Abstract
The COVID-19 pandemic has fundamentally altered daily life across the globe, and the stress associated with these changes is likely to impact sleep. Sleep is critical for physical and mental health; thus, understanding the factors which may contribute to poor sleep during the pandemic represents a first step in identifying behavioral health targets for intervention efforts during and following the pandemic. This review first summarizes the developing research on sleep during the pandemic. The impact of the pandemic on sleep is then examined through the lens of the 3P model of insomnia by proposing pandemic-specific predisposing, precipitating, and perpetuating factors. The potential consequences of sleep disturbance on physical and mental health conditions most relevant to the pandemic are also reviewed. Finally, recommendations for reducing or eliminating pandemic-specific perpetuating factors are detailed, highlighting the potential utility of behavioral sleep medicine interventions in the integration of behavioral health responses and public health initiatives during and following the pandemic.
Keywords: sleep, insomnia, pandemic, coronavirus, COVID-19
The COVID-19 pandemic has fundamentally altered daily life across the globe. In addition to the physical and emotional toll of the virus, efforts to mitigate spread have resulted in reduced social interactions, financial strain, and transitions to working and schooling from home. The stress associated with these dramatic changes to daily life are likely to impact sleep, as both chronic (Hall et al., 2015) and daily stress are associated with sleep disturbance (Voellmin et al., 2014), and stressful life events are associated with insomnia onset (Healey et al., 1981). Though brief sleep loss in the face of an acute stressor may be adaptive, prolonged sleep disturbance may have notable consequences for physical and psychological health, as sleep is involved in modulating neuroendocrine function (Van Cauter et al., 2015), clearing metabolic waste from the brain (Xie et al., 2013), and memory consolidation (Walker & Stickgold, 2004). Poor sleep has likewise been linked to adverse outcomes for both physical (Itani et al., 2017) and mental health (Baglioni et al., 2016).
A common form of sleep disturbance is insomnia, which is characterized by difficulties with sleep initiation (i.e., falling asleep) and maintenance (i.e., staying sleep) (American Psychiatric Association, 2013). The coronavirus pandemic represents a unique combination of physical and emotional stressors and behavioral changes that may contribute to new or worsening insomnia symptoms. Notably, the development of insomnia has been well-characterized through the 3P model (Spielman et al., 1987). Here, the potential effects of the coronavirus pandemic on sleep will be examined through the lens of the 3P model by proposing predisposing, precipitating, and perpetuating factors that are unique to the pandemic. Such examination may delineate potential consequences of pandemic-related sleep disturbance for physical and mental health outcomes and inform recommendations for sleep health during the pandemic, consistent with recent calls for the integration of behavioral health with public health response to the pandemic (Kaslow et al., 2020).
The 3P model of insomnia
The 3P model of insomnia represents an extension of a diathesis-stress model to describe how acute insomnia onsets and transitions to chronic insomnia over time through a combination of predisposing, precipitating, and perpetuating factors (Spielman, Caruso, & Glovinsky, 1987; see Figure 1). Predisposing factors represent diatheses, or pre-existing vulnerability factors that increase risk of eventual insomnia. Common predisposing factors include genetic risk (Drake et al., 2011), prior insomnia symptoms, neuroticism, pain, and pre-existing history of medical and psychological comorbidities (C. J. Harvey et al., 2014; Leblanc et al., 2009; Perlis et al., 2005). Increasing attention has also been giving to the predisposing role of sleep reactivity, which describes the tendency to experience poor sleep in the face of stress (Drake et al., 2011). Evening chronotype, or the tendency to be an “evening type” person with associated later timing of sleep/wake activities (Adan et al., 2012), may also predispose insomnia by creating a mismatch between the individual “body clock” and societal demands. Consistent with a typical diathesis-stress model, these predisposing factors are not thought to cause insomnia in isolation; rather, they contribute to elevated risk for eventual insomnia in interaction with precipitating and perpetuating factors.
Figure 1.
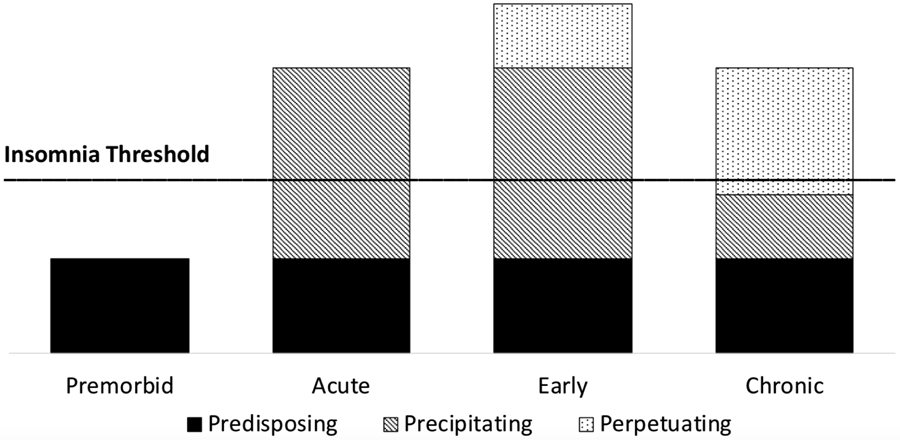
3P model of insomnia adapted from Spielman et al., 1987.
Precipitating factors in the 3P model of insomnia correspond to stress in a diathesis-stress model. Stressful life events, such as divorce, death of a loved one, birth of a child, or a new job, are associated with the onset of acute insomnia symptoms (Bastien et al., 2004). Importantly, experiencing acute insomnia during stress is relatively common and typically resolves without intervention. Indeed, previous research estimates that over 30% of individuals experience acute insomnia in a given year, and of those only slightly over 20% transition into chronic insomnia (Ellis et al., 2012). These estimates then raise an important question- what is maintaining insomnia over time in the 20%?
The 3P model answers this question by articulating perpetuating factors, or behaviors that exacerbate and maintain sleep disturbance, even following the offset of the precipitating factor (Spielman et al., 1987). Classic perpetuating factors include excessive time in bed awake, going to bed early, sleeping in, napping, and engaging in non-sleep behaviors in the bed or bedroom. Such behaviors are thought to maintain insomnia through multiple mechanisms, including weakening learned associations between the bed and sleep, conditioned arousal, and diminished homeostatic sleep drive at bedtime (Perlis et al., 2005; Spielman et al., 1987). Over time, the bed/bedroom is inconsistently paired with sleep, which results in the bed being a weak associative cue for sleep. Further, the bed/bedroom is frequently paired with wakefulness, which eventually results in the bed being a conditioned stimulus for arousal. Counter-fatigue measures, such as nicotine and caffeine, in high amounts and/or late in the day may also perpetuate sleep difficulties. Though classic perpetuating factors are largely behavioral, cognitive perpetuating factors have also been proposed, such as excessive worry, attentional bias to internal or external signals that may disturb sleep (e.g., road noise), and cognitive distortions about sleep onset and maintenance (e.g., underestimating total sleep time) (A. G. Harvey, 2005). Perpetuating factors are thought to be the critical factors that maintain insomnia and are therefore the primary target of intervention in cognitive behavior therapy for insomnia (CBTI).
Sleep during the coronavirus pandemic
There is increasing evidence to support the view that numerous physical and emotional consequences of the pandemic are likely to alter sleep. Indeed, estimates of clinically significant insomnia symptoms during lockdown periods in Greece, China, Israel, and France range from 7.2-37.6% (Kokou-Kpolou et al., 2020; L. yu Lin et al., 2020; Ren et al., 2020; Voitsidis et al., 2020; Zreik et al., 2020), suggesting a higher prevalence of insomnia symptoms during the pandemic relative to the estimated 4.37% one-month incidence rate of acute insomnia established prior to the pandemic (Ellis et al., 2012). Further, these estimates appear similar to rates of sleep disturbance observed following the September 11, 2001 terrorist attacks, after which 25% of adults in Manhattan reported insomnia symptoms (Galea et al., 2002), and 11% of adults nationwide reported severe difficulty falling or staying asleep (Schuster et al., 2001). Among healthcare workers in China, 32% report clinically significant insomnia symptoms (K. Lin et al., 2020; Zhou et al., 2020), and increased insomnia symptoms are associated with frontline work, working with COVID-19 patients, longer hours, and fewer years of work experience (Cai et al., 2020; K. Lin et al., 2020; H. Wang et al., 2020).
