Abstract
Objectives:
Long-acting reversible contraceptives are effective contraceptives for women with HIV, but there are limited data on etonogestrel implant and antiretroviral therapy pharmacokinetic drug-drug interactions. We evaluated etonogestrel/antiretroviral therapy drug-drug interactions, and the effects of etonogestrel on ritonavir-boosted-atazanavir, ritonavir-boosted-lopinavir, and efavirenz pharmacokinetics.
Methods:
We enrolled postpartum women using etonogestrel implants and receiving ritonavir-boosted-atazanavir, ritonavir-boosted-lopinavir, or efavirenz-based regimens between 2012 and 2015. Etonogestrel implants were inserted 2 to 12 weeks postpartum. We performed pharmacokinetic sampling pre-etonogestrel insertion and 6 to 7 weeks post-insertion. We measured antiretroviral concentrations pre and post-etonogestrel insertion, and compared etonogestrel concentrations between antiretroviral regimens. We considered a minimum serum etonogestrel concentration of 90 pg/ml adequate for ovulation suppression.
Results:
We collected pharmacokinetic data for 74 postpartum women, 22 on ritonavir-boosted-atazanavir, 26 on ritonavir-boosted-lopinavir, and 26 on efavirenz. The median serum concentrations of etonogestrel when co-administered were highest with etonogestrel/ritonavir-boosted-atazanavir (604pg/mL) and etonogestrel/ritonavir-boosted-lopinavir (428pg/mL), and lowest with etonogestrel/efavirenz (125pg/mL); p<0.001. Minimum concentration (Cmin) of ritonavir-boosted-atazanavir and ritonavir-boosted-lopinavir were lower after etonogestrel implant insertion, but overall exposure, pre-dose concentrations, clearance, and half-lives were unchanged. We found no significant change in efavirenz exposure after etonogestrel insertion.
Conclusions:
Unlike efavirenz, ritonavir-boosted-atazanavir and ritonavir-boosted-lopinavir were not associated with significant decreases in etonogestrel concentrations. Efavirenz was associated with a significant decrease in etonogestrel concentrations.
Implications:
The findings demonstrate no interactions between etonogestrel and ritonavir-boosted-lopinavir or ritonavir-boosted-atazanavir, but confirm the decreased efficacy of etonogestrel with efavirenz-based antiretrovirals. This information should be used to counsel women with HIV who desire long-acting reversible contraceptives.
Keywords: HIV, Long-acting reversible contraceptives, Etonogestrel, Efavirenz, Atazanavir, Lopinavir, Pharmacokinetics
INTRODUCTION:
Human immunodeficiency virus (HIV) infection during pregnancy and postpartum continues to be a significant public health problem [1, 2]. According to the Joint United Nations Program on HIV and AIDS, more than half of people with HIV worldwide are women, the majority of whom are of reproductive age [3]. In 2019, women accounted for 48% of all new HIV infections, and most resided in low and middle-income countries [3]. Many women with HIV experience disproportionately high rates of unintended pregnancy [4]. Therefore, addressing the family planning needs of women living with HIV is of clinical and public health importance.
Long-acting reversible contraceptives such as etonogestrel-containing progestin-only implants (containing 68 mg etonogestrel), are currently favored due to their high efficacy, tolerability, and continuation rates compared to other forms of reversible contraceptives [5, 6]. Etonogestrel efficacy is directly related to its pharmacologic properties. Following subdermal insertion, mean peak serum etonogestrel concentration ranges between 781 and 894 pg/mL within the first few weeks, then decreases gradually to 192 – 261 pg/mL at 12 months, 154 – 194 pg/mL at 24 months, and 156 – 177 pg/mL at 36 months [7, 8]. Etonogestrel is approximately 66% bound to albumin, and 32% bound to sex-hormone-binding-globulin in plasma [8], and is released at approximately 60 micrograms/day after 3 months, with the release rate slowly decreasing to 30 micrograms/day by the end of 2 years.[9] A minimum serum etonogestrel concentration of 90 pg/ml is required to prevent ovulation.and a single etonogestrel implant is expected to provide contraception for three years before being removed. [8] Etonogestrel is metabolized in liver microsomes by the cytochrome P450 3A4 (CYP3A4) isoenzyme [8, 9].
There have been several pharmacokinetic studies evaluating the interactions between etonogestrel-releasing contraceptives and antiretroviral therapy. While etonogestrel contraceptive implants are highly efficacious, their metabolism and efficacy can be affected by pharmacokinetic drug-drug interactions with hepatic enzyme inducers of CYP3A4, notably efavirenz and ritonavir. Ritonavir, a potent inhibitor of CYP3A4, impedes the metabolism of etonogestrel, thereby increasing the plasma concentrations of both medications, while efavirenz, a substrate and a potent inducer of CYP3A4, increases the metabolism of etonogestrel, decreasing its plasma concentration.[10–12] These reductions in plasma concentration of etonogestrel may be of sufficient magnitude to compromise contraceptive efficacy, resulting in increased rates of unintended pregnancies, with medical, psychosocial, and economic implications.[13, 14] Thus, characterizing the pharmacokinetic drug-drug interactions between most used antiretrovirals and etonogestrel implants is critical.
Using a sparse pharmacokinetic sampling scheme, Chappell and colleagues demonstrated an 82% reduction in plasma concentrations of etonogestrel in 19 women using efavirenz-based antiretrovirals compared to 20 antiretroviral-naïve women.[10] Other efavirenz-etonogestrel drug-drug interaction pharmacokinetic studies including 25 and 30 women using etonogestrel implants, demonstrated reductions of 49% and 63% in plasma etonogestrel concentrations respectively when used concomitantly with efavirenz.[11]–[12] In contrast, use of the protease-inhibitor combinations of lopinavir/ritonavir including 45 women with etonogestrel contraceptive implant was associated with a 52% increase in the bioavailability of etonogestrel, suggesting that ritonavir-boosted lopinavir does not impair etonogestrel contraceptive implant efficacy.[15] Newer studies have evaluated drug-drug. interactions between atazanavir/ritonavir and etonogestrel. In the AIDS Clinical Trials Group A5316 study, a three-arm multicenter pharmacokinetic study of 25 antiretroviral-naïve women (arm-1, control), 25 women on efavirenz-based antiretrovirals (arm-2), and 24 women on ritonavir-boosted atazanavir (arm-3), efavirenz lowered plasma concentrations of etonogestrel by 79% when etonogestrel was administered as a vaginal ring, and ritonavir-boosted atazanavir increased etonogestrel concentrations by 71% compared to controls.[16]
These prior pharmacokinetic drug-drug interaction studies between etonogestrel and the antiretrovirals efavirenz and ritonavir-boosted lopinavir are limited by sparse sampling designs. Intensive plasma sampling strategies are critically important in pharmacokinetic studies to provide a better understanding of intra and inter-individual variability that will allow for robust pharmacokinetic predictions. [17, 18] No prior studies have evaluated the potential drug-drug interactions between ritonavir-boosted atazanavir and etonogestrel subdermal implant. Given these knowledge gaps, our goal was to describe the pharmacokinetic drug-drug interactions between etonogestrel and efavirenz, ritonavir-boosted atazanavir and ritonavir-boosed lopinavir in women with HIV during the postpartum period, using intensive plasma sampling data from the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network P1026s protocol.
