Abstract
Human ventral temporal cortex (VTC) contains category-selective regions that respond preferentially to ecologically-relevant categories such as faces, bodies, places, and words, which are causally involved in the perception of these categories. How do these regions develop during childhood? We used functional MRI to measure longitudinal development of category-selectivity in school-age children over 1 to 5 years. We discovered that from young childhood to the teens, face- and word-selective regions in VTC expand and become more category-selective, but limb-selective regions shrink and lose their preference for limbs. Critically, as a child develops, increases in face- and word-selectivity are directly linked to decreases in limb-selectivity, revealing that during childhood limb-selectivity in VTC is repurposed into word- and face-selectivity. These data provide evidence for cortical recycling during childhood development. This has important implications for understanding typical as well as atypical brain development and necessitates a rethinking of how cortical function develops during childhood.
A central question in neuroscience is how does cortical function develop? Ventral temporal cortex (VTC) is an excellent model system to address this question as it contains regions selective for ecological categories such as faces1, bodies2, places3, and words4 that are critical for human behavior and can be identified in each individual. When infants first open their eyes, they are inundated with faces, body-parts, their surrounding room, and objects. This visual input may begin to shape VTC representations in infancy and lead to the emergence of face representations in the first year of life5–7. However, experience with other categories, such as words, does not begin until later in childhood when children learn to read.
Two main theories regarding the development of category-selective regions have been proposed. The theory of functional refinement predicts that category-selective regions emerge from raw, general-purpose cortex8,9, which has some basic property10 that is present early in development such as foveal bias11,12. For example, a longitudinal study that tracked development of word-selectivity during the first year of school, found that word-selectivity emerged upon previously unspecialized cortex9. Similarly, developmental studies of face-selective regions reported that the growing part of face-selective regions showed less specificity in children than adults8 and also in younger than older infant monkeys7.
Alternatively, the theory of competition posits that rivalry for cortical resources may constrain development13,14. In particular, as both face and word recognition require fine visual acuity afforded by foveal vision, emerging representations for these categories may compete over foveal resources in lateral VTC15. This competition for foveal cortical territory may lead to recycling of cortex selective to one category earlier in childhood (e.g., faces) to be selective to another category (e.g., words) with later demands for reading14,16. For example, comparison of brains of illiterate or late literate adults to literate adults suggests that increased literacy in adulthood is associated with reduced responses to faces in the left fusiform gyrus14,17. Because reading acquisition is specific to humans, and the fusiform gyrus, which is the anatomical structure where face-selective regions reside, is hominoid-specific, addressing this intense theoretical debate necessitates longitudinal brain measurements in school-age children to examine the development of multiple category representations.
Here, we addressed this key gap in knowledge using longitudinal fMRI in 29 children (initially 5–12 years old) to measure development of cortical representations of multiple categories as well as their relationship. Children were scanned repeatedly over the course of 1 to 5 years (mean±SD: 3.75±1.5 years, Supplementary Fig. 1A), with an average of 4.4±1.92 fMRI sessions per child and 128 included sessions overall (Methods). During fMRI, children viewed images from 10 categories spanning five domains: characters (pseudowords, numbers), body parts (headless bodies, limbs), faces (adult faces, child faces), objects (string instruments, cars), and places (houses, corridors, Supplementary Fig. 1D). Analyses were performed in each individual’s native brain space, which allows precise tracking of the developing cortex in each participant and prevents blurring of responses from different categories due to normalization to a standard brain template18.
Results
How does category-selectivity develop in VTC?
To assess the development of category-selectivity in VTC, we first quantified the volume of category-selective activations in VTC as a function of age. Selectivity was computed by contrasting responses to each category vs. all other categories except the other category from the same domain (e.g., limbs vs. all other categories except bodies, t>3, voxel level). VTC was anatomically defined on the cortical surfaces of each child and divided into lateral and medial partitions (Fig. 1B). This division captures the center-periphery organization of VTC12,15, where lateral VTC represents the central visual field, and medial VTC represents the peripheral visual field19. To test for age-related changes in the volume of category-selective activations, we used linear mixed models (LMMs) with age as a fixed effect and participant as a random effect. LMMs with intercepts that varied across participants and a constant slope fit the data best in the majority of cases (Methods). Fig. 1A shows examples of linear mixed model fits for words, limbs, and faces (all LMMs in Supplementary Fig. 2). Fig. 1C summarizes the LMM slopes and confidence intervals (CI) for all categories and VTC partitions. The slopes (βage) indicate the rate of change (mm3/month) of category-selective volume with age (Fig. 1C). Significant development after FDR correction20 is indicated by asterisks in Fig. 1C (full statistics in Supplementary Tables 1–2).
Fig. 1. Developmental increases and decreases in category-selective activation in lateral VTC.
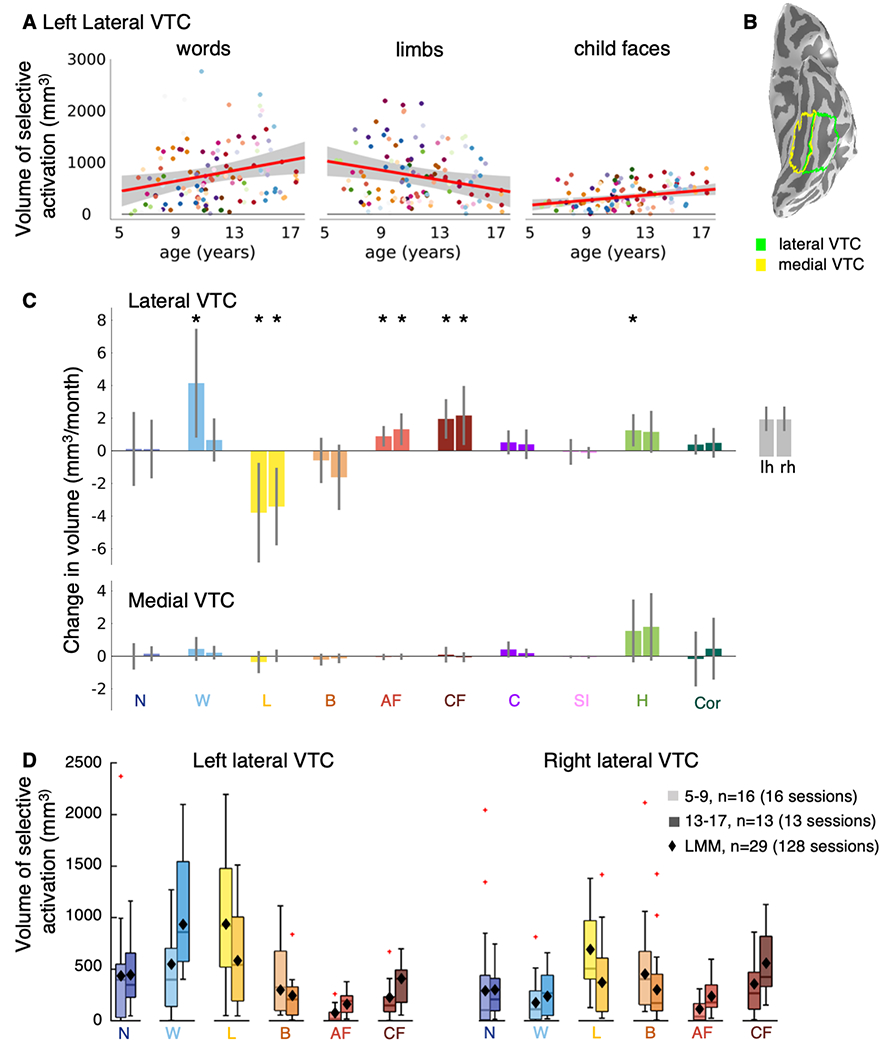
(A) Volume of word-, limb- and child-face-selective activation by age. Each dot is a session and colored by participant. Red line: Linear mixed model (LMM) prediction of category-selective activation by age. Shaded gray: 95% confidence interval (CI). All scatterplots in Supplementary Fig. 2. (B) Lateral and medial VTC on the inflated cortical surface of a 5-year-old. (C) LMM slopes indicating change in category-selective activation volume per month (n=128 sessions, 29 children). Error bars: 95% CI. If the CI does not cross the y=0 line, the slope is significantly different than 0 before FDR-correction. Asterisks: Significant development after FDR-correction (p<0.05). (D) Category-selective activation by age group. The boxplots illustrate the average category-selective volume for two age groups: 5-9- and 13-17-year-olds. One session per child is included per boxplot (see legend for number of sessions). The diamonds represent the LMM estimate for category-selective volume for the mean age of each age group. LMMs are based on data from all sessions, same as panels A,C. Boxplots show the 75% and 25% percentiles (colored areas) and the median (horizontal lines). Whiskers extend to the most extreme data points not considered outliers (minimum, maximum). Crosses: outliers (values more than 1.5 times the interquartile range away from the bottom or top of the box). All categories in Supplementary Fig. 3. Acronyms: N: numbers; W: words; L: limbs; B: bodies; AF: adult faces; CF: child faces, C: Cars, SI: String instruments, H: Houses, Cor: Corridors.
Results reveal variable development of category-selectivity across VTC partitions and categories. Changes in category-selectivity were significant in lateral VTC, but were not statistically significant in medial VTC (Fig. 1C, Supplementary Fig. 2, Supplementary Tables 1–2). Surprisingly, there were both developmental increases and decreases in the volume of category-selective activation in lateral VTC: word- and face-selective activation significantly increased, but limb-selective activation significantly decreased (Fig. 1A,C, Supplementary Fig. 2).
Interestingly, during childhood, the volume of word-selective activation significantly increased in left lateral VTC (Fig. 1C-light blue, βage=4.14 mm3/month (95%CI:0.81,7.48), t(126)=2.46, pFDR=0.044). However, there was no statistically significant change in word-selective activation in right lateral VTC (Fig. 1C,D, Supplementary Fig. 2, βage=0.66 mm3/month (−0.66,1.99), t(126)=0.99, pFDR=0.46), or of number-selective activations bilaterally (Fig. 1C, Supplementary Fig. 2, Supplementary Table 1, left: βage=0.11 mm3/month (−2.15, 2.38), t(126)=0.10, pFDR=0.92; right: βage=0.11 mm3/month (−1.69, 1.90), t(126)=0.12, pFDR=0.92). Examining the average measured volume of activations (Fig. 1D-boxplots, Supplementary Fig. 3 for all categories) as well as LMM predictions of the mean volume (Fig. 1D-diamonds), revealed that word-selectivity doubled on average from ~500 mm3 in 5–9-year-olds to ~1000 mm3 in 13–17-year-olds. Notably, as the volume of word-selective activation doubled, the volume of limb-selective activation halved (Fig. 1D-yellow). In fact, limb-selective activation significantly decreased over childhood in both hemispheres (Fig. 1C-yellow, left: βage=−3.79 mm3/month (−6.84,−0.74), t(126)=−2.46, pFDR=0.044; right: βage=−3.42 mm3/month (−5.79,−1.04), t(126)=−2.85, pFDR=0.04). However, there was no statistically significant change in body-selective activation (Fig. 1C-orange, left: βage=−0.59 mm3/month (−1.98, 0.80), t(126)=−0.84, pFDR=0.50; right: βage=−1.63 mm3/month (−3.63, 0.38), t(126)=−1.61, pFDR=0.22). At the same time, the volume of face-selective activation significantly increased over childhood bilaterally for both adult faces (Fig. 1C-bright red: left: βage=0.89 mm3/month (0.26,1.52), t(126)=2.79, pFDR=0.04; right: βage=1.32 mm3/month (0.34,2.29), t(126)=2.68, pFDR=0.042) and child faces (Fig. 1C-dark red, left: βage=1.95 mm3/month (0.74,3.17), t(126)=3.18, pFDR=0.037; right: βage=2.16 mm3/month (0.35,3.97), t(126)=2.37, pFDR=0.049). Similar longitudinal development in lateral VTC was observed for other contrasts, such as contrasting responses by domain (Supplementary Fig. 4) and for other metrics, such as the magnitude of category-selectivity within constant-sized regions (Supplementary Fig. 5).
