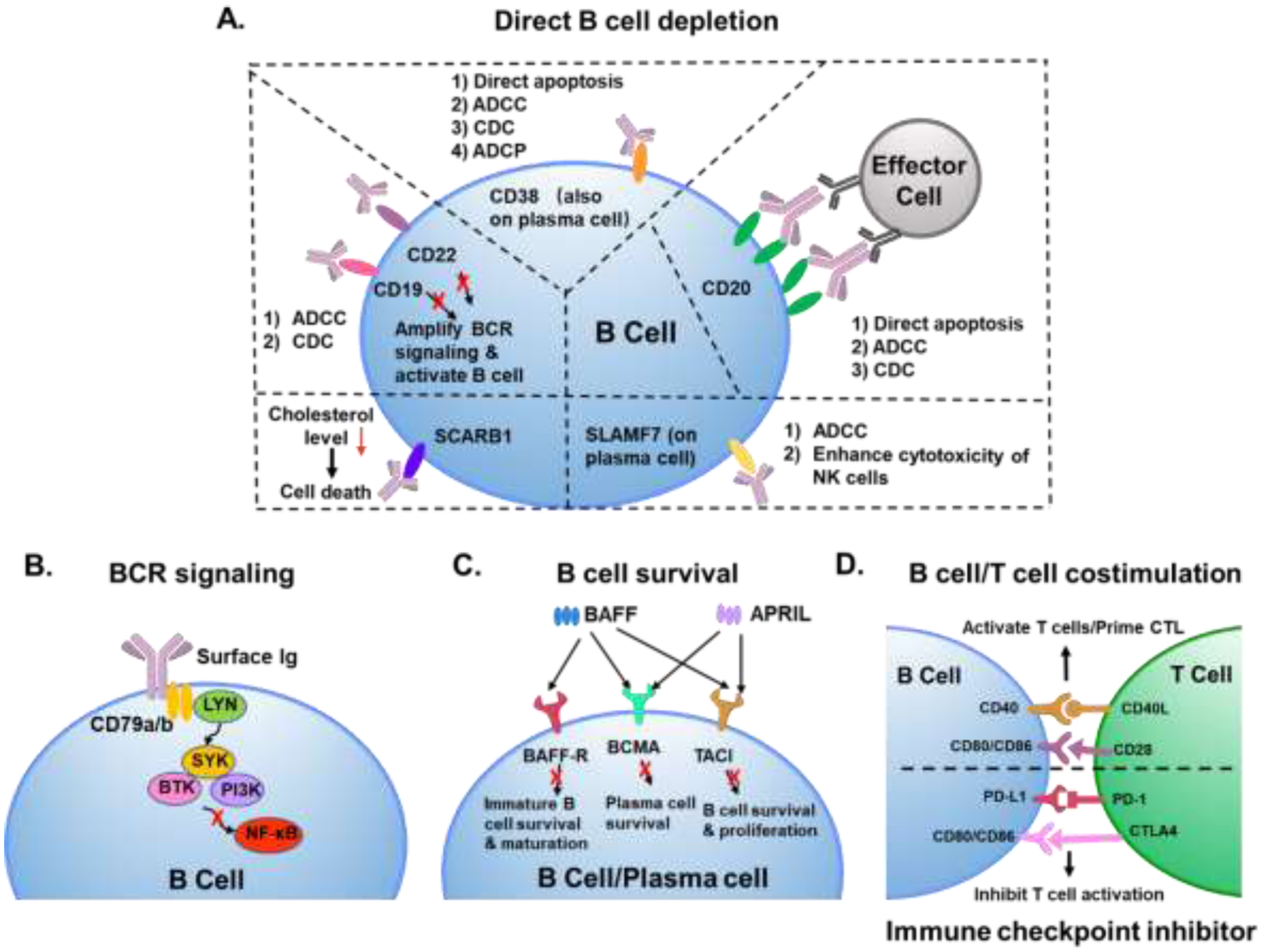
B cell targeting approaches. A. Mechanisms for direct B cell depletion. ADCC: antibody-dependent cellular cytotoxicity; CDC: complement-dependent cytotoxicity; ADCP: antibody-dependent cellular phagocytosis; BCR: B cell antigen receptor signaling; SCARB1: scavenger receptor type B-1; SLAMF7: surface antigen signaling lymphocytic activation molecule-F7; NK cells: natural killer cells. B. Through BCR signaling. LYN: tyrosine-protein kinase; SYK: spleen tyrosine kinase; BTK: Bruton’s tyrosine kinase; PI3K: phosphoinositide 3-kinase; NF-κB: nuclear factor kappa B. C. Targeting B cell survival. BAFF: B cell activating factor; APRIL: a proliferation-inducing ligand; BAFF-R: B cell activating factor receptor; BCMA: B cell maturation antigen; TACI: transmembrane activator and CAML interactor. D. Targeting B cell/T cell costimulation and immune checkpoint inhibition. CTL: cytotoxic T lymphocyte; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; CTLA4: cytotoxic T lymphocyte associated protein 4.
Table 1.1.
Examples of nanomedicines for B cell targeting.
| Mechanisms | Targets | Diseases/Applications | Clinical Options | Targeting Moieties | Nano-strategies |
|---|---|---|---|---|---|
| Direct B cell depletion | CD20 | Non-Hodgkin’s lymphoma (NHL) [105–107, 114, 115, 120], chronic lymphocytic leukemia (CLL) [110, 111], rheumatoid arthritis (RA) [109], leukemia [125], melanoma [126], etc | mAb: rituximab (Rituxan®), obinutuzumab (Gazyva®), ofatumumab (Kesimpta®), ibritumomab (Zevalin®), ocrelizumab (Ocrevus®), etc. More details in [288]. | Antibodies [92–104], Fab’ fragment [105–115, 120], scFv [122, 124, 125], aptamers [126] | PLA-b-PEG-COOH, PCL-COOH [93, 94], PLGA [95], caveosphere [96], albumin [97], chitosan/quantum dots [98], liposome [99, 100, 126], micelles [101], iron oxide [102–104], PHPMA [105–111], HSA [114, 115, 120], ELP [122], polystyrene-PEG-biotin [124], PEG-coated iron oxide [125] |
| CD19 | Burkitt’s lymphoma [130], acute lymphoblastic leukemia (ALL) [131–133, 135], rheumatoid arthritis [289] | mAb: inebilizumab (Uplizna®); BiTE: blinatumomab (Blincyto®); CAR-T therapies: axicabtagene ciloleucel (Yescarta®), tisagenlecleucel (Kymriah®), lisocabtagene maraleucel (Breyanzi®). More details in [290]. | Antibodies [130–132, 135, 137, 140, 289], CD19L [136] | Liposome [130, 132, 133], gold NPs [131], PEG-PCL [135], mPEG-bPEI-PEBP [137], iron oxide [140] | |
| CD22 | ALL [150], NHL [151], diffuse large B-cell lymphoma (DLBCL) [153] |
mAb: epratuzumab in clinical trials [291, 292]; ADC: inotuzumab ozogamicin (Besponsa®) [293]; CAR-T therapy: moxetumomab pasudotox (Lumoxiti®) [294] |
Antibodies [150, 151], Fab’ fragment [152], sialic acid [153], N-linked glycan scaffold [154] | Iron oxide [150], quantum dot [151], liposome [152], ChNP [153] | |
| CD38 | Multiple myeloma [157, 158] | mAb: daratumumab (Darzalex®); isatuximab-irfc (Sarclisa®) [155]. | Antibody [157, 158] | Chitosan NP [157], PEO-b-PBCL NP [158] | |
| Surface antigen signaling lymphocytic activation molecule-F7 | Multiple myeloma [161, 223] | mAb: elotuzumab (Empliciti®) [162] | Antibody [161, 223] | Silica/Gd NP [223] | |
| Idiotypic sequences | NHL [168, 169] | DNA vaccines failed several clinical trials [166, 167] | Immunogenic self-antigen expressing plasmid DNA [168, 169] | PEI-PLGA NP [168, 169] | |
| Scavenger receptor type B-1 | DLBCL [170] | N/A | Cholesterol-poor high-density lipoprotein [170] | Lipoprotein-like NP [170] | |
| Modulation of B cell receptor signaling | CD79a/b | NHL [179, 180], RA [182] | ADC: polatuzumab vedotin (Polivy®) [181] | Antibody [179, 180, 182] | N/A |
| Bruton tyrosine kinase | CLL, mantle cell lymphoma, small lymphocytic lymphoma, marginal zone lymphoma, RA [187] | Kinase inhibitors: ibrutinib (Imbruvica®), acalabrutinib (Calquence®), zanubrutinib (Brukinsa®) [295] | Inhibitor (Ibrutinib) [186], siRNA [187] | Chitosan/sulfobutylether-b-cyclodextrin NP [185], PEG-b-PLGA [187] | |
| Spleen tyrosine kinase | ALL [191], mantel cell lymphoma [192], B-precursor acute lymphoblastic leukemia [193], RA [296], CLL [297] | Kinase inhibitor: fostamatinib (Tavalisse®); entospletinib in clinical trials Phase I/II [189] | Inhibitors (C61 [191–193], R406 and R788 [296, 297]) | Liposome [191–193] | |
| Phosphoinositide 3 kinase | Pancreatic adenocarcinoma [200], breast cancer [201, 207], multiple myeloma [208], etc | Kinase inhibitor: alpelisib (Piqray®), idelalisib (Zydelig®), copanlisib (Aliqupa®), duvelisib (Copiktra®), umbralisib (Ukoniq®). More information provided in [298]. | Inhibitors (IPI-549 [200, 201], GDC-0941 [206], AS101 [207], Walterinnesia aegyptia venom [207, 208]) | PLGA [200], PEGylated PCL NP [201], silica NP [207, 208] | |
| Targeting B cell survival | B cell maturation antigen (BCMA) | Multiple myeloma [222, 223] |
ADC: belantamab mafodotin (Blenrep®); CAR T therapy: idecabtagene vicleucel (Abecma®). More in [299]. |
BiTE, CAR-T, BCMA72–80 peptide [222], antibody [223] | PLGA NP [222], silica/Gd NP [223] |
| B cell activating factor receptor (BAFF-R) | ALL [227], atherosclerosis [228], RA [230], NHL [231], myasthenia [232] |
mAb: belimumab (Benlysta®); tabalumab/bortezomib/ianalumab in clinical trials [217, 300]; CAR-T therapy PMB-101 got IND (investigational new drug) clearance for ALL. |
CAR-T [227], antibody [228, 229], CRISPR-Cas9 [230], BAFF competitor (mBAFF) [231], siRNA [232, 233], RNA aptamer [234] | (PEG-b-PLGA)-based cationic lipid-assisted NP [230], PEGylated liposome [231] | |
| Transmembrane activator and CAML interactor (TACI) | Systemic lupus erythematosus [235], RA [236], multiple myeloma [239–241] | Recombinant fusion proteins: atacicept failed in clinical trials [237]. More discussion in [242]. | TACI-Ig [235, 236], anti-APRIL antibody [239], CAR-T [240, 241] (usually combined with BCMA-targeting) | N/A | |
| Targeting B cell/T cell costimulation and immune checkpoint blockade | CD80/CD86 | RA [249], renal transplant [251] | Recombinant fusion proteins: abatacept (Orencia®); FPT155 in clinical trial Phase Ia/Ib [301]. | CTLA-Ig [249], antibody [251], ovalbumin (targeting dendritic cells) [252, 303, 304] | Liposome [302], carbon nanotube [303] |
| CD40 | RA [257], activate APCs [259], melanoma [260] | mAb: sotigalimab in clinical trials phase I/II. More discussion on CD40 targeting in [263]. | Antibody [257, 259, 260, 303] | Silicon NP [259], PLGA NP [260], carbon nanotube [303] | |
| Programmed cell death ligand 1 and 2 (PD-L1/L2) | Various type of tumors including melanoma [273], colorectal cancel [274], breast cancer [275, 278, 284], glioma [280], melanoma [283], DLBCL [286], etc. |
αPD-1 mAb: permbrolizumab (Keytruda®), nivolumab (Opdivo®), cemiplimab (Libtayo®); αPD-L1 mAb: atezolizumab (Tecentriq®), avelumab (Bavencio®), durvalumab (Imfinzi®), etc. More in [304]. |
Antibody [265–268, 272–274], peptide antagonist PPA [278, 280], scFv [282], plasmid DNA encoding CRISPR/Cas13a [283], siRNA [284, 285], microRNA [286] | PLGA NP [273], gold NP [274], liposome [275], PHPMA [278], PEG-PCL NP [280], iTEP [253], PEI-HPBA [254], lipid-coated calcium phosphate NP [284], lipid NP [286] |
2. DIRECT B CELL DEPLETION
Direct B cell depletion is one of the most used strategies targeting B cells to treat cancer and autoimmune diseases (Figure 2A) [67, 73, 74]. Monoclonal antibodies are the major agents used for B cell depletion such as rituximab (Rituxan®), obinutuzumab (Gazyva®) and ofatumumab (Kesimpta®) targeting CD20 [75], blinatumomab (Blincyto®) and inebilizumab (Uplizna®) targeting CD19 [76], inotuzumab ozogamicin (Besponsa®) targeting CD22 [77] and daratumumab (Darzalex®) targeting CD38 [78]. Depletion strategies targeting CD19 can kill plasma cells, which are responsible for antibody production, while those targeting CD20 and CD22 do not diminish plasma cells. CD38 targeting approaches can specifically reduce the number of plasma cells as CD38 is particularly expressed on plasma cells [79]. Patients with chronic inflammation usually require repeated B cell depletion treatment; adverse effects including infusion reactions, immune response to chimeric antibody and infections may happen. Following repeated depletion, B cells can be restored in up to 6 months as a passive process once treatment is terminated [80]. Different disease types also affect the repopulation process. The repopulation speeds after rituximab depletion of B cell are significantly differentiated between patients with rheumatoid arthritis, connective tissue diseases, and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) [81].
2.1. CD20
The CD20 receptor is widely expressed on the surface of most B cells except pro B cells and plasma cells. Rituximab (Rituxan®, RTX), the first clinical anti-CD20 monoclonal antibody (mAb) to be developed, was approved to treat B cell non-Hodgkin’s lymphoma (NHL) by the FDA in 1997 [82]. Rituximab is a chimeric antibody with murine variable regions of αCD20. The second generation of anti-CD20 antibodies with fully humanized sequences and engineered Fc fragments, include ocrelizumab (Ocrevus®), velruzumab, obinutuzumab and ofatumumab, and are created to be more effective with higher tolerance and minimized immunogenicity [83].
The anti-CD20 mAbs are effective via three mechanisms: antibody-dependent cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and CD20 crosslinking-mediated apoptosis [84, 85]. Immune effector cells (macrophages, neutrophils, NK cells, eosinophils, etc) are required for all these three mechanisms to function [86]. Notably, the interaction between Fc fragments and Fc gamma receptors (FcγR) triggers effector functions mediated by neutrophils and results in infusion related reactions [87] and RTX-induced interstitial lung injury [88]. Due to the murine-derived variable regions in RTX, production of human anti-chimeric antibodies was reported in 27% of patients treated with RTX [89]. Moreover, intrinsic resistance to RTX in patients has been observed which may due to RTX internalization [90]. Blocking of FcγRIIb could efficiently inhibit the internalization of RTX, demonstrating that the absence of Fc fragment could impede the development of resistance to therapeutic antibodies [91]. CD20-negative B cells resulte from FcγR mediated trogocytosis; they contribute to resistance development to CD20 mAb therapy [85]. Also, resistance to CDC induced by RTX can be initiated by depletion of necessary complement effector molecules due to RTX exposure [90].
To further enhance therapeutic outcomes of anti-CD20 mAbs, antibody or antibody derivate-decorated nanocarriers have been extensively investigated. N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-epirubicin (EPI) conjugate attached to RTX (RTX-P-EPI) presented superior anti-tumor efficacy in comparison with RTX combined with small molecule drug epirubicin (RTX+EPI) and RTX combined with polymer-EPI (RTX+P-EPI) in treatment of lymphoma in a mouse model [92]. Carboxylic acid-terminated biodegradable polymers, polylactic acid-block-polyethylene glycol-COOH (PLA-b-PEG-COOH) and polycaprolactone-COOH (PCL-COOH) modified with RTX were used for tumor diagnosis as well as treatment when loaded with hydroxychloroquine sulfate and chlorambucil [93, 94]. Poly(lactic-co-glycolic acid) (PLGA) nanoparticle core coated with poly-L-arginine layers and CD20/CD44 antibody dual-targeting outer layer was applied to deliver siRNA, which can silence the B-cell lymphoma 2 (Bcl-2) protein thus inducing cell apoptosis [95]. In addition to polymer-based nanocarriers, versatile caveospheres [96], albumin [97], chitosan/quantum dots (QD) [98], liposomes [99, 100] and micelles [101] have been conjugated with anti-CD20 mAbs to enhance targeting and therapeutic efficiency. Nanoparticles containing iron oxide conjugated with anti-CD20 antibody have been used to enhance imaging and diagnosis of tumors [102–104].
