Abstract
Objective:
Macrophage to foam cell transition and their accumulation in the arterial intima are the key events that trigger atherosclerosis, a multifactorial inflammatory disease. Previous studies have linked arterial stiffness and cardiovascular disease and have highlighted the use of arterial stiffness as a potential early-stage marker. Yet the relationship between arterial stiffness and atherosclerosis in terms of macrophage function is poorly understood. Thus, it is pertinent to understand the mechanobiology of macrophages to clarify their role in plaque advancement.
Approach and Results:
We explore how substrate stiffness affects proliferation of macrophages and foam cells, traction forces exerted by macrophages and uptake of native and oxidized low-density lipoproteins. We demonstrate that stiffness influences foam cell proliferation under both naïve and inflammatory conditions. Naïve foam cells proliferated faster on the 4 kPa polyacrylamide gel and glass whereas under inflammatory conditions, maximum proliferation was recorded on glass. Macrophage and foam cell traction forces were positively correlated to the substrate stiffness. Furthermore, the influence of stiffness was demonstrated on the uptake of lipoproteins on macrophages treated with lipopolysaccharide + interferon gamma. Cells on softer 1 kPa substrates had a significantly higher uptake of low-density lipoproteins and oxidized low-density lipoproteins compared to stiffer substrates.
Conclusion:
The results herein indicate that macrophage function is modulated by stiffness and help better understand ways in which macrophages and foam cells could contribute to the development and progression of atherosclerotic plaque.
Keywords: Stiffness, proliferation, cell area, traction forces, low density lipoprotein
Introduction
Despite recent advances, cardiovascular diseases (CVDs) continue to be the number one cause of mortality, accounting for 31% of worldwide deaths[1]. While cardiovascular disease is an umbrella term used to describe a myriad of conditions such as coronary artery disease, hypertension, and myocardial infarction[2], the common underlying cause is a chronic inflammatory disease called atherosclerosis. Atherosclerosis is a focal, multifactorial disease that is characterized by the retention of low-density lipoproteins (LDL) in the arterial intima. Oxidative modifications of the LDL trigger an inflammatory response that leads to the recruitment of monocyte-derived macrophages, which proliferate and internalize oxidized LDL (oxLDL), forming lipid laden foam cells[3]. While the functional role of recruited macrophages is lipid clearance, this beneficial process is rendered maladaptive, since the transition of macrophages to foam cells prevent further critical immune function from these cells[4]. Formation and retention of such foam cells in the arterial intima is a hallmark feature of atherosclerotic lesions and contributes directly to inflammation and plaque progression.
The advent of advanced diagnostic technology together with detailed epidemiological and clinical studies have highlighted the importance of alterations in vascular mechanics as a biomarker for disease progression in atherosclerosis[5]. Multiple reports suggest alterations in arterial stiffness, in both human and animal models, lead to an increased incidence of atherosclerotic disease and point to pathological changes in the arterial wall[6]–[11]. Macrophages are modulated by the surrounding matrix stiffness, causing changes to phenotype and function in vitro and in vivo, and have been previously shown to undergo changes in polarization[12], cell adhesion[13], phagocytosis[14], and migration[15]. Since macrophages are susceptible to varying tissue stiffness and actively respond to alterations in the environment, vascular stiffness may play a role in directly modulating the form and function of macrophages.
Several studies have investigated the role of substrate stiffness in modulating macrophage activity leveraging macrophages cultured on synthetic substrates in the form of polyacrylamide (PA) gels of varying stiffness. Patel et al. (2012) cultured murine derived RAW264.7 and human derived U937 macrophage-like cells on 0.3 – 76.8 kPa gels and assessed the phagocytotic activity of both opsonized and IgG opsonized latex beads in the presence or absence of exogenously administered inflammatory molecules such as lipopolysaccharide (LPS) and interferon gamma (INF-γ)[16]. Similarly, Goswami et al. (2017) reported on the uptake of oxLDL of cultured RAW264.7 murine macrophages on 0.5 – 8 kPa[17]. Both inflamed and non-treated studies found that macrophages were primed on stiffer substrates and induced greater uptake of oxLDL and increased phagocytotic activity of beads on stiffer substrates. Conversely, Sridharan et al. (2019) reported remarkably low levels of phagocytotic activity of THP-1 monocyte cells on stiffer substrates (323 kPa), an effect not present on soft-medium gels (11–88 kPa)[18]. Taken together, these data suggest that the effect of substrate stiffness on macrophage activity remains inconsistent.
In order to recapitulate key physiological function and phenotype of cellular processes in vitro, the cell model is an important factor. Detailed transcriptomic and proteomic profiling of differing sources of macrophages have revealed significant differential gene expression when human-derived macrophages are compared to murine-derived RAW 264.7 and THP-1 monocytic cell line[19]. Additionally, the overlap between the conserved mRNA and protein signatures across murine and human-derived macrophage tissue is extremely low, with only 231 genes shared between the species out of 489 genes in human macrophages and 459 genes in murine and, out of 977 and 1038 genes detected on human and murine, only 513 proteins were detected in both species[20]. As a result, the use of murine and/or macrophage-like human cell lines may not fully recapitulate the effect of stiffness due to a lack of complete genetic and/or proteomic repertoire and may indicate the lack of consistent macrophage function in vitro present in prior reports.
In the present study, we aimed to reconcile such differences by culturing human peripheral blood monocyte derived macrophages to elucidate the effect of matrix stiffness on various critical macrophage function in the context of atherosclerotic disease progression. We evaluated the effect of substrate stiffness on the proliferation capacity of macrophages on PA gels tuned to physiological ranges encountered by macrophages in vivo. Next, we assessed traction forces exerted by macrophages and foam cells on a range of matrix stiffnesses. Lastly, we report on the role of matrix stiffness on the uptake of oxLDL, a key event in the formation of foam cells which lead to the formation of plaque in the arterial wall. Our data suggest that human foam cell proliferation, macrophage and foam cell traction forces, and uptake of oxLDL by human macrophages is regulated by biomechanical cues and provides important insights into modelling macrophage function and phenotype in vitro.
2. Materials and Methods
2.1. Primary human macrophages cell culture
Peripheral blood derived primary human macrophages (PHM) were obtained from Celprogen, Torrance, CA, (Cat No. 36070–01) and cultured according to manufacturer’s protocol. Briefly, P3 macrophages were expanded in human macrophage medium (Celprogen, Cat No. M36070–01) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Gaithersburg, MD, Cat No. 26140079) and cultured in tissue culture treated T75 flasks until 75–80% confluency. Following, cells were treated with 0.25% trypsin-EDTA solution (Gibco, Gaithersburg, MD, Cat No. 25300062), isolated via centrifugation (1200 RPM for 5 min) and resuspended in fresh complete medium. A total of 5000 – 20000 viable cells were counted using a hemocytometer and plated on polyacrylamide (PA) gel multi-well plates (Matrigen, LLC) pretreated with 0.1 mg/ml fibronectin to aid cellular adhesion. In all experimental cases, cells were either maintained in complete media for vehicle/non-treated conditions or pretreated with 10 ng/ml lipopolysaccharide (LPS) (Invivogen, San Diego, CA, Cat No. NC0202558) and 20 ng/ml interferon-γ (INF-γ) (Invitrogen, Waltham, MA, Cat No. RHIFN-G CF 100 UG) for 24hrs for treated conditions. In all cases, cells were maintained at 37°C, 5% CO2, and 95% humidity, and media was exchanged every 48 hours. To visualize cell nuclei, cells were fixed in 4% paraformaldehyde at room temperature (RT) for 20 minutes. Following fixation, 1 μg/ml DAPI, reconstituted in ice cold phosphate buffer saline (PBS) was added and allowed to incubate at RT for 5 minutes. Following incubation, a triple wash was performed with PBS and cells were visualized using epifluorescence imaging. Images were acquired at 20X using an Olympus microscope (model IX83) with wavelength specific filters for DAPI (nuclei) and TRITC (native LDL/oxLDL). Unless otherwise stated, all phase contrast imaging was carried out at 20X magnification.
