Abstract
During moments involving selective attention, the thalamus orchestrates the preferential processing of prioritized information by coordinating rhythmic neural activity within a distributed frontoparietal network. The timed release of neuromodulators from subcortical structures dynamically sculpts neural synchronization in thalamocortical networks to meet current attentional demands. In particular, noradrenaline modulates the balance of cortical excitation and inhibition, as reflected by thalamocortical alpha synchronization (~8–12 Hz). These neuromodulatory adjustments facilitate the selective processing of prioritized information. Thus, by disrupting effective rhythmic coordination in attention networks, age-related locus coeruleus degeneration can impair higher levels of neural processing. In sum, findings across different levels of analysis and modalities shed light on how the noradrenergic modulation of neural synchronization helps to shape selective attention.
Keywords: noradrenaline, norepinephrine, locus coeruleus, rhythmic neural activity, selective attention, cognitive aging
Neuromodulation sculpts neural synchronization in attention networks
Daily life confronts us with a multitude of sensations that far exceeds neural processing resources. It thus poses a complex computational problem: What aspects of the environment are most relevant for current and future goal-directed actions and how can these be selected for further analysis [1]? A distributed network including frontal and parietal cortical structures supports the preferential processing of relevant information [2]. The thalamus orchestrates communication within this network via a transient synchronization of its rhythmic neural activity (see Glossary) [3,4], while neuromodulators tune synchronization in thalamocortical networks to correspond with current demands [5-11]. However, while evidence about the roles of neuromodulation and neural synchronization in the selective processing of prioritized information is accumulating, their interactions are often overlooked. Bridging levels of analysis, in this review we synthesize animal and human research indicating that neuromodulation supports selective attention by dynamically sculpting patterns of thalamocortical synchronization.
Rhythmic neural activity coordinates information processing in distributed cortical networks [2-4]. A thalamic pathway mediates frontoparietal modulations of alpha activity (~8–12 Hz) [12] that promote flexible information gating by temporarily disengaging task-irrelevant brain regions [13,14]. Note that functional equivalents of the primate alpha rhythm may manifest at lower frequencies in rodents [15,16]. Selective information processing in frontoparietal networks moreover depends on the timed release of neuromodulators from subcortical structures [8,17-21]. This opinion article focuses specifically on noradrenergic neuromodulation based on recent methodological advancements that open the door for non-invasive assessment (Box 1). A transient activation of the noradrenergic system increases the signal-to-noise ratio of currently relevant representations and biases the competition for processing resources across levels of the cortical hierarchy [22,23]. Recent studies indicate that activation within the locus coeruleus-noradrenaline (LC–NA) system translates into dynamic changes in alpha synchronization on a neural network level, presumably mediated via a thalamic mechanism [5-7,9,10,24,25] (Figure 1, Key Figure). Thus, noradrenergic neuromodulation may act as a dynamic switch to modulate the sensitivity of cortical networks for processing high-priority inputs. In the following, we first summarize recent research on noradrenergic neuromodulation and neural synchronization before proposing how the noradrenergic modulation of rhythmic neural activity shapes attention.
Box 1: Novel non-invasive proxies reflecting noradrenergic neuromodulation.
Technical challenges in reliably targeting the small brainstem nucleus have long impeded in-vivo research exploring LC’s effects on attention in humans [105,106]. However, using recently developed MR sequences [107-111], an elevated signal or hyperintensity can be observed bordering the fourth ventricle (Figure I). Applying a combination of MRI and histology of human post-mortem samples, a recent study investigated whether this hyperintensity is an indicator for LC integrity (i.e., a proxy for cell density) [107]. Excitingly, the hyperintensities detected on LC-MRI scans indeed closely matched the location of noradrenergic cells that contained neuromelanin, as confirmed by subsequent histology [107] (cf. [112]). The dark, insoluble pigment neuromelanin is a by-product of catecholamine synthesis and thus accumulates in the LC across the lifespan [113]. Within LC cells, neuromelanin scavenges metals and, as a compound, produces paramagnetic effects [114]. Therefore, neuromelanin–metal compounds may serve as endogenous contrast agent that allows the non-invasive assessment of LC integrity using MRI [114]. Nevertheless, unraveling the precise contrast mechanisms underlying LC-MRI is an active area of research [109,111]. Recent findings of elevated MR contrast in rodents naturally lacking neuromelanin suggest LC’s high water content and abundance of metal ions as sources of hyperintensity [110]. Irrespective of the precise contrast mechanisms, transgenic mice with a ~70 % reduction of noradrenergic neurons lack the elevated LC-MRI signal evident in wild-type mice, indicating noradrenergic cells as a source of the hyperintensity [110]. Lower LC-MRI contrast has consistently been reported in diseases associated with noradrenergic neurodegeneration like Alzheimer’s [109,111,115].
In addition to structural measures, studies in rodents and primates established luminance-independent pupil changes as a proxy for noradrenergic activity [28,40,41,98,116-118]. Pupil dilation reflects the interaction of two opposing muscles: the dilator and sphincter pupillae, innervated by the sympathetic and parasympathetic nervous systems, respectively. Critically, the LC functions as a premotor nucleus for both, by stimulating/inhibiting sympathetic and parasympathetic preganglionic neurons, respectively [119]. Indeed, optogenetic activation of the LC caused the pupil to dilate, whereas inhibition reliably constricted the pupil [28,41]. In combination with studies that demonstrated LC spiking and noradrenergic axon activity before pupil dilations [98,116], this suggests that pupil dilation may serve as a moment-to-moment index of noradrenergic activity [117]. However, cholinergic and serotonergic influences on pupil dilation have also been reported [98,120] and further research is needed to disentangle their relative contributions. Taken together, LC-MRI and pupil dilation are valuable non-invasive tools to advance our understanding of the noradrenergic system across species.
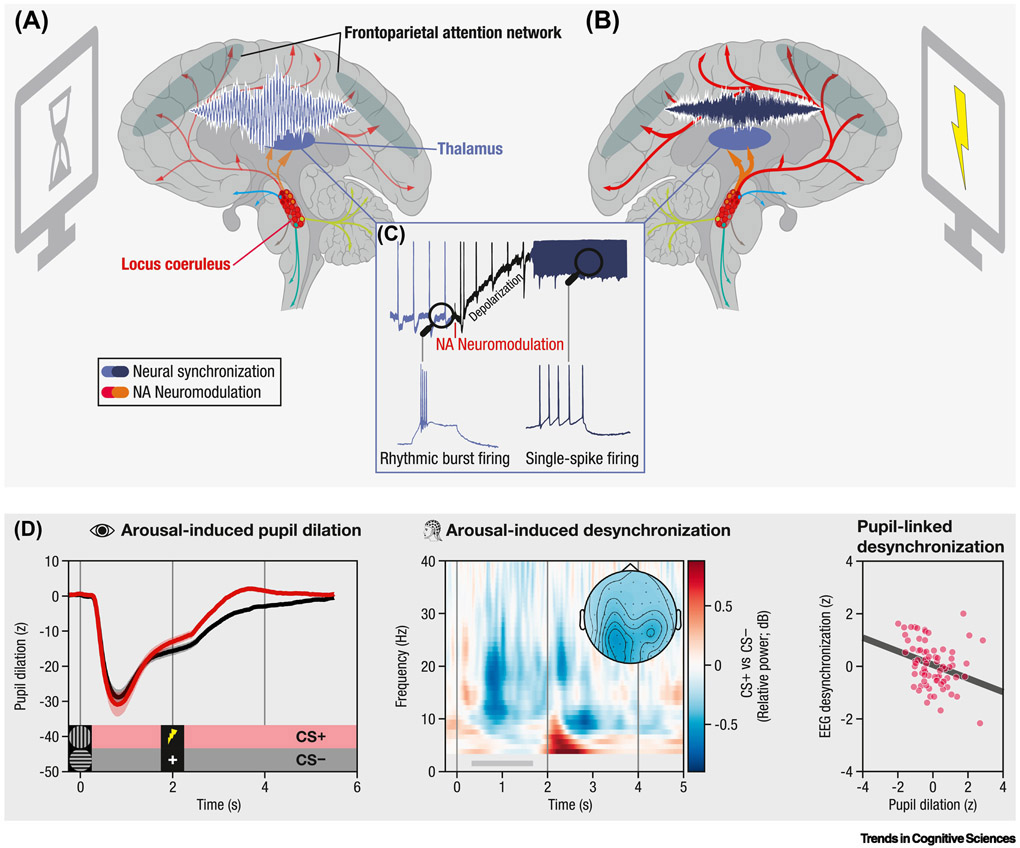
Figure 1, Key Figure. Locus coeruleus–noradrenergic neuromodulation shapes patterns of thalamocortical synchronization during attention.
