Abstract
Full-thickness wounds to the eye can lead to serious vision impairment. Current standards of care (from suturing to tissue transplantation) usually require highly skilled surgeons and use of an operating theater. In this study, we report the synthesis, optimization, and in vitro and ex vivo testing of photocrosslinkable hydrogel-based adhesive patches that can easily be applied to globe injuries or corneal incisions. According to the type and concentration of polymers used in the adhesive formulations, we were able to finely tune the physical properties of the bioadhesive including viscosity, elastic modulus, extensibility, ultimate tensile strength, adhesion, transparency, water content, degradation time, and swellability. Our in vitro studies showed no sign of cytotoxicity of the hydrogels. Moreover, the hydrogel patches showed higher adhesion on freshly explanted pig eyeballs compared to a marketed ocular sealant. Finally, ex vivo feasibility studies showed that the hydrogel patches could seal complex open-globe injuries such as large incision, cruciform injury, and injury associated with tissue loss. These results suggest that our photocrosslinkable hydrogel patch could represent a promising solution for the sealing of open-globe injuries or surgical incisions.
Keywords: Eye, cornea, injury, laceration, incision, adhesive hydrogel, patch
Graphical Abstract
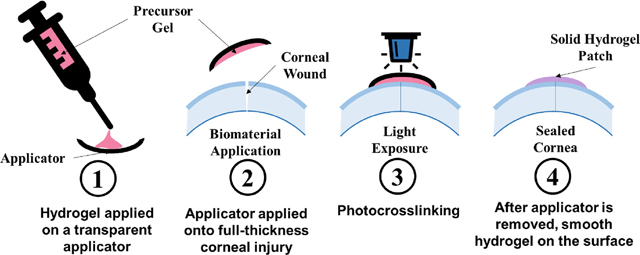
1. INTRODUCTION
With almost 2.5 million new cases each year in the US alone, ocular injuries represent a major burden for patients and healthcare providers [1,2]. Severe ocular injuries can induce scarring and cause corneal opacification, which is one of the leading causes of vision impairment worldwide [3]. Among them, open-globe injuries (full-thickness wounds) are particularly challenging to be treated due to breakage in the ocular protective barriers, thus compromising the eye integrity [4]. However, injuries are not the only cause of full-thickness breaks in the integrity of the eye; in addition to trauma, inflammation (auto-immune diseases) or iatrogenic causes (surgical incisions from ocular surgeries such as cataract surgery) are common [5,6]. While small, clear and linear incisions may seal spontaneously or close by using a bandage contact lens [7], larger and complex ocular wounds and incisions require surgical intervention. Suturing, conjunctival flap [8], amniotic membrane grafting [9], and corneal transplantation (keratoplasty) [10] are the main approaches to treat open-globe injuries. However, these approaches also require advanced surgical skills and an operating theater.
For these reasons, the use of surgical adhesives has been of interest for assisting in the closure of full-thickness ocular lacerations and cuts, especially in emergency settings. Cyanoacrylate glues, fibrin glues, and polyethylene glycol (PEG)-based sealants (i.e. ReSure® sealant) are the main commercially available product categories currently used in ophthalmology. Cyanoacrylate glues (i.e. Histoacryl®, Nexacryl® or Dermabond®) have been used off-label for many years by ophthalmologists [11,12]. They have high adhesive strength to the corneal tissue and can rapidly polymerize, resulting in good sealing properties. Nevertheless, cyanoacrylate glues release toxic by-products such as formaldehyde, which induces cytotoxicity, inflammation, and neovascularization [13,14]. Fibrin glues (i.e. Evicel®, Tisseel® or Artiss®) are composed of human fibrinogen and human thrombin that are packaged separately and polymerize once they are mixed together [12]. Due to their biological origin, fibrin glues are more biocompatible and well-tolerated by the body, including the eye, compared with cyanoacrylate glues [14]. However, their adhesive strength is low which can be not ideal for closure of full-thickness cuts [12,14]. Therefore, fibrin glues are usually used off-label as an adjunct to amniotic membrane [15–17] and conjunctival flaps or grafts [18,19]. PEG-based sealants are synthetic but well-tolerated surgical adhesives with fast polymerization times ranging from 20 to 60 sec [12]. Among this category, ReSure® is the only FDA-approved sealant for sealing clear corneal incisions (up to 3.5 mm) following cataract surgery [20]. However, ReSure® sealant typically persists for only 1 to 3 days [21], limiting its use for mid- to long-term sealing of very small incisions only. Due to these limitations, none of the surgical glues represent an optimal treatment to seal open-globe injuries, especially larger ocular wounds.
As a result, many studies have been published over the last decade on the development of new types of adhesives for ocular injuries [22]. Among these studies, only few studies have described adhesive properties specifically designed for ocular injuries [23,24]. Bayat et al. reported the use of a thermoresponsive sealant based on poly(N-isopropylacrylamide) (PNIPAM) for temporary closure of ocular injury [23]. Liquid at 4°C, PNIPAM sealant polymerized at physiologic temperature, occluding open-globe injuries. However, this sealant was tested only on 3-mm scleral linear incisions, which can often self-seal. More recently, McTiernan et al. developed a collagen/PEG-based sealant, called LiQD cornea, for sealing full-thickness corneal lacerations [24]. However, the sealant has been tested only on even a smaller 1-mm corneal full-thickness perforations. Our team has recently developed a naturally derived adhesive hydrogel, called GelCORE (gel for corneal regeneration), based on gelatin methacryloyl (GelMA) [25]. Despite its high biocompatibility and adhesion with corneal tissue, GelCORE was too liquid to be retained on full-thickness injuries without runoff during crosslinking time; thus, it could only be used on partial-thickness injuries (i.e. stromal defects).
In this study, we show the development, characterization, and in vitro and ex vivo testing of a hydrogel-based adhesive patch designed for sealing open-globe injuries. The patch is based on three photocrosslinkable polymers, GelMA, hyaluronic acid glycidyl methacrylate (HAGM) and PEG diacrylate (PEGDA). Each of these polymers present advantages which can be beneficial for the patch efficiency. First, GelMA has shown remarkable adhesive properties on ocular tissues [25]. Second, the high viscosity of HAGM can improve the retention of hydrogel prepolymer on the leaking injuries prior photopolymerization. Finally, the addition of PEGDA can improve the flexibility and stretchability as well as control the in vivo biodegradation of the resulting bioadhesives [26].
Easily applicable using a contact lens, the viscous precursor could be solidified upon exposure to visible light. We describe the synthesis of each polymer and the preparation of the hydrogel precursors. We then present data on the effects of each polymer on mechanical and other physical properties of the resulting patches, and the in vitro cytocompatibility and ex vivo adhesive properties of the patches in order to identify the best formulation for sealing open-globe injuries.
2. MATERIALS AND METHODS
2.1. Pre-polymer Synthesis
HAGM was synthetized using a protocol previously described [27–29]. Briefly, 10% (v/w) of hyaluronic acid sodium salt (1.6 MDa, Sigma Aldrich) was dissolved in 200 mL deionized water for 12 h under vigorous stirring. Once dissolved, 8.0 mL triethylamine (Sigma Aldrich), 8.0 mL glycidyl methacrylate (Sigma Aldrich), and 4.0 g of tetrabutyl ammonium bromide (TBAB) (Sigma Aldrich) were added separately in the mentioned order. After fully mixed, the resulted solution was incubated at 55 °C for 1 h. After cooling, the solution was then precipitated in 20 times excess volume of acetone (4 L), resulting in the formation of white solid fibers. The precipitate was then dissolved in ultrapure water, dialyzed, and freeze-dried.
GelMA was also synthetized using a protocol previously described [25,30,31]. Briefly, 10% (w/v) porcine gelatin (Instagel®, Bloom 240–250, PB Leiner USA, Davenport, IA) was dissolved in 200 mL deionized water for 1 h at 50°C. Then, the solution was reacted with 8 mL of methacrylic anhydride (Sigma Aldrich) for 3.5 h at 50°C. The solution was then dialyzed and freeze-dried.
PEGDA was synthetized as previously described with some modifications [32,33]. Briefly, 10 % (w/v) of PEG (35 kDa, Sigma Aldrich) was dissolved in dichloromethane at 4 °C. Next, triethylamine was added to the PEG solution under N2 environment. Acryloyl chloride (Sigma Aldrich) was then added to the solution and stirred overnight under dry N2 gas. The molar ratio of PEG, acryloyl chloride and triethylamine was 1:4:4. Finally, the insoluble salt (triethylamine-HCl) was filtered (using celite 545 powder and an alumina column), and the product was precipitated by adding ice-cold ether. The crude product was filtered with a 9-μm paper filter and freeze-dried.