As limb representations are located between face- and word-activations, and the latter show developmental increases, it is possible that the developmental decrease in volume of limb-selective activation may result from the inclusion of faces and words as control categories when defining category contrasts. To test this possibility, we repeated the analyses in Fig. 1, excluding faces, words and limbs from the control categories in the contrast. Results of these analyses reveal a qualitative similar pattern of results though they do not survive FDR correction (Extended Data Fig. 1A,C, Supplementary Fig. 6, Supplementary Tables 3, 4). That is, word-selective volume increased in the left hemisphere, face-selective volume increased bilaterally, while limb-selective volume decreased bilaterally with childhood development.
Another concern is that age-related differences in data quality may conflate developmental effects. Thus, we performed several controls to test if factors that may affect data quality contribute to these findings. First, behavioral performance on the oddball task performed during scanning was overall high (median performance=91%, SD=18%), indicating that children were attending to the stimuli. Second, to test if developmental effects may be related to temporal signal-to-noise (tSNR) or motion during scan, we ran new LMMs, which included in addition to age, tSNR and average motion during scan as predictors. Motion was not a significant predictor of the volume of category-selective activation (Supplementary Table 5). While tSNR was a significant predictor, it was independent from age. That is, LMMs that included tSNR as an additional predictor revealed the same pattern of results showing significant age-related increases in face- and word-selective activations as well as significant age-related decreases in limb-selective activations (Extended Data Fig. 1B,C, Supplementary Tables 6, 7). Third, to test if developmental effects are driven by overall lower reliability of responses in younger children compared to older children, we assessed response reliability in V1 as a control ROI. Response reliability in V1 was numerically high (left: median=0.74, SD=0.14; right: median=0.56, SD=0.16) and it was not statistically significant related to age (Supplementary Fig. 7). Together, these analyses validate that age-related changes in category-selective activations reflect longitudinal development rather than differences in measurement quality across years.
Is development anatomically specific?
To determine the anatomical specificity of the observed development, we defined limb-, word-, and face-selective regions of interest (ROIs) in each participant and session (Fig. 2A–C, t-value > 3 voxel-level) and examined them longitudinally. Category-selective ROIs remained largely within the same anatomical structures as ROIs expanded or shrank across childhood (Fig. 2A–C). We used LMMs to examine the effect of age on ROI volume (age: fixed effect, subject: random effect, change of ROI volume per month (βage) in Fig. 2D, full statistics in Supplementary Table 8).
Fig. 2. Development of category-selective ROIs.
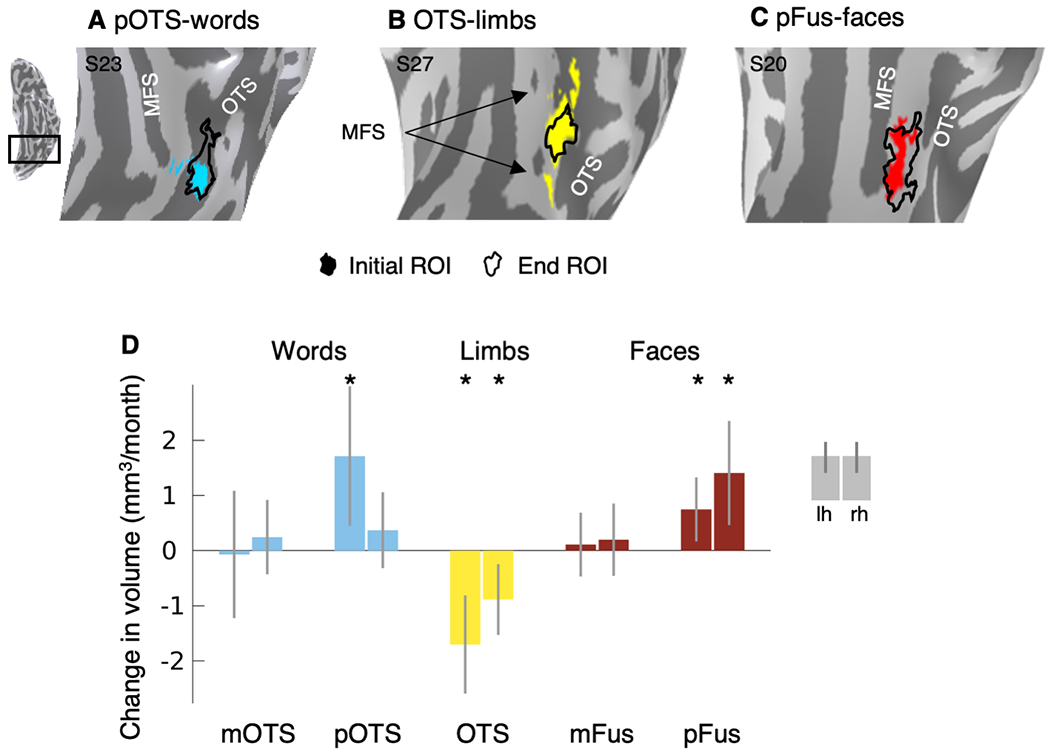
Initial ROIs (colored) and end ROIs (outline) in 3 example children: (A) left pOTS-words at age 10 and 15, (B) left OTS-limbs at age 11 and 13, (C) left pFus-faces at age 9 and 14. MFS: mid fusiform sulcus; OTS: occipito-temporal sulcus. (D) LMM slopes: change in volume of category-selective regions per month. Error bars: 95% CI. If the CI does not cross the y=0 line, this indicates that the slope is significantly different than 0 (before FDR-correction). Asterisks: significant development, p<0.05, FDR-corrected. The number of sessions per ROI is as follows: left mOTS-words: n=103, right mOTS-words: n=35, left pOTS-words: n=119, right pOTS-words: n=73, left OTS-limbs: n=126, right OTS-limbs: n=126, left mFus-faces: n=107, right mFus-faces: n=102, left pFus-faces: n=120, right pFus-faces: n=98.
We found that posterior ROIs significantly expanded with age. In contrast, the volume of anterior ROIs did not significantly change with age (Fig. 2D). Activation for words grew significantly in the left posterior occipitotemporal sulcus (Fig. 2D-pOTS-words, βage=1.71 mm3/month (0.45,2.98), t(117)=2.68, pFDR=0.02), but there was no statistically significant effect of age on activation in the mid occipitotemporal sulcus (Fig. 2D-mOTS-words, left: βage=−0.07 mm3/month (−1.22,1.08), t(101)=−0.12, pFDR=0.90, right: βage=0.25 mm3/month (−0.43,0.92), t(33)=0.74, pFDR=0.66). Similarly, bilateral activation for faces grew significantly in the posterior fusiform (Fig. 2D-pFus-faces, left: βage=0.75 mm3/month (0.17,1.33), t(118)=2.55, pFDR=0.02, right: βage=1.41 mm3/month (0.46,2.35), t(96)=2.96, pFDR=0.019). However, there was no statistically significant effect of age on activation in the mid fusiform (Fig. 2D-mFus-faces, left: βage=0.11 mm3/month (−0.47,0.69), t(105)=0.38, pFDR=0.79, right: βage=0.20 mm3/month (−0.46,0.85), t(100)=0.60, pFDR=0.69). In contrast to the growth of pOTS-words and pFus-faces, OTS-limbs shrank significantly in both hemispheres (Fig. 2D-OTS-limbs, Supplementary Table 8, left: βage=−1.70 mm3/month (−2.59,−0.81), t(124)=−3.78, pFDR=0.002, right: βage=−0.89 mm3/month (−1.53,−0.24), t(124)=−2.73, pFDR=0.02). Control analyses showed that (i) the surface area of face- and word-selective regions significantly expand and the surface area of limb-selective regions significantly shrinks (Supplementary Fig. 8) and (ii) the expansion of face-selective regions and the shrinking of limb-selective regions is also significant for ROIs defined from domain contrasts (Supplementary Fig. 9).
What functional changes occur in the developing regions?
We next assessed the functional properties of the developing regions of category-selective ROIs in each participant. For words and faces, where selective voxels emerge during development, we call the difference between the end and initial ROIs the emerging pOTS-words and emerging pFus-faces. For limbs, we call the difference between the initial and end ROIs the waning OTS-limbs. We focus on the left hemisphere due to the observed left lateralized development of word-selectivity (right hemisphere in Extended Data Fig. 2). LMMs were used to assess the effect of age on selectivity in emerging and waning ROIs (age: continuous fixed effect; participant: random effect). LMM slopes (change in selectivity, t/month) and their significance are shown in Fig. 3-left (statistics in figure caption and Supplementary Table 9). Since developing regions are not completely independent from the original ROIs, we repeated the analysis in independent ring-shaped ROIs centered on the initial functional ROIs, yielding similar findings (Supplementary Fig. 10).
Fig. 3. Age-related increases in word- and face-selectivity parallel decreases in limb-selectivity in the developing regions.
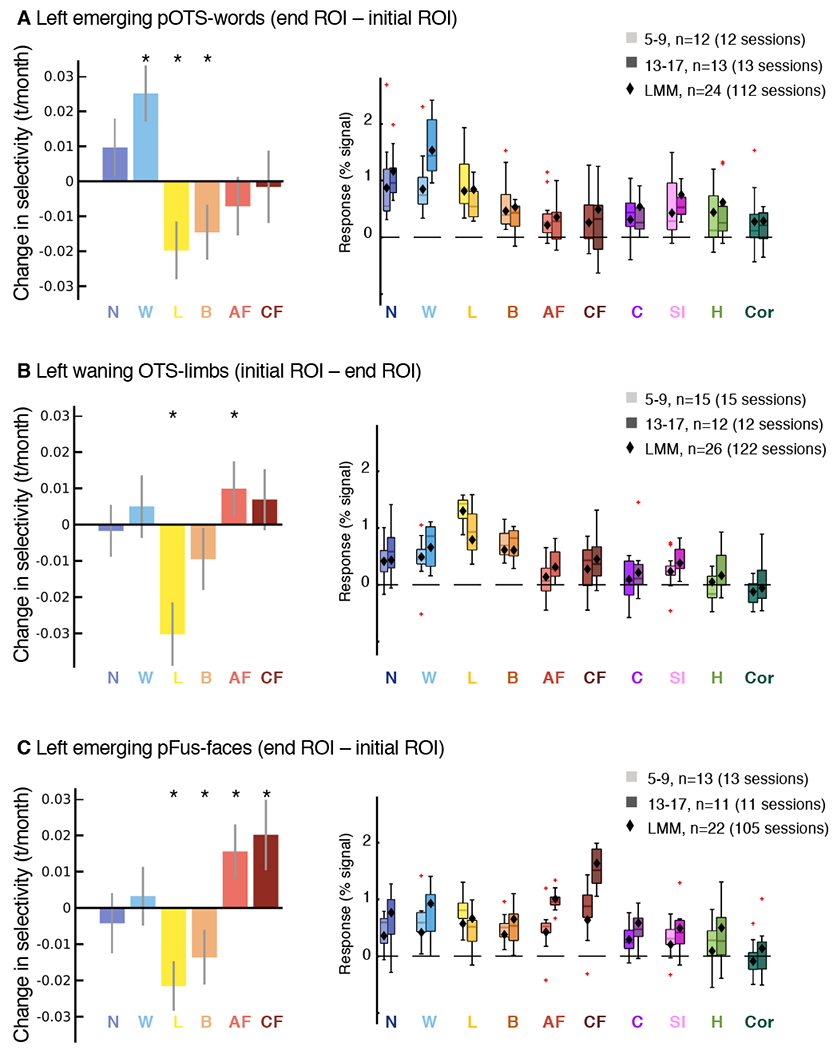
(A-C) Left: LMM slopes indicating changes in selectivity by age. Error bars: 95% CI. If the CI does not cross the y=0 line, the slope is significantly different than 0 (before FDR-correction). Asterisks: Significant development after FDR-correction (p<0.05). All ROIs and categories in Extended Data Fig. 2. Full statistics in Supplementary Tables 9–10. Right: Response amplitudes for the 10 categories. The boxplots illustrate response amplitudes by age group for 5-9-year-olds and 13-17-year-olds. One session per child is included per boxplot; Legend indicates number of sessions. LMMs on response amplitudes using data from all available sessions for each ROI were used to estimate development of response amplitudes. Diamonds represent LMM estimates for response amplitudes for the mean age of each age group. Full statistics in Supplementary Table 11. Boxplots show the 75% and 25% percentiles (colored areas), and median (horizontal lines). Whiskers extend to the most extreme data points not considered outliers (minimum, maximum). Crosses: outliers (values more than 1.5 times the interquartile range away from the bottom or top of the box).