(A) Anatomically and functionally defined locus coeruleus ensembles innervate different cortical and subcortical targets (see colored cells/projections [circles/arrows]), including the thalamus and frontoparietal network. (blue; grey). During inattentiveness, low noradrenaline levels are linked to rhythmic burst firing in thalamic alpha generators (see C). Alpha-rhythmic activity (~8–12 Hz) in thalamocortical attention networks indicates a state of relative inhibition (see high amplitude low-frequency oscillation [light blue]) (B) Behaviorally relevant events elicit a transient increase in locus coeruleus activity. Elevated noradrenaline (NA) levels modulate cortical synchronization by shifting thalamic pacemakers from a rhythmic firing pattern to a mode of activity that allows for reliable information transfer (single-spike firing; see C). Neural activity in thalamocortical attention networks is desynchronized (dark blue), supporting the processing of attended stimuli. (C) Noradrenaline depolarizes thalamic neurons and abolishes the rhythmic burst firing that is linked to thalamocortical alpha rhythms (reproduced, with permission, from [9]). (D) Pupil-indexed noradrenergic neuromodulation is related to cortical low-frequency desynchronization. (left) Compared to perceptually matched control stimuli (CS−), fear-conditioned stimuli (CS+) elicit a transient pupil dilation, a marker of locus coeruleus activity (Box 1). (middle) Concurrent electroencephalography recordings reveal an arousal-related alpha-beta desynchronization at posterior electrodes (topography shows averaged activity between 0.5–1.5 s and 8–20 Hz [grey horizontal bar]). (right) Larger pupil dilation is associated with more alpha-beta desynchronization (i.e., more negative values), indicating an association between proxies of noradrenergic neuromodulation and cortical synchronization (reproduced, with permission, from [7]). Credits: Sagittal brain section, adapted from Patrick J. Lynch under a Creative Commons Attribution 2.5 License 2006.
Noradrenergic neuromodulation supports selective attention
In humans, noradrenergic cells cluster in distinct, pigmented nuclei in the brainstem. The largest of these is the locus coeruleus (LC), with a length of about 15 mm adjacent to the fourth ventricle [26]. The LC receives afferent input from several subcortical structures and, crucially, the prefrontal cortex [27-29]. The LC thus integrates information from evolutionarily conserved regions controlling autonomic functions and arousal as well as phylogenetically younger areas implicated in higher-order cognition [30]. Via an ascending pathway, the LC in turn provides noradrenergic innervation for the thalamus and almost all areas of neocortex [25,29-31]. This connectivity pattern grants prefrontal regions a top-down regulatory influence on noradrenergic neuromodulation with its pronounced effect on thalamic- and forebrain functioning [9,10,27,28,32].
A new look at the blue spot: More than diffuse arousal
Researchers had characterized the LC as a homogenous structure that uniformly broadcasts noradrenaline throughout the entire cortex via diffuse and highly arborized projections [33]. Importantly, such an arrangement would argue against a noradrenergic role in selective attention, which requires a spatially highly specific innervation of cortical networks [21] as well as differential innervation of representations that are the focus of attention versus those that should be ignored [22]. However, recent evidence indicates a more differentiated structural and functional organization of the LC [17,28,29,34-36]. For instance, a recent study [34] demonstrated that functionally distinct LC populations with connections to the amygdala and prefrontal cortex are involved in the acquisition and extinction of fear memories, respectively (see [17] for similar observations regarding attention). In addition, contrary to earlier assumptions of a synchronous activation of all LC cells [32], multiple distinct LC ensembles (i.e., subsets of synchronized LC neurons) were identified with the potential for a more targeted noradrenergic modulation [35]. Together with reports of a direct noradrenergic modulation of thalamic processing [31,37], these findings suggest an anatomically and functionally specialized neuromodulatory system that may be ideally suited to bias processing in thalamocortical networks corresponding to attentional demands [31,33].
Noradrenaline biases processing towards prioritized representations
Noradrenergic neuromodulation influences selective attention via several mechanisms: First, LC activity is associated with the transition to, and the maintenance of a vigilant behavioral state [23,38] that allows for the optimal processing of sensory information [39]. More specifically, transient (phasic) LC activations—in response to salient stimuli or recruited via top-down mechanisms [27,32]—are associated with rapid attention allocation and essential for the signaling of sensory inputs [8,10,19]. Reliable phasic LC responses are observed against the backdrop of an ongoing (tonic) firing rhythm, whereby too low or high tonic LC firing is linked to drowsiness and stress, respectively (i.e., an inverted-u shaped association [38,40,41]). Second, in the prefrontal cortex, noradrenaline acts on α2A-adrenoceptors to boost activity in attention networks [17,42,43]. Specifically, noradrenaline increases persistent firing in prefrontal circuits that code task-relevant information in the absence of external stimulation, a neural correlate of attention and working memory [42]. In line with this, α2Λ-agonists have been applied to ameliorate senescent attention deficits in aged monkeys, hinting at noradrenergic neurodegeneration with advancing age (Box 2) [43] (but see [44]). Third, in sensory cortices, noradrenaline enhances the selective processing of currently relevant representations, probably in interaction with local glutamate levels [22,45]. Seminal work in monkeys found that noradrenaline application to the auditory cortex preceding sensory stimulation decreased the rate of spontaneous firing while mostly sparing stimulus-evoked responses (i.e., increased signal-to-noise ratios [46]). More recently, LC activation paired with auditory stimulation in rats was shown to induce long-lasting increases in tone-evoked neural responses [47]. In addition, in the same study LC activation led to stimulus-evoked activity in previously unresponsive auditory cortex neurons and improved perceptual detection abilities [47]. Crucially, recent evidence indicates that a noradrenergic modulation of thalamic information transmission may play a prominent role in LC’s amplification of sensory processing [31,37]. Finally, on a network level, phasic LC activity has been conceptualized as a circuit breaker that interrupts ongoing processing in the dorsal attention network and supports the reorienting of attention, mediated via activation of the ventral attention network [48,49].
Box 2: Age-related changes in noradrenergic neuromodulation.
Recent cross-sectional investigations of large lifespan samples report a decline in LC-MRI signal, a proxy for noradrenergic cell density, starting around 60 years of age [121-123]. For instance, in over 600 participants (18–88 years), a negative quadratic relationship between age and LC-MRI contrast was detected, compatible with a loss of noradrenergic cells in later life [121]. Additionally, in-vivo PET imaging revealed a lower availability of the noradrenergic transporter in later life, prominently in the thalamus [106]. Taken together, the available evidence suggests that aging impairs the noradrenergic system at multiple points (i.e., LC cell bodies, LC terminal regions) resulting in less reliable noradrenergic neuromodulation in later life (but see [124] for a discussion).
Recent theories of cognitive aging posit a high susceptibility of LC neurons to neurodegeneration, presumably resulting from their exposure to natural stressors [124,125]. For instance, LC’s anatomical location adjacent to the ventricular system and its widespread, unmyelinated axons expose it to toxins from cerebrospinal fluid (CSF) and blood circulation [124-126]. Neuromelanin accumulating in the LC may become toxic. While neuromelanin initially traps free metals and thus counters their toxicity, when a cell dies it releases its accumulated neuromelanin, potentially accelerating neuronal decline [113]. Further, LC’s constant, tonic firing rhythm produces a high bioenergetic demand associated with an increased risk of oxidative stress and subsequent cell damage [124-126]. Analyses of post-mortem samples moreover identified the LC as one of the first brain sites in which abnormally phosphorylated tau proteins accumulate, a hallmark of age-related neurodegenerative diseases like Alzheimer’s [127,128]. Tau burden in the LC linearly increases with Alzheimer’s disease progression and is associated with LC shrinkage, already at presymptomatic stages of the disease [111,128]. Besides its influence on cognitive processing [23,50], noradrenergic neuromodulation subserves critical neuroprotective functions (e.g., anti-inflammatory effects mediated via action on β-adrenoceptors on microglia) [124,126] and thus a loss of LC cells may exacerbate the progression of age-related neurodegenerative diseases [124].