2.2. Preparation of precursor gels and hydrogel samples
A visible light-sensitive photoinitatiator (PI) system was used to crosslink the pre-polymers into solid adhesive hydrogels. The PI solution was prepared by dissolving 0.5 mM Eosin Y disodium salt (photoinitiator), 1.875 % (w/v) triethanolamine (co-initiator) and 1.25 % N-Vinylcaprolactam (co-monomer) in phosphate buffered saline (PBS). Hydrochloric acid was used to adjust the pH of the final solution to around 7.4, as recommended for eye drops. Different formulations of hydrogel precursors were prepared by dissolving various concentrations of HAGM (1 %, 2 %, 3 %), GelMA (2%, 4 %) and PEGDA (0.5 %, 1 %) pre-polymers in the PI solution (Table 1). After complete dissolution, the final precursor gels were loaded into a 1-mL syringe and protected from light until use. Then, precursor gels were prepared following protocol previously described [25]. Briefly, precursor gels were injected into polydimethylsiloxane cylindrical molds (6-mm diameter; 2.5-mm height) and crosslinked for 4 min using a dental blue light (450-nm wavelength, 300-mW intensity, with 1 cm between the light and the sample).
Table 1.
Composition of formulations of hydrogel precursors prepared from different pre-polymer 17 concentrations.
| Formulation name | Pre-Polymer concentration (%) | ||
|---|---|---|---|
| HAGM (H) | PEGDA (P) | GelMA (G) | |
| H1 | 1% | - | - |
| H2 | 2% | - | - |
| H3 | 3% | - | - |
| H3P0.5 | 3% | 0.5% | - |
| H3P1 | 3% | 1% | - |
| H3G2 | 3% | - | 2% |
| H3G4 | 3% | - | 4% |
| H3G4P0.5 | 3% | 0.5 % | 4% |
| H3G4P1 | 3% | 1 % | 4% |
2.3. Rheological tests
Fifty μL of precursor solutions were pipetted between the parallel plates of an Anton-Paar 302 Rheometer. Steady shear rate sweeps were conducted by varying the shear rate from 1 to 500 s−1 at 37 °C to determine the shear stress and viscosity of the precursor gels. The data were analyzed by Carreau-Yasuda model. All rheological tests were repeated at least three times (n≥3).
2.4. Tensile tests
The tensile tests were conducted using an Instron 5542 mechanical tester. Prior the test, the photocrosslinked hydrogel samples (at least 3 per group) were placed between two pieces of double-sided tape within the instrument tension grips (14 mm length and 5-mm width rectangle, 8-mm gauge distance) and extended at a rate of 2 mm/min until failure. The slope of the stress-strain curves was obtained and reported as elastic modulus. The stress and the strain at the point of failure were reported as ultimate stress and extensibility.
2.5. Transparency tests
Transparency of hydrogel samples prepared from the different formulations (Table 1) was assessed using a photographic-based method as previously described [34]. A resolution test chart (R2L2S1P1, Thorlabs) was placed under a stereomicroscope equipped with a CMOS camera (S9i, Leica). Hydrogel samples (n=3 per group) were placed on the resolution test chart and a photograph of each sample was taken. Photographs were then analyzed using ImageJ software. For this, a 3×2-mm2 region of interest (ROI) of the hydrogel sample was selected and the minimal and maximal pixel intensity of this ROI was measured. Contrast was calculated using Eq. 1, where Imax and Imin are the maximal and the minimal pixel intensity of the ROI, respectively.
| (Eq. 1) |
2.6. Water content measurement
Hydrogel samples were prepared as described in section 2.2. Immediately after photocrosslinking, samples (n=6 per formulation) were incubated in PBS for 24h at 37 °C. The samples were then freeze-dried and weighed. The water content was calculated according to Eq. 2, where W1 is the weight of the sample after 1-day incubation in PBS and W2 is the weight of the dried sample.
| (Eq. 2) |
2.7. In vitro degradation tests
Hydrogel samples were prepared as described in section 2.2. After photocrosslinking, samples (n=6 per formulation) were then incubated at 37°C in PBS supplemented with 2U/mL of hyaluronidase and collagenase I, two enzymes naturally present in the eye. The enzyme solutions were refreshed every week to maintain the enzymatic activity. Each day, the presence of the hydrogel was visually assessed by the same operator. The degradation time was determined by the first day where the hydrogel was not found present in the enzymatic solution.
2.8. In vitro swellability tests
Hydrogel samples were prepared as described in section 2.2. The weight of each hydrogel sample (n = 9 per formulation) was measured following photocrosslinking and after 1 h, 2 h, 6 h, 1 day, 2 days, 3 days, 1 week, 2 weeks, 3 weeks, and 4 weeks incubation in PBS at 37 °C. Swelling ratio was then calculated according to Eq. 3, where W0 is the weight of the sample just after photocrosslinking and W1 is the final weight of the sample after 24 h incubation.
| (Eq. 3) |
2.9. In vitro toxicology tests
In vitro toxicology tests were performed to assess biocompatibility of the hydrogel patches using human corneal epithelial cells (HCECs, provided from Dr. Argueso lab, Schepens Eye Research Institute, Mass. Eye and Ear). The elution test method was used according to ISO10993–1 standard concerning the cytotoxicity of medical devices. HCECs were cultivated in 48-well plates (for Viable/Dead test) or 96-well plates (for MTT assay). Extracts were obtained by incubating photocrosslinked hydrogel samples in 1 mL of keratocyte serum-free medium (K-SFM kit, 17005042, ThermoFisher) supplemented with bovine pituitary extract (50 μg/mL) and human recombinant epithelial growth factor (5 ng/mL) at 37 °C for 24 h. Fluid extracts were then applied to a confluent HCEC monolayer. Control groups were prepared similarly by incubating the cells with fresh medium. After 3 days of incubation at 37 °C, cells were stained with calcein-AM (viable dye) and propidium iodide (dead dye) (Cell Viability Imaging Kit, 06432379001, Sigma Aldrich). Then, cells were observed using an inverted fluorescence microscope (n=3 per formulation). In vitro PrestoBlue assay (PrestoBlue™ Cell Viability reagent, A13261, ThermoFisher) was also performed on each group following manufacturer protocols (n=3 per formulation).
2.10. Ex vivo adhesion tests
In order to correctly seal ocular injuries, hydrogel patches must have high adhesive properties, especially in a wet environment. We assessed the adhesive properties of the different formulations (Table 1) ex vivo by usingfreshly explanted pig eyeballs (Sierra Medical, Whittier, CA). A 4-mm linear full-thickness incision was created on the cornea of each pig eyeball using a surgical 15° stab knife. An infusion cannula was placed in the eye to reproduce the physiologic intraocular pressure (IOP) and to monitor success of a proper incision. Once incision success was confirmed via leaking from the incision, the hydrogel patches were applied on the incision using a contact lens (methafilcon A, 55% water, diameter 15, base curve 8.30, Kontur Kontact, Hercules, CA) manually trephined at 8 mm as shown in Fig. 1C (n=10 per formulation). Images from the cross-sections of the sealed cornea were acquired using Optical Coherence Tomography (OCT). Marketed ReSure® sealant was used as a control (n=5). IOP was then increased by injecting PBS via an infusion cannula until the hydrogel detached and the incision leaked. The pressure at which the leakage was observed from the defect site was recorded by using a pressure sensor (PS-3203, PASCO Scientific, Roseville, CA).
Fig. 1. Synthesis and application of photocrosslinkable hydrogel patch.
