Abstract
Objective:
To evaluate associations between time since amputation (TSAmp) and mobility outcomes of adults with lower-limb amputation (LLA).
Design:
Secondary analysis of a cross-sectional dataset, including 109 community-dwelling adults, ≥1 year after unilateral transfemoral (N=39; mean age=54±15years) or transtibial amputation (N=70; mean age=58±14years). Participants attended standardized clinical evaluations and completed mobility-related outcome measures: Prosthesis Evaluation Questionnaire-Mobility Subscale (PEQ-MS), Timed Up and Go (TUG), 10-Meter Walk Test (10MWT), and 6-Minute Walk Test (6MWT).
Results:
After controlling for age, sex, amputation level and etiology, TSAmp was significantly associated with each mobility outcome. PEQ-MS and TSAmp were linearly associated, with TSAmp explaining 10.6% of the overall variance. TUG time and TSAmp were linearly associated, with TSAmp and an interaction term (levelxTSAmp) explaining 8.4% of the overall variance; 10MWT speed and 6MWT distance had non-linear associations with TSAmp, with TSAmp and non-linear terms (TSAmp2) explaining 12.1% and 13.2% of the overall variance, respectively.
Conclusions:
Based on the findings, longer TSAmp may be associated with better PEQ-MS score and TUG time, while longer TSAmp may be associated with better or worse 10MWT speed and 6MWT distance depending upon time elapsed since LLA. Estimations of post-amputation mobility among adults with LLA should consider TSAmp.
Keywords: amputation, prostheses, outcome measures, walking speed, walk test
Introduction
Following a lower-limb amputation (LLA), individuals demonstrate reduced mobility1 and report lower quality-of-life.2 After undergoing rehabilitation with a prosthesis, adults with LLA may be expected to regain mobility and re-integrate into their communities. Post-rehabilitation success relies on a tailored treatment approach that considers an individual’s demographics, current health status, amputation-specific factors (e.g., amputation level and etiology), and pre-amputation mobility.3,4 Additionally, rehabilitative success relies on clinicians’ ability to anticipate potential changes in an individual’s mobility as he/she gains experience with his/her amputation and prosthesis. Current literature, however, provides little clinical guidance concerning the impact of time since amputation (TSAmp) or an individual’s prosthesis experience on functional mobility following LLA.
Knowledge of expected mobility outcomes at various time-points following amputation may enhance patient care. First, by identifying post-amputation time points corresponding with mobility status decline, clinicians may better anticipate the need for intervention to mitigate declines leading to loss of independence. Post-amputation time points that are associated with mobility decline may become standard periods after LLA for mobility screening. Second, for clinicians evaluating a new patient following LLA, prognosing functional mobility based on available literature is challenging. Consequently, clinicians rely on their past experiences with similar patients, resulting in considerable inter-clinician variability due to differences in practice years and patient population exposure. Thus, the ability to accurately and objectively project future mobility through data obtained from clinical outcome measures based on other patients’ trajectories is quite appealing, particularly for novice clinicians. With respect to prosthesis prescription, accurately estimating future mobility may be vital for (a) establishing prosthesis candidacy3,4 and (b) anticipating changes in mobility status, for example, change in the patient’s Medicare Functional Classification Level (MFCL).5 The MFCL classifies individuals with amputation into five groups (K0 to K4) based on their mobility potential with a prosthesis. Considering the differences in ambulatory potential between MFCL levels,6 prosthesis prescription for each level can vary greatly.7 Hence, when a K3-classified individual with LLA continues to use a prosthesis more suitable for an individual with a lower mobility level (i.e., K2-classified) despite improvements in walking speed and function, he/she may not achieve peak performance. Anticipating a change in mobility status (or MFCL) may provide the justification necessary to make timely and necessary upgrades to an individual’s prosthesis. Lastly, for the individual after LLA, estimates based on peer data, may provide a realistic target for mobility performance post-amputation, which can be motivating, as well as align with patient preference of being compared to peers with LLA.
