Abstract
Methamphetamine (MA) abuse remains a public health issue. Prenatal MA exposure (PME) poses a significant health problem, as we know very little about the drug's long-term physiological impact on the developing human brain. We investigated the long-term consequences of early MA exposure using a mouse model that targets the brain growth spurt, which occurs during human third-trimester. Adult mice previously subjected to acute MA during post-natal days 4–9 exhibited hyperactivity during the Open-Field Test, while exhibiting no motor coordination changes during the Rotarod Test. Neonatal MA exposure reduced basal dopamine (DA) uptake rates in adult nucleus accumbens slices compared with saline-injected controls. Although slices from neonatal MA-exposed mice showed no change in evoked DA signals in the presence of MA, they exhibited potentiated non-evoked DA release through DA efflux in response to MA. These data suggest that developmental MA exposure alters brain development to produce long-lasting physiological changes to the adult mesolimbic DA system, as well as altering responses to acute MA exposure in adulthood. This study provides new insights into an important, under-investigated area in drugs of abuse research.
Keywords: Dopamine, Methamphetamine, Development, Prenatal methamphetamine exposure, Fast-scan cyclic voltammetry
Introduction
Methamphetamine (MA) is a high-efficacy psychostimulant with devastating health effects and high potential for abuse (Krasnova and Cadet 2009). MA abuse has re-emerged as a serious public health concern in the United States over the past decade (Hedegaard et al. 2018; Jones et al. 2020). Usage rates amongst pregnant women are particularly concerning as they range from 0.7% to 4.8% (Wright et al. 2015). While the teratogenic effects of drugs like alcohol and cocaine have been extensively investigated, our knowledge of prenatal methamphetamine exposure (PME) is still limited (Kwiatkowski et al. 2018). A growing body of literature suggests that PME causes behavioral and psychiatric disturbances in humans (Abar et al. 2013; Diaz et al. 2014; Kiblawi, 2014). Dopamine (DA) dysfunctions during development may contribute to these effects (Kwiatkowski et al. 2014; Thompson et al. 2009). PME may also cause a heightened sensitivity to MA and other drugs of abuse later in life (Glantz and Chambers 2006; Macuchova and Slamberova 2017a). Rodent studies have revealed long-term effects of developmental MA exposure including memory and cognition impairment (Jablonski et al. 2017; Siegel et al. 2011), altered stress response (Zuloaga et al. 2015), and heightened sensitivity to cocaine in adulthood (McFadden et al. 2011). Additionally, the vulnerability of the developing rodent brain to MA-induced oxidative damage (Tsai et al. 2019) and DA terminal degradation (Kaewsuk et al. 2009) has been described. Although some studies have assessed the effects of developmental MA administration on the DA system (Graham et al. 2013), the long-term impact remains under-investigated.
To investigate MA’s impact on the developing DA system, we exposed mice to MA postnatally during the ‘brain growth spurt’, which occurs during the third trimester of human pregnancy (Dobbing and Sands 1979). MA exposure during this critical period of intense neural cell proliferation and differentiation may cause long-lasting changes to the DA system (Patten et al. 2014). MA elevates extracellular DA by inhibiting uptake via the dopamine transporter (DAT) as well as by causing the reverse transport of DA through DAT, a phenomenon known as ‘DA efflux’ (Seiden et al. 1993; Sulzer et al. 1992). We employed fast-scan cyclic voltammetry (FSCV) to measure dopaminergic activity in brain slices from mice neonatally exposed to MA. Our results suggest neonatal MA exposure alters striatal DA transmission and may enhance the response to MA later in life.
Methods
Animals and Experimental Design
Wild-type C57/BL6 mice from one litter were subcutaneously injected with MA (5 mg/kg) (3 males, 4 females) or phosphate-buffered saline (PBS) (1 male, 2 females) once daily during post-natal days (PND) 4–9. Behavior was tested at 5 months of age, and FSCV experiments performed in months 7–9. Successful FSCV experiments were only obtained from 2 controls and 6 MA-treated mice. Therefore, two additional female mice from a second litter that had received PBS injection were used as controls to increase the sample size. Additionally, non-evoked FSCV data from 1 subject in the MA-treated group was excluded due to lack of a stable baseline recording.