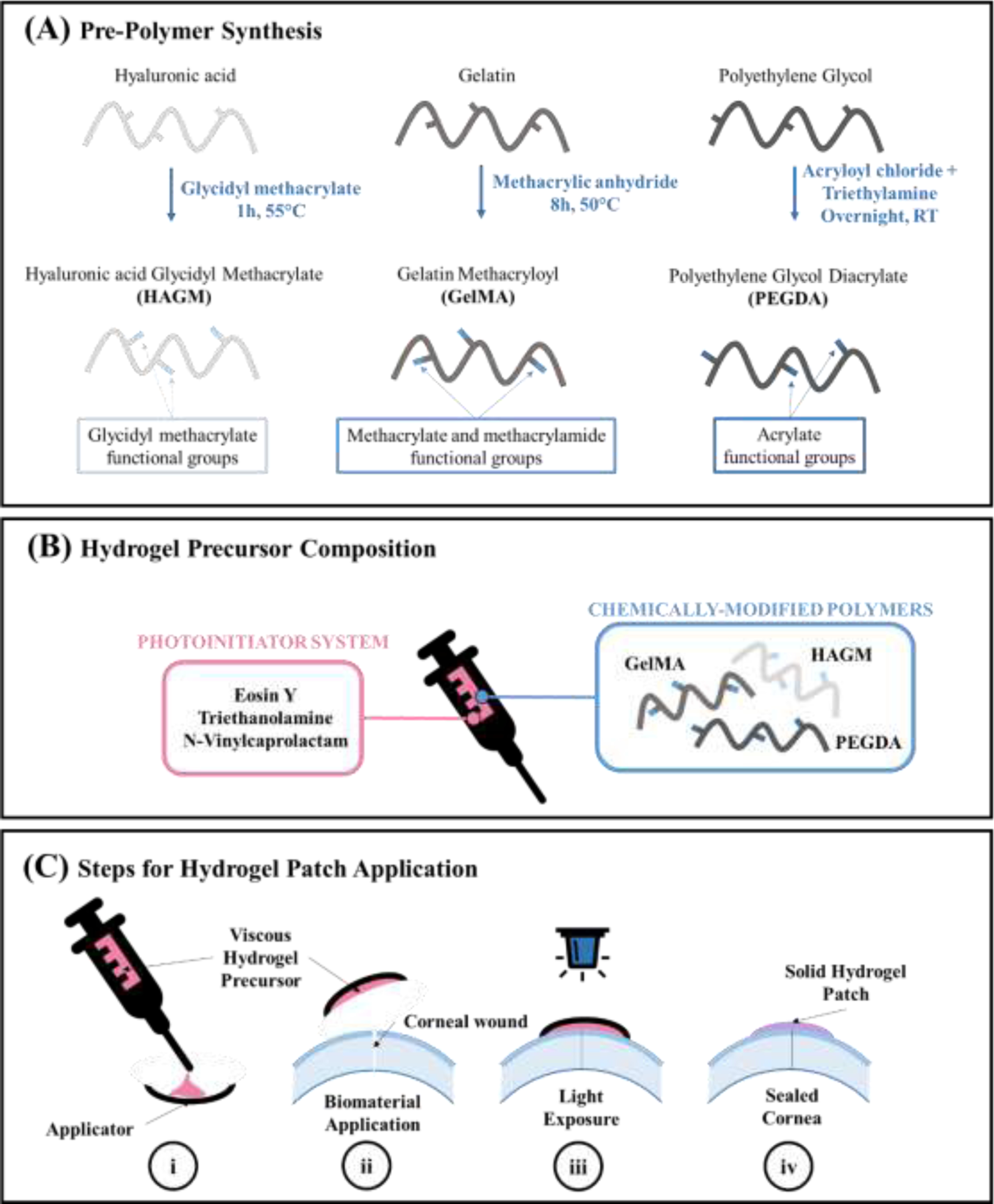
(A) Synthesis of each polymer that forms the hydrogel precursors. (B) Hydrogel precursors are composed of three polymers (HAGM, GelMA and PEGDA) and a photoinitiator system (Eosin Y, triethanolamine and N-vinylcaprolactam) and loaded into a syringe for easy application. (C) (i) Hydrogel precursors are first applied on a transparent applicator similar to a contact lens. (ii) Then, the applicator containing the hydrogel precursor is directly applied onto the full-thickness injury. (iii) Precursors are photocrosslinked for 4 min using blue light into solid hydrogels. (iv) After photocrosslinking, the applicator can be removed, leaving behind a smooth adhesive hydrogel on the surface of the eye.
2.11. Ex vivo feasibility studies
Dependent on the cause of injury, ocular wounds can have various sizes and shapes. While cataract surgeries induce small clear linear incisions, projectile impacts can lead to large and complex injuries that are particularly challenging to seal. Therefore, a feasibility study was performed to determine if the hydrogel patches can efficiently seal large or complex injuries. Three types of corneal full-thickness injuries were assessed in this study: large linear incision, cruciform injury and injury associated with tissue loss. Large linear incision and cruciform injury were created using a surgical 15° stab knife, and the injury associated with tissue loss was performed using a 3-mm surgical trephine. After, approximatively 100 μL of H3G4P1 precursor gel was applied directly on injuries using a contact lens (methafilcon A, 55% water, diameter 15, base curve 8.30, Kontur Kontact, Hercules, CA). In order to spread the hydrogel precursor all over the injury, circular movement of the contact lens over the ocular surface were performed manually. Then the hydrogel precursors were photocrosslinked for 4 min on the injury until patch formation. The contact lens was then removed, and dimensional assessments were performed using macroscopic and OCT imaging.
2.12. Statistical analysis
At least three samples were tested per group for each experiment. Six samples were tested for the measurement of water content, degradation tests and MTT-based assays. Nine samples were testing for the measurement of swellability and adhesion tests. Mann-Whitney tests were performed to determine significant differences between groups. All statistical analyses and figures were realized using GraphPad Prism 6.0 (GraphPad Software). All data were presented as means ± SD.
3. RESULTS
3.1. Synthesis and physical characterization of bioadhesive
We developed a biomaterial based on three polymers (HA, gelatin and PEG), that were chemically modified to become photocrosslinkable (Fig. 1A). The hydrogel precursors were prepared by mixing different concentrations of each polymer (see Table 1) with photoinitiators. The final precursor solutions were loaded into a syringe, allowing precise application (Fig. 1B). Using an applicator such as a contact lens, the hydrogel precursor can be applied onto the ocular injury. After 4-min crosslinking using visible light, the precursor turned into a solid hydrogel that adhered onto the injury. After photocrosslinking, the contact lens can be removed easily using a forceps, leaving behind a smooth and adherent hydrogel layer on the cornea (Fig. 1C).
The viscosity of the hydrogel precursor solution formed by using different concentrations of HAGM, GelMA and PEGDA (Table 1) was measured using rheological analysis. Results showed the higher the concentration of HAGM, the more viscous the resulting precursor solution (Fig. 2A-B). However, precursors containing GelMA and PEGDA did not show significant differences in term of viscosity (Fig. 2B). Based on these results, 3% HAGM was selected as the optimal concentration that gave optimal viscosity to the hydrogel precursors, providing an initial retention on the injury site prior and during photocrosslinking.
Figure 2. Mechanical characterization of the hydrogel precursors and the photocrosslinked hydrogels.
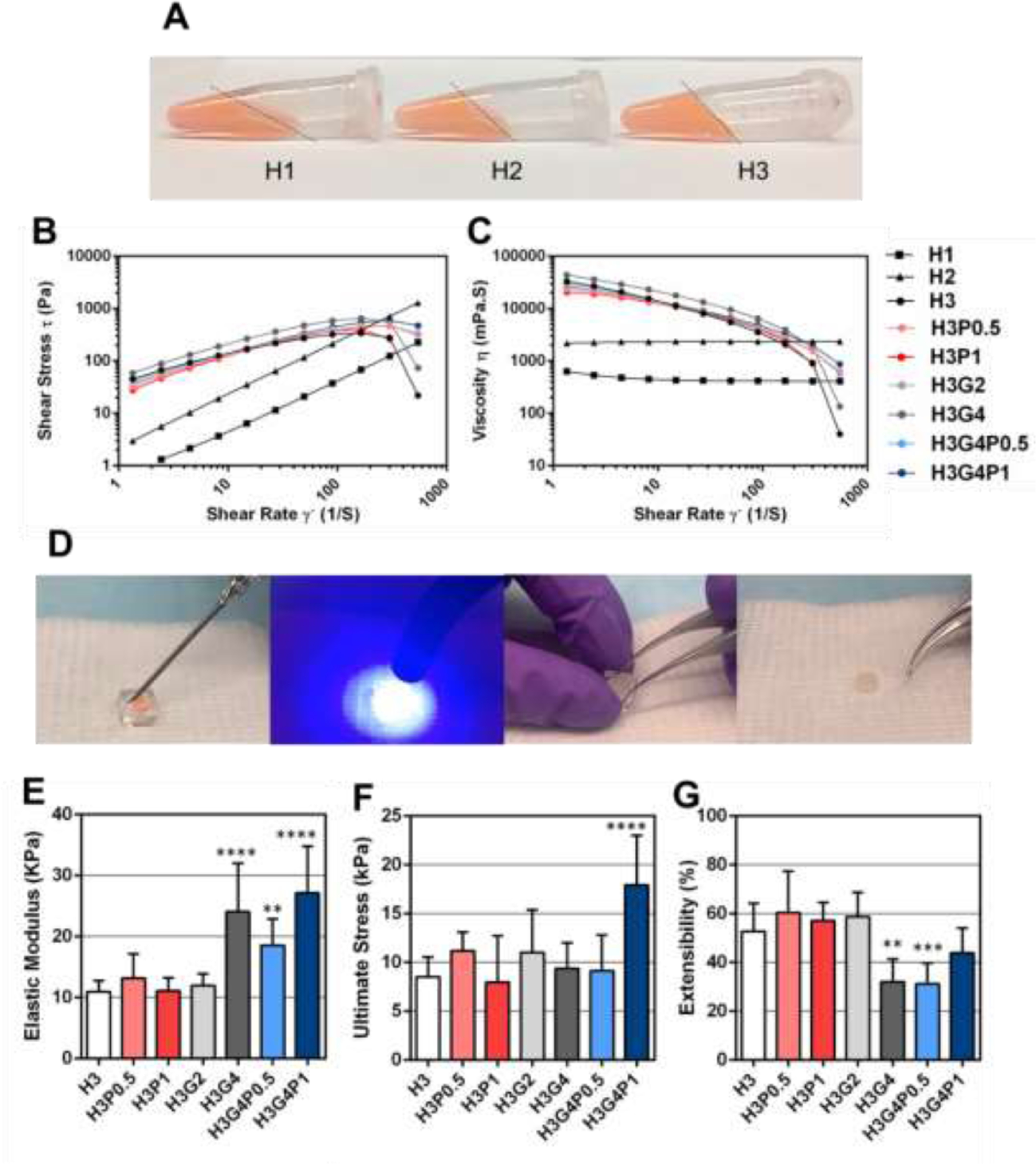
(A) Representative images of hydrogel precursors prepared with 1% (H1), 2% (H2) and 3% (H3) HAGM. (B) Shear stress and (C) steady-shear viscosity of hydrogel precursors prepared with different concentrations of HAGM, GelMA and PEGDA. (D) Steps for the preparation of photocrosslinked hydrogel samples. Hydrogel precursors were injected into polydimethylsiloxane cylindrical molds (6-mm diameter; 2.5-mm height) and crosslinked for 4 min using blue light. (E) Elastic modulus, (F) ultimate tensile strength and (G) Extensibility of photocrosslinked hydrogel samples prepared with different concentrations of HAGM, GelMA and PEGDA. Data is represented as mean ± SD (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 and n ≥ 3).