In the emerging left pOTS-words, selectivity to words significantly increased with age, as expected by the definition of the ROI (Fig. 3A-left blue bar). At the same time, selectivity to limbs and bodies significantly decreased (Fig. 3A-left), but we found no statistically significant changes in selectivity to other categories (Extended Data Fig. 2, Supplementary Table 9). As a control, we repeated these analyses excluding adult faces, child faces, limbs and words as control categories from all contrasts, finding similar results (Fig. 3A-left, Extended Data Fig. 2 -open bars, Supplementary Table 10).
To elucidate if the changes in selectivity in the emerging ROI were due to increased responses to the preferred category or decreased responses to nonpreferred categories, we used LMMs to quantify the response amplitude to each category as a function of age (age: continuous fixed effect; participant: random effect, change in %-signal/month (βage), Supplementary Fig. 11, statistics in Supplementary Table 11). Results show that developmental changes in word-selectivity were associated with significant increases in the responses to words (βage=0.008 %-signal/month (0.004,0.012), t(110)=3.82, pFDR=0.002) with no statistically significant changes in responses to other categories (Supplementary Fig. 11, Supplementary Table 11) except that responses to string instruments also significantly increased (βage=0.004 %-signal/month (0.001,0.006), t(110)=2.98, pFDR=0.025). Indeed, average responses to words in pOTS-words are higher in teens (13-17-year-olds) than children (5-9-year-olds; Fig. 3A-right-boxplots, Supplementary Fig. 11, Supplementary Table 11) in correspondence with LMM predictions (Fig. 3A-right-diamonds).
Similarly, in the emerging pFus-faces, selectivity to faces increased (Fig. 3C-left-red bars, Extended Data Fig. 2). At the same time, selectivity to limbs significantly decreased bilaterally (Fig. 3C-left-yellow bar) and this development was significant in the left hemisphere also when faces and words were excluded as control categories from the contrast (Fig. 3C-left, Extended Data Fig. 2-open bars); selectivity to bodies decreased significantly only in the left hemisphere (Fig. 3C-orange). We found no statistically significant changes in selectivity to other categories (Extended Data Fig. 2, Supplementary Table 9). Increases in selectivity to faces were associated with significant increases in responses to faces (Supplementary Fig. 11, Supplementary Table 11, adult faces: βage=0.006 %-signal/month (0.004,0.009), t(103)=5.33, pFDR<0.001; child faces: βage=0.011 %-signal/month (0.007,0.015), t(103)=5.79, pFDR<0.001). Indeed, average responses to both adult and child faces were higher in teens than children in correspondence with LMM predictions (Fig. 3C-right). Additionally, responses to words and string instruments also significantly increased in the left emerging pFus-faces (words: βage=0.006 %-signal/month (0.002,0.01), t(103)=2.78, pFDR=0.04, string instruments: βage=0.003 %-signal/month (7.7×10−4,0.006), t(103)=2.62, pFDR=0.04), and there was a trend for an increase in responses to cars (Fig. 3C-right, Supplementary Fig. 11, Supplementary Table 11). Thus, developmental increases in word- and face-selectivity in emerging word and face ROIs, respectively, are largely driven by increased response amplitudes to the preferred category, rather than decreased response amplitudes to nonpreferred categories.
As OTS-limbs is located between pOTS-words and pFus-faces, we asked if increased responses to faces and words also occur in the waning OTS-limbs. We found that not only did responses to adult faces significantly increase with age (Supplementary Fig. 11, βage=0.002 %-signal/month (4.6×10−4,0.003), t(120)=2.64, pFDR=0.043), but also responses to limbs significantly decrease with age (Fig. 11, βage=−0.005 %-signal/month (−0.008,−0.003), t(120)=−4.47, pFDR<0.001). This is an intriguing phenomenon in which this waning region responds more strongly to limbs in 5-9-years-olds than in 13-17-year-olds (Fig. 3B-right-yellow). There were no statistically significant changes in responses to other categories (Fig. 3B-right, Supplementary Fig. 11, Supplementary Table 11-full statistics). These changes in responses amplitudes resulted in significant decreases in limb-selectivity (Fig. 3B-left, Extended Data Fig. 2) as well as significant increases in face-selectivity (Fig. 3B-left). In the right waning OTS-limbs these increases in face-selectivity were also significant when limbs and words were excluded as control categories from the contrast (Extended Data Fig. 2). There were no statistically significant changes in selectivity to other categories including bodies (Extended Data Fig. 2). Therefore, developmental decreases in limb-selectivity of the waning OTS-limbs reflect decreased response amplitudes to the preferred category together with increased response amplitudes to faces.
Given the profound developmental decreases in limb-selectivity in both emerging and waning ROIs, we tested if this is a general phenomenon across lateral VTC. Analyses of lateral VTC excluding voxels that were selective in the first session to categories showing development, revealed no statistically significant decreases in limb-selectivity in the remainder of lateral VTC (Supplementary Fig. 12).
Are changes in face-, word-, and limb-selectivity linked?
While the theory of competition does not make predictions about limb representations, it predicts that development of face and word representations are linked as they compete over cortical territory that shows a foveal bias13,14. Thus, we tested if there is a quantitative relationship between selectivities to faces, words, and limbs in the emerging and waning ROIs. Model comparison of LMMs relating the selectivity to the preferred category to selectivity of the other categories revealed that in all developing ROIs, selectivity to the preferred category was better predicted by the selectivity to the other two categories rather than just one of them (likelihood ratio tests comparing a one-predictor-LMM with a two-predictor-LMM, left hemisphere: all χ2≥4.76, p≤0.029, Supplementary Table 12). Moreover, in all developing ROIs, selectivity to the preferred category was significantly and negatively related to selectivity to the other two categories (βcategory1, βcategory2: fixed effects, subject: random effect, Fig. 4, left hemisphere; Extended Data Fig. 3, right hemisphere; statistics in figure caption and full statistics in Supplementary Table 13). E.g., in the emerging pOTS-words, higher word-selectivity is significantly linked with both lower face- and limb-selectivity (Fig. 4A, Supplementary Table 13). The negative relationship between the preferred category of an ROI and the other two categories was also observed at the voxel level (Supplementary Fig. 13).
Fig. 4. Developmental changes in word-, face-, and limb-selectivity are linked.
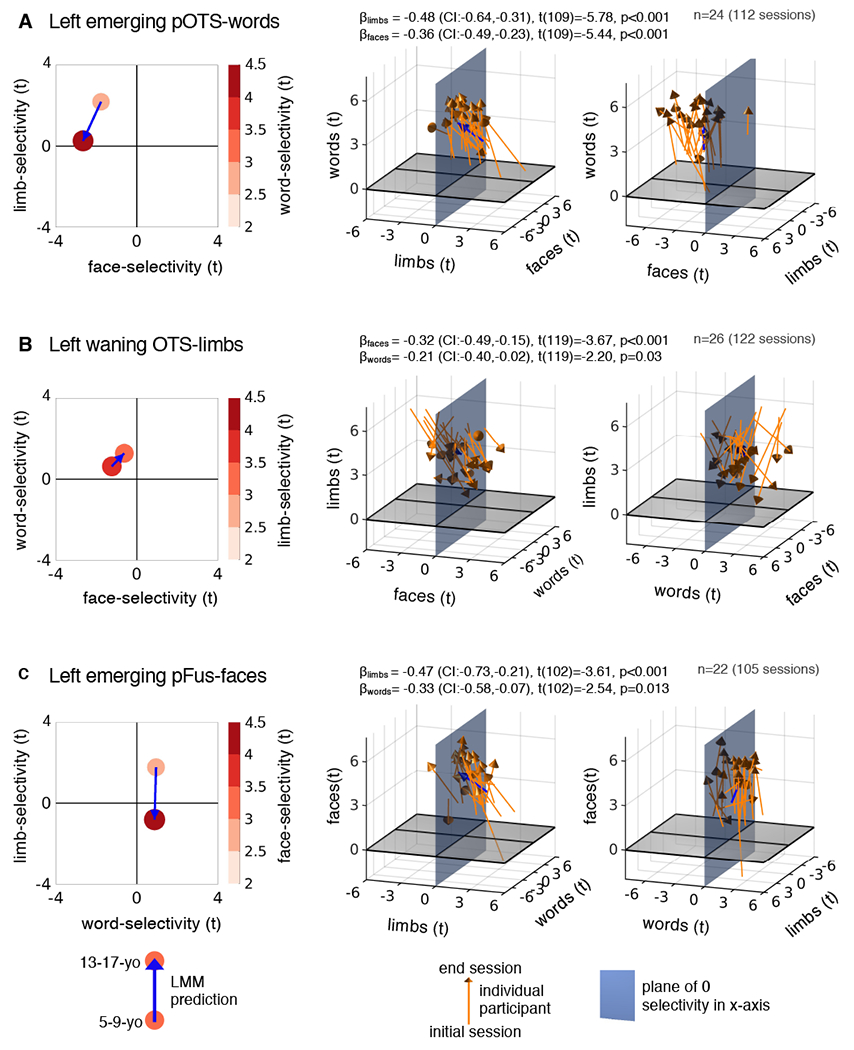
Left: LMM prediction (circle) of category selectivity to words (A), limbs (B), and faces (C) vs. selectivity to the other two categories in 5-9-year-olds and 13-17-year-olds. Middle: Individual participant data visualized in 3D. In each panel the variable on the z-axis is related to the x- and y-variables, this relationship is quantified in the model; βs, 95%-CI, t-values, degrees of freedom, and p-values are shown at the top. Full statistics in Supplementary Table 13; Orange arrows: Individual child data. Blue arrows: LMM prediction (same as left panel). Right: Rotated version of the plots in the middle column to enhance visibility of positive and negative values along the other horizontal axis. Right hemisphere in Extended Data Fig. 3.
We visualized how selectivity to words, faces, and limbs changes in emerging and waning ROIs. Using the LMM, we related the selectivity to the preferred category with the selectivity to the other two categories for 5-9-year-olds and for 13-17-year-olds (Fig. 4-left, Supplementary Table 14). In the emerging pOTS-words, 5-9-year-olds have positive selectivity to limbs and negative selectivity to faces (Fig. 4A). By age 13-17, word-selectivity has increased while limb-selectivity has reduced to zero and face-selectivity has become even more negative (Fig. 4A-left). That is, after development, selectivity to words in pOTS-words has replaced the initial selectivity for limbs, not faces. Notably, this developmental pattern is visible in individual children from their initial age (Fig. 4A-middle & right, arrow bases) to their end age (Fig. 4A-middle & right, arrow heads). Similarly, in the emerging pFus-faces, 5-9-year-olds have positive limb-selectivity but no clear preference for words (Fig. 4C-left). By age 13-17, as face-selectivity has increased, limb-selectivity is lost and there is little change to word-selectivity (Fig. 4C, Extended Data Fig. 3, Supplementary Table 14).
