Abstract
Opioids are the frontline analgesics in pain management. However, chronic use of opioid analgesics causes paradoxical pain that contributes to the decrease of their efficacy in pain control and the escalation of dose in long-term management of pain. The underling pathogenic mechanism is not well understood. Microglia have been commonly thought to play a critical role in the expression of opioid-induced hyperalgesia (OIH) in animal models. We performed microglial ablation experiments using either a genetic (CD11b-diphtheria toxin receptor transgenic mouse) or pharmacological (colony-stimulating factor 1 receptor inhibitor PLX5622) approaches. Surprisingly, ablating microglia using these specific and effective approaches did not cause detectable impairment in the expression of hyperalgesia induced by morphine. We confirmed this conclusion with behavioral test of mechanical and thermal hyperalgesia, in male and female mice, and with different species (mouse and rat). These findings raise caution about the widely assumed contribution of microglia to the development of OIH.
Introduction
Morphine and other opioid analgesics are the front-line medicine for managing severe pain[26]. The broad use of opioids for pain management may contribute to the current opioid epidemic, a sharp rise in the incidence of addiction and death by opioid overdose[44]. Clinical data indicate that chronic use of opioids in fact can paradoxically cause a pain state called opioid-induced hyperalgesia (OIH)[11]. The development of OIH is a critical factor driving for opioid dose escalation to effectively control pain. However, the dose escalation may eventually lead to potential overdose[3; 12]. Understanding the pathogenic mechanism of OIH is essential for the development of effective strategies to mitigate OIH and improve safety of opioid analgesics.
Neuronal malplasticity in the pain transmission and modulatory pathways has been considered as the main OIH mechanism. However, emerging evidence indicates a key contribution of glia[36]. Among different types of glia, microglia have been a focal point of interest[36]. Microglia are the major CNS resident immune cells, and become activated in the pain pathways of various models of pathological pain [10; 45]. In their reactive states, microglia can release pro-inflammatory cytokines to stimulate pain-processing neurons and facilitate the expression of central sensitization [10; 36]. In addition, they also play a critical role in structural alterations of pain neuronal circuits, as suggested by the recent studies on microglia-mediated synaptic degeneration in the spinal dorsal horn (SDH) induced by mouse models of HIV-associated pain [37].
Microglia are activated in the spinal cords of animals administered with opioids[13; 18; 24], and implicated in morphine-induced prolonging of neuropathic pain[19]. Both minocycline, a commonly used microglial inhibitor (but see Discussion), and anti-MAC1 (CD11B)-saporin, an antibody-conjugated toxin that targets microglia, impaired the expression of thermal hyperalgesia induced by morphine[18; 21]. In addition, conditional knockout (CKO) of BDNF induced by CD11b-Cre and CKO of Mu opioid receptor (MOR) driven by Cx3cr1-Cre were reported to impair thermal OIH[18; 34]. It was also reported that opioids simulated microglia via Toll-like receptor 4 (TLR4) [22], and activated microglia may promote OIH expression by releasing signaling molecules such as cytokines, BDNF and ATP[36]. These lines of evidence appear consistent with the popular conception that microglia support the development of OIH and are an attractive therapeutic target for the paradoxical pain.
However, glial expression of MOR remains controversial[13; 27; 30] (and refs cited therein). Studies by Corder et al. suggest that morphine acts through peripheral nociceptor but not microglia to induce OIH. They reported a significant increase in Iba1 after chronic morphine with no behavioral signs of OIH in the global MOR knockout line, which indicates a dissociation of morphine-induced microglial activation from MOR and OIH [13]. These findings evoked considerable discussion in the field about the contribution of microglia to OIH[39].
In this study, we determined the effect of microglial ablation by two specific approaches on the expression of OIH in mice or rats. Our results showed that these approaches did not cause significant impairment of mechanical and thermal hyperalgesia induced by repeated administration of morphine. These findings suggest that microglia are dispensable for OIH development.
Results
As microglia probably modulate pain pathogenesis specifically in males but not in females[31; 40], we first performed experiments with C57BL/6 male mice. We repeatedly administered the animals with morphine (20 mg/kg/day for 4 days; i.p.). Microglial activation in response to morphine treatment was evaluated by measuring the protein level of microglial marker Iba1. Similar to previous studies[14; 18; 33; 41], we observed Iba1 increase in the spinal cord of WT mice collected at day 7 (Fig. 1A). To determine the role of spinal microglia in the expression of OIH induced by morphine, we used the CD11b-diphtheria toxin receptor (DTR) transgenic mouse backcrossed to a C57BL/6 genetic background to ablate microglia[16]. Morphine induced microglial activation at a magnitude similar to that of WT mice (Fig. 1A; Fig. 1B). Administration of diphtheria toxin (DT) to the transgenic mice (0.8 μg/kg/day for 2 days, i.t.) at the beginning of morphine treatment abolished morphine-induced increase of Iba1 protein (Fig. 1B) and microglial cells in the SDH (Fig. 1C). These results suggest that the DT administration efficiently ablated activated microglia in DTR transgenic mice. It was interesting to observe that DT administration did not affect the up-regulation of GFAP induced by morphine exposure (Supp. Fig). This finding indicates that this genetic approach specifically ablates microglia and that the microglial ablation does not affect morphine-induced astrocyte reaction. To determine the temporal profile of OIH development, we monitored the expression of mechanical hyperalgesia by von Frey tests, induced by repeated morphine administration (20 mg/kg/day, i.p.) for 4 days in WT and DTR transgenic mice. We observed that mechanical hyperalgesia started to express following the first day of morphine administration, and peaked at day 5 (Fig. 2A). The OIH maintained even after morphine administration stopped (Fig. 2A). The DTR transgenic mice followed a temporal profile of OIH expression similar to that of WT mice (Fig. 2A; Fig. 2B), indicating the DTR transgenic mice develop OIH normally as WT mice.
FIGURE 1. Microglial ablation in DTR transgenic mice.
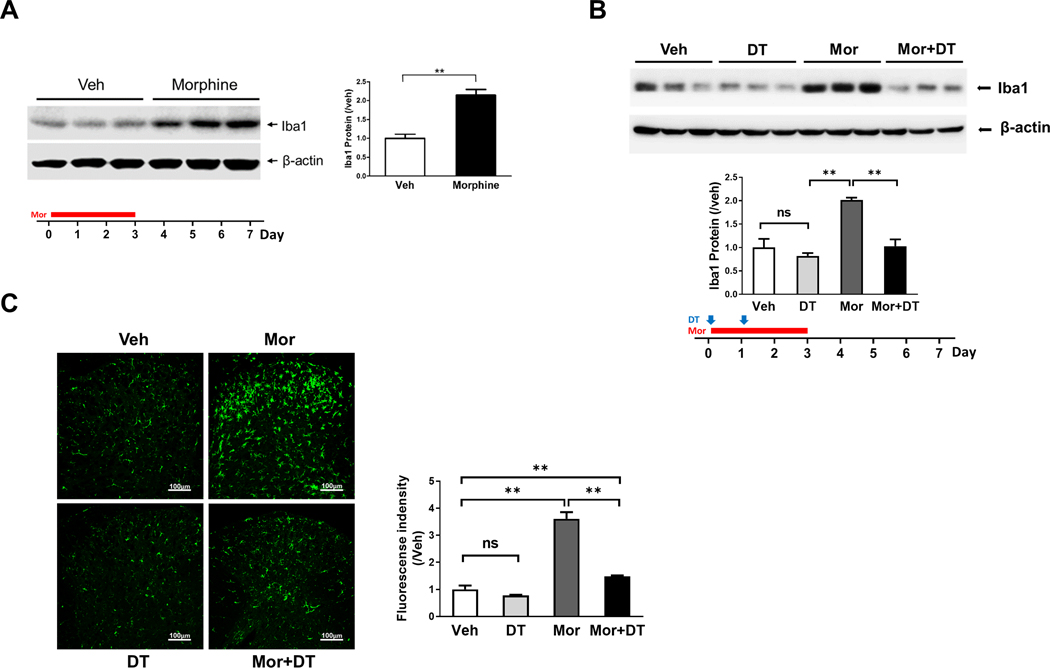
A. Immunoblotting analysis of Iba1 protein in the spinal cord of morphine (Mor)-treated WT (C57BL/6) mice (i.p. daily for first 4 days) at day 7 indicated in bottom paradigm (**, p<0.01). B. Immunoblotting analysis of Iba1 protein in the spinal cord of CD11b-DTR transgenic mice at day 7, treated by vehicle (Veh), morphine (Mor) or Mor+DT (diphtheria toxin) ( **, p <0.01). DT was injected (i.t.) daily at the first two days and morphine administration is similar to Fig.1A to evaluate the role of microglia in OIH expression. C. Immunofluorescent images of microglia in the spinal cord dorsal horn of the CD11b-DTR transgenic mice at day 7, treated by vehicle (Veh), DT, Mor) or Mor+DT.