Tensile tests were performed on the hydrogels prepared with different concentrations of HAGM, GelMA and PEGDA (Table 1) after photocrosslinking for 4 min using blue light (Fig. 2D). Elastic modulus was found significantly higher for all hydrogels containing 4% GelMA (24 ± 7 kPa for H3G4, 19 ± 4 kPa for H3G4P0.5 and 27 ± 7 kPa for H3G4P1) compared with hydrogels containing 2% GelMA or no GelMA (e.g. 11 ± 2 kPa for H3) (Fig. 2E). The value for ultimate tensile stress was found significantly higher only for hydrogels containing 4% GelMA and 1% PEGDA (18 ± 2 kPa for H3G4P1) compared with all other hydrogels (e.g. 9 ± 2 kPa for H3) (Fig. 2F). On the contrary, extensibility was found significantly lower for some hydrogels containing 4% GelMA (32 ± 8 % for H3G4 and 31 ± 8 % for H3G4P0.5) compared to the hydrogels containing 2% GelMA or no GelMA (e.g. 53 ± 10 % for H3) (Fig. 2G). However, no significant difference was found for the hydrogels containing both 4% GelMA and 1% PEGDA (44 ± 9 %) compared to the hydrogels with 2% GelMA or no GelMA (Fig. 2G).
These results suggest that the addition of 4% GelMA to hydrogels gave more resistance to deformation, but also less extensibility. By adding 1% PEGDA, extensibility can be restored, and a higher material resistance can be achieved. Eyelid movement induces shear stresses on the ocular surface. Having a hydrogel patch with adaptive characteristics that is flexible enough to endure increased shear stress and return to its initial shape can also decrease the sensation of a foreign body on the ocular surface.
Other physical properties, including transparency, water content, degradation time and swelling ratio have been assessed on the photocrosslinked hydrogels prepared with different concentrations of HAGM, GelMA and PEGDA (Table 1). While hydrogels prepared without GelMA were found to be highly transparent (between 98–100% for H3, H3P0.5 and H3P1), transparency was significantly lower for hydrogels prepared with 2% GelMA (~78% for H3G2) or 4% GelMA (between 24–31% for H3G4, H3G4P0.5 and H3G4P1) (Fig. 3A-B). Transparency was also found significantly lower when 1% PEGDA was added (~24% for H3G4P1) compared with hydrogels with 0.5% PEGDA or no PEGDA (~31% for H3G4 and H3G4P0.5) (Fig. 3A-B).
Figure 3. Physical characterization of hydrogels after photocrosslinking.
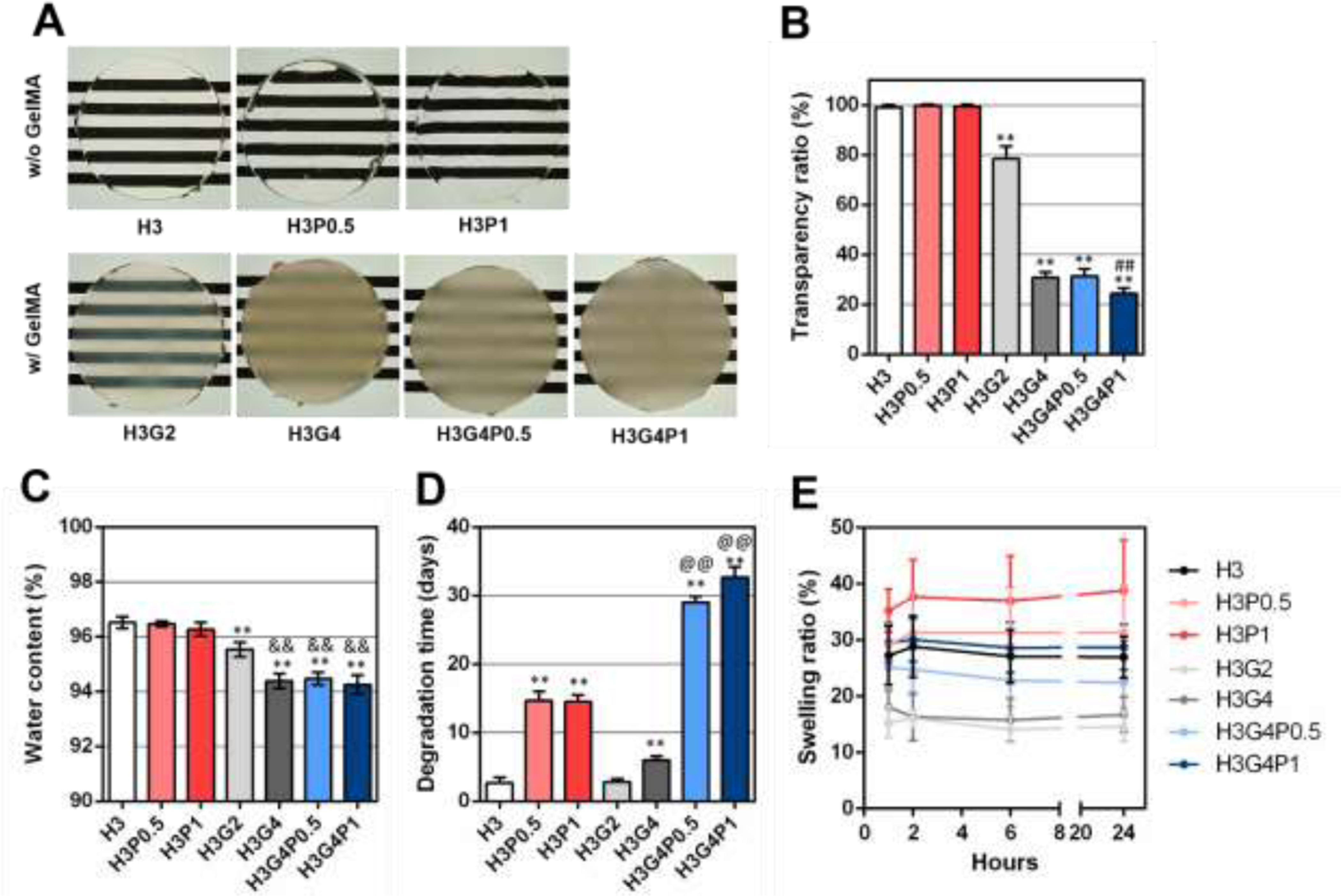
(A) Macroscopic images of photocrosslinked hydrogel sample, prepared with 3% HAGM and different concentrations of GelMA and PEGDA, placed on a resolution test chart. (B) Transparency ratios (%), (C) water content (%), (D) degradation time (days) and (E) swelling ratio over 24 h after photocrosslinking (%) of the photocrosslinked hydrogels. Data is represented as mean ± SD (**p<0.005 compared to H3, ##p<0.005 compared to H3G4P0.5, &&p<0.005 compared to H3G2, @@p<0.005 compared to H3P1, n=6).