In sum, due to its exposure to various physiological stressors (e.g., toxins, oxidative stress, and aberrant tau) the LC shows a high vulnerability for age-related neurodegeneration with probably deleterious consequences for late-life cognitive abilities. Following this notion, a growing number of in-vivo [122,129-132] and post-mortem [133] studies demonstrate the importance of LC integrity for the maintenance of cognitive performance in aging.
These lines of evidence indicate that noradrenergic neuromodulation biases neural information processing towards prioritized representations at multiple brain sites, including the thalamus and cortical regions. Although its effect varies across release and binding sites as well as LC firing modes (i.e., tonic or phasic), noradrenaline generally increases signal-to-noise ratios of salient or prioritized stimuli and thereby supports attention [21-23,50]. On a neural ensemble level, noradrenaline release is closely linked to changes in neural activity [5,10,24,25] within frequency bands that play a prominent role in selective attention.
Modulations of alpha-oscillatory activity support selective attention
Neural synchronization provides a mechanism for routing prioritized information through thalamocortical networks [3,4]. A long line of research implicates the most prominent oscillation in the waking state, the alpha rhythm, in attention [13,14]. Lesion studies suggest the thalamus as a pacemaker of the alpha rhythm [15,51,52]. However, intracranial recordings in primates also point to cortical generators in the parietal and occipital lobes [53-55]. Most likely, the alpha rhythm constitutes a complex product of thalamic and cortical generators, regions that are densely linked via reciprocal connections [4,15,51,55].
Alpha desynchronization: A domain-general attentional selection mechanism
Functionally, alpha oscillations are conceptualized as frontoparietal-mediated inhibition that temporarily disengages task-irrelevant brain regions and thus distributes processing resources to support selective attention [2,52,56]. This interpretation is based on the observation that shifts of attention—for instance to a cued location of a visual display in a spatial orientation task—reliably decrease alpha activity in regions processing the attended space. In contrast, brain areas that code for the visual space occupied by distractors typically exhibit increased alpha-rhythmic activity [57,58]. Such attention-related, spatially targeted patterns of alpha modulation have commonly been investigated in the visual domain. However, they are also evident in somatosensory and auditory paradigms, suggesting a domain-general selection mechanism [59,60]. Please note that our discussions mainly concern within-region alpha modulations, for a discussion of inter-regional alpha dynamics, see [61].
Underlining their functional relevance, event-related increases (synchronization) and decreases (desynchronization) of alpha-rhythmic activity in distractor- and target-coding brain regions have been associated with attention performance on a single-trial level [62,63]. Moreover, several studies suggest that attention-related modulations of alpha rhythms may provide a causal mechanism for the flexible selection of relevant information [64-66] (reviewed in [56]). For instance, using rhythmic transcranial magnetic stimulation to alter synchronization in the visual cortex revealed a frequency- and spatially-specific modulation of perception [64]. Stimulation within the alpha but not other control frequencies impaired performance contralateral to the stimulated hemisphere, while increasing ipsilateral target detection. Hence, artificially increasing synchronization of alpha-rhythmic activity biased attention away from the visual space processed by the stimulated brain site [1,64]. In a recent neurofeedback training study, participants learned to manipulate alpha synchronization in left relative to right parietal areas [65]. The training-induced shift in alpha rhythms led to a corresponding alteration in visually evoked responses during the training as well as a change in alpha asymmetry after training. Importantly, interindividual differences in alpha asymmetry were associated with a sustained modulation of attention [65], underlining the functional relevance of alpha rhythms [65,67]. Notably, artificially reinstating alpha synchronization in distractor-processing brain regions may aid older adults in overcoming their attentional deficits [66] (Box 3).
Box 3: Noradrenergic neurodegeneration may disrupt rhythmic coordination within the aging brain.
With age, brain structure and function change, resulting in declining attention [70,104]. Such cases of non-optimal performance can shed light on the dynamics of complex systems [134]. In particular changes in neuromodulation have been identified as crucial determinants of aging cognition [124,135]. However, age differences in the modulation of neural rhythms are also linked to cognitive decline.
With advancing age, alpha rhythms occur less often and, when they do, have diminished amplitudes and lower peak frequencies [103,136]. In addition, older adults show differential alpha modulations during attention tasks [62,137-140]. For example, older compared to younger adults show a delayed and diminished alpha synchronization for task-irrelevant stimuli, which suggests a less reliable attentional filter [62,141,142]. Accumulating evidence links age-related changes in alpha dynamics and cognitive decline [62,138-140,143]. However, a recent study showed comparable attentional alpha modulations in middle-aged and older adults, which did not influence behavior [70]. Taken together, inhibitory processes, as expressed by alpha modulations, may operate less reliably in older adults, at least at high levels of task difficulty [143,144].
Integrating neuromodulatory and oscillatory changes, in this opinion article we propose a hypothetical link bridging levels of analysis [135]: Noradrenergic neurodegeneration (Box 2) may lead to age-related neuronal loss in the LC [121,122]. As a consequence, older adults may be less able to recruit noradrenergic neuromodulation to bias information processing according to current goals [21,22]. Neural synchronization in thalamocortical networks is dynamically modulated via noradrenergic inputs [9,10,24,31]. Hence, age-related impairments in modulating alpha synchronization to selectively gate relevant information may partly reflect diminishing noradrenergic drive [5-7].
This proposal is compatible with the notion that a shortage of neuromodulators in aging and disease impairs thalamic alpha generation [103]. Initial empirical support comes from a study that recorded cortical EEG activity during participants’ lifetime and subsequent post-mortem concentrations of neuromodulators in the thalamus of Alzheimer’s patients and controls [145]. Compared with controls, Alzheimer’s patients' specimens showed lower noradrenaline levels in the thalamus [145] (Box 2). Among patients, cortical alpha rhythms were linked to cognition and, importantly, thalamic noradrenaline levels, while other neuromodulators showed no reliable association [145]. Finally, another investigation in healthy older adults approximated noradrenergic responsiveness using pupil dilation and EEG desynchronization and revealed an association with attentional performance as well as a decline in later life [7]. While several questions remain to be answered, we propose that age-related noradrenergic decline disrupts the rhythmic coordination in thalamocortical networks necessary for attention.
However, several recent studies indicate that attention-related alpha modulations may not be associated with markers of early sensory gain control [68-70]. This suggests that early sensory gain control and alpha-rhythmic inhibition may represent complementary mechanisms of selective attention [70,71]. Subsequent localizations of cortical alpha activity close to the parietal lobe suggest that, upstream of early sensory processing, alpha synchronization may help gate information flow through thalamocortical networks [13,56,71].
Taken together, while alpha activity likely constitutes one among several mechanisms, attention-related alpha modulation consistently emerges as a domain-general, behaviorally relevant mechanism for the selective processing of relevant information. But how do alpha modulations help shape attention?
Routing prioritized information via alpha-mediated inhibition
First, desynchronization of alpha-rhythmic activity may facilitate the firing of neuronal ensembles coding selected stimuli by releasing these ensembles from inhibition [13,60]. Thereby, decreases in alpha activity dynamically regulate the windows for processing and facilitate the timed expression of a neural code necessary for the representation of prioritized information [72]. In particular, within an alpha oscillation, inhibitory and excitatory phases alternate in a cyclic succession indicated by the peaks and troughs of the oscillation [13,14]. Local increases in alpha amplitude during the inhibitory up-phases elevate neural response thresholds, resulting in a reduced likelihood of action potential initiation [60]. Massed neural firing is therefore more likely during release from inhibition during the down-phases of the corresponding alpha cycle [3,6,73,74]. With increasing alpha amplitude, the duty cycle for neuronal processing becomes shorter [13], whereas decreases in alpha activity allow for longer processing windows (i.e., wider troughs). On an information-theoretical level, synchronization of alpha rhythms increases the redundancy of neuronal firing and thereby reduces the information carried in the transmitted neural code [72]. Desynchronization of low-frequency activity, in turn, enhances the information-coding capacity of neural ensembles representing prioritized stimuli [72,75]. In sum, by adjusting excitation and inhibition in thalamocortical networks, dynamic modulations of alpha-oscillatory activity support the selective processing of attended stimuli.
While the previous sections separately outlined how noradrenergic and alpha-oscillatory mechanisms support selectivity, there are indications they interact [5-7]. Such interactions were envisioned by Steriade and colleagues [76] almost thirty years ago: “Neuromodulatory systems are capable of shifting thalamocortical networks between different oscillatory states. Synchrony and other network properties could be exploited for controlling the flow of information between brain areas and for deciding where to store important information.” Excitingly, the tools to explore neuromodulatory influences on thalamocortical synchronization during attention have become increasingly available (Box 1).