In the waning OTS-limbs, 5-9-year-olds exhibit largely negative face-selectivity and mild positive word-selectivity (Fig. 4B-left, Supplementary Table 14). As limb-selectivity declines by age 13-17, both word- and face-selectivity increase (Fig. 4B-left). While limb-selectivity consistently declined across individuals, there was more variability in individual developmental trajectories compared to the emerging ROIs (Fig. 4B- middle, right). In some children limb-selectivity was replaced by word-selectivity and in others with face-selectivity.
As multiple control categories were used to determine selectivity in the prior analysis, we also sought to directly measure if there are changes in preference between the ROI-defining category vs. each of the two other developing categories. For instance, in the emerging pOTS-words, we directly measured preference to words vs. faces and words vs. limbs in each session. We plotted this preference for each child and used LMMs to estimate the significance of developmental effects as well as estimate this preference from age 5-17.
Results of pairwise selectivity analyses in the developing ROIs replicate results of Fig. 4, both at the individual subject level (Fig. 5, Extended Data Fig. 4, thin lines) and group level (all LMM slopes significant, except for faces vs. words, in left pFus-faces, stats in Fig. 5, Extended Data Fig. 4, Supplementary Table 15). In the emerging left pOTS-words, there is a significant age-related increase of preference to both words vs. limbs and words vs. faces (stats in Fig. 5, Supplementary Table 15). It is interesting that preference to words vs. limbs estimated from the LMM is negative at age 5 and flips to be positive around age 9 (Fig. 5A). However, there is no such flip in word vs. face preference, which steadily becomes more positive from age 5 onwards. This suggests that in the emerging left pOTS-words initial preference for limbs vs. words is flipped to a later preference for words vs. limbs. Likewise, in the left emerging pFus-faces, negative preference to faces vs. limbs (but not faces vs. words) at age 5 is flipped to a positive preference later in childhood (Fig. 5C, Extended Data Fig. 4). Consistent with the prior analyses, the waning OTS-limbs showed significant age-related decrease in both limbs vs. faces (Fig. 5B-left) and limbs vs. words (Fig. 5B-right, stats in Fig. 5, Supplementary Table 15). In fact, the waning OTS-limbs starts with positive preference to limbs vs. faces and limbs vs. words at age 5, and loses both preferences by age 17. That is, at age 17 this waning region has no clear preference for these three categories. In sum, these analyses provide evidence that in emerging word- and face-selective regions, earlier limb-selectivity is repurposed into word- and face-selectivity, respectively.
Fig. 5. In emerging category-selective ROIs, selectivity flips from preference to limbs at age 5 to preference to either words or faces, respectively, later in childhood.
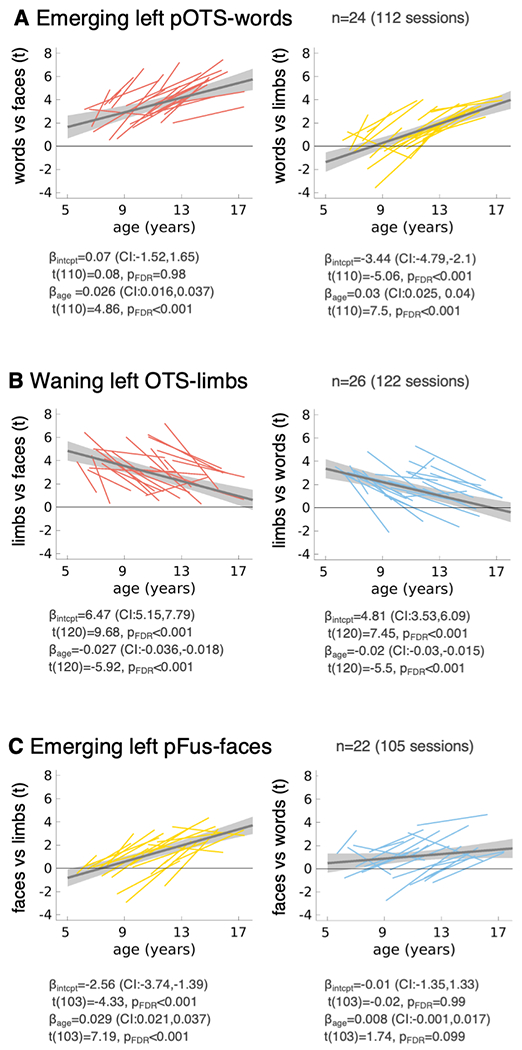
In each plot we show the pairwise preference of the ROI-defining category vs. each of the other two developing categories as a function of age. Thin lines: individual participant data showing the pairwise preference from the initial to end session. Gray line: LMM prediction of pairwise preference based on data from all sessions. Shaded gray: 95%-CI. LMM results (intercept: βintercept and slope: βage (rate of change in preference, t/month) and their significance are reported under each panel. (A) Emerging left pOTS-words (n=24 subjects, n=112 sessions). Left: Words vs. faces (red). Right: Words vs. limbs (yellow). (B) Waning left OTS-limbs (n=26 subjects, n=122 sessions). Left: Limbs vs. faces (red). Right: Limbs vs. Words (blue). (C) Emerging pFus-faces (n=22 subjects, n=105 sessions). Left: Faces vs. limbs (yellow). Right: Faces vs. Words (blue). Right hemisphere data in Extended Data Fig. 4. Statistics in Supplementary Table 15.
Discussion
Our longitudinal measurements in children using a large range of ecologically-relevant categories reveal new insights about the functional development of high-level visual cortex. We show that while face- and word-selective regions grow and become more selective, limb-selective regions shrink during childhood development and lose their selectivity to limbs. Importantly, the decrease in limb-selectivity is directly linked to the increase in word- and face-selectivity providing evidence for cortical recycling during childhood development. We discuss each of these findings and their implications below.
A surprising finding from our study is that childhood development is not only associated with growth of category-selective regions and increases in selectivity, but also involves loss of selectivity. That is, in addition to finding growth and increased selectivity of face- and word-selective regions in VTC as individual children develop, consistent with cross-sectional studies8,12,16,21–23, we find that limb-selective regions in VTC shrink during childhood development and lose their selectivity to limbs. As such, our results show that young children’s VTC is actually more selective for limbs than it is later in childhood. No significant developmental changes were found for body-selective volume (Fig. 1C), consistent with previous findings9,22.
Our results provide empirical evidence for recycling14 of category-selectivity in high-level visual cortex during childhood. However, contrary to previous predictions13,14 that face-selectivity is recycled to word-selectivity during development, our results show that limb-selectivity is recycled to both word- and face-selectivity. This recycling occurs via a mechanism of decreasing responses to limbs and increasing responses to both faces and words at the ROI and voxel levels. While we find that in the emerging left pFus-faces, not only responses to faces, but also responses to words increase with age (Fig. 3C-right), future research is required to determine whether this finding reflects competition for foveal resources between words and faces11,13. Crucially, our results reveal no evidence that initial face-selectivity is flipped into word-selectivity with age but show that limb-selectivity is repurposed into word- and face-selectivity with development. Future research is needed to determine if this recycling also occurs at the single neuron level24.
Critically, our results require a rethinking of prevailing developmental theories that propose that cortical development involves sculpting of new representations upon general-purpose cortex8,9. First, in contrast to the prevailing view suggesting that children’s VTC is indistinctive8–10,16,25, the present data shows that young children’s’ VTC is more selective to limbs than it is later in childhood. Second, our data suggest that during childhood, cortical selectivity can change from one category to another.
These results generate new questions for future research. First, why do young children exhibit large VTC representations for limbs? Some clues to this question can be gleaned from behavioral studies that examined what infants and toddlers look at in natural settings. These studies discovered that young infants (≤ 6 months) look at faces more than hands, but older infants and toddlers (1- 2 year-olds) look at hands more than faces26–28. Developmental psychologists have hypothesized that this change in the child’s “visual diet” may be related to multiple factors including the child’s mobility28, dexterity at manipulating objects26,28, and use of communicative information in gestures29. Based on this developmental literature and our findings, we speculate that much like baby teeth changing to permanent teeth, as children’s diet and size changes, cortical recycling in VTC may reflect adjustment to changing visual demands during childhood. Thus, we propose an intriguing new hypothesis that cortical recycling in VTC co-occurs with changes in the saliency and frequency of visual stimuli that are socially and communicatively relevant, moving from hands and gestures in toddlers to faces and words in school age children and teens. Future research can test this hypothesis by determining what children and teens look at together with computational modeling to test the effect of different visual diets on emerging VTC representations30–32.
Second, what are the behavioral implications of this cortical recycling? Prior research has found that developmental improvements in face recognition ability8,33 and face discriminability34 are linked with developmental increases in face selectivity8,33 and neural sensitivity to faces34 in VTC, respectively. Likewise, developmental improvements in reading proficiency are linked with (i) increases in neural selectivity to words9,35,36 and (ii) increases in the informativeness of distributed responses to words in VTC12. These studies suggest that the developmental decreases in responses to limbs in VTC may also have behavioral ramifications, a hypothesis that can be tested in future developmental research combining neuroimaging and behavioral measurements.
The present findings have important implications for understanding typical37 and atypical38–41 brain development. First, these data fill a key gap in knowledge by quantifying the rate of the development of category-selectivity from young children to teens. Thus, they offer a foundation for using fMRI to assess developmental and learning deficiencies, especially those related to reading42 and social perception38,39. We acknowledge that a limitation of the current study is the variability in data acquisition with regard to the time interval between scans and number of scans per child. Thus, we emphasize the necessity for future longitudinal research with more regular age sampling spanning a large duration in both typical and atypical populations, which will allow further in-depth analyses of cortical recycling at finer spatial scales, such as on the level of individual voxels. Second, it will be important to determine if there is a critical period during development in which cortical recycling can occur10,43 and if this period is particularly protracted in lateral VTC, which overlaps foveal representations11,15,19. Third, childhood visual deprivation40 or brain lesions41 may affect cortical recycling and the emergence of category representations in VTC in atypical populations44–47. Future longitudinal studies measuring cortical development in children who have vastly different visual experience with faces, hands, and written words (e.g., congenital blind, sighted, and congenital deaf), will be important for determining how differing visual inputs and behavioral demands during childhood affect cortical recycling. Finally, an open question for future research is whether cortical recycling occurs in other brain systems in which earlier representations may be altered by schooling48 or prolonged childhood experience. Some examples include learning new languages after one’s mother tongue49,50 or learning complex math51 upon an earlier numerosity system52,53.
In sum, the surprising finding from our study is that contrary to the prevailing hypothesis that during childhood new cortical representations are formed upon unspecified cortex, we find evidence for cortical recycling in which limb representations are repurposed during childhood to represent faces and words. The discovery of cortical recycling is important because it not only provides a key advancement in understanding cortical development, but also necessitates a rethinking of how cortical function develops during childhood.
Methods
Statement on ethical regulations
This study was approved by the Institutional Review Board of Stanford University and complies with all relevant ethical regulations. Prior to the start of the study, parents gave written consent, and children gave written assent. For their participation children received $30 per hour for scanning, as well as a small toy.
Participants
Children with normal or corrected-to-normal vision were recruited from local schools in the Bay Area. The diversity of the participants reflects the makeup of the Bay Area population. 62.5% of children were Caucasian, 20% were Asian, 5% were Native Hawaiian, 5% were Hispanic, and 7.5% were multiracial or from other racial/ethnic groups.
Prior to their first MRI session, children were trained in a scanner simulator to acclimate them to the scanner environment and to enhance quality of MRI data. In the simulator, children practiced laying still while watching a short movie and receiving live feedback on their motion. For subsequent scans, simulator training was repeated if necessary.
We collected data from 40 (26 female) children (onset age=5-12 years, M=8.66 years, SD=2.34 years, Supplementary Fig. 1). We selected this age range because (i) it captures the phase in which children start learning to read and (ii) it covers a broad age range spanning childhood and adolescence in which previous studies have documented VTC development9,21.