Several studies have attempted to quantify changes in sleep since the onset of the pandemic by comparing measures of sleep during lockdown/stay at home periods in China, Italy, Israel, India, Canada, the US, Austria, Germany, Switzerland, and Argentina to data collected prior to the pandemic (Gao & Scullin, 2020; Leone et al., 2020; Wright Jr et al., 2020), to retrospective estimates of sleep prior to the pandemic (Blume et al., 2020; Cellini et al., 2020; Dey et al., 2020; Innocenti et al., 2020; L. yu Lin et al., 2020; Robillard et al., 2020; Zreik et al., 2020), or to pre-existing data (Casagrande et al., 2020). From this developing literature, several points of convergence have emerged. One of the most robust findings thus far is that sleep timing has shifted later since the onset of the pandemic (Cellini et al., 2020; Dey et al., 2020; Gao & Scullin, 2020; Innocenti et al., 2020; Leone et al., 2020; Robillard et al., 2020; Wright Jr et al., 2020), likely reflecting increased schedule flexibility among individuals now working from home. Similarly, several studies have found that social jetlag, or the mismatch in sleep timing between work days and free days (Wittmann et al., 2006), has decreased during the pandemic (Blume et al., 2020; Leone et al., 2020; Wright Jr et al., 2020), which again likely reflects an increased ability to sleep on a schedule more consistent with biological timing among those working from home. Likewise, several studies have found small increases in sleep duration (Blume et al., 2020; Gao & Scullin, 2020; Leone et al., 2020) and time in bed (Cellini et al., 2020; Wright Jr et al., 2020), though one study found no change in sleep duration (Innocenti et al., 2020). Though later sleep timing is frequently linked to negative outcomes (e.g., Nota et al., 2020), evidence for decreased social jetlag and increased sleep duration suggest that sleep may benefit from the reduced demands associated with the lockdown periods. However, these benefits may not be universal, as one study found an association between increased subjective burden and decreased sleep duration during lockdown (Blume et al., 2020).
Despite apparent improvements in sleep duration and social jetlag, several studies have found that subjective sleep quality has decreased since the pandemic onset relative to retrospective estimates of sleep quality prior to the pandemic (Blume et al., 2020; Casagrande et al., 2020; Cellini et al., 2020; Innocenti et al., 2020; Robillard et al., 2020). Interestingly, available evidence suggests that subjective sleep quality during the pandemic is unchanged relative to subjective sleep quality measured prior to the pandemic (Leone et al., 2020), and one study even found that subjective sleep quality during the pandemic is decreased relative to a retrospective report of sleep quality prior to the pandemic, but not relative to sleep quality measured prior to the pandemic (Gao & Scullin, 2020). These divergent findings suggest that contextual factors experienced during the pandemic may bias the perception of subjective sleep quality. Indeed, decreased subjective sleep quality during the pandemic is associated with increased stress and family responsibilities (Robillard et al., 2020), lower socioeconomic status (Beck et al., 2020), adverse life impact of the pandemic (Gao & Scullin, 2020), death of a loved one due to COVID-19, and being unemployed (Casagrande et al., 2020). Thus, although the evidence for a reduction in sleep quality from prior to the pandemic is mixed, there is a robust association between increased pandemic-related stress and decreased sleep quality, highlighting the importance of contextual factors in understanding sleep during the pandemic.
The developing literature suggests mixed impacts of the pandemic on sleep. Decreased social jetlag and increased sleep duration represent benefits of reduced schedule demands, while decreased sleep quality may reflect the detrimental impact of stress on sleep among those suffering most during the pandemic. Together these findings suggest that interactions between potential predisposing (e.g., frontline workers) and precipitating (e.g., pandemic-related stress) factors may influence sleep outcomes during the pandemic, consistent with the 3P model of insomnia. These results highlight the utility of examining sleep during the pandemic through the lens of the 3P model to further characterize potential pandemic-specific predisposing, precipitating, and perpetuating factors (see Figure 2) that can inform a coordinated public health response (Kaslow et al., 2020).
Figure 2.
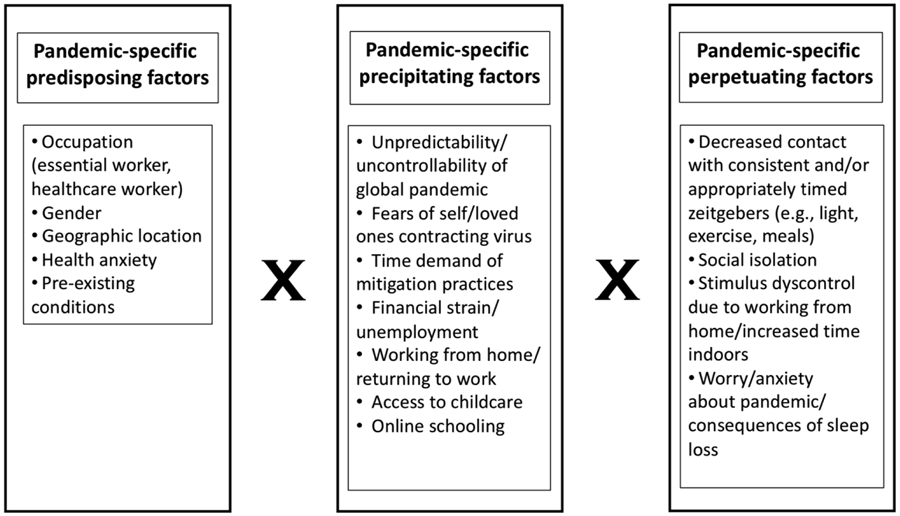
Depiction of interactions between potential pandemic-specific predisposing, precipitating, and perpetuating factors.
Predisposing factors
The common predisposing factors in the 3P model likely also predispose individuals to poor sleep during the pandemic. However, a few possible predisposing factors may be particularly relevant to understanding pandemic-related sleep disturbance. First, essential workers may be vulnerable to sleep disturbance due to increased work hours, risk of virus exposure, concerns about personal protective equipment supplies, and particularly for healthcare workers, increased emotional burden. Indeed, the developing literature indicates healthcare workers may be particularly vulnerable to sleep disturbance and insomnia during the pandemic (Pappa et al., 2020). Healthcare workers also frequently engage in shift work, which demands circadian misalignment and may result in insomnia symptoms (Wright, Bogan, et al., 2013). Thus, many healthcare workers likely experienced sleep disturbance and insomnia symptoms prior to the pandemic, conferring vulnerability for further sleep impairment during the pandemic.
Increased rates of insomnia in women compared to men (Theorell-Haglöw et al., 2018) suggest gender may also be a predisposing factor. Gender may also be particularly relevant to sleep during the pandemic, as women are more likely to lose employment during the pandemic and more likely to shoulder increased unpaid work, such as childcare and at-home schooling (King et al., 2020). Indeed, several studies have found elevated sleep disturbance in women compared to men during the pandemic (Beck et al., 2020; Casagrande et al., 2020; Robillard et al., 2020), as well as increased insomnia symptoms (L. yu Lin et al., 2020; Voitsidis et al., 2020) (but see also Kokou-Kpolou et al., 2020).