Behavior
Rotarod
Mice were placed onto a rotating cylinder (Stoelting) that began rotating at 4 rpm and increased to 40 rpm over 5 min. Mice were given 4 trials a day with a 30 min inter-trial period for 2 consecutive days.
Open Field
Mice were placed into an open field apparatus (50 × 50 cm) for 5 min. Movement was tracked and analyzed via camera using video-tracking software (VideoMot 2, TSE Systems).
Fast-Scan Cyclic Voltammetry
Brains were extracted and coronal slices (350 μm) made in ice-cold artificial cerebral spinal fluid (ACSF) as previously described (Torres et al. 2021). DA release was evoked in the nucleus accumbens (NAc) shell with trains of 10 stimuli at 10 Hz, 370 µA, every 2 min. Voltammetric recordings were collected and analyzed using the LabVIEW (RRID:SCR_014325; National Instruments, Austin, TX)-based software Demon Voltammetry and Analysis Software (RRID:SCR_014468) and fitted to a Michaelis–Menten-based kinetic model as previously reported (Yorgason et al. 2011), where Vmax represents the rate of DA uptake and Km represents the apparent affinity of DA for DAT. Vmax was measured at baseline and kept constant throughout the experiment while Km was set to 160 nM at baseline (Wu et al. 2001) and increased to model uptake inhibition by MA.
Following 30 min of stable baseline recording, MA (10 µM, 2 times the measured EC50 when applied to mouse NAc slices) (Hedges, 2018) was bath applied in ACSF for 30 min. DA efflux was monitored by sampling and averaging current readouts for 5-s epochs just prior to each stimulation train.
Statistics
Differences between groups were compared using the non-parametric assigned-rank Mann–Whitney U test. Pearson correlation coefficients were calculated using Vmax and Km values from individual experiments. Time-course data are shown as mean ± S.E.M. and data used for direct comparisons between groups are shown as box-whisker plots displaying median and quartiles.
Results and Discussion
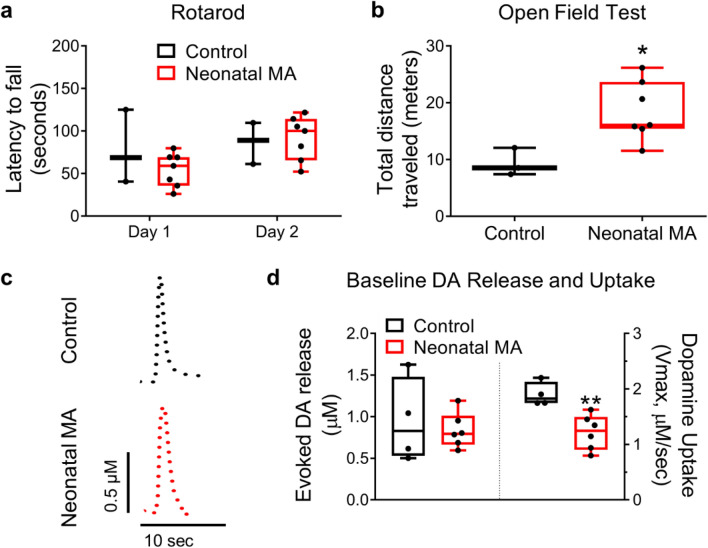
Motor coordination appeared unaffected in neonatal MA-exposed mice as they performed similarly to controls in the Rotarod Test (Fig. 1a), however, they displayed increased locomotor activity in the Open Field test (Mann–Whitney U test: p = 0.024) (Fig. 1b). Although the amplitude of stimulated DA release measured in brain slices extracted in adulthood was unchanged, DA uptake rates (Vmax) in the neonatal MA group were decreased to ~ 63% of controls (Mann–Whitney U test: p = 0.0095) (Fig. 1c, d). Past studies correlated less-than-two-fold changes in Vmax with significant alterations in behavior (Kaplan, 2016; Salahpour, 2008).