Data from 4 children were excluded because they dropped out of the study after participating only once, and thus did not provide longitudinal data. Data from 7 children were excluded because their data did not pass inclusion criteria (see below). In the remaining 29 children, 29 functional sessions were excluded due to motion, 1 session due to a technical error during acquisition, and 1 session due to aliasing artifacts during acquisition. Therefore, data from 128 functional sessions of 29 neurotypical children (18 female, 11 male) are reported in this study (Supplementary Fig. 1A,B has an overview of the included and excluded sessions). Initial ages of the included children ranged from 5 to 12 years (mean=9.19, SD=2.13). No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous cross-sectional publications33,54 and larger than previous longitudinal studies on VTC development9.
Participants were scanned using functional and structural MRI for 1 to 5 years. When possible, children participated in 1 to 2 functional scans per year. Additionally, children participated in 1 structural MRI session per year. Each child participated in at least 2 and up to 10 fMRI sessions (mean=4.41, SD=1.92) with the time interval between the first and last fMRI scan ranging from 10 months to 5 years (mean=45 months, SD=18 months, Supplementary Fig. 1C). Functional and anatomical scans were typically conducted on different days to avoid fatigue.
Magnetic resonance imaging
Structural imaging
Data were acquired at the Center for Cognitive Neurobiological Imaging at Stanford University on a 3 Tesla GE Discovery MR750 scanner (GE Medical Systems) using a phase-array 32 channel head coil. Whole brain anatomical scans were collected using quantitative MRI (qMRI55) with a spoiled gradient echo sequence using multiple flip angles (α=4°, 10°, 20°, 30°), TR=14ms and TE=2.4ms. The scan resolution was 0.8x0.8x1.0mm3 (later resampled to 1mm isotropic). For T1-calibration we acquired spin-echo inversion recovery scans with an echo-planar imaging read-out, spectral spatial fat suppression and a slab inversion pulse. These scans were acquired at TR=3s, inplane resolution=2mmx2mm, slice thickness=4mm and 2x acceleration, echo time=minimum full.
Functional imaging
Functional data were collected using the same scanner and head coil as the structural images. Slices were oriented parallel to the parieto-occipital sulcus. We used a simultaneous multi-slice, one-shot T2* sensitive gradient echo EPI sequence with a multiplexing factor of 3 to acquire near whole brain coverage (48 slices), FOV=192mm, TR=1s, TE=30ms, and flip angle=76°. Resolution was 2.4 mm isotropic.
10 category experiment
Participants completed three runs of a 10-category experiment11,12,54. During each run, participants viewed images from five domains each comprising images of two categories: faces (adult faces, child faces), body parts (headless bodies, limbs), objects (cars, string instruments), places (corridors, houses), and characters (pseudowords, numbers). Following prior work on visual representations, we define a visual category as a set of exemplars sharing the same parts and configuration, e.g., limbs56–59 and domain as a grouping of one or more categories that share semantic association (whether or not they share visual features, e.g., houses and corridors are both places, but are visually dissimilar) and are thought to require distinct cortical processing mechanisms60. Examples of stimuli are shown in Supplementary Fig. 1D.
Images were presented in 4 s blocks, at a rate of 2 Hz and did not repeat across the course of the experiment. Image blocks were intermixed with gray luminance screen baseline blocks. Blocks were counterbalanced across categories and baseline. Stimuli were grayscale and contained a phase-scrambled background generated from randomly selected images. Participants were instructed to view the images while fixating on a central dot and to perform an oddball task. Oddball task: Participants pressed a button whenever an image comprising only the phase-scrambled background appeared. Due to occasional button box malfunction behavioral responses in the oddball task were recorded in 98 out of 128 sessions used in the analyses.
Data analysis
Data analysis was performed in MATLAB version 2017b (The MathWorks, Inc.) and using the mrVista software package (https://github.com/vistalab/vistasoft/wiki/mrVista).
Inclusion criteria
In each functional session children participated in three runs of the 10 category-experiment. Criteria for inclusion of data were (i) at least 2 runs per session where within-run motion < 2 voxels and between-run motion < 3 voxels, and (ii) the child participated in at least two fMRI sessions that were at least six months apart.
Because only two of the three runs survived motion quality thresholds for several fMRI sessions, analyses include two runs per child per session to ensure equal amounts of data across participants and sessions. For sessions with all 3 runs passing motion quality criteria, 2 runs with lowest within-run motion were included.
Structural MRI data analysis and individual template
Quantitative whole brain images of each child and timepoint were processed with the mrQ pipeline (https://github.com/mezera/mrQ55) to generate synthetic T1 brain volumes. For each child, the synthetic T1 brain volumes from their multiple timepoints were used to generate a within-subject brain volume template. Each participant’s brain anatomical template was generated using the FreeSurfer Longitudinal pipeline implemented in FreeSurfer version 6.0. (https://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing61. The gray-white matter segmentation of each participant’s within-subject brain template was manually edited to fix segmentation errors (e.g., holes and handles) to generate an accurate cortical surface reconstruction of each participant’s brain. The motivation for aligning the functional data to the within-subject-template were to: (i) enable comparison of regions of interest (ROIs) from different timepoints in the same brain volume for each participant, and (ii) minimize potential biases which can occur from aligning longitudinal data to the anatomical volume from a single timepoint61. On average 2.48 (SD=0.69) synthetic T1s were used to generate the within-subject-template (min=2, max=5). Functional data from all sessions of a participant were aligned to their within-subject brain template. In 17 participants the last fMRI session that was included was conducted after the within-subject template had been created. These functional sessions were acquired on average 11± 2 months after acquisition of the last synthetic T1 that was included in the within-subject-template (excluding 2 participants whose last T1 could not be used because of technical error during acquisition and subject motion).
Definition of anatomical regions of interest (VTC ROIs)
On the inflated surface of each hemisphere in each participant, we defined anatomical regions of interest (ROIs) of the lateral and medial VTC (Fig. 1B) as in previous publications12. We first defined the VTC anatomically and then separated it to lateral and medial VTC. The posterior border of VTC was the posterior transverse collateral sulcus (ptCoS) and the anterior border was aligned to the posterior end of the hippocampus, which typically aligns with the anterior tip of the mid fusiform sulcus (MFS). The lateral border of VTC was the inferior temporal gyrus (ITG) and the medial border of VTC was the medial border of the collateral sulcus (CoS). Finally, VTC was divided into its lateral and medial partitions along the MFS (example in Fig. 1B).
fMRI data analysis
Functional data from each session were aligned to the individual within-subject template. Motion correction was performed both within and across functional runs. No spatial smoothing and no slice-timing correction were performed. Time courses were transformed into percentage signal change by dividing each timepoint of each voxel’s data by the average response across the entire run. To estimate the contribution of each of the 10 conditions (corresponding to the 10 image categories) a general linear model (GLM) was fit to each voxel by convolving the stimulus presentation design with the hemodynamic response function (as implemented in SPM, www.fil.ion.ucl.ac.uk/spm).
Definition of selectivity in lateral and medial VTC
We used a data-driven approach to examine the development of category selectivity in VTC. The motivation for this type of analysis was to (i) use an automated, observer-independent approach and (ii) use an approach that does not require clustered activations. In each participant, we assessed selectivity to each category in anatomically defined lateral and medial VTC ROIs (Fig. 1B). Selectivity was defined as t-value > 3 (voxel-level) for the contrast of interest. We also performed a complementary threshold-independent analysis in constant-sized regions (see Control analysis below). Category contrasts (Fig. 1) were computed by contrasting responses to each category with all other categories from all other domains (i.e., words vs. all other categories except numbers). We used this approach for defining contrasts to ensure that all categories are contrasted to the same number of control categories and contrasts are not biased to any particular category. We also computed contrasts for domains (faces, body parts, characters, objects, places), see supplemental analyses (Supplementary Fig. 4). For domain contrasts, responses to both categories of each domain were contrasted with stimuli from all the other domains (i.e., characters vs. all others).
Control analysis on categories included in the contrasts
Results of the analysis related to Fig. 1 revealed a bilateral decrease in limb-selectivity in addition to increases in face- and word-selectivity with age. Given that face-, limb-, and word-selective regions neighbor in the brain, it is possible that the observed decrease in limb-selectivity may partially be driven by the inclusion of categories showing age-related increases in selectivity (e.g., faces and words) as control categories in the contrast (see above). Therefore, to ensure that the decrease in limb-selectivity and the increase in face- and word-selectivity are not driven by including the respective other two categories as controls in the contrasts, we repeated all analyses related to Figs. 1 and 3, excluding these three categories from being control categories in all contrasts. That is, we contrasted each category vs. all other categories excluding limbs, words, adult faces, child faces, and the other category from the same domain. For instance, responses to limbs were contrasted with responses to cars, string instruments, houses, corridors, and numbers. Results are shown in Extended Data Fig. 1 and 2 and Supplementary Fig. 6.
Definition of functional regions of interest
To examine the anatomical specificity of the observed development of selectivity (Fig. 1), we defined word-, limb-, and face-selective functional regions in each participant (see Fig. 2A–C). For the definition of functional ROIs, a threshold of a t-value > 3 (voxel-level) was used. All ROIs were defined in each participant’s native cortical surface generated from the within-subject brain template. Word-selective regions were defined as above-threshold clusters for the contrast words vs. all categories except numbers that straddled the occipito-temporal-sulcus (OTS). The more anterior cluster was defined as mOTS-words and the posterior cluster as pOTS-words. These clusters are also referred to as the visual word form area4 (VWFA 1 and 2, respectively). The limb-selective region was defined as above-threshold cluster of voxels for the contrast limbs vs. all categories except bodies straddling the OTS and was labelled OTS-limbs. This cluster is also referred to as the fusiform body area2 (FBA). Face-selective regions were defined using the contrast faces (adult and child) vs. all other categories, because we observed similar development for both face types in the analysis related to Fig. 1. Face-selective clusters were defined as above-threshold clusters on the lateral fusiform gyrus. The more anterior cluster typically aligns with the anterior tip of the MFS, and was defined as mFus-faces19, while the more posterior cluster was defined as pFus-faces19. These two face-selective clusters are also referred to as the fusiform face area1 (FFA 1 and 2, respectively). For supplemental analyses (Supplementary Figs. 4,9) we also defined place-, character-, and combined body-part-selective regions. Place-selective regions were defined as above threshold clusters on the collateral sulcus that respond to houses and corridors vs. all other categories. This region is also referred to as parahippocampal place area3 (PPA). Character-selective regions were defined as above-threshold clusters on the OTS that responded more strongly to words and numbers vs. all other categories. Similarly, a body-part-selective region was defined as above-threshold clusters on the OTS that responded more strongly to bodies and limbs than the other categories.
Emerging and waning ROIs
To characterize the selectivity and responses within VTC regions that changed with development, we defined in each participant’s VTC ROIs that: (i) either gained selectivity to faces or words or (ii) lost selectivity to limbs during childhood. To do so, we defined emerging face and word ROIs as well as waning limb ROIs. For words and faces, where selective voxels emerge during development, the emerging ROI is the region that was selective to faces (or words) in the end timepoint but was not selective to that category in the initial timepoint (Fig. 2A,C). We call the region that is the difference between the end and initial ROIs the emerging pOTS-words and emerging pFus-faces, respectively. For limbs, where selectivity declines during development, we call the difference between the initial and end ROIs the waning OTS-limbs. That is, the waning OTS-limbs is the region that was within the ROI in the initial timepoint but not in the end timepoint (Fig. 2B). For a given participant, the initial ROI corresponds to the first fMRI session in which the ROI could be identified, and end ROI corresponds to the last fMRI session in which the ROI could be identified.