Spontaneous alpha oscillations are under tonic noradrenergic control
The degree of cortical synchronization fluctuates across behavioral states. Strongly synchronized cortical regimes reflect reduced vigilance and slow-wave sleep [51], whereas desynchronized modes of activity reflect alert wakefulness [45]. Rhythmic firing in the LC shows an inverse, state-dependent pattern with the rate of tonic discharge increasing from sleep to active wakefulness [38,41]. Importantly, these two patterns are linked, as activation of the LC (and related ascending neuromodulatory systems) abolishes rhythmic firing in the thalamus and thereby modulates cortical synchronization [9,25,31,76].
Seminal studies [77,78] investigated the effect of pharmacological noradrenaline blockade and selective lesions in the ascending noradrenergic tract on alpha-rhythmic activity in sensory cortices in awake cats. After suppressing noradrenergic neuromodulation, alpha activity showed a marked increase, suggesting an inhibitory effect of the LC on thalamocortical alpha generators [77,78]. Suppressing noradrenaline also made the cats stand motionless. Without the ability to modulate cortical synchronization their behavior had become detached from the environment [79]. Further investigations [25] extended these findings by revealing a dual effect of noradrenergic drugs on cortical synchronization in awake rats: while α1-antagonists and α2-agonists promoted synchronization at around 8 Hz in a dose-dependent manner, α2-antagonists reduced synchronization and were associated with increased behavioral activation. Whereas α1 receptors are often excitatory, α2-agonists tend to be inhibitory. These observations, together with the fact that α1 receptors require more noradrenaline to activate than do α2 receptors [80], suggest that the release of large amounts of noradrenaline elicits cortical desynchronization, mediated via thalamic α1-adrenoceptors, and allows for reliable thalamocortical information transfer [9,76]. Low noradrenaline levels during states of inattentiveness in turn are presumed to promote synchronization via action on α2-adrenoceptors in line with their higher affinity for noradrenaline [25].
Mechanistically, via depolarization of the membrane potential, noradrenaline suppresses rhythmic burst activity in thalamic neurons and promotes a mode of neural activity that allows for more precise information transfer [9,76,81] (Figure 1). Note that rhythmic firing within the thalamus is crucially implicated in the generation of alpha rhythms [51,52]. Hence, tonic noradrenergic innervation of the thalamus promotes cortical desynchronization. Accordingly, in two recent studies in rats tonic LC activation abolished thalamic burst firing [31,37] (also see [82]). This noradrenergic modulation enhanced the information transmitted in the thalamic firing and improved perceptual sensitivity. Control experiments revealed that noradrenaline’s action on thalamic α1-adrenoceptors enhanced information transmission irrespective of sensory and cortical influences [31]. Taken together, initial invasive animal studies demonstrate a direct modulation of ongoing alpha oscillations by tonic changes in noradrenergic neuromodulatory drive.
Tonic noradrenergic neuromodulation shapes cortical and behavioral states: New animal studies of an old mechanism
Corroborating earlier observations, optogenetic LC activation in rodents reduced low-frequency EEG synchronization (<10 Hz) and stimulated transitions towards more activated behavioral states (e.g., sleep-to-wake transitions, locomotion) [38,41,83]. Spontaneous fluctuations in pupil-indexed noradrenergic tone (Box 1) also co-vary with thalamic bursting [82] and cortical activity in awake mice [39]. Specifically, constricted pupils, as observed during periods of drowsiness or inattentiveness, were associated with increased bursting and cortical low frequency (<10 Hz) fluctuations. In contrast, with greater pupil diameters, suggestive of stronger noradrenergic drive, there was a shift towards increased activity at higher frequencies (50–100 Hz; [39]). The mice in this study additionally completed a challenging auditory detection task. Optimal performance occurred at intermediate pupil dilation, in line with an inverted U-shaped relationship between tonic noradrenaline levels and performance [32,39] (for investigations in humans, see [84]). Intermediate noradrenaline levels thus appear to promote more reliable processing of sensory stimuli in thalamocortical circuits via low frequency desynchronization [39].
A recent animal study set out to explicitly test how pupil-indexed neuromodulation shapes patterns of thalamocortical communication [5]. In states of low noradrenergic tone, indicated by constricted pupils, synchronization between the thalamus and parietal cortex was governed by the alpha frequency. In contrast, pupil dilation was associated with alpha desynchronization and increased high frequency oscillations (>30 Hz) [5]. On a behavioral level, in periods of thalamocortical alpha synchronization, animals engaged less with their environment, indicating that neuromodulatory inputs allow dynamic switching between behavioral states [3,5,9].
Non-invasive tools indicate tonic noradrenergic influences on alpha rhythms in humans
Direct investigations of noradrenergic influences on cortical and behavioral states in humans are scarce [84-89]. However, a non-invasive brain stimulation technique known to increase LC discharge (vagus nerve stimulation [90,91]), robustly increased pupil dilation and diminished alpha oscillations [87]. This complements a recent investigation in rats demonstrating that vagus nerve stimulation abolished rhythmic burst firing and improved information transmission in the thalamus [37]. Notably, vagus nerve stimulation-induced modulations of thalamic firing were similar to those induced by direct LC activation [31]. In another study, intake of a drug that enhances cortical noradrenaline levels increased pupil dilation, alpha desynchronization, and behavioral effects suggestive of cortical excitation [86,88]. Finally, during rest, periods of high pupil-indexed neuromodulation coincided with low-frequency desynchronization as well as high-frequency synchronization [89]. Interestingly, pupil dilation showed an inverted U-shaped relationship with alpha rhythms [89], as suggested by previous animal research [25]. Taken together, across species and measures, the level of tonic LC activity modulates the level of cortical synchronization, presumably mediated via a thalamocortical mechanism.
Phasic noradrenergic neuromodulation elicits transient alpha desynchronization
While acting on a more circumscribed spatial and temporal scale, the neural processes that mediate selective attention are similar to those underpinning global changes in cortical synchronization (i.e., cortical state) [11,45]. In particular, local cortical desynchronization, as observed during selective attention, has been suggested to arise as a result of phasic neuromodulation that gains spatial specificity by focused glutamatergic inputs [22,45].
Several animal studies have directly investigated the influence of phasic LC activation on circumscribed changes in cortical oscillatory dynamics. Specifically, naturally occurring [10] and artificially induced [24,83] phasic LC activity have been related to modulations of cortical excitability [36]. After pulsed trains of LC stimulation that mimicked naturalistic LC bursts, a transient decrease in low-frequency activity (<11 Hz) and a concomitant increase in high frequency (>45 Hz) rhythms in the prefrontal cortex were observed [24]. A pharmacological noradrenaline blockade abolished these effects, confirming an underlying noradrenergic mechanism [24]. A follow-up study that investigated sensory-evoked phasic LC activity replicated and extended these findings [10]. Sensory stimulation produced robust phasic LC activity that preceded prefrontal low-frequency desynchronization (<15 Hz) and high-frequency synchronization (>30 Hz) by about 150–200 ms [10]. Importantly, these prefrontal changes linearly scaled with the amplitude of LC responses and were eliminated after a pharmacological disruption of LC discharge [10].
Taken together, phasic LC activation rapidly provokes localized cortical excitation. This encompasses transient desynchronization of low-frequency activity as well as the increased high-frequency synchronization that is essential for the selective processing of salient information [10,24,92]. These noradrenaline-induced changes in cortical synchronization are thought to arise via a thalamic mechanism and a noradrenergic activation of cholinergic and GABAergic neurons in the basal forebrain [9,10,24,25,93].