2.2. Foam cell culture
500,000 PHMs were seeded in a T25 flask and cultured in human macrophage medium (Celprogen, Cat No. M36070–01) supplemented with 10% Fetal Bovine Serum (FBS). Macrophages were allowed to adhere for 4 hours and following which, 50 μg/ml of DiL-oxLDL was exogenously added to the media. Cells were maintained at 37°C, 5% CO2, and 95% humidity for 72 hours to allow PHMs to transition to foam cells. Following the 72-hour incubation period, foam cells were treated with 0.25% trypsin-EDTA solution, isolated via centrifugation (1200 RPM for 5 min) and resuspended in 10 ml complete medium. Cells were counted using a hemocytometer and 5000 – 20000 viable cells were plated on polyacrylamide (PA) gel multi-well plates.
2.3. In vitro proliferation Assay
To assess the proliferation of PHMs and presumptive foam cells in response to varying substrate stiffness, cells were counted using flow cytometry. Both macrophages and foam cells were plated at a density of 5,000–20,000/well on fibronectin coated glass bottom 24 well plates (Cellvis, Cat No. NC0397150) or PA gels (Matrigen, Cat No. SW24-EC-1) of varied stiffness (1 kPa, 4 kPa, and 8kPa). For inflammatory experimental conditions, media was supplemented with 10 ng/ml LPS + 20 ng/ml INF-γ or vehicle (double deionized water) and cells were pretreated for 24 hours before the assay. Cells were allowed to proliferate in complete medium up to 96 hours. Cells were assayed every 24 hours using flow cytometry (refer section 2.7).
2.4. In vitro traction force measurement of PHMs
PHMs were plated on 0.1 mg/ml fibronectin coated PA gels of stiffness 1kPa - 50kPa, purchased from Matrigen, LLC, Irvine, CA, Cat no. SV3520-EC-ST1YG, at a density of 1,000 cells/gel. Using an Olympus microscope at 20x magnification, a location with a single cell was chosen. A phase contrast image was taken of the cell, and a GFP fluorescent image was taken of the beads. The cell was then trypsinized, and a second set of phase contrast and fluorescent images were taken. The bead displacements and tangential stresses were measured using a custom particle image velocimetry (PIV) MATLAB script (generously provided by Dr. Adam J Engler, University of California, San Diego) [21].
2.5. Uptake of native LDL and ox-LDL
To determine the uptake of native LDL (nLDL) (Kalen Biomedical, LLC, Germantown, MD, Cat no. 770230–9) and oxLDL (Kalen Biomedical, Germantown, MD, LLC, Cat no. 770262–9) by PHMs in the presence or absence of inflammatory mediators, 20,000 cells/well were seeded on pretreated PA gels of stiffness ranging from 1kPa – 8 kPa and glass (control). For experimental conditions exposed to inflammatory mediators, cells were incubated with complete medium supplemented with 10 ng/ml LPS + 20 ng/ml INF-γ and cells were pretreated for 24 hours before the assay. After initial exposure to inflammatory mediators, cells were treated with 20 μg/ml nLDL or oxLDL and incubated for additional 96 hours. To assess the formation of foam cells in vitro, cells were assayed every 24 hours via flow cytometry (refer section 2.7).
2.6. Flow cytometry and Data Analysis
For the cell proliferation assays, the cells were trypsinized carefully using 0.25%Trypsin-EDTA, centrifuged at 1200 RPM for 5mins and the pellet was resuspended in 200μl of fresh complete medium at each time point. The cells were analyzed by BD Fortessa (BD Biosciences) to obtain the cell counts. For the lipoprotein uptake studies, macrophages were incubated with nLDL and oxLDL at 37°C. At each time point, the cells were trypsinized, pelleted and resuspended in fresh medium before analyzing them by flow cytometry (BD Fortessa). The data was analyzed using FlowJo software v.10. The populations were gated to omit dead and apoptotic cells and live population cell counts, median forward scatter and medium front scatter data was obtained. All experiments were conducted at least 3 times.
2.7. Statistical Analysis
Unless otherwise stated, all data are represented as mean ± standard error of the mean (SEM). Comparisons between multiple groups were performed using a two- way ANOVA followed by a post-hoc Tukey’s test. Prior to implementation of statistical tests, a normality test was performed on the data sets using the Shapiro-Wilk test. In all cases, we found that the data are normally distributed (Shapiro-Wilk test, p > 0.05). Comparisons between two groups were performed using the Student t-test. In all cases, p-values ≤ 0.05 were considered as statistically significant. All statistical tests were performed in GraphPad Prism (V8).
3. Results
3.1. Uptake profile of oxLDL by in vitro preparation of cultured naïve human macrophages.
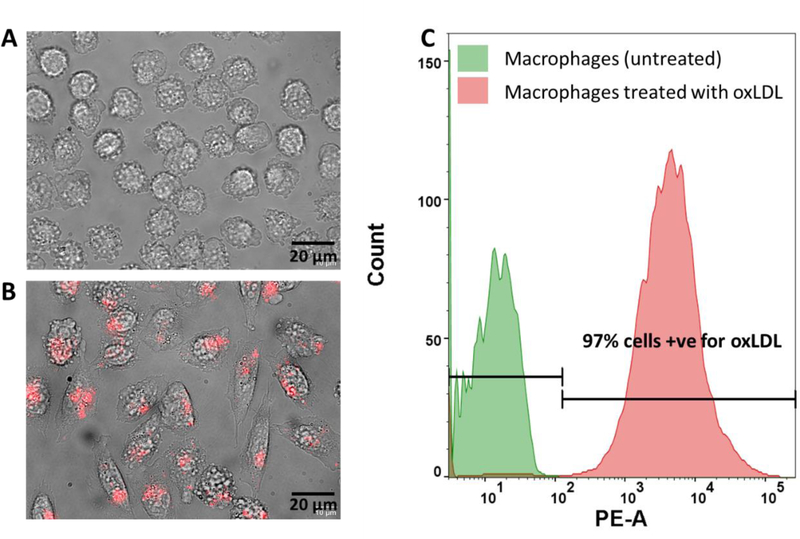
In order to model a physiologically relevant paradigm of macrophage function in vitro, peripheral blood derived human macrophages were used to study foam cell formation. While prior studies have focused on cellular models ranging from murine-derived sources and macrophage-like tumorigenic cell lines which may not recapitulate key pathophysiological events relevant to the uptake of lipoproteins, we hypothesized that the use of a human-based, more physiologically relevant system will lead to improved modelling of the in vivo scenario. Therefore, PHM’s were cultured for 4 days in vitro, assessed for viability (>97%, Figure S1), and seeded at a density of ~20,000 cells/well on glass bottom 24-well plates. Cells were exposed to fluorescently tagged oxLDL (DiL-oxLDL) for 72 hours in vitro and uptake profiles were measured as a function of available DiL fluorescence signal in the cytosol via flow cytometry. As is seen in (Figure 1 A), phase contract imaging revealed that PHM cultures formed focal adhesions and assumed a circular morphology. Interestingly, exposure to DiL-oxLDL induced a morphological change in cellular shape, wherein, cells were more spread and polarized (Figure 1B). Post exposure to 50 μg/ml of DiL-oxLDL for 72 hours in vitro, we observed 97% of PHMs developed into lipid laden foam cells, using fluorescence as a proxy to functional cellular uptake of lipoproteins. (Figure 1C). Interestingly, the cell volume and granularity decreased when PHMs transition to foam cells (Figure S2), yet the cell area of foam cells increased by 56% compared to naïve macrophages (n=300 cells/condition, p < 0.0001 by Student t test). We observed no significant change in the viability of the cells treated with oxLDL and the viability was over 90% in all cases. Taken together, the results suggest that PHMs can be successfully cultured at high viability and display uptake of ox-LDL in vitro, warranting further investigation of key events in macrophage form and function.