METHODS
The study protocol, the informed consent documents, and all subsequent modifications were reviewed and approved by the local institutional review boards/ethics committees. The study followed all relevant human subject research guidelines. All participants signed informed consent before participation, and the study was registered in ClinicalTrials.gov [NCT00042289]. This study was done as part of IMPAACT P1026s, an ongoing, non-blinded international opportunistic study of antiretroviral pharmacokinetics in pregnant and postpartum women. From May 2012 to July 2015 we enrolled postpartum women with HIV, who desired to use etonogestrel implants and were on efavirenz, ritonavir-boosted atazanavir or ritonavir-boosted lopinavir based regimens for at least 2 weeks.
Eligible women were receiving one of these antiretroviral regimens and desired postpartum contraception with an etonogestrel implant by prescription at the specified doses listed in the protocol. Women continued to take their prescribed medications throughout the course of the study. We excluded women on medications known to interfere with absorption, metabolism, or clearance of the drugs being evaluated and those with clinical or laboratory toxicity that would likely require a change in the medication regimen during the study. The participant’s physician determined the choice of antiretrovirals and contraceptives and prescribed all medications and remained responsible for her clinical management throughout the study.
Clinical and Laboratory Monitoring:
Maternal data obtained for this analysis were maternal age, ethnicity, weight, concomitant medications, CD4 and plasma viral load assay results. Local labs performed the plasma viral load assays and had lower limits of detection of fewer than 50 copies per milliliter. We assessed maternal clinical and laboratory toxicities through history and physical examination and laboratory assays (alanine aminotransferase, aspartate aminotransferase, creatinine, blood urea nitrogen, albumin, bilirubin, hemoglobin) on each pharmacokinetic sampling day. We used the Division of AIDS/National Institute of Allergy and Infectious Diseases Toxicity Table for Grading Severity of Adult Adverse Experiences to report adverse events for study participants.[19] We followed all toxicities through resolution.
Sample collection and drug assays:
The etonogestrel implant was inserted between 2 and 12 weeks postpartum. We performed pharmacokinetic sampling was performed before, and 6 to 7 weeks after implant insertion. We collected plasma samples at 0, 1, 2, 6, 8, 12 hours post-dose and a 24 hours post-dose sample in women receiving efavirenz or atazanavir. We measured Antiretroviral therapy and etonogestrel concentrations using liquid chromatography-mass spectrometry. The lower limits of quantitation were atazanavir: 0.047 mcg/mL, lopinavir: 0.09 mcg/mL, ritonavir: 0.049 mcg/mL, efavirenz: 0.039 mcg/mL, and etonogestrel: 4 pg/mL. The P1026s target minimum area under the curve for atazanavir, lopinavir and efavirenz were 29.4, 52 and 40 μg*hr/mL (10th percentile in non-pregnant historical controls), respectively. Mean (± SD) etonogestrel concentrations within the first few weeks of use in women not receiving antiretrovirals was 1145 (± 577) pg/mL. We collected serum samples for the assessment of etonogestrel once during intensive antiretroviral sampling and were frozen at −70 °C until measurement.
Pharmacokinetic and statistical analytic plan
We calculated pharmacokinetic parameters with standard non-compartmental methods. Each antiretroviral arm had a target enrollment of 25 women with evaluable pharmacokinetic data to provide reasonably precise estimates of pharmacokinetic parameters and differences in antiretroviral exposure before and after etonogestrel initiation. We summarized etonogestrel plasma concentrations (both continuous and categorized by the threshold of 90 pg/mL concentrations) and compared among the three study arms using the Kruskal-Wallis test and Fisher’s exact test, respectively (α=0.05). We compared antiretroviral therapy pharmacokinetic parameters before and after etonogestrel initiation at the within-participant level using Wilcoxon signed-rank test. Two-tailed Wilcoxon signed-rank tests compared within-subject pharmacokinetic parameters with a two-sided–value <0.1. We considered a two-sided p-value less than 0.10 statistically significant. We calculated within-participant geometric mean ratios and 90% confidence intervals (CIs) for pharmacokinetic parameters in the before versus after etonogestrel initiation conditions for the antiretrovirals of interest to describe the range of relative differences that were consistent with the observed data and help assess whether there was a clinically significant difference in exposure. We also summarized descriptive statistics of pharmacokinetic parameters during each study period. In addition, we created figures for antiretrovirals of interest to show the change in concentration before and after etonogestrel initiation.
RESULTS:
Demographic characteristics
We enrolled seventy-four postpartum women (22 on ritonavir-boosted atazanavir; 26 on ritonavir-boosted lopinavir and 26 on efavirenz) in the study with pharmacokinetic data obtained prior to and after etonogestrel implant insertion. Table 1 summarizes the demographic characteristics of the study population. The timing of implant insertion ranged from 2.6–11.7 weeks post-delivery, median 7.4 weeks.