FIGURE 2. Microglial ablation in DTR transgenic mice did not affect the expression and maintenance of mechanical OIH.
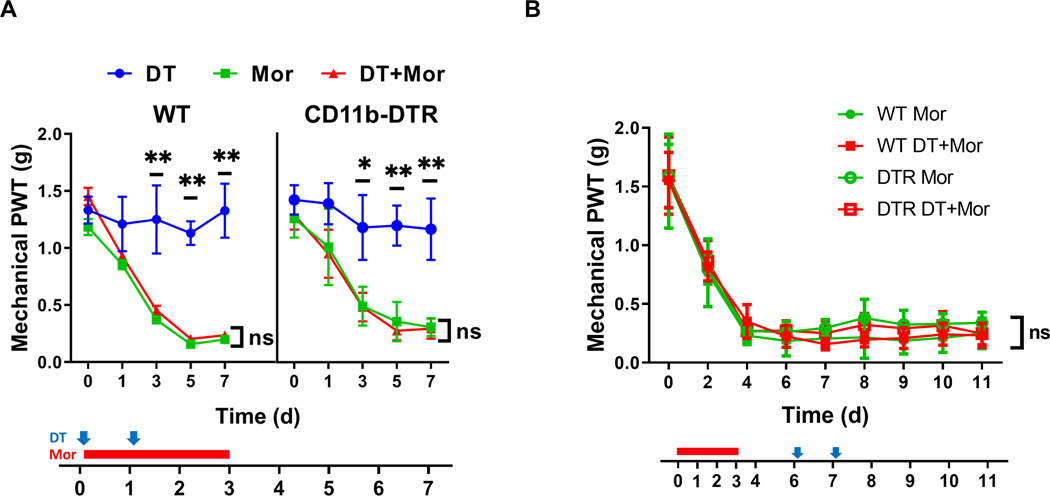
A. Effect of early DT administration on mechanical sensitivity of WT and CD11b-DTR transgenic mice with or without morphine (Mor) treatment. Von Frey tests were performed to measure the hind paw withdrawal threshold (PWT). Von Frey testing was performed 2 hours prior to morphine (i.p. daily for 4 days, indicated by the red bar) and DT (i.t.; indicated by blue arrows) treatment, to avoid the interference of potential acute analgesic effects of morphine administration. DT treatment of the DTR transgenic mice (red triangle) did not cause detectable effect on the expression of morphine-induced hypersensitivity compared with the OIH profiles of various control groups, including morphine-treated WT (red triangle) and DTR transgenic mice without DT (green square). N=6 mice/group; p>0.05 (DTR+DT+Mor vs. DTR+Mor, DTR+DT+Mor vs. WT+DT+Mor, or DTR+DT+Mor vs. WT+Mor); B. Effect of late DT administration on mechanical sensitivity of WT and CD11b-DTR transgenic mice with morphine (Mor) treatment. DT was injected (i.t.; indicated by blue arrows) after the completion of morphine administration (i.p. daily for 4 days, indicated by the red bar) to evaluate the role of microglia in OIH maintenance. No detectable effects on mechanical sensitivity were observed for DT-treated DTR transgenic mice (empty red square), compared with various control groups. N=6 animals/group; p>0.05 (DTR+DT+Mor vs. DTR+Mor, DTR+DT+Mor vs. WT+DT+Mor, or DTR+DT+Mor vs. WT+Mor).
To determine the effect of microglial ablation on the expression of mechanical OIH induced by morphine, we compared the temporal OIH expression profiles of WT and DTR transgenic mice following DT administration (0.8 μg/kg/day for 2 days, i.t.) (Fig. 2A). Interestingly, DT treatment did not cause a detectable change of OIH profiles of the transgenic mice (Fig. 2A),viz, the OIH expression profile of the transgenic mice treated with DT was not significantly different from that of the transgenic mice without DT treatment, similar to the OIH profiles of WT mice with or without DT treatment (Fig. 2A). These observations suggest that microglial ablation in the transgenic mice does not affect the expression of mechanical hyperalgesia induced by morphine.
To assess if microglia were involved in OIH maintenance, we performed additional experiments to determine the effect of DT administered after OIH was established. We observed that injection of DT at days 6 and 7 when OIH was established, had no detectable effect on the established OIH, compared with the control groups, including WT mice with or without DT administration and DTR transgenic mice without DT treatment (Fig. 2B). These data indicate that ablation of microglia in the transgenic mice does not affect the maintenance of OIH.
We were cautious about the results from the DTR transgenic mice because there were detectable microglia remaining after DT treatment (Fig. 1B, 1C). To validate the observations from DTR transgenic mice, we determined the effect of microglial depletion using a recently developed colony-stimulating factor 1 receptor (CSF1R) inhibitor PLX5622, which was reported to almost completely deplete microglia [17; 20; 23; 35], after three days of PLX5622 administration[1]. We confirmed that almost complete ablation of mouse spinal microglia was achieved within 5 days on PLX5622 diets (data not shown). To evaluate microglial contribution to OIH expression, we used a feeding paradigm with PLX5622-containing diets that started 5 days prior to morphine treatment and continued afterward. Spinal cords were collected after morphine administrations 20 mg/kg/day for 4 days; i.p.) to confirm microglial ablation. We did not detect Iba1 protein on Western blots, indicating nearly complete depletion of microglia (Fig. 3A). The nearly complete microglial depletion was confirmed by additional approaches, viz, fluorescent immunostaining (Fig. 3B), or flow cytometry (Fig.3C).
FIGURE 3. Microglial depletion by PLX5622.
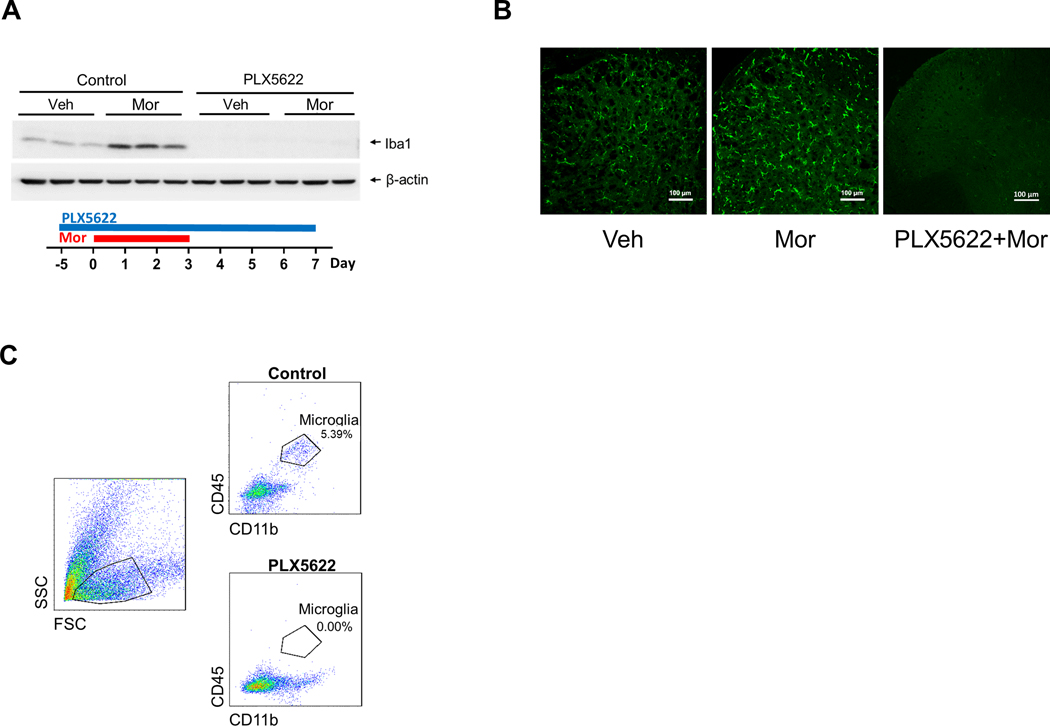
A-C. Microglial depletion in the spinal cord verified by immunoblotting (A), immunostaining (B) and flow cytometry analysis (C). A feeding paradigm with PLX5622-containing diets for mice (C57BL/6 males) was started 5 days prior to morphine treatment (one i.p./day for 4 days), and continued afterward. Spinal cords were collected from mice that were sacrificed at day 3 after last morphine injection, for immunoblotting (A, Iba1), immunostaining (B, Iba1) and flow cytometry analysis (C, microglia are identified as CD45-high and CD11b-high cells). All three approaches confirm the almost complete depletion of microglia in the spinal cord and the cortex (not shown), as indicated by the barely detectable Iba1 on Western blots (A), few of Iba1 positive cells on fluorescent images (B) and lack of microglia revealed by flow cytometry (C). D. Effect of microglial depletion on the expression of mechanical hyperalgesia induced by morphine in C57BL/6 males.
We performed von Frey tests to determine the OIH expression profiles in mice after PLX5622-mediated microglial depletion, and found no evident differences between the temporal profiles of mechanical OIH expressed by the WT mice (C57Bl/6) with or without PLX5622 treatment (Fig. 4A). We also observed that the microglial depletion did not alter mechanical sensitivity of mice without morphine treatment (Fig. 4A).
FIGURE 4. Microglial depletion by PLX5622 did not affect mechanical and thermal OIH Drug administration paradigms are indicated beneath the graphs.
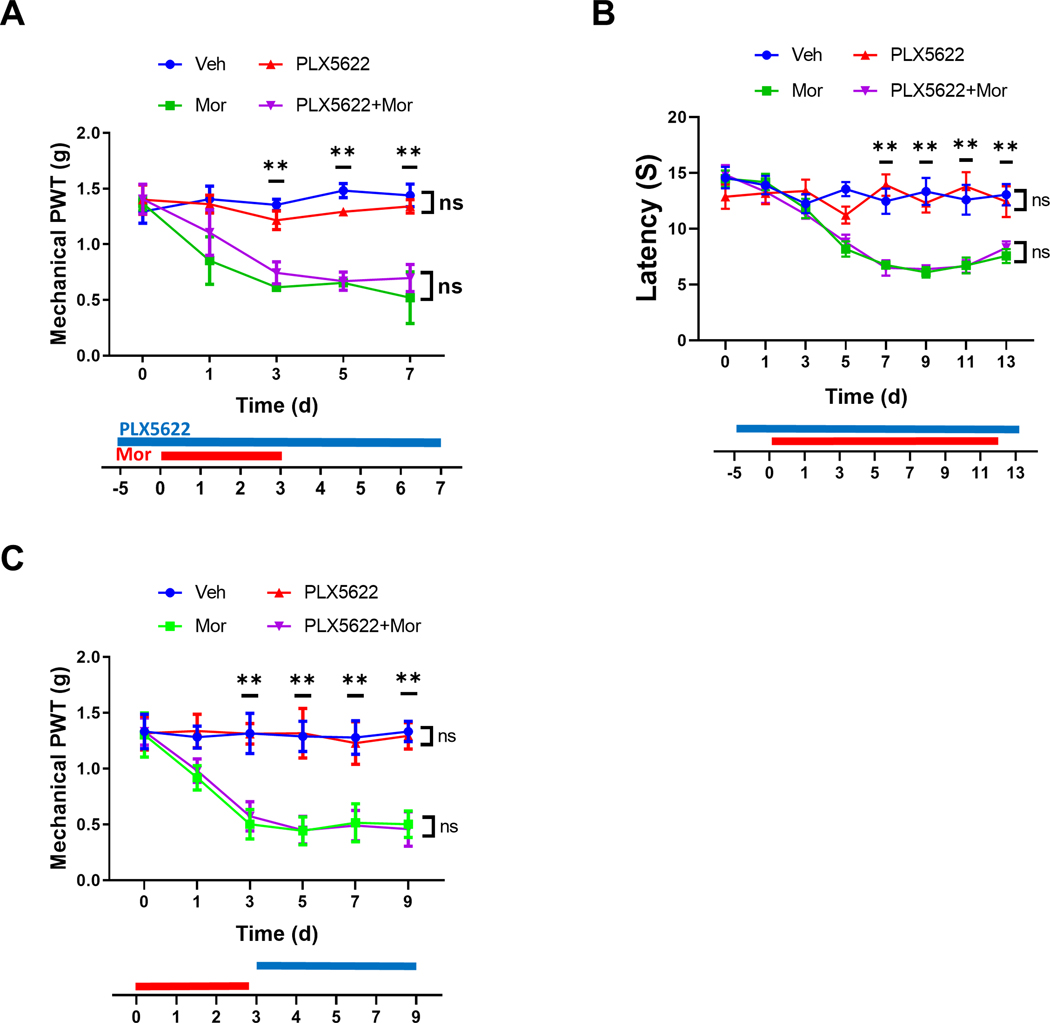
A. Effect of microglial depletion on the expression of mechanical OIH.Von Frey testing was performed to measure mechanical hyperalgesia induced by morphine treatment. PLX5622 administration did not impair mechanical OIH expressionN=6 male mice/group. B. Effect of microglial depletion on the expression of thermal OIH. Hot plate tests were performed to measure the latency of hindpaw withdrawal behaviors. PLX5622-mediated microglial depletion did not affect the thermal OIH expression. N=6 mice/group; p>0.05 for PLX6522+Mor vs. Mor and Veh vs. PLX6522. C. Effect of microglial depletion on mechanical OIH maintenance. Von Frey tests revealed that the PLX5622 administration did not affect maintenance of morphine-induced mechanical hypersensitivity. N=6 mice/group; p>0.05 for PLX6522+Mor vs. Mor and Veh vs. PLX6522 in Fig. 4A and 4C.