Figure 1.
Uptake profile of oxLDL by in vitro preparations of cultured naïve human macrophages on polyacrylamide gels. (A) (top panel) Representative phase image of day in vitro (DIV) 3 human macrophages grown on polyacrylamide (PA) gels under normal growth conditions. (B) Human macrophages post-treatment with 50 μg/ml Dil-oxLDL for 72 hours. (C) Flow cytometry analysis of naïve (left) and Dil-oxLDL (right) treated macrophages in terms of fluorescence intensity as a function of cell count. Bars represent percentage of the population positively identified as lipid laden foam cells post-treatment with ox-LDL.
3.2. Effects of substrate stiffness on proliferation of macrophages and foam cells under naïve and LPS + INF-γ stimulated conditions
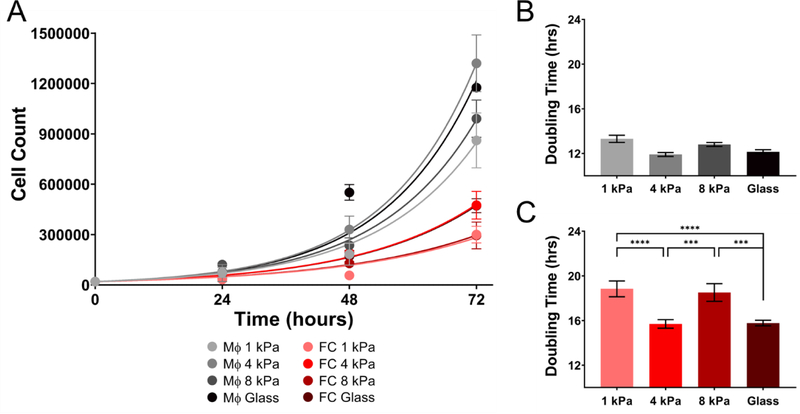
Macrophages are the key population known to play a critical role in early-stage atherosclerosis. Implications of arterial stiffness in atherosclerotic plaque progression have previously been demonstrated. Therefore, we investigated the role of substrate stiffness on proliferation of primary human macrophages and oxLDL induced foam cells. Macrophages and foam cells were seeded on PA gels of stiffness range 1 kPa – 8 kPa and glass. Briefly, PHMs and foam cells were cultured for 4 days on varying stiffness and cell counts were quantified every 24 hours by flow cytometry. As shown in Figure 2A, the growth rates of macrophages were significantly higher than foam cells on all stiffnesses assayed. While the doubling times acquired for macrophages on 1 kPa, 4 kPa, 8 kPa and glass (13.312 ± 1.122, 11.91 ± 0.61, 12.8 ± 0.616 and 12.148 ± 0.65 hours) (mean ± SEM, n=3) indicated that stiffness did not play a role on proliferation of naïve macrophages (Two-way ANOVA, p>0.05) (Figure 2B), foam cells cultured on 1 kPa and 8 kPa (doubling times: 18.84 ± 2.45 and 18.51 ± 2.73 hours) gels proliferated significantly slower than those on 4 kPa and glass (15.7 ± 1.32 and 15.78 ± 0.864 hours) (mean ± SEM, n=3) ( Two-way ANOVA, P > 0.05) (Figure 2C). These results suggest that foam cells are less proliferative and more preferrentially mechanosensitive to specific matrix stifnesses when compared to macrophages.
Figure 2.
Proliferation of human macrophages under naïve condition is higher than presumptive foam cells. (A) Proliferation of naïve macrophages and foam cells assayed at discreet time points from 24–72 hours under varying substrate stiffness (1 kPa, 4 kPa, 8 kPa, and glass) and (B) Doubling times for macrophages on various stiffnesses. (C) Doubling time for foam cells on various stiffnesses. Mean ± SEM, *** p < 0.001, **** p < 0.0001 Two-way ANOVA with Tukey Post-test. n=3
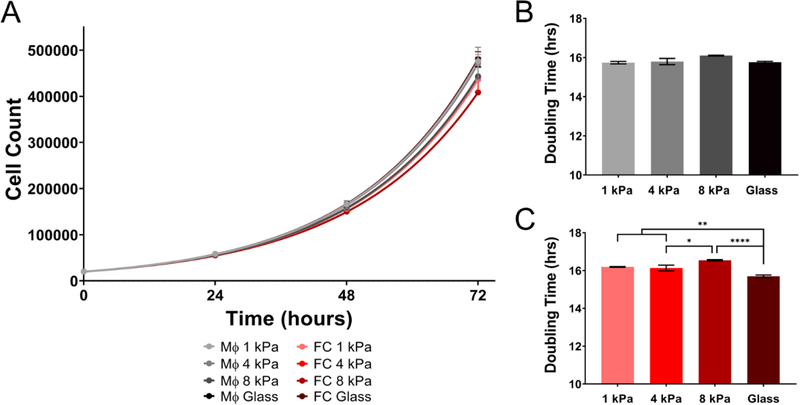
To assess the effect of inflammation on the proliferative capacity of macrophages and foam cells cultured on varying substrate stiffness, cells were pretreated with 10ng/ml LPS + 20ng/ml INF-γ for 24 hours prior to seeding on PA gels of varying stiffness (1 – 8 kPa and glass). Under inflammatory conditions the differential growth rates between cell types was abolished, as macrophages had a doubling time of (15.74 ± 0.22, 15.79 ± 0.55, 16.01 ± 0.06 and 15.76 ± 0.164 hours) on 1, 4, 8 kPa and glass, compared to foam cells (16.189 ± 0.074, 16.13 ± 0.524, 16.54 ± 0.11 and 15.69 ± 0.24 hours) (Two-way ANOVA and p > 0.05) (mean ± SEM, n=3) (Figure 3A). Similar to macrophages cultured under naïve conditions, stiffness did not modulate proliferation rates of macrophages under inflammatory conditions (Figure 3B). However, foam cells cultured on glass proliferated significantly faster than those on 1, 4, and 8 kPa gels (Two-way ANOVA and p < 0.05). Taken together, naïve macrophages proliferate faster than naïve foam cells (average doubling time for macrophages 12.54 ± 0.55 hours and foam cells 17.20 ± 1.47 hours), whereas inflammation (i.e., LPS + INF-γ) synchronized the proliferation rates of macrophages and foam cells (average doubling time for macrophages 15.85 ± 0.14 hours and foam cells 16.14 ± 0.3 hours). The mechanosensitive capabilities were only observed in activated foam cells and in both naïve and inflamed conditions, foam cells displayed increased proliferation on stiffer substrates.