The Kopeček lab has been working on targeting CD20 utilizing polymer-based nanomedicines for more than 10 years. Drug-free macromolecular therapeutics (DFMT) is a new paradigm in nanomedicine. Apoptosis is induced by biorecognition at the cell surface and crosslinking of receptors; no small molecule drug is needed. DFMT platform has two components: 1) a bispecific engager, comprising of Fab’-MORF1 (Fab’ fragment of αCD20 conjugated with designated morpholino oligonucleotide sequence MORF1), and 2) a crosslinking (effector) molecule, P-(MORF2)X (N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer decorated with multiple copies of complementary MORF2). The hybridization of MORF1/MORF2 induces CD20 crosslinking due to the macromolecular character of the MORF2 containing effector. The B cell depletion efficacy of this DFMT platform has been proven in vitro [105–107], in vivo (lymphoma mouse model [105, 106, 108], collagen-induced arthritis mouse model [109]), and in cells from patients diagnosed with different subtypes of B cell malignancies [110, 111]. Chu et al investigated the effect of multivalency of DFMT on apoptosis and proved that high valency P-MORF2 containing 10 MORF2 per macromolecule showed significantly higher B cell apoptosis rate in DFMT system compared to low valency P-MORF2 containing 3 MORF2 [105]. The superior efficacy was likely due to higher avidity to B-cells as well as more effective CD20 clustering contributed by high valence of P-MORF2. To enhance the performance of DFMT in RTX resistant lymphoma cells, gemcitabine or HPMA copolymer-gemcitabine conjugate were used to pretreat cells or mice as gemcitabine was proven to increase CD20 expression levels by activating NF-κB pathway as NF-κB binds to the CD20 promoter to induce CD20 transcriptional activation [106, 112]. As gemcitabine could induce p53-dependent cell apoptosis by itself in cancer cells [113], the combination of gemcitabine with our DFMT system may have syngenetic effect in apoptosis induction of CD20-positive B cells. In addition to using a synthetic polymer backbone as crosslinking component, human serum albumin (HSA) was also evaluated and conjugated with MORF2 to provide the HSA-(MORF2)X effector [114, 115].
As a large number of patients have experienced side effect induced by RTX due to chimeric components, fully humanized type II antibodies such as obinutuzumab (OBN) have been developed to be less immunogenic [116]. CD20 mAbs are categorized into Type I or Type II depending on their mechanisms-of-action (Figure 3A). Both of the two types could elicit antibody-dependent cellular cytotoxicity (ADCC). However, type I mAbs rearrange CD20 into lipid rafts and the Fc fragment efficiently trigger the complement cascade while type II mAbs induce homotypic adhesion and direct programmed cell death via a caspase–independent pathway [117, 118] (Figure 3B). Unlike type I antibodies (rituximab and ofatumumab) that work primarily via CDC, OBN induces enhanced apoptosis by lysosome disruption and actin remodeling and ADCC, with only weak CDC activity [83, 116, 119]. The superior efficacy of OBN compared to RTX in preclinical studies may be due to 1) OBN inducing less CD20 internalization compared to RTX and 2) Fc fragment of OBN demonstrating better binding to FcγRIIIa [85]. The design of second-generation DFMT aims to augment the therapeutic efficacy by combining the activation pathways of Type I mAb and Type II mAb [120] (Figure 3C). This new design is composed of two components: a) bispecific engager, OBN-MORF1 (designated morpholino oligonucleotide sequence MORF1 conjugated to OBN); and b) a crosslinking (effector) nanoconjugate HSA-(MORF2)X (HSA decorated with multiple copies of complementary sequence MORF2). The binding of OBN to CD20 is not hampered by attachment of MORF1. Type II pathways are activated upon OBN-MORF1 binding to CD20. Subsequent exposure to multivalent crosslinking effector HSA-(MORF2)X leads to clustering the OBN-MORF1-CD20 complexes into lipid rafts, which triggers the Type I pathway (Figure 3C). This new “clustered OBN (cOBN)” strategy integrates mechanism-of-actions of both Type I and Type II αCD20, contributing to enhanced apoptotic levels of tumor cells [120]. Recently, it was demonstrated that DFMT is effective in crosslinking of CD38 receptors with concomitant induction of apoptosis in lymphoma and multiple myeloma cells [121].
Figure 3.
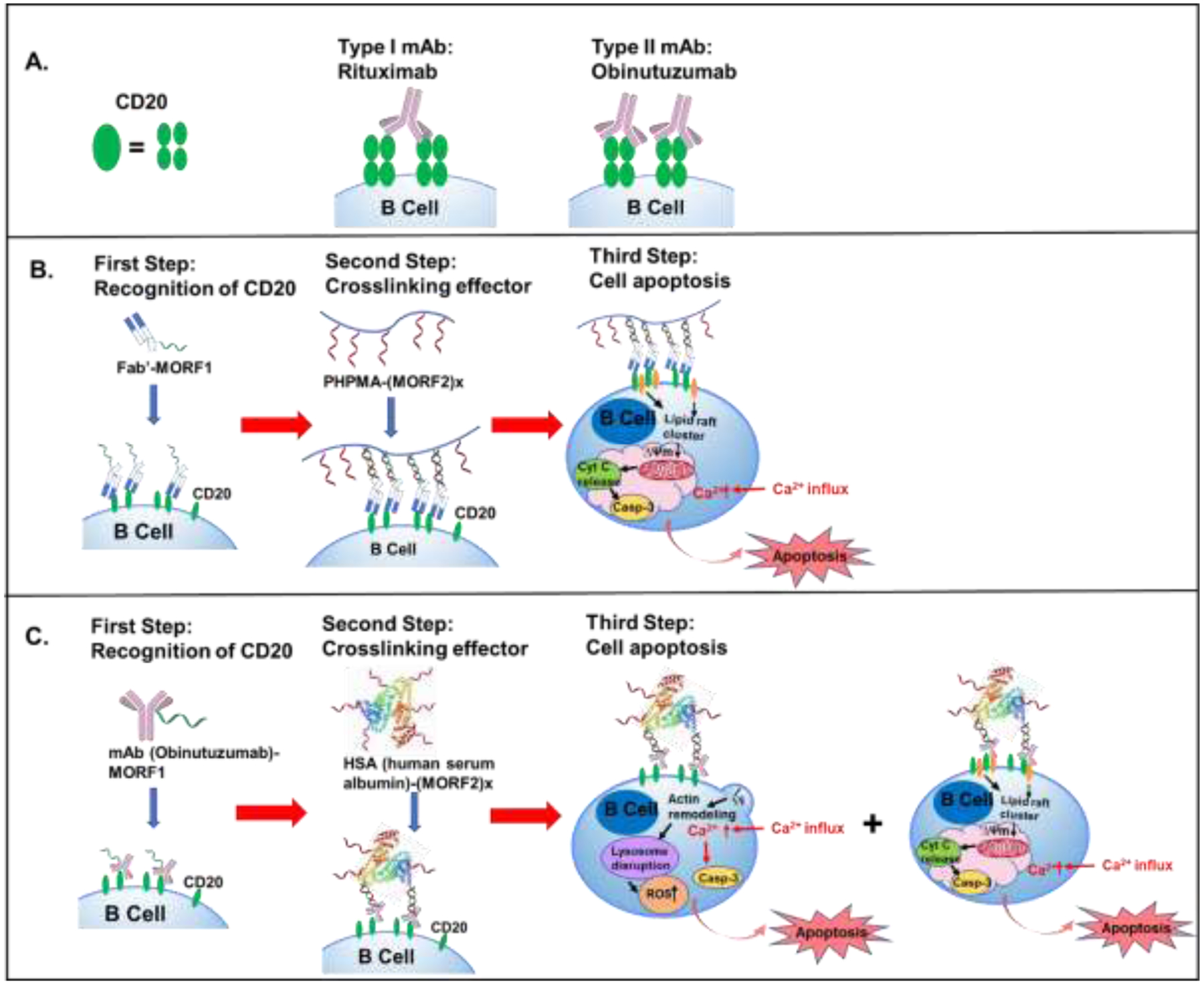
A. CD20 receptor is composed as a tetramer. Type I antibody like rituximab (RTX) binds to two different tetramers (inter-tetramer binding), inducing CD20 redistribution in lipid rafts. Type II antibody like obinutuzumab (OBN) binds within the same tetramer (intra-tetramer binding), which doesn’t cause CD20 redistribution. B. Treatment of Fab’-MORF1 followed by P-(MORF2)x causes raarangement of CD20 complexes in lipid rafts, leading to calcium influx and caspase activation and finally cell apoptosis. C. Second generation of DFMT: binding to CD20 of OBN-MORF1 conjugate initiates actin remodeling, thus trigger lysosome disruption and reactive oxygen species (ROS) production, resulting in onset of Type II apoptosis. In addition, crosslinking of the CD20-OBN-MORF1 complex with HSA-(MORF2)X leads to clustering the CD20 complexes in lipid rafts thus triggering Type I apoptotic effects.
Similarly, the MacKay lab utilized a polypeptide-based system conjugated with antibody derivates to initiate CD20 crosslinking [122]. The single chain variable regions (scFv) which can recognize and bind to CD20 receptors were expressed as a fusion with an elastin-like polypeptide (ELP), functioning as the crosslinking component. The ELP is a hydrophilic, stimulus-responsive biopolymers comprised of pentameric repeats of [Val-Pro-Gly-Xaa-Gly]n, in which Xaa could be different amino acid. One important property of these polypeptides is that they undergo a reversible phase transition at certain temperature, which depends on Xaa and number of repeats [122, 123]. The therapeutic efficacy of this design was proven in lymphoma models [122]. In other studies, scFv fused with streptavidin (SA) was used to pre-target B cells, followed by fluorescent polystyrene-PEG-biotin [124] or chitosan-biotinylated PEG-coated iron oxide [125] nanoparticles which could bind to SA via biotin-SA interaction. Pre-targeting with CD20 specific fusion protein significantly increased tumor targeting of nanoparticles. Besides antibody or antibody fragments, the use of anti-CD20 aptamers was also studied to kill CD20+ melanoma stem cells with salinomycin-loaded lipid-polymer nanoparticles as the carrier [126].
In summary, crosslinking of CD20 receptors expressed on most B cell surface could induce apoptosis, leading to B cell death. Anti-CD20 antibodies, Fab’, scFvs as well as aptamers were utilized in nanotechnology to target CD20 as a B cell depletion strategy. Furthermore, this is a platform technology applicable to crosslinking of other receptors.
2.2. CD19
Compared to CD20, CD19 has a broader expression across the stages of B cell development, including pro-B cells where CD20 is absent [127]. Thus, a strategy designed to deplete CD19+ B cells can remove not only mature B cells but also antibody-secreting cells. Inebilizumab (Uplizna®) [128] and blinatumomab (Blincyto®) [129] are two examples of clinical antibodies targeting CD19.
PEGylated recombinant hybrid αCD19 antibodies enhanced the binding of compounds to CD19+ cell lines but not to CD19− cell lines [130]. In most cases, αCD19 mAb was used as a targeting agent combined with small molecule drugs. PEGylated gold nanoparticles conjugated with αCD19 antibody achieved higher cytotoxicity compared to free antibody due to high oxidative stress as well as trapping cells in the growth phases [131]. Imatinib-encapsulated CD19-targeting liposomes attained excellent efficacy in killing cells from patients diagnosed with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL), overcoming the resistance and system toxicity of imatinib [132]. A similar idea was reported using CD19-targeting liposomes loaded with compound “C61”, a tyrosine kinase (SYK) P-site inhibitor to initiate apoptosis of B-precursor leukemia cells [133]. SYK phosphorylation is involved in activation of proliferation and survival. Inhibition of SYK suppresses anti-apoptotic pathways such as PI3K and STAT3 [134]. Modification of polymeric nanocarriers encapsulating doxorubicin (DOX) with αCD19 mAb significantly increased cellular uptake as well as cytotoxicity [135]. The Uckun lab developed a recombinant human CD19-ligand protein (CD19L). This ~54 kDa CD19L has a great potential to serve as the targeting moiety for nanoscale delivery [136].
As the strategy of chimeric antigen receptor T cells (CAR-T) targeting CD19 antigen has been approved by the FDA to treat acute lymphoblastic leukemia (ALL) in 2017, a three-segment amphiphilic copolymer, methoxy polyethylene glycol-branched polyethyleneiminepoly(2-ethylbutyl phospholane) (mPEG-bPEI-PEBP), was reported to encapsulate DNA plasmids of CAR-T to overcome low transfection efficiency and loading capacity as well as biosafety issues [137]. Antigen loss or antigen-low escape has been recognized as one of the major mechanisms for ALL relapse, which is likely due to the down-regulation of antigen expression caused by therapeutic pressure of CAR T therapies [138]. Although antigen loss in lymphoma is less reported than in leukemia, sequential loss of tumor surface antigens was observed. A patient bearing DLBCL lost CD19, CD20 and CD22 expression after CD19 CAR T and CD22 CAR T treatment [139]. Dual or multi-targeted CAR T therapies may be needed to overcome such tumor immune issues. More discussion regarding strategies overcoming antigen loss could be found in [138].
Similar to CD20, iron oxide nanoparticles conjugated with αCD19 mAb could be used for imaging, for example, to track B cells spreading from the bone marrow and spleen [140]. αCD3 and αCD16 are commonly used antibodies for dual targeting with CD19 [141, 142]. CD3 antigen can associate with T cell receptor (TCR). The use of αCD3 mAb was capable to stimulate T cell proliferation with tumor cells presence. Blinatumomab (a bispecific T cell engager specific for CD19 and CD3) has been evaluated in several clinical trials for the treatment of NHL and ALL [143]. CD19 × CD3 × IL12 bispecific T cell engaging-cytokines transported by magnetic iron oxide nanoparticles were able to redirect T cells to leukemic B cells and co-deliver T cell-stimulating IL-12 simultaneously. The screened nanoparticles showed ex vivo lytic effect towards leukemia [144]. A similar strategy is described in [145]. CD16 also called FcγRIII is expressed on the surface of NK cells. The use of αCD16 mAb helped enrich NK cells to enhance the killing efficacy of CD19+ lymphoma cells [142]. Bispecific killer engagers (BiKE) containing a single variable portion (VH and VL) of αCD19 mAb and of αCD16 mAb linked together showed enhanced NK cell-mediated targeting of tumor cells [146]. CD19 can also be targeted by αCD19 scFV fusion protein as a B-cell based tumor vaccine treatment to induce anti-tumor immunity [147].
Anti-CD19 antibodies and CD19 ligand protein were commonly used as B cell targeting moieties. Dual targeting of CD19 with pathway inhibitors or receptor ligands such as αCD3 and αCD16 enhanced the therapeutic efficacy of nano-systems [141, 142].
2.3. CD22
CD22, a transmembrane protein which specifically binds to sialic acid, is a immunoglobulin-like lectin highly expressed on mature B lymphocytes as well as their malignant counterparts [148]. As a regulatory endocytic receptor, CD22 constitutively recycles between the cell surface and endosomes. Once the receptor is bound to the ligands, a rapid internalization happens and the ligands can be released in the acidic endosome. These superior properties favor CD22 to be a suitable molecular target for drug delivery [149].
Anti-CD22 mAb was conjugated to conjugate with superparamagnetic iron oxide nanoparticles to enhance the delivery efficacy of siRNA to treat ALL. The siRNA targeted the MAX dimerization protein 3 (MDX3) transcription factor, which is anti-apoptotic in pre-B ALL. Dual targeting of CD22 and MDX3 has great potential in the treatment of ALL [150]. Cadmium–tellurium quantum dots (CdTe QDs) conjugated with αCD22 mAbs and co-loaded with doxorubicin (DOX) and gambogic acid (GA) were developed as a nanoparticle-based strategy in combination with targeting moiety and chemotherapy. This delivery regimen can specifically target and preferentially deliver DOX and GA into B lymphocytic tumor cells [151]. Liposome loaded with doxorubicin derivative, AD198, was conjugated with anti-CD22 Fab’ to target CD22 positive B malignant lymphocytes. The CD22 targeted liposomal formulation of the liposome was more specific and effective when compared to untargeted formulation [152]. Sialic acid (SA) has been also used to initiate the receptor-mediated internalization into CD22-expressing cells. Kwon’s group utilized adeno-associated virus (AAV) with an acid-degradable, siRNA-encapsulating polyketal shell, producing core-shell viral/nonviral chimeric nanoparticles (ChNPs) to treat B cell lymphoma. The ChNPs were modified with SA on the surface to enhance the siRNA release in the cytoplasm of target cells [153]. Besides using SA and αCD22 mAb as targeting moieties, the Paulson lab developed a chemically distinct natural N-linked glycan scaffolds which showed high affinity to CD22 glycan ligand. These glycan scaffolds can efficiently deliver auristatin and saporin to kill B lymphocytic cells via CD22-mediated internalization [154].
As CD22 could mediate internalization once bound to ligands, various targeting moieties such as αCD22 mAb, sialic acid as well as natural N-linked glycan scaffolds have been used to enhance nanocarrier endocytosis, along with subsequent drug release in acidic endosomes for killing B cells.
2.4. CD38
CD38 is a transmembrane glycoprotein expressed on the surface of many immune cells including T cells, B cells and nature killer (NK) cells. While the high and uniform expression of CD38 is observed on multiple myeloma (MM) cells, much lower expression levels of CD38 are observed on normal lymphoid cells, myeloid cells, red blood cells and platelets [155]. The αCD38 mAb including daratumumab, isatuximab (Sarclisa®) and MOR202 function via multiple mechanisms: Fc-dependent immune effector mechanisms including ADCC, CDC and ADCP, inhibition of ectoenzymatic function, direct apoptotic induction and immunomodulatory effects by the depletion of CD38+ immune suppressor cells (regulatory T cells, regulatory B cells, and MDSCs) [155]. CD38 is involved in calcium homeostasis by acting as cyclic ADP ribose hydrolase. Antibodies such as isatuximab inhibit the cyclic ADP-ribosyl cyclase activity by binding to CD38 [156]. Similar to CD20-targeting therapy in NHL, αCD38 mAb has been applied as a combination strategy with standard chemotherapy to treat MM.