Table 1:
Demographic characteristics of postpartum women living with HIV on atazanavir, lopinavir, and efavirenz and using etonogestrel implants, 2012–2015 (N=74).
| Characteristic | N (%) | Median (range) |
|---|---|---|
| Patients included | 74 | |
| Age (years) | 26.7 (16–41) | |
| Weight (kg) | 63 (38.7 – 141) | |
| Country | ||
| USA | 7 (9) | |
| Brazil | 55 (74) | |
| Argentina | 5 (7) | |
| Thailand | 7 (9) | |
| Race/ethnicity | ||
| Black Non-Hispanic | 4 (5) | |
| Hispanic | 63 (85) | |
| Asian, Pacific Islander | 7 (9) | |
| Duration of ARVs before implant insertion (weeks) | ||
| Atazanavir/ritonavir | 21 | 32 (2.1– 363) |
| Efavirenz | 26 | 4.4 (0.1– 267) |
| Lopinavir/ritonavir | 26 | 29.1 (6.9–323) |
| Timing of Implant insertion (weeks after delivery) | 7.4 (2.6–11.7) | |
| CD4+ (cells/μL) before implant insertion | 621 (79 –1578) | |
| Plasma HIV-1 RNA concentration (copies/mL) before implant insertion | 40 (20– 127310) | |
| Undetectable (< 400) | 58 (78.4) | |
| (< 50) | 49 (66.2) |
Etonogestrel pharmacokinetics
Table 2 summarizes etonogestrel plasma concentration data for all three arms (etonogestrel /ritonavir-boosted atazanavir; etonogestrel/efavirenz, and etonogestrel/ritonavir boosted lopinavir). The median serum concentrations of etonogestrel when co-administered with ritonavir-boosted atazanavir, efavirenz, and ritonavir boosted lopinavir were highest with etonogestrel/ritonavir-boosted-atazanavir (604pg/mL) and etonogestrel/ritonavir-boosted-lopinavir (428pg/mL), and lowest with etonogestrel/efavirenz (125pg/mL). These differences in plasma etonogestrel concentrations were statistically significant (p<0.001).
Table 2.
Etonogestrel serum concentrations, by antiretroviral use, in postpartum women living with HIV on atazanavir, lopinavir, and efavirenz, 2012–2015 (N=74).
| Study arm | |||||
|---|---|---|---|---|---|
| Characteristic | ATV/r+ENG (N=22) |
EFV+ENG (N=26) |
LPV/r+ENG (N=26) |
P-Value | |
| Concentration (pg/mL) | Min, Max | 260, 2,400 | 2.0, 2,330.0 | 224.1, 3,680.0 | <.001* |
| Median (Q1, Q3) | 604 (436, 838) | 125.0 (41.5, 202.0) | 428 (340, 563) | ||
| Concentration < 90 pg/mL | Yes | 0 (0%) | 11 (42%) | 0 (0%) | <.001** |
| No | 22 (100%) | 15 (58%) | 26 (100%) | ||
P values were determined by using the Kruskal-Wallis Test and the
Fisher’s Exact Test. A two-sided p-value less than 0.10 was considered statistically significant. Min, minimum concentration; Max, maximum concentration, Q1, lower (25th percentile); Q3, upper (75th percentile); ATV, Atazanavir; EFV, Efavirenz; LPV, Lopinavir; ENG, Etonogestrel.
Antiretroviral pharmacokinetics
Table 3 shows Atazanavir parameters. Atazanavir minimum plasma concentrations (Cmin) were higher pre-etonogestrel implant (geometric mean ratio, GMR 2.33 (CI 1.12, 4.86; p=0.09) compared to post-etonogestrel implant insertion. Atazanavir plasma concentrations at 24 hours post-dose (C24) [(GMR 1.39 (CI 0.97–2.00); p=0.006) were lower post-etonogestrel insertion. There were no significant differences between pre- and post-implant insertion efavirenz pharmacokinetic parameters, as shown in Table 4. Table 5 shows lopinavir pharmacokinetic data are shown in. Lopinavir Cmin was higher pre-etonogestrel implant (GMR 2.78 (CI 1.49, 5.21; p=0.056) compared to post-etonogestrel implant insertion. Figures 1–3 show the concentration-time curves for median atazanavir concentrations (Figure 1); median efavirenz concentrations (Figure 2); and median lopinavir concentrations (Figure 3) before and after etonogestrel implant insertion. The proportions of women meeting antiretroviral pharmacokinetic targets before and after etonogestrel insertion were: 77% and 66% for ritonavir-boosted atazanavir, 84% and 84% for ritonavir-boosted lopinavir and 90% and 81% for efavirenz.
Table 3.
Atazanavir pharmacokinetic comparison before versus after etonogestrel implant initiation in postpartum women living with HIV, 2012–2015 (N=74).
| Parameter | Before ENG Median (range) N=22 | After ENG Median (range) N=22 | Geometric mean of before/after initiation ratio [90% CI] | *P-value of Wilcoxon signed rank test comparison |
|---|---|---|---|---|
| AUC0–24 (μg*hr/mL) | 53.962 (8.739,157.300) | 55.254 (9.471,157.567) | 1.101 [0.840, 1.443] | 0.3669 |
| CL/F (L/hr) | 5.560 (1.907,34.331) | 5.445 (1.904,31.676) | 0.908 [0.693, 1.191] | 0.5184 |
| Tmin (hr) | 10 (0, 24) | 0 (0,24) | 0.3096 | |
| Tmax (hr) | 3 (2,4) | 2 (1,6) | 0.9077 | |
| T1/2 (hr) | 17.154 (9.076,67.577) | 18.206 (6.510,152.105) | 0.888 [0.681, 1.156] | 0.4980 |
| Vd/F (L) | 157.238 (43.345,505.124) | 185.475 (61.989,1,866.663) | 0.732 [0.550, 0.975] | 0.1054 |
| Cmin (μg/mL) | 0.929 (0.024,4.673) | 0.411 (0.024,4.539) | 2.333 [1.121, 4.855] | 0.0921 |
| Cmax (μg/mL) | 4.392 (0.638,10.402) | 4.647 (0.643,8.563) | 1.167 [0.835, 1.630] | 0.4780 |
| C0(μg/mL) | 1.078 (0.024,6.572) | 0.593 (0.024,5.129) | 2.505 [1.070, 5.864] | 0.1074 |
| C12(μg/mL) | 1.792 (0.290,5.763) | 1.463 (0.276,6.309) | 1.211 [0.871, 1.682] | 0.5184 |
| C24h (μg/mL) | 1.206 (0.116,4.673) | 1.064 (0.126, 4.539) | 1.391 [0.965, 2.003] | 0.0600 |
p-value for Wilcoxon signed rank test. Geometric means are not calculated for Tmin and Tmax, and ties (differences of zero) are excluded from the median calculation since the Wilcoxon test ignores ties.
AUC0–24 = area under concentration (AUC) vs time curve (0 to 24 hours post-dose); CL/F = apparent oral clearance; Tmin = time to achieve minimum (trough) plasma concentration; Tmax = time to achieve maximum plasma concentration; T1/2 = elimination half-life; Vd/F = apparent volume of distribution; Cmin= minimum plasma concentration; Cmax = maximum plasma concentration; C0 = initial concentration at time zero; C12 = concentration at 12 hours post-dose; C24 = concentration at 24 hours post-dose.