Figure 3.
Proliferation rates between macrophages and foam cells are alike under inflammation. (A) Proliferation of macrophages and foam cells assayed at discreet time points from 24–72 hours under varying substrate stiffness (1 kPa, 4 kPa, 8 kPa, and glass). (B) Doubling time of macrophages. (C) Doubling time of foam cells. Mean ± SEM, * p < 0.1, ** p < 0.01, **** p < 0.0001 Two-way ANOVA with Tukey Post-test. n=3
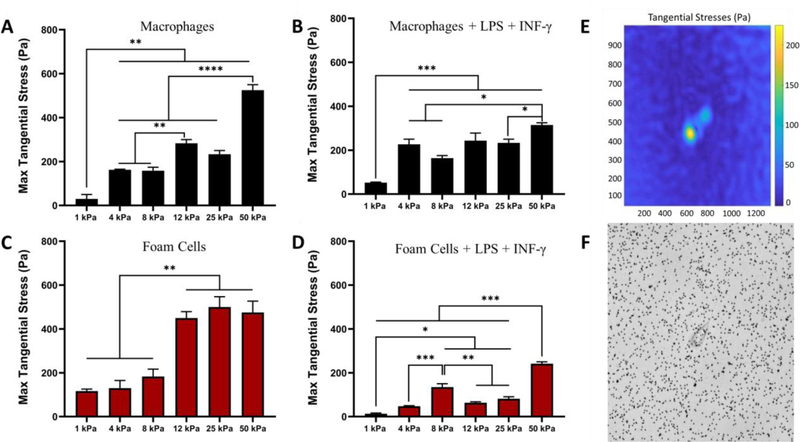
3.3. Traction forces of primary human macrophages are biphasic with gel stiffness.
To further quantify the effects of stiffness on macrophage behaviour, the forces exerted by the cells were quantified using traction force microscopy (TFM). We investigated the forces applied by macrophages and foam cells treated with or without LPS + INF-γ, on a stiffness range of 1 kPa to 50 kPa PA gels embedded with 1μm fluorescent beads. TFM analysis indicated that traction forces increase with increasing stiffness (Figure 4). Similar traction forces were observed between naive macrophages (Figure 4A) and naive foam cells (Figure 4C). Naive macrophages showed maximum tangential stress of 525 ± 17.67 pascals on 50 kPa substrates and naive foam cells 475 ± 42.49 pascals on 50 kPa substrates. When treated with LPS + INF-γ, traction forces of both macrophages and foam cells was significantly reduced (Figure 4B, D). At 50 kPa stiffness, macrophages under inflammatory conditions had maximum tangential stress of 315 ± 8.90 pascals and foam cells, 241 ± 6.8 pascals (mean ± SEM, n=3 to 5). Foam cells treated with LPS + INF-γ showed the lowest traction forces compared to all conditions.
Figure 4.
Traction Force Microscopy (TFM) shows traction forces increase with stiffness.
(A) Maximum tangential stress in pascals of naïve macrophages on substrate stiffness 1– 50 kPa. (B) Maximum tangential stress in pascals macrophages treated with inflammatory cytokines. (C) Max tangential stress in pascals of foam cells and (D) Max tangential stress in pascals of foam cells under inflammation. (E) and (F) Representative tangential stress heat map and phase image of cell on PA gels with fluorescent beads embedded. Mean ± SEM, * p < 0.1, ** p < 0.01, *** p < 0.001, **** p < 0.0001 One-way ANOVA with Tukey Post-test. n=3 to 5
3.4. Stiffness dependent uptake of lipoproteins in macrophage to foam cell transition
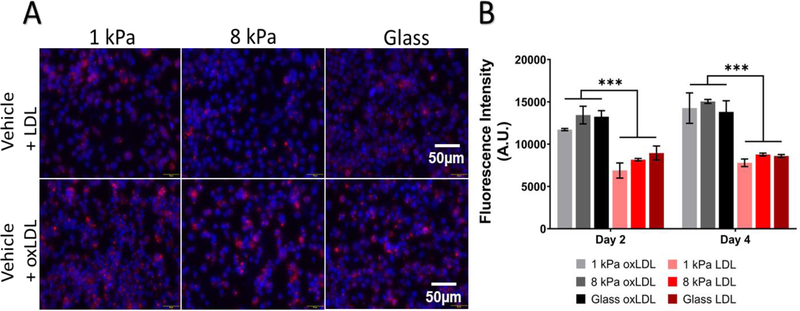
Lastly, we investigated the effect of stiffness on native and oxidized LDL uptake during macrophage to foam cell transition. 20μg/ml DiL-LDL or DiL-oxLDL was exogenously added to macrophages cultured on PA gels. Cells were trypsinized and analyzed by flow cytometry for lipoprotein content every 24 hours up to 3 days. The average median fluorescence intensities (average MFI) of oxLDL and LDL on all substrates on day 4 were 14373.89 ± 511.28 and 8386 ± 422.971 (mean ± SEM, n=3) respectively. Irrespective of substrate stiffness, we observed that macrophages showed preferential uptake of oxLDL compared to native LDL (unpaired Student’s T test, p< 0.0001) (Figure 5B). While uptake of oxLDL was not mediated by stiffness, macrophages on 8 kPa and glass had significantly higher amount of LDL at the 48-hour timepoint compared to those on 1 kPa gels (1 kPa: 6880.333 ± 895.666, 8 kPa: 8154.667 ± 150.568 and glass: 8949 ± 837.565) (Two-way ANOVA and p < 0.01) but this effect was not observed at day 4. The results indicate that under naïve conditions, stiffness dose not influence the uptake mechanisms of lipoproteins. In addition, PHMs displayed higher preference for oxLDL compared to native LDL.
Figure 5.