Post-LLA, estimating mobility potential can be challenging, as various factors are known to impact mobility, including age, amputation level, amputation etiology, comorbidities, and physical fitness.3,4 TSAmp, however, remains a largely unexplored factor in estimating mobility in this population. Current evidence on TSAmp is limited to small-scale studies (N ≤ 20), primarily examining biomechanical measures (e.g., gait deviation index8 and prosthetic knee angular impulse9), which evaluate specific gait-related impairments but may not directly reflect functional mobility. Ideally, to most accurately estimate mobility with respect to TSAmp, a large-scale, longitudinal cohort study examining mobility-specific outcomes is needed. To inform a future, prospective study, we propose first to evaluate associations between TSAmp and mobility measures [self-reported (i.e., Prosthesis Evaluation Questionnaire-Mobility Subscale) and performance-based (i.e., Timed Up and Go test, 10-Meter Walk Test, 6-Minute Walk Test)], using a cross-sectional dataset from an outpatient Amputee Clinic. Using mobility outcome data obtained from adults with varied TSAmp, while considering factors known to impact mobility (e.g., age, sex amputation level and etiology3,4), we may begin to understand mobility outcome trajectories following LLA, which may ultimately help with predicting patient outcomes.
Methods
Study Sample
Data for this retrospective analysis were extracted from a pre-existing cross-sectional dataset obtained from an outpatient interdisciplinary Amputee Clinic at the University of Delaware. Participants attended the clinic from April 2014 to September 2019 and were considered for inclusion if they were ≥18 years-old, community-dwelling, and had undergone a unilateral transfemoral (TFA) or transtibial amputation (TTA) at least 1 year prior to their clinical evaluation. Individuals were excluded if they did not use a prosthesis, had an amputation of the contralateral limb, or if they had a neurological disorder (e.g., stroke), which limited their walking ability. Included participants signed an informed consent form approved by the Institutional Review Board for Human Subjects Research at the University of Delaware at the start of their Amputee Clinic appointment. This study conforms to all STROBE guidelines and reports the required information accordingly (see Supplementary Checklist).
Procedures
As part of the standardized clinical evaluation, participants provided demographic information, including age, sex, amputation level and etiology. TSAmp was calculated as the number of years from the date of amputation. Medical comorbidities, and specifically those that might impact functional outcomes, were recorded for various systems, including the following: cardiac, vascular, respiratory, gastrointestinal, musculoskeletal, neurological, and endocrine. The principal investigator trained all personnel in administering standardized clinical evaluations. Participant mobility-level (i.e., K-level) was determined by the interdisciplinary team (i.e., prosthetist, physical therapist, and physician) through group consensus based on data obtained from the standardized clinical examination.
Self-Reported Outcome Measure
During the one-time clinical evaluation, to reduce reporting bias, participants completed the Prosthetic Evaluation Questionnaire-Mobility Subscale (PEQ-MS) prior to performance-based outcome measures. The PEQ-MS measures an individual’s perceived ability to ambulate with a prosthesis and consists of 12 items, scored on a 0 to 4 scale, which are totaled.10 Higher scores indicate greater perceived mobility. Reliability, validity and responsiveness of the questionnaire have been previously reported.10,11
Performance-based Outcome Measures
The Timed Up and Go (TUG) assesses mobility, balance ability, and fall risk.12 Reliability and validity among individuals with LLA has been previously reported.12 Participants were instructed to stand up from a chair with armrests, walk three meters (at their self-selected speed), turn 180°, walk back to the chair, and sit down. Time to complete the TUG was recorded in seconds.
The 10-Meter Walk Test (10MWT) is a valid measure of walking speed among individuals with LLA.13 Participants were instructed to “walk” along a straight 10-meter path “as fast and as safely as possible”, speed was calculated over the middle six meters to allow for acceleration and deceleration at either end.14 Fast walking speed was chosen, as it better reflects an individual’s ability to accommodate speed changes necessary for community ambulation.15
The 6-Minute Walk Test (6MWT) measures walking endurance and aerobic capacity; reliability and validity among people with LLA has been previously reported.16 After assessing vital signs to establish participant safety for testing, participants walked along a rectangular path, with an assistive device if needed, and were instructed to cover as much ground as possible in 6 minutes. An examiner trailed the participant to avoid pacing the participant and recorded the distance covered in meters using a rolling measurement tool.