Geographic location may also represent a unique predisposing factor for sleep disturbance during the pandemic. That is, individuals living in locations with higher COVID-19 cases, that are more densely populated, and/or with overburdened healthcare facilities may be vulnerable to worse sleep. Though few studies have addressed this question thus far, one study found higher insomnia symptoms in individuals living in urban versus rural settings during the lockdown in Greece (Voitsidis et al., 2020). Likewise, another study conducted during the lockdown in Italy found that sleep quality was stratified by region, such that those living in North Italy (considered the epicenter of the pandemic in Italy) reported worse sleep quality compared to those living in South Italy (Casagrande et al., 2020). Thus, living in areas more severely impacted by COVID-19 may represent a vulnerability for sleep disturbance during the pandemic.
Neuroticism, worry, and pre-existing psychopathology are common predisposing factors for insomnia (C. J. Harvey et al., 2014; Perlis et al., 2005). Relatedly, health anxiety may be especially relevant during the pandemic. Health anxiety is the tendency to perceive physical signs and symptoms as indicators of disease and overestimate the likelihood and consequences of illness/disease (Rachman, 2012; Salkovskis & Warwick, 1986). At a modest level, health anxiety is arguably adaptive to prevent and efficiently respond to potential illness. However, high health anxiety can be maladaptive, as in hypochondriasis/illness anxiety disorder (American Psychiatric Association, 2013). Importantly, worries about coronavirus are associated with increased insomnia symptoms during the pandemic (Kokou-Kpolou et al., 2020; Voitsidis et al., 2020). Thus, those with prior elevated health anxiety may be particularly vulnerable to experiencing increased distress during the pandemic, including disruptions to sleep. Relatedly, those with a pre-existing health condition may experience increased anxiety that interferes with sleep during the pandemic. Indeed, one study found that having a chronic illness was associated with increased sleep difficulties during the pandemic (Robillard et al., 2020).
Interestingly, although evening chronotype is typically a predisposing factor for insomnia, it may be protective against sleep disturbances during the pandemic. Specifically, those working from home and/or those with increased flexibility during the pandemic may be able to adopt a sleep/wake schedule aligned with their circadian preference, which may facilitate longer sleep duration and improved sleep quality. Indeed, Blume and colleagues (2020) found that evening chronotypes experienced decreased social jetlag and increased sleep duration during lockdown in Germany. However, given the high socioeconomic status in this sample, it will be important to replicate these findings in diverse samples to determine the role of eveningness in sleep during the pandemic among those with consistent or increased schedule/work demands. Likewise, though increasing age is typically associated with increased insomnia symptoms (Salo et al., 2012), the developing literature on sleep during the pandemic indicates increased insomnia symptoms (Zhou et al., 2020), increased sleep disturbance (Beck et al., 2020), later sleep timing, and long sleep duration (Leone et al., 2020) in younger adults compared to older adults (but see also Gao & Scullin, 2020). These findings are somewhat surprising, as older adults could be expected to be particularly vulnerable to poor sleep during the pandemic given the association between age and COVID-19 mortality (Cummings et al., 2020). However, younger adults, who are more likely to be working, may be experiencing more changes in their sleep/wake schedules than older adults, which may be contributing to this unexpected effect.
Precipitating factors
Sleep is known to be vulnerable to stress (Dimsdale, 2014), and there are numerous stressors associated with the pandemic that may disrupt sleep. First, individuals may be experiencing stress about the pandemic itself, including concerns about contracting the virus, the time demand and burden of mitigation practices, and fears for the safety of loved ones. Similarly, the stress of contracting the virus may also disrupt sleep, as one study found an elevated prevalence of insomnia among COVID-19 patients in China (Y. Wang et al., 2020). Life events that are uncontrollable and unpredictable are experienced as most stressful (Mineka & Kihlstrom, 1978). A global event like a pandemic is likely to be perceived as beyond individual control. Likewise, the lack of knowledge in the early weeks of the pandemic (e.g., the mode of transmission, what activities were safe, etc.) likely contributed to perceived unpredictability, further amplifying stress and potentially disrupting sleep. Indeed, one study found that over 50% of participants reported moderate to extremely severe stress during the early stages of the pandemic (Cellini et al., 2020).
Stressful aspects of mitigation efforts may also disrupt sleep. Financial strain from lost employment has been well-documented, while others experienced a sudden transition to working from home and learning new teleworking technologies. As the pandemic continues, returning to work may also generate stress about exposure to the virus. Indeed, in a study of individuals returning to work in China, those who viewed returning to work as a health risk reported increased stress and insomnia symptoms (Tan et al., 2020). Further, essential workers are tasked with facing increased risk of contracting the virus while frequently working longer and harder hours. The effects of stress on sleep for essential workers are evident in a study of healthcare workers in China, which found that increased stress is associated with increased sleep disturbance during the pandemic (Xiao et al., 2020). A study of physicians also found a relation between increased stress and sleep disturbance, and those treating COVID-19 patients reported higher levels of sleep disturbance (Abdulah & Musa, 2020). These stressors are further compounded for individuals with school-aged children attempting to finish the school year at home and having reduced access to childcare and other activities during the summer. Many college students experienced converging stressors such as working jobs to contribute to household income and managing younger siblings online schooling while finishing their semester online. Indeed, one study found the effect of distress on sleep disturbance during the pandemic was strongest among college students (Cellini et al., 2020).
Though not exhaustive, these stressors may precipitate sleep disturbances during the pandemic. The 3P model suggests that acute sleep problems in the face of pandemic-related stress will likely resolve following the resolution of the stressor(s). However, pandemic-precipitated sleep disturbance may transition into chronic insomnia if combined with perpetuating factors, which are considered next.
Perpetuating factors
Several features of the pandemic may perpetuate sleep disturbance, the majority of which center around decreased exposure to circadian entrainment cues, or zeitgebers (a German term for “time giver”). Although human circadian rhythms are endogenously generated, these rhythms run slightly longer than 24 hours on average and therefore require external time cues to synchronize the internal clock with environmental time (Moore-Ede et al., 1983). The most significant zeitgeber is light, which has been consistently shown to entrain circadian rhythms (Golombek & Rosenstein, 2010). Indeed, after 1 week of exposure to only solar light, humans demonstrate a phase advance towards increased synchrony between circadian timing and the natural light/dark cycle (Wright, McHill, et al., 2013). Light exposure also alters sleep timing, such that morning light exposure results in advanced sleep timing (Corbett et al., 2012; Dijk et al., 1989), and evening light exposure results in delayed sleep timing (Komada et al., 2000). Further, later first exposure to morning light is associated with more fragmented sleep the following night (Wams et al., 2017), highlighting the impact of timing of light exposure on subsequent sleep continuity. Thus, sleep timing may be delayed and sleep continuity disrupted during the pandemic, as individuals spend more time indoors while social distancing and those working from home are no longer exposed to morning sunlight during their morning commute. This assertion is supported by a recent finding that participants on lockdown in Germany reported spending less time outdoors, but more time spent outdoors was associated with increased sleep duration (Blume et al., 2020).
Relatedly, there is some evidence that evening light exposure from electronic devices may disrupt homeostatic sleep pressure (Chellappa et al., 2013). Decreased access to social activities may contribute to increased electronic usage, particularly at night given evidence for delayed sleep timing during the pandemic (Blume et al., 2020). Indeed, studies conducted during the first surge of the pandemic in Italy and Canada found that increased electronic use before bed is associated with small increases in sleep onset latency and delays in sleep timing (Cellini et al., 2020), and increased TV exposure is associated with worse sleep quality (Robillard et al., 2020), respectively. These findings indicate that altered timing and amount of light exposure as a result of social distancing measures may perpetuate pandemic-related sleep disturbance by delaying sleep timing and disrupting sleep continuity.