The water content of all hydrogel formulations was found to be between 94 % and 96%, which is higher than the water content of the native cornea (~80%) (Fig. 3C). Water content was found significantly lower when 2% GelMA (96 ± 0.2 % for H3G2) or 4% GelMA (94 ± 0.2 % for H3G4) was added compared with hydrogels prepared without GelMA (97 ± 0.2 % for H3). On the contrary, the addition of PEGDA (0.5% or 1%) did not induce any significant difference of water content of the hydrogel samples (Fig. 3C).
The degradation time of photocrosslinked hydrogels was also assessed in the presence of enzymes naturally present in the eye. The addition of 0.5% or 1% PEGDA significantly and largely delayed the degradation time from ~3 days (for H3) to ~15 days (for H3P0.5 and H3P1), respectively (Fig. 3D). While the addition of 2% GelMA did not affect the degradation time (~3 days for H3G2), it was found that 4% GelMA slightly delayed the degradation time (~6 days for H3G4). Moreover, the simultaneous use of GelMA and PEGDA significantly and largely delayed the degradation time until ~29 days for H3G4P0.5 and ~33 days for H3G4P1 (Fig. 3D).
Finally, the swelling ratio of each hydrogel formulation was determined after incubation in PBS at different time points. Results showed that the swelling ratio of all hydrogels increased in the first hour of incubation and remained stable afterwards for 24 h (Fig. 3E). While the addition of 0.5% and 1% PEGDA increased the swelling ratio after 1 h (29 ± 3 % for H3P0.5 and 35 ± 3 % for H3P1), the addition of 2% and 4% GelMA decreased the swelling ratio after 1 h (15 ± 3 % for H3G2 and 18 ± 3 % for H3G4). Interestingly, the simultaneous use of GelMA and PEGDA allowed for swelling ratios (25 ± 4 % for H3G4P0.5 and 30 ± 3 % for H3G4P1) similar to the hydrogel with no PEGDA and GelMA (27 ± 5 for H3) (Fig. 3E).
All these results suggest that the physical properties of the photocrosslinked hydrogels could be tuned according to the concentrations of GelMA and PEGDA used. An ideal hydrogel for corneal sealing should have a high transparency to preserve the patient vision and a water content similar or higher compared to the native cornea, to maintain an optimal oxygenation and nutrients renewal on the ocular surface. Moreover, a slow degradation rate of the hydrogel would allow for longer retention on the injury. Finally, a limited swellability can keep the initial volume of the hydrogel, which could provide better comfort for the patient. On one hand, GelMA seems to reduce the transparency and swelling. On the other hand, PEGDA seems to delay the degradation rate and induce swelling.
3.2. In vitro cytocompatibility
In vitro cytotoxicity studies of photocrosslinked hydrogels were assessed following the ISO10993–1 standard concerning the cytotoxicity of medical devices. Results showed that no change was observed in the Viable/Dead staining and density between HCECs incubated with the culture medium alone or with the extracts of the photocrosslinked hydrogels, regardless of the addition of GelMA or PEGDA (Fig. 4A). Moreover, PrestoBlue assays showed no significant difference in absorbance among all groups tested after 3-days incubation with hydrogels (Fig. 4B). These results suggested that all photocrosslinked hydrogels tested were not toxic for human cells and thus, could be well-tolerated on the human ocular surface.
Figure 4. In vitro cytocompatibility of the hydrogels after photocrosslinking.
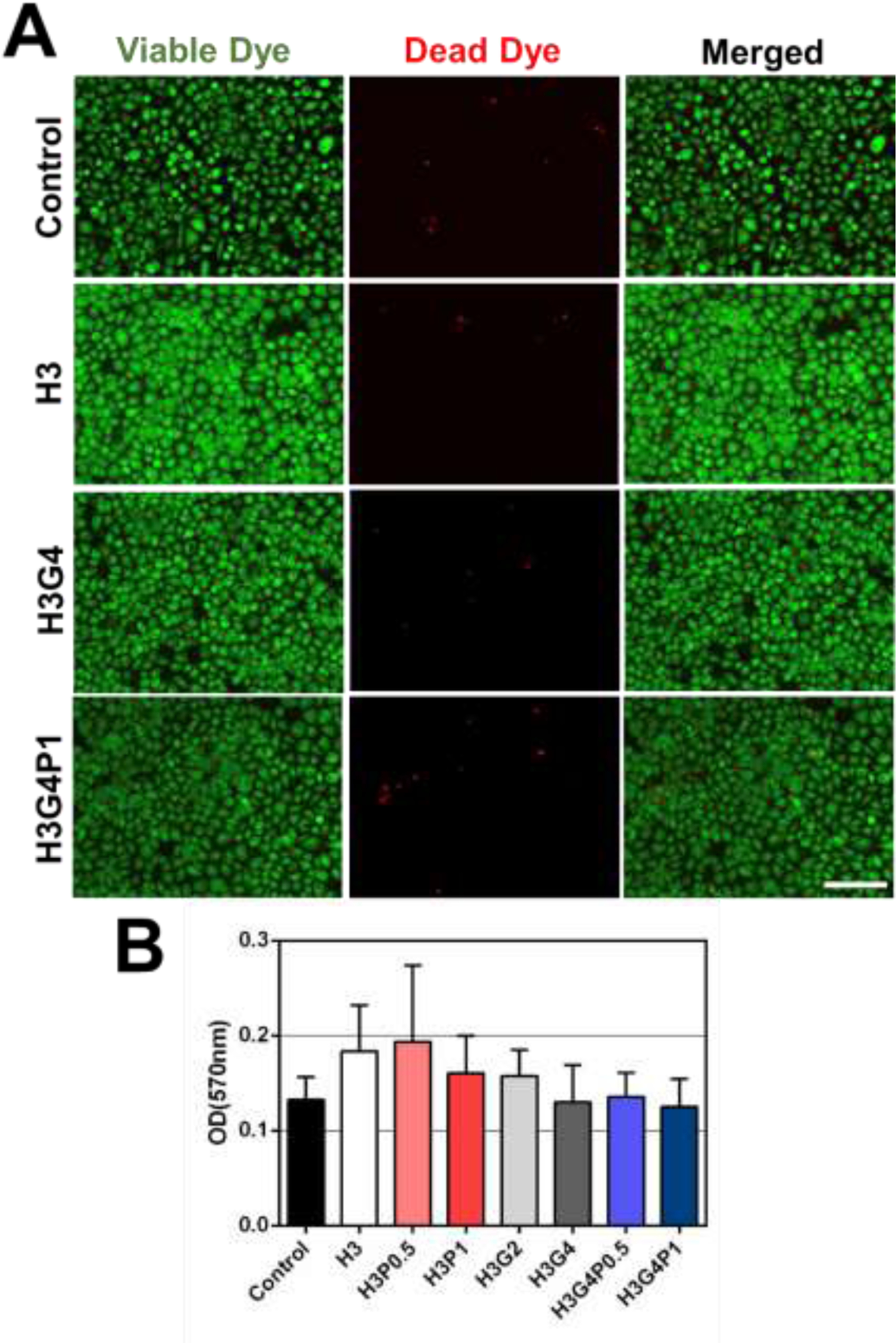
(A) Microscopic images of human corneal epithelial cells (HCECs) incubated for 24 h with culture medium or fluid extracts of photocrosslinked hydrogels prepared with different concentrations of HAGM, GelMA and PEGDA and then stained with calcein-AM (green - viable dye) and propidium iodide (red - dead dye) (n=3 per group), scale bar = 100 μm. (B) Cell viability measured by PrestoBlue assay after 3-days incubation with hydrogels prepared with different concentrations of HAGM, GelMA and PEGDA. Data is represented as mean ± SD (n=3 per group).