Table 4.
Efavirenz pharmacokinetic comparison before versus after etonogestrel implant initiation in postpartum women living with HIV, 2012–2015 (N=74).
| Parameter | Before ENG Median (range) N=26 | After ENG Median (range) N=26 | Geometric mean of before/after initiation ratio [90% CI] | *P-value of Wilcoxon signed rank test comparison |
|---|---|---|---|---|
| AUC0–24 (μg*hr/mL) | 53.636 (26.294,216.550) | 56.651 (26.279,299.551) | 1.015 [0.915, 1.126] | 0.5609 |
| CL/F (L/hr) | 11.188 (2.771,22.819) | 10.593 (2.003,22.832) | 0.985 [0.888, 1.093] | 0.3253 |
| Tmin (hr) | 24 (0, 24) | 12.500 (0,24) | 0.4463 | |
| Tmax (hr) | 2 (1,12) | 2 (0,8) | 0.7574 | |
| T1/2 (hr) | 33.901 (12.930,493.404) | 37.383 (18.368,182.112) | 0.925 [0.740, 1.155] | 0.3509 |
| Vd/F (L) | 557.930 (166.366,8,387.456) | 572.872 (289.556,2,802.635) | 0.911 [0.706, 1.177] | 0.7956 |
| Cmin (μg/mL) | 1.547 (0.544,7.877) | 1.432 (0.020,10.135) | 1.187 [0.874, 1.611] | 0.8532 |
| Cmax (μg/mL) | 4.108 (2.232,11.326) | 4.233 (1.337,14.050) | 1.058 [0.938, 1.193] | 0.7019 |
| C0(μg/mL) | 1.586 (0.544,9.137) | 1.653 (0.020,10.135) | 1.139 [0.836, 1.552] | 0.4055 |
| C12(μg/mL) | 1.962 (0.963,9.128) | 2.017 (0.913,12.473) | 1.054 [0.929, 1.195] | 0.7956 |
| C24h (μg/mL) | 1.604 (0.717,7.877) | 1.555 (0.697,11.561) | 1.004 [0.892, 1.130] | 0.8532 |
p-value for Wilcoxon signed rank test. Geometric means are not calculated for Tmin and Tmax, and ties (differences of zero) are excluded from the median calculation since the Wilcoxon test ignores ties.
AUC0–24 = area under concentration (AUC) vs time curve (0 to 24 hours post-dose); CL/F = apparent oral clearance; Tmin = time to achieve minimum (trough) plasma concentration; Tmax = time to achieve maximum plasma concentration; T1/2 = elimination half-life; Vd/F = apparent volume of distribution; Cmin= minimum plasma concentration; Cmax = maximum plasma concentration; C0 = initial concentration at time zero; C12 = concentration at 12 hours post-dose; C24 = concentration at 24 hours post-dose.
Table 5.
Lopinavir pharmacokinetic comparison before versus after etonogestrel implant initiation in postpartum women living with HIV, 2012–2015 (N=74).
| Parameter | Before ENG Median (range) N=26 | After ENG Median (range) N=26 |
Geometric mean of before/after initiation ratio [90% CI] | *P-value of Wilcoxon signed rank test comparison |
|---|---|---|---|---|
| AUC0–24 (μg*hr/mL) | 115.967 (15.778,259.477) | 100.203 (3.392,159.927) | 1.242 [0.968, 1.592] | 0.1140 |
| CL/F (L/hr) | 3.450 (1.542,25.353) | 3.992 (2.501,117.925) | 0.805 [0.628, 1.033] | 0.3253 |
| Tmin (hr) | 7(0, 12) | 0.500 (0,12) | 0.3447 | |
| Tmax (hr) | 4 (1,8) | 4 (0,12) | 0.6635 | |
| T1/2 (hr) | 11.554 (4.512,72.673) | 11.742 (2.602,89.322) | 1.015 [0.837, 1.231] | 0.9063 |
| Vd/F (L) | 62.557 (23.559,2,658.654) | 60.321 (22.028,2,290.847) | 0.924 [0.776, 1.099] | 0.9063 |
| Cmin (μg/mL) | 6.023 (0.045,17.694) | 5.339 (0.028,10.217) | 2.784 [1.489, 5.206] | 0.0559 |
| Cmax (μg/mL) | 12.049 (2.120,23.921) | 10.551 (0.555,16.719) | 1.179 [0.956, 1.453] | 0.1550 |
| C0(μg/mL) | 7.445 (0.045,21.525) | 6.302 (0.045,13.222) | 2.676 [1.415, 5.061] | 0.2176 |
| C12(μg/mL) | 7.060 (1.929,17.694) | 6.623 (0.555,12.438) | 1.201 [0.958, 1.505] | 0.1628 |
p-value for Wilcoxon signed rank test. Geometric means are not calculated for Tmin and Tmax, and ties (differences of zero) are excluded from the median calculation since the Wilcoxon test ignores ties.
AUC0–24 = area under concentration (AUC) vs time curve (0 to 24 hours post-dose); CL/F = apparent oral clearance; Tmin = time to achieve minimum (trough) plasma concentration; Tmax = time to achieve maximum plasma concentration; T1/2 = elimination half-life; Vd/F = apparent volume of distribution; Cmin= minimum plasma concentration; Cmax = maximum plasma concentration; C0 = initial concentration at time zero; C12 = concentration at 12 hours post-dose; C24 = concentration at 24 hours post-dose.