Statistics
All analyses were conducted using SPSS Statistics 26 (IBM Corp., Armonk, NY, USA). Demographic characteristics were examined using descriptive statistics. Correlations (negligible: 0.0 – 0.3; low: 0.3 – 0.5; moderate: 0.5 – 0.7; high: 0.7 – 0.9; very high: 0.9 – 1.0)17 between demographics (e.g., age, TSAmp) and the four outcome measures were examined to identify significant associations. Correlations, residual analyses, and scatter plots were used to examine assumptions of multicollinearity, normality, linearity and homoscedasticity. Non-linear associations with TSAmp were examined using a squared term. Multiple regression analyses were conducted for each of the four outcome measures. Suspected covariates of age and sex were entered into Block I. Additional suspected covariates, i.e., amputation level and etiology, were entered in Block II. TSAmp was entered in Block III. Lastly, when appropriate, a squared (TSAmp2) or interaction term (levelxTSAmp) was entered in Block IV. Significance of each coefficient was determined at an alpha of 0.05. Post hoc simple slope analyses were conducted for non-linear associations.18
Results
Participants
Of the 227 participants in the cross-sectional dataset, 105 were excluded based on eligibility criteria and 13 were excluded as they were missing data on 3 of the 4 outcome measures of interest (Figure 1). In the final analysis, 109 participants were included, of whom 39 had a TFA and 70 had a TTA. Participant characteristics by amputation level are presented in Table 1. Mean TSAmp, K-levels (MFCL), and Houghton Scale scores (a measure of prosthesis use and stability when wearing a prosthesis19) indicate participants were long-term prosthesis users and community-ambulators.20 Trauma was the most common cause of amputation. Due to the small number of cases under some etiologies, for regression analyses, amputation etiology was dichotomized as traumatic versus non-traumatic. Comorbidities are provided in Table 2, with the most prevalent being high blood pressure.
Figure 1.
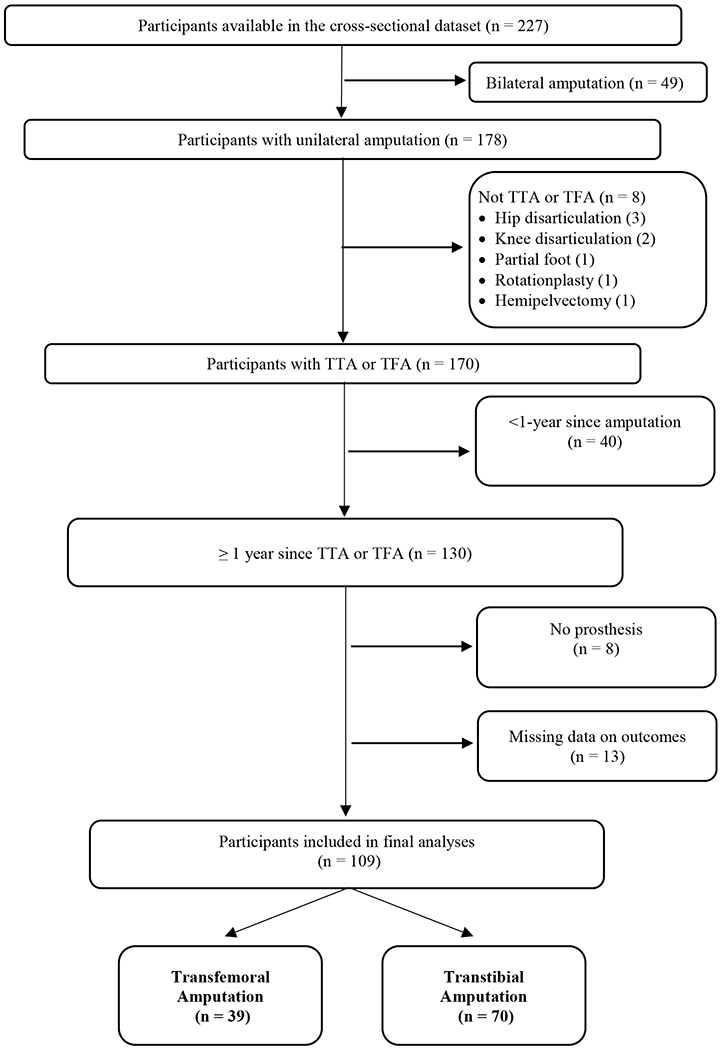
Participant selection based on exclusion and inclusion criteria
Table 1.