Control analysis in independent ring-shaped ROIs
The emerging and waning ROIs were defined from individual participant’s functional ROIs on their native brain anatomy and thus capture precisely the part of cortex that undergoes development. As these ROIs are not completely independent from the original ROIs, we sought to validate these results in an independent manner. Thus, we conducted a complementary analysis to evaluate responses in independent ring ROIs. First, we created two disk ROIs centered at the center coordinate of the initial functional ROI. One ROI was sized to match the surface area of the initial ROI, and the other, sized to match the corresponding end ROI. Then, we defined the area in between these two disk ROIs as the independent ring-shaped ROI.
Within each ring-shaped ROI, we measured responses to the 10 categories as well as selectivity to each category (Supplementary Fig. 10). Importantly, this analysis replicated the significant decrease in limb-selectivity in the waning limb-ROIs, emerging left pOTS-words, and emerging left pFus-faces. In right pFus-faces, we found a similar trend for decreasing limb-selectivity (see figure legend of Supplementary Fig. 10 for statistics). In left pOTS-words, we find a similar trend for increasing word-selectivity (Supplementary Fig. 10A) and in right pFus-faces we find a similar trend for increasing face-selectivity (Supplementary Fig. 10D,E). We note that a limitation of this approach is that while the ring analysis guarantees independence, it does not capture exactly the developing tissue, as the actual developing ROIs are not ring shaped (Fig. 2A–C, see example ROIs). Therefore, this approach is less accurate for assessing developmental changes in VTC.
Control analysis in a constant number of lateral VTC voxels
In the analyses in Fig. 1, we evaluated category-selectivity by estimating the number of voxels within an anatomical lateral VTC ROI that significantly responded to one category (vs. all other categories except the other category from the same domain, threshold of t>3, voxel-level). While this threshold guarantees significant selectivity, ultimately the threshold value is a number decided by the experimenter.
To ensure that findings of developmental effects do not depend on the threshold used, we performed a complementary analysis of category-selectivity in lateral VTC across development which did not depend on a threshold. Across development, we kept the number of voxels constant and evaluated their mean selectivity (t-value) to a category. Specifically, at each timepoint we selected the 20% most selective voxels (i.e., the voxels with the highest t-values) for each category contrast in lateral VTC and calculated their mean t-value (Supplementary Fig. 5). The 20% most selective voxels are determined in each session independently, to avoid biasing the selection of voxels to a specific timepoint. Then, we used LMMs to determine if the mean selectivity of these voxels changes over time. LMMs can be expressed as: t-value ~ age in months + (1|participant).
Results of this analysis largely replicate the main finding presented in Fig. 1C, and reveal: (i) a significant increase in word-selectivity in the left hemisphere (see legend of Supplementary Fig. 5 for statistics), (ii) a bilateral decrease in limb-selectivity, (iii) an increase in selectivity to adult faces in the right hemisphere, (iv) a bilateral increase in selectivity to child faces, and (v) an increase in selectivity to houses in the left hemisphere.
Control analysis on response reliability in V1
To assess the reliability of responses, we conducted a multivariate pattern analysis (MVPA62) in V1 ROIs defined by the Glasser atlas63. For each voxel, the response amplitude (beta value) for each category was estimated from the GLM of each run. These vectors of responses - also called multivoxel patterns (MVPs) - were generated independently for each of the two runs in each session. We then calculated correlations between pairs of MVPs (run1 to run2) to each category combination, resulting in 10x10 representational similarity matrices. We calculated the mean of reliability across all categories (mean of the on-diagonal correlations) as a measure for the reliability of responses, as it provides a measurement for the similarity of responses to the same category from one functional run to another. Next, we tested if there is a relationship between the reliability of responses and age. To this end, we ran linear mixed models predicting the response reliability by age using random intercept models with the grouping variable subject (response reliability ~age + (1|subject)). Results of this analysis are shown in Supplementary Fig. 7.
Is the decrease in limb-selectivity uniform in lateral VTC?
As we found accumulating evidence for reductions in limb-selectivity in lateral VTC, an open question is whether the decrease in limb-selectivity occurs across the entire lateral VTC or whether it is restricted to the regions in which selectivity changes across development. To this end, we aimed to assess development of limb-selectivity in VTC excluding voxels that were selective to categories showing significant development (Fig. 1). Thus, we identified in each participant’s first session the voxels selective for these categories, excluded them from lateral VTC, and then measured limb-selectivity across development in the remaining lateral VTC voxels. Next, we used LMMs to test if there was a significant decrease in the mean selectivity to limbs in these remaining lateral VTC voxels (Supplementary Fig. 12). While there was a trend for a decrease in selectivity in both hemispheres, the effects were not significant after FDR correction (left: slope=−0.0039 t/month, pFDR=0.16; right: slope=−0.0036 t/month, pFDR=0.16, FDR corrected).
Can cortical recycling be measured at the voxel level?
To assess if cortical recycling is also observable at the voxel level, we measured the selectivity for limbs, faces, and words in each session for each voxel of the waning and emerging parts of category-selective ROIs. Using this data, we examined the relationship between the voxel selectivity to the category that defines the ROI as a function of voxel selectivity to the two other categories (i.e., in emerging pOTS-words we related word-selectivity to limb- and face-selectivity: word-selectivity ~limb-selectivity + face-selectivity +(1|session). To quantify the relationship, we ran a separate linear mixed model (LMM) for each participant and ROI. Thus, the number of independent measurements in each LMM is the number of voxels in the emerging/waning ROI and the grouping variable in the LMM is ‘session’. We then summarized the individual subject slopes from the LMM for each ROI in a boxplot (Supplementary Fig. 13)
Statistical Analyses
Linear mixed models (LMMs) were used for statistical analyses because: (i) the data has a hierarchical structure with sessions being nested within each participant, and (ii) sessions were unevenly distributed across time (Supplementary Fig. 1A). Models were fitted using the ‘fitlme’ function in MATLAB version 2017b (The MathWorks, Inc.). In initial analyses the fit of random-intercept models, which allow intercepts to vary across participants, were compared with the fit of random-slope models, which allow both intercepts and slopes to vary across participants. Results revealed that a random-intercept model fitted the data best in the majority of cases. Thus, to enable comparability across analyses, LMMs with random intercepts were used throughout the analyses presented here. Due to the relatively large number of samples, data distribution was assumed to be normal, but this was not formally tested.
LMMs related to Figure 1 can be expressed as: volume of selective activation for a category in mm3 ~ age in months + (1|participant), in which volume of selective activation is the response variable, age in months is a continuous predictor (fixed effect), and the term (1|participant) indicates that random intercepts are used for the grouping variable participant. The slopes of the LMMs are plotted in Fig. 1C. Statistics were run on the complete data set including 128 sessions. Boxplots in Fig. 1D showing volume of selective activation in different age groups based on a subset of the data are used for visualization purposes only, and not to evaluate statistical significance. One session per child per age group is included in the boxplot. For 5-9-yo, each participant’s first session was included and for 13-17-yo, each participant’s last session was included. To confirm the validity of the choice of these age groups for the boxplots in Fig. 1D (5-9yo and 13-17yo), we used the LMMs (shown in Fig. 1C) to predict the size of category-selective activation for the mean age in years of the participants in each of the two age groups. This predicted size is indicated as a black diamond in the boxplots. Estimated mean size (diamonds) from the LMM corresponded well with the medians of the boxplots, thus, validating the grouping of the participants in the boxplots.
To estimate changes in the size of category- and domain-selective ROIs (Fig. 2D, Supplementary Fig. 9) we used LMMs specified as: ROI size in mm3 ~ age in months + (1|participant). Similarly, to estimate changes in the surface area of category-selective ROIs (Supplementary Fig. 8) we used LMMs specified as: ROI surface area in mm2 ~ age in months + (1|participant).
To evaluate significance of the developmental changes in emerging and waning ROIs, we used two separate LMMs: (i) selectivity changes across development (Fig. 3-left) were modeled as t-value ~ age in months + (1|participant), and (ii) changes in responses across development (Fig. 3-right) as % signal (β)~ age in months + (1|participant). Percent signal refers to the change in response for a certain condition relative to a blank baseline. Selectivity refers to contrasting responses to different categories (see above definition of contrasts and thresholds). Selectivity and response values were obtained for each voxel and LMMs were fit on the average value in an ROI. Boxplots in Fig. 3 show mean responses for each of the 10 categories in 5-9-year-olds and 13-17-year-olds. One session per child per age group is included in the boxplot. For 5-9-yo, each participant’s first session was included and for 13-17-yo, each participant’s last session was included. Boxplots are used for visualizing response amplitudes (units of % signal) in different age groups, but are not used to evaluate statistical significance, which was done using the LMM. The selection of age groups in the boxplots is validated by plotting the LMM’s prediction of the parameter of interest for the mean age of participants in each age group (compare black diamonds to measured median).
LMM analyses related to Fig. 4 tested if changes in limb-, face-, and word-selectivity in the developing ROIs were significantly related to each other. For each emerging and waning ROI, we tested if selectivity for one category (e.g., word-selectivity in the emerging pOTS-words) was predicted by selectivity to the other categories (e.g., limb- and face-selectivity in the emerging pOTS-words). We also tested if an LMM with two predictors (e.g., predicting word-selectivity from both limb- and face-selectivity) is a better model than an LMM with one predictor (e.g., predicting word-selectivity just from face-selectivity) using a likelihood ratio test. If the likelihood ratio test confirmed that both predictors contributed significantly to the model fit, both predictors were included in the analysis. Parameters of LMMs and likelihood ratio tests are reported in Supplementary Tables 12–14. Tables are grouped by analysis type.
We also performed an analysis in which we directly evaluated the pairwise contrasts between words, faces, and limbs in the developing ROIs for each child and session. For instance, in the emerging pOTS-words, we directly contrasted (i) words vs. faces, and (ii) words vs. limbs. We used LMMs to test whether and how a region’s preference for one category over the other changes with age. For each emerging and waning ROI and each contrast we ran a LMM of the format: t-value ~ age in months + (1|participant). Left hemisphere data are presented in Fig. 5, right hemisphere data are presented in Extended Data Fig. 4, and full statistics are in Supplementary Table 15.
The reported statistical tests are two-tailed. False-discovery rate (FDR) correction following the procedure by Benjamini and Hochberg20 as implemented in MATLAB version 2017b (The MathWorks, Inc.) was applied to correct the statistical significance taking into account multiple comparisons related to each analysis.
Extended Data
Extended Data Figure 1. Control analyses examining developmental increases and decreases in category-selective activation in lateral VTC.