Fig. 1.
Neonatal exposure to methamphetamine elevates locomotor activity and decreases dopamine (DA) uptake measured in the nucleus accumbens in adulthood. (a) No difference was detected between Neonatal MA and Control groups during the Rotarod Test. (b) C57 wild-type mice subcutaneously injected with methamphetamine (MA, 5 mg/kg daily) during prenatal days 4–9 (Neonatal MA) traveled a greater distance on average (18.5 ± 2.0 m) than vehicle-injected (Control) mice (9.3 ± 1.4 m) during the Open Field Test. Mann–Whitney U test: *p = 0.024. (c) Representative evoked DA release traces from nucleus accumbens (NAc) brain slices measured with fast-scan cyclic voltammetry. (d) Neonatal exposure to MA had no significant impact on the amplitude of evoked DA. Kinetic modeling of DA release traces revealed that neonatal MA exposure caused a decrease in DA uptake rates (1.2 ± 0.1 µM/sec), represented by ‘Vmax,’ compared to the control group (1.9 ± 0.1 µM/sec). Mann–Whitney U test: **p = 0.0095. Boxplots show data as median, first and third quartiles, minimum and maximum values; ‘n’ of Control and Neonatal MA = 3 and 7 for behavioral data, and 4 and 6 for FSCV data
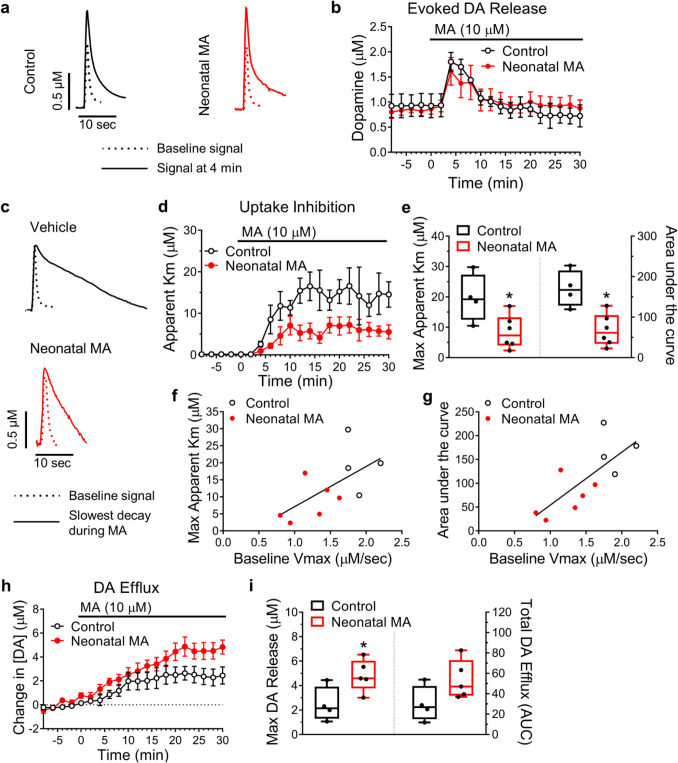
Exposing brain slices to MA (10 µM) augmented evoked DA release roughly two-fold, followed by a gradual return to baseline. This effect did not differ between groups (Fig. 2a, b). The impairment of DA uptake caused by MA (Km) was reduced in slices taken from mice neonatally exposed to MA, however (Mann–Whitney U test of Max Km: p = 0.04; Area under the curve: *p = 0.02) (Fig. 2c-e). This could be due to altered DAT availability or functionality, or altered DA metabolism dynamics (Schaefer et al. 2006). Baseline uptake rates correlated positively with the degree of MA-induced uptake inhibition (Fig. 2f, g), indicating a robust selectivity of MA for DAT similar to that previously demonstrated with amphetamine (Calipari et al. 2015). Surprisingly, slices from neonatal MA-exposed mice exhibited greater MA-induced DA efflux than controls (Mann–Whitney U test comparing peak response: p = 0.03) (Fig. 2h, i). Whether this represents a greater susceptibility to the psychostimulant or addictive effects of MA remains a worthy course of investigation.