Sample characteristics presented by amputation level
| Transfemoral amputation (N = 39) |
Transtibial amputation (N = 70) |
||||
|---|---|---|---|---|---|
| Age (years) | 54 ± 15 | 58 ± 14 | |||
|
| |||||
| TSAmp (years) | 18 ± 17 | 14 ± 16 | |||
|
| |||||
| Houghton Scale (0 – 12) | 10 ± 2 | 10 ± 2 | |||
|
| |||||
| Frequency | % | Frequency | % | ||
|
| |||||
| Sex (F) | 10 | 25 | 20 | 29 | |
| Etiology | Trauma | 19 | 48.7 | 21 | 30.0 |
| Vascular/Diabetes | 9 | 23.1 | 30 | 42.9 | |
| Cancer | 3 | 7.7 | 6 | 8.6 | |
| Congenital | 5 | 12.8 | 2 | 2.9 | |
| Infection | 0 | 0.0 | 5 | 7.1 | |
| Other | 3 | 7.7 | 6 | 8.6 | |
|
| |||||
| MFCL | K0 | 0 | 0.0 | 1 | 1.4 |
| K1 | 0 | 0.0 | 2 | 2.9 | |
| K2 | 7 | 17.9 | 8 | 11.4 | |
| K3 | 19 | 48.7 | 35 | 50.0 | |
| K4 | 13 | 33.3 | 24 | 34.3 | |
|
| |||||
| Suspension | Pinlock | 14 | 35.9 | 31 | 44.3 |
| Suction | 17 | 43.5 | 30 | 42.9 | |
| Lanyard | 6 | 15.4 | 1 | 1.4 | |
| Other | 2 | 5.1 | 8 | 11.4 | |
|
| |||||
| Prosthetic foot | SACH | 7 | 17.9 | 10 | 14.3 |
| Multi-axial | 6 | 15.4 | 9 | 12.9 | |
| Dynamic response | 26 | 66.7 | 50 | 71.4 | |
| Microprocessor | 0 | 0.0 | 1 | 1.4 | |
Continuous data presented as mean ± SD.
TSAmp: time since amputation; F: female; SACH: solid ankle cushion heel; MFCL: mobility functional classification level.
Table 2.
Participant comorbidities
| Transfemoral amputation (N = 39) |
Transtibial amputation (N = 70) |
|||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Cardiac | ||||
| Heart disease | 1 | 2.6 | 5 | 7.1 |
| Angina | 0 | 0 | 2 | 2.9 |
| CHF | 1 | 2.6 | 2 | 2.9 |
|
| ||||
| Vascular | ||||
| High BP | 15 | 38.5 | 41 | 58.6 |
| Low BP | 1 | 2.6 | 2 | 2.9 |
| Anemia | 2 | 5.1 | 6 | 8.6 |
| Leukemia | 0 | 0 | 1 | 1.4 |
|
| ||||
| Respiratory | ||||
| SOB | 3 | 7.7 | 13 | 18.6 |
| Lung disease | 0 | 0 | 4 | 5.7 |
| Asthma | 2 | 5.1 | 6 | 8.6 |
|
| ||||
| Gastrointestinal | ||||
| Nausea/vomiting | 0 | 0 | 3 | 4.3 |
| Hernia | 0 | 0 | 2 | 2.9 |
| Bowell/Bladder problems | 3 | 7.7 | 9 | 12.9 |
|
| ||||
| Musculoskeletal | ||||
| Rheumatoid Arthritis | 2 | 5.1 | 8 | 11.4 |
| Osteoarthritis | 7 | 17.9 | 8 | 11.4 |
| Osteoporosis | 0 | 0 | 4 | 5.7 |
|
| ||||
| Neurological | ||||
| Numbness | 3 | 7.7 | 24 | 34.3 |
| Peripheral neuropathy | 2 | 5.1 | 14 | 20.0 |
|
| ||||
| Endocrine | ||||
| Diabetes | 5 | 12.8 | 34 | 48.6 |
CHF: congestive heart failure; BP: blood pressure; SOB: shortness of breath.
Main Results
Mean TSAmp and outcome measure scores are presented (Table 3). Significant correlations were observed between TSAmp and all outcome measures (PEQ-MS: r = 0.33; TUG: r = −0.23; 10MWT: r = 0.30; 6MWT: r = 0.37).
Table 3.