Initial studies suggest phasic noradrenergic influences on neural desynchronization during attention in humans, as well [6,7,94] (Figure 1). For example, in one study [7], participants completed an arousal-manipulated attention task while pupil dilation and EEG were recorded concurrently. During the attention task, arousing or perceptually matched control stimuli were presented on a trial-by-trial basis to dynamically modulate arousal-related noradrenergic drive [18]. Crucially, larger pupil dilation in response to arousing stimuli was associated with a stronger transient alpha-beta desynchronization (9–30 Hz), indicating a common underlying dependence on phasic noradrenaline release [10]. On a behavioral level, noradrenergic responsiveness, as approximated by greater pupil dilation and EEG desynchronization, was linked to better attention across several tasks [7]. Similarly, attention-related pupil dilation has recently been linked to spatially circumscribed alpha desynchronization on a single-trial level [94]. Finally, in another study larger pupil dilation and EEG desynchronization were associated with increasing difficulty in a parametric attention task [6]. Specifically, with increasing attentional demands, alpha activity desynchronized whereas theta and gamma rhythms increased [3,5]. Again, pupil-indexed neuromodulation tracked oscillatory changes. To probe a potential thalamic contribution to these attention-related effects, participants completed the task a second time during functional MRI. Importantly, multivariate analyses revealed that behaviorally relevant thalamic BOLD modulations were indeed associated with both pupil dilation and EEG desynchronization [6]. Together, this supports a role of noradrenergic neuromodulation in attention-related cortical excitability adjustments, likely mediated via the thalamus.
To summarize, early investigations in animals linked tonic noradrenaline levels to the degree of spontaneous alpha synchronization [9,25,79], a marker of cortical excitability [64]. Specifically, they elucidated that noradrenaline modulates cortical synchronization by shifting thalamic neurons from a rhythmic firing pattern to a mode of activity that allows for reliable information transfer [9,31,81,82]. As rhythmic firing of thalamic pacemaker cells contributes to the generation of the cortical alpha rhythm [4,51,52], spontaneous alpha oscillations are under tonic noradrenergic control. More recent investigations, also in humans, extended these findings by revealing an association between ongoing, pupil-indexed noradrenergic activity and low-frequency synchronization in thalamocortical circuits [5,15,39,82,87,89]. Importantly, the processes mediating selective attention are thought to be similar to those underpinning such global changes in cortical synchronization [11,45]. Accordingly, transient bursts of LC activity have been shown to induce cortical desynchronization, likely again via a thalamic mechanism [10,24,31,83]. In summary, phasic LC activation may facilitate the selective processing of relevant stimuli by transiently modulating thalamocortical alpha synchronization which in turn adjusts local cortical excitability [5-9,15,25].
Is there a specific noradrenergic modulation of alpha desynchronization?
Noradrenaline is one of multiple neuromodulators that influences attention [21] and state-dependent thalamocortical synchronization [11,45,76]. Especially cholinergic neuromodulation originating from the nucleus basalis contributes to attentional functions [20]. Stimulation [95] and lesion [93] studies revealed that acetylcholine abolishes rhythmic burst firing in the thalamus and desynchronizes the cortex. This cholinergic modulation of thalamic firing patterns [9] facilitates reliable thalamocortical information transfer [81]. Note that thalamic burst firing is implicated in the generation of the cortical alpha rhythm [51,52] (for cholinergic modulation of thalamic alpha-rhythms, see [96,97]). Hence, acetylcholine promotes cortical desynchronization, similar to the action of noradrenaline [9,11,31,83]. The overlapping and unique features of cholinergic and noradrenergic neuromodulation during attention are an area of active research [11,21,86,88]. This endeavor is complicated by the fact that several non-invasive proxies of noradrenergic neuromodulation are also influenced by cholinergic activity (e.g., pupil dilation [98]; vagus nerve stimulation [91,99]), likely reflecting dense and reciprocal connections between the nuclei [100]. Interestingly, however, a study exploring neuromodulatory determinants of state-dependent cortical synchronization revealed that noradrenergic but not cholinergic influences control the dynamics of cortical networks [101]. The neural processes underpinning cortical state are similar to those regulating attention [11,45]. Thus, noradrenergic influences on thalamocortical synchronization likely play a mechanistic role in attention [31,102], presumably in concert with other neuromodulators [100].
State and species differences may explain differences in frequency bands
Notably, several animal studies reviewed in the preceding sections report LC-dependent decreases in low-frequency activity that only partly overlap with the alpha frequency [10,24,39,83]. Species and state differences may explain these lower frequency effects. First, a cortical 3–5 Hz rhythm in rodents has been suggested as an evolutionary precursor and functional homologue of the primate alpha rhythm [15,16]. Second, the thalamocortical mechanisms underlying the alpha rhythm can also generate lower frequency rhythms, although in different behavioral states [103]. Several of the reported animal experiments were conducted during reduced vigilance states (anesthesia) [8,10,24] that are characterized by relative thalamocortical synchronization (i.e., dominated by slower rhythms [10,45,51]). In sum, recent findings demonstrate that LC activation abolishes rhythmic bursting in the thalamus [31,37,82] and thus shows a mechanistic relation to generators of the cortical alpha rhythm [15,51,52]. State (anesthesia vs waking) and species (rodents vs primates) differences may underlie the differences in frequency bands observed across studies.
Noradrenergic effects on low and high frequencies may act in concert
In addition to decreases in alpha rhythms, most LC-stimulation studies report a synchronization in high frequencies [10,24]. While alpha rhythms are generated by thalamocortical interactions [15,51], gamma oscillations are considered the product of cortical interneurons [52]. Recently, the glutamate amplifies noradrenergic effects model [22] outlined how noradrenaline activates cortical interneurons and induces local gamma synchronization (cf. [101]). Alpha rhythms constrain the time windows for high-frequency activity [6,13,73]. Thus, noradrenaline’s alpha-desynchronizing action in the thalamus [25,31] and gamma-synchronizing action in the cortex [22] may act in concert to promote attention [13,92].
Concluding remarks
Rhythmic neural activity within a frontoparietal network interconnected by the thalamus supports the preferential processing of prioritized information needed for selective attention [3,48]. Prefrontal control over noradrenergic nuclei provides a powerful tool to bias processing in thalamocortical networks according to attentional demands [17,27,28]. While earlier conceptualizations considered the noradrenergic LC as a homogenous structure that uniformly broadcasts its output throughout the brain, recent evidence reveals a more targeted system suited for attention-related forebrain modulation [33]. Novel technological advances that enable non-invasive assessments of the LC-NA system contribute to this refined understanding (Box 1). Noradrenergic neuromodulation biases information processing towards prioritized representations across multiple brain sites, prominently including the thalamus [25,31]. On a neural ensemble level, fluctuations in noradrenergic neuromodulation translate into changes in rhythmic neural activity [9-11,76,83]. In particular, modulations of alpha-oscillatory activity, mediated via a thalamocortical circuit, temporarily disengage task-irrelevant brain areas and thus facilitate the flexible gating of information [3,5,13]. Hence, because the prefrontal cortex provides input to the LC [27,28], noradrenergic influences on thalamic pacemakers grant prefrontal-brainstem circuits control over cortical alpha synchronization [5,9,25,87]. Prefrontal LC activation may thus subserve the selective processing of prioritized information by dynamically adjusting the balance of cortical excitation and inhibition via alpha synchronization [6-8,10].
With advancing age, our ability to filter out relevant pieces of information wanes [104]. We highlight evidence suggesting that age-related degeneration of the LC (Box 2) may impair thalamocortical alpha synchronization (Box 3) and contribute to attention deficits in later life [7]. While several questions remain to be answered (see Outstanding Questions), the reviewed findings highlight how the noradrenergic modulation of neural synchronization shapes attention.
Outstanding questions:
How do different neuromodulatory systems (e.g., noradrenaline, acetylcholine) interact to shape cortical excitability according to attentional demands?
Can noradrenergic influences on low-frequency desynchronization be disentangled from their association with (concurrently observed) high-frequency synchronization? Does noradrenergic neuromodulation exert its effects on synchronization primarily indirectly via the thalamus or are there also direct effects in the cortex?
What is the relative contribution of noradrenergic and other neuromodulatory systems to non-luminance induced changes in pupil diameter? Can we solve the inverse problem (inferring neural activity from pupil recordings)?
Can non-invasive stimulation techniques that influence noradrenergic neurotransmission (e.g., vagus nerve stimulation) be exploited to optimize cortical alpha synchronization during attention? Can we disentangle different neuromodulatory correlates of vagus nerve stimulation?