The first study to utilize CD38 as a target for nanomedicine drug delivery system to treat MM was reported by the Azab lab in 2017. They decorated bortezomib (BTZ) loaded polymeric chitosan nanoparticles with αCD38 mAb to enhance targeting efficacy and therapeutic results. BTZ is a proteasome inhibitor used to treat MM. The advantages of using chitosan nanoparticles include large drug loading capacity, superior adsorption capabilities and long shelf life. Conjugation of αCD38 mAb provides a better tumor penetration of the nanoparticles. The acidic tumor environment accelerates drug release from chitosan nanoparticles and enhances proteasome activity inhibition [157]. A similar study reported utilizing αCD38 mAb to conjugate with STAT3 (signal transducer and activator of transcription 3) inhibitor loaded poly(ethylene oxide)-block-poly(α-benzyl carboxylate-ε-caprolactone) (PEO-b-PBCL) backbone [158]. To avoid the NK cell killing side effect of daratumumab, F(ab’)2 fragments derived from daratumumab were used to pre-block NK cells to augment the anti-tumor effects [159]. A more detailed summary of anti-CD38 mAbs was provided in [156].
CD38 is mainly selected as a targeting receptor in therapies of multiple myeloma. αCD38 mAb were utilized with polymeric nanoparticles in this field.
2.5. Other Potential Targets
Besides the receptors mentioned above, there are other potential targets under investigation which have not been extensively utilized in nano-delivery systems for B cell depletion strategies.
The surface antigen signaling lymphocytic activation molecule-F7 (SLAMF7; CD319) is a marker of normal and malignant plasma cells. Besides plasma cells, SLAMF7 is also expressed on NK cells, CD8+ T cells and mature dendritic cells [160]. The humanized anti-SLAMF7 mAb elotuzumab (Empliciti®) has been investigated in clinical trials to treat MM [161] and approved by the FDA in 2017 [162]. The function of elotuzumab includes two mechanisms. One is the Fc-mediated ADCC on MM cells and NK cells. The other one activates natural cytotoxicity of NK cells, from which IFN-γ is secreted to stimulate other immune cells [163]. In addition, the strong expression of SLAMF7 on CD20- plasmablast and plasma cells in RA synovium makes it a promising target for RA treatment. Humanized monoclonal antibody PDL241 could inhibit IgM production by depleting SLAMF7+ plasmablast and plasma cells in CIA mouse models [164].
One special target of B cell lymphomas is idiotypic sequences, which are specific to the hypervariable regions of immunoglobulin expressed by malignant B cells. Tumor-specific idiotype vaccines have been developed to induce anti-idiotypic immune activation as the lymphoma idiotype vaccines are the first-in-class to show prominent anti-tumor efficacy in human cancer [165]. Tumor associated, poorly immunogenic self-antigen expressing plasmid DNA (pDNA) has been used as DNA vaccine to treat B cell lymphoma [166]. Several clinical trials have been performed with limited therapeutic effect due to low transfection efficiency [166, 167]. To optimize the delivery strategy, synthetic polymer was utilized as a carrier. Branched polyethylenimine (PEI) conjugated with PLGA has been proven to enhance adjuvant effects in phagocytic cells and protect the anti-tumor effect in B cell lymphoma animal models [168]. PEI loaded with pDNA has been evaluated in patients with non-Hodgkin’s lymphoma (NHL). The cationic polymer PEI enhanced the transfection efficiency of pDNA [169].
There are also cholesterol depletion strategies reported in the literature. Scavenger receptor type B-1 (SCARB1), highly expressed on the surface of diffuse large B cell lymphoma (DLBCL) cells, is significantly involved in cholesterol and cholesteryl ester homeostasis. Cholesterol-poor high-density lipoprotein (HDL)-like NPs (HDL NPs) targeting SCARB1 reduced cellular cholesterol levels, ultimately leading to lymphoma cell death [170].
Most of the targets discussed in this section are receptors expressed on B cell surfaces. The mechanisms of action include ADCC, CDC, crosslinking-mediated apoptosis, receptor-mediated internalization, etc. Corresponding antibodies and their derivates as well as natural or synthetic ligands were applied as targeting/therapeutic moieties combined with nanotechnology to enhance therapeutic outcomes.
3. MODULATION OF B CELL ANTIGEN RECEPTOR (BCR) SIGNALING
The B cell antigen receptor signaling pathway is involved in B cell survival. The BCR complex is comprised of a membrane-bound immunoglobulin associated with CD79a(Igα)/CD79b(Igβ) (Figure 2B). CD79a and CD79b comprise a heterodimeric signal-transduction component of the B cell receptor. The immunoreceptor tyrosine-based activation motif (ITAM) within the cytoplasmic domain of CD79a/79b complex is vital for signal transduction. Functional BCR is essential for B cell maturation, activation, development and maintenance [171]. Once bound to the antigen, the CD79a/b recruits the Src family kinases Lyn, Blk, Fyn, which phosphorylate the tyrosine residues in ITAM. The phosphorylation mediates recruitment of various kinases and adaptor proteins including the spleen tyrosine kinase (SYK) and Bruton tyrosine kinase (BTK), initiating the formation of signalosome complex [172]. BCR aggregation is stimulated by the costimulatory receptor CD19 and activation of BTK [171]. The signalosome is responsible to activate three main downstream pathways: BTK, phospholipase C-γ2 (PLC-γ2) and phosphatidalyinositol-3-kinase (PI3K). The mitogen-activated protein kinase (MAPK) and AKT pathway contribute to the BCR-induced survival and proliferation [173, 174]. Along with activation of these pathways, multiple effectors are activated including NF-κB, Jun and nuclear factor of activated T cells (NFAT). Activation of NF-κB signaling pathway protects B cells from apoptosis and is critical for mature B cell survival [175]. Thus, modulation of BCR signaling is another commonly used method to treat B cell-related diseases. Inhibitors targeting SYK, BTK, PI3K, Lyn, etc. have been well studied.
3.1. CD79a/b
CD79a was reported to play a vital role in maintaining the immature, immune suppressive phenotype of myeloid derived suppressor cells (MDSCs) and in induction of protumorigenic cytokines secretion, resulting in tumor promotion effects. These functions make CD79a a novel target for cancer therapy [176]. High expression levels of CD79a were observed in most B lineage acute lymphoblastic leukemias (ALL) [177]. Compared to CD20 receptor, CD79a is expressed on a wider range of B cells from pre-B stage to the plasma cell stage of differentiation. However, expression loss of CD79b before differentiation of plasma cells has been reported [178]. Thus, CD79b could be considered as a potential anti-tumor target. Anti-CD79b mAb can be used as a component of antibody-drug conjugates to induce a prolonged depletion of proliferating B cells to treat NHL as it can be transported into the B cells [179, 180]. Antibody drug conjugate polatuzumab vedotin (Polivy®), an anti-CD79b-monomethyl auristatin E (MMAE) conjugate, has been approved by the FDA in 2019 for treatment of diffuse large B-cell lymphoma [181]. The anti-CD79b mAb also has great therapeutic efficacy in autoimmunity such as in mouse model of rheumatoid arthritis [182]. Those fundamental studies focusing on antibodies of CD79a/b indicate that this complex possesses great potential as an ideal target for nano-scale drug delivery system.
3.2. Bruton Tyrosine Kinase (BTK)
BTK is a non-receptor kinase which is essential for B cell proliferation and survival. Ibrutinib (Imbruvica®), a small molecule drug, the first-in-class BTK inhibitor, has been studied in the treatment of B cell malignancies. Clinical trials demonstrated the therapeutic efficacy of ibrutinib in CLL patients [183]. The FDA approved the use of ibrutinib in combination treatment of mantle cell lymphoma (MCL), CLL, small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), Waldenström’s macroglobulinemia (WM) and chronic graft versus host disease (cGVHD). Due to the poor water solubility and hepatic first pass effect of ibrutinib [184], formulation with nanotechnology has been investigated to enhance bioavailability. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles were used to deliver ibrutinib to maintain the drug activity and prolong the drug release [185]. Other BTK inhibitors such as acalabrutinib, zanubrutinib, evobrutinib, tirabrutinib, SNS-062, etc. are also under investigation [186].
Besides BTK inhibitors, small interfering RNA (siRNA) are being investigated to silence BTK genes. PEG-b-PLGA was used to encapsulate siRNA targeting BTK genes in B cells and macrophages to treat rheumatoid arthritis in collagen-induced arthritis model. The systemic administration of siRNA loaded NPs significantly reduced expression of inflammatory cytokines and alleviated arthritis symptoms [187].
3.3. Spleen Tyrosine Kinase (SYK)
SYK is an intracellular cytoplasmic tyrosine kinase, which is important for immunoreceptor signaling in B cells, neutrophils and macrophages. Not limited to the crucial role in adaptive immune receptor signaling, SYK is also involved in multiple biological functions such as cellular adhesion, innate immune recognition, osteoclast maturation, platelet activation and vascular development [134]. The prodrug fostamatinib (Tavalisse®) as the first oral SYK inhibitor has been approved by FDA in 2019 for treatment of chronic immune thrombocytopenia (ITP) [188]. Entospletinib (GS-9973), cerdulatinib (PRT062070) and TAK-659 are also SYK inhibitors currently under investigation in clinical trials for various B cell lymphomas [189]. The Uckun lab did a wide range of research targeting SYK pathway. They developed C61 as an inhibitor for substrate binding targeting SYK P-site, which can inactivate SYK pathway and directly induce B cell apoptosis [190]. Interestingly, C61-loaded liposomal NPs demonstrated a promising pharmacokinetic and safety profile in mouse models and showed remarkable capacity to induce apoptosis in primary B-precursor ALL blast cells from patients [191, 192]. This formulation of C61 also significantly augmented the antitumor efficacy of low-dose total body irradiation (TBI) against B-precursor acute lymphoblastic leukemia (BPL) in mouse models [193].
3.4. Phosphatidylinositide-3-Kinase (PI3K)
Besides highly involved in B cells, the PI3K pathway is overactive in various cancer cells, such as breast cancer, colorectal cancer [194], glioblastoma [195] and lung cancer [196]. Activation of the PI3K pathway reduces apoptosis and enhances cell proliferation. Among various PI3K isoforms (α, β, γ, and δ), PI3K-γ is essential in immune suppression during inflammation and cancer and is also important for cellular activation and migration [197]. PI3K-γ is highly expressed in tumor-associated B cells. Eganelisib (IPI-549) was designed by Infinity Pharmaceuticals as a PI3K-γ inhibitor posseing clinical potential [198]. It can also be used in combination with checkpoint blockade antibodies to overcome treatment resistance [199]. The Huang lab has utilized IPI-549 embedded in poly(lactic-co-glycolic-acid) (PLGA) NPs modified with aminoethylanisamide (AEAA) to reshape the tumor immune microenvironment and mitigate immune suppression in desmoplastic tumors more effectively than free drug [200]. Similarly, the targeting carrier aminoethyl aminoethylanisamide-polyethylene glycol-polycaprolactone (AEAA-PEG-PCL) was used for co-treatment of breast cancer by combination of IPI-549 and silibinin [201]. AEAA is a ligand of sigma receptor, which is expressed on surface of many tumor cells, thus promoting tumor cell uptake [202]. Silibinin is an anti-tumor agent, which can significantly inhibit tumor-associated fibroblasts [203]. Co-treatment of IPI-549 and silibinin were proved to remodel the tumor microenvironment thus enhancing anti-tumor efficacy by decreasing myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) as well as change the stromal structure of tumor [201]. There are numerous other molecules designed for different PI3K isoforms. Idelalisib (Zydelig®) is the first-in-class PI3K- δ inhibitor approved by the FDA for the treatment of multiple B cell lymphomas including CLL, relapsed FL and relapsed small lymphocytic lymphoma [204]. Pictilisib (GDC-0941) and AS101 are also general inhibitors of PI3K/Akt pathway; they are able to sensitize B lymphocytes to chemotherapy such as paclitaxel [205, 206]. Besides synthetic inhibitors, the natural product snake venom extracted from Walterinnesia aegyptia (WEV) was reported to inhibit the PI3K/AKT pathway. Silica NPs have been used as a carrier for the snake venom [207, 208].
In this section, some of the major components of the B cell signaling pathway are discussed including CD79a/b, bruton tyrosine kinase, spleen tyrosine kinase and phosphatidylinositide-3-kinase. For CD79a/b receptor, corresponding antibodies were used to deplete B cells. For those three kinases, inhibitors such as chemical drugs and siRNA were widely employed in nanotechnology to block those pathways.
4. TARGETING B CELL SURVIVAL
The tumor-necrosis factor (TNF) superfamily plays numerous roles in regulating cell functions including cell activation, apoptosis and tumor homeostasis [209]. B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) as two members of the TNF family, are responsible for B cell generation and preservation. BAFF can activate B cells and plasma cells via three transmembrane receptors: 1) transmembrane activator, calcium modulator, and cyclophilin ligand interactor (TACI), 2) B cell maturation antigen (BCMA), and 3) BAFF receptor (BAFF-R) (Figure 2C). TACI and BCMA can recognize and bind both BAFF and APRIL while BAFF-R only recognizes BAFF [210]. BAFF and APRIL are essential for the survival, proliferation, and differentiation of B cells and plasma cells. Overexpression of BAFF may cause expanded mature B cell compartment and autoimmunity in mice [211]. APRIL participates in augmentation of B-cell antigen presentation, stimulation of B cell proliferation and augmentation of B cell survival [212]. BAFF and APRIL are involved in B cell malignancies, including CLL [213], multiple myeloma [214], and non-Hodgkin’s lymphoma [215]. Thus, inhibition of the BAFF/APRIL system has been investigated as a potential strategy in treatment of B-cell associated diseases.
4.1. B Cell Maturation Antigen (BCMA)
BCMA, a transmembrane glycoprotein, also known as tumor necrosis factor-receptor superfamily member 17 (TNFRSF17), recognizes BAFF and APRIL. It participates in regulation of B cell proliferation, differentiation and survival [216–218]. BCMA is preferentially expressed on mature B cells, especially highly expressed on all MM cells. It plays a vital role in transformation of plasma cell and progression of MM [219]. Overexpression of BCMA or APRIL binding to BCMA notably enhance MM cells growth and survival [220]. BCMA targeting strategies include antibody-drug conjugates (ADCs), bispecific T-cell engagers (BiTEs), chimeric antigen receptor T cells (CAR-T) and bispecific molecules [216]. Besides those treatment modalities, the Anderson lab has developed immunogenic heteroclitic peptides, BCMA54–62 (YILWTCLGL) and BCMA72–80 (YLMFLLRKI), which are able to induce MM-specific immune responses with high anti-tumor abilities [221]. The highly effective peptide BCMA72–80 (YLMFLLRKI) was encapsulated into PLGA-based NPs to enhance its delivery to APCs. The presence of the BCMA72–80 peptide induced antigen-specific CD8+ cytotoxic T lymphocytes (CTL), which are capable of maintaining a long-lasting immunity against MM cells [222].
To utilize BCMA and SLAMF7 (described in subsection 2.5) dual targeting for whole-body MM detection, ultra-small, silica-comprised and gadolinium-containing nanoparticles were conjugated with αBCMA mAb or αSLAMF7 mAb to enhance MRI imaging contrast [223].
4.2. B Cell Activating Factor Receptor (BAFF-R)
BAFF-R is expressed on B cell surface and frequently overexpressed in B cell malignancies [213, 224]. Also, excessive BAFF-R initiates severe autoimmune disorders. Overexpression of BAFF-R in transgenic mice initiated emerging autoreactive B cells secreting rheumatoid factor and anti–DNA autoantibodies, resulting in lupus-like autoimmune manifestations [225]. BAFF/BAFF-R complex is rapidly internalized and translocated to lysosomes once bound to each other [226]. CAR T cells targeting BAFF-R were able to target acute lymphocytic leukemia (ALL) cells lacking CD19, which provides a potential therapeutic approach for patients with this tumor escape variant [227]. The use of anti-BAFF-R mAb selectively depleted mature B2 cells and enhanced atherosclerosis development in hyperlipidemic ApoE−/− mice. Consequently, these data suggest a potential for management of cardiovascular diseases [228].
Besides strategies with antibodies or CAR T cells, gene therapies are also utilized in BAFF-R targeting. The clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 gene editing system has been extensively utilized for DNA deletion, insertion, and replacement [229]. A library of poly(ethylene glycol)-block-poly(lactide-co-glycolide) (PEG-b-PLGA)-based cationic lipid-assisted nanoparticles (CLANs) with different surface PEG density and zeta potential were used to encapsulate CRISPR-Cas9 for in vivo B cell targeting. The treatment decreased the number of B cells and possessed therapeutic efficacy in mouse model of rheumatoid arthritis [230]. mBAFF, a soluble BAFF mutant, is a competitor of BAFF. mBAFF can block BAFF’s biological activities, suppressing lymphocyte activation and proliferation. PEGylated liposomes were used to deliver mBAFF and vincristine to treat NHL mouse models [231]. An RNA aptamer and siRNA targeting BAFF-R have been used as targeting agents. An anti-BAFF-R antibody was used to deliver siRNA against BAFF-R [232]. RNA aptamer of BAFF-R was utilized in combination with siRNA against signal transducer and activator of transcription 3 (STAT3) as a dual functional conjugate to provide enhanced effect in inhibiting B lymphocyte survival in vitro [233].