To evaluate the potential role of microglia in the development of thermal OIH, we performed experiments to measure paw withdrawal latency to thermal stimulation. Compared to the mechanical OIH, the thermal OIH developed slower. No significant thermal hyperalgesia was observed before day 7 (Fig. 4B), while significant mechanism hyperalgesia was observed at day 3 (Fig. 4A). In addition, we did not observe detectable differences between the temporal profiles of thermal OIH expression of the groups with or without PLX6522 administration (Fig. 4B). The microglial depletion induced by PLX5622 did not change thermal sensitivity of mice without morphine treatment (Fig. 4B).
To assess the potential role of microglia in OIH maintenance, we administered PLX5622 after mechanical OIH was established. Von Frey tests showed that PLX5622 had no detectable effect on mechanical OIH expressed in this experimental paradigm (Fig. 4C). Thus, the data collectively suggest that PLX5622, although almost completely depleted microglia, did not affect both expression and maintenance of mechanical OIH and the expression of thermal OIH. The data
Recent evidence suggests a sex dimorphism of microglial contribution to the pathogenesis of neuropathic pain, with a key role of microglia in male but not female rodents [31; 40]. However, the above results indicate that microglia in male mice are dispensable for the development of OIH induced by morphine. This surprising finding prompted us to test the effect of microglial ablation in females. Without PLX5622 treatment, males and females displayed indistinguishable temporal profile of mechanical OIH induced by morphine (Fig. 5A), indicating there is no detectable gender difference in the OIH expression. In addition, the female mice treated with PLX5622 developed mechanical OIH following a temporal profile that was similar to that of males treated with PLX5622 (Fig. 5A). We found no significant difference in OIH expressed between females treated with PLX5622 and without PLX5622 at day 0 (Fig. 5B) and day 7 (Fig. 5C). These findings indicate that microglia are dispensable for mechanical OIH in both males and females.
FIGURE 5. Microglial depletion did not affect the expression of mechanical OIH in female mice.
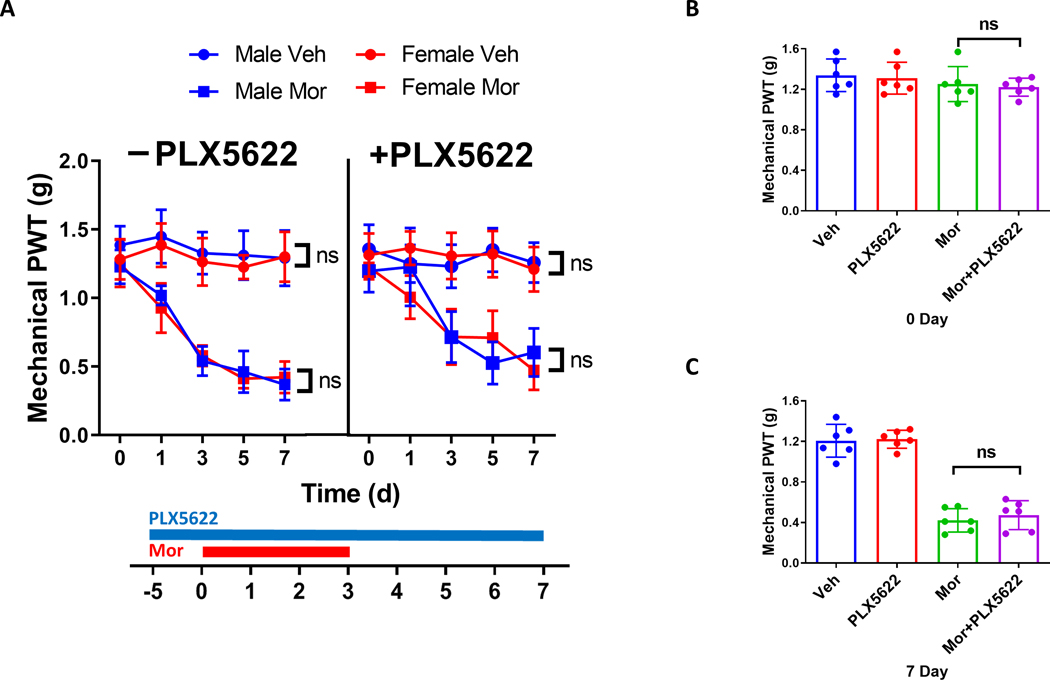
A. Comparison of the effect of PLX5622 administration on mechanical OIH in male and female mice. Von Frey tests showed that male and females displayed similar temporal expression profiles of mechanical OIH, with or without PLX5622 treatment. B, C. PLX5622 administration did not affect the expression of mechanical OIH in female mice at days 0 (B) and 7 (C). N=6 mice/group; p>0.05 for PLX6522+Mor vs. Mor and Veh vs. PLX6522.
To test whether the above finding sustains in other species, we also tested the effect of PLX6522 on rats (male, Sprague-Dawley). We observed that PLX5622 administration reversed microglial increase induced by morphine, although the ablation was not as complete as in mice (Fig.6A). Von Frey tests showed no detectable effect of PLX6522 on mechanical OIH induced in rats (Fig.6B).
FIGURE 6. Microglial ablation by PLX6522 did not affect mechanical OIH in rats.
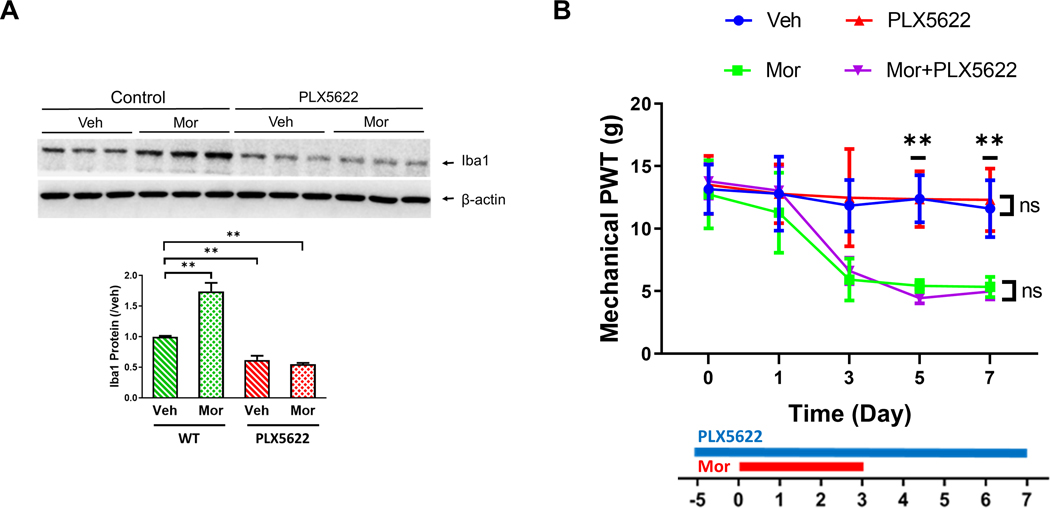
A. PLX6522-mediated microglial ablatin in rat spinal cords. Male rats (Sprague-Dawley, 180–200g) were on PLX6522-containing diets for 5 days before the start (day 0) of morphine administration (20mg/kg; i.p./daily for 4 days). Spinal cords were collected at day 7. Marked decrease of spinal microglia (as indicated by Iba1 levels) was induced by PLX6522. B. Effect of PLX6522-mediated microglial ablation on OIH expression in male rats. PLX5622 administration did not affect the development of morphine-induced mechnical hypersensitivity in rats, measured by Von Frey tests. N=6 male rats/group; p>0.05 for PLX6522+Mor vs. Mor and Veh vs. PLX6522.