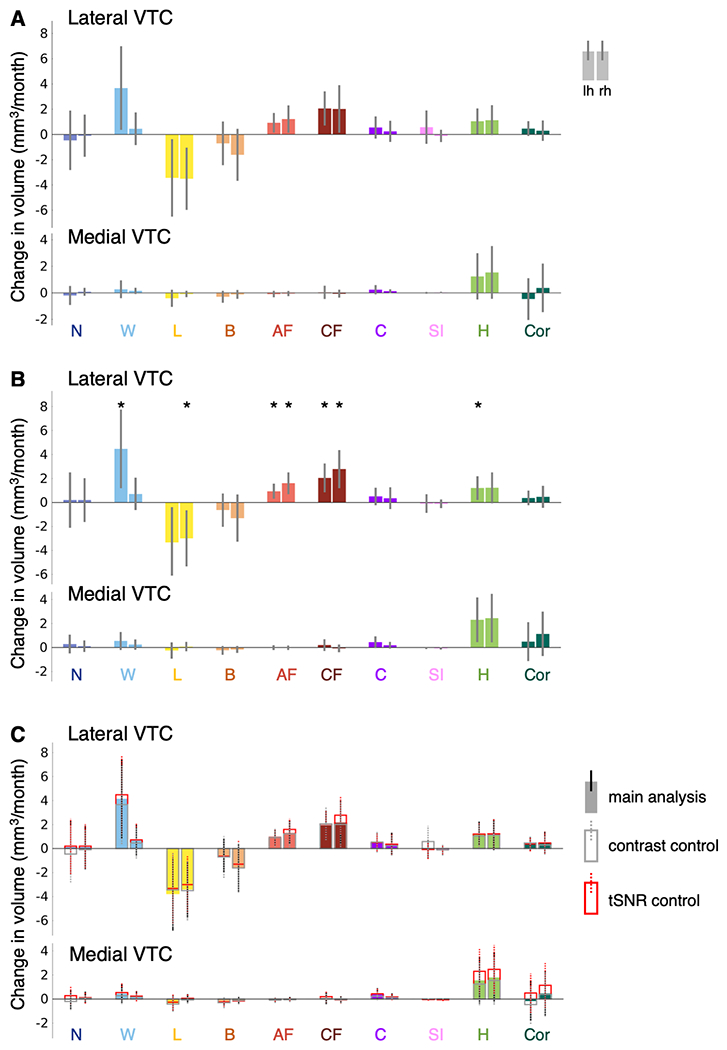
LMM slopes indicating change in category-selective activation volume per month (n=128 sessions, 29 children). Error bars: 95% CI. If the CI does not cross the y=0 line, this indicates that the slope is significantly different than 0 (before FDR-correction). (A) Slopes for the age predictor for models in which adult faces, child faces, limbs, and words are excluded as control categories from the contrast. No effects survive FDR-correction. (B) Slopes for the age predictor for models including both age and time series signal-to-noise ratio (tSNR) as predictors. Significant development after FDR-correction (p<0.05) is indicated by asterisks. (C) Slopes for the main analysis (filled bars), the contrast control (open bars with gray outline) and the tSNR control (open bars with red outline) are overlaid to illustrate the changes in effect size across the different analyses. Full statistics in Supplementary Tables 3–4,6–7. Related to Fig. 1.
Extended Data Figure 2. Control analyses examining functional changes underlying the development of category-selective ROIs.
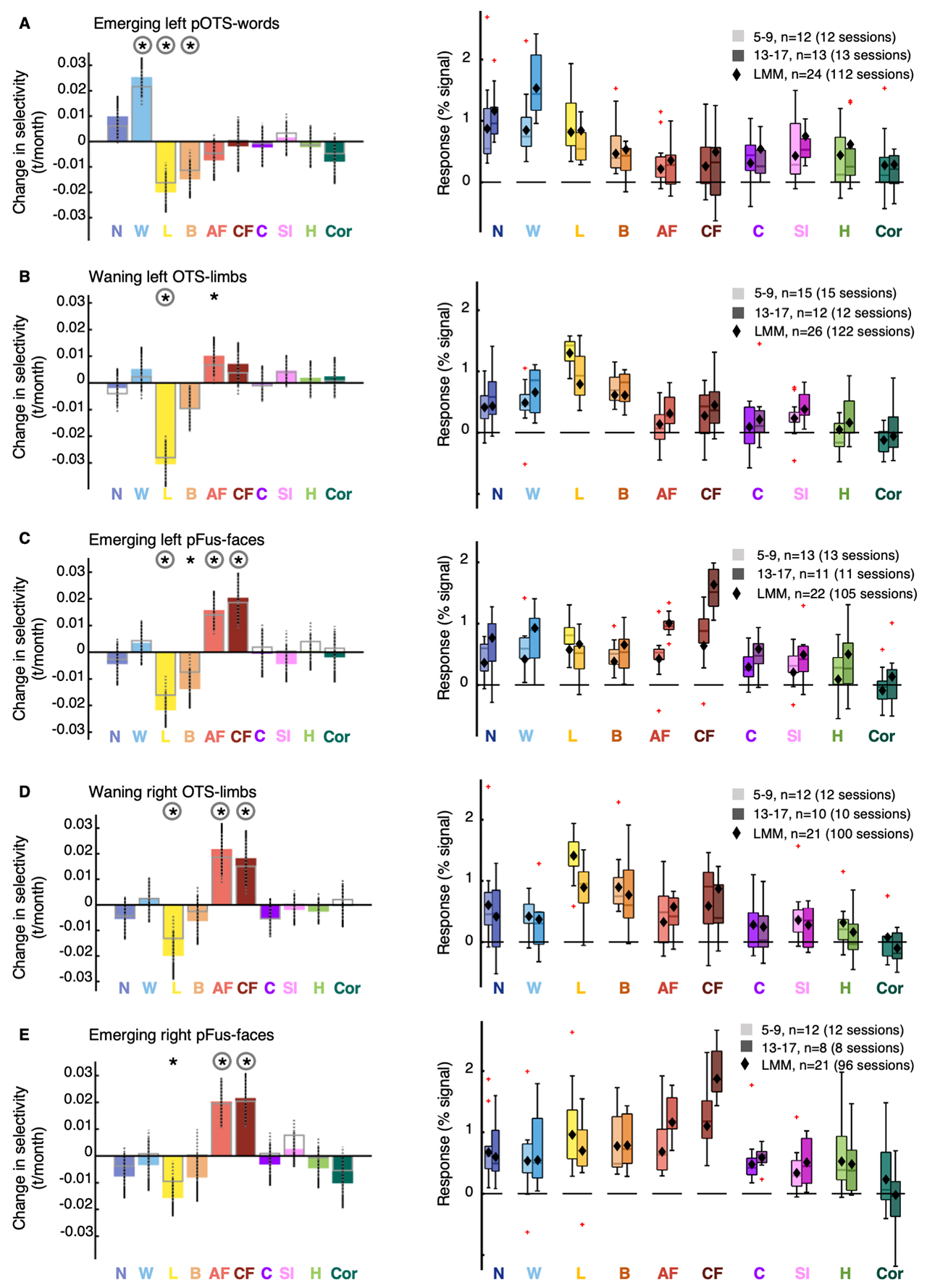
Left panel: Colored bars: Slopes of LMMs indicating changes in selectivity by age for all 10 categories in emerging and waning ROIs. Open bars with gray outline: LMM slopes for contrasts in which adult faces, child faces, limbs, and words are excluded as control categories in contrasts. Error bars: 95% CI. If the CI does not cross the y=0 line, this indicates that the slope is significantly different than 0 (before FDR-correction). Asterisks: significant development after FDR-correction (p<0.05) for colored bars, circles: significant after FDR-correction for open bars. Right panel: Response amplitudes for 5-9-year-olds and 13-17-year-olds. Lighter colors indicate younger ages. One functional session per child is included per boxplot. Boxplots show the 75% and 25% percentiles (colored areas) and median (horizontal lines). Whiskers extend to the most extreme data points not considered outliers (minimum, maximum). Crosses: outliers (values more than 1.5 times the interquartile range away from the bottom or top of the box). Black diamonds: LMM prediction for the response at the mean age of each age group. (A) Left emerging pOTS-words. Left panel: n=24 (112 sessions). (B) Left waning OTS-limbs. n=26, 122 sessions. (C) Left emerging pFus-faces. n=22, 105 sessions. (D) Right waning OTS-limbs. n=21, 100 sessions. (E) Right emerging pFus-faces. n=21, 96 sessions. Full statistics in Supplementary Tables 9–10. As we observed a significant decrease in limb-selectivity in emerging parts of word- and face-selective regions and word-, face- and limb-selective regions neighbor, we tested if emerging parts of word- and face-selective regions overlap with waning parts of the limb-selective regions. However, the overlap between the developing parts of the ROIs (difference between initial and end ROIs) assessed by the dice coefficient (DC) was small (overlap between developing parts of OTS-limbs and pFus-faces, left: DC=0.025±0.01 (mean± SD), n=22; right: DC=0.026±0.01, n=18; overlap between developing parts of left OTS-limbs and pOTS-words: DC=0.006±SD=0.004, n=21). Related to Fig 3.
Extended Data Figure 3. Developmental changes in word-, face-, and limb-selectivity are also linked in the right hemisphere.
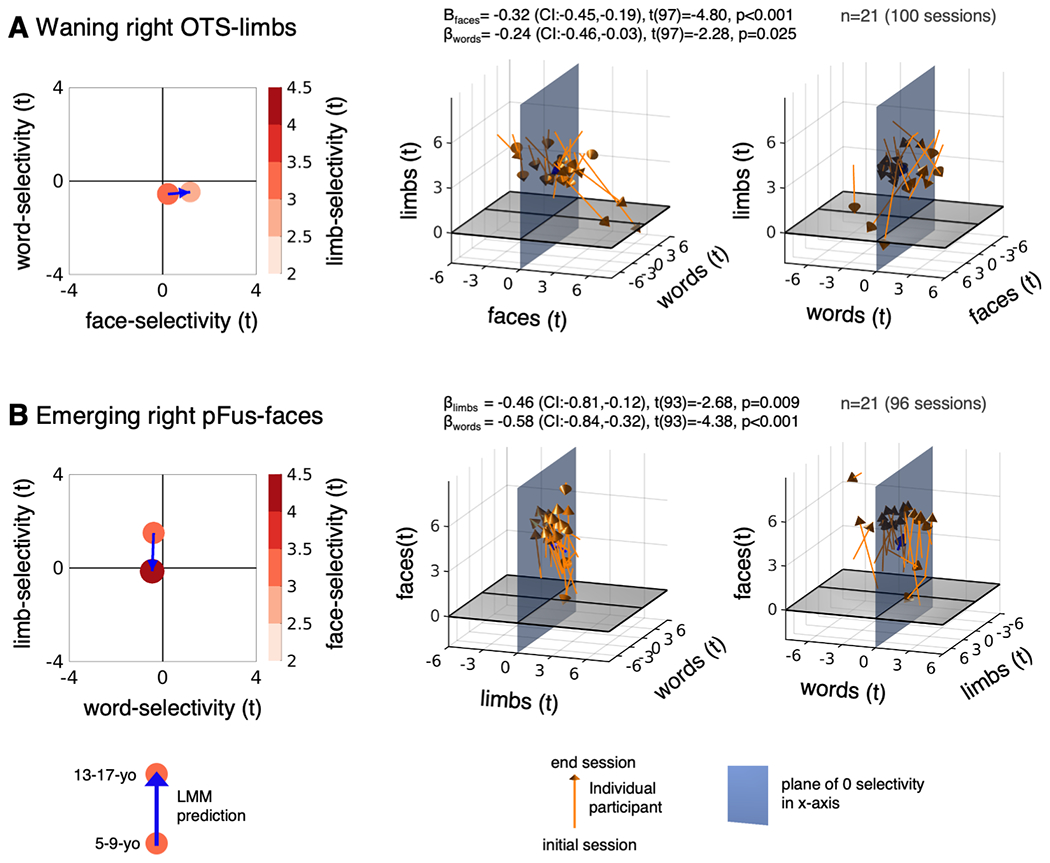
(A) Limb-selectivity vs face- and word-selectivity in the waning right OTS-limbs. (B) Face-selectivity vs. limb- and word-selectivity in the emerging right pFus faces. Left: Model prediction for 5-9-year-olds and 13-17-year-olds for the selectivity that defines the ROI as a function of the selectivity to the other two variables. Middle: Individual participant data visualized in 3D. In each panel the variable on the z-axis is related to the x- and y-variables. LMM βs, 95%-CIs, t-values, df, and p-values are shown on top. Full statistics are reported in Table S11. Orange arrows: Individual child data. Blue arrows: LMM, same as left panel. Right: Rotated version of the plots in the middle column to increase visibility of changes along the horizontal axes. Related to Fig. 4.
Extended Data Figure 4. Pairwise preferences of the ROI-defining category in the right hemisphere.