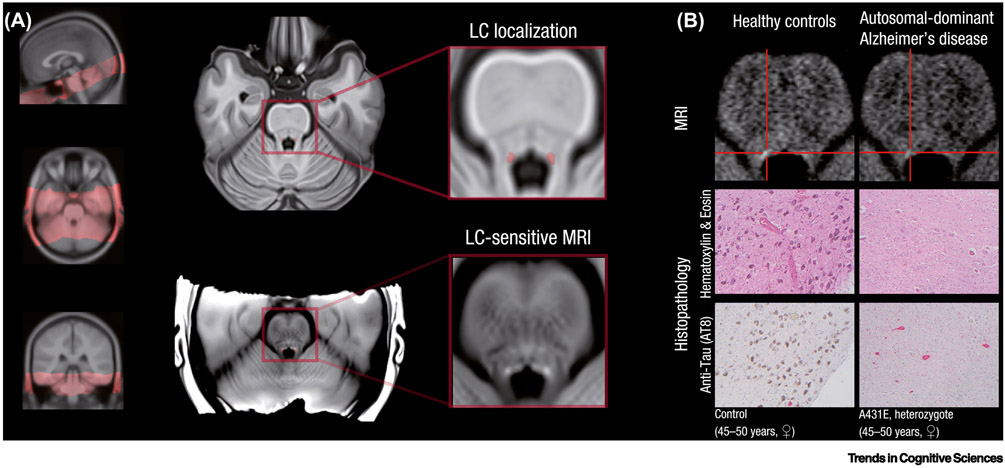
Figure I. Locus coeruleus magnetic resonance imaging (LC-MRI) in healthy aging and neurodegenerative disease.
(A) Orientation of a typical locus coeruleus-sensitive sequence covering the brainstem (left). The localization of the locus coeruleus, bordering the fourth ventricle, is highlighted in red on an axial slice of standard anatomical scan (middle, upper panel). In LC-MRI, the locus coeruleus can be detected as a cluster of bright, hyperintense voxels (middle, lower panel) (taken with permission from [122]). (B) In neurodegenerative diseases, noradrenergic cells decline. Lower in-vivo LC-MRI contrast in autosomal-dominant Alzheimer’s disease (upper panel) corresponds to noradrenergic neurodegeneration in participants who died with the same disease-causing mutation (A431E). In particular, Hematoxylin and Eosin staining reveals locus coeruleus depigmentation (i.e., absence of the dark, granular neuromelanin). In addition, immunostained slides with anti-tau (AT8) show neurofibrillary tangles within noradrenergic neurons as well as the presence of tau positive threads (red) (reproduced, with permission, from [115,122]).
Highlights:
Neural synchronization, particularly in the alpha frequency band (~8–12 Hz), supports routing prioritized information through thalamocortical attention networks.
The noradrenergic locus coeruleus has long been regarded as nexus of a global arousal system, unable to innervate attention-relevant specific cortical networks. Recent evidence reveals a more specialized neuromodulatory system, which biases processing in thalamocortical circuits according to attentional demands.
Transient locus coeruleus activations facilitate the selective processing of relevant information by flexibly adjusting local cortical excitability via thalamocortical alpha synchronization.
However, with advancing age, the locus coeruleus harbors toxins and Alzheimer’s-related tau precursors, making it susceptible to neurodegeneration, which may disrupt synchronization in the aging brain.
Acknowledgements:
MJD is recipient of a stipend from the GA. Lienert-Foundation and was supported by a stipend from the Davis School of Gerontology, University of Southern California. MM’s work was supported by National Institutes of Health (NIH) grant R01AG025340, an Alexander von Humboldt fellowship, and a Max Planck Sabbatical Award. M.W.-B.'s work on this manuscript was partially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; WE 4269/5-1), and a Jacobs Foundation Early Career Research Fellowship. We thank Julia Delius for editorial assistance.
Glossary:
- Adrenoceptor
Binding site for noradrenaline and noradrenergic drugs. The pre- and postsynaptically located α2-receptors mediate inhibition, whereas the postsynaptic α1- and β-adrenoceptors typically have excitatory effects.
- Affinity
A receptor’s affinity indicates what concentration of the ligand (e.g., noradrenaline) is sufficient to trigger a physiological response. α2-Adrenoceptors have the highest affinity for noradrenaline followed by moderate-affinity α1- and low-affinity β-adrenoceptors.
- Agonist
Chemical that imitates the action of noradrenaline at adrenoceptors.
- Alpha activity
Alpha activity, rhythmic neural activity between 8 and 12 Hz, constitutes the dominant rhythm in the waking state that can non-invasively be recorded using electroencephalography and magnetoencephalography.
- Antagonist
Chemical that blocks the action of noradrenaline at adrenoceptors.
- Ascending neuromodulatory system (ascending reticular activating system; ARAS)
A group of subcortical neuromodulatory nuclei that sends ascending tracts to the thalamus and cortex and has an activating influence on behavioral and cortical state.
- Burst activity
When thalamic cells fire in a burst mode, the cortical electroencephalogram (EEG) is synchronized. Neuromodulators like noradrenaline can shift thalamic cells to a single-spike, or transfer, mode of neuronal firing which is associated with a desynchronized EEG.
- Contralateral
On the opposite side. The left visual field, for instance, is processed in the contralateral, right, hemisphere.
- Ipsilateral
On the same side (also see contralateral).
- Locus coeruleus–noradrenaline
The locus coeruleus, Latin for the blue spot, is the largest cluster of noradrenergic cells in the human brain. In the locus coeruleus, noradrenaline is synthesized from dopamine (by dopamine beta-hydroxylase) and then released via widespread axons throughout the brain.
- Neuromodulator
The main neuromodulators—serotonin, acetylcholine, dopamine, and noradrenaline—are released via diffuse projections from subcortical cell clusters (nuclei). In target neurons, neuromodulators modify cellular properties and the effectiveness of their synaptic transmission.
- Phasic
Brief, burst-like firing pattern of noradrenergic neurons elicited in response to salient (e.g., aversive) stimuli or recruited internally via top-down mechanisms to support processing in the forebrain.
- Rhythmic neural activity
Rhythmic neural activity, also called neural oscillations or brainwaves, reflects the spatial summation (i.e., ensemble activity) of rhythmic postsynaptic potentials originating from apical dendrites.
- Selective attention
Selective attention denotes the preferential processing of relevant information by enhancing the neural processing of prioritized stimuli while at the same suppressing irrelevant stimuli.
- Tonic
Locus coeruleus cells fire in a state-dependent, slow (0.5–5 Hz) rhythm with the highest discharge during active wakefulness, followed by drowsy, inattentive states, slow-wave sleep, and, finally, minimal release during rapid-eye-movement sleep.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desimone R and Duncan JS (1995) Neural mechanisms of selective visual attention. Annu. Rev. Neurosci 18,193–222 [DOI] [PubMed] [Google Scholar]
- 2.Buschman TJ and Kastner S (2015) From behavior to neural dynamics: An integrated theory of attention. Neuron 88,127–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiebelkorn IC et al. (2019) The mediodorsal pulvinar coordinates the macaque frontoparietal network during rhythmic spatial attention. Nat. Commun 10, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saalmann YB et al. (2012) The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 337, 753–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stitt I et al. (2018) Arousal dependent modulation of thalamo-cortical functional interaction. Nat. Commun 9, 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosciessa JQ et al. (2021) Thalamocortical excitability modulation guides human perception under uncertainty. Nat. Commun. 12, 2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl MJ et al. (2020) Noradrenergic responsiveness supports selective attention across the adult lifespan. J. Neurosci 40, 4372–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safaai H et al. (2015) Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc. Natl. Acad. Sci. U. S. A 112, 12834–12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick DA (1989) Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221 [DOI] [PubMed] [Google Scholar]
- 10.Neves RM et al. (2018) Locus coeruleus phasic discharge is essential for stimulus-induced gamma oscillations in the prefrontal cortex. J. Neurophysiol 119, 904–920 [DOI] [PubMed] [Google Scholar]
- 11.McCormick DA et al. (2020) Neuromodulation of Brain State and Behavior. Annu. Rev. Neurosci 43, 391–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketz NA et al. (2015) Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends Neurosci. 38, 3–12 [DOI] [PubMed] [Google Scholar]
- 13.Jensen O and Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klimesch W et al. (2007) EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev 53, 63–88 [DOI] [PubMed] [Google Scholar]
- 15.Nestvogel DB and Mccormick DA (2021) Visual thalamocortical mechanisms of waking state dependent activity and alpha oscillations. bioRxiv Prepr. DOI: 10.1101/2021.04.14.439865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senzai Y et al. (2019) Layer-specific physiological features and interlaminar interactions in the primary visual cortex of the mouse. Neuron 101, 500–513.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bari A et al. (2020) Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proc. Natl. Acad. Sci. U. S. A 117, 29080–29089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T-H et al. (2018) Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat. Hum. Behav 2, 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vazey EM et al. (2018) Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl. Acad. Sci. U. S. A 115, E9439–E9448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everitt BJ and Robbins TW (1997) Central cholinergic systems and cognition. Annu. Rev. Psychol 48, 649–684 [DOI] [PubMed] [Google Scholar]
- 21.Thiele A and Bellgrove MA (2018) Neuromodulation of attention. Neuron 97, 769–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mather M et al. (2016) Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci 39, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge CW and Waterhouse BD (2003) The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev 42, 33–84 [DOI] [PubMed] [Google Scholar]
- 24.Marzo A et al. (2014) Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J. Neurophysiol 111, 2570–2588 [DOI] [PubMed] [Google Scholar]
- 25.Buzsáki G et al. (1991) Noradrenergic control of thalamic oscillation: The role of α-2 receptors. Eur. J. Neurosci 3, 222–229 [DOI] [PubMed] [Google Scholar]
- 26.Fernandes P et al. (2012) The human locus coeruleus 3-D stereotactic anatomy. Surg. Radiol. Anat 34, 879–885 [DOI] [PubMed] [Google Scholar]