In addition to light exposure, other behavioral zeitgebers disrupted by social distancing may perpetuate sleep difficulties during the pandemic. One such behavioral zeitgeber is exercise (Mistlberger & Skene, 2005). Timing of exercise is linked to phase shifts in the melatonin rhythm (Buxton et al., 2003; Yamanaka et al., 2015), and may facilitate entrainment to a phase-advanced sleep schedule (Miyazaki et al., 2001). Timing of physical activity is also associated with sleep timing, such that a higher proportion of morning activity is associated with earlier bedtime and rise time (Quante et al., 2019). Likewise, delayed sleep timing is associated with decreased moderate to vigorous physical activity (Shechter & St-Onge, 2014). Increased sedentary behavior during social distancing and potential avoidance of exercise due to concerns about contracting the virus may contribute to delayed sleep timing during the pandemic. In addition to a potential circadian effect on sleep timing, exercise is also implicated in sleep architecture, such that regular exercise is associated with decreased sleep onset latency, wake after sleep onset, and REM sleep and increased total sleep time, sleep efficiency, and slow wave sleep (see Chennaoui, Arnal, & Sauvet, 2015 for a review). Indeed, participants in one study reported decreased exercise during the pandemic; however, increased exercise was associated with increased sleep duration (Blume et al., 2020), while decreased exercise was associated with increased insomnia risk during the pandemic (Fu et al., 2020). Thus, decreased exercise during the pandemic disrupt sleep continuity and architecture.
Changes in eating behaviors may also contribute to sleep disturbance during the pandemic. First, meal timing, another behavioral zeitgeber (Mistlberger & Skene, 2005), may be altered by disrupted or inconsistent daytime schedules during the pandemic. Studies of meal timing indicate early versus late meal timing shifts the timing of some circadian outputs, including core body temperature (Kräuchi et al., 2002) and circadian gene expression (Wehrens et al., 2017). Likewise, later meal timing is associated with later sleep timing (Quante et al., 2019). Thus, changes in meal timing during the pandemic may contribute to variability in sleep timing. Changes in the content of food intake may also contribute to sleep disturbances. Individuals may consume higher calorie food during the pandemic due to stress or may choose high carbohydrate foods with longer shelf lives to reduce trips to the grocery store (e.g., pasta). Nutrient content in evening meals is linked to subsequent sleep continuity and architecture, such that higher calorie and carbohydrate evening food intake is associated with decreased sleep efficiency and REM sleep and increased sleep onset latency and wake after sleep onset (Crispim et al., 2011). Thus, variability in meal timing and nutritional content may contribute to delayed sleep timing and sleep disturbances during the pandemic.
Although social activity has been posited as a non-photic zeitgeber, evidence for such an effect is mixed and may be better accounted for by the pairing of social activity with other zeitgebers (i.e., light, exercise; Mistlberger & Skene, 2005). However, social activity has been implicated in sleep independent of circadian rhythms. Indeed, decreased engagement in social activity is associated with poor sleep quality (Carney et al., 2006), and increased loneliness is associated with decreased sleep efficiency (Cacioppo et al., 2002). One study also found that loneliness is associated with increased insomnia symptoms during the pandemic (Kokou-Kpolou et al., 2020). Thus, social isolation during social distancing and working from home may contribute to poor sleep quality and continuity during the pandemic. Whether these effects may be offset by virtual social engagement remains unknown. Finally, research on the regularity of daily activities such as exercise, eating, social activities, and work suggest that increased irregularity is associated with poor sleep quality (Monk et al., 2003). There are numerous ways in which daily activities are more irregular during the pandemic, such as working at irregular times to meet childcare demands, altering exercise schedules to decrease exposure to others, and lack of regular social contact. Such irregularity likely results in inconsistent contact with circadian entrainment cues and a weak association between time of day (i.e., bedtime) and sleep, which in turn may disrupt sleep.
In addition to decreased exposure to circadian entrainment cues, other non-circadian features of the pandemic may also perpetuate sleep disturbances. One such feature is poor stimulus control. Behavioral theories of insomnia propose that difficulties with sleep initiation are due in part to the bed and/or bedroom being a weak discriminative stimulus for sleep. That is, consistent pairing of the bed/bedroom with responses other than sleep, such as wakefulness, reading, watching TV, etc., results in a weak association between the bed and sleep, such that the bed/bedroom do not reliably cue a sleep response (Bootzin & Stevens, 2005). Social distancing practices and working from home may thus contribute to poor stimulus control during the pandemic. For some, the bed or bedroom may be the only location in the home that affords privacy for working, particularly for those sharing small living spaces with others working from home and/or those with children who are now at home during business hours. Even under ideal circumstances (e.g., access to a designated home office space), unusually large amounts of time spent in the home with reduced exposure to discriminative cues (e.g., leaving the home for work) may weaken the association between the bed/bedroom and sleep. Individuals who have lost work due to the pandemic may develop poor stimulus control through a similar mechanism, as well as potential increased time spent in bed without the demands of a work day. Such diminished associative cueing may then perpetuate sleep disturbances during the pandemic.
Finally, the pandemic is likely to be associated with increased worry, which may also perpetuate sleep disturbances. Indeed, previous research suggests that worry prior to bed predicts subsequent poor sleep, including increased sleep onset latency and decreased total sleep time and sleep efficiency (McGowan et al., 2016). A host of pandemic-related worries may therefore perpetuate sleep problems, such as worries about finances, the health and safety of loved ones, and/or the long-term consequences of the pandemic. Indeed, the developing research indicates that pandemic-related anxiety, including uncertainty about the infection status of self and loved ones (Voitsidis et al., 2020) and uncertainty about having been exposed to COVID-19 (Casagrande et al., 2020), as well as general anxiety (Cellini et al., 2020; Gao & Scullin, 2020; Kokou-Kpolou et al., 2020) are associated with sleep disturbance during the pandemic. Interestingly, research from the insomnia literature suggests that sleep-specific worry (that is, worry about sleep problems) may be particularly potent for maintaining sleep difficulties (Lancee et al., 2017). Sleep-specific worries may take unique forms during the pandemic, such as worries about insufficient sleep resulting in increased vulnerability to infection or impairing the ability to provide effective instruction for children completing online schooling. Thus, both general and sleep-specific worry may perpetuate sleep problems during the pandemic.
Implications for physical and mental health outcomes
Considerable research has delineated the negative consequences of poor sleep for both physical and mental health. Importantly, sleep disturbance is linked to conditions that may be particularly relevant during the pandemic. Previous research suggests that insufficient sleep is a risk factor for obesity, insulin resistance, and diabetes (Reutrakul & Van Cauter, 2018), and obesity and diabetes have likewise been identified as predictors of COVID-19 morbidity and mortality (Muniyappa & Gubbi, 2020). Similarly, insomnia is linked to subsequent hypertension (Jarrin et al., 2018), and hypertension has also been implicated as a risk factor for COVID-19 mortality (Zuin et al., 2020). Further, considerable research has delineated a bidirectional relationship between sleep health and immune system function (see Besedovsky, Lange, & Haack, 2019; Irwin & Opp, 2017 for reviews), including reduced immune responses to viral exposure among sleep deprived individuals and habitual short sleepers (Besedovsky et al., 2019). Likewise, accumulating evidence suggests immune responses are under circadian control (Scheiermann et al., 2018). These findings suggest that sleep and circadian rhythm disturbance may impair the ability of the immune system to mount an effective response to a viral threat, such as the coronavirus. Thus, disrupted sleep and circadian rhythms during the pandemic may contribute to poor physical health outcomes linked to COVID-19 and possibly vulnerability to COVID-19 itself.
Poor sleep during the pandemic may similarly be linked to pandemic-related mental health outcomes. For example, mood disorders may be particularly vulnerable to sleep disruptions during the pandemic. Disrupted sleep continuity and architecture feature prominently in major depressive disorder (Palagini et al., 2013; Soehner et al., 2014), and non-depressed individuals with insomnia are twice as likely to subsequently develop depression compared to non-depressed individuals without insomnia (Baglioni et al., 2011), highlighting a potential causal effect of sleep disturbance on the development of mood disorders. Likewise, in bipolar disorder, decreased need for sleep and altered circadian rhythms are observed in manic episodes (American Psychiatric Association, 2013; Murray & Harvey, 2010), and changes in sleep and contact with zeitgebers predict the onset of a mood episode (Murray & Harvey, 2010). These findings suggest that sleep and circadian rhythm disruptions may precipitate mood episode onset during the pandemic.