Human Macrophages preferentially uptake more oxLDL than LDL in vitro. (A) Top panel: Blue: DAPI, Red: Dil-oxLDL. uptake of LDL by macrophages exposed to 20 μg/ml Dil-LDL for 48 hours on 1 kPa, 8 kPa, and glass (left to right). Bottom panel: uptake of oxLDL by DIV2 macrophages exposed to 20 μg/ml Dil-oxLDL for 48 hours on 1 kPa, 8 kPa, and glass (left to right). (B) Summarized average median fluorescence intensity (A.U.) from DIV 0–4 on varying substrate stiffness on LDL uptake and oxLDL. Average MFI ± SEM, *** p < 0.001, unpaired Student’s T test, p < 0.0001. n=3
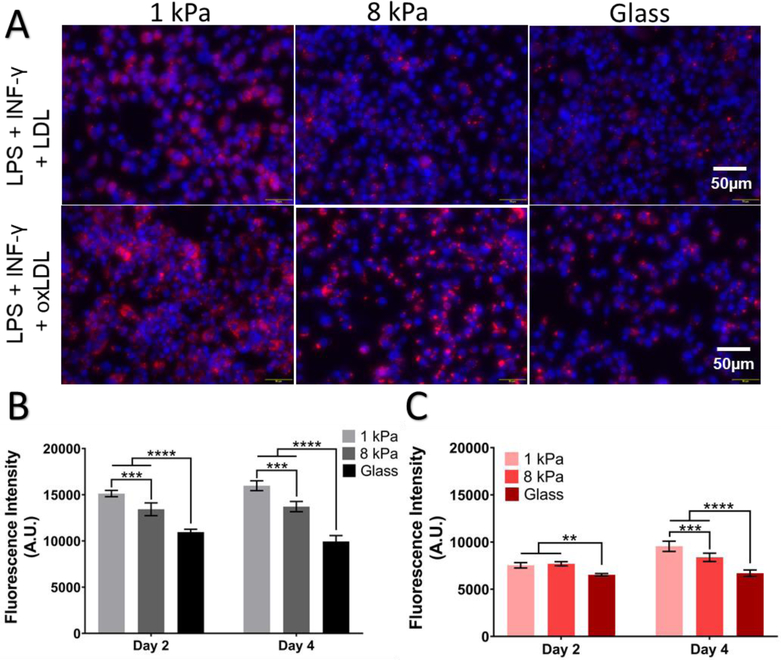
Furthermore, we assessed the effects of stiffness on lipoprotein uptake of macrophages under inflammatory conditions. Prior to the addition of LDL or oxLDL, cells were pretreated with 10ng/ml LPS + 20ng/ml INF-γ for 24 hours. The average MFI of oxLDL on 1 kPa, 8 kPa and glass was observed to be 15976.33 ± 536.2004, 13717 ± 552.4225 and 9946 ± 638.4862 and LDL 9550.667 ± 545.762, 8379.667 ± 440.586 and 6698.333 ± 338.641 (mean ± SEM, n=3). In the presence of inflammatory mediators, we observed uptake of both native LDL and oxLDL (Figure 6) was modulated by stiffness and in all experimental conditions, macrophages showed increased uptake of lipoproteins on softer 1 kPa gels compared to the stiffer conditions (Two-way ANOVA and p < 0.001). Altogether, these results strongly suggest a key role of inflammatory mediators in modulating the effect of matrix stiffness on lipoprotein uptake.
Figure 6.
Substrate stiffness modulates LDL and oxLDL uptake under inflammatory conditions. (A) Top panel: Blue: DAPI, Red: Dil-oxLDL. Uptake of LDL by macrophages exposed to 20 μg/ml Dil-LDL and a combination of 10 ng/ml LPS and 20 ng/ml INF-γ at 48 hours time points on 1 kPa, 8 kPa, and glass (left to right). Bottom panel: uptake of oxLDL by DIV2 macrophages exposed to 20 μg/ml Dil-oxLDL and a combination of 10 ng/ml LPS and 20 ng/ml INF-γ at 48 hours time points on 1 kPa, 8 kPa, and glass (left to right). Summarized average median fluorescence intensity (A.U.) at 48 hour and 96-hour time point on varying substrate stiffness on (B) oxLDL uptake and (C) LDL uptake. Average MFI ± SEM, ** p < 0.01, *** p < 0.001, **** P < 0.0001 Two-way ANOVA with Tukey Post-test. n=3
4. Discussion
Numerous studies have highlighted that arterial stiffness measurements could be used as a novel cardiovascular disease risk factor[22]–[25] but, the role of stiffness on key events such as macrophage to foam cell transition during atherosclerosis is yet to be fully understood. In vivo studies have been crucial to understanding the role of stiffness in vascular diseases, such as understanding the relationship between pulse wave velocity and arterial stiffness[26], [27] to better inform patient health. Although informative, some of the major drawbacks of these studies is that they use disease endpoints as final results and often require prior knowledge of molecular targets and pathways involved. Attempts to produce animal models with altered vascular stiffness have failed to isolate stiffness alone, as a controllable variable and face the challenge of inducing global changes in biochemical properties of the extracellular matrix due to exacerbated inflammatory response, thus making it difficult to interpret results[28],[29]. Thus, in vitro models are important to gain better understanding of the molecular pathways and targets involved in stiffness dependent immune cell functions. To this end, we have developed an in vitro model leveraging peripheral blood derived primary human macrophages to test the effects of stiffness in a physiologically relevant range of 1–8 kPa [30]–[34] and ~70GPa (glass). We also used a lipoprotein concentration that is similar to the concentration found in plasma [35]. In this study we aimed to understand the effects of substrate stiffness on primary human macrophage function in terms of proliferation, forces applied and their transition to foam cells under naïve and inflammatory conditions.
Prior studies have found that macrophage proliferation is a key event in atherosclerotic plaque formation and progression. Robbins et al. 2013 showed that local macrophage proliferation dominated replenishment of immune cells rather than monocyte infiltration in ApoE−/− mouse aortic macrophages[36]. Sakai et al. 2000 and Lamharzi et all. 2004 have demonstrated that the presence of oxLDL causes activation of protein kinase C which influences macrophage proliferation in mouse peritoneal macrophages and glucose oxidized LDL lead to the phosphorylation of protein kinase B/Akt inducing macrophage proliferation in murine cells, respectively[37], [38]. Evidence of stiffness dependent macrophage proliferation has revealed varying results. Experiments conducted by Chen et al. 2020 demonstrated that stiffness did not affect proliferation of murine Raw 264.7 cells that were cultured on low, medium and high stiffness polyacrylamide gels [12]. Conversely, Adlerz et al. 2015 found that human monocyte derived macrophages cultured on softer 13 kPa substrates had significantly slower proliferation rates compared to cells on stiffer 280 kPa gels [15]. Similarly, Scott et al. 2020 found that macrophage proliferation increases with increasing substrate stiffness. While the previous two studies did not evaluate the proliferation of macrophages under stiffness and inflammatory conditions, Scott et al. 2020 did observe that macrophage proliferative capacities were reduced by 1.8 fold when treated with INF-γ and LPS [39]. Thus, we aimed to understand the effects of substrate stiffness on macrophage and foam cell proliferation in our in vitro system. Our results suggest that under both naïve and inflammatory conditions, stiffness does not significantly affect the rate of proliferation of macrophages on PA gels of 1 – 8 kPa and glass, but interestingly, stiffness modulated the rate of proliferation of foam cells under both conditions. Under naïve conditions we observed decreased proliferative capacities of foam cells on 1 kPa and 8 kPa substrates compared to 4 kPa and glass. When inflammatory conditions were added to the cultures, we saw that foam cells on softer 1 – 8 kPa substrates proliferated slower than glass. We also observed that foam cells had lower proliferative capacities compared to naïve macrophages. When macrophages were treated with inflammatory mediators (i.e., LPS + INF-γ) they also displayed lower proliferation tendencies as compared to naïve macrophages. These results suggest that foam cells are more mechanically tuned both in the presence of oxLDL (i.e., naïve foam cells) and inflammation.
The simplest theory follows that macrophage-derived foam cells retain analogous mechanosensation, since presumably ox-LDL uptake may not have any effect on surface receptor expression and therefore show no difference in stiffness-dependent behavior. However, it is important to note that, firstly, the lipid accumulation, proinflammatory activation, and proliferation of macrophages is not straightforward. Spann et al. (2012) has shown via lipodomic and transcriptomic studies in vivo that macrophage-derived foam cells lose expression of proinflammatory factors[40]. Therefore, the cellular function of foam cells may be modulated as a function of lipid uptake and lead to gross genotypic and phenotypic changes which are not conserved across the two cell types. Additionally, prior reports have demonstrated differential expression between macrophages and foam cells. Song et al. (2020) found 167 differentially expressed genes between macrophages and foam cells of which, 102 were significantly upregulated and 65 downregulated[41]. Similarly, Shiffman et al. (2000) demonstrated that 268 genes were 2 fold regulated in ox-LDL treated macrophages compared to control cultures. More importantly, of the 268 genes surveyed, 71 genes known to control cell division and replication were significantly downregulated in foam cells[42]. This could explain why in our study we observe decreased proliferative capacities in naive foam cells as compared to naive macrophages. Additionally, they also observed 127 genes involved in ECM production, ECM modification, cell adhesion and migration. Integrin subunits α2, α5, αX, β3, β5, and β7 were upregulated by 2–3 fold and fibronectin was upregulated 3.8 fold in foam cells. Such changes might inform why we observe more mechanosensing capabilities in foam cells compared to macrophages in our results and could explain why foam cells persist in atherosclerotic lesions and plaque progression.
To the best of our knowledge, no previous study has explored the substrate-mediated effect on foam cell proliferation or compared it to macrophages. Due to the evidence of differential gene expression between macrophage and foam cells, we hypothesize that matrix stiffness may modulate differential responsiveness across macrophages and foam cells, and key regulators of proliferation may be modulated differentially across cell types. Additionally, in vivo models have demonstrated that alterations in vascular stiffness exacerbates the disease. Drew et al. showed proliferating cells in early plaque formation of cholesterol-fed rabbit aortic intima were predominantly macrophage-derived foam cells [43]. Studies quantifying the stiffness of arterial intimal and plaque compositions has shown that lipid rich and foam cell rich areas of the early plaque are in the range of 3–6 kPa [44]–[46]. Our foam cell proliferation results indicate that foam cells (naïve and inflammatory) are more proliferative at 4 kPa and their proliferation slows down when the stiffness is 1kPa and 8 kPa. These results are important in understanding how changes in stiffness during atherosclerosis affect the function of these cells. Increased foam cell proliferation at 4kPa aligns with the in vivo scenario showing that these cells are prominent and proliferative in the early intimal plaque regions where the stiffness is typically in the range of 3–6 kPa [43]–[46].
The traction forces exerted by macrophages is dependent on stiffness of the PA gel substrate. We observed that traction forces of macrophages and foam cells under naïve and inflammatory conditions is positively correlated to the stiffness of the substrate. Numerous prior studies have shown that cell traction forces of different cell types from tumor cells, fibroblasts, stem cells and immune cells, increase with increasing substrate stiffness [47]–[56]. Observation made by Hind et al. 2015 suggest that primary human macrophages exert forces that increase with increase in substrate stiffness [57]. They quantified traction forces of human blood derived macrophages on substrates with modulus ranging from 2.5 kPa to 15 kPa and measured forces exerted by a population of 1 × 104 cells. Additionally, Rougerie et al. 2020 studies the traction forces exerted by bone marrow derived macrophages of mice and found that macrophage average stress linearly correlated with the stiffness of the substrate [58]. Our results agree with prior studies where we see increasing forces with increasing substrate stiffnesses. Additionally, we quantified traction forces of macrophages and foam cells under inflammatory conditions by polarizing the cells to a pro inflammatory phenotype by treating them with LPS and INF-γ. Under induced inflammatory conditions, we observed that traction forces of both macrophages and foam cells decreased compared to the naïve conditions. These results are similar to that of observations made by Hind et al. 2016 where they showed traction stresses exerted by M1 macrophages (treated with LPS and INF-γ) was significantly lesser than M0 (untreated) macrophages.
The role of oxidized LDL in the mechanisms leading to atherosclerosis are well understood. Exposure of macrophages to oxLDL particles leads to an inflammatory response and uptake of these particles and their transformation into lipid laden foam cells which is believed to orchestrate lesion formation and progression of atherosclerosis[59]. We investigated how changes in stiffness affect the rate of uptake of lipoproteins by human macrophages. Without the presence of inflammatory cues, macrophages on all substrates showed similar uptake without any preference to a certain stiffness range. Inflammation is a key physiological characteristic of atherosclerosis and thus, next we sought to understand the lipoprotein uptake properties of macrophages under inflammatory conditions. Interestingly, our results show that in both LDL and oxLDL uptake, macrophages on soft 1 kPa substrates had maximum lipoprotein uptake. The uptake rate reduced along the stiffness gradient. Several studies have reported that oxLDL uptake by macrophages increases with increased stiffness. R. Goswami et al. 2017 demonstrated using murine resident macrophages that uptake of oxLDL was higher on 8 kPa hydrogels compared to 0.5 kPa hydrogels[17]. Another recent study by Li et al. 2020 reported that differentiated human monocytic THP-1 macrophages contained higher lipid contend in cells cultured on stiffer 30 kPa substrates compared to 4 and 13 kPa matrices[60]. While our results are in odds with the above-mentioned studies, the difference could possibly be explained by the genomic and proteomic differences between human, cancerous, and murine cells. Spiller et al. 2015 compared gene expression in commonly used macrophage phenotypes to study in vitro macrophage function[19]. In comparing the expressions of murine bone marrow, human monocytes from peripheral blood, human monocytic cell line THP-1 and iPSC derived macrophages, they found that murine derived macrophages and THP-1 cells were the most dissimilar to human peripheral derived macrophages. Additionally, Goswami et al. 2017 observed a strong link between the mechanosensitive ion channel, TRPV4, and the modulation of oxLDL uptake, an effect which is lost in both pharmacological and/or genetic perturbation of TRPV4. This is further bolstered by in vivo studies [4], where ApoE −/− mice demonstrate uptake of oxLDL despite genetic deletion of canonical receptor-mediated pathways such as SR-A and/or CD36, which have been strongly implicated in uptake mechanisms. Thus, we hypothesize that there must be other mechanisms involved in the uptake of oxLDL, specifically under inflammation. Prior reports [61] have shown in mice deficient in both ApoE and Cav-1 (caveolae protein marker) a 70% reduction in atherosclerotic plaques. The exact mechanisms remain to be tested in our in vitro model.
In conclusion, we find that primary human macrophages and foam cells show increasing traction forces on increasing stiffness and traction forces are reduced under inflammatory conditions. Our findings suggest that inflammation potentiates matrix stiffness mediated uptake of lipoproteins in primary human macrophages on softer substrates. Additionally, we also find that foam cells have a higher mechanosensing capacity to affect their proliferation than macrophages suggesting the importance of matrix stiffness on foam cell behavior during early-stage atherosclerosis.
Supplementary Material
Acknowledgements
The authors thank laboratory members Maziyar Keshavarzian, Alyssa Lamberti, Kaylie Kruppa, Dhivya Addula, Julia Mach for their assistance in imaging and FACS experimentation and analysis. We also thank Jacob Henderson from the Flow cytometry Core for helping with the FACS experiments. Lastly, we are extremely grateful to Dr. Adam J Engler and Jaimie Mayner for providing us with the TimelapseTFMcode for the traction force data analysis.