Fig. 2.
Neonatal exposure to methamphetamine alters the response to methamphetamine measured in the nucleus accumbens (NAc) in adulthood. (a) Representative traces of evoked dopamine (DA) release in NAc brain slices from C57 wild-type mice subcutaneously injected with methamphetamine (MA, 5 mg/kg daily; Neonatal MA) or vehicle (Control) during prenatal days 4–9. Sample traces show the amplitude of DA release prior to the application of MA (10 µM) to the brain slice (dotted line) and immediately following MA application (solid line) when DA release reaches a maximal value. (b) Time course of the amplitude of DA release in slices before and after MA application; ‘n’ per group were 4 and 6 for Control and Neonatal MA, respectively. (c) Sample traces showing baseline DA release transients (dotted line) and DA release transients after MA application (solid line) when the rate of decay of the signal reached a maximal value. Following MA application, the rate of decay increases as a result of MA inhibiting DA uptake via the dopamine transporter (DAT). The shorter rate of decay displayed by the Neonatal MA group represents a lesser degree of inhibition of the (DAT) by MA. (d, e) Quantification of the inhibition of DA uptake caused by MA, represented by ‘apparent Km’, was greater in slices from the Control group compared to the Neonatal MA group. Mann–Whitney U test of Max Km: *p = 0.04; Area under the curve: *p = 0.02. A higher baseline Vmax correlated positively with (f) the maximum Km value observed in the presence of MA (r10 = 0.6164, p = 0.0577), and (g) the total inhibition (area under the curve, AUC) (r10 = 0.7451, p = 0.0134) caused by 30 min of MA exposure. The linear regression slopes for max Km and AUC, were 11.8 ± 5.33 (trended towards significantly non-zero: F1,8 = 4.902) and 111.1 ± 35.16 (significantly non-zero: F1,8 = 9.982), respectively. These F values were derived from the linear regression analysis. (h) Time course representation of DA efflux, the tonic change in extracellular DA concentration in brain slices resulting from MA application. Extracellular DA concentration was sampled every 2 min, in the absence of and just prior to electrical stimulation. (i) The maximal change in extracellular DA concentration following MA application was greater in slices from mice neonatally exposed to MA (4.8 ± 0.6 µM) than in slices from control mice (2.5 ± 0.7 µM). Mann–Whitney U test: *p = 0.03. Total DA efflux (area under the curve) trended upwards in the Neonatal MA group (53.9 ± 8.5) compared to the Control group (29.8 ± 8.8). Mann–Whitney U test: p = 0.11. Time-course graphs show data as mean ± S.E.M. Boxplots show data as median, first and third quartiles, minimum and maximum values; ‘n’ Control = 4, ‘n’ Neonatal MA = 5
Human studies have reported reduced DAT levels in MA abusers, taken to indicate DA terminal loss (Volkow, 2001). In rodents, hyperactivity results from DAT knockout (Spielewoy et al. 2000) and hypofunction (Mereu et al. 2017). However, animal models with increased DAT activity can also exhibit hyperlocomotion, likely through enhanced DA release (Yorgason et al. 2016, 2013). Thus, different mechanisms of increasing extracellular DA can increase locomotion. Dopamine D2 receptors, which can be desensitized by neonatal MA exposure (Graham et al. 2013), may down-regulate DAT surface expression via direct protein–protein interactions (Lee et al. 2007). Collectively, our data demonstrate that MA exposure during PND 4–9 results in long-lasting changes to mesolimbic DA activity.