Figure 1:
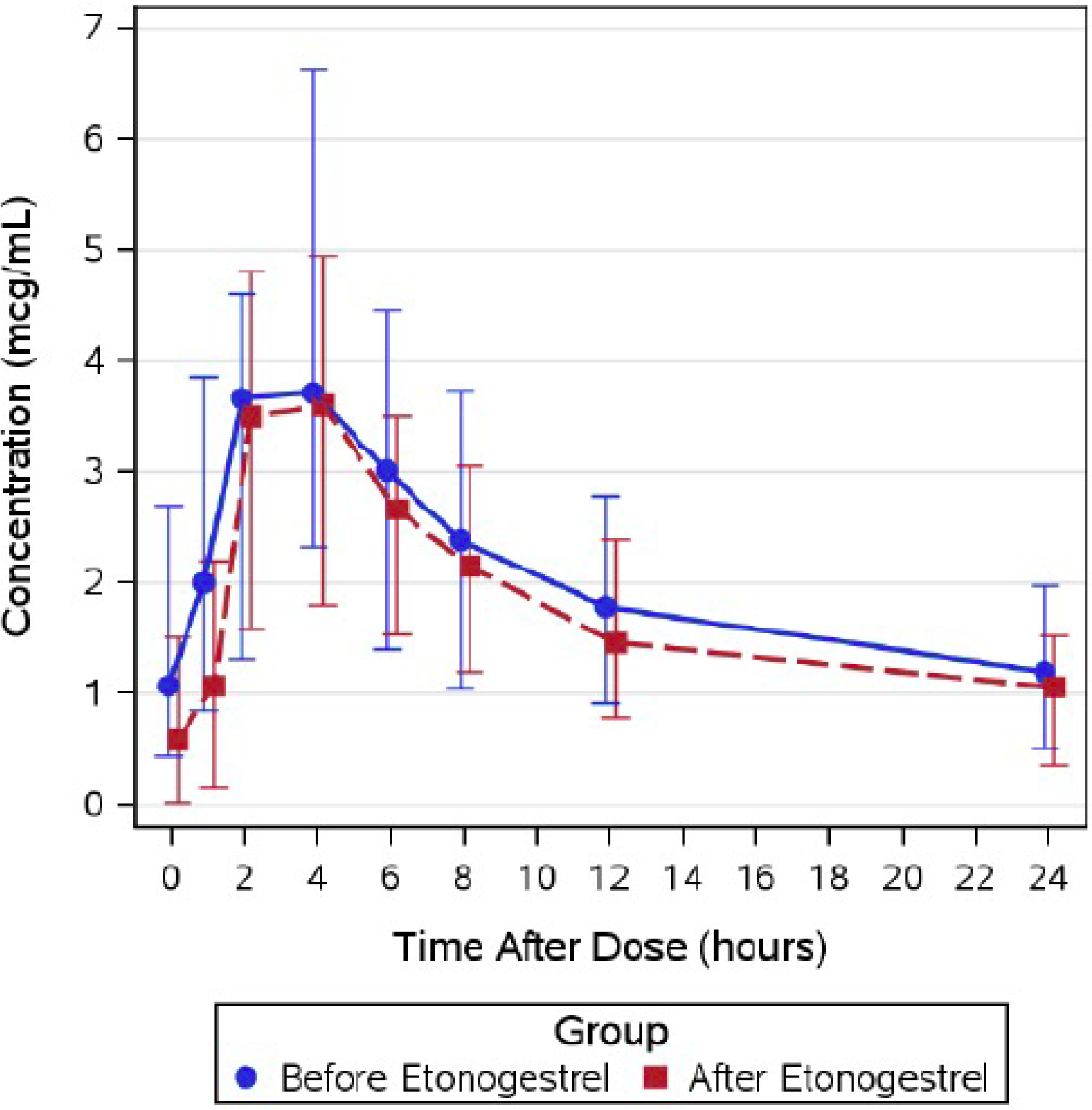
Summary of median (interquartile range) atanazavir concentrations before and after etonogestrel implant in postpartum women living with HIV, 2012–2015 (N=74).
Figure 3:
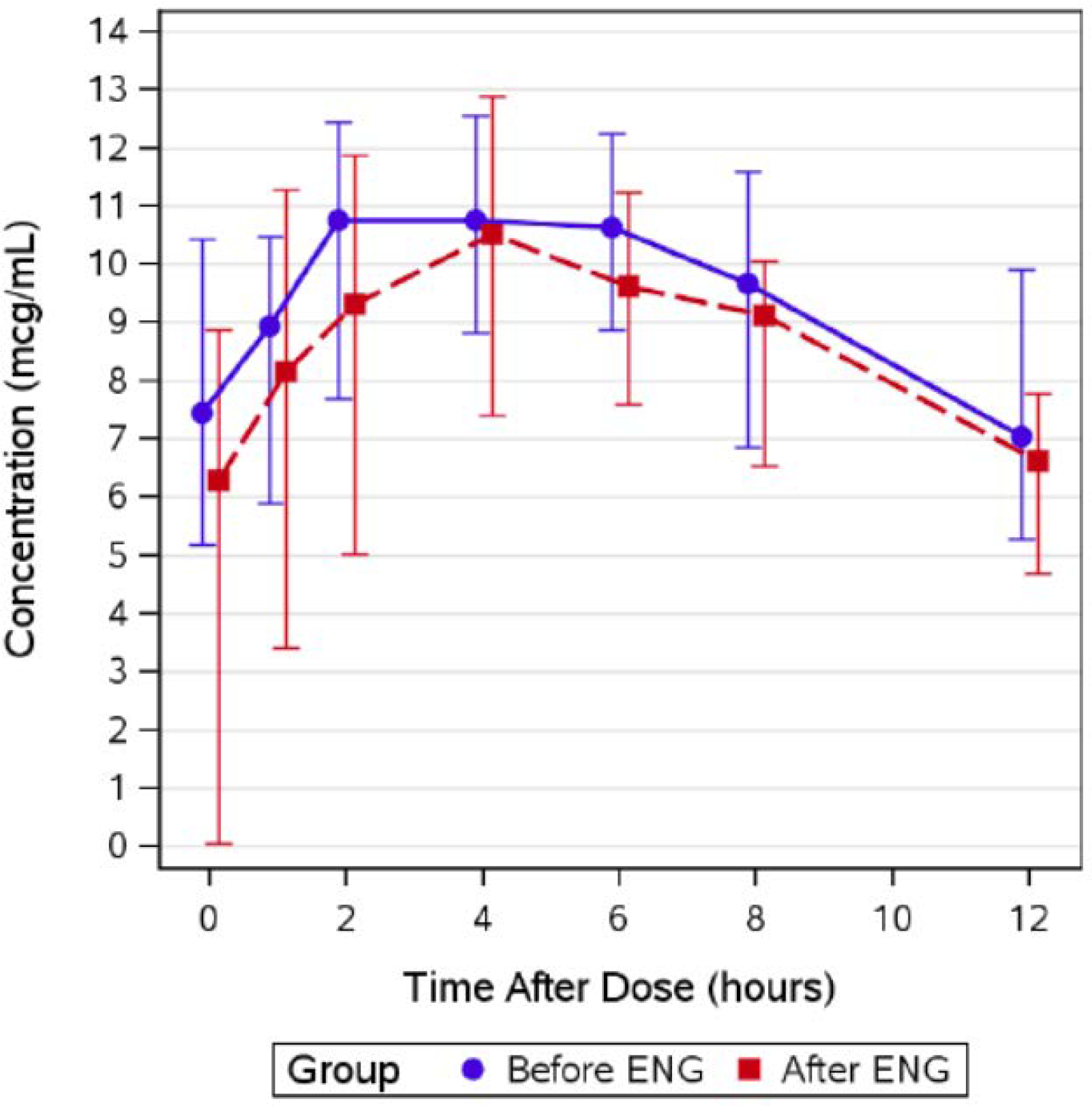
Summary of median (interquartile range) lopinavir concentrations before and after etonogestrel implant in postpartum women living with HIV, 2012–2015 (N=74).