Outcome measure descriptives presented by amputation level
| Transfemoral amputation | ||||
|---|---|---|---|---|
|
| ||||
| Measure | PEQ-MS | TUG (sec) | 10MWT (m/sec) | 6MWT (m) |
| Mean | 34.7 | 14.19 | 1.22 | 351 |
| SD | 10.2 | 7.76 | 0.42 | 129 |
| Total valid cases | 37 | 36 | 36 | 32 |
|
| ||||
| Transtibial amputation | ||||
|
| ||||
| Measure | PEQ-MS | TUG (sec) | 10MWT (m/sec) | 6MWT (m) |
|
| ||||
| Mean | 35.0 | 11.18 | 1.30 | 379 |
| SD | 10.3 | 4.55 | 0.48 | 149 |
| Total valid cases | 67 | 64 | 68 | 56 |
Total valid cases represent the total number of participants who completed the test.
PEQ-MS: Prosthesis Evaluation Questionnaire - Mobility Subscale; TUG: Timed Up and Go;10MWT: 10-Meter Walk Test; 6MWT: 6-Minute Walk Test.
PEQ-MS and TSAmp had a linear association. Overall, the model explained 17.5% of the variance in PEQ-MS scores (Table 4). Age and TSAmp significantly contributed to the model. Age, sex, amputation level and etiology explained 6.9% of the total variance. Above and beyond these variables, TSAmp explained 10.6% of the total variance. For each year of age, there was a 0.14-point reduction in PEQ-MS, while for each year elapsed since amputation there was a 0.22-point increase in PEQ-MS.
Table 4.
Regression results for mobility outcome measures
| PEQ-MS | TUG (sec) | |||
|---|---|---|---|---|
|
| ||||
| B | p-value | B | p-value | |
| Intercept | 36.26 | 0.00 | 5.63 | 0.02 |
| Age | −0.14 | 0.03 | 0.23 | 0.00 |
| Sex | 3.15 | 0.14 | −0.63 | 0.57 |
| Level | 2.04 | 0.31 | −6.78 | 0.00 |
| Etiology | −0.15 | 0.95 | −0.12 | 0.91 |
| TSAmp | 0.22 | 0.00 | −0.17 | 0.00 |
| LevelxTSAmp | 0.14 | 0.03 | ||
| R2 | 0.175* | 0.432* | ||
| R2 Change | ||||
| Block I | 0.060* | 0.246* | ||
| Block II | 0.009 | 0.102* | ||
| Block III | 0.106* | 0.053* | ||
| Block IV | 0.031* | |||
|
| ||||
|
| ||||
| 10MWT (m/sec) | 6MWT (m) | |||
|
| ||||
| B | p-value | B | p-value | |
|
| ||||
| Intercept | 1.55 | 0.00 | 490.07 | 0.00 |
| Age | -0.01 | 0.00 | -4.70 | 0.00 |
| Sex | 0.19 | 0.03 | 9.79 | 0.72 |
| Level | 0.19 | 0.01 | 74.13 | 0.00 |
| Etiology | -0.04 | 0.61 | -7.14 | 0.79 |
| TSAmp | 0.02 | 0.00 | 8.78 | 0.00 |
| TSAmp2 | -0.000252 | 0.04 | -0.113 | 0.03 |
| R2 | 0.413* | 0.474* | ||
| R2 Change | ||||
| Block I | 0.261* | 0.294* | ||
| Block II | 0.031 | 0.048 | ||
| Block III | 0.096* | 0.099* | ||
| Block IV | 0.025* | 0.033* | ||
R2 refers to the total variance explained by the model.
R2 change illustrates the variance explained by each block.
Block I: Age & Sex; Block II: Amputation level; Block III: TSAmp; Block IV (TUG): An interaction term, LevelxTSAmp, was used for the TUG; Block IV (6MWT & 10MWT): A squared term, TSAmp2, was used to examine the curvilinear relationship between TSAmp and 10MWT & 6MWT.
B = Unstandardized beta coefficient
Sex: 0 = female; 1 = male; Level: 0 = transfemoral amputation; 1 = transtibial amputation
p < .05
TUG and TSAmp had a linear association. Overall, the model explained 43.2% of the variance in TUG scores (Table 4). Age, amputation level, TSAmp and LevelxTSAmp significantly contributed to the model. For each year of age, there was a corresponding 0.23 sec increase in TUG time. Age, sex, amputation level and etiology explained 34.8% of the total variance. Above and beyond these variables, TSAmp and the LevelxTSAmp interaction term explained 5.3% and 3.1% of the total variance, respectively.
10MWT and TSAmp had a curvilinