Abstract
Implantable loop recorder (ILR) is recommended to detect subclinical atrial fibrillation (AF) following cryptogenic stroke, but the clinical outcomes of this practice is unclear. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate 12-month AF detection, change in oral anticoagulation (OAC), and recurrent stroke, in ILR versus usual care after ischemic stroke. We searched Medline, Embase, Web of Science, Cochrane Library for randomized controlled trials (RCTs) comparing ILR to usual care following any ischemic stroke. Primary outcomes were cumulative AF detection and recurrent stroke (ischemic or hemorrhagic) or transient ischemic attack (TIA) over 12 months. Secondary outcome was OAC initiation. Meta-analysis was performed with Mantel-Haenszel pooled odds ratios (ORs) and random effects models. Of 200 identified articles, 3 trials were included (1233 participants). CRYSTAL AF included cryptogenic stroke only, STROKE-AF included small- or large-vessel stroke only, and PER DIEM included all ischemic strokes. The 12-month AF detection was 13% in the ILR group and 2.4% in controls. ILR was more likely to detect AF compared to usual care (OR 5.8, 95% CI, 3.2–10.2). Stroke or TIA occurred in 7% with ILR and 9% with usual care (OR 0.8, 95% CI 0.5–1.2). Among patients with detected AF, 97% and 100% were started on OAC in CRYSTAL AF and PER DIEM, respectively, compared to 68% in STROKE-AF. In conclusion, ILR was superior to usual care in AF detection, but the relative low incidence of AF and the non-differential risk of stroke between the ILR and usual care arms may suggest that most patients do not benefit from ILR implantation. Further studies are warranted to understand if patient selection can be improved to increase the diagnostic yield of ILR.
Keywords: atrial fibrillation, stroke, implantable loop recorder
Of 795,000 ischemic strokes occurring annually in the US, 20–25% are recurrent.1 Detection of subclinical AF as the etiology of the index stroke is critical for prevention of recurrent stroke because it identifies patients in whom oral anticoagulation (OAC) is highly effective for secondary stroke prophylaxis.1 Implantable loop recorder (ILR) placement was endorsed by the 2019 American Heart Association /American College of Cardiology /Heart Rhythm Society AF guideline for detection of subclinical AF in cryptogenic stroke.2 However, cryptogenic stroke is a diagnosis of exclusion and its definition is nebulous. Clinically, the decision to implant ILRs may be partly arbitrary and made at the clinician’s discretion. It is also not well known if cryptogenic stroke in reality is enriched for AF compared to other stroke subtypes. Importantly, although it is posited that ILR reduces the risk of recurrent stroke through greater AF detection followed by OAC, the clinical significance of occult AF detected by extended rhythm monitoring is uncertain3,4 and the randomized clinical trials (RCTs) were not powered to answer this question. Therefore, we conducted a meta-analysis of RCTs to quantify the incremental change in AF detection, initiation of OAC, and subsequent risk of stroke associated with ILR use versus usual care in post-stroke settings.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files. We performed our meta-analysis of RCTs using a protocol designed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.5 The protocol including our search strategies is registered in International Prospective Register of Systematic Reviews (PROSPERO). We searched Medline, Embase, Web of Science, and the Cochrane Library for RCTs comparing ILR to usual care in adults aged 18 years or older who received a diagnosis of ischemic stroke or transient ischemic attack (TIA) and without known history of AF. Original studies published in the English language in 1990 or later and those with AF detection and stroke (ischemic or hemorrhagic) or TIA as outcomes with minimum of 12 months of follow-up were eligible for inclusion. We included all ischemic stroke subtypes and any form of non-ILR external rhythm monitoring performed in the control group (e.g. 12-lead electrocardiogram, Holter monitor, 30-day event monitor, Patch monitor, external loop recorder, mobile cardiac outpatient telemetry). D.K. and D.F. designed the search strategies using the Peer Review of Electronic Search Strategies standard. All titles and abstracts were independently reviewed for inclusion by two investigators (D.K. and Q.D.). We used Endnote (Endnote; Clarivate Analytics, Philadelphia, PA), and Rayyan (Qatar Computing Research Institute [Data Analytics], Doha, Qatar)6 for the screening process.