Figure 2:
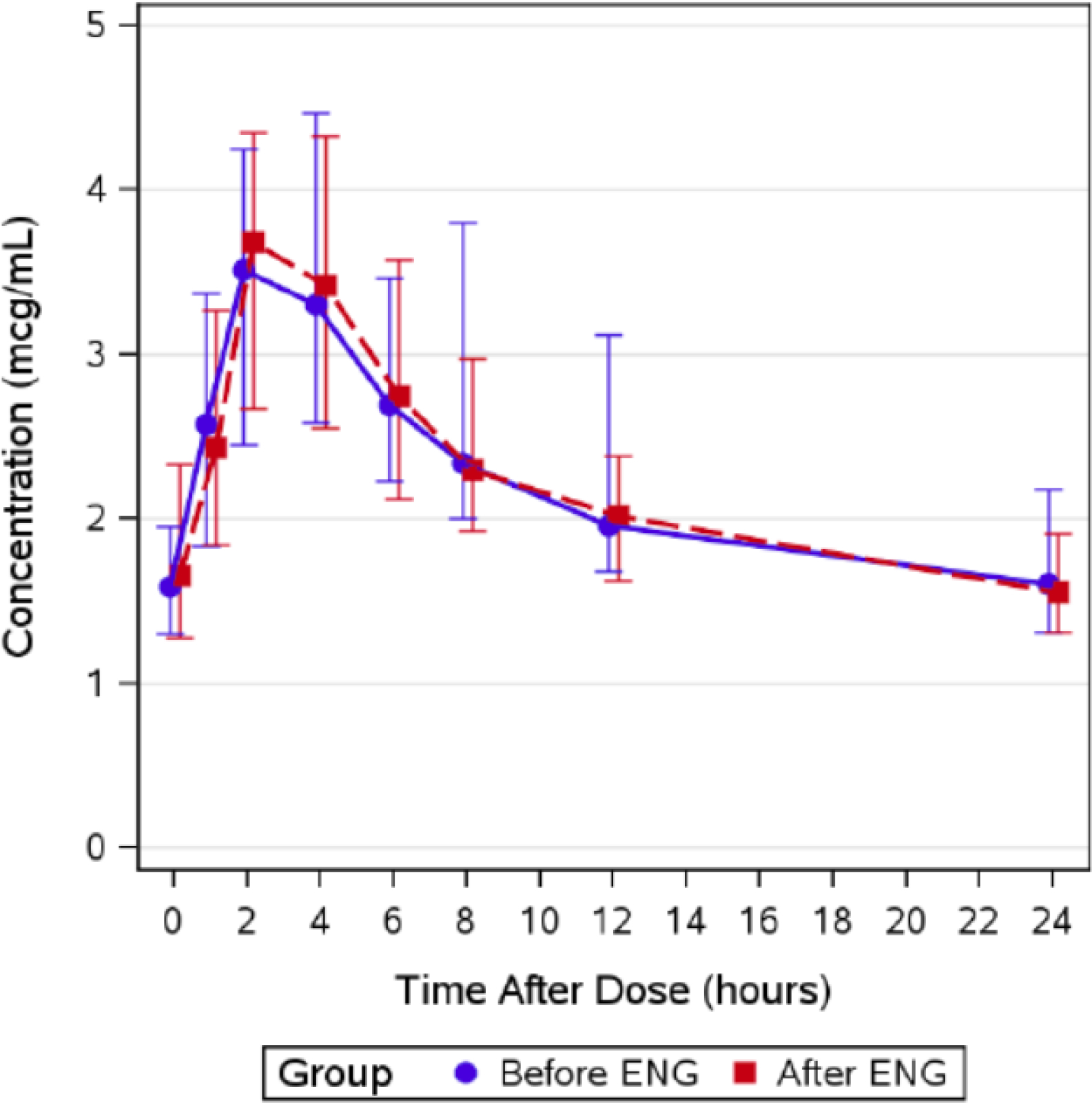
Summary of median (interquartile range) efavirenz concentrations before and after etonogestrel implant in postpartum women living with HIV, 2012–2015 (N=74).
We also evaluated ritonavir pharmacokinetic data (for both ritonavir-boosted atazanavir and ritonavir-boosted lopinavir) (data not shown). While there were no significant differences between pre- and post-etonogestrel implant insertion in ritonavir pharmacokinetic parameters in the ritonavir-boosted atazanavir arm, in women on ritonavir-boosted lopinavir, ritonavir Cmin was higher pre- etonogestrel implant (GMR 1.19 (CI 0.92, 1.54; p=0.030) compared to post- etonogestrel implant insertion.
Treatment related adverse events
There were 14 treatment-related adverse events in the study. Eleven were of moderate-intensity (grade 2) and 3 of severe intensity (grade 3). All Grade 3 events were increased bilirubin levels in participants receiving ritonavir-boosted atazanavir. Grade 2 events in the ritonavir-boosted atazanavir arm were: increased bilirubin (7) and increased serum glutamate pyruvate kinase (1) and irregular vaginal bleeding (1). Grade 2 events in the ritonavir-boosted lopinavir arm were increased amylase (1) and lower abdomen cramps (1). A twin pregnancy occurred in the efavirenz arm 16 months after implant insertion; the implant was removed; pregnancy was continued and the patient delivered healthy infants.