- 27.Totah NK et al. (2021) Synchronous spiking associated with prefrontal high γ oscillations evokes a 5-Hz rhythmic modulation of spiking in locus coeruleus. J. Neurophysiol 125, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 28.Breton-Provencher V and Sur M (2019) Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci 22, 218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz LA et al. (2015) Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabadi E (2013) Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol 27, 659–693 [DOI] [PubMed] [Google Scholar]
- 31.Rodenkirch C et al. (2019) Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci 22, 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aston-Jones G and Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450 [DOI] [PubMed] [Google Scholar]
- 33.Poe GR et al. (2020) Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci 21, 644–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uematsu A et al. (2017) Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci 20, 1602–1611 [DOI] [PubMed] [Google Scholar]
- 35.Totah NK et al. (2018) The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 99, 1055–1068.66 [DOI] [PubMed] [Google Scholar]
- 36.Chandler DJ et al. (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl. Acad. Sci. U. S. A 111, 6816–6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodenkirch C and Wang Q (2020) Rapid and transient enhancement of thalamic information transmission induced by vagus nerve stimulation. J. Neural Eng 17, 026027. [DOI] [PubMed] [Google Scholar]
- 38.Carter ME et al. (2010) Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci 13, 1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGinley MJ et al. (2015) Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerbi V et al. (2019) Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 [DOI] [PubMed] [Google Scholar]
- 41.Hayat H et al. (2020) Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv 6, eaaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnsten AFT (2020) Guanfacine’s mechanism of action in treating prefrontal cortical disorders: Successful translation across species. Neurobiol. Learn. Mem 176, 107327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M et al. (2011) Neuronal basis of age-related working memory decline. Nature 476, 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barcelos NM et al. (2018) Guanfacine treatment for prefrontal cognitive dysfunction in older participants: a randomized clinical trial. Neurobiol. Aging 70, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris KD and Thiele A (2011) Cortical state and attention. Nat. Rev. Neurosci 12, 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foote SL et al. (1975) Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 86, 229–242 [DOI] [PubMed] [Google Scholar]
- 47.Martins ARO and Froemke RC (2015) Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci 18, 1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corbetta M et al. (2008) The reorienting system of the human brain: From environment to theory of mind. Neuron 58, 306–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouret S and Sara SJ (2005) Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 [DOI] [PubMed] [Google Scholar]
- 50.Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci 10, 211–223 [DOI] [PubMed] [Google Scholar]
- 51.Crunelli V et al. (2018) Dual function of thalamic low-vigilance state oscillations: Rhythm-regulation and plasticity. Nat. Rev. Neurosci 19, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen O et al. (2019) Human brain oscillations: From physiological mechanisms to analysis and cognition. In Magnetoencephalography: From Signals to Dynamic Cortical Networks 2nd edn. (Supek S and Aine CJ, eds), pp. 471–517, Springer International Publishing [Google Scholar]
- 53.Halgren M et al. (2019) The generation and propagation of the human alpha rhythm. Proc. Natl. Acad. Sci. U. S. A 116, 23772–23782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bollimunta A et al. (2008) Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J. Neurosci 28, 9976–9988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bollimunta A et al. (2011) Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J. Neurosci 31, 4935–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peylo C et al. Cause or consequence? Alpha oscillations in visuospatial attention., Trends in Neurosciences, 44. June-(2021) , Elsevier BV, 705–713 [DOI] [PubMed] [Google Scholar]
- 57.Samaha J et al. (2016) Decoding and reconstructing the focus of spatial attention from the topography of alpha-band oscillations. J. Cogn. Neurosci 28, 1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Popov T et al. (2019) Spatial specificity of alpha oscillations in the human visual system. Hum. Brain Mapp 40, 4432–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frey JN et al. (2014) Selective modulation of auditory cortical alpha activity in an audiovisual spatial attention task. J. Neurosci 34, 6634–6639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haegens S et al. (2011) α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl. Acad. Sci. U. S. A 108, 19377–19382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palva S and Palva JM (2011) Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol 2, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahl MJ et al. (2019) Diminished pre-stimulus alpha-lateralization suggests compromised self-initiated attentional control of auditory processing in old age. Neuroimage 197, 414–424 [DOI] [PubMed] [Google Scholar]
- 63.Wöstmann M et al. (2019) Alpha oscillations in the human brain implement distractor suppression independent of target selection. J. Neurosci 39, 9797–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romei V et al. (2010) On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: Correlation or causation? J. Neurosci 30, 8692–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagherzadeh Y et al. (2020) Alpha synchrony and the neurofeedback control of spatial attention. Neuron 105, 577–587.e5 [DOI] [PubMed] [Google Scholar]
- 66.Borghini G et al. (2018) Alpha oscillations are causally linked to inhibitory abilities in ageing. J. Neurosci 38, 4418–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gundlach C and Forschack N (2020) Commentary: Alpha synchrony and the neurofeedback control of spatial attention. Front. Neurosci 14, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antonov PA et al. (2020) Too little, too late, and in the wrong place: Alpha band activity does not reflect an active mechanism of selective attention. Neuroimage 219, 117006. [DOI] [PubMed] [Google Scholar]
- 69.Keitel C et al. (2019) Stimulus-driven brain rhythms within the alpha band: The attentional-modulation conundrum. J. Neurosci 39, 3119–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tune S et al. (2021) Neural attentional-filter mechanisms of listening success in middle-aged and older individuals. Nat. Commun 12, 4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhigalov A and Jensen O (2020) Alpha oscillations do not implement gain control in early visual cortex but rather gating in parieto-occipital regions. Hum. Brain Mapp 41, 5176–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanslmayr S et al. (2016) Oscillations and episodic memory: Addressing the synchronization/desynchronization conundrum. Trends Neurosci. 39, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spaak E et al. (2012) Layer-specific entrainment of gamma-band neural activity by the alpha rhythm in monkey visual cortex. Curr. Biol 22, 2313–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kloosterman NA et al. (2019) Humans strategically shift decision bias by flexibly adjusting sensory evidence accumulation. Elife 8, e37321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffiths BJ et al. (2019) Alpha/beta power decreases track the fidelity of stimulus specific information. Elife 8, e49562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steriade M et al. (1993) Thalamocortical oscillations in the sleeping and aroused brain. Science. 262, 679–685 [DOI] [PubMed] [Google Scholar]
- 77.Delagrange P et al. (1989) Effect of DSP4, a neurotoxic agent, on attentive behaviour and related electrocortical activity in cat. Behav. Brain Res 33, 33–43 [DOI] [PubMed] [Google Scholar]
- 78.Delagrange P et al. (1993) Effects of locus coeruleus lesions on vigilance and attentive behaviour in cat. Behav. Brain Res 53, 155–165 [DOI] [PubMed] [Google Scholar]
- 79.Rougeul-Buser A and Buser P (1997) Rhythms in the alpha band in cats and their behavioural correlates. Int. J. Psychophysiol 26, 191–203 [DOI] [PubMed] [Google Scholar]
- 80.Mather M (2021) Noradrenaline in the aging brain: Promoting cognitive reserve or accelerating Alzheimer’s disease? Semin. Cell Dev. Biol 116,108–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sherman SM (2001) Tonic and burst firing: Dual modes of thalamocortical relay. Trends Neurosci. 24, 122–126 [DOI] [PubMed] [Google Scholar]
- 82.Crombie D et al. (2021) Pupil size dynamics predict dLGN firing mode over a wide range of timescales. bioRxiv Prepr. DOI: 10.1101/2021.04.30.442134 [DOI] [Google Scholar]
- 83.Liu Y et al. (2017) Dynamic lateralization of pupil dilation evoked by locus coeruleus activation results from sympathetic, not parasympathetic, contributions. Cell Rep. 20, 3099–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waschke L et al. (2019) Local cortical desynchronization and pupil-linked arousal differentially shape brain states for optimal sensory performance. Elife 8, e51501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gelbard-Sagiv H et al. (2018) Noradrenaline modulates visual perception and late visually evoked activity. Curr. Biol 28, 2239–2249.e6 [DOI] [PubMed] [Google Scholar]