Previous studies reported microglial activation induced by different morphine doses [12;17], raising the possibility that the dispensability of microglia in OIH we observed might be only for a specific microglial state activated by the morphine dosage we used. To evaluate this possibility, we induced OIH with escalating morphine doses, from 20 to 50mg/kg). Compared with mice treated with a constant dose of 20mg/kg, mice receiving the Escalating morphine doses developed heightened mechanical OIH (Fig. 7). Importantly, we observed that similar to the result of PLX5622 treatment on the former group of mice, the OIH development induced by the escalating morphine dosage displayed similar temporal profiles in mice with or without PLX5622 administration (Fig. 7).
FIGURE 7. Effects of microglial depletion on OIH expression induced by different dosaging paradigms of morphine administration.
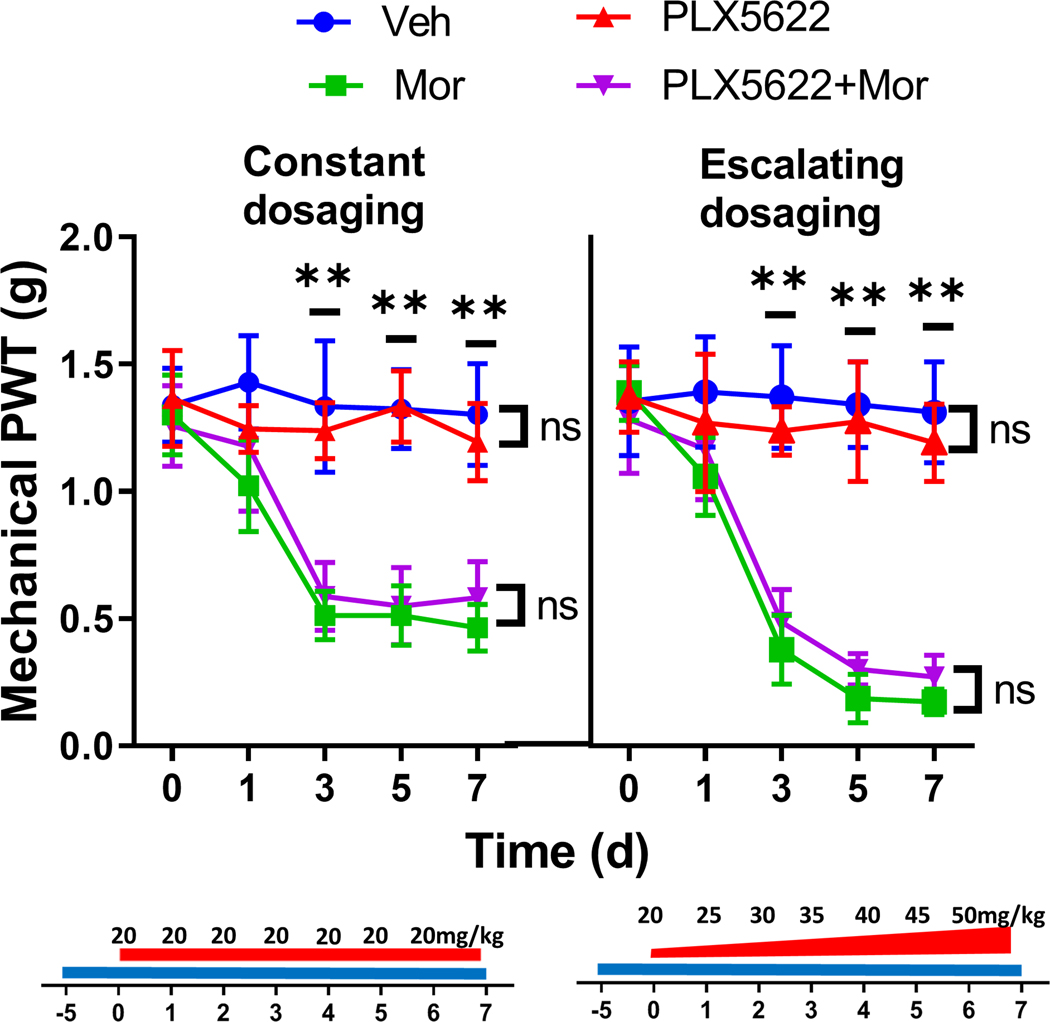
PLX5622 treatment did not change the temporal expression of mechanical OIH induced by either constant or escalating dosaging of morphine. Constant morphine dosaging (20mg/kg, i.p./day for 7 days) or escalating dosaging (from 20, 25, 30, 35, 40, 45, 50mg/kg/day) were used to induce OIH in C57BL/6 male mice. PLX5622 treatment started at 5 days before the first dose of morphine. (N=6 mice/group).
When we compared the results of tests on mechanical nociceptive sensitivity between control male mice received sham treatment with vehicles and the corresponding groups treated only for ablating microglia, by either genetic (Fig. 2A) or pharmacological approaches (Fig. 4A; Fig. 2C), we observed no detectable differences in nociceptive responses between the two groups (Fig. 2A; Fig. 4A; Fig. 4C). Similar results were observed for female mice (Fig. 5) and rats (Fig. 6B). We did not observe difference of thermal nociceptive sensitivity between the control and PLX6522-treated mice neither (Fig. 4B). Collectively, these data suggest microglial ablation did not affect the basal nociceptive responses.
Neuroinflammation is considered to play an important role in OIH expression [10; 36]. To assess the potential contribution of microglia to neuroinflammation in the OIH models, we determined the effect of microglial depletion on the expression levels of IL-1β and GFAP, which are commonly used to evaluate neuroinflammation. We found that PLX5622 treatment did not affect morphine-induced up-regulation of IL-1β and astrocyte activation (measured by GFAP levels) in mice (Fig. 8A). Similar results were obtained from rat OIH models (Fig. 8B). These data suggest that microglia are dispensable for neuroinflammation implicated in OIH expression.
FIGURE 8. Microglial ablation by PLX6522 did not affect morphine-induced astrocyte activation and IL-1β upregulationin mice and rats.
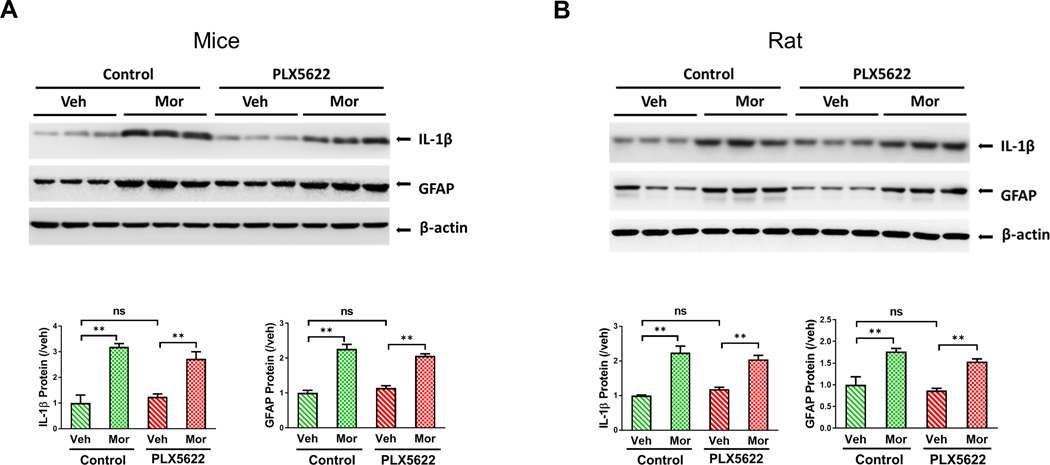
A. Immunoblotting analysis of IL-1β and GFAP protein in the spinal cord of PLX5622-feeding C57BL/6 mice(**, p <0.01). B. Immunoblotting analysis of IL-1β and GFAP protein in the spinal cord of PLX5622-feeding rats Spinal cords were collected at day 7 in the morphine administration paradigm shown in Fig. 3A. (**, p <0.01).