Poor sleep during the pandemic may also be linked to posttraumatic stress symptoms (PTSS). Considerable research has linked sleep disturbance to trauma, PTSS, and posttraumatic stress disorder (PTSD; see Zhang et al., 2019 for a review), and pre-trauma sleep disturbances are linked to the subsequent development of PTSS and PTSD (Koffel et al., 2013; Short et al., 2019). Indeed, the pandemic may be conceptualized as a mass trauma event (Horesh & Brown, 2020), which may then be followed by PTSS. Though research on mental health outcomes during the pandemic is nascent, preliminary findings indicate elevated PTSS following the pandemic onset (C. H. Liu et al., 2020), and subjective sleep disturbances are associated with higher levels of PTSS during the pandemic (Casagrande et al., 2020; N. Liu et al., 2020). Together these findings suggest that sleep difficulties may exacerbate PTSS in response to the pandemic and suggest the need for research examining the role of sleep problems in pandemic-related PTSS.
There has also been speculation that the pandemic may contribute to new or worsening cases of obsessive-compulsive disorder (OCD) as a result of heightened focus on contamination and public health measures that parallel common contamination-related compulsions (Fineberg et al., 2020; Rivera et al., 2020). Indeed, one study of patients with OCD found increased OCD symptoms following the pandemic onset, particularly among those with contamination fears prior to the pandemic (Davide et al., 2020). Notably, both sleep and circadian rhythm disturbances have been implicated in OCD (Cox & Olatunji, 2020; Nota et al., 2015), including links between delayed sleep timing and increased next day OCD symptoms (Schubert et al., 2019). Thus, disturbances in sleep continuity and timing and variable circadian entrainment during the pandemic may contribute to pandemic-related OCD symptom exacerbations. Though no study to date has examined this effect, there is preliminary evidence that increased insomnia symptoms prior to the pandemic predict increased OCD symptoms following the pandemic onset (Cox & Olatunji, under review), highlighting potential impacts of sleep disturbance on OCD symptoms during the pandemic.
Recommendations and future directions
The 3P model emphasizes the role of perpetuating factors in the transition from acute to chronic insomnia (see Figure 1). Thus, a conceptualization of sleep disturbance during the pandemic through the 3P framework (see Figure 2) suggests that acute sleep problems experienced during the pandemic can be offset by eliminating or reducing the existence of perpetuating factors, such as those detailed above. The recommendations in Table 1 are provided to reduce the likelihood that acute insomnia during the pandemic transitions to chronic insomnia.
Table 1.
Recommendations for sleep health during the coronavirus pandemic
| Recommendations for sleep health during the coronavirus pandemic |
|---|
| 1. Maintain a consistent bedtime and wake time, and only go to bed when feeling sleepy and ready to sleep. |
| 2. Refrain from using the bed/bedroom for activities other than sleep and sex. Avoid spending any more time in the bed/bedroom than was spent there prior to the pandemic. |
| 3. If having trouble sleeping, get out of bed, and engage in some pleasant, relaxing activity outside of the bedroom for 15-20 minutes or until sleepiness has increased. Repeat this procedure as needed. |
| 4. If working from home, establish a dedicated workspace that is clearly separate from the bed and bedroom. |
| 5. Get natural daylight exposure during the day, particularly in the morning (e.g., step outside in the morning). Bright light boxes may also be used if access to natural light is limited. A minimum of 30 minutes of bright light exposure with 1-2 hours after awakening is recommended. |
| 6. Limit exposure to bright light, particularly blue light close to bedtime. |
| 7. Maintain a regular exercise regimen, ideally in natural daylight. |
| 8. Maintain regular meal timing. Avoid high calorie/carbohydrate/fat foods close to bedtime. |
| 9. Limit exposure to pandemic-related news close to bedtime. |
| 10. If worry is interfering with sleep, schedule a daily “worry time” devoted to thinking through and writing out worry content. |
| 11. Limit prescription sleep medication to short-term use to avoid side effects, tolerance, and/or dependence. Only use prescription sleep medications as directed by a prescriber. |
| 12. If sleep problems are severe, persistent, and interfering with daytime functioning, consider seeking a referral for cognitive behavior therapy for insomnia (CBTI). CBTI is recommended as the first-line treatment for insomnia by the American College of Physicians (Qaseem et al., 2016). |
The full impact of the coronavirus pandemic on sleep remains to be determined, and there is a critical need for immediate and sustained research on sleep throughout the duration of the pandemic and in its aftermath. One such example is the International COVID-19 Sleep Study (ICOSS), an international collaboration to examine sleep and circadian rhythm changes during the pandemic (Partinen et al., 2020). Relatedly, funding initiatives are needed to facilitate the initiation of new sleep-related projects during the pandemic and/or the addition of pandemic-oriented questions to existing projects. For example, studies utilizing prospective monitoring of sleep continuity (e.g., sleep diaries), particularly in more socioeconomically diverse samples, may provide more insight into nightly variability in sleep without the limitations of retrospective reporting. Relatedly, ecological momentary assessment studies sampling daily exposure to zeitgebers are also needed to examine the predictive effect of variable exposure to circadian entrainment cues on the subsequent night’s sleep. However, the extant literature suggests divergent sleep responses to the pandemic that depend on stress and pre-existing risk factors. Extant findings are consistent with the 3P model of insomnia, which proposes that chronic insomnia develops through interacting predisposing, precipitating, and perpetuating factors, and suggest that the 3P model may also account for sleep changes during the pandemic. In addition to identifying who may be most vulnerable to poor sleep in the face of pandemic-related stress, a significant strength of the 3P model is the articulation of malleable perpetuating factors that may maintain sleep disruption over time.
The pandemic-specific perpetuating factors proposed here (see Figure 2) are likely not exhaustive; thus, continued research is needed to fully elucidate behavioral mechanisms to target in sleep interventions during the pandemic. The necessity of such intervention is supported by previous research linking sleep disturbances to poor physical and mental health outcomes over time. Indeed, the pandemic highlights a critical need for behavioral health interventions to complement public health initiatives (Kaslow et al., 2020). Notable organizations related to public health (e.g., American Psychological Association, American Medical Association, etc.) should include sleep health dissemination in their pandemic response campaigns. Healthcare professionals should screen for sleep disturbance during mental and physical health visits. CBT-I principles, reflected in the recommendations provided here, are an ideal component of behavioral health responses to the pandemic, as online CBT-I is highly efficacious for sleep problems and has secondary benefits for comorbid mental and physical health conditions (Drerup & Ahmed-Jauregui, 2019). Further, CBT-I has been shown to be effective when administered in primary care settings by providers from a range of disciplines (e.g., nurses, social workers, counselors) (Davidson et al., 2019), and a “one-shot” CBT-I administration has also been shown to reduce insomnia symptoms (Randall et al., 2019), highlighting the potential for application beyond the traditional therapy framework. Given the significant overlap between sleep and physical and mental health and the efficacy of CBT-I, psychology training programs should place increased emphasis on training students in sleep and behavioral sleep interventions. The available literature suggests sleep disturbances may develop during the pandemic through interactions between pandemic-specific predisposing, precipitating, and perpetuating factors, and behavioral sleep interventions are well-positioned to target such factors during the pandemic, which may offset potential consequences of sleep disruption on physical and mental health.
Public Significance Statement.
This article reviews the developing literature on the effects of the pandemic on sleep and highlights potential features of the pandemic that may predispose, precipitate, and perpetuate sleep disturbance. Sleep disturbance may contribute to poor physical and mental health outcomes during the pandemic, and interventions for healthy sleep should be integrated into behavioral and public health responses to the pandemic.
Acknowledgements.
The authors would like to thank Dr. Kenneth P. Wright, Jr. and Dr. Lance A. Riley for their comments on drafts of this manuscript.