Two investigators (D.K. and Q.D.) independently extracted data from the selected RCTs. All quantitative data were extracted from the trials’ intention-treat-analyses. We extracted relevant data into a pre-defined data extraction form. Extracted data include number of sites and location, the specific ILR used for intervention, types of external rhythm monitoring used in control group, primary, secondary, and post-hoc end points, inclusion and exclusion criteria, and follow-up duration. We performed a meta-analysis comparing the relative risks of AF detection and stroke (ischemic or hemorrhagic) or TIA in post-stroke patients monitored with ILR vs. those without ILR using Mantel-Haenszel pooled ORs with alpha 0.05 from random effects models (Review Manager Version 5.4.1,The Cochrane Collaboration, Copenhagen, Denmark).7 We used the intention-to-treat principle for analyses. We used random effects models to account for variation in the true effect sizes and the size of study sample across the studies.
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the quality of the evidence, 8 and our assessment was summarized using GRADEpro.9 We qualitatively assessed for risk of bias using the Cochrane Collaboration’s risk of bias tool10 based on the following elements: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias
RESULTS
Among the 200 records identified from search strategy, 3 RCTs including 1233 participants (613 ILR and 620 control) using the intention-to-treat principle for analyses met inclusion criteria (Supplemental Figure I).11–13 Study characteristics are shown in Table 1. Patients had median or mean age of 61–68 years and 467 (38%) were women. One trial reported race/ethnicity of the participants, and 87% were White. In 2 of the trials, there was no specific criteria for definition of usual care for control group,11,12 whereas the third trial specifically compared ILR to 30-day external loop recorder.13 In all 3 trials, ischemic strokes were manually subtyped by study investigators using the Trial of ORG in Acute Stroke Treatment classification system,14 with CRYSTAL AF (Cryptogenic Stroke and Underlying Atrial fibrillation) including only cryptogenic strokes,11 STROKE-AF (Stroke of Known Cause and Underlying Atrial Fibrillation) including only small- and large-artery strokes12 and PER DIEM (Post-Embolic Rhythm Detection with Implantable vs External Monitoring) including all ischemic stroke subtypes, 25% of which were small- or large-artery strokes and 66% of which were cryptogenic.13
Table 1.
Characteristics of the Included Randomized Controlled Trials
| Trial Name | Enrollment Period | No. Sites & Countries | No. of Participants | Inclusion criteria | Control group | Primary (1°), Secondary (2°), Post-hoc Outcomes | |
|---|---|---|---|---|---|---|---|
| CRYSTAL AF, 201411 | 6/2009 – 4/2012 | 55 sites, Europe, Canada, US | ILR: 221 Control: 220 |
≥40 yrs, CS | ECG, Holter, event monitor | 1°: AF at 6-mo. | 2°: AF at 12-mo., stroke/TIA, change in OAC use |
| STROKE-AF , 202112 | 5/2015–11/2017 | 33 sites, US | ILR: 242 Control: 250 |
≥60 yrs LAA or lacunar |
ECG, Holter, event monitor, MCT | 1°: AF at 12-mo. | 2°: AF at 36-mo. Post-hoc: AF at 6-mo., stroke/TIA, OAC use |
| PER DIEM , 202113 | 4/2016–7/2019 | 3 sites, Canada | ILR: 150 Control: 150 |
≥18 yrs All strokes |
30-day external loop recorder | 1°: AF at 12-mo. | 2°: AF or death, time to AF, stroke/TIA, ICH, MB ≤12-mo. |
Abbreviations: AF, atrial fibrillation; CRYSTAL AF, Cryptogenic Stroke and Underlying Atrial fibrillation; CS, cryptogenic stroke; ECG, electrocardiogram; ICH, intracranial hemorrhage; ILR, implantable loop recorder; LAA, large-artery atherosclerosis; MB, major bleeding; MCT, mobile cardiac telemetry; PER DIEM, Post-Embolic Rhythm Detection with Implantable vs External Monitoring; OAC, oral anticoagulation; STROKE-AF, Stroke of Known Cause and Underlying Atrial Fibrillation; TIA, transient ischemic attack; US, United States; Yrs, years.
Figure 1 shows the results of pooled analyses for AF and stroke (ischemic or hemorrhagic) or TIA. The 12-month AF occurrence was 13% among ILR and 2.4% among controls, with ranges in individual studies from 11% to 15% for ILR and 1.6% to 4.7% for control (pooled OR 5.8, 95% CI, 3.2–10.2, p < 0.001) (Supplemental Table I). Number needed to monitor for 12 months to detect one additional AF event with ILR was 10 (pooled risk difference 0.10, 95% CI, 0.08–0.13).
Figure 1.
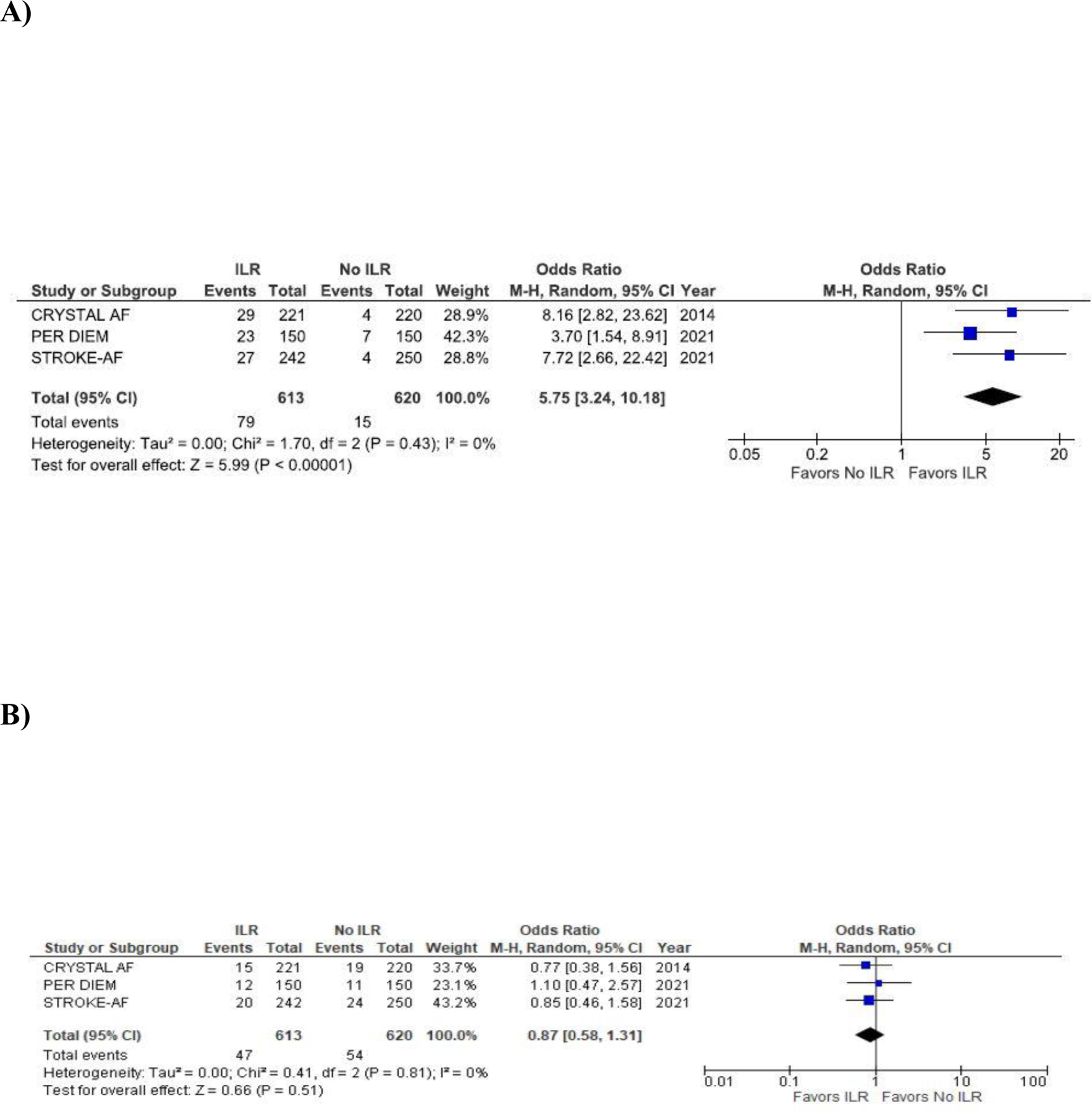
Pooled analyses comparing ILR to control in the odds of (A) AF detection (B) stroke (ischemic or hemorrhagic) or TIA. The forest plot shows that ILR is more likely to detect AF compared to control but there is no statistical difference in the risk of stroke or TIA between ILR and control.
Two trials reported OAC initiation:11,12 at 12 months, 15% of ILR and 6% of controls were started on OAC. One trial reported that among the patients started on OAC in the ILR and usual care arms, 53% and 79% did not have AF, respectively.12 After AF detection with either ILR or usual care, OAC was started in 97% of cryptogenic stroke in CRYSTAL AF,11 68% small- or artery strokes in STROKE-AF,12 and 100% of all ischemic strokes in PER DIEM13 (Supplemental Table I).