Several factors should be considered while interpreting the findings of this study. First, although the results may have been influenced by sex, there were not sufficient numbers to detect statistical differences and no apparent correlations were noted. While a single dose concentration was chosen for neonatal injections and challenge of brain slices, including varying doses might provide additional insight. We exposed mice to MA during PND 4–9 in order to target the brain growth spurt, which occurs postnatally in rodents and during the third trimester in humans (Dobbing and Sands 1979). However, although some similarity in neurodevelopmental events exists between rodents and humans, they are not exactly the same and, thus, the specific molecular processes involved must be further dissected. Importantly, although neonatal MA exposure induced hyperactivity in adulthood, exposure did not seem to alter motor coordination in our study despite MA being toxic to nigrostriatal neurons (Ares-Santos et al. 2014). Further voltammetric studies should include the caudate-putamen to determine whether changes are specific to the mesolimbic system. Finally, an important caveat is the fact that, although numerous rodent studies have reported effects of developmental MA lasting into adulthood, studies in humans have focused on children. To our knowledge, however, this is the first study to use FSCV to investigate the long-term neurological effects of developmental MA exposure in mice and provides important physiological insight on PME.
The notion that PME increases sensitivity to MA and other drugs of abuse later in humans has been proposed by previous studies (Macuchova and Slamberova 2017b). Our results are evidence of physiological changes to the DA system, however it is not possible to relate the current findings to abuse liability. Future studies should, therefore, include assays of abuse liability. Since PME studies in humans are often confounded by sociological and genetic factors (Kwiatkowski et al. 2014), substantial work with animal models is required to investigate the mechanisms involved and guide human studies accordingly.
Acknowledgements
The authors wish to thank Ann Hashimoto and for technical assistance.
Author Contributions
FPB, DJT, and MAA designed the research; DJT performed the research; DJT, JTY and FPB analyzed the data; DJT and FPB wrote the manuscript; MAA and JTY were involved in manuscript revisions.
Funding
This work was supported by the National Institutes of Health [Grant Numbers R24 DA027318, P20-RR016467, G12MD007601, and F32 DK124963].
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All mouse care and experimental procedures were approved by the UH Manoa Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abar B et al (2013) Examining the relationships between prenatal methamphetamine exposure, early adversity, and child neurobehavioral disinhibition. Psychol Addict Behav 27:662–673. 10.1037/a0030157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Espadas I, Martinez-Murillo R, Moratalla R (2014) Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology 39:1066–1080. 10.1038/npp.2013.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano CA, Jones SR (2015) Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci 6:155–162. 10.1021/cn500262x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SD et al (2014) Effects of prenatal methamphetamine exposure on behavioral and cognitive findings at 7.5 years of age. J Pediatr 164:1333–1338. 10.1016/j.jpeds.2014.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3:79–83 [DOI] [PubMed] [Google Scholar]
- Glantz MD, Chambers JC (2006) Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol 18:893–922 [DOI] [PubMed] [Google Scholar]
- Graham DL et al (2013) Neonatal +-methamphetamine exposure in rats alters adult locomotor responses to dopamine D1 and D2 agonists and to a glutamate NMDA receptor antagonist, but not to serotonin agonists. Int J Neuropsychopharmacol 16:377–391. 10.1017/S1461145712000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M (2018) Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep 67:1–14 [PubMed] [Google Scholar]
- Hedges DM et al (2018) Methamphetamine induces dopamine release in the nucleus accumbens through a sigma receptor-mediated pathway. Neuropsychopharmacology 43:1405–1414. 10.1038/npp.2017.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Williams MT, Vorhees CV (2017) Learning and memory effects of neonatal methamphetamine exposure in rats: Role of reactive oxygen species and age at assessment. Synapse. 10.1002/syn.21992 [DOI] [PubMed] [Google Scholar]