4.3. Transmembrane Activator and CAML Interactor (TACI)
Unlike BCMA and BAFF-R which are predominantly expressed on B cells, TACI is expressed on both B cells and activated T cells [234]. A TACI-Ig fusion protein was developed to block the BAFF/APRIL pathway. TACI-Ig was made by fusing the TACI extracellular domains to the Fc portion of human Ig heavy chain γ1 (IgG1). Treatment with TACI-Ig significantly reduced B cell counts in systemic lupus erythematosus (SLE) mouse model [235]. Another study using collagen-induced arthritis (CIA) mouse models also indicated that treatment with TACI-Ig could inhibit proliferation response as well as differentiation of T and B cells, along with the alleviation of arthritis scores and other clinical symptoms [236]. However, the fusion protein TACI-Ig named “atacicept” failed to show superior efficacy in clinical trials of MM, rheumatoid arthritis and SLE [237]. In patients with SLE and multiple sclerosis (MS), atacicept-led inflammation was observed [237, 238]. Besides TACI-Ig, antagonistic anti-APRIL antibody [239] and TACI-incorporating CAR-T cells [240, 241] were also developed to treat MM. TACI can be a potential cell surface marker for development of targeting strategies as a supplement to BCMA-targeted therapies [242].
To utilize the nano-strategies targeting B cell survival, BCMA could be targeted by antibodies and immunogenic heteroclitic peptides; BAFF-R was blocked with antibodies, gene therapies such CRISPR gene editing system, RNA aptamer and siRNA as well as BAFF mutant (mBAFF) in the literature; TACI were mainly targeted by TACI-Ig fusion protein [237].
5. TARGETING B CELL AND T CELL COSTIMULATION AND IMMUNE CHECKPOINT BLOCKADE
In addition to activation of antigen receptors, costimulation between B and T cells is essential to fully activate lymphocytes for sustaining proliferation and differentiation (Figure 2D). The first cell surface costimulatory molecule is CD28, which is the major molecule for the activation of T cells [243]. Another class of costimulatory receptors are members of the TNF receptor family such as CD40 and CD27. There are also inhibitory molecules in contrast to costimulatory molecules like cytotoxic T lymphocyte associated protein 4 (CTLA4) and programmed cell death protein 1 (PD-1) receptor. CD80 and CD86 on B cells interact with both of the costimulatory receptors, CD28 and CTLA4 on T cells [244]. Notably, CTLA4 and PD-1 inhibit T cell activation and proliferation [245, 246].
5.1. CD80/CD86
Binding of CD80/CD86 on APCs to CD28 on the surface of CD4+ T cells, stimulates T cell proliferation and cytokine release [244]. Tada et al. reported that CD28-deficient mice were resistant to collagen-induced arthritis (CIA) as their T cell activation was severely impaired [247]. Consequently, blockade of CD28 on T cells provides a potential target for rheumatoid arthritis therapy. CTLA4 inhibits T cells activation by associating to CD80/CD86 as it has a higher binding affinity of CTLA4-CD80/CD86 compared to CD28-CD80/CD86 [248]. Abatacept (CTLA-Ig) is a fusion protein of the extracellular domain of human CTLA4 and Fc region of human IgG1. Abatacept binds to CD80/86 on APCs and so scavenges the costimulatory signal from APCs to CD4+ T cells. This suppresses the proliferation and cytokine production of T cells [249]. Besides abatacept, antibodies specific for CD80/CD86 and combination with sirolimus can also be utilized to prolong allograft survival in cynomolgus monkey renal transplant recipients [250]. Treatment of ovalbumin loaded NPs can promote the maturation of dendritic cells by enhancing the expression level of costimulatory factors CD80/CD86 [251].
5.2. CD40
CD40 is located on APCs including B cells, macrophages and dendritic cells. Ligation of CD40 on dendritic cells enhances production of IL-12 and augments T cell stimulation. CD40 and its ligand CD40L, expressed on activated CD4+ T helper cells, can activate APCs to prime CD8+ cytotoxic T lymphocytes (CTLs) [252]. Thus, the CD40-CD40L interaction plays a vital role in regulation of both B cell and T cell immune responses. Blockade of CD40L inhibits CTL priming [253].
The agonistic CD40 mAb FGK45 could be used in combination with antigens or vaccines to promote immune responses [254]. As immunosuppressive tumor microenvironments can restrain antitumor efficacy, CD40 activation by CD40 agonist mAb can enhance T cell response and reverse the unfavorable condition in many cancer types such as pancreatic carcinoma and melanoma [255, 256]. In addition, CD40 agonist mAb elicits protective immune responses in the treatment of autoimmune inflammatory condition in CIA mouse models [257]. However, due to the high dose needed for antibody treatment, potentially lethal side effects could occur [258]. Luminescent porous silicon NPs conjugated with multivalent CD40 mAb have been developed to lower systemic toxicity and enhance APC activation. Polymer-based NPs allow the multivalent CD40 mAb to amplify the potency of immune responses [259]. The poly(lactic-co-glycolic acid) (PLGA)-based NPs loaded with ovalbumin and CD40 agonistic mAb have been evaluated in melanoma mouse model. CD40-targeting improves efficient in vivo delivery of these NPs via enhanced binding and uptake of NP to DC, resulting in significant tumor control [260].
Interactions of CD40 with other ligands are also under investigation. The CD40-CD40L interaction is involved in development of atherosclerosis, leading to cardiovascular diseases [261]. But the inhibition of CD40-CD40L can also cause immune suppression. When the interaction between CD40 and tumor necrosis factor receptor-associated factor (TRAF) in macrophages was blocked while the CD40-TRAF2/3/5 interactions remained intact, reduction in atherosclerosis but preservation of CD40-mediated immunity was observed [262]. More detailed discussion of CD40 B cells in development of cancer vaccines is covered in [263].
5.3. Programmed Cell Death Ligands 1 and 2 (PD-L1/L2)
PD-1 is expressed on activated T cells, B cells and myeloid cells [264]. PD-1 can bind to two different ligands PD-L1 and PD-L2. hPD-L1 and PD-L2 is not limited to APCs but are expressed also on nonlymphoid tissues such as heart, lung and pancreatic islets [265]. As PD-1-PD-L1/L2 interaction can suppress the activation of T cells, overexpression of PD-L1/L2 in cancers leads to progression of tumors, resulting in poor clinical outcomes.
Multiple immunotherapy strategies are based on blockade of PD-1 associated pathways. αPD-1 mAb or αPD-L1 mAb have been evaluated in different types of cancers including non-small cell lung cancer, melanoma, renal cell cancer or Hodgkin’s lymphoma [266–269]. The FDA has approved permbrolizumab (Keytruda®), nivolumab (Opdivo®) and cemiplimab (Libtayo®) as αPD-1 mAbs as well as αPD-L1 mAbs atezolizumab (Tecentriq®), avelumab (Bavencio®) and durvalumab (Imfinzi®). Due to the off-target effects and toxicity of mAb treatment [270–272], nanocarriers such as PLGA NPs [273], gold NPs [274] and liposomes [275] have been conjugated with αPD-1 or αPD-L1 mAb in order to enhance tumor suppression effects and minimize side effects.
Peptides have been developed as small molecule candidates for immunotherapy. PPA, a dodecapeptide antagonist to PD-L1, possesses binding affinity to PD-L1 high enough to disrupt the PD-1-PD-L1 pathway [276]. Interestingly, multivalent HPMA copolymer-PPA conjugates combine checkpoint inhibition with PD-L1 crosslinking (Figure 4). The latter results in the change of PD-L1 subcellular fate; instead of recycling, PD-L1 uses the lysosomal route resulting in its degradation and downregulation. The multivalent polymer-peptide PD-L1 antagonist demonstrated higher antitumor activity than anti-PD-L1 Abs in combination with chemotherapy (multivalent HPMA copolymer-epirubicine conjugate [277]) and immunotherapy [278, 279]. Sun et al. combined the PPA peptide with the tumor angiogenesis affinity molecule CGKRK producing a new molecule, named CD peptide. PEG-PCL NPs loaded with paclitaxel and decorated with CD possessed higher antitumor activity than controls [280]. Recombinant single-chain variable fragment (scFv) of αPD-1 fusion protein-expressed with an amphiphilic immune-tolerant elastin-like polypeptide (iTEP) can self-assemble into multivalent nanoparticles, which efficiently block the PD-1 immune checkpoint in vitro and in vivo [281]. The role of PD-L2 in PD-1/PD-L1 axis targeting was also explored. Co-expression of PD-L2 with PD-L1 could inhibit PD-1/PD-L1 binding as well as enhance CD3 and inducible T cell co-stimulator (ICOS) on T cells. Administration of soluble multimeric PD-L2-Fc fusion protein was able to regulate immune response thus enhancing mice survival rate [282].
Figure 4.
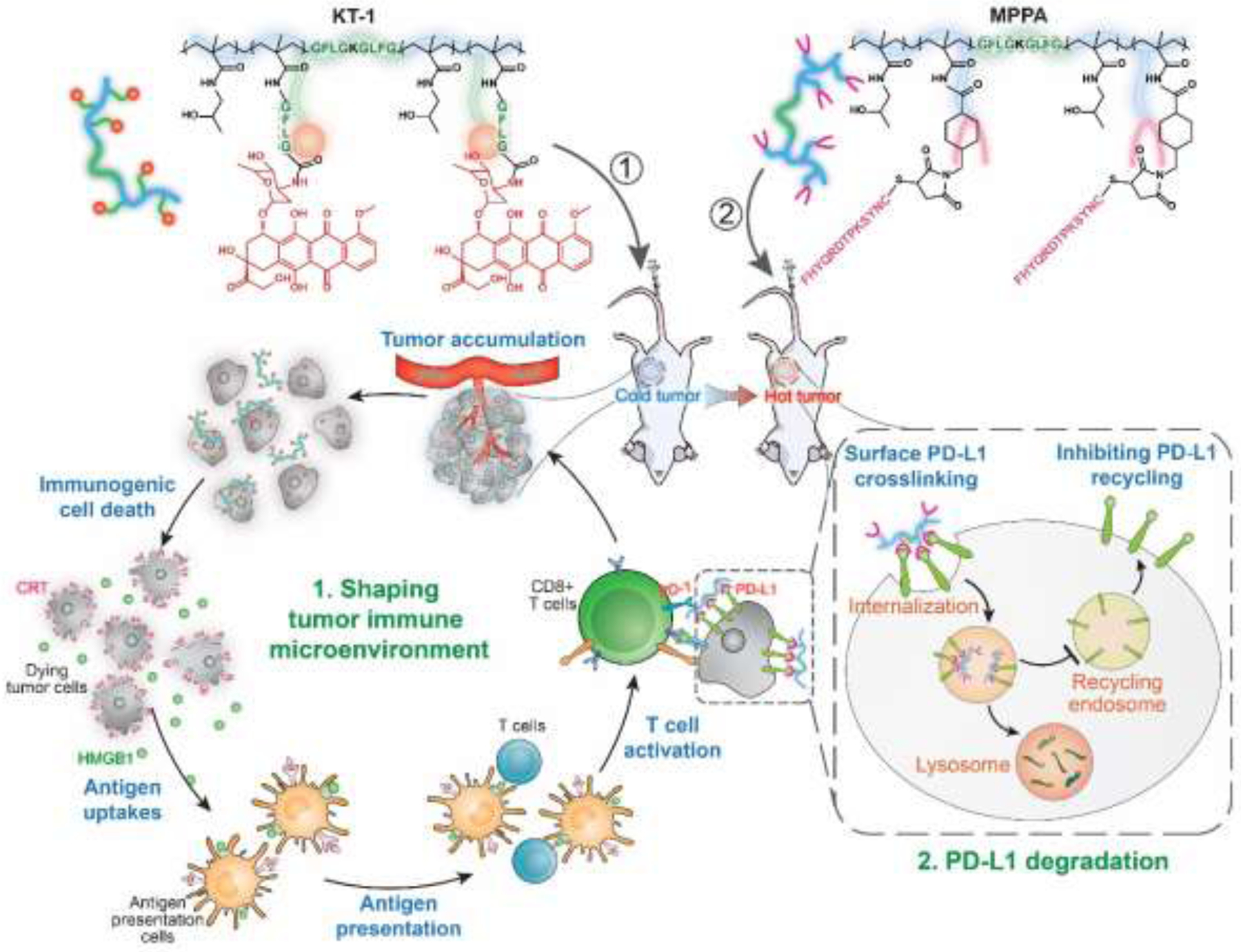
Schematic illustration of the combination strategy targeting PD-L1. Degradable HPMA copolymer-epirubicin (EPI) conjugate (KT-1) [277] induces ICD and activates the immune response of tumor microenvironment. Sequentially, the multivalent polymer peptide PD-L1 antagonist (MPPA) mediates PD-L1 crosslinking; this redirects PD-L1 to lysosome degradation instead of recycling back to cell surface. This consecutive two-step treatment regimen activates and recruits the peripheral T cells into tumor microenvironment in a durable way. Reprinted from ref [278].
Besides strategies using PD-pathway targeting antibodies, methods of gene editing are also under investigation. Dual-locking nanoparticles (DLNPs) were formed with 4-(hydroxyethyl) phenylboronic acid (HPBA)-modified polyethyleneimine (PEI1.8k–HPBA) and decorated by PEG on the surface. Plasmid DNA (pDNA) encoding the CRISPR/Cas13a system targeting PD-L1 was encapsulated in the core of DLNPs. The CRISPR/Cas13a system can only be released when an acidic microenvironment and high H2O2 concentration are both present; these enhances accumulation and gene-editing efficacy of the CRISPR/Cas13a system at the tumor sites. Besides superior blood circulation stability, the pDNA-loaded DLNP showed significant tumor suppression and improved survival rates in mouse melanoma model [283].
Small RNA molecules such as siRNA and miRNA have been also utilized in PD-1/PD-L1 targeting strategies. SiRNAs targeting PD-1 and PD-L1 were delivered by lipid-based NPs. These therapies were proven to be effective to increase tumor infiltrating lymphocytes cytotoxicity to cancer cells by silencing PD-1 and PD-L1 [284, 285]. MicroRNAs (miRs) play an important role in regulating interaction of tumor cells with microenvironment. As overexpressed in diffuse large B-cell lymphoma (DLBCL), miR155 sensitized B lymphocytes to anti-PD-L1 antibody via PD-1/PD-L1 interaction [286].
In short, this section summarized the costimulation and immune checkpoint blockade between B cells and T cells. To block the interactions between B cells and T cells, antibodies against CD80/CD86 and fusion protein (CTLA-Ig) were reported to block the CD80/CD86-CD28 pathway. CD40 ligand was applied to block CTL priming. On the other hand, CD40 agonist mAb can enhance T cell response. There are numerous studies targeting the PD-1-PD-L1 checkpoint pathway for cancer therapy; besides antibodies, dodecapeptide antagonist to PD-L1 (PPA-1), scFv of αPD-1 fusion protein, CRISPR gene editing systems as well as siRNA/mRNA have been extensively used with platforms of nanotechnology.
6. SUMMARY
B cells play vital roles in the development of numerous diseases, including B cell lymphomas, autoimmune diseases, diverse carcinomas and cardiovascular diseases. This review summarized the major targeting molecules of B cell therapies and corresponding applications of nanotechnologies.
B cell targeting strategies are widely studied based on diverse mechanisms. Direct B cell depletion, modulation of BCR signaling, targeting B cell survival factors, targeting the costimulation of B cells and T cells, and immune checkpoint blockade are the major strategies discussed in this review. Selected surface receptors, pathways and cytokine factors which can be targeted in B cell therapy have been included. In addition, the field of B cell targeting vaccines is developing rapidly. CD19 (section 2.2), idiotypic sequences (section 2.5) and CD40 (section 5.2) related conjugates are currently under investigation. As DNA, mRNA, peptide/protein are the common agents used for cancer vaccines, a wide array of biomaterials has been employed as carriers such as lipid nanoparticles, PLGA, gold nanoparticles, and liposomes. A more comprehensive summary of applications of biomaterials in vaccine-based cancer immunotherapy is provided in [287].
Targeting agents including antibodies and their derivatives, ligands, small molecular inhibitors, gene editing with the CRISPR technique, siRNAs and miRNAs are commonly used in B cell targeting. Applications of nanocarriers in delivery of these agents has shown enhanced blood circulating time, improved targeting efficacy, reduced adverse effects as well as improved therapeutic outcomes. These advantages are enabled by the biophysical properties of nanocarriers. The multivalence of functional molecules introduced by nanocarriers delivery augments the density of targeting moieties and enhances their performance. It is noteworthy that the emerging area of using nanocarriers with the thriving CRISPR techniques cannot be overlooked [283].
Furthermore, single targeting may not be strong enough to attain ideal outcomes. Dual or triple functional nanocarriers can be developed in a single composition to achieve better therapeutic results. The multi-functionality could be achieved by combination either with therapeutic agents such as chemotherapy or with multiple targeting moieties/ligands. As reported in the literature, dual targeting of CD20/CD37 [100] or CD19/CD3 or CD16 [129, 142] could enhance direct B cell depletion efficacy of CD20 and CD19, while dual targeting of TACI/BCMA [240] or BCMA/SLAMF7 [223] significantly augmented plasma cell clearance. Besides therapeutic applications, nanomedicines provide advantages for tumor diagnosis and imaging [93, 102, 103, 223].
Table 1 provides a concise toolbox to locate a proper target for a variety of diseases. For example, multiple myeloma is a plasma cell disorder; consequently, receptors over-expressed on plasma cells such as BCMA, CD38 and SLAMF7 may have a great potential serving as targets. Regarding each specific receptor, the frequently used targeting agents and nanocarriers can be located. The current first-line treatments in clinical or in clinical trials are also provided as references.
Statement of Significance.
As B cells are engaged significantly in the development of many kinds of diseases, utilization of nanomedicines in B cell depletion therapies have been rapidly developed. Although numerous studies focused on B cell targeting have already been done, there are still various potential receptors awaiting further investigation. This review summarizes the most relevant studies that utilized nanotechnologies associated with different B cell depletion approaches, providing a useful tool for selection of receptors, agents and/or nanocarriers matching specific diseases. Along with uncovering new targets in the function map of B cells, there will be a growing number of candidates that can benefit from nanoscale drug delivery.
Acknowledgment
The research in authors’ laboratory was supported in part by the National Institutes of Health, grants RO1 GM95606 and CA246716 (to JK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest
J.K. and J.Y. are co-inventors of US Patent 9,289,510 a patent application related to combination chemo- and immunotherapy. The University of Utah licensed both to TheraTarget, Inc. J.K. is Chief Scientific Advisor and J.Y. Scientific Advisor for Bastion Biologics. Otherwise, the authors declare no competing financial interests.
7. REFERENCES
- [1].Pietersz GA, Wang X, Yap ML, Lim B, Peter K, Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies, Nanomedicine 12 (2017) 1873–1889. [DOI] [PubMed] [Google Scholar]
- [2].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Biotechnol 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ghosh P, Han G, De M, Kim C, Rotello V, Gold nanoparticles in delivery applications, Adv. Drug Deliv. Rev 60 (2008) 1307–1315. [DOI] [PubMed] [Google Scholar]
- [4].Probst CE, Zrazhevskiy P, Bagalkot V, Gao X, Quantum dots as a platform for nanoparticle drug delivery vehicle design, Adv. Drug Deliv. Rev 65 (2013) 703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wahajuddin, Arora S, Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers, Int. J. Nanomed 7 (2012) 3445–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI, Mesoporous silica nanoparticles in biomedical applications, Chem. Soc. Rev 41 (2012) 2590–2605. [DOI] [PubMed] [Google Scholar]
- [7].Kopeček J, Yang J, Polymer nanomedicines, Adv. Drug Deliv. Rev 156 (2020) 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liechty WB, Kryscio DR, Slaughter BV, Peppas NA, Polymers for drug delivery systems, Ann. Rev. Chem. Biomol. Eng 1 (2010) 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang J, Kopeček J, The light at the end of the tunnel – second generation HPMA conjugates for cancer treatment, Curr. Opin. Colloid Interface Sci 31 (2017) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hwang D, Ramsey JD, Kabanov AV, Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval, Adv. Drug Deliv. Rev 156 (2020) 80–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cabral H, Miyata K, Osada K, Kataoka K, Block copolymer micelles in nanomedicine applications, Chem. Rev 118 (2018) 6844–6892. [DOI] [PubMed] [Google Scholar]
- [12].Malam Y, Loizidou M, Seifalian AM, Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer, Trends Pharmacol. Sci 30 (2009) 592–599. [DOI] [PubMed] [Google Scholar]
- [13].Anselmo AC, Mitragotri S, Nanoparticles in the clinic, Bioeng. Transl. Med 1 (2016) 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anselmo AC, Mitragotri S, Nanoparticles in the clinic: an update, Bioeng. Transl. Med 4 (2019) e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gordillo-Galeano A, Mora-Huertas CE, Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release, Eur. J. Pharm. Biopharm 133 (2018) 285–308. [DOI] [PubMed] [Google Scholar]
- [16].Al-mahallawi AM, Abdelbary AA, Aburahma MH, Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam, Int. J. Pharm 485 (2015) 329–340. [DOI] [PubMed] [Google Scholar]
- [17].Farooq MU, Novosad V, Rozhkova EA, Wali H, Ali A, Fateh AA, Neogi PB, Neogi A, Wang Z, Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells, Sci. Rep 8 (2018) 2907–2928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [18].Al-Jamal WT, Al-Jamal KT, Bomans PH, Frederik PM, Kostarelos K, Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer, Small 4 (2008) 1406–1415. [DOI] [PubMed] [Google Scholar]
- [19].Kopeček J, Kopečková P, Minko T, Lu Z-R, HPMA copolymer–anticancer drug conjugates: design, activity, and mechanism of action, Eur. J. Pharm. Biopharm 50 (2000) 61–81. [DOI] [PubMed] [Google Scholar]
- [20].Walling M, Novak J, Shepard JRE, Quantum dots for live cell and in vivo imaging, Int. J. Mol. Sci 10 (2009) 441–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neuwelt A, Sidhu N, Hu C-AA, Mlady G, Eberhardt SC, Sillerud LO, Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation, AJR Am. J. Roentgenol 204 (2015) W302–W313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS, Stealth coating of nanoparticles in drug-delivery systems, Nanomaterials (Basel) 10(4) (2020) doi: 10.3390/nano10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv. Drug Deliv. Rev 99 (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pérez-Herrero E, Fernández-Medarde A, Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy, Eur. J. Pharm. Biopharm 93 (2015) 52–79. [DOI] [PubMed] [Google Scholar]
- [25].Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs, Cancer Res. 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- [26].Maeda H, Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects, Bioconj. Chem 21 (2010) 797–802. [DOI] [PubMed] [Google Scholar]
- [27].Golombek SK, May J-N, Theek B, Appold L, Drude N, Kiessling F, Lammers T, Tumor targeting via EPR: strategies to enhance patient responses, Adv. Drug Deliv. Rev 130 (2018) 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Islam W, Fang J, Imamura T, Etrych T, Šubr V, Ulbrich K, Maeda H, Augmentation of the enhanced permeability and retention effect with nitric oxide–generating agents improves the therapeutic effects of nanomedicines, Mol. Cancer Ther 17 (2018) 2643–2653. [DOI] [PubMed] [Google Scholar]
- [29].Moody PR, Sayers EJ, Magnusson JP, Alexander C, Borri P, Watson P, Jones AT, Receptor crosslinking: a general method to trigger internalization and lysosomal targeting of therapeutic receptor: ligand complexes, Mol. Ther 23 (2015) 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang J, Tian S, Petros RA, Napier ME, Desimone JM, The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies, J. Am. Chem. Soc 132 (2010) 11306–11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tesauro D, Accardo A, Diaferia C, Milano V, Guillon J, Ronga L, Rossi F, Peptide-based drug-delivery systems in biotechnological applications: recent advances and perspectives, Molecules 24 (2019) 351–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peng Z-H, Kopeček J, Enhancing accumulation and penetration of HPMA copolymer–doxorubicin conjugates in 2D and 3D prostate cancer cells via iRGD conjugation with an MMP-2 cleavable spacer, J. Am. Chem. Soc 137 (2015) 6726–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arruebo M, Valladares M, González-Fernández Á, Antibody-conjugated nanoparticles for biomedical applications, J. Nanomater 2009 (2009) 439389. [Google Scholar]
- [34].Lu Z-R, Kopečková P, Kopeček J, Polymerizable Fab’ antibody fragments for targeting of anticancer drugs, Nat. Biotechnol 17 (1999) 1101–1104. [DOI] [PubMed] [Google Scholar]
- [35].Jo H, Ban C, Aptamer–nanoparticle complexes as powerful diagnostic and therapeutic tools, Exp. Mol. Med 48 (2016) e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY, Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications, FEBS Lett. 584 (2010) 2670–2680. [DOI] [PubMed] [Google Scholar]
- [37].Radford DC, Yang J, Doan MC, Li L, Dixon AS, Owen SC, Kopeček J, Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery, J. Control. Release 319 (2020) 285–299. [DOI] [PubMed] [Google Scholar]
- [38].Heuer-Jungemann A, Feliu N, Bakaimi I, Hamaly M, Alkilany A, Chakraborty I, Masood A, Casula MF, Kostopoulou A, Oh E, Susumu K, Stewart MH, Medintz IL, Stratakis E, Parak WJ, Kanaras AG, The role of ligands in the chemical synthesis and applications of inorganic nanoparticles, Chem. Rev 119 (2019) 4819–4880. [DOI] [PubMed] [Google Scholar]
- [39].Saleem K, Khursheed Z, Hano C, Anjum I, Anjum S, Applications of nanomaterials in leishmaniasis: A focus on recent advances and challenges, Nanomaterials 9 (2019) 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hardy RR, Hayakawa K, B cell development pathways, Annu. Rev. Immunol 19 (2001) 595–621. [DOI] [PubMed] [Google Scholar]
- [41].Cooper MD, The early history of B cells, Nat. Rev. Immunol 15 (2015) 191–197. [DOI] [PubMed] [Google Scholar]
- [42].Lebien TW, Tedder TF, B lymphocytes: how they develop and function, Blood 112 (2008) 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsai D-Y, Hung K-H, Chang C-W, Lin K-I, Regulatory mechanisms of B cell responses and the implication in B cell-related diseases, J. Biomed. Sci 26 (2019). 10.1186/s12929-019-0558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rodríguez-Pinto D, B cells as antigen presenting cells, Cellular Immunol. 238 (2005) 67–75. [DOI] [PubMed] [Google Scholar]
- [45].Edwards JCW, Cambridge G, B-cell targeting in rheumatoid arthritis and other autoimmune diseases, Nat. Rev. Immunol 6 (2006) 394–403. [DOI] [PubMed] [Google Scholar]
- [46].Harvey BP, Gee RJ, Haberman AM, Shlomchik MJ, Mamula MJ, Antigen presentation and transfer between B cells and macrophages, Eur. J. Immunol 37 (2007) 1739–1751. [DOI] [PubMed] [Google Scholar]
- [47].Hernández-Pérez S, Vainio M, Kuokkanen E, Sustar V, Petrov P, Fórsten S, Paavola V, Rajala J, Awoniyi L, Sarapulov A, Vihinen H, Jokitalo E, Bruckbauer A, Mattila P, B cells rapidly target antigen and surface-derived MHCII into peripheral degradative compartments, J Cell Sci. 133 (2020) jcs235192. [DOI] [PubMed] [Google Scholar]
- [48].Roche PA, Furuta K, The ins and outs of MHC class II-mediated antigen processing and presentation, Nat. Rev. Immunol 15 (2015) 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang W, Adler LN, Macmillan H, Mellins ED, Synergy between B cell receptor/antigen uptake and MHCII peptide editing relies on HLA-DO tuning, Sci. Rep 9 (2019) 13877–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Weyand CM, Goronzy JJ, Ectopic germinal center formation in rheumatoid synovitis, Ann. N. Y. Acad. Sci 987 (2003) 140–149. [DOI] [PubMed] [Google Scholar]
- [51].Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, Yoshie O, Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus, J. Virol 78 (2004) 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vazquez MI, Catalan-Dibene J, Zlotnik A, B cells responses and cytokine production are regulated by their immune microenvironment, Cytokine 74 (2015) 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gunderson AJ, Coussens LM, B cells and their mediators as targets for therapy in solid tumors, Exp. Cell Res 319 (2013) 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J, The B-side of cancer immunity: the underrated tune, Cells 8 (2019) 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hampe CS, B cells in autoimmune diseases, Scientifica 2012 (2012) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Harbers SO, Crocker A, Catalano G, D’Agati V, Jung S, Desai DD, Clynes R, Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance, J. Clin. Inv 117 (2007) 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ehlers M, Allelein S, Schott M, TSH-receptor autoantibodies: pathophysiology, assay methods, and clinical applications, Minerva Endocrinol. 43 (2018) 323–332. [DOI] [PubMed] [Google Scholar]
- [58].Michalek K, Morshed SA, Latif R, Davies TF, TSH receptor autoantibodies, Autoimmun. Rev 9 (2009) 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ding C, Wang L, Marroquin J, Yan J, Targeting of antigens to B cells augments antigen-specific T-cell responses and breaks immune tolerance to tumor-associated antigen MUC1, Blood 112 (2008) 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sarvaria A, Madrigal JA, Saudemont A, B cell regulation in cancer and anti-tumor immunity, Cell. Mol. Immunol 14 (2017) 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hofmann K, Clauder A-K, Manz RA, Targeting B cells and plasma cells in autoimmune diseases, Front. Immunol 9 (2018) 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bohula EA, Giugliano RP, Leiter LA, Verma S, Park J-G, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS, Inflammatory and cholesterol risk in the FOURIER trial, Circulation 138 (2018) 131–140. [DOI] [PubMed] [Google Scholar]
- [63].Galkina E, Ley K, Immune and inflammatory mechanisms of atherosclerosis, Annu. Rev. Immunol 27 (2009) 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ait-Oufella H, Herbin O, Bouaziz J-D, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z, B cell depletion reduces the development of atherosclerosis in mice, J. Exp. Med 207 (2010) 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh B-H, Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis, J. Immunol 185 (2010) 4410–4419. [DOI] [PubMed] [Google Scholar]
- [66].Kyaw T, Tipping P, Toh B-H, Bobik A, Current understanding of the role of B cell subsets and intimal and adventitial B cells in atherosclerosis, Curr. Opin. Lipidol 22 (2011) 373–379. [DOI] [PubMed] [Google Scholar]
- [67].Lee DSW, Rojas OL, and Gommerman JL, B cell depletion therapies in autoimmune disease: advances and mechanistic insights, Nat. Rev. Drug Discov 20 (2021) 179–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gorman C, Leandro M, Isenberg D, B cell depletion in autoimmune disease, Arthritis Res. Ther 5 (2003) S17–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burger JA, Wiestner A, Targeting B cell receptor signalling in cancer: preclinical and clinical advances, Nat. Rev. Cancer 18 (2018) 148–167. [DOI] [PubMed] [Google Scholar]
- [70].Magliozzi R, Marastoni D, Calabrese M, The BAFF / APRIL system as therapeutic target in multiple sclerosis, Expert Opin. Ther. Targets 24 (2020) 1135–1145. [DOI] [PubMed] [Google Scholar]
- [71].Gizinski AM, Fox DA, Sarkar S, Co-stimulation and T cells as therapeutic targets, Best Pract. Res. Clin. Rheumatol 24 (2010) 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Goodman A, Patel SP, Kurzrock R, PD-1–PD-L1 immune-checkpoint blockade in B-cell lymphomas, Nat. Rev. Clin. Oncol 14 (2017) 203–220. [DOI] [PubMed] [Google Scholar]
- [73].Plosker GL and Figgitt DP, Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia, Drugs. 63 (2003) 803–43. [DOI] [PubMed] [Google Scholar]
- [74].Reiser M, Dörfel SS, Hensel M, Hoesl M, Jordan WO, Koenigsmann M, Meyer D, Reichert D, Schwarzer A, Marquardt M, Kellershohn K, Jentsch-Ullrich K, Rituximab in combination with chemotherapy for the treatment of chronic lymphocytic leukaemia in clinical practice, Eur. J. Haematol 100 (2018) 455–464. [DOI] [PubMed] [Google Scholar]
- [75].Du FH, Mills EA, Mao-Draayer Y, Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment, Autoimmun. Highlights 8 (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Naddafi F, Davami F, Anti-CD19 monoclonal antibodies: a new approach to lymphoma therapy, Int. J. Mol. Cell Med 4 (2015) 143–151. [PMC free article] [PubMed] [Google Scholar]
- [77].Lamb YN, Inotuzumab ozogamicin: first global approval, Drugs 77 (2017) 1603–1610. [DOI] [PubMed] [Google Scholar]
- [78].Raedler LA, Darzalex (Daratumumab): first anti-CD38 monoclonal antibody approved for patients with relapsed multiple myeloma, Am. Health Drug Benefits 9 (2016) 70–73. [PMC free article] [PubMed] [Google Scholar]
- [79].Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F, CD38: a target for immunotherapeutic approaches in multiple myeloma, Front. Immunol 9 (2018) 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen DR, Cohen PL, Living life without B cells: is repeated B-cell depletion a safe and effective long-term treatment plan for rheumatoid arthritis? Int. J. Clin. Rheumtol 7 (2012) 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Thiel J, Rizzi M, Engesser M, Dufner A-K, Troilo A, Lorenzetti R, Voll RE, Venhoff N, B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients, Arthritis Res. Therapy 19(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Grillo-Lopez AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, Leonard JE, McClure A, Weaver R, Cairelli S, Rosenberg J, Rituximab the first monoclonal antibody approved for the treatment of lymphoma, Curr. Pharm. Biotechnol 1 (2000) 1–9. [DOI] [PubMed] [Google Scholar]