DISCUSSION:
Use of effective contraceptives such as progestin-only long-acting reversible methods in women with HIV allows for optimal birth spacing; and reduces unplanned pregnancies, leading to reduced maternal morbidity and mortality.[20] Despite these advantages of long-acting contraceptive methods, drug-drug interaction studies have raised concerns that co-administration of some antiretrovirals may alter etonogestrel-based contraceptive efficacy.[10–12] Due to these potential drug-drug interactions, current guidelines often advise alternative methods of contraception or dual-use of barrier contraceptives.[21] In addition, the World Health Organization recommends the use of a particular contraceptive method when the advantages of using that method outweigh the theoretical or proven risks. (Medical Eligibility for contraception, Category 2).[22]
Our study demonstrated decreased etonogestrel concentrations when co-administered with efavirenz. Our etonogestrel data are consistent with findings from other studies, most of which were not yet reported while our study was in progress. [10–12] Previous research demonstrated a reduction of 49–63% [11] in plasma etonogestrel concentrations when used concomitantly with efavirenz. Forty-two percent of women using etonogestrel /efavirenz in our study had etonogestrel concentrations below the minimum required to suppress ovulation. Although prior data have consistently demonstrated that etonogestrel concentrations are decreased when used with antiretroviral therapy, the highly variable reductions in concentrations of etonogestrel in the blood are likely due to differences in assay methods (use of radioimmunoassay versus liquid chromatography-mass spectrometry) and assay matrix (plasma vs serum).[23] Etonogestrel is primarily metabolized by CYP3A4 enzyme,[8] and efavirenz is both a substrate and a potent inducer of CYP3A4.[8, 9, 11] Therefore, it would be expected that concomitant administration of etonogestrel with efavirenz would lead to decreased etonogestrel by CYP450 enzyme induction, thus accelerating the metabolism of etonogestrel.
Prior studies of drug-drug interactions between atazanavir and combined oral contraceptive pills (ethinyl-estradiol and norethindrone) have demonstrated enhanced effects and increased plasma concentrations of ethinyl-estradiol and norethindrone by atazanavir.[24] The mechanism of this interaction is via inhibition of uridine diphospho-glucoronsyltransferase 1A1-mediated metabolism by atazanavir. Although data exist in the literature on the drug-drug interactions between atazanavir and combined contraceptives in the form of pills [25] and vaginal rings,[16] our study is the first to describe the drug-drug interactions between ritonavir-boosted atazanavir and subdermal etonogestrel. Atazanavir is a potent inhibitor of uridine diphospho-glucoronsyltransferase 1A1, and is extensively metabolized by CYP3A4, and is both a substrate and inhibitor of the CYP3A4 iso-enzyme.[25] Hence, boosting of atazanavir with ritonavir increases its serum concentration by inhibition of CYP3A. Therefore, it is expected that etonogestrel plasma concentrations would be increased when co-administered with atazanavir due to atazanavir-mediated inhibition of CYP3A4. This was consistent with the findings from our study, as none of the women in the ritonavir-boosted atazanavir arm had etonogestrel concentrations below the minimal threshold to suppress ovulation (90 pg/mL); and the median serum concentrations of etonogestrel when co-administered with ritonavir-boosted atazanavir, efavirenz, and ritonavir-boosted lopinavir were highest with etonogestrel/ritonavir-boosted atazanavir (604pg/mL), suggesting that ritonavir-boosted atazanavir does not reduce etonogestrel contraceptive efficacy.
We demonstrated etonogestrel concentrations above 90 pg/mL (the threshold for ovulation suppression) in women on ritonavir-boosted lopinavir, with median etonogestrel concentration of 428pg/mL. Lopinavir is primarily metabolized by CYP3A, and when co-administered with ritonavir (as ritonavir-boosted lopinavir), inhibits CYP3A-mediated metabolism.[26] The high etonogestrel concentration observed with concomitant ritonavir-boosted lopinavir-based antiretrovirals in this study is likely because ritonavir also inhibits CYP3A4 dependent hepatic metabolism of etonogestrel.
Our study has strengths. This is the first study to describe etonogestrel/ritonavir-boosted atazanavir drug-drug interactions. We monitored postpartum participants enrolled in the IMPAACT 1026s study, during which evaluation of clinical findings related to etonogestrel exposure occurred at regular time intervals. This study also had its limitations. First, we sampled participants twice between 2 and 12 weeks postpartum: prior to implant insertion in the postpartum period, and sampled at 6 to 7 weeks after implant insertion. Since a single etonogestrel implant is expected to provide contraception for three years post-insertion, we could not determine the effect of these antiretrovirals on etonogestrel plasma concentrations after the 12th postpartum week in our cohort. Second, we did not assess the pharmacogenomic relationship between ritonavir-boosted atazanavir, efavirenz, and ritonavir-boosted lopinavir which might affect etonogestrel plasma exposure.
In conclusion, we demonstrated that ritonavir-boosted atazanavir and ritonavir-boosted lopinavir do not impair etonogestrel efficacy. Our findings with etonogestrel/efavirenz drug-drug interactions are consistent with previous research suggesting that women using the etonogestrel contraceptive implant and efavirenz-based antiretroviral regimens could have decreased contraceptive efficacy. Women taking efavirenz should not use etonogestrel implants due to the increased risk of contraceptive failure. Etonogestrel implants can be offered to women on ritonavir-boosted atazanavir or ritonavir-boosted lopinavir.