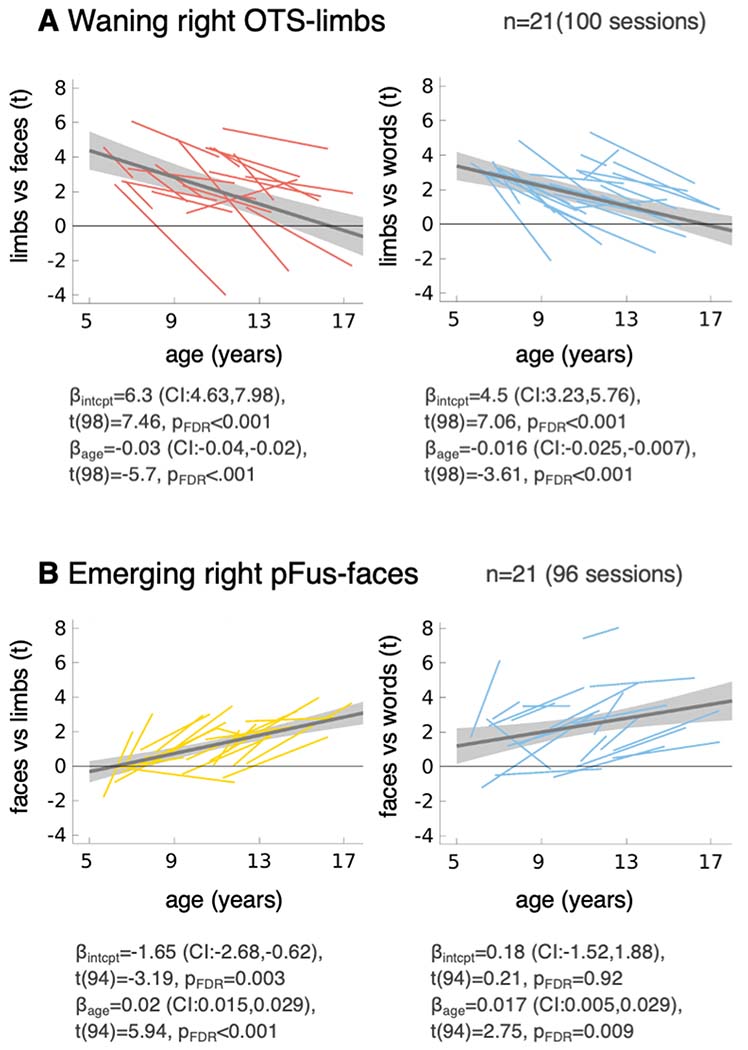
In each plot we show the pairwise preference of the ROI-defining category vs. each of the other two developing categories as a function of age. Thin lines: individual participant data showing the pairwise preference from the initial to end session. Gray line: LMM prediction of pairwise preference based on data from all sessions. Shaded gray: 95%-CI. LMM results (intercept: βintercept and slope: βage (rate of change in preference, t/month) and their significance are reported under each panel. (A) Waning right OTS-limbs (n=21 subjects, n=100 sessions). Left: Limbs vs faces. Right: Limbs vs Words. (B) Emerging right pFus-faces (n=21 subjects, n=96 sessions). Left: Faces vs limbs. Right: Faces vs Words. Related to Fig. 5. Statistics in Supplementary Table 15.
Supplementary Material
Acknowledgements
We thank Laura Villalobos, Erica Yeawon Hwang, Savana Huskins, Alema Fitisemanu, and Philip Eykamp for manually editing gray-white matter brain segmentations. We thank Caitlyn Estrada and Nancy Lopez-Alvarez for help with data collection, and Rachel Hinds for help with data entry and management. We thank Jon Winawer for his constructive review of our manuscript.
Funding:
A fellowship of the German National Academic Foundation NO 1448/1-1 (MN); NIH grant 2RO1 EY 022318 (KGS); NIH training grant 5T32EY020485 (VSN); NSF Graduate Research Development Program DGE-114747 (JG); Ruth L. Kirschstein National Research Service Award F31EY027201 (JG). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Code availability
Code is available at: https://github.com/VPNL/corticalRecycling.
Competing interests
The authors declare no competing interests.
Data availability
The data required to generate the main figures are available in the GitHub repository (see code availability). Due to the large size of the raw data, it will be made available from the corresponding author upon request.
References
- 1.Kanwisher N, McDermott J & Chun MM The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17, 4302–4311 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peelen MV & Downing PE Selectivity for the human body in the fusiform gyrus. J. Neurophysiol 93, 603–608 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Epstein R & Kanwisher N A cortical representation of the local visual environment. Nature 392, 598–601 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Cohen L et al. The visual word form area. Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123, 291–307 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Deen B et al. Organization of high-level visual cortex in human infants. Nat. Commun 8, 13995 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Heering A & Rossion B Rapid categorization of natural face images in the infant right hemisphere. Elife 4, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingstone MS et al. Development of the macaque face-patch system. Nat. Commun 8, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golarai G et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci 10, 512–522 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehaene-Lambertz G, Monzalvo K & Dehaene S The emergence of the visual word form: Longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLoS Biol. 16, 1–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srihasam K, Vincent JL & Livingstone MS Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat. Neurosci 17, 1776–1783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez J, Natu V, Jeska B, Barnett M & Grill-Spector K Development differentially sculpts receptive fields across early and high-level human visual cortex. Nat. Commun 9, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordt M et al. Learning to Read Increases the Informativeness of Distributed Ventral Temporal Responses. Cereb. Cortex 1–16 (2019). doi: 10.1093/cercor/bhy178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrmann M & Plaut DC A vision of graded hemispheric specialization. Ann. N. Y. Acad. Sci 1359, 30–46 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Dehaene S, Cohen L, Morais J & Kolinsky R Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci 16, 234–244 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Levy I, Hasson U, Avidan G, Hendler T & Malach R Center-periphery organization of human object areas. Nat Neurosci 4, 533–539 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Cantlon JF, Pinel P, Dehaene S & Pelphrey KA Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex 21, 191–199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehaene S et al. How learning to read changes cortical networks for vision and language. Science 1359, 1359–64 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Frost MA & Goebel R Measuring structural-functional correspondence: Spatial variability of specialised brain regions after macro-anatomical alignment. Neuroimage 59, 1369–1381 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Weiner KS et al. The mid-fusiform sulcus: A landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage 84, 453–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y & Hochberg Y Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 57, 289–3300 (1995). [Google Scholar]
- 21.Scherf KS, Behrmann M, Humphreys K & Luna B Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci 10, (2007). [DOI] [PubMed] [Google Scholar]
- 22.Peelen MV, Glaser B, Vuilleumier P & Eliez S Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev. Sci 12, 16–25 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Golarai G, Liberman A, Yoon JM & Grill-Spector K Differential development of the ventral visual cortex extends through adolescence. Front. Hum. Neurosci 3, 80 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobatake E, Wang G & Tanaka K Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J. Neurophysiol 80, 324–330 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Arcaro MJ, Schade PF, Vincent JL, Ponce CR & Livingstone MS Seeing faces is necessary for face-domain formation. Nat. Neurosci 20, 1404–1412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fausey CM, Jayaraman S & Smith LB From faces to hands: Changing visual input in the first two years. Cognition 152, 101–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank MC, Vul E & Saxe R Measuring the Development of Social Attention Using Free-Viewing. Infancy 17, 355–375 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Long B, Kachergis G, Agrawal K, & Frank MC Detecting social information in a dense database of infants’ natural visual experience. (2020). [Google Scholar]
- 29.Liszkowski U, Carpenter M & Tomasello M Pointing out new news, old news, and absent referents at 12 months of age. Dev. Sci 10, 1–7 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Haber N, Mrowca D, Wang S, Fei-Fei L & Yamins DLK Learning to play with intrinsically-motivated, self-aware agents. Adv. Neural Inf. Process. Syst.2018-Decem, 8388–8399 (2018). [Google Scholar]
- 31.Khaligh-Razavi SM & Kriegeskorte N Deep Supervised, but Not Unsupervised, Models May Explain IT Cortical Representation. PLoS Comput. Biol 10, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang Chengxu; Zhai Alex Lin; Yamins D. Local Aggregation for Unsupervised Learning of Visual Embeddings. in Proceedings of the IEEE/CVF International Conference on Computer Vision (2019). [Google Scholar]
- 33.Gomez J et al. Microstructural proliferation in human cortex is coupled with the development of face processing. Science (80−.). 355, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natu VS et al. Development of Neural Sensitivity to Face Identity Correlates with Perceptual Discriminability. J. Neurosci 36, 10893–10907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wandell BA, Rauschecker AM & Yeatman JD Learning to see words. Annu. Rev. Psychol 63, 31–53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Shachar M, Dougherty RF, Deutsch GK & Wandell BA The Development of Cortical Sensitivity to Visual Word Forms. J. Cogn. Neurosci 23, 2387–2399 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldstein Ewing SW, Bjork JM & Luciana M Implications of the ABCD study for developmental neuroscience. Dev. Cogn. Neurosci 32, 161–164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constantino JN et al. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature 547, 340–344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duchaine BC & Nakayama K Developmental prosopagnosia: a window to content-specific face processing. Curr. Opin. Neurobiol 16, 166–173 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Amedi A, Raz N, Pianka P, Malach R & Zohary E Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci 6, 758–766 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Liu TT et al. Successful Reorganization of Category-Selective Visual Cortex following Occipito-temporal Lobectomy in Childhood. Cell Rep. 24, 1113–1122.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norton ES, Beach SD & Gabrieli JDE Neurobiology of dyslexia. Curr. Opin. Neurobiol 30, 73–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srihasam K, Mandeville JB, Morocz IA, Sullivan KJ & Livingstone MS Behavioral and Anatomical Consequences of Early versus Late Symbol Training in Macaques. Neuron 73, 608–619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Büchel C, Price C & Friston K A multimodal language region in the ventral visual pathway. Nature 394, 274–277 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Emmorey K, McCullough S & Weisberg J Neural correlates of fingerspelling, text, and sign processing in deaf American sign language–English bilinguals. Lang. Cogn. Neurosci 30, 749–767 (2015). [Google Scholar]
- 46.Bi Y, Wang X & Caramazza A Object Domain and Modality in the Ventral Visual Pathway. Trends Cogn. Sci 20, 282–290 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Reich L, Szwed M, Cohen L & Amedi A A ventral visual stream reading center independent of visual experience. Curr. Biol 21, 363–368 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Dehaene S & Cohen L Cultural recycling of cortical maps. Neuron 56, 384–398 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Lucas TH, McKhann GM & Ojemann GA Functional separation of languages in the bilingual brain: A comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J. Neurosurg 101, 449–457 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Green DW, Crinion J & Price CJ Convergence, Degeneracy, and Control. Lang. Learn 56, 99–125 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amalric M & Dehaene S Origins of the brain networks for advanced mathematics in expert mathematicians. Proc. Natl. Acad. Sci 113, 4909 LP–4917 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kersey AJ & Cantlon JF Neural Tuning to Numerosity Relates to Perceptual Tuning in 3–6-Year-Old Children. J. Neurosci 37, 512 LP–522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pica P, Lemer C, Izard V & Dehaene S Exact and approximate arithmetic in an Amazonian indigene group. Science (80-.). 306, 499–503 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Natu VS et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc. Natl. Acad. Sci. U. S. A 116, 20750–20759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezer A et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat. Med 19, 1667–1672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grill-Spector K & Kanwisher N Visual recognition: as soon as you know it is there, you know what it is. Psychol Sci 16, 152–160 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Weiner KS & Grill-Spector K Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage 52, 1559–1573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiner KS, Sayres R, Vinberg J & Grill-Spector K fMRI-adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J. Neurophysiol 103, 3349–3365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stigliani A, Weiner KS & Grill-Spector K Temporal Processing Capacity in High-Level Visual Cortex Is Domain Specific. J. Neurosci 35, 12412–12424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanwisher N Domain specificity in face perception. Nat. Neurosci 3, 759–763 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Reuter M, Schmansky NJ, Rosas HD & Fischl B Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haxby JV et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science (80-.). 293, 2425–2430 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Glasser MF et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data required to generate the main figures are available in the GitHub repository (see code availability). Due to the large size of the raw data, it will be made available from the corresponding author upon request.