3.3. Ex vivo adhesion
Adhesive properties of the hydrogel patches were assessed on freshly explanted pig eyeballs (Fig. 5A). Different hydrogel precursors, prepared with different concentrations of HAGM, GelMA and PEGDA, have been compared to the marketed ReSure® sealant. OCT images showed that, just after 4-min photocrosslinking, the hydrogel patches were found firmly adherent to the corneal surface and thus able to seal the 4-mm incision without visual leaking (Fig. 5B). Adhesion of each adhesive was then quantified by injecting PBS into the eye and measuring the resulting burst pressure. Results demonstrated that all formulation exhibited burst pressures higher than 22 mmHg, which corresponds to the normal intraocular pressure of the human eye (Fig. 5C). In addition, the hydrogels composed of 4% GelMA significantly increased the burst pressure values (121 ± 27 mmHg for H3G4) compared with hydrogels without GelMA (79 ± 30 mmHg for H3). However, no significant difference of burst pressures was observed with the addition of 0.5 or 1% PEGDA. The burst pressures obtained for all the hydrogel patches, regardless of polymer concentrations, were found to be higher compared with ReSure® sealant (37 ± 18 mmHg) (Fig. 5C). Interestingly, while ReSure® burst via a break in the center of the material (cohesion failure), hydrogel patches usually burst by detachment at the adhesive and ocular surface interface while staying intact (adhesion failure).
Figure 5. Adhesion assessment of the hydrogel patches on an ex vivo pig corneal injury.
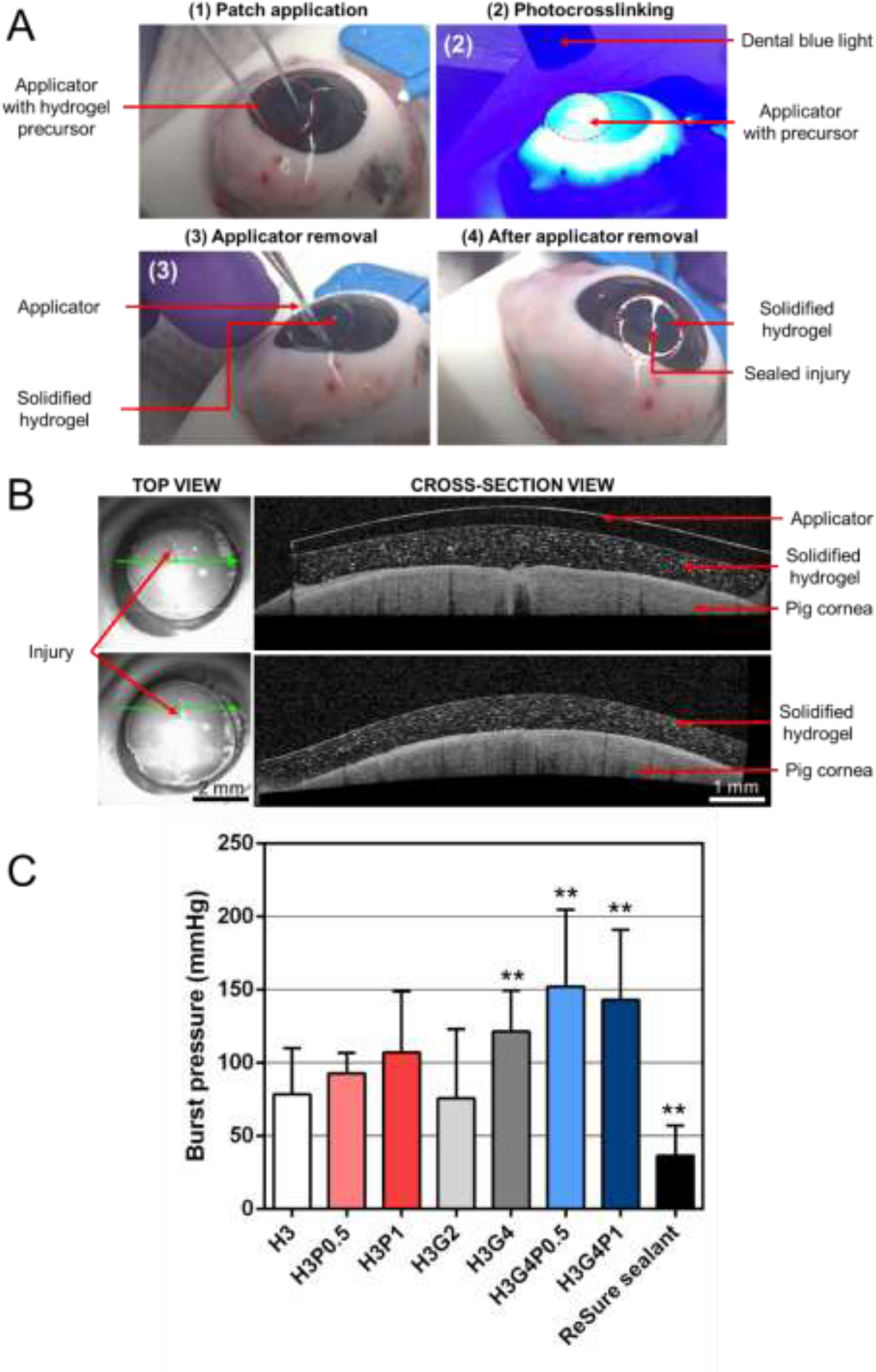
(A) Representative images for hydrogel patch application (formulation H3). (B) OCT images of the hydrogel patch after photocrosslinking before and after applicator removal (formulation H3). (C) Average burst pressures of the photocrosslinked hydrogel patches prepared with different concentrations of HAGM, GelMA and PEGDA (n=10 per group) compared with ReSure® (Control, n=5). Graph is presented as mean ± SD (**p<0.005 compared to H3).
3.4. Ex vivo feasibility
Feasibility studies were performed to estimate the scope of open-globe injuries that could be sealed by our hydrogel patch technology. According to the previous results, the hydrogel containing 3% HAGM, 4% GelMA and 1% PEGDA (H3G4P1) exhibited the highest burst pressure with the slowest degradation rate. Therefore, this formulation has been tested on three different types of open-globe injuries (Fig. 6A). The first model was a large linear injury that could occur when a sharp object hit the eye, the second one was a cruciform injury, that could occur when a blunt object hit the eye, and the third model was an injury associated with tissue loss, that could occur after auto-immune diseases or infections. Results demonstrated that the patches were able to cover the width of the entire injuries (Fig. 6B). OCT images showed that the hydrogel finely fitted the borders of the injury and provided a smooth surface (Fig. 6C). No leaking was visually observed. These results suggest that the hydrogel patch technology could be efficient in sealing different types of open-globe injuries.
Fig. 6. Application and dimensional assessment of hydrogel patches on different types of open-globe injuries.

(A) Models of open-globe injuries models tested, (B) Macroscopic images and (C) OCT images of pig eyeballs with different injury models after application and photocrosslinking for 4 min (n=3 per group). Blue asterisks correspond to corneal tissue and pink asterisks to the hydrogel patch. Scale bar = 3 mm.
4. Discussion
Despite the existence of treatments readily available for superficial injuries and small ocular incisions, larger open-globe injuries can be challenging to seal, especially in suboptimal or emergent settings where access to qualified surgeons, materials, or operating theaters can be challenging or non-existent [35]. In some settings, the average time between point of injury to the eye and surgical repair has been reported to be as long as several weeks [36]. If not correctly treated, open-globe injuries can lead to a myriad of complications including infection, significant inflammation, neovascularization and scar formation, all of which can profoundly diminish the chance of visual recovery [37]. The ideal characteristics for an ocular sealant are the following: (1) ease of application, (2) high viscosity before crosslinking to rapidly and efficiently seal a wound without run-off, (3) strong adhesiveness on the ocular surface, (4) high tensile strength, (5) low degradation time to assure retention until complete healing or surgical intervention, (6) excellent biocompatibility, (7) high water content (up to 75% as recommended for contact lenses) to preserve corneal oxygenation, (8) minimal swellability to prevent foreign body sensation and thus increase patient comfort and (9) transparent to preserve patient vision.