Acknowledgments
We thank the patients for participating in the studies. We thank the staff from the centres participating in the IMPAACT network: IMPAACT investigators: 2802 New Jersey Medical School CRS (Linda Bettica, RN; Charmane Calilap-Bernardo, MA, PNPC; Arlene Bardeguez, MD, MPH); 3801 Texas Children’s Hospital CRS (Shelley Buschur, RN, CNM; Chivon Jackson, RN, BSN, ADN; Mary Paul, MD); 4201 University of Miami Pediatric Perinatal HIV/AIDS CRS ( Claudia Florez, MD; Patricia Bryan, BSN, MPH; Monica Stone, MD); 4601 University of California San Diego Mother-Child-Adolescent Program CRS (Andrew D. Hull, MD; Mary Caffery, RN, MSN; Stephen A. Spector, MD); 4701 Duke University Medical Center CRS (Joan Wilson, RN, BSN, MPH; Julieta Giner, RN, ACRN; Margaret A. Donnelly, PA-C); 5013 Jacobi Medical Center Bronx NICHD CRS (Mindy Katz, MD; Raphaelle Auguste, RN; Andrew Wiznia, MD); 5017 Seattle Children’s Hospital NICHD CRS (Jane Hitti, MD, MPH; Corry Venema-Weiss, ARNP; Joycelyn Thomas, RN); 5018 University of South Florida - Tampa NICHD CRS (Karen L. Bruder, MD; Gail Lewis, RN; Denise Casey, RN); 5023 Washington Hospital Center NICHD CRS (Rachel Scott, MD; Patricia Tanjutco, MD; Vanessa Emmanuel, BA); 5048 University of Southern California School of Medicine–Los Angeles County NICHD CRS (Françoise Kramer, MD; LaShonda Spencer, MD;James Homans, MD); 5052 University of Colorado Denver NICHD CRS (Emily Barr, CPNP, CNM, MSN; Jenna Wallace, MSW; Torri Metz, MD); 5072 Hospital dos Servidores Rio de Janeiro NICHD CRS (Esau C. Joao MD, PhD; Plinio Tostes Berardo Carneiro da Cunha, MD, PhD; Camile Medeiros Braga, MD); 5082 Hospital General de Agudos Buenos Aires NICHD CRS (Marcelo H. Losso, MD; Silvina A. Ivalo, MD; Alejandro Hakim, MD); 5093 Miller Children’s Hospital NICHD CRS (Jagmohan Batra, MD; Tempe Chen, MD; Janielle Jackson Alvarez, RN); 5098 Hospital Santa Casa Porto Alegre Brazil NICHD CRS (Regis Kreitchmann, PhD, MD; Debora Fernandes Coelho, MN, PhD; Marcelo Comerlato Scotta, MSc, MD); 6501 St Jude CRS (Katherine M. Knapp, MD; Nina Sublette, FNP, PhD; Thomas Wride, MS); 6701 The Children’s Hospital of Philadelphia (Steven D. Douglas, MD; Carol A. Vincent, PhD, CRNP; Samuel Parry, MD); 6901 Bronx-Lebanon Hospital CRS (Jenny Gutierrez, MD; Mary Elizabeth Vachon, MPH; Murli Purswani, MD).
Financial Support
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
Footnotes
Conflicts of Interest
The authors report no conflicts of interest related to this work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Clinical Trial Registration Number: The study is registered in ClinicalTrials.gov [NCT00042289].
REFERENCES
- [1].Pitts CJ. Update on Clinical Practice Guidelines for Human Immunodeficiency Virus. The Nursing clinics of North America. 2020;55:417–27. [DOI] [PubMed] [Google Scholar]
- [2].Eke AC, Olagunju A, Best BM, et al. Innovative Approaches for Pharmacology Studies in Pregnant and Lactating Women: A Viewpoint and Lessons from HIV. Clin Pharmacokinet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. 2020.
- [4].Joshi B, Velhal G, Chauhan S, et al. Contraceptive Use and Unintended Pregnancies Among HIV-Infected Women in Mumbai. Indian journal of community medicine : official publication of Indian Association of Preventive & Social Medicine. 2015;40:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception. 2016;94:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ali M, Akin A, Bahamondes L, et al. Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Human reproduction (Oxford, England). 2016;31:2491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennink HJ. The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant. The European journal of contraception & reproductive health care : the official journal of the European Society of Contraception. 2000;5 Suppl 2:12–20. [PubMed] [Google Scholar]
- [8].FDA. Implanon™ (Etonogestrel 68 mg subdermal implant).
- [9].Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception. 1998;58:283–8. [DOI] [PubMed] [Google Scholar]
- [10].Chappell CA, Lamorde M, Nakalema S, et al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS (London, England). 2017;31:1965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patel RC, Stalter RM, Thomas KK, et al. A pharmacokinetic and pharmacogenetic evaluation of contraceptive implants and antiretroviral therapy among women in Kenya and Uganda. AIDS (London, England). 2019;33:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Landolt NK, Phanuphak N, Ubolyam S, et al. Significant decrease of ethinylestradiol with nevirapine, and of etonogestrel with efavirenz in HIV-positive women. Journal of acquired immune deficiency syndromes (1999). 2014;66:e50–2. [DOI] [PubMed] [Google Scholar]
- [13].Yazdkhasti M, Pourreza A, Pirak A, Abdi F. Unintended Pregnancy and Its Adverse Social and Economic Consequences on Health System: A Narrative Review Article. Iranian journal of public health. 2015;44:12–21. [PMC free article] [PubMed] [Google Scholar]
- [14].Matiluko AA, Soundararjan L, Hogston P. Early contraceptive failure of Implanon in an HIV-seropositive patient on triple antiretroviral therapy with zidovudine, lamivudine and efavirenz. The journal of family planning and reproductive health care. 2007;33:277–8. [DOI] [PubMed] [Google Scholar]
- [15].Vieira CS, Bahamondes MV, de Souza RM, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. Journal of acquired immune deficiency syndromes (1999). 2014;66:378–85. [DOI] [PubMed] [Google Scholar]
- [16].Scarsi KK, Cramer YS, Rosenkranz SL, et al. Antiretroviral therapy and vaginally administered contraceptive hormones: a three-arm, pharmacokinetic study. The lancet HIV. 2019;6:e601–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tam VH, Kabbara S, Yeh RF, Leary RH. Impact of sample size on the performance of multiple-model pharmacokinetic simulations. Antimicrobial agents and chemotherapy. 2006;50:3950–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eke AC, Shoji K, Best BM, et al. Population Pharmacokinetics of Tenofovir in Pregnant and Postpartum Women Using Tenofovir Disoproxil Fumarate. Antimicrobial agents and chemotherapy. 2021;65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. 2014.
- [20].Sitruk-Ware R, Nath A, Mishell DR Jr. Contraception technology: past, present and future. Contraception. 2013;87:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Patel RC, Onono M, Gandhi M, et al. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: a retrospective cohort study. The lancet HIV. 2015;2:e474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].WHO. Medical eligibility criteria for contraceptive use: A WHO family planning cornerstone. 5th Edition ed: World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland: 2015. [Google Scholar]
- [23].Nair SG, Patel DP, Gonzalez FJ, Patel BM, Singhal P, Chaudhary DV. Simultaneous determination of etonogestrel and ethinyl estradiol in human plasma by UPLC-MS/MS and its pharmacokinetic study. Biomedical chromatography : BMC. 2018;32:e4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tittle V, Bull L, Boffito M, Nwokolo N. Pharmacokinetic and pharmacodynamic drug interactions between antiretrovirals and oral contraceptives. Clinical pharmacokinetics. 2015;54:23–34. [DOI] [PubMed] [Google Scholar]
- [25].Wood R Atazanavir: its role in HIV treatment. Expert review of anti-infective therapy. 2008;6:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van Waterschoot RA, ter Heine R, Wagenaar E, et al. Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. British journal of pharmacology. 2010;160:1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