Sources of Funding
This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) under award number R01HL136776, and American Heart Association (AHA) under award number 17SDG33400239. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHA.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- [1].Benjamin EJ et al. , Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association, vol. 135, no. 10. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mangge H, “Antioxidants, inflammation and cardiovascular disease,” World J. Cardiol, vol. 6, no. 6, p. 462, 2014, doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ross R, “Inflammation or Atherogenesis,” pp. 115–126, 1999. [Google Scholar]
- [4].Moore KJ, Sheedy FJ, and Fisher EA, “Macrophages in atherosclerosis: A dynamic balance,” Nat. Rev. Immunol, vol. 13, no. 10, pp. 709–721, 2013, doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, and London GM, “Carotid arterial stiffness as a predictor of cardiovascular and all- cause mortality in end-stage renal disease,” Hypertension, vol. 32, no. 3, pp. 570–574, 1998, doi: 10.1161/01.HYP.32.3.570. [DOI] [PubMed] [Google Scholar]
- [6].Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, and O’Rourke MF, “Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community,” Circulation, vol. 68, no. 1, pp. 50–58, 1983, doi: 10.1161/01.CIR.68.1.50. [DOI] [PubMed] [Google Scholar]
- [7].London GM et al. , “Cardiac and arterial interactions in end-stage renal disease,” Kidney Int, vol. 50, no. 2, pp. 600–608, 1996, doi: 10.1038/ki.1996.355. [DOI] [PubMed] [Google Scholar]
- [8].Wada T et al. , “Correlation of ultrasound-measured common carotid artery stiffness with pathological findings,” Arterioscler. Thromb, vol. 14, no. 3, pp. 479–482, 1994, doi: 10.1161/01.atv.14.3.479. [DOI] [PubMed] [Google Scholar]
- [9].Gotschy A et al. , “Local arterial stiffening assessed by MRI precedes atherosclerotic plaque formation,” Circ. Cardiovasc. Imaging, vol. 6, no. 6, pp. 916–923, 2013, doi: 10.1161/CIRCIMAGING.113.000611. [DOI] [PubMed] [Google Scholar]
- [10].Hansen L and Taylor WR, “Is increased arterial stiffness a cause or consequence of atherosclerosis?,” Atherosclerosis, vol. 249, pp. 226–227, 2016, doi: 10.1016/j.atherosclerosis.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tedla YG, Yano Y, Carnethon M, and Greenland P, “Association between Long-Term Blood Pressure Variability and 10-Year Progression in Arterial Stiffness,” Hypertension, vol. 69, no. 1, pp. 118–127, 2017, doi: 10.1161/HYPERTENSIONAHA.116.08427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen M et al. , “Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-κB signaling pathway,” Bioact. Mater, vol. 5, no. 4, pp. 880–890, 2020, doi: 10.1016/j.bioactmat.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pelham RJ and Wang YL, “Cell locomotion and focal adhesions are regulated by substrate flexibility,” Proc. Natl. Acad. Sci. U. S. A, vol. 94, no. 25, pp. 13661–13665, 1997, doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okamoto T et al. , “Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression,” Exp. Cell Res, vol. 367, no. 2, pp. 264–273, 2018, doi: 10.1016/j.yexcr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- [15].Adlerz KM, Aranda-Espinoza H, and Hayenga HN, “Substrate elasticity regulates the behavior of human monocyte-derived macrophages,” Eur. Biophys. J, vol. 45, no. 4, pp. 301–309, 2016, doi: 10.1007/s00249-015-1096-8. [DOI] [PubMed] [Google Scholar]
- [16].Patel NR et al. , “Cell Elasticity Determines Macrophage Function,” PLoS One, vol. 7, no. 9, pp. 1–10, 2012, doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goswami R et al. , “TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation,” Free Radic. Biol. Med, vol. 110, no. April, pp. 142–150, 2017, doi: 10.1016/j.freeradbiomed.2017.06.004. [DOI] [PubMed] [Google Scholar]
- [18].Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, and O’Brien FJ, “Material stiffness influences the polarization state, function and migration mode of macrophages,” Acta Biomater, vol. 89, pp. 47–59, 2019, doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- [19].Spiller KL et al. , “Differential gene expression in human, murine, and cell line-derived macrophages upon polarization,” Exp. Cell Res, vol. 347, no. 1, pp. 1–13, 2016, doi: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- [20].Martinez FO et al. , “Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences,” Blood, vol. 121, no. 9, pp. 57–69, 2013, doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- [21].Lo Sardo V et al. , “Unveiling the Role of the Most Impactful Cardiovascular Risk Locus through Haplotype Editing.,” Cell, vol. 175, no. 7, pp. 1796–1810.e20, December. 2018, doi: 10.1016/j.cell.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang AYM, “Cardiovascular risk in diabetic end-stage renal disease patients,” J. Diabetes, vol. 3, no. 2, pp. 119–131, 2011, doi: 10.1111/j.1753-0407.2011.00113.x. [DOI] [PubMed] [Google Scholar]
- [23].Mattace-Raso FUS et al. , “Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study,” Circulation, vol. 113, no. 5, pp. 657–663, 2006, doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- [24].Cecelja M and Chowienczyk P, “Role of arterial stiffness in cardiovascular disease,” JRSM Cardiovasc. Dis, vol. 1, no. 4, pp. 1–10, 2012, doi: 10.1258/cvd.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stehouwer CDA, Henry RMA, and Ferreira I, “Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease,” Diabetologia, vol. 51, no. 4, pp. 527–539, 2008, doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- [26].Wilkinson IB and Mceniery CM, “Annual Scientific Meeting of ASCEPT 2003 ARTERIAL STIFFNESS, ENDOTHELIAL FUNCTION AND NOVEL PHARMACOLOGICAL APPROACHES Ian B Wilkinson and Carmel M McEniery,” Pulse, no. December 2003, pp. 795–799, 2004. [DOI] [PubMed] [Google Scholar]
- [27].Vappou J, Luo J, and Konofagou EE, “Pulse wave imaging for noninvasive and quantitative measurement of arterial stiffness in vivo,” Am. J. Hypertens, vol. 23, no. 4, pp. 393–398, 2010, doi: 10.1038/ajh.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moeller A, Ask K, Warburton D, Gauldie J, and Kolb M, “The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis?,” Int. J. Biochem. Cell Biol, vol. 40, no. 3, pp. 362–382, 2008, doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thumkeo D et al. , “Targeted Disruption of the Mouse Rho-Associated Kinase 2 Gene Results in Intrauterine Growth Retardation and Fetal Death,” Mol. Cell. Biol, vol. 23, no. 14, pp. 5043–5055, 2003, doi: 10.1128/mcb.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].D. G. C.&K. Irwin EHEF, Saha K, Rosenbluth M, Gamble LJ, “Modulus-dependent macrophage adhesion and behavior,” J. Biomater. Sci. Polym. Ed, vol. 19, no. 10, 2008. [DOI] [PubMed] [Google Scholar]
- [31].Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, and Truskey GA, “Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy,” J. Biomech, vol. 34, no. 12, pp. 1545–1553, 2001, doi: 10.1016/S0021-9290(01)00149-X. [DOI] [PubMed] [Google Scholar]