- 86.Pfeffer T et al. (2018) Catecholamines alter the intrinsic variability of cortical population activity and perception. PLoS Biol. 16, e2003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharon O et al. (2021) Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J. Neurosci 41, 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfeffer T et al. (2021) Circuit mechanisms for the chemical modulation of cortex-wide network interactions and behavioral variability. Sci. Adv 7, eabf5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfeffer T et al. (2021) Coupling of pupil- and neuronal population dynamics reveals diverse influences of arousal on cortical processing. bioRxiv Prepr. DOI: 10.1101/2021.06.25.449734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hulsey DR et al. (2017) Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol 289, 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collins L et al. (2021) Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr. Biol 31, 2088–2098.e3 [DOI] [PubMed] [Google Scholar]
- 92.Fries P (2015) Rhythms for cognition: Communication through coherence. Neuron 88, 220–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buzsáki G et al. (1988) Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci 8, 4007–4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitmarsh S et al. (2021) Neuronal correlates of the subjective experience of attention. Eur. J. Neurosci DOI: 10.1111/ejn.15395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goard M and Dan Y (2009) Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci 12, 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bauer M et al. (2012) Cholinergic enhancement of visual attention and neural oscillations in the human brain. Curr. Biol 22, 397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hughes SW et al. (2004) Synchronized oscillations at α and θ frequencies in the lateral geniculate nucleus. Neuron 42, 253–268 [DOI] [PubMed] [Google Scholar]
- 98.Reimer J et al. (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun 7, 13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mridha Z et al. (2021) Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat. Commun 12, 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Briand LA et al. (2007) Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Prog. Neurobiol 83, 69–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Constantinople CM and Bruno RM (2011) Effects and mechanisms of wakefulness on local cortical networks. Neuron 69, 1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robbins TW and Arnsten AFT (2009) The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu. Rev. Neurosci 32, 267–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hughes SW and Crunelli V (2005) Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neurosci. 11, 357–372 [DOI] [PubMed] [Google Scholar]
- 104.Cabeza R et al. , eds. (2017) Cognitive neuroscience of aging: Linking cognitive and cerebral aging, 2nd edn.Oxford University Press. [Google Scholar]
- 105.Astafiev SV et al. (2010) Comment on “Modafinil shifts human locus coeruleus to low- tonic, high-phasic activity during functional MRI” and “Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area.” Science. 328, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding Y-S (2021) Progress in PET imaging of the norepinephrine transporter system. In PET and SPECT of Neurobiological Systems (Dierckx RAJO et al. , eds), pp. 713–747, Springer International Publishing [Google Scholar]
- 107.Keren NI et al. (2015) Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. Neuroimage 113, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sasaki M et al. (2006) Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 17, 1215–1218 [DOI] [PubMed] [Google Scholar]
- 109.Liu KY et al. (2017) Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neurosci. Biobehav. Rev 83, 325–355 [DOI] [PubMed] [Google Scholar]
- 110.Watanabe T et al. (2019) Magnetic resonance imaging of noradrenergic neurons. Brain Struct. Funct 224, 1609–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Betts MJ et al. (2019) Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cassidy CM et al. (2019) Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc. Natl. Acad. Sci. U. S. A 116, 5108–5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zecca L et al. (2004) The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. U. S. A 101, 9843–9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trujillo P et al. (2017) Contrast mechanisms associated with neuromelanin-MRI. Magn. Reson. Med 78, 1790–1800 [DOI] [PubMed] [Google Scholar]
- 115.Dahl MJ et al. (2020) Lower MR-indexed locus coeruleus integrity in autosomal-dominant Alzheimer’s disease is related to cortical tau burden and memory deficits. medRxiv Prepr. DOI: 10.1101/2020.11.16.20232561 [DOI] [Google Scholar]
- 116.Joshi S et al. (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Privitera M et al. (2020) A complete pupillometry toolbox for real-time monitoring of locus coeruleus activity in rodents. Nat. Protoc 15, 2301–2320 [DOI] [PubMed] [Google Scholar]
- 118.de Gee JW et al. (2017) Dynamic modulation of decision biases by brainstem arousal systems. Elife 6, e23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joshi S and Gold JI (2020) Pupil size as a window on neural substrates of cognition. Trends Cogn. Sci 24, 466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cazettes F et al. (2020) Phasic activation of dorsal raphe serotonergic neurons increases pupil size. Curr. Biol 31, 192–197.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu KY et al. (2019) In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging 74, 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dahl MJ et al. (2019) Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav 3, 1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bachman SL et al. (2020) Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiol. Aging 100, 72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mather M and Harley CW (2016) The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends Cogn. Sci 20, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weinshenker D (2018) Long road to ruin: Noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci. 41, 211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chalermpalanupap T et al. (2017) Down but not out: The consequences of pretangle tau in the locus coeruleus. Neural Plast. 2017, 7829507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braak H et al. (2011) Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 [DOI] [PubMed] [Google Scholar]
- 128.Theofilas P et al. (2017) Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer’s Dement. 13, 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elman JA et al. (2021) MRI-assessed locus coeruleus integrity is heritable and associated with cognition, Alzheimer’s risk, and sleep-wake disturbance. Alzheimer’s Dement. 17, 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu KY et al. (2020) Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat. Commun 11, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dutt S et al. (2021) Brainstem substructures and cognition in prodromal Alzheimer’s disease. Brain Imaging Behav. DOI: 10.1007/s11682-021-00459-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacobs HIL et al. (2021) In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci. Transl. Med 13, eabj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson RS et al. (2013) Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80, 1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lindenberger U et al. (2006) Delineating brain-behavior mappings across the lifespan: Substantive and methodological advances in developmental neuroscience. Neurosci. Biobehav. Rev 30, 713–717 [DOI] [PubMed] [Google Scholar]
- 135.Li S-C et al. (2001) Aging cognition: From neuromodulation to representation. Trends Cogn. Sci 5, 479–486 [DOI] [PubMed] [Google Scholar]
- 136.Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev 29, 169–195 [DOI] [PubMed] [Google Scholar]
- 137.Sander MC et al. (2012) Lifespan age differences in working memory: A two-component framework. Neurosci. Biobehav. Rev 36, 2007–2033 [DOI] [PubMed] [Google Scholar]
- 138.Wöstmann M et al. (2015) Neural alpha dynamics in younger and older listeners reflect acoustic challenges and predictive benefits. J. Neurosci 35, 1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leenders MP et al. (2018) Diminished alpha lateralization during working memory but not during attentional cueing in older adults. Cereb. Cortex 28, 21–32 [DOI] [PubMed] [Google Scholar]
- 140.Deiber M-P et al. (2013) Age-associated modulations of cerebral oscillatory patterns related to attention control. Neuroimage 82, 531–546 [DOI] [PubMed] [Google Scholar]
- 141.Deiber M-P et al. (2010) Aging effects on selective attention-related electroencephalographic patterns during face encoding. Neuroscience 171, 173–186 [DOI] [PubMed] [Google Scholar]
- 142.Gazzaley A et al. (2005) Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci 8, 1298–1300 [DOI] [PubMed] [Google Scholar]
- 143.Sander MC et al. (2012) Amplitude modulations and inter-trial phase stability of alpha-oscillations differentially reflect working memory constraints across the lifespan. Neuroimage 59, 646–654 [DOI] [PubMed] [Google Scholar]
- 144.Tune S et al. (2018) Probing the limits of alpha power lateralisation as a neural marker of selective attention in middle-aged and older listeners. Eur. J. Neurosci 48, 2537–2550 [DOI] [PubMed] [Google Scholar]
- 145.Soininen H et al. (1992) Slowing of the dominant occipital rhythm in electroencephalogram is associated with low concentration of noradrenaline in the thalamus in patients with Alzheimer’s disease. Neurosci. Lett 137, 5–8 [DOI] [PubMed] [Google Scholar]