- Jones CM, Olsen EO, O’Donnell J, Mustaquim D (2020) Resurgent Methamphetamine Use at Treatment Admission in the United States, 2008–2017. Am J Public Health 110:509–516. 10.2105/AJPH.2019.305527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int 55:397–405. 10.1016/j.neuint.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Kaplan SV et al (2016) Impaired brain dopamine and serotonin release and uptake in Wistar rats following treatment with carboplatin. ACS Chem Neurosci 7:689–699. 10.1021/acschemneuro.5b00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiblawi ZN et al (2014) Prenatal methamphetamine exposure and neonatal and infant neurobehavioral outcome: results from the IDEAL study. Subst Abus 35:68–73. 10.1080/08897077.2013.814614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407. 10.1016/j.brainresrev.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski MA, Roos A, Stein DJ, Thomas KG, Donald K (2014) Effects of prenatal methamphetamine exposure: a review of cognitive and neuroimaging studies. Metab Brain Dis 29:245–254. 10.1007/s11011-013-9470-7 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski MA, Donald KA, Stein DJ, Ipser J, Thomas KGF, Roos A (2018) Cognitive outcomes in prenatal methamphetamine exposed children aged six to seven years. Compr Psychiatry 80:24–33. 10.1016/j.comppsych.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26:2127–2136. 10.1038/sj.emboj.7601656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macuchova E, Slamberova R (2017a) Does prenatal methamphetamine exposure induce sensitization to drugs in adulthood? Physiol Res 66:S457–S467 [DOI] [PubMed] [Google Scholar]
- McFadden L, Yamamoto BK, Matuszewich L (2011) Alterations in adult behavioral responses to cocaine and dopamine transporters following juvenile exposure to methamphetamine. Behav Brain Res 216:726–730. 10.1016/j.bbr.2010.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M et al (2017) Dopamine transporter (DAT) genetic hypofunction in mice produces alterations consistent with ADHD but not schizophrenia or bipolar disorder. Neuropharmacology 121:179–194. 10.1016/j.neuropharm.2017.04.037 [DOI] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR (2014) A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr 2:93. 10.3389/fped.2014.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A et al (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A 105:4405–4410. 10.1073/pnas.0707646105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT (2006) Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/-)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/-)methylphenidate administration in the neonatal rat. J Neurochem 98:1369–1378. 10.1111/j.1471-4159.2006.04034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA (1993) Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33:639–677. 10.1146/annurev.pa.33.040193.003231 [DOI] [PubMed] [Google Scholar]
- Siegel JA, Park BS, Raber J (2011) Long-term effects of neonatal methamphetamine exposure on cognitive function in adolescent mice. Behav Brain Res 219:159–164. 10.1016/j.bbr.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B (2000) Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol 11:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S (1992) Weak base model of amphetamine action. Ann N Y Acad Sci 654:525–528 [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD (2009) Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10:303–312. 10.1038/nrn2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DJ et al (2021) Selenoprotein P Modulates Methamphetamine Enhancement of Vesicular Dopamine Release in Mouse Nucleus Accumbens Via Dopamine D2 Receptors. Front Neurosci 15:631825. 10.3389/fnins.2021.631825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SA, Bendriem RM, Lee CD (2019) The cellular basis of fetal endoplasmic reticulum stress and oxidative stress in drug-induced neurodevelopmental deficits Neurobiol. Stress 10:100145. 10.1016/j.ynstr.2018.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND et al (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TE, Schuetter R, Tellei J, Sauvage L (2015) Methamphetamines and pregnancy outcomes. J Addict Med 9:111–117. 10.1097/ADM.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA (2001) Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods 112:119–133 [DOI] [PubMed] [Google Scholar]
- Yorgason JG, Luxford W, Kalinec F (2011) vitro and in vivo models of drug ototoxicity: studying the mechanisms of a clinical problem. Expert Opin Drug Metab Toxicol 7:1521–1534. 10.1517/17425255.2011.614231 [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR (2013) Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci 37:1022–1031. 10.1111/ejn.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, Jones SR (2016) Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology 101:471–479. 10.1016/j.neuropharm.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Jacobskind JS, Raber J (2015) Methamphetamine and the hypothalamic-pituitary-adrenal axis. Front Neurosci 9:178. 10.3389/fnins.2015.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.