The 12 month incidence of stroke or TIA was 7.8% in the ILR group versus 8.9% among controls, with trial ranges from 6.8% to 8.7% for ILR and 7.3% to 10% for control. (pooled OR 0.9, 95% CI 0.6–1.3, p=0.52). There was a minimal heterogeneity among the included studies for both outcomes (AF detection: I2 = 0%, p=0.43; stroke/TIA: I2 = 0%, p=0.82). Excluding STROKE-AF, in which a significantly lower proportion of patients with AF received OAC compared to the other two trials, did not significantly change the OR.
GRADE ratings for the pooled analyses for AF detection and stroke or TIA are shown in Supplemental Table II. The quality of evidence for AF detection was high. The quality of evidence for stroke or TIA was moderate because the trials were not powered to study the outcome. Based on the Cochrane Collaboration’s risk of bias assessment tool, risk of bias ranged from low to moderate for the 3 trials (Supplemental Table III). The trials were all open-label studies, and outcome assessments were not blinded to group assignment in at least 2 trials.12,13 One study changed its definition of AF as an end point after the trial was started, and reported only 8 out of 15 pre-specified secondary outcomes because the data were not collected or deemed unreliable.13 Risk of bias for stroke or TIA was higher than risk of bias for AF detection because stroke or TIA was either a secondary or post hoc outcome; 2 studies did not report how stroke or TIA were defined as endpoints and how the events were adjudicated.11,13
DISCUSSION
Our systematic review of RCTs has 3 main findings. First, 1-year risks of AF were low and comparable across trials that include only cryptogenic versus small- and large-artery strokes. Second, the OR for AF detection with ILR was approximately 6 times greater than with usual care. Third, ILR did not significantly reduce the risk of stroke or TIA compared to usual care.
The findings from our study have important clinical implications. The 2019 American Heart Association /American College of Cardiology /Heart Rhythm Society AF guideline currently recommends (Class IIa) extended AF surveillance with ILR following cryptogenic stroke.2 However, our finding that the risk of AF detection is similar regardless of stroke subtypes indicate that the current system of using of ischemic stroke subtypes to guide AF evaluation for secondary prevention of AF-related stroke is of limited clinical impact. Alternative methods that identify patients at highest risk for undetected AF - and most likely to benefit from ILR monitoring - are needed.
Despite additional data from recent trials,12,13 the significance of ILR-detected occult AF on secondary stroke prevention remains uncertain. None of the RCTs on the use of ILR for post-stroke AF surveillance were powered to examine recurrent stroke as a primary outcome. Patients with ischemic stroke often have coexisting cardiovascular risk factors predisposing them to AF and atherosclerotic disease, and whether the detected AF is the culprit for the index stroke or future stroke or simply an innocent bystander remains unknown. Pooled AF prevalence at the end of 1-year of follow-up with ILR was 13% in our study, and we do not know whether this represents background AF prevalence in patients without stroke.
OAC initiation after AF detection was nearly universal in the CRYSTAL AF and PER DIEM trials whereas 68% of small- or large-artery strokes enrolled in STROKE-AF received OAC after AF detection. The latter finding was unexpected since the guidelines and CHA2DS2-VASc risk score recommend OAC in patients with prior history of stroke regardless of the etiology of the index stroke. In addition, in STROKE-AF, more than half of the patients who were started on OAC did not have AF diagnosis even though OAC has no benefit15,16 for secondary prevention or can be harmful16 when there is no AF. Further studies are needed to understand these OAC prescribing behaviors and how they may impact clinic outcome in real-world practice.
Our meta-analysis should be interpreted in the context of its limitations. Stroke events in the 3 trials were low and well-below the optimal information criterion necessary to detect a clinically significant reduction in stroke or TIA. However, it is unlikely that additional RCTs will produce the ~500 stroke events needed to adequately power a definitive evaluation of stroke reduction with ILR.17 Second, Second, we were unable to perform a subgroup analysis of patients with embolic stroke of undetermined source because cryptogenic strokes in CRYSTAL AF and PER DIEM were not further categorized. Third, we were unable to determine AF detection rates in racially/ethnically diverse patient population because only one trial reported race/ethnicity of the study participants.11 Accurate determination of racial/ethnic variation in the risk of post-stroke AF is important because prior studies have shown that Black individuals have higher short-term risk of recurrent stroke compared to White,18–20 despite having a seemingly lower risk of AF.21,22 Additional studies are needed to identify those who are most likely to benefit from post-stroke AF surveillance and shed insight into the clinical impact of post-stroke ILR use.
In conclusion, our systematic review and meta-analysis of RCTs demonstrate that ILR is more likely to detect AF following ischemic stroke compared to usual care, but the clinical impact of improved detection of relatively low AF rates after stroke remains unclear.
Supplementary Material
Sources of Funding
Dr. Ko is supported by American College of Cardiology Foundation/Merck Research Fellowship in Cardiovascular Diseases and Cardiometabolic Disorders and NHLBI 1K23 HL151903–01A1. Dr. Anderson is supported by NIH (R01NS103924, U01NS069763) and the American Heart Association. Dr. Walkey is supported by NIH (R01 HL136660, R01 HL139751, R01 HL151607, UL54 TR004130, OTA-20–011E). Dr. Anderson reports sponsored research support from the Bugher Foundation, Bayer AG, and Massachusetts General Hospital, and has consulted for ApoPharma and Invitae. Dr. Walkey reports sponsored research support from Gilead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The remainder of the authors report no disclosures
References
- 1.Hart RG, Pearce LA and Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM and Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation 2019:Cir0000000000000665. [DOI] [PubMed]
- 3.Katsanos AH, Kamel H, Healey JS and Hart RG. Stroke Prevention in Atrial Fibrillation. Circulation 2020;142:2371–2388. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns H, Doehner W, Engstrom G, Fauchier L, Friberg L, Gladstone DJ, Glotzer TV, Goto S, Hankey GJ, Harbison JA, Hobbs FDR, Johnson LSB, Kamel H, Kirchhof P, Korompoki E, Krieger DW, Lip GYH, Lochen ML, Mairesse GH, Montaner J, Neubeck L, Ntaios G, Piccini JP, Potpara TS, Quinn TJ, Reiffel JA, Ribeiro ALP, Rienstra M, Rosenqvist M, Sakis T, Sinner MF, Svendsen JH, Van Gelder IC, Wachter R, Wijeratne T and Yan B. Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation 2019;140:1834–1850. [DOI] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P and Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouzzani M, Hammady H, Fedorowicz Z and Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Review Manager Web (RevMan Web). Version 5.4.1. The Cochrane Collaboration, September 2020. Available at revman.cochrane.org.
- 8.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook. . [Google Scholar]
- 9.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020. (developed by Evidence Prime, Inc.). Available from gradepro.org. [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K and Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, Vives CA, Ziegler PD, Franco NC, Schwamm LH and Investigators S-A. Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA 2021;325:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck BH, Hill MD, Quinn FR, Butcher KS, Menon BK, Gulamhusein S, Siddiqui M, Coutts SB, Jeerakathil T, Smith EE, Khan K, Barber PA, Jickling G, Reyes L, Save S, Fairall P, Piquette L, Kamal N, Chew DS, Demchuk AM, Shuaib A and Exner DV. Effect of Implantable vs Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients With Ischemic Stroke: The PER DIEM Randomized Clinical Trial. JAMA 2021;325:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL and Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 15.Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C, Brueckmann M, Chernyatina M, Donnan G, Ferro JM, Grond M, Kallmunzer B, Krupinski J, Lee BC, Lemmens R, Masjuan J, Odinak M, Saver JL, Schellinger PD, Toni D and Toyoda K. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N Engl J Med 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 16.Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y, Davalos A, Shamalov N, Mikulik R, Cunha L, Lindgren A, Arauz A, Lang W, Czlonkowska A, Eckstein J, Gagliardi RJ, Amarenco P, Ameriso SF, Tatlisumak T, Veltkamp R, Hankey GJ, Toni D, Bereczki D, Uchiyama S, Ntaios G, Yoon BW, Brouns R, Endres M, Muir KW, Bornstein N, Ozturk S, O’Donnell MJ, De Vries Basson MM, Pare G, Pater C, Kirsch B, Sheridan P, Peters G, Weitz JI, Peacock WF, Shoamanesh A, Benavente OR, Joyner C, Themeles E and Connolly SJ. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 17.Tirschwell D and Akoum N. Detection of Subclinical Atrial Fibrillation After Stroke: Is There Enough Evidence to Treat? JAMA 2021;325:2157–2159. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy BS. Does race predict stroke readmission? An analysis using the truncated negative binomial model. J Natl Med Assoc 2005;97:699–713. [PMC free article] [PubMed] [Google Scholar]
- 19.Albright KC, Huang L, Blackburn J, Howard G, Mullen M, Bittner V, Muntner P and Howard V. Racial differences in recurrent ischemic stroke risk and recurrent stroke case fatality. Neurology 2018;91:e1741–e1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB and Tsao CW. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr. and Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25:71–76, 76.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewland TA, Olgin JE, Vittinghoff E and Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 23.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M and Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2010;41:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