- [32].Sato M, Nagayama K, Kataoka N, Sasaki M, and Hane K, “Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress,” J. Biomech, vol. 33, no. 1, pp. 127–135, 2000, doi: 10.1016/S0021-9290(99)00178-5. [DOI] [PubMed] [Google Scholar]
- [33].Radmacher M, Fritz M, Kacher CM, Cleveland JP, and Hansma PK, “Measuring the viscoelastic properties of human platelets with the atomic force microscope,” Biophys. J, vol. 70, no. 1, pp. 556–567, 1996, doi: 10.1016/S0006-3495(96)79602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, and Discher DE, “Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments,” J. Cell Biol, vol. 166, no. 6, pp. 877–887, 2004, doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Holvoet P et al. , “Identifying Patients With Coronary Artery Disease,” Atheroscler. Thromb. Vasc. Biol, vol. 21, no. Ldl, pp. 844–848, 2001. [DOI] [PubMed] [Google Scholar]
- [36].Robbins CS et al. , “Accumulation in Atherosclerosis,” Nat. Med, vol. 19, no. 9, pp. 1166–1172, 2014, doi: 10.1038/nm.3258.Local. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sakai M, Kobori S, Miyazaki A, and Horiuchi S, “No Title,” Curr. Opin. Lipidol, vol. 11, no. 5, pp. 503–509, 2000. [DOI] [PubMed] [Google Scholar]
- [38].Lamharzi N et al. , “Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized LDL,” Diabetes, vol. 53, no. 12, pp. 3217–3225, 2004, doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- [39].Scott RA, Kiick KL, and Akins RE, “Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages,” Acta Biomater, vol. 122, pp. 220–235, 2021, doi: 10.1016/j.actbio.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Spann NJ et al. , “Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses,” vol. 151, no. 1, pp. 138–152, 2013, doi: 10.1016/j.cell.2012.06.054.Regulated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Song Z et al. , “Identification of foam cell biomarkers by microarray analysis,” BMC Cardiovasc. Disord, vol. 20, no. 1, pp. 1–9, 2020, doi: 10.1186/s12872-020-01495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shiffman D et al. , “Large scale gene expression analysis of cholesterol-loaded macrophages,” J. Biol. Chem, vol. 275, no. 48, pp. 37324–37332, 2000, doi: 10.1074/jbc.M004732200. [DOI] [PubMed] [Google Scholar]
- [43].Drew AF and Tipping PG, “T Helper Cell Infiltration and Foam Cell Proliferation Are Early Events in the Development of Atherosclerosis in Cholesterol-Fed Rabbits,” Arterioscler. Thromb. Vasc. Biol, vol. 15, no. 10, pp. 1563–1568, October. 1995, doi: 10.1161/01.ATV.15.10.1563. [DOI] [PubMed] [Google Scholar]
- [44].Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, and Riou L, “Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy,” J. Struct. Biol, vol. 174, no. 1, pp. 115–123, 2011, doi: 10.1016/j.jsb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- [45].Le Master E, Ahn SJ, and Levitan I, “Mechanisms of endothelial stiffening in dyslipidemia and aging: Oxidized lipids and shear stress,” Curr. Top. Membr, vol. 86, pp. 185–215, 2020, doi: 10.1016/bs.ctm.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Peloquin J, Huynh J, Williams RM, and Reinhart-King CA, “Indentation measurements of the subendothelial matrix in bovine carotid arteries,” J. Biomech, vol. 44, no. 5, pp. 815–821, 2011, doi: 10.1016/j.jbiomech.2010.12.018. [DOI] [PubMed] [Google Scholar]
- [47].McKenzie AJ, Hicks SR, Svec KV, Naughton H, Edmunds ZL, and Howe AK, “The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation,” Sci. Rep, vol. 8, no. 1, pp. 1–21, 2018, doi: 10.1038/s41598-018-25589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Razafiarison T et al. , “Biomaterial surface energy-driven ligand assembly strongly regulates stem cell mechanosensitivity and fate on very soft substrates,” Proc. Natl. Acad. Sci. U. S. A, vol. 115, no. 18, pp. 4631–4636, 2018, doi: 10.1073/pnas.1704543115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kraning-Rush CM, Califano JP, and Reinhart-King CA, “Cellular traction stresses increase with increasing metastatic potential,” PLoS One, vol. 7, no. 2, 2012, doi: 10.1371/journal.pone.0032572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hui KL, Balagopalan L, Samelson LE, and Upadhyaya A, “Cytoskeletal forces during signaling activation in Jurkat T-cells,” Mol. Biol. Cell, vol. 26, no. 4, pp. 685–695, 2015, doi: 10.1091/mbc.E14-03-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhou DW, Lee TT, Weng S, Fu J, and García AJ, “Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions,” Mol. Biol. Cell, vol. 28, no. 14, pp. 1901–1911, 2017, doi: 10.1091/mbc.E17-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mekhdjian AH et al. , “Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix,” Mol. Biol. Cell, vol. 28, no. 11, pp. 1467–1488, 2017, doi: 10.1091/mbc.E16-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hall MS et al. , “Fibrous nonlinear elasticity enables positive Mechanical feedback between cells and ECMs,” Proc. Natl. Acad. Sci. U. S. A, vol. 113, no. 49, pp. 14043–14048, 2016, doi: 10.1073/pnas.1613058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Müller C and Pompe T, “Distinct impacts of substrate elasticity and ligand affinity on traction force evolution,” Soft Matter, vol. 12, no. 1, pp. 272–280, 2015, doi: 10.1039/c5sm01706h. [DOI] [PubMed] [Google Scholar]
- [55].Marinkovic A, Mih JD, Park JA, Liu F, and Tschumperlin DJ, “Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness,” Am. J. Physiol. - Lung Cell. Mol. Physiol, vol. 303, no. 3, 2012, doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Trichet L et al. , “Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness,” Proc. Natl. Acad. Sci. U. S. A, vol. 109, no. 18, pp. 6933–6938, 2012, doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hind LE, Dembo M, and Hammer DA, “Macrophage motility is driven by frontal-towing with a force magnitude dependent on substrate stiffness,” Integr. Biol. (United Kingdom), vol. 7, no. 4, pp. 447–453, 2015, doi: 10.1039/c4ib00260a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rougerie P and Cox D, “Spatio-temporal mapping of mechanical force generated by macrophages during FcγR-dependent phagocytosis reveals adaptation to target stiffness,” bioRxiv, p. 2020.04.14.041335, January. 2020, doi: 10.1101/2020.04.14.041335. [DOI] [Google Scholar]
- [59].Libby P, Ridker PM, and Maseri A, “Inflammation and atherosclerosis,” Circulation, vol. 105, no. 9, pp. 1135–1143, 2002, doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- [60].Li J et al. , “miRNA-mediated macrophage behaviors responding to matrix stiffness and ox-LDL,” J. Cell. Physiol, vol. 235, no. 9, pp. 6139–6153, 2020, doi: 10.1002/jcp.29543. [DOI] [PubMed] [Google Scholar]
- [61].Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, and Lisanti MP, “Genetic Ablation of Caveolin-1 Confers Protection Against Atherosclerosis,” Arterioscler. Thromb. Vasc. Biol, vol. 24, no. 1, pp. 98–105, 2004, doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.