Discussion
We are interested in interrogating the potential role of microglia in OIH, by determine the effect of microglial ablation by different approaches, in different genders, and different rodent species (mouse and rat). We observed that microglial ablation during the induction of mechanical OIH did not affect OIH expression in male mice (Fig. 2A; Fig. 4A). Similarly, we observe no detectable changes of mechanical OIH expression in female mice with microglial ablation (Fig. 5). When ablating microglia was performed after mechanical OIH had been established, we did not observe changes of OIH maintenance (Fig. 2B; Fig. 4C). Furthermore, microglial ablation also did not change the expression of thermal OIH (Fig. 4B). Finally, microglial ablation in rats did not change the expression of mechanical OIH (Fig. 6B). These findings suggest that microglia are dispensable for the expression and maintenance of OIH. Our data are consistent with recent randomized studies with both laboratory animals and human patients on the effect of the glial inhibitor minocycline[2; 4].
Our data seem to be inconsistent with some of the previous reports that show the critical roles of microglia in opioid-induced hyperalgesia [18; 21]. Because the expression of OIH is modulated by many molecular and cellular processes[36], it is well possible that studies from different laboratories may be performed under different conditions that differentially affected these processes. Different studies may differ in the animals used (ages, genders and species), the forms and doses of opioids, and/or the routes or paradigms of drug administration. There are several main differences in experimental design in the current study in comparison with Ferrini et al. influential work [18]. Our microglial ablation experiments were mainly performed on mice. Because microglial ablation on rats with PLX5622 was incomplete (Fig. 6), we cannot exclude the slim possibility that the residual microglia rescue OIH. Compared with the Ferrini study, we used higher morphine dosages (20mg/kg or higher vs. 10mg/kg in Ferrini et al.) to induce OIH. In addition, we administered morphine with a paradigm of single intraperitoneal injection daily, while Ferrini et al used a paradigm of two subcutaneous injections daily. These types of experimental manipulations may induce different subtypes of microglia that could contribute differently to OIH.
Another major difference is that the strategies employed in the current study differ from what was used by Ferrini et al. We used two complementary approaches to ablate microglia, namely the genetic approach with CD11b-DTR transgenic mouse and the pharmacological approach with PLX5622 in both mice and rats, and obtained comparable results from both approaches. We tested the effect of microglial ablation from either approach on mechanical OIH and the effect of microglial ablation by PLX5622 on thermal OIH, and did not observe significant impairment of either forms of OIH. Mac1-Saporin was used in the Ferrini study to ablate microglia in rats [18]. Non-specific effects of saporin-mediated cell ablation on astrocytes were reported[29], which may complicate result interpretation when saporin-based approaches are used to ablate microglia. In consistence with a role of microglia in OIH, CD11b-Cre-driven CKO of BDNF and Cx3cr1-Cre-driven CKO of MOR were observed to impair thermal OIH [18; 34]. However, because CD11b and Cx3cr1 are also expressed in multiple other cell types in addition to microglia [25; 28; 32], the possibility that the CKOs in non-microglial cells caused the observed impairment of thermal OIH in mutant mice has not been conclusively excluded.
Microglia are only required for the development of mechanical pain hypersensitivity induced by nerve injury in male but not female mice and rats[31; 40]. This sex dimorphism of microglia raises the interesting possibility that mechanical pain hypersensitivity induced by different pathogenic causes in male mice may differentially depend on microglia. In this context, it is important to note that both males and female mice were used in this study. Our data suggest that microglia are dispensable in both males and female mice for the development of mechanical hypersensitivity induced by morphine.
In conclusion, we have used two different approaches to ablate microglia. Our results show that the development of OIH induced by morphine is not affected even after most of microglia are depleted by PLX5622 (Fig. 3A–3C). Both mechanical and thermal OIH can still be induced by morphine, with no detectable impairment, when microglia are ablated. Both male and female mice can still develop OIH, with no detectable defect, after microglia ablation. In addition, similar to mice, rats with microglial ablation also still express OIH as controls. These findings strongly suggest that microglia are not essential for the development of OIH. As shown in (Fig. 1A and Fig. 8A), morphine exposure also activates astrocyte activation [42; 43]. Blocking astrocytic inflammatory signal transduction pathways impairs OIH development [5; 38], Our results show that astrocyte activation and IL-1β up-regulation are not affected by microglial ablation (Fig. 8; Supp Fig. 1). It would be of interest to test the potential role of reactive astrocytes in OIH development. Although our data indicate microglia are dispensable for the development of OIH in rodents, the findings do not exclude the possibility that microglia are involved in the expression of other forms of opioid-induced abnormalities such as tolerance, withdrawal, and addiction, which would be of interest for future studies.
Materials & Methods
Animals
Adult male or female C57BL/6 mice (8–10 weeks) and CD11b-DTR (006000; B6. FVB-Tg (ITGAM-DTR/EGFP)34Lan/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The CD11b-DTR mice were backcrossed with C57BL/6 for at least six generations before experimentation. The expression of diphtheria toxin receptor in CD11b-DRT mice is controlled by the human ITGAM (integrin alpha M) promoter (CD11b), and infusion of diphtheria toxin induces the reversible depletion of microglia/macrophages in the transgenic mice[16]. Additionally, adult male, Sprague-Dawley rats (180–200 g) were also used. Animal procedures were performed following protocols approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Materials
Morphine sulfate was purchased from West-Ward (Eatontown, NJ, USA). PLX5622-containing rodent diet (each kilogram contains 1200 mg PLX5622) was purchased from Research Diets (New Brunswick, NJ, USA). Diphtheria toxin (DT) from Sigma; HibernateA and HibernateA-Ca from BrainBits (Springfield, IL, USA); papain from Worthington Biochemical (Lakewood, NJ, USA); Optiprep Density Gradient Medium from Sigma (St. Louis, MO, USA). Antibodies used for immunoblotting were: anti-IBa1 (1:1000, Wako: 016–20001); anti-GFAP (1:1000, Millipore: MAB360); anti-IL-1β (1:500, Novus Biologicals: NB600–633) and anti-β-actin (1:1000, Santa Cruz Biotechnology: sc-1616-R). The antibody for immunohistochemistry was anti-IBa1 (1:200, Wako: 016–20001). Antibodies used for flow cytometry were: APC-anti-CD11b (Biolegend: 101211), FITC-anti-CD45 (Biolegend: 103107) and anti-CD16/32 (Biolegend: 101302).
Drug administration
In this study, animals were intraperitoneally (i.p.) injected with morphine sulfate (20 mg/kg) daily for 4 consecutive days to lead to and maintain hyperalgesia as well as at constant dose 20mg/kg or escalating dose from 20mg to 50mg/kg daily for 7 days. For investigating the effect of microglia on the development of morphine-induced hyperalgesia, DT (20 ng) was intrathecally (i.t.) injected to CD11b-DRT or wild type mice daily for 2 days. To establish the PLX5622-induced microglia ablation model, C57BL/6 mice or SD rats were fed with rodent diets containing PLX5622 for 5 days prior to administration of morphine. For identifying the influence of microglia on the maintenance of morphine-induced hyperalgesia (OIH), the DT injection schedule was delayed until the 7th day after the first administration of morphine in CD11b-DRT or wild type mice; in the PLX5622-induced model, mice were not fed with PLX5622-containing diets until after the last injection of morphine. Mice treated with vehicle were used as controls.