- [83].Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CHT, Tutt A, Beers SA, Glennie MJ, Cragg MS, Illidge TM, Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies, Blood 117 (2011) 4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Safdari Y, Ahmadzadeh V, Farajnia S, CD20-targeting in B-cell malignancies: novel prospects for antibodies and combination therapies, Invest. New Drugs 34 (2016) 497–512. [DOI] [PubMed] [Google Scholar]
- [85].Boross P, Leusen JHW, Mechanisms of action of CD20 antibodies, Am. J. Cancer Res 2 (2012) 676–690. [PMC free article] [PubMed] [Google Scholar]
- [86].Kellner C, Otte A, Cappuzzello E, Klausz K, Peipp M, Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy, Transfus. Med. Hemother 44(5) (2017) 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gürcan HM, Keskin DB, Stern JNH, Nitzberg MA, Shekhani H, Ahmed AR, A review of the current use of rituximab in autoimmune diseases, Int. Immunopharmacol 9 (2009) 10–25. [DOI] [PubMed] [Google Scholar]
- [88].Liote H, Liote F, Seroussi B, Mayaud C, Cadranel J, Rituximab-induced lung disease: a systematic literature review, Eur. Respir. J 35 (2010) 681–687. [DOI] [PubMed] [Google Scholar]
- [89].Pijpe J, Van Imhoff GW, Spijkervet FKL, Roodenburg JLN, Wolbink GJ, Mansour K, Vissink A, Kallenberg CGM, Bootsma H, Rituximab treatment in patients with primary Sjögren’s syndrome: an open-label phase II study, Arthritis Rheum. 52 (2005) 2740–2750. [DOI] [PubMed] [Google Scholar]
- [90].Rezvani AR, Maloney DG, Rituximab resistance, Best Pract. Res. Clin. Haematol 24 (2011) 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Teige I, Mårtensson L, Frendéus BL, Targeting the antibody checkpoints to enhance cancer immunotherapy–focus on FcγRIIB, Front. Immunol 10 (2019) 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhang L, Fang Y, Kopeček J, Yang J, A new construct of antibody-drug conjugates for treatment of B-cell non-Hodgkin’s lymphomas, Eur. J. Pharm. Sci 103 (2017) 36–46. [DOI] [PubMed] [Google Scholar]
- [93].Capolla S, Garrovo C, Zorzet S, Lorenzon A, Rampazzo E, Spretz R, Pozzato G, Nunez L, Macor P, Tripodo C, Biffi S, Targeted tumor imaging of anti-CD20-polymeric nanoparticles developed for the diagnosis of B-cell malignancies, Int. J. Nanomed 22 (2015) 4099–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mezzaroba N, Zorzet S, Secco E, Biffi S, Tripodo C, Calvaruso M, Mendoza-Maldonado R, Capolla S, Granzotto M, Spretz R, Larsen G, Noriega S, Lucafò M, Mansilla E, Garrovo C, Marín GH, Baj G, Gattei V, Pozzato G, Núñez L, Macor P, New potential therapeutic approach for the treatment of B-cell malignancies using chlorambucil/hydroxychloroquine-loaded anti-CD20 nanoparticles, PLoS One 8 (2013) e74216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Choi KY, Correa S, Min J, Li J, Roy S, Laccetti KH, Dreaden E, Kong S, Heo R, Roh YH, Lawson EC, Palmer PA, Hammond PT, Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles, Adv. Funct. Mater 29 (2019) 1900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Glass JJ, Yuen D, Rae J, Johnston APR, Parton RG, Kent SJ, De Rose R, Human immune cell targeting of protein nanoparticles – caveospheres, Nanoscale 8 (2016) 8255–8265. [DOI] [PubMed] [Google Scholar]
- [97].Nevala WK, Butterfield JT, Sutor SL, Knauer DJ, Markovic SN, Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20+ B-cell lymphoma, Sci. Rep 7 (2017) 45682–45690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mansur HS, Mansur AAP, Soriano-Araújo A, Lobato ZIP, De Carvalho SM, Leite MDF, Water-soluble nanoconjugates of quantum dot-chitosan-antibody for in vitro detection of cancer cells based on “enzyme-free” fluoroimmunoassay, Mater.Sci. Eng C52 (2015) 61–71. [DOI] [PubMed] [Google Scholar]
- [99].Saesoo S, Sathornsumetee S, Anekwiang P, Treetidnipa C, Thuwajit P, Bunthot S, Maneeprakorn W, Maurizi L, Hofmann H, Rungsardthong RU, Saengkrit N, Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma, Colloids Surf. B 161 (2018) 497–507. [DOI] [PubMed] [Google Scholar]
- [100].Yu B, Mao Y, Yuan Y, Yue C, Wang X, Mo X, Jarjoura D, Paulaitis ME, Lee RJ, Byrd JC, Lee LJ, Muthusamy N, Targeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cells, Biomaterials 34 (2013) 6185–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yin H, Meng T, Shu L, Mao M, Zhou L, Chen H, Song D, Novel reduction-sensitive micellar nanoparticles assembled from Rituximab-doxorubicin conjugates as smart and intuitive drug delivery systems for the treatment of non-Hodgkin’s lymphoma, Chem. Biol. Drug Des 90 (2017) 892–899. [DOI] [PubMed] [Google Scholar]
- [102].Song L, Chen Y, Ding J, Wu H, Zhang W, Ma M, Zang F, Wang Z, Gu N, Zhang Y, Rituximab conjugated iron oxide nanoparticles for targeted imaging and enhanced treatment against CD20-positive lymphoma, J. Mater. Chem. B 8 (2020) 895–907. [DOI] [PubMed] [Google Scholar]
- [103].Song L, Zhang W, Chen H, Zhang X, Wu H, Ma M, Wang Z, Gu N, Zhang Y, Apoptosis-promoting effect of rituximab-conjugated magnetic nanoprobes on malignant lymphoma cells with CD20 overexpression, Int. J. Nanomedicine 14 (2019) 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang T, Kievit FM, Veiseh O, Arami H, Stephen ZR, Fang C, Liu Y, Ellenbogen RG, Zhang M, Targeted cell uptake of a noninternalizing antibody through conjugation to iron oxide nanoparticles in primary central nervous system lymphoma, Tumor 80 (2013) 134–141. [DOI] [PubMed] [Google Scholar]
- [105].Chu T-W, Yang J, Zhang R, Sima M, Kopeček J, Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis, ACS Nano 8 (2014) 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Li L, Yang J, Wang J, Kopeček J, Amplification of CD20 cross-linking in rituximab-resistant B-lymphoma cells enhances apoptosis induction by drug-free macromolecular therapeutics, ACS Nano 12 (2018) 3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li L, Yang J, Wang J, Kopeček J, Drug-free macromolecular therapeutics induce apoptosis via calcium influx and mitochondrial signaling pathway, Macromol. Biosci 18 (2018) 1700196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chu T-W, Zhang R, Yang J, Chao MP, Shami PJ, Kopeček J, A two-step pretargeted nanotherapy for CD20 crosslinking may achieve superior anti-lymphoma efficacy to rituximab, Theranostics 5 (2015) 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang J, Li Y, Li L, Yang J, Kopeček J, Exploration and evaluation of therapeutic efficacy of drug-free macromolecular therapeutics in collagen-induced rheumatoid arthritis mouse model, Macromol. Biosci 20 (2020) 1900445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chu T-W, Kosak KM, Shami PJ, Kopeček J, Drug-free macromolecular therapeutics induce apoptosis of patient chronic lymphocytic leukemia cells, Drug Deliv. Transl. Res 4 (2014) 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang J, Li L, Yang J, Clair PM, Glenn MJ, Stephens DM, Radford DC, Kosak KM, Deininger MW, Shami PJ, Kopeček J, Drug-free macromolecular therapeutics induce apoptosis in cells isolated from patients with B cell malignancies with enhanced apoptosis induction by pretreatment with gemcitabine, Nanomedicine: NBM 16 (2019) 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hayashi K, Nagasaki E, Kan S, Ito M, Kamata Y, Homma S, Aiba K, Gemcitabine enhances rituximab-mediated complement-dependent cytotoxicity to B cell lymphoma by CD20 upregulation, Cancer Sci. 107 (2016) 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hill R, Rabb M, Madureira PA, Clements D, Gujar SA, Waisman DM, Giacomantonio CA, Lee PWK, Gemcitabine-mediated tumour regression and p53-dependent gene expression: implications for colon and pancreatic cancer therapy, Cell Death Dis 4 (2013) e791–e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang L, Fang Y, Li L, Yang J, Radford DC, Kopeček J, Human serum albumin-based drug-free macromolecular therapeutics: apoptosis induction by coiled-coil-mediated cross-linking of CD20 antigens on lymphoma B cell surface, Macromol. Biosci 18 (2018) 1800224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li L, Yang J, Soodvilai S, Wang J, Opanasopit P, Kopeček J, Drug-free albumin-triggered sensitization of cancer cells to anticancer drugs, J. Control. Release 293 (2019) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Seyfizadeh N, Seyfizadeh N, Hasenkamp J, Huerta-Yepez S, A molecular perspective on rituximab: a monoclonal antibody for B cell non Hodgkin lymphoma and other affections, Crit. Rev. Oncol. Hematol 97 (2016) 275–290. [DOI] [PubMed] [Google Scholar]
- [117].Meyer S, Evers M, Jansen JHM, Buijs J, Broek B, Reitsma SE, Moerer P, Amini M, Kretschmer A, Ten Broeke T, Den Hartog MT, Rijke M, Klein C, Valerius T, Boross P, Leusen JHW, New insights in type I and II CD20 antibody mechanisms-of-action with a panel of novel CD20 antibodies, Brit. J. Haematol 180 (2018) 808–820. [DOI] [PubMed] [Google Scholar]
- [118].Lim SH, Beers SA, French RR, Johnson PWM, Glennie MJ, Cragg MS, Anti-CD20 monoclonal antibodies: historical and future perspectives, Haematologica 95 (2010) 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tobinai K, Klein C, Oya N, Fingerle-Rowson G, A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies, Adv. Ther 34 (2017) 324–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Li L, Wang J, Li Y, Radford DC, Yang J, Kopeček J, Broadening and enhancing functions of antibodies by self-assembling multimerization at cell surface, ACS Nano 13 (2019) 11422–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gambles MT, Li J, Wang J, Sborov D, Yang J, Kopeček J, Crosslinking of CD38 receptors triggers apoptosis of malignant B cells, Molecules 26 (2021) 4658; https://doi.org/10/3390/molecules26154658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Aluri SR, Shi P, Gustafson JA, Wang W, Lin Y-A, Cui H, Liu S, Conti PS, Li Z, Hu P, Epstein AL, Mackay JA, A hybrid protein–polymer nanoworm potentiates apoptosis better than a monoclonal antibody, ACS Nano 8 (2014) 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Urry DW, Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers, J. Phys. Chem. B 101 (1997) 11007–11028. [Google Scholar]
- [124].Yang Q, Parker CL, Lin Y, Press OW, Park SI, Lai SK, Pretargeting with bispecific fusion proteins facilitates delivery of nanoparticles to tumor cells with distinct surface antigens, J. Control. Release 255 (2017) 73–80. [DOI] [PubMed] [Google Scholar]
- [125].Gunn J, Park SI, Veiseh O, Press OW, Zhang M, A pretargeted nanoparticle system for tumor cell labeling, Mol. Biosyst 7 (2011) 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zeng Y-B, Yu Z-C, He Y-N, Zhang T, Du L-B, Dong Y-M, Chen H-W, Zhang Y-Y, Wang W-Q, Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20+ melanoma stem cells, Acta Pharmacol. Sin 39 (2018) 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang K, Wei G, Liu D, CD19: a biomarker for B cell development, lymphoma diagnosis and therapy, Exp. Hematol. Oncol 1 (2012) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chen D, Gallagher S, Monson N, Herbst R, Wang Y, Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies, J. Clin. Med 5 (2016) 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hoffman LM, Gore L, Blinatumomab, a bi-specific anti-CD19/CD3 BiTE® antibody for the treatment of acute lymphoblastic leukemia: perspectives and current pediatric applications, Front. Oncol 4 (2014) 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tung HY, Su YC, Chen BM, Burnouf PA, Huang WC, Chuang KH, Yan YT, Cheng TL, Roffler SR, Selective delivery of PEGylated compounds to tumor cells by anti-PEG hybrid antibodies, Mol. Cancer Ther 14 (2015) 1317–1326. [DOI] [PubMed] [Google Scholar]
- [131].Tatar A-S, Jurj A, Tomuleasa C, Florea A, Berindan-Neagoe I, Cialla-May D, Popp J, Astilean S, Boca S, CD19-targeted, Raman tagged gold nanourchins as theranostic agents against acute lymphoblastic leukemia, Colloids Surf. B 184 (2019) 110478. [DOI] [PubMed] [Google Scholar]
- [132].Harata M, CD19-targeting liposomes containing imatinib efficiently kill Philadelphia chromosome-positive acute lymphoblastic leukemia cells, Blood 104 (2004) 1442–1449. [DOI] [PubMed] [Google Scholar]
- [133].Myers DE, Yiv S, Qazi S, Ma H, Cely I, Shahidzadeh A, Arellano M, Finestone E, Gaynon PS, Termuhlen A, Cheng J, Uckun FM, CD19-antigen specific nanoscale liposomal formulation of a SYK P-site inhibitor causes apoptotic destruction of human B-precursor leukemia cells, Integr. Biol 6 (2014) 766–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mócsai A, Ruland J, Tybulewicz VLJ, The SYK tyrosine kinase: a crucial player in diverse biological functions, Nat. Rev. Immunol 10 (2010) 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Krishnan V, Xu X, Kelly D, Snook A, Waldman SA, Mason RW, Jia X, Rajasekaran AK, CD19-targeted nanodelivery of doxorubicin enhances therapeutic efficacy in B-cell acute lymphoblastic leukemia, Mol. Pharm 12 (2015) 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Uckun FM, Sun L, Qazi S, Ma H, Ozer Z, Recombinant human CD19-ligand protein as a potent anti-leukaemic agent, Brit. J. Haematol 153 (2011) 15–23. [DOI] [PubMed] [Google Scholar]
- [137].Fan J, He Q, Jin Z, Chen W, Huang W, A novel phosphoester-based cationic copolymer nanocarrier delivers chimeric antigen receptor plasmid and exhibits anti-tumor effect, RSC Adv. 8 (2018) 14975–14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Majzner RG, Mackall CL, Tumor antigen escape from CAR T-cell therapy, Cancer Disc. 8 (2018) 1219–1226. [DOI] [PubMed] [Google Scholar]
- [139].Shalabi H, Kraft IL, Wang H-W, Yuan CM, Yates B, Delbrook C, Zimbelman JD, Giller R, Stetler-Stevenson M, Jaffe ES, Lee DW, Shern JF, Fry TJ, Shah NN, Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma, Haematologica 103 (2018) e215–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Siegers GM, Krishnamoorthy S, Gonzalez-Lara LE, McFadden C, Chen Y, Foster PJ, Pre-labeling of immune cells in normal bone marrow and spleen for subsequent cell tracking by MRI, Tomography 2 (2016) 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Robinson HR, Qi J, Cook EM, Nichols C, Dadashian EL, Underbayev C, Herman SEM, Saba NS, Keyvanfar K, Sun C, Ahn IE, Baskar S, Rader C, Wiestner A, A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era, Blood 132 (2018) 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kipriyanov SM, Cochlovius B, Schafer HJ, Moldenhauer G, Bahre A, Le Gall F, Knackmuss S, Little M, Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 × CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma, J. Immunol 169 (2002) 137–144. [DOI] [PubMed] [Google Scholar]
- [143].Wu J, Fu J, Zhang M, Liu D, Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia, J. Hematol. Oncol 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Do P, Perdue LA, Chyong A, Hunter R, Dougan J, Henry CJ, Porter CC, Dreaden EC, Rapid assembly and screening of multivalent immune cell-redirecting therapies for leukemia, ACS Combin. Sci 22 (2020) 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Schütz C, Varela JC, Perica K, Haupt C, Oelke M, Schneck JP, Antigen-specific T cell redirectors: a nanoparticle based approach for redirecting T cells, Oncotarget 7 (2016) 68503–68512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA, Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells, Methods Mol. Biol 1441 (2016) 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ma Y, Xiang D, Sun J, Ding C, Liu M, Hu X, Li G, Kloecker G, Zhang H-G, Yan J, Targeting of antigens to B lymphocytes via CD19 as a means for tumor vaccine development, J. Immunol 190 (2013) 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Moyron-Quiroz JE, Partida-Sanchez S, Donis-Hernandez R, Sandoval-Montes C, Santos-Argumedo L, Expression and function of CD22, a B-cell restricted molecule, Scand. J. Immunol 55 (2002) 343–351. [DOI] [PubMed] [Google Scholar]
- [149].O’Reilly MK, Tian H, Paulson JC, CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells, J. Immunol 186 (2011) 1554–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Satake N, Duong C, Chen C, Barisone GA, Diaz E, Tuscano J, Rocke DM, Nolta J, Nitin N, Targeted therapy with MXD3 siRNA, anti-CD22 antibody and nanoparticles for precursor B-cell acute lymphoblastic leukaemia, Br. J. Haematol 167 (2014) 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Xu P, Zuo H, Chen D, Peng M, Jiang Y, Liu X, Ouyang J, Chen B, Anti-CD22-conjugated CdTe QDs co-loaded with doxorubicin and gambogic acid: a novel platform for lymphoma treatment, RSC Adv. 7 (2017) 33905–33913. [Google Scholar]
- [152].Mittal N, Mandal B, Balabathula P, Setua S, Janagam D, Lothstein L, Thoma L, Wood G, Formulation, development, and in vitro evaluation of a CD22 targeted liposomal system containing a non-cardiotoxic anthracycline for B cell malignancies, Pharmaceutics 10 (2018) 50–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cho SK, Kwon YJ, Simultaneous gene transduction and silencing using stimuli-responsive viral/nonviral chimeric nanoparticles, Biomaterals 33 (2012) 3316–3323. [DOI] [PubMed] [Google Scholar]