In this study, we describe the development and characterization of a photocrosslinkable hydrogel patch designed for sealing open-globe injuries. Each of the three pre-polymers (HAGM, GelMA and PEGDA) give specific properties to the resulting hydrogel. First, HAGM makes the precursor viscous enough for application, and most importantly, for retention on the injury during polymerization without run-off. This is especially beneficial when compared to cyanoacrylate glues, due to their low viscosity, can only be applied on patients in the supine position to prevent significant run-off [38]. The high viscosity of our hydrogels also allows for control of the thickness of adhesive applied resulting in better sealing. Moreover, HA is already used in ophthalmology and known to be well-tolerated in the eye [39–41]. Secondly, we show that the addition of GelMA results in a higher stiffness (higher elastic modulus) of the hydrogel as well as a higher adhesion onto the ocular surface, both parameters susceptible to improve sealing efficacy. The gelatin backbone contains RGD motif which promotes cell adhesion and binding [42]. Moreover, the use of GelMA seems to decrease the swellability of the resulting hydrogel following photocrosslinking, producing a patch that could be more comfortable for the patient. In previous studies, we showed that GelMA can promote ex vivo and in vivo re-epithelialization of the cornea improving the wound healing process [25,31]. However, GelMA also lead to a loss in transparency, which can potentially reduce the patient vision during the healing process. Gelatin contains hydrophobic groups which may be attributed to the loss of transparency when mixed with hydrophilic polymer. Although placement of a transparent patch would be ideal, it is worth noting that the presence of ocular injuries already result in a significant loss of vision for the patient. Therefore, any benefits of transparency are forfeited as vision is already compromised at application. Third, the addition of PEGDA tends to increase resistance to deformation of the hydrogel as well as substantially delaying degradation time. PEGDA is a derivative of a synthetic polymer known to have a lower degradation rate compared natural polymers such as HAGM or GelMA [26]. Moreover, PEG is well-tolerated in the human body and are already used in various pharmaceutical formulations [43]. With all these polymers combined, it produces a hydrogel with suitable properties for sealing open-globe injuries. In particular, the formulation H3G4P1 exhibited the higher adhesion strength and lower biodegradation rate compared to all formulations tested in this study. Moreover, in vitro cytotoxicity tests show no sign of toxicity by using these three polymers.
The feasibility study performed on freshly explanted pig eyeballs showed that our hydrogel patch can be sealed on various models of open-globe injuries, induced by sharp or blunt objects, as well as injury associated with tissue loss, suggesting that our technology can be adjusted according to the type of injury. In order to assess the efficiency of the hydrogel by itself, the contact lens has been removed in this study. However, the contact lens could also help to maintain the hydrogel on the ocular surface. We expect the patch to be fully degraded or detached by the end of the wound healing process, or able to be manually removed using forceps prior surgical intervention. However, it is important to assess the degradation rate of the engineered adhesive in vivo which will be the focus of our future work.
This study establishes the first proof-of-concept assessing in vitro and ex vivo feasibility, efficiency, and safety of a photocrosslinkable hydrogel-based patch for sealing open-globe injuries. We acknowledge that the 4-min photocrosslinking time could be long to maintain for an eye. However, the eye need not be fixated onto the light. We are also working to optimize the photoinitiators to lower the time needed for crosslinking. Therefore, this technology could represent a promising solution to stabilize ocular injuries in emergency settings before surgical repair.
5. Conclusion
Contrary to commercially available adhesives, our technology can be easily applied underneath a contact lens, so that no specific surgical skills or operating theater is required. Moreover, crosslinking of the patch can also easily be triggered using a simple portable visible LED system. Other adhesives mentioned such, as fibrin glues or cyanoacrylate glues, polymerize upon contact with the body or by mixing two components prior to application. Therefore, it requires the operator to apply the adhesive in a very short time, making it challenging to apply precisely with optimal control. Polymerization using visible light gives the added benefit to the operator to have the adequate time to correctly apply the hydrogel patch on the injury before solidification. The use of in vitro and ex vivo models allowed us to test multiple formulations to optimize various properties to determine the most suitable formulation for future in vivo testing and clinical applications. Despite these promising in vitro and ex vivo results, the efficiency of our technology still needs to be tested in vivo.
Statement of Significance:
Current management of severe ocular injuries require advanced surgical skills and access to an operating theater. To address the need for emergent management of wounds that cannot be handled in the operating room, surgical adhesives have gained popularity, but none of the currently available adhesives have optimal bioavailability, adhesive or mechanical properties. This study describes the development, optimization and testing of a light-sensitive adhesive patch that can easily be applied to the eye. After solidification using visible light, the patch shows no toxicity and is more adherent to the tissue than a marketed sealant. Thus this technology could represent a promising solution to stabilize ocular injuries in emergency settings before definitive surgical repair.
6. Acknowledgments
This work is supported by National Institutes of Health (NIH) (R01EB023052, R01HL140618), Department of Defense Vision Research Program Technology/Therapeutic Development Award (W81XWH-18-1-0654), Research to Prevent Blindness (RPB) Stein Innovation Award and The Tej Kohli Cornea Program at Mass Eye and Ear, located at Mass Eye and Ear, Boston, US. Senne Seneca is an SB PhD Fellow at the Research Foundation Flanders (FWO).
Footnotes
Conflicts of interest
Dr. Dana and Dr. Annabi have equity interest in Gelmedix Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Whitcher JP, Srinivasan M, Upadhyay MP, Corneal blindness: a global perspective, Bull. World Health Organ 79 (2001) 214–221. [PMC free article] [PubMed] [Google Scholar]
- [2].Négrel AD, Thylefors B, The global impact of eye injuries, Ophthalmic Epidemiol. 5 (1998) 143–169. 10.1076/opep.5.3.143.8364. [DOI] [PubMed] [Google Scholar]
- [3].Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Resnikoff S, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Bourne R, Ackland P, Arditi A, Barkana Y, Bozkurt B, Braithwaite T, Bron A, Budenz D, Cai F, Casson R, Chakravarthy U, Choi J, Cicinelli MV, Congdon N, Dana R, Dandona R, Dandona L, Das A, Dekaris I, Del Monte M, Deva J, Dreer L, Ellwein L, Frazier M, Frick K, Friedman D, Furtado J, Gao H, Gazzard G, George R, Gichuhi S, Gonzalez V, Hammond B, Hartnett ME, He M, Hejtmancik J, Hirai F, Huang J, Ingram A, Javitt J, Jonas J, Joslin C, Keeffe J, Kempen J, Khairallah M, Khanna R, Kim J, Lambrou G, Lansingh VC, Lanzetta P, Leasher J, Lim J, Limburg H, Mansouri K, Mathew A, Morse A, Munoz B, Musch D, Naidoo K, Nangia V, Palaiou M, Parodi MB, Pena FY, Pesudovs K, Peto T, Quigley H, Raju M, Ramulu P, Resnikoff S, Robin A, Rossetti L, Saaddine J, Sandar M, Serle J, Shen T, Shetty R, Sieving P, Silva JC, Silvester A, Sitorus RS, Stambolian D, Stevens G, Taylor H, Tejedor J, Tielsch J, Tsilimbaris M, van Meurs J, Varma R, Virgili G, Volmink J, Wang YX, Wang N-L, West S, Wiedemann P, Wong T, Wormald R, Zheng Y, Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis, The Lancet Global Health. 5 (2017) e888–e897. 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- [4].Kong GYX, Henderson RH, Sandhu SS, Essex RW, Allen PJ, Campbell WG, Wound-related complications and clinical outcomes following open globe injury repair, Clin. Experiment. Ophthalmol 43 (2015) 508–513. 10.1111/ceo.12511. [DOI] [PubMed] [Google Scholar]
- [5].Jhanji V, Young AL, Mehta JS, Sharma N, Agarwal T, Vajpayee RB, Management of Corneal Perforation, Survey of Ophthalmology. 56 (2011) 522–538. 10.1016/j.survophthal.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [6].Pieramici DJ, MacCumber MW, Humayun MU, Marsh MJ, de Juan E, Open-globe Injury: Update on Types of Injuries and Visual Results, Ophthalmology. 103 (1996) 1798–1803. 10.1016/S0161-6420(96)30424-7. [DOI] [PubMed] [Google Scholar]