Measurement of mechanical and thermal nociception
Mechanical nociceptive hypersensitivity in mice was measured as described[7]. The plantar surface of the hind paw of mice in the resting state were stimulated by the calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA) and paw withdrawal thresholds (PWT) were recorded using the Dixon up and down paradigm. In rat case, PWT were assessed on the ventral surface of the hind paw using von Frey filaments (Stoelting, Wood Dale, IL, USA) and the up-down method[9; 15; 46]. For thermal sensitivity test, paw withdrawal latency to a thermal stimulus was measured using a solid state laser method as described [8]. The latency to hindpaw licking and/or shaking, or jumping were recorded. All tests were conducted 2 hours before treatment by an experimenter who did not know the details on the treatments.
Spinal cell dissociation
HibernateA (HABG) media containing 30mL HibernateA, 600μL, 88μL 0.5mM Glutamax and 2μL of 1% penicillin and streptomycin was prepared prior to dissection. Papain mixture containing 12mg papain, 6 ml HibernateA-Ca media and 15μL 0.5mM Glutamax was placed in a 37℃ water bath for 20 minutes and then placed on ice until use. Animals were anesthetized and cardiac perfusion was carried out followed by rapidly dissecting lumber segment of spinal cord. Tissues were placed in 6ml HABG on ice and cut into 0.5mm pieces before transferred into 6ml papain mixture. The mixture with tissues was incubated in 37℃ for 30 minutes with occasional shaking. After incubation, tissues were transferred to 2ml HABG as little papain as possible and triturated 15–20 times using polished glass Pasteur pipettes avoiding bubbles followed by settling in room temperature for 1 minute. The supernatant was transferred to an empty 15ml tube. 2ml HABG was added to the sediment and two times of repeats for triturating, settling and transferring supernatant were carried out and finally got 6ml cell suspension. To separate the debris from the cell suspension, a four layers of density gradient media was created as described[6]. Cell suspension was carefully pipetted on top of the density gradient with little mixing. The density gradient was centrifuged at 800g for 15 minutes at 22℃ and then the top layer was aspirated and discarded. Another 5ml HABG was added to dilute the density gradient and centrifuged for 10 minutes at 1000rmp at 22℃. After centrifuging, supernatant was discarded and 2ml HABG was added to resuspend the cell pellet.
Flow cytometry staining
Dissociated spinal cells were mixed with FACS buffer containing 1xPBS, 5%FBS and 2mM EDTA and centrifuged at 800g for 5 minutes at 4℃. Cell pellet was resuspended by FACS buffer and then counted to adjust cell number to a concentration of 2×106 cell/mL with FACS buffer. Cell suspension was centrifuged at 400g for 8 minutes in 4℃ and then resuspended by 100μL FACS buffer containing 1μL Fc blocker and incubated for 5 minutes avoiding light. Antibodies were added to cell suspension and mixed well followed by incubating at 4℃ for 30 minutes. After incubation, samples were washed using FACS buffer twice and centrifuged at 400g for 8 minutes in 4℃ and resuspended in 300μL FACS buffer for flow test.
Western blotting analysis
Mice were anesthetized with 3% isoflurane and sacrificed for collecting the L4-L6 lumbar spinal cord segments. Tissues were homogenized in RIPA lysis buffer (1% Nonidet P-40, 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, and 1 mM EDTA pH 8.0) with a protease inhibitor cocktail (Sigma). BCA Protein Assay kits (Thermo) were used to determine protein concentrations. Equal amounts of protein were loaded and separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% nonfat milk in TBST buffer for 1 hour at room temperature and then incubated with anti-IBa1 (1:1000, Wako: 016–20001); anti-GFAP (1:1000, Millipore: MAB360); anti-IL-1β (1:500, Novus Biologicals: NB600–633) and anti-β-actin (1:1000, Santa Cruz Biotechnology: sc-1616-R) in TBST buffer overnight at 4°C. After washing with TBST buffer, membranes were incubated with HRP-conjugated secondary antibody. Enhanced Chemiluminescence kits (Pierce) were used to visualize protein bands, and NIH ImageJ software was used for quantification. β-actin was used here as a reference control.
Immunohistochemistry
Mice were anesthetized with 3% isoflurane and transcardially perfused first with 20 ml of 0.01 M PBS and then with 30 ml of 4% paraformaldehyde in 0.01 M phosphate buffer. The L4 and L5 lumbar spinal cord sections were dissected out and fixed in 4% PFA solution for 12 hours at 4°C, dehydrated with 30% sucrose solution in PBS for 24 hours at 4°C, and frozen in optimal cutting temperature medium (Tissue-Tek). Tissues were cut into 15 μm sections on a cryostat (Leica CM 1900) and mounted onto Superfrost Plus microscope slides. The sections were next incubated in blocking solution containing 5% BSA and 0.3% Triton X-100 in 0.01 M PBS for 1 h at room temperature and then in rabbit anti-IBa1 (1:200, Wako: 016–20001) overnight. Sections were washed with 0.01 M PBS and incubated in FITC-conjugated secondary antibody (1:200, Jackson ImmunoResearch Laboratories). They were then stained with DAPI (Sigma). IgG from the same animal source was used as a negative control for immunostaining. Images were collected with a laser confocal microscope (Zeiss).
Statistical analysis
Statistical analysis was conducted with Prism 5 (GraphPad) software. Data were represented as mean ± SEM from three independent experiments. One-way ANOVA was used for immunoblotting data and two-way ANOVA with a Bonferroni post hoc test was performed to analyze animal pain behavior.
Supplementary Material
Supplementary Figure. Microglial ablation in DTR transgenic mice did not affect morphine-induced astrocytes reaction. Immunoblotting analysis of GFAP protein was performed on the spinal cord of CD11b-DTR transgenic mice at day 7 (DT and morphine administartion paradigm shown in Fig.1B), treated with vehicle (Veh), Diphtheria toxin (DT), morphine (Mor) or Mor+DT (left) (**, p <0.01).
Acknowledgement
We thank Plexxikon Inc. for the use of PLX5622. SJT was supported by NIH grants R01NS079166, R01DA036165, R01NS095747 and R01DA050530, and by the Cecil H. and Ida M. Green Distinguished Chair in Neuroscience and Cell Biology. QY was supported by NIH grant R01CA208765.