- [154].Peng W, Paulson JC, CD22 ligands on a natural N-glycan scaffold efficiently deliver toxins to B-lymphoma cells, J. Am. Chem. Soc 139 (2017) 12450–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Van De Donk NWCJ, Richardson PG, Malavasi F, CD38 antibodies in multiple myeloma: back to the future, Blood 131 (2018) 13–29. [DOI] [PubMed] [Google Scholar]
- [156].Wong SW, Comenzo RL, CD38 monoclonal antibody therapies for multiple myeloma, Clin. Lymphoma Myeloma Leuk 15 (2015) 635–645. [DOI] [PubMed] [Google Scholar]
- [157].De La Puente P, Luderer MJ, Federico C, Jin A, Gilson RC, Egbulefu C, Alhallak K, Shah S, Muz B, Sun J, King J, Kohnen D, Salama NN, Achilefu S, Vij R, Azab AK, Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma, J. Control. Release 270 (2018) 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Huang YH, Vakili MR, Molavi O, Morrissey Y, Wu C, Paiva I, Soleimani AH, Sanaee F, Lavasanifar A, Lai R, Decoration of anti-CD38 on nanoparticles carrying a STAT3 inhibitor can improve the therapeutic efficacy against myeloma, Cancers (Basel) 11 (2019) 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Cherkasova E, Espinoza L, Kotecha R, Reger RN, Berg M, Aue G, Attar RM, Sasser AK, Carlsten M, Childs RW, Treatment of ex vivo expanded NK cells with daratumumab F(ab’)2 fragments protects adoptively transferred NK cells from daratumumab-mediated killing and augments daratumumab-induced antibody dependent cellular toxicity (ADCC) of myeloma, Blood 126 (2015) 4244–4244. [Google Scholar]
- [160].Malaer JD, Mathew PA, CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma, Am. J. Cancer Res 7 (2017) 1637–1641. [PMC free article] [PubMed] [Google Scholar]
- [161].Jakubowiak AJ, Benson DM, Bensinger W, Siegel DSD, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DEH, Singhal AK, Anderson KC, Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma, Clin. Trial 30 (2012) 1960–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gormley NJ, Ko C-W, Deisseroth A, Nie L, Kaminskas E, Kormanik N, Goldberg KB, Farrell AT, Pazdur R, FDA drug approval: Elotuzumab in combination with lenalidomide and dexamethasone for the treatment of relapsed or refractory multiple myeloma, Clinic. Cancer Res 23 (2017) 6759–6763. [DOI] [PubMed] [Google Scholar]
- [163].Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA, CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function, Mol. Immunol 39 (2002) 1–8. [DOI] [PubMed] [Google Scholar]
- [164].Woo J, Vierboom MP, Kwon H, Chao D, Ye S, Li J, Lin K, Tang I, Belmar NA, Hartman T, Breedveld E, Vexler V, ‘T Hart BA, Law DA, Starling GC, PDL241, a novel humanized monoclonal antibody, reveals CD319 as a therapeutic target for rheumatoid arthritis, Arthritis Res. Ther 15 (2013) R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Inoges S, Idiotype vaccines for lymphoma: Potential factors predicting the induction of immune responses, World J. Clin. Oncol 2(6) (2011) 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, Rajapaksa R, Caspar CB, van Beckhoven A, Levy R, Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma, Cancer Res. 62 (2002) 5845–5852. [PubMed] [Google Scholar]
- [167].Rice J, Ottensmeier CH, Stevenson FK, DNA vaccines: precision tools for activating effective immunity against cancer, Nat. Rev. Cancer 8 (2008) 108–120. [DOI] [PubMed] [Google Scholar]
- [168].Pai Kasturi S, Qin H, Thomson KS, El-Bereir S, Cha S-C, Neelapu S, Kwak LW, Roy K, Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles, J. Control. Release 113 (2006) 261–270. [DOI] [PubMed] [Google Scholar]
- [169].Meleshko AN, Petrovskaya NA, Savelyeva N, Vashkevich KP, Doronina SN, Sachivko NV, Phase I clinical trial of idiotypic DNA vaccine administered as a complex with polyethylenimine to patients with B-cell lymphoma, Hum. Vaccin. Immunother 13 (2017) 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Rink J, Yang S, Cen O, Karmali R, Thaxton CS, Gordon LI, Inhibition of pro-survival pathways in DLBCL cells by functional lipoprotein-like nanoparticles, Blood 132 (2018) 1668–1668. [Google Scholar]
- [171].Woyach JA, Johnson AJ, Byrd JC, The B-cell receptor signaling pathway as a therapeutic target in CLL, Blood 120 (2012) 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WWA, Zürn C, Reth M, Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop, Mol. Cell 10 (2002) 1057–1069. [DOI] [PubMed] [Google Scholar]
- [173].Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, Ionescu C, Berindan-Neagoe I, A comprehensive review on MAPK: a promising therapeutic target in cancer, Cancers 11 (2019) 1618–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Madhunapantula SV, Mosca PJ, Robertson GP, The Akt signaling pathway, Cancer Biol. Ther 12 (2011) 1032–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Karin M, Lin A, NF-κB at the crossroads of life and death, Nat. Immunol 3 (2002) 221–227. [DOI] [PubMed] [Google Scholar]
- [176].Luger D, Yang Y-A, Raviv A, Weinberg D, Banerjee S, Lee M-J, Trepel J, Yang L, Wakefield LM, Expression of the B-cell receptor component CD79a on immature myeloid cells contributes to their tumor promoting effects, PLoS One 8 (2013) e76115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lai R, Juco J, Lee SF, Nahirniak S, Etches WS, Flow cytometric detection of CD79a expression in T-cell acute lymphoblastic leukemias, Am. J. Clin. Pathol 113 (2000) 823–830. [DOI] [PubMed] [Google Scholar]
- [178].Huang X, Takata K, Sato Y, Tanaka T, Ichimura K, Tamura M, Oka T, Yoshino T, Downregulation of the B-cell receptor signaling component CD79b in plasma cell myeloma: A possible post transcriptional regulation, Pathology Int. 61 (2011) 122–129. [DOI] [PubMed] [Google Scholar]
- [179].Fuh FK, Looney C, Li D, Poon KA, Dere RC, Danilenko DM, McBride J, Reed C, Chung S, Zheng B, Mathews WR, Polson A, Prabhu S, Williams M, Anti-CD22 and anti-CD79b antibody-drug conjugates preferentially target proliferating B cells, Br. J. Pharmacol 174 (2017) 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, Slaga DS, Giere L, Du C, Tan C, Hongo JA, Gogineni A, Cole MJ, Vandlen R, Stephan JP, Young J, Chang W, Scales SJ, Ross S, Eaton D, Ebens A, Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma, Blood 110 (2007) 616–623. [DOI] [PubMed] [Google Scholar]
- [181].Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ, Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma, J. Clin. Oncol 38 (2020) 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Brühl H, Cihak J, Talke Y, Gomez MR, Hermann F, Goebel N, Renner K, Plachý J, Stangassinger M, Aschermann S, Nimmerjahn F, Mack M, B-cell inhibition by cross-linking CD79b is superior to B-cell depletion with anti-CD20 antibodies in treating murine collagen-induced arthritis, Eur. J. Immunol 45 (2015) 705–715. [DOI] [PubMed] [Google Scholar]
- [183].Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S, Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia, N. Engl. J. Med 369 (2013) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Scheers E, Leclercq L, De Jong J, Bode N, Bockx M, Laenen A, Cuyckens F, Skee D, Murphy J, Sukbuntherng J, Mannens G, Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men, Drug Metabol. Disposition 43 (2015) 289–297. [DOI] [PubMed] [Google Scholar]
- [185].Zhao L, Tang B, Tang P, Sun Q, Suo Z, Zhang M, Gan N, Yang H, Li H, Chitosan/sulfobutylether-β-cyclodextrin nanoparticles for ibrutinib delivery: a potential nanoformulation of novel kinase inhibitor, J. Pharm. Sci 109 (2020) 1136–1144. [DOI] [PubMed] [Google Scholar]
- [186].Burger JA, Bruton tyrosine kinase inhibitors: present and future, Cancer J. 25 (2019) 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Zhao G, Liu A, Zhang Y, Zuo ZQ, Cao ZT, Zhang HB, Xu CF, Wang J, Nanoparticle-delivered siRNA targeting Bruton’s tyrosine kinase for rheumatoid arthritis therapy, Biomater. Sci 7 (2019) 4698–4707. [DOI] [PubMed] [Google Scholar]
- [188].Connell NT, Berliner N, Fostamatinib for the treatment of chronic immune thrombocytopenia, Blood 133 (2019) 2027–2030. [DOI] [PubMed] [Google Scholar]
- [189].Liu D, Mamorska-Dyga A, Syk inhibitors in clinical development for hematological malignancies, J. Hematol. Oncol 10 (2017) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Uckun FM, Qazi S, Ma H, Tuel-Ahlgren L, Ozer Z, STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 2902–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Uckun FM, Qazi S, Cely I, Sahin K, Shahidzadeh A, Ozercan I, Yin Q, Gaynon P, Termuhlen A, Cheng J, Yiv S, Nanoscale liposomal formulation of a SYK P-site inhibitor against B-precursor leukemia, Blood 121 (2013) 4348–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Cely I, Yiv S, Yin Q, Shahidzadeh A, Tang L, Cheng J, Uckun FM, Targeting mantle cell lymphoma with anti-SYK nanoparticles, J. Anal. Oncol 1 (2012) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Uckun FM, Myers DE, Cheng J, Qazi S, Liposomal nanoparticles of a spleen tyrosine kinase P-site inhibitor amplify the potency of low dose total body irradiation against aggressive B-precursor leukemia and yield superior survival outcomes in mice, EBioMedicine 2 (2015) 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JKV, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PVK, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B, The genomic landscapes of human breast and colorectal cancers, Science 318 (2007) 1108–1113. [DOI] [PubMed] [Google Scholar]
- [195].The Cancer Genome Atlas Research Network, Comprehensive genomic characterization defines human glioblastoma genes and core pathways, Nature 455 (2008) 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK, Somatic mutations affect key pathways in lung adenocarcinoma, Nature 455 (2008) 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA, PI3Kγ is a molecular switch that controls immune suppression, Nature 539 (2016) 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Evans CA, Liu T, Lescarbeau A, Nair SJ, Grenier L, Pradeilles JA, Glenadel Q, Tibbitts T, Rowley AM, Dinitto JP, Brophy EE, O’Hearn EL, Ali JA, Winkler DG, Goldstein SI, O’Hearn P, Martin CM, Hoyt JG, Soglia JR, Cheung C, Pink MM, Proctor JL, Palombella VJ, Tremblay MR, Castro AC, Discovery of a selective phosphoinositide-3-kinase (PI3K)-γ inhibitor (IPI-549) as an immuno-oncology clinical candidate, ACS Med. Chem. Lett 7 (2016) 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T, Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells, Nature 539 (2016) 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Zhang X, Shen L, Liu Q, Hou L, Huang L, Inhibiting PI3 kinase-γ in both myeloid and plasma cells remodels the suppressive tumor microenvironment in desmoplastic tumors, J. Control. Release 309 (2019) 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Jiang M, He K, Qiu T, Sun J, Liu Q, Zhang X, Zheng H, Tumor-targeted delivery of silibinin and IPI-549 synergistically inhibit breast cancer by remodeling the microenvironment, Int. J. Pharm 581 (2020) 119239. [DOI] [PubMed] [Google Scholar]
- [202].Van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RAJO, Potential applications for sigma receptor ligands in cancer diagnosis and therapy, Biochim. Biophys. Acta-Biomembr 1848 (2015) 2703–2714. [DOI] [PubMed] [Google Scholar]
- [203].Ko J-W, Shin N-R, Park S-H, Lee I-C, Ryu J-M, Kim H-J, Cho Y-K, Kim J-C, Shin I-S, Silibinin inhibits the fibrotic responses induced by cigarette smoke via suppression of TGF-β1/Smad 2/3 signaling, Food Chem. Toxicol 106 (2017) 424–429. [DOI] [PubMed] [Google Scholar]
- [204].Yang Q, Modi P, Newcomb T, Quéva C, Gandhi V, Idelalisib: First-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma, Clin. Cancer Res 21 (2015) 1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX, Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941, Cancer Cell 15 (2009) 429–440. [DOI] [PubMed] [Google Scholar]
- [206].Danoch H, Kalechman Y, Albeck M, Longo DL, Sredni B, Sensitizing B- and T- cell lymphoma cells to paclitaxel/abraxane–induced death by AS101 via inhibition of the VLA-4–IL10–survivin axis, Mol. Cancer Res 13 (2015) 411–422. [DOI] [PubMed] [Google Scholar]
- [207].Badr G, Al-Sadoon MK, El-Toni AM, Daghestani M, Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFkappaB and ERK signaling, Lipids Health Dis. 11 (2012) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Al-Sadoon MK, Rabah DM, Badr G, Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: molecular targets for cell cycle arrest and apoptosis induction, Cell Immunol. 284 (2013) 129–138. [DOI] [PubMed] [Google Scholar]
- [209].Bodmer J-L, Schneider P, Tschopp J, The molecular architecture of the TNF superfamily, Trends Biochem. Sci 27 (2002) 19–26. [DOI] [PubMed] [Google Scholar]
- [210].Schneider P, The role of APRIL and BAFF in lymphocyte activation, Curr. Opin. Immunol 17 (2005) 282–289. [DOI] [PubMed] [Google Scholar]
- [211].Steri M, Orrù V, Idda ML, Pitzalis M, Pala M, Zara I, Sidore C, Faà V, Floris M, Deiana M, Asunis I, Porcu E, Mulas A, Piras MG, Lobina M, Lai S, Marongiu M, Serra V, Marongiu M, Sole G, Busonero F, Maschio A, Cusano R, Cuccuru G, Deidda F, Poddie F, Farina G, Dei M, Virdis F, Olla S, Satta MA, Pani M, Delitala A, Cocco E, Frau J, Coghe G, Lorefice L, Fenu G, Ferrigno P, Ban M, Barizzone N, Leone M, Guerini FR, Piga M, Firinu D, Kockum I, Lima Bomfim I, Olsson T, Alfredsson L, Suarez A, Carreira PE, Castillo-Palma MJ, Marcus JH, Congia M, Angius A, Melis M, Gonzalez A, Alarcón Riquelme ME, Da Silva BM, Marchini M, Danieli MG, Del Giacco S, Mathieu A, Pani A, Montgomery SB, Rosati G, Hillert J, Sawcer S, D’Alfonso S, Todd JA, Novembre J, Abecasis GR, Whalen MB, Marrosu MG, Meloni A, Sanna S, Gorospe M, Schlessinger D, Fiorillo E, Zoledziewska M, Cucca F, Overexpression of the cytokine BAFF and autoimmunity risk, N. Eng. J. Med 376 (2017) 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Dillon SR, Gross JA, Ansell SM, Novak AJ, An APRIL to remember: novel TNF ligands as therapeutic targets, Nat. Rev. Drug Disc 5 (2006) 235–246. [DOI] [PubMed] [Google Scholar]
- [213].Kern C, Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway, Blood 103 (2004) 679–688. [DOI] [PubMed] [Google Scholar]
- [214].Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF, Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival, Blood 103 (2004) 689–694. [DOI] [PubMed] [Google Scholar]
- [215].Novak AJ, Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: Correlation with disease activity and patient outcome, Blood 104 (2004) 2247–2253. [DOI] [PubMed] [Google Scholar]
- [216].Cho S-F, Anderson KC, Tai Y-T, Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy, Front. Immunol 9 (2018) 1821–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Samy E, Wax S, Huard B, Hess H, Schneider P, Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases, Int. Rev. Immunol 36 (2017) 3–19. [DOI] [PubMed] [Google Scholar]
- [218].Lee WS, Amengual O, B cells targeting therapy in the management of systemic lupus erythematosus, Immunol. Med 43 (2020) 16–35. [DOI] [PubMed] [Google Scholar]
- [219].Peperzak V, Vikström I, Walker J, Glaser SP, Lepage M, Coquery CM, Erickson LD, Fairfax K, Mackay F, Strasser A, Nutt SL, Tarlinton DM, Mcl-1 is essential for the survival of plasma cells, Nat. Immunol 14 (2013) 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Tai YT, Acharya C, An G, Moschetta M, Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, Van Eenennaam H, Van Elsas A, Qiu L, Richardson P, Munshi N, Anderson KC, APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment, Blood 127 (2016) 3225–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Bae J, Samur M, Richardson P, Munshi NC, Anderson KC, Selective targeting of multiple myeloma by B cell maturation antigen (BCMA)-specific central memory CD8+ cytotoxic T lymphocytes: immunotherapeutic application in vaccination and adoptive immunotherapy, Leukemia 33 (2019) 2208–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Bae J, Parayath N, Ma W, Amiji M, Munshi N, Anderson KC, BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8(+) cytotoxic T lymphocytes against multiple myeloma: clinical applications, Leukemia 34 (2020) 210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Detappe A, Reidy M, Yu Y, Mathieu C, Nguyen HV, Coroller TP, Lam F, Jarolim P, Harvey P, Protti A, Nguyen QD, Johnson JA, Cremillieux Y, Tillement O, Ghobrial IM, Ghoroghchian PP, Antibody-targeting of ultra-small nanoparticles enhances imaging sensitivity and enables longitudinal tracking of multiple myeloma, Nanoscale 11 (2019) 20485–20496. [DOI] [PubMed] [Google Scholar]
- [224].He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A, Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL, J. Immunol 172 (2004) 3268–3279. [DOI] [PubMed] [Google Scholar]