- [7].Hugkulstone CE, Use of a bandage contact lens in perforating injuries of the cornea., J R Soc Med. 85 (1992) 322–323. [PMC free article] [PubMed] [Google Scholar]
- [8].Khodadoust A, Quinter AP, Microsurgical approach to the conjunctival flap, Arch. Ophthalmol 121 (2003) 1189–1193. 10.1001/archopht.121.8.1189. [DOI] [PubMed] [Google Scholar]
- [9].Grau AE, Durán JA, Treatment of a large corneal perforation with a multilayer of amniotic membrane and TachoSil, Cornea. 31 (2012) 98–100. 10.1097/ICO.0b013e31821f28a2. [DOI] [PubMed] [Google Scholar]
- [10].Roozbahani M, Hammersmith KM, Nagra PK, Ma JF, Rapuano CJ, Therapeutic Penetrating Keratoplasty: A Retrospective Review, Eye Contact Lens. 44 Suppl 2 (2018) S433–S441. 10.1097/ICL.0000000000000522. [DOI] [PubMed] [Google Scholar]
- [11].Webster RG, Slansky HH, Refojo MF, Boruchoff SA, Dohlman CH, The Use of Adhesive for the Closure of Corneal Perforations: Report of Two Cases, Arch Ophthalmol. 80 (1968) 705–709. 10.1001/archopht.1968.00980050707004. [DOI] [PubMed] [Google Scholar]
- [12].Guhan S, Peng S-L, Janbatian H, Saadeh S, Greenstein S, Al Bahrani F, Fadlallah A, Yeh T-C, Melki SA, Surgical adhesives in ophthalmology: history and current trends, Br J Ophthalmol. 102 (2018) 1328–1335. 10.1136/bjophthalmol-2017-311643. [DOI] [PubMed] [Google Scholar]
- [13].Yin J, Singh RB, Al Karmi R, Yung A, Yu M, Dana R, Outcomes of Cyanoacrylate Tissue Adhesive Application in Corneal Thinning and Perforation, Cornea. (2019). 10.1097/ICO.0000000000001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sharma A, Kaur R, Kumar S, Gupta P, Pandav S, Patnaik B, Gupta A, Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations, Ophthalmology. 110 (2003) 291–298. 10.1016/S0161-6420(02)01558-0. [DOI] [PubMed] [Google Scholar]
- [15].Borroni D, Wowra B, Romano V, Boyadzhieva M, Ponzin D, Ferrari S, Ahmad S, Parekh M, Simple limbal epithelial transplantation: a review on current approach and future directions, Surv Ophthalmol. 63 (2018) 869–874. 10.1016/j.survophthal.2018.05.003. [DOI] [PubMed] [Google Scholar]
- [16].Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B, Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases, Cornea. 24 (2005) 369–377. 10.1097/01.ico.0000151547.08113.d1. [DOI] [PubMed] [Google Scholar]
- [17].Duchesne B, Tahi H, Galand A, Use of Human Fibrin Glue and Amniotic Membrane Transplant in Corneal Perforation, Cornea. 20 (2001) 230–232. [DOI] [PubMed] [Google Scholar]
- [18].Chung H-W, Mehta JS, Fibrin glue for Gundersen flap surgery, Clin Ophthalmol. 7 (2013) 479–484. 10.2147/OPTH.S42105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Romano V, Cruciani M, Conti L, Fontana L, Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery, Cochrane Database Syst Rev. 2016 (2016). 10.1002/14651858.CD011308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nallasamy N, Grove KE, Legault GL, Daluvoy MB, Kim T, Hydrogel ocular sealant for clear corneal incisions in cataract surgery, J Cataract Refract Surg. 43 (2017) 1010–1014. 10.1016/j.jcrs.2017.05.035. [DOI] [PubMed] [Google Scholar]
- [21].Product and Safety Information, (n.d.). https://www.resuresealant.com/safety-efficacy/ (accessed August 1, 2020).
- [22].Trujillo-de Santiago G, Sharifi R, Yue K, Sani ES, Kashaf SS, Alvarez MM, Leijten J, Khademhosseini A, Dana R, Annabi N, Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications, Biomaterials. 197 (2019) 345–367. 10.1016/j.biomaterials.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bayat N, Zhang Y, Falabella P, Menefee R, Whalen JJ, Humayun MS, Thompson ME, A reversible thermoresponsive sealant for temporary closure of ocular trauma, Sci Transl Med. 9 (2017). 10.1126/scitranslmed.aan3879. [DOI] [PubMed] [Google Scholar]
- [24].McTiernan CD, Simpson FC, Haagdorens M, Samarawickrama C, Hunter D, Buznyk O, Fagerholm P, Ljunggren MK, Lewis P, Pintelon I, Olsen D, Edin E, Groleau M, Allan BD, Griffith M, LiQD Cornea: Pro-regeneration collagen mimetics as patches and alternatives to corneal transplantation, Science Advances. 6 (2020) eaba2187. 10.1126/sciadv.aba2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sani ES, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, Khademhosseini A, Dana R, Annabi N, Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels, Science Advances. 5 (2019) eaav1281. 10.1126/sciadv.aav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Natural and Synthetic Biomedical Polymers, Elsevier, 2014. 10.1016/C2011-0-07330-1. [DOI] [Google Scholar]
- [27].Tücking K-S, Vasani RB, Cavallaro AA, Voelcker NH, Schönherr H, Prieto‐Simon B, Hyaluronic Acid–Modified Porous Silicon Films for the Electrochemical Sensing of Bacterial Hyaluronidase, Macromolecular Rapid Communications. 39 (2018) 1800178. 10.1002/marc.201800178. [DOI] [PubMed] [Google Scholar]
- [28].Oudshoorn MHM, Rissmann R, Bouwstra JA, Hennink WE, Synthesis of methacrylated hyaluronic acid with tailored degree of substitution, Polymer. 48 (2007) 1915–1920. 10.1016/j.polymer.2007.01.068. [DOI] [Google Scholar]
- [29].Baier Leach J, Bivens KA, Patrick CW, Schmidt CE, Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds, Biotechnol Bioeng. 82 (2003) 578–589. 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- [30].Khalil IA, Saleh B, Ibrahim DM, Jumelle C, Yung A, Dana R, Annabi N, Ciprofloxacin-loaded bioadhesive hydrogels for ocular applications, Biomater. Sci 8 (2020) 5196–5209. 10.1039/D0BM00935K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jumelle C, Sani ES, Taketani Y, Yung A, Gantin F, Chauhan SK, Annabi N, Dana R, Growth factor-eluting hydrogels for management of corneal defects, Materials Science and Engineering: C. 120 (2021) 111790. 10.1016/j.msec.2020.111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baek K, Jeong JH, Shkumatov A, Bashir R, Kong H, In Situ Self-Folding Assembly of a Multi-Walled Hydrogel Tube for Uniaxial Sustained Molecular Release, Advanced Materials. 25 (2013) 5568–5573. 10.1002/adma.201300951. [DOI] [PubMed] [Google Scholar]
- [33].Jeong JH, Chan V, Cha C, Zorlutuna P, Dyck C, Hsia KJ, Bashir R, Kong H, “Living” microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry, Adv Mater. 24 (2012) 58–63, 1. 10.1002/adma.201103207. [DOI] [PubMed] [Google Scholar]
- [34].Gonzalez-Andrades M, de la J.Cardona C, Ionescu AM, Mosse CA, Brown RA, Photographic-Based Optical Evaluation of Tissues and Biomaterials Used for Corneal Surface Repair: A New Easy-Applied Method, PLoS ONE. 10 (2015) e0142099. 10.1371/journal.pone.0142099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Snider EJ, Cornell LE, Acevedo JM, Gross B, Edsall PR, Lund BJ, Zamora DO, Development and Characterization of a Benchtop Corneal Puncture Injury Model, Sci Rep. 10 (2020) 4218. 10.1038/s41598-020-61079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thach AB, Ward TP, Dick JSB, Bauman WC, Madigan WP, Goff MJ, Thordsen JE, Intraocular Foreign Body Injuries during Operation Iraqi Freedom, Ophthalmology. 112 (2005) 1829–1833. 10.1016/j.ophtha.2005.04.024. [DOI] [PubMed] [Google Scholar]
- [37].Colyer MH, Weber ED, Weichel ED, Dick JSB, Bower KS, Ward TP, Haller JA, Delayed Intraocular Foreign Body Removal without Endophthalmitis during Operations Iraqi Freedom and Enduring Freedom, Ophthalmology. 114 (2007) 1439–1447. 10.1016/j.ophtha.2006.10.052. [DOI] [PubMed] [Google Scholar]
- [38].Bhatia SS, Ocular Surface Sealants and Adhesives, The Ocular Surface. 4 (2006) 146–154. 10.1016/S1542-0124(12)70041-1. [DOI] [PubMed] [Google Scholar]
- [39].Huynh A, Priefer R, Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology, Carbohydr Res. 489 (2020) 107950. 10.1016/j.carres.2020.107950. [DOI] [PubMed] [Google Scholar]
- [40].Schulz A, Rickmann A, Wahl S, Germann A, Stanzel BV, Januschowski K, Szurman P, Alginate- and Hyaluronic Acid-Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation, Transl Vis Sci Technol. 9 (2020) 34. 10.1167/tvst.9.13.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Balazs EA, [Use of hyaluronic acid in eye surgery], Annee Ther Clin Ophtalmol. 33 (1982) 95–110. [PubMed] [Google Scholar]
- [42].Davidenko N, Schuster CF, Bax DV, Farndale RW, Hamaia S, Best SM, Cameron RE, Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry, J Mater Sci Mater Med. 27 (2016) 148. 10.1007/s10856-016-5763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].D’souza AA, Shegokar R, Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications, Expert Opin Drug Deliv. 13 (2016) 1257–1275. 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]