References
- [1].Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, Le MT, Kawashita T, Giedzinski E, Parihar VK, West BL, Baulch JE, Limoli CL. Elimination of microglia improves cognitive function following cranial irradiation. Scientific reports 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aguado D, Abreu M, Benito J, García-Fernández J, Gómez de Segura IA. Amitriptyline, minocycline and maropitant reduce the sevoflurane minimum alveolar concentration and potentiate remifentanil but do not prevent acute opioid tolerance and hyperalgesia in the rat: A randomised laboratory study. European Journal of Anaesthesiology (EJA) 2015;32(4):248–254. [DOI] [PubMed] [Google Scholar]
- [3].Angst MD Martin S, Clark MDPDJD. Opioid-induced HyperalgesiaA Qualitative Systematic Review. Anesthesiology 2006;104(3):570–587. [DOI] [PubMed] [Google Scholar]
- [4].Arout CA, Waters AJ, MacLean RR, Compton P, Sofuoglu M. Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients. Psychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu JT. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neuroscience bulletin 2014;30(6):936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nature protocols 2007;2(6):1490–1498. [DOI] [PubMed] [Google Scholar]
- [7].Callahan BL, Gil ASC, Levesque A, Mogil JS. Modulation of Mechanical and Thermal Nociceptive Sensitivity in the Laboratory Mouse by Behavioral State. The Journal of Pain 2008;9(2):174–184. [DOI] [PubMed] [Google Scholar]
- [8].Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009;147(1–3):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [10].Chen G, Zhang Y-Q, Qadri YJ, Serhan CN, Ji R-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018;100(6):1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chu LF, Angst MS, Clark D. Opioid-induced Hyperalgesia in Humans: Molecular Mechanisms and Clinical Considerations. The Clinical Journal of Pain 2008;24(6):479–496. [DOI] [PubMed] [Google Scholar]
- [12].Collett BJ. Opioid tolerance: the clinical perspective. British Journal of Anaesthesia 1998;81(1):58–68. [DOI] [PubMed] [Google Scholar]
- [13].Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nature Medicine 2017;23:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cui Y, Chen Y, Zhi J-L, Guo R-X, Feng J-Q, Chen P-X. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Research 2006;1069(1):235–243. [DOI] [PubMed] [Google Scholar]
- [15].Dixon WJ. Staircase bioassay: the up-and-down method. Neuroscience and biobehavioral reviews 1991;15(1):47–50. [DOI] [PubMed] [Google Scholar]
- [16].Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. The Journal of clinical investigation 2005;115(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014;82(2):380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferrini F, Trang T, Mattioli AM, Laffray T-S, Del’Guidice T, Lorenzo L-E, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu J-M, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nature Neuroscience 2013;16:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences 2016;113(24):E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hilla AM, Diekmann H, Fischer D. Microglia Are Irrelevant for Neuronal Degeneration and Axon Regeneration after Acute Injury. The Journal of Neuroscience 2017;37(25):6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 2010;167(3):880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain, Behavior, and Immunity 2010;24(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Janova H, Arinrad S, Balmuth E, Mitjans M, Hertel J, Habes M, Bittner RA, Pan H, Goebbels S, Begemann M, Gerwig UC, Langner S, Werner HB, Kittel-Schneider S, Homuth G, Davatzikos C, Völzke H, West BL, Reif A, Grabe HJ, Boretius S, Ehrenreich H, Nave K-A. Microglia ablation alleviates myelin-associated catatonic signs in mice. The Journal of clinical investigation 2017;128(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, Tuke J, Hutchinson MR. Codeine-induced hyperalgesia and allodynia: investigating the role of glial activation. Translational Psychiatry 2014;4:e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and cellular biology 2000;20(11):4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kalso E, Edwards JE, Moore AR, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. PAIN 2004;112(3):372–380. [DOI] [PubMed] [Google Scholar]
- [27].Kao S-C, Zhao X, Lee C-Y, Atianjoh FE, Gauda EB, Yaster M, Tao Y-X. Absence of μ opioid receptor mRNA expression in astrocytes and microglia of rat spinal cord. NeuroReport 2012;23(6):378–384 3 10.1097/WNR.1090b1013e3283522e3283521b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li H, Zhang G-X, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol 2008;181(4):2483–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin L-H, Moore SA, Jones SY, McGlashon J, Talman WT. Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013;33(47):18608–18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maduna T, Audouard E, Dembélé D, Mouzaoui N, Reiss D, Massotte D, Gaveriaux-Ruff C. Microglia Express Mu Opioid Receptor: Insights From Transcriptomics and Fluorescent Reporter Mice. Frontiers in psychiatry 2019;9:726–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. PAIN 2018;159(9):1752–1763. [DOI] [PubMed] [Google Scholar]
- [32].McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. The Journal of Immunology 1992;149(4):1326–1333. [PubMed] [Google Scholar]
- [33].Raghavendra V, Rutkowski MD, DeLeo JA. The Role of Spinal Neuroimmune Activation in Morphine Tolerance/Hyperalgesia in Neuropathic and Sham-Operated Rats. The Journal of Neuroscience 2002;22(22):9980–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reiss D, Maduna T, Maurin H, Audouard E, Gaveriaux-Ruff C. Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice. Journal of Neuroscience Research 2020;n/a(n/a). [DOI] [PubMed] [Google Scholar]
- [35].Reshef R, Kudryavitskaya E, Shani-Narkiss H, Isaacson B, Rimmerman N, Mizrahi A, Yirmiya R. The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb. eLife 2017;6:e30809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roeckel L-A, Le Coz G-M, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016;338:160–182. [DOI] [PubMed] [Google Scholar]
- [37].Ru W, Liu X, Bae C, Shi Y, Walikonis R, Mo Chung J, Tang SJ. Microglia Mediate HIV-1 gp120-Induced Synaptic Degeneration in Spinal Pain Neural Circuits. J Neurosci 2019;39(42):8408–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sanna MD, Ghelardini C, Galeotti N. Activation of JNK pathway in spinal astrocytes contributes to acute ultra-low-dose morphine thermal hyperalgesia. Pain 2015;156(7):1265–1275. [DOI] [PubMed] [Google Scholar]
- [39].Soleiman M Mu-Opioid Receptors on Nociceptors, Not Microglia, Drive Morphine Tolerance and Hyperalgesia in Mice. Pain Research Forum 2017;16 Feb. 2017. Available at: https://www.painresearchforum.org/news/77806-mu-opioid-receptors-nociceptors-not-microglia-drive-morphine-tolerance-and-hyperalgesia. [Google Scholar]
- [40].Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience 2015;18:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tai Y-H, Wang Y-H, Wang J-J, Tao P-L, Tung C-S, Wong C-S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. PAIN 2006;124(1):77–86. [DOI] [PubMed] [Google Scholar]
- [42].Tumati S, Largent-Milnes TM, Keresztes AI, Yamamoto T, Vanderah TW, Roeske WR, Hruby VJ, Varga EV. Tachykinin NK(1) receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation. European journal of pharmacology 2012;684(1–3):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vacca V, Marinelli S, Luvisetto S, Pavone F. Botulinum toxin A increases analgesic effects of morphine, counters development of morphine tolerance and modulates glia activation and mu opioid receptor expression in neuropathic mice. Brain Behav Immun 2013;32:40–50. [DOI] [PubMed] [Google Scholar]
- [44].Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain — Misconceptions and Mitigation Strategies. New England Journal of Medicine 2016;374(13):1253–1263. [DOI] [PubMed] [Google Scholar]
- [45].Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in neurosciences 2001;24(8):450–455. [DOI] [PubMed] [Google Scholar]
- [46].Wu Z, Li L, Xie F, Du J, Zuo Y, Frost JA, Carlton SM, Walters ET, Yang Q. Activation of KCNQ Channels Suppresses Spontaneous Activity in Dorsal Root Ganglion Neurons and Reduces Chronic Pain after Spinal Cord Injury. Journal of neurotrauma 2017;34(6):1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Microglial ablation in DTR transgenic mice did not affect morphine-induced astrocytes reaction. Immunoblotting analysis of GFAP protein was performed on the spinal cord of CD11b-DTR transgenic mice at day 7 (DT and morphine administartion paradigm shown in Fig.1B), treated with vehicle (Veh), Diphtheria toxin (DT), morphine (Mor) or Mor+DT (left) (**, p <0.01).