- [225].Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL, Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations, J. Exp. Med 190 (1999) 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sellam J, Miceli-Richard C, Gottenberg JE, Ittah M, Lavie F, Lacabaratz C, Gestermann N, Proust A, Lambotte O, Mariette X, Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjogren’s syndrome and systemic lupus erythematosus, Ann. Rheum. Dis 66 (2007) 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Qin H, Dong Z, Wang X, Cheng WA, Wen F, Xue W, Sun H, Walter M, Wei G, Smith DL, Sun X, Fei F, Xie J, Panagopoulou TI, Chen C-W, Song JY, Aldoss I, Kayembe C, Sarno L, Müschen M, Inghirami GG, Forman SJ, Kwak LW, CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies, Science Transl. Med 11 (2019) eaaw9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh B-H, BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(−/−) mice, PLoS One 8 (2013) e60430–e60430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, Genome engineering using the CRISPR-Cas9 system, Nat. Protocols 8 (2013) 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Li M, Fan Y-N, Chen Z-Y, Luo Y-L, Wang Y-C, Lian Z-X, Xu C-F, Wang J, Optimized nanoparticle-mediated delivery of CRISPR-Cas9 system for B cell intervention, Nano Res. 11 (2018) 6270–6282. [Google Scholar]
- [231].Zhang L, Gao H, Chen L, Wu B, Zheng Y, Liao R, Jiang Y, He F, Tumor targeting of vincristine by mBAFF-modified PEG liposomes in B lymphoma cells, Cancer Lett. 269 (2008) 26–36. [DOI] [PubMed] [Google Scholar]
- [232].Ibtehaj N, Anderson KE, Huda R, High-dose BAFF receptor specific mAb-siRNA conjugate generates Fas-expressing B cells in lymph nodes and high-affinity serum autoantibody in a myasthenia mouse model, Clin. Immumol 176 (2017) 122–130. [DOI] [PubMed] [Google Scholar]
- [233].Zhou J, Tiemann K, Chomchan P, Alluin J, Swiderski P, Burnett J, Zhang X, Forman S, Chen R, Rossi J, Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells, Nucleic Acids Res. 41 (2013) 4266–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wang H, Marsters SA, Baker T, Chan B, Lee WP, Fu L, Tumas D, Yan M, Dixit VM, Ashkenazi A, Grewal IS, TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice, Nat. Immunol 2 (2001) 632–637. [DOI] [PubMed] [Google Scholar]
- [235].Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH, TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease, Nature 404 (2000) 995–999. [DOI] [PubMed] [Google Scholar]
- [236].Liu Y, Zhang L, Wu Y, Tong T, Zhao W, Li P, Huang M, Wang W, Fang J, Wei W, Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice, Eur. J. Pharmacol 654 (2011) 304–314. [DOI] [PubMed] [Google Scholar]
- [237].Kaegi C, Steiner UC, Wuest B, Crowley C, Boyman O, Systematic review of safety and efficacy of atacicept in treating immune-mediated disorders, Front. Immunol 11 (2020) 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Hartung HP, Kieseier BC, Atacicept: targeting B cells in multiple sclerosis, Ther. Adv. Neurol. Disord 3 (2010) 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Tai Y-T, Lin L, Xing L, Cho S-F, Yu T, Acharya C, Wen K, Hsieh PA, Dulos J, Van Elsas A, Munshi N, Richardson P, Anderson KC, APRIL signaling via TACI mediates immunosuppression by T regulatory cells in multiple myeloma: therapeutic implications, Leukemia 33 (2019) 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lee L, Draper B, Chaplin N, Philip B, Chin M, Galas-Filipowicz D, Onuoha S, Thomas S, Baldan V, Bughda R, Maciocia P, Kokalaki E, Neves MP, Patel D, Rodriguez-Justo M, Francis J, Yong K, Pule M, An APRIL-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma, Blood 131 (2018) 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Schmidts A, Ormhøj M, Choi BD, Taylor AO, Bouffard AA, Scarfò I, Larson RC, Frigault MJ, Gallagher K, Castano AP, Riley LS, Cabral ML, Boroughs AC, Velasco Cárdenas RMH, Schamel W, Zhou J, Mackay S, Tai Y-T, Anderson KC, Maus MV, Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma, Blood Adv. 3 (2019) 3248–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Xu S, Lam K-P, Transmembrane activator and CAML interactor (TACI): another potential target for immunotherapy of multiple myeloma? Cancers 12 (2020) 1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Azuma M, Co-signal molecules in T-cell activation, Adv. Exp. Med. Biol 1189 (2019) 3–23. [DOI] [PubMed] [Google Scholar]
- [244].Waldman AD, Fritz JM, Lenardo MJ, A guide to cancer immunotherapy: from T cell basic science to clinical practice, Nat. Rev. Immunol 20 (2020) 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL, CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms, Mol. Cell. Biol 25 (2005) 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Patsoukis N, Sari D, Boussiotis VA, PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A, Cell Cycle 11 (2012) 4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW, CD28-deficient mice are highly resistant to collagen-induced arthritis, J. Immunol 162 (1999) 203–208. [PubMed] [Google Scholar]
- [248].Bhatia S, Sun K, Almo SC, Nathenson SG, Hodes RJ, Dynamic equilibrium of B7–1 dimers and monomers differentially affects immunological synapse formation and T cell activation in response to TCR/CD28 stimulation, J. Immunol 184 (2010) 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Korhonen R, Moilanen E, Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis, Basic Clin. Pharmacol. Toxicol 104 (2009) 276–284. [DOI] [PubMed] [Google Scholar]
- [250].Bîrsan T, Hausen B, Higgins JP, Hubble RW, Klupp J, Stalder M, Celniker A, Friedrich S, O’Hara RM, Morris RE, Treatment with humanized monoclonal antibodies against CD80 and CD86 combined with sirolimus prolongs renal allograft survival in cynomolgus monkeys, Transplantation 75 (2003) 2106–2113. [DOI] [PubMed] [Google Scholar]
- [251].Jia J, Zhang Y, Xin Y, Jiang C, Yan B, Zhai S, Interactions between nanoparticles and dendritic cells: from the perspective of cancer immunotherapy, Front. Oncol 8 (2018) 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Vonderheide RH, CD40 agonist antibodies in cancer immunotherapy, Annu. Rev. Med 71 (2020) 47–58. [DOI] [PubMed] [Google Scholar]
- [253].Ara A, Ahmed KA, Xiang J, Multiple effects of CD40-CD40L axis in immunity against infection and cancer, Immunotargets Ther. 7 (2018) 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Pardoll DM, Spinning molecular immunology into successful immunotherapy, Nat. Rev. Immunol 2 (2002) 227–238. [DOI] [PubMed] [Google Scholar]
- [255].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH, CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans, Science 331 (2011) 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ, Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody, J. Clin. Oncol 25 (2007) 876–883. [DOI] [PubMed] [Google Scholar]
- [257].Mauri C, Mars LT, Londei M, Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process, Nat. Med 6 (2000) 673–679. [DOI] [PubMed] [Google Scholar]
- [258].Barr TA, McCormick AL, Carlring J, Heath AW, A potent adjuvant effect of CD40 antibody attached to antigen, Immunology 109 (2003) 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ, Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody, Adv. Mater 24 (2012) 3981–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Rosalia RA, Cruz LJ, Van Duikeren S, Tromp AT, Silva AL, Jiskoot W, De Gruijl T, Löwik C, Oostendorp J, Van Der Burg SH, Ossendorp F, CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses, Biomaterials 40 (2015) 88–97. [DOI] [PubMed] [Google Scholar]
- [261].Lutgens E, Lievens D, Beckers L, Donners M, Daemen M, CD40 and its ligand in atherosclerosis, Trends Cardiovasc. Med 17 (2007) 118–123. [DOI] [PubMed] [Google Scholar]
- [262].Seijkens TTP, Van Tiel CM, Kusters PJH, Atzler D, Soehnlein O, Zarzycka B, Aarts SABM, Lameijer M, Gijbels MJ, Beckers L, Den Toom M, Slütter B, Kuiper J, Duchene J, Aslani M, Megens RTA, Van ‘T Veer C, Kooij G, Schrijver R, Hoeksema MA, Boon L, Fay F, Tang J, Baxter S, Jongejan A, Moerland PD, Vriend G, Bleijlevens B, Fisher EA, Duivenvoorden R, Gerdes N, De Winther MPJ, Nicolaes GA, Mulder WJM, Weber C, Lutgens E, Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis, J. Am. College Cardiol 71 (2018) 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Wennhold K, Shimabukuro-Vornhagen A, von Berwelt-Baildon M, B cell-based cancer immunotherapy, Transfus. Med. Hemother 46 (2019) 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Han Y, Liu D, Li L, PD-1/PD-L1 pathway: current researches in cancer, Am. J. Cancer Res 10 (2020) 727–742. [PMC free article] [PubMed] [Google Scholar]
- [265].Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH, Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses, Eur. J. Immunol 33 (2003) 2706–2716. [DOI] [PubMed] [Google Scholar]
- [266].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti–PD-1 antibody in cancer, N. Engl. J. Med 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A, Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma, N. Engl. J. Med 369 (2013) 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Brahmer JJR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM, Safety and activity of anti–PD-L1 antibody in patients with advanced cancer, N. Engl. J. Med 366 (2012) 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Chen L, Han X, Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future, J. Clin. Invest 125 (2015) 3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Johansen A, Christensen SJ, Scheie D, Højgaard JLS, Kondziella D, Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies, Neurology 92 (2019) 663–674. [DOI] [PubMed] [Google Scholar]
- [271].Iwai Y, Hamanishi J, Chamoto K, Honjo T, Cancer immunotherapies targeting the PD-1 signaling pathway, J. Biomed. Sci 24 (2017) 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD, Adverse renal effects of immune checkpoint inhibitors: a narrative review, Am. J. Nephrol 45 (2017) 160–169. [DOI] [PubMed] [Google Scholar]
- [273].Ordikhani F, Uehara M, Kasinath V, Dai L, Eskandari SK, Bahmani B, Yonar M, Azzi JR, Haik Y, Sage PT, Murphy GF, Annabi N, Schatton T, Guleria I, Abdi R, Targeting antigen-presenting cells by anti–PD-1 nanoparticles augments antitumor immunity, JCI Insight 3 (2018) e122700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Emami F, Banstola A, Vatanara A, Lee S, Kim JO, Jeong J-H, Yook S, Doxorubicin and anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy, Mol. Pharm 16 (2019) 1184–1199. [DOI] [PubMed] [Google Scholar]
- [275].Du Y, Liang X, Li Y, Sun T, Jin Z, Xue H, Tian J, Nuclear and fluorescent labeled PD-1-liposome-DOX-64Cu/IRDye800CW allows improved breast tumor targeted imaging and therapy, Mol. Pharm 14 (2017) 3978–3986. [DOI] [PubMed] [Google Scholar]
- [276].Chang H-N, Liu B-Y, Qi Y-K, Zhou Y, Chen Y-P, Pan K-M, Li W-W, Zhou X-M, Ma W-W, Fu C-Y, Qi Y-M, Liu L, Gao Y-F, Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy, Ang. Chem. Int. Ed 54 (2015) 11760–11764. [DOI] [PubMed] [Google Scholar]
- [277].Yang J, Zhang R, Radford DC, Kopeček J, FRET-trackable biodegradable HPMA copolymer-epirubicin conjugates for ovarian carcinoma therapy, J. Control. Release 218 (2015) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Li L, Li Y, Yang CH, Radford DC, Wang J, Janát-Amsbury M, Kopeček J, Yang J, Inhibition of immunosuppressive tumors by polymer-assisted inductions of immunogenic cell death and multivalent PD-L1 crosslinking, Adv. Funct. Mater 30 (2020) 1908961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Li L, Wang J, Radford DC, Kopeček J, Yang J, Combination treatment with immunogenic and anti-PD-L1 polymer-drug conjugates of advanced tumors in a transgenic MMTV-PyMT mouse model of breast cancer, J. Control. Release 332 (2021) 652–659; 10.1016/j.jconrel.2021.02.011 [DOI] [PubMed] [Google Scholar]
- [280].Sun Z, Zhang Y, Cao D, Wang X, Yan X, Li H, Huang L, Qu X, Kong C, Qin H, Wang M, Xu W, Liang L, PD-1/PD-L1 pathway and angiogenesis dual recognizable nanoparticles for enhancing chemotherapy of malignant cancer, Drug Deliv. 25 (2018) 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Zhao P, Atanackovic D, Dong S, Yagita H, He X, Chen M, An anti-programmed death-1 antibody (αPD-1) fusion protein that self-assembles into a multivalent and functional αPD-1 nanoparticle, Mol. Pharm 14 (2017) 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ, Stacey KJ, Yarski M, Poh CM, Lee JS, Cooper MA, Rénia L, Richard D, McCarthy JS, Sharpe AH, Wykes MN, Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4 + T Cell immunity, Immunity 45 (2016) 333–345. [DOI] [PubMed] [Google Scholar]
- [283].Zhang Z, Wang Q, Liu Q, Zheng Y, Zheng C, Yi K, Zhao Y, Gu Y, Wang Y, Wang C, Zhao X, Shi L, Kang C, Liu Y, Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy, Adv. Mater 31 (2019) 1905751. [DOI] [PubMed] [Google Scholar]
- [284].Wu Y, Gu W, Li J, Chen C, Xu ZP, Silencing PD-1 and PD-L1 with nanoparticle-delivered small interfering RNA increases cytotoxicity of tumor-infiltrating lymphocytes, Nanomedicine 14 (2019) 955–967. [DOI] [PubMed] [Google Scholar]
- [285].Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, Liu J, Schaap N, van der Voort R, Dolstra H, Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation, Cancer Immunol. Immunother 62 (2013) 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Zheng Z, Sun R, Zhao H-J, Fu D, Zhong H-J, Weng X-Q, Qu B, Zhao Y, Wang L, Zhao W-L, MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+T cells, Mol. Cancer 18 (2019) 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Zhang R, Billingsley MM, Mitchell MJ, Biomaterials for vaccine-based cancer immunotherapy, J. Control. Release 292 (2018) 256–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Casan JML, Wong J, Northcott MJ, Opat S, Anti-CD20 monoclonal antibodies: reviewing a revolution, Hum. Vaccines Immunother 14 (2018) 2820–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Tedder TF, CD19: a promising B cell target for rheumatoid arthritis, Nat. Rev. Rheumatol 5 (2009) 572–577. [DOI] [PubMed] [Google Scholar]
- [290].Frigault MJ, Maus MV, State of the art in CAR T cell therapy for CD19+ B cell malignancies, J. Clin. Invest 130 (2020) 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Geh D, Gordon C, Epratuzumab for the treatment of systemic lupus erythematosus, Exp. Rev. Clin. Immunol 14 (2018) 245–258. [DOI] [PubMed] [Google Scholar]
- [292].Franca R, Favretto D, Granzotto M, Decorti G, Rabusin M, Stocco G, Epratuzumab and blinatumomab as therapeutic antibodies for treatment of pediatric acute lymphoblastic leukemia: current status and future perspectives, Curr. Med. Chem 24 (2017) 1050–1065. [DOI] [PubMed] [Google Scholar]
- [293].Deangelo DJ, Advani AS, Marks DI, Stelljes M, Liedtke M, Stock W, Gökbuget N, Jabbour E, Merchant A, Wang T, Vandendries E, Neuhof A, Kantarjian H, O’Brien S, Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: outcomes by disease burden, Blood Cancer J 10 (2020) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Dhillon S, Moxetumomab Pasudotox: first global approval, Drugs 78 (2018) 1763–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Kim H-O, Development of BTK inhibitors for the treatment of B-cell malignancies, Arch. Pharm. Res 42 (2019) 171–181. [DOI] [PubMed] [Google Scholar]
- [296].Pine PR, Chang B, Schoettler N, Banquerigo ML, Wang S, Lau A, Zhao F, Grossbard EB, Payan DG, Brahn E, Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor, Clin. Immumol 124 (2007) 244–257. [DOI] [PubMed] [Google Scholar]
- [297].Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L, Efremov DG, The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eμ- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling, Blood 116 (2010) 4894–4905. [DOI] [PubMed] [Google Scholar]
- [298].Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X, Targeting PI3K in cancer: mechanisms and advances in clinical trials, Mol. Cancer 18 (2019) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Shah N, Chari A, Scott E, Mezzi K, Usmani SZ, B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches, Leukemia 34 (2020) 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Smulski CR, Eibel H, BAFF and BAFF-receptor in B cell selection and survival, Front. Immunol 9 (2018) 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Millward M, Gan HK, Joshua AM, Kuo JC-Y, Prawira A, Richardson GE, Barbee SD, Inamdar SP, Pierce KL, Qureshi M, Horvath L, FPT155–001: A phase Ia/Ib study of FPT155 (CD80-FC) in patients with advanced solid tumors, J. Clin. Oncol 37(8_suppl) (2019) TPS42–TPS42. [Google Scholar]
- [302].Van Broekhoven CL, Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy, Cancer Res. 64 (2004) 4357–4365. [DOI] [PubMed] [Google Scholar]
- [303].Hassan HAFM, Smyth L, Wang JTW, Costa PM, Ratnasothy K, Diebold SS, Lombardi G, Al-Jamal KT, Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy, Biomaterials 104 (2016) 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB, Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence, Cancers 12 (2020) 738. [DOI] [PMC free article] [PubMed] [Google Scholar]