Funding.
This work was supported by the National Institute of Mental Health of the National Institutes of Health [F31MH113271]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest. None
References
- Abdulah DM, & Musa DH (2020). Insomnia and stress of physicians during COVID-19 outbreak. Sleep Medicine. 10.1016/j.sleepx.2020.100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, & Randler C (2012). Circadian typology: A comprehensive review. Chronobiology International, 29(9), 1153–1175. 10.3109/07420528.2012.719971 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, & Riemann D (2011). Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135, 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, Reynolds CF, & Riemann D (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological Bulletin, 142(9), 969–990. 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, Morin CM, Bastien CH, Vallieres A, Precipitating CMM, Bastien CH, Vallières A, & Morin CM (2004). Precipitating factors of insomnia. Behavioral Sleep Medicine, 2(1), 50–62. 10.1207/s15402010bsm0201 [DOI] [PubMed] [Google Scholar]
- Beck F, Léger D, Fressard L, Peretti-Watel P, Verger P, & Group TC (2020). Covid-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. Journal of Sleep Research. 10.1111/jsr.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, & Haack M (2019). The sleep-immune crosstalk in health and disease. Physiological Reviews, 99, 1325–1380. 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume C, Schmidt MH, & Cajochen C (2020). Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Current Biology, 30, R783–R801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootzin RR, & Stevens SJ (2005). Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review, 25, 629–644. 10.1016/j.cpr.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, & Van Cauter E (2003). Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. American Journal of Physiology, 284, 714–724. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, & Hobson JA (2002). Do loneley days invade the nights? Potential social modulation of sleep efficiency. Psychological Science, 13(4), 384–388. [DOI] [PubMed] [Google Scholar]
- Cai Q, Feng H, Huang J, Wang M, Wang Q, & Lu X (2020). The mental health of frontline and non-frontline medical workers during the coronavirus disease 2019 (COVID-19) outbreak in China: A case-control study. Journal of Affective Disorders, 275, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Edinger JD, Meyer B, Lindman L, & Istre T (2006). Daily activities and sleep quality in college students. Chronobiology International, 23(3), 623–637. 10.1080/07420520600650695 [DOI] [PubMed] [Google Scholar]
- Casagrande M, Favieri F, Tambelli R, & Forte G (2020). The enemy who sealed the world: Effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Medicine, 75, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N, Canale N, Mioni G, & Costa S (2020). Changes in sleep pattern, sense of time, and digital media use during COVID-19 lockdown in Italy. Journal of Sleep Research. 10.1111/jsr.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Gotz T, Krebs J, & Cajochen C (2013). Acute exposure to evening blue-enriched light impacts on human sleep. Journal of Sleep Research, 22, 573–580. 10.1111/jsr.12050 [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Arnal PJ, & Sauvet F (2015). Sleep and exercise: A reciprocal issue? Sleep Medicine Reviews, 20, 59–72. 10.1016/j.smrv.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Corbett RW, Middleton B, & Arendt J (2012). An hour of bright white light in the early morning improves performance and advances sleep and circadian phase during the Antarctic winter. Neuroscience Letters, 525(2), 146–151. 10.1016/j.neulet.2012.06.046 [DOI] [PubMed] [Google Scholar]
- Cox RC, & Olatunji BO (2020). Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Medicine Reviews, 101282. 10.1016/j.smrv.2020.101282 [DOI] [PubMed] [Google Scholar]
- Crispim CA, Zimberg IZ, dos Reis BG, Diniz R ., Tufik S, & de Mello MT (2011). Relationship between food intake and sleep pattern in healthy individuals. Journal of Clinical Sleep, 7(6), 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, & O’Donnell MR (2020). Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet, 395, 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davide P, Andrea P, Martina O, Andrea E, Davide D, & Mario A (2020). The impact of the COVID-19 pandemic on patients with OCD: Effects of contamination symptoms and remission state before the quarantine in a preliminary naturalistic study. Psychiatry Research, 291, 113213. 10.1016/j.psychres.2020.113213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR, Dickson C, & Han H (2019). Cognitive behavioural treatment for insomnia in primary care: A systematic review of sleep outcomes. British Journal of General Practice, 69(686), E657–E664. 10.3399/bjgp19X705065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Majumdar P, Saha A, & Sahu S (2020). COVID-19 pandemic lockdown-induced altered sleep/wake circadian rhythm, health complaints and stress among traffic police personnel in India. Chronobiology International, 00(00), 1–9. 10.1080/07420528.2020.1831524 [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DGM, Daan S, & Lewy A (1989). Bright morning light advances the human circadian system without affecting NREM sleep homeostasis. American Journal of Physiology, 256, R106–111. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE (2014). The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behavioral Sleep Medicine, 5(4), 256–278. 10.1080/15402000701557383.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Friedman NP, Wright KP, & Roth T (2011). Sleep reactivity and insomnia: Genetic and environmental influences. Sleep, 34(9), 1179–1188. 10.5665/sleep.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drerup ML, & Ahmed-Jauregui S (2019). Online delivery of cognitive behavioral therapy-insomnia: Considerations and controversies. Sleep Medicine Clinics, 14, 283–290. 10.1016/j.jsmc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Ellis JG, Perlis ML, Neale LF, Espie CA, & Bastien CH (2012). The natural history of insomnia: Focus on prevalence and incidence of acute insomnia. Journal of Psychiatric Research, 46(10), 1278–1285. 10.1016/j.jpsychires.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Van Ameringen M, Drummond L, Hollander E, Stein DJ, Geller D, Walitza S, Pallanti S, Pellegrini L, Zohar J, Rodriguez CI, Menchon JM, Morgado P, Mpavaenda D, Fontenelle LF, Feusner JD, Grassi G, Lochner C, Veltman DJ, … Dell’Osso B (2020). How to manage obsessive-compulsive disorder (OCD) under COVID-19: A clinician’s guide from the International College of Obsessive Compulsive Spectrum Disorders (ICOCS) and the Obsessive-Compulsive and Related Disorders Research Network (OCRN) of the Euro. Comprehensive Psychiatry, 100, 152174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Wang C, Zou L, Guo Y, Lu Z, Yan S, & Mao J (2020). Psychological health, sleep quality, and coping styles to stress facing the COVID-19 in Wuhan, China. Translational Psychiatry, 10, 225. 10.1038/s41398-020-00913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Resnick H, Ahern J, Gold J, Bucuvalas M, Kilpatrick D, Stuber J, & Vlahov D (2002). Posttraumatic stress disorder in Manhattan, New York City, after the September 11th terrorist attacks. Journal of Urban Health, 79(3), 340–353. 10.1093/jurban/79.3.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, & Scullin MK (2020). Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: Integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Medicine, 73, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, & Rosenstein RE (2010). Physiology of circadian entrainment. Physiological Reviews, 90, 1063–1102. 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, Kravitz HM, Krafty RT, & Buysse DJ (2015). Chronic stress is prospectively associated with sleep in midlife women: The SWAN sleep study. Sleep, 38(10), 1645–1654. 10.5665/sleep.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2005). A Cognitive Theory and Therapy for Chronic Insomnia. Journal of Cognitive Psychotherapy, 19(1), 41–59. 10.1891/jcop.19.1.41.66332 [DOI] [Google Scholar]
- Harvey CJ, Gehrman P, & Espie CA (2014). Who is predisposed to insomnia: A review of familial aggregation, stress-reactivity, personality and coping style. Sleep Medicine Reviews, 18(3), 237–247. 10.1016/j.smrv.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, & Soldatos CR (1981). Onset of insomnia: Role of life-stress events. Psychosomatic Medicine, 43(5), 439–451. 10.1097/00006842-198110000-00007 [DOI] [PubMed] [Google Scholar]
- Horesh D, & Brown AD (2020). Traumatic stress in the age of COVID-19: A call to close critical gaps and adapt to new realities. Psychological Trauma: Theory, Research, Practice, and Policy, 12(4), 331–335. [DOI] [PubMed] [Google Scholar]
- Innocenti P, Puzella A, Mogavero MP, Bruni O, & Ferri R (2020). Letter to editor: CoVID-19 pandemic and sleep disorders—a web survey in Italy. Neurological Sciences, 41(8), 2021–2022. 10.1007/s10072-020-04523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Opp MR (2017). Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology Reviews, 42, 129–155. 10.1038/npp.2016.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani O, Jike M, Watanabe N, & Kaneita Y (2017). Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Medicine, 32, 246–256. 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Jarrin SD, Drake CL, & Morin CM (2018). Insomnia and hypertension: A systematic review. Sleep Medicine Reviews, 41, 3–38. 10.1016/j.smrv.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Kaslow NJ, Friis-Healy EA, Cattie JE, Cook SC, Crowell AL, Cullum KA, del Rio C, Marshall-Lee ED, LoPilato AM, VanderBroek-Stice L, Ward MC, White DT, & Farber EW (2020). Flattening the emotional distress curve: A behavioral health pandemic response strategy for COVID-19. American Psychologist. [DOI] [PubMed] [Google Scholar]
- King T, Hewitt B, Crammond B, Sutherland G, Maheen H, & Kavanagh A (2020). Reordering gender systems: Can COVID-19 lead to improved gender equality and health? The Lancet, 396(10244), 80–81. 10.1016/S0140-6736(20)31418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel E, Polusny MA, Arbisi PA, & Erbes CR (2013). Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. Journal of Anxiety Disorders, 27(5), 512–519. 10.1016/j.janxdis.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Kokou-Kpolou CK, Megalakaki O, Laimou D, & Kousouri M (2020). Insomnia during COVID-19 pandemic and lockdown: Prevalence, severity, and associated risk factors in French population. Psychiatry Research, 290, 113128. 10.1016/j.psychres.2020.113128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada Y, Tanaka H, Yamamoto Y, Shirakawa S, & Yamazaki K (2000). Effects of bright light pre-exposure on sleep onset process. Psychiatry and Clinical Neurosciences, 54, 365–366. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Werth E, & Wirz-Justice A (2002). Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. Journal of Biological Rhythms, 17(4), 364–376. [DOI] [PubMed] [Google Scholar]
- Lancee J, Eisma MC, Van Zanten KB, & Topper M (2017). When thinking impairs sleep: Trait, daytime, and nighttime repetitive thinking in insomnia. Behavioral Sleep Medicine, 15(1), 53–69. 10.1080/15402002.2015.1083022 [DOI] [PubMed] [Google Scholar]
- Leblanc M, Mérette C, Savard J, Ivers H, Baillargeon L, & Morin CM (2009). Incidence and risk factors of insomnia in a population-based sample. SLEEP, 32(8), 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone MJ, Sigman M, & Golombek DA (2020). Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Current Biology, 30, R930–R931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Yang BX, Luo D, Liu Q, Ma S, Huang R, Lu W, Majeed A, Lee Y, Lui LMW, Mansur RB, Nasri F, Subramaniapillai M, Rosenblat JD, Liu Z, & McIntyre RS (2020). The mental health effects of COVID-19 on health care providers in China. American Journal of Psychiatry, 177(7), 635–636. 10.1176/appi.ajp.2020.20040374 [DOI] [PubMed] [Google Scholar]
- Lin L. yu, Wang J, Ou-yang X. yong, Miao Q, Chen R, Liang F. xia, Zhang Y. pu, Tang Q, & Wang T (2020). The immediate impact of the 2019 novel coronavirus (COVID-19) outbreak on subjective sleep status. Sleep Medicine, xxxx, 18–24. 10.1016/j.sleep.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Zhang E, Tin G, Wong F, Hyun S, & Hahm HC (2020). Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Research, 113172. 10.1016/j.psychres.2020.113172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhang F, Wei C, Jia Y, Shang Z, & Sun L (2020). Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: Gender differences matter. Psychiatry Research, 287, 112921. 10.1016/j.psychres.2020.112921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan SK, Behar E, & Luhmann M (2016). Examining the relationship between worry and sleep: A daily process approach. Behavior Therapy, 47(4), 460–473. 10.1016/j.beth.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Mineka S, & Kihlstrom JF (1978). Unpredictable and uncontrollable events: A new perspective on experimental neurosis. Journal of Abnormal Psychology, 87(2), 256–271. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, & Skene DJ (2005). Nonphotic entrainment in humans? Journal of Biological Rhythms, 20(4), 339–352. 10.1177/0748730405277982 [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto S, Masubuchi S, Hashimoto S, Ma- S, Honma S, & Phase-ad- KH (2001). Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. American Journal of Physiology, 281, 197–205. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Buysse DJ, DeGrazia JM, & Kupfer DJ (2003). The relationship between lifestyle regularity and subjective sleep quality. Chronobiology International, 20(1), 97–107. 10.1081/CBI-120017812 [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Czeisler CA, & Richardson GS (1983). Circadian timekeeping in health and disease. Part 1. Basic properties of circadian pacemakers. The New England Journal of Medicine, 309(8), 469–476. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, & Gubbi S (2020). COVID-19 pandemic, coronaviruses, and diabetes mellitus. American Journal of Physiology. Endocrinology and Metabolism, 318, E736–E741. 10.1152/ajpendo.00124.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, & Harvey A (2010). Circadian rhythms and sleep in bipolar disorder. Bipolar Disorders, 12(5), 459–472. 10.1111/j.1399-5618.2010.00843.x [DOI] [PubMed] [Google Scholar]
- Nota JA, Potluri S, Kelley KN, Elias JA, & Krompinger JW (2020). Delayed Bedtimes Are Associated With More Severe Obsessive-Compulsive Symptoms in Intensive Residential Treatment. Behavior Therapy, xxxx. 10.1016/j.beth.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Nota JA, Sharkey KM, & Coles ME (2015). Sleep, arousal, and circadian rhythms in adults with obsessive-compulsive disorder: A meta-analysis. Neuroscience and Biobehavioral Reviews, 51, 100–107. 10.1016/j.neubiorev.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, & Riemann D (2013). REM sleep dysregulation in depression: State of the art. Sleep Medicine Reviews, 17(5), 377–390. 10.1016/j.smrv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, & Katsaounou P (2020). Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behavior and Immunity. 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partinen M, Bjorvatn B, Holzinger B, Chung F, Penzel T, Espie CA, Morin CM, & ICOSS-collaboration group. (2020). Sleep and circadian problems during the coronavirus disease 2019 (COVID-19) pandemic: The International COVID-19 Sleep Study (ICOSS). Journal of Sleep Research, July, e13206. 10.1111/jsr.13206 [DOI] [PubMed] [Google Scholar]
- Perlis ML, Jungquist C, Smith MT, & Posner D (2005). Cognitive behavioral treatment of insomnia: A session by session guide. Springer US. [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165, 125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- Quante M, Mariani S, Weng J, Marinac CR, Kaplan ER, Rueschman M, Mitchell JA, Hipp JA, Feliciano EMC, & Wang R (2019). Zeitgebers and their association with rest-activity patterns. Chronobiology International, 36(2), 203–213. 10.1080/07420528.2018.1527347.Zeitgebers [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S (2012). Health anxiety disorders: A cognitive construal. Behaviour Research and Therapy, 50, 502–512. 10.1016/j.brat.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Randall C, Nowakowski S, & Ellis JG (2019). Managing acute insomnia in prison: Evaluation of a “one-shot” cognitive behavioral therapy for insomnia (CBT-I) intervention. Behavioral Sleep Medicine, 17(6), 827–836. 10.1080/15402002.2018.1518227 [DOI] [PubMed] [Google Scholar]
- Ren Y, Qian W, Li Z, Liu Z, Zhou Y, Wang R, Qi L, Yang J, Song X, Zeng L, & Zhang X (2020). Public mental health under the long-term influence of COVID-19 in China: Geographical and temporal distribution. Journal of Affective Disorders, 277, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, & Van Cauter E (2018). Sleep in fluences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism, 84, 56–66. 10.1016/j.metabol.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Rivera RM, Carballea D, Rivera RM, & Carballea D (2020). Coronavirus: A trigger for OCD and illness anxiety disorder? Psychological Trauma: Theory, Research, Practice, and Policy. [DOI] [PubMed] [Google Scholar]
- Robillard R, Dion K, Pennestri M-H, Solomonova E, Lee E, Saad M, Murkar A, Godbout R, Edwards JD, Quilty L, Daros AR, Bhatla R, & Kendzerska T (2020). Profiles of sleep changes during the COVID-19 pandemic: Demographic, behavioural and psychological factors. Journal of Sleep Research, September, e13231. 10.1111/jsr.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkovskis PM, & Warwick HMC (1986). Morbid preoccupations, health anxiety, and reassurance: A cognitive-behavioural approach to hypochondriasis. Behaviour Research and Therapy, 24(5), 597–602. [DOI] [PubMed] [Google Scholar]
- Salo P, Vahtera J, Ferrie JE, Akbaraly T, Goldberg M, Zins M, Pentti J, Virtanen M, Shipley MJ, Singh-Manoux A, Dauvilliers Y, & Kivimaki M (2012). Trajectories of sleep complaints from early midlife to old age: Longitudinal modeling study. Sleep, 35(11), 1559–1568. 10.5665/sleep.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, & Loudon A (2018). Clocking in to immunity. Nature Reviews Immunology, 18(7), 423–437. 10.1038/s41577-018-0008-4 [DOI] [PubMed] [Google Scholar]
- Schubert JR, Stewart E, & Coles ME (2019). Later bedtimes predict prospective increases in symptom severity in individuals with obsessive compulsive disorder (OCD): An initial study. Behavioral Sleep Medicine, 2002(May). 10.1080/15402002.2019.1615490 [DOI] [PubMed] [Google Scholar]
- Schuster MA, Stein BD, Jaycox LH, Collins RL, Marshall GN, Elliott MN, Zhou AJ, Kanouse DE, Morrison JL, & Berry SH (2001). A national survey of stress reactions after the September 11, 2001 terrorist attacks. New England Journal of Medicine, 345, 1507–1512. [DOI] [PubMed] [Google Scholar]
- Shechter A, & St-Onge M-P (2014). Delayed sleep timing is associated with low levels of free-living physical activity in normal sleeping adults. Sleep Medicine, 15(12), 1586–1589. 10.1016/j.sleep.2014.07.010.Delayed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NA, Boffa JW, Wissemann K, & Schmidt NB (2019). Insomnia symptoms predict the development of post-traumatic stress symptoms following an experimental trauma. Journal of Sleep Research, May, 1–9. 10.1111/jsr.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehner AM, Kaplan KA, & Harvey AG (2014). Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. Journal of Affective Disorders, 167, 93–97. 10.1016/j.jad.2014.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Caruso LS, & Glovinsky PB (1987). A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America, 10(4), 541–553. 10.1016/S0193-953X(18)30532-X [DOI] [PubMed] [Google Scholar]
- Tan W, Hao F, McIntyre RS, Jiang L, Jiang X, Zhang L, Zhao X, Zou Y, Hu Y, Luo X, Zhang Z, Lai A, Ho R, Tran B, Ho C, & Tam W (2020). Is returning to work during the COVID-19 pandemic stressful? A study on immediate mental health status and psychoneuroimmunity prevention measures of Chinese workforce. Brain Behavior and Immunity, 87, 84–92. 10.1016/j.bbi.2020.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell-Haglöw J, Miller CB, Bartlett DJ, Yee BJ, Openshaw HD, & Grunstein RR (2018). Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults – What do we know? A clinical update. Sleep Medicine Reviews, 38, 28–38. 10.1016/j.smrv.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, & Leproult R (2015). Metabolic consequences of sleep and sleep loss. Sleep Medicine, 9, S23–S28. 10.1016/S1389-9457(08)70013-3.Metabolic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmin A, Schäfer V, Cajochen C, Meyer AH, Bader K, Winzeler K, & Wilhelm FH (2014). Daily stress, presleep arousal, and sleep in healthy young women: a daily life computerized sleep diary and actigraphy study. Sleep Medicine, 15(3), 359–366. 10.1016/j.sleep.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Voitsidis P, Gliatas I, Bairachtari V, Papadopoulou K, Papageorgiou G, Parlapani E, Syngelakis M, Holeva V, & Diakogiannis I (2020). Insomnia during the COVID-19 pandemic in a Greek population. Psychiatry Research, 289, 113076. 10.1016/j.psychres.2020.113076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, & Stickgold R (2004). Sleep-dependent learning and memory consolidation. Neuron, 44(1), 121–133. 10.1016/j.neuron.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Wams EJ, Woelders T, Marring I, Van Rosmalen L, Beersma DGM, Gordijn MCM, & Hut RA (2017). Linking light exposure and subsequent sleep: A field polysomnography study in humans. SLEEP, 40(12), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang D, Huang H, Zhang J, Guo L, Liu Y, Ma H, & Geng Q (2020). The psychological impact of COVID-19 pandemic on medical staff in Guangdong, China: A cross-sectional study. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu L-Y, Ma Y-F, Bo H-X, Deng H-B, Cao J, Wang Y, Wang X-J, Xu Y, Lu Q-D, Wang H, & Wu X-J (2020). Association of insomnia disorder with sociodemographic factors and poor mental health in COVID-19 inpatients in China. Sleep Medicine. 10.1016/j.sleep.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, & Johnston JD (2017). Meal timing regulates the human circadian system. Current Biology, 27(12), 1768–1775.e3. 10.1016/j.cub.2017.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social jetlag: Misalignment of biological and social time. Chronobiology International, 23(1–2), 497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- Wright KP Jr, Linton SK, Withrow D, Casiraghi L, de la Iglesia H, Vetter C, & Depner CM (2020). Sleep in university students prior to and during COVID-19 stay-at-home orders. Current Biology, 30, R797–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Bogan RK, & Wyatt JK (2013). Shift work and the assessment and management of shift work disorder (SWD). Sleep Medicine Reviews, 17, 41–54. 10.1016/j.smrv.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, & Chinoy ED (2013). Entrainment of the human circadian clock to the natural light-dark cycle. Current Biology, 23(16), 1554–1558. 10.1016/j.cub.2013.06.039.Entrainment [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Zhang Y, Kong D, Li S, & Yang N (2020). The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 ( COVID-19 ) in January and February 2020 in China. Medical Science Monitor, 26, e923549. 10.12659/MSM.923549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, & Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide S, Honma S, & Honma K (2015). Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. American Journal of Physiology, 309, 1112–1121. 10.1152/ajpregu.00127.2015 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren R, Sanford LD, Yang L, Zhou J, Zhang J, Wing Y, Shi J, Lu L, & Tang X (2019). Sleep in posttraumatic stress disorder: A systematic review and meta-analysis of polysomnographic findings. Sleep Medicine Reviews, 48, 101210. 10.1016/j.smrv.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang W, Sun Y, Qian W, Liu Z, Wang R, & Qi L (2020). The prevalence and risk factors of psychological disturbances of frontline medical staff in china under the COVID-19 epidemic: Workload should be concerned. Journal of Affective Disorders, 277, 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zreik G, Asraf K, Haimov I, & Tikotzky L (2020). Maternal perceptions of sleep problems among children and mothers during the coronavirus disease 2019 (COVID-19) pandemic in Israel. Journal of Sleep Research, July, 1–7. 10.1111/jsr.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuin M, Rigatelli G, Zuliani G, Rigatelli A, Mazza A, & Roncon L (2020). Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. Journal of Infection, 81, e84–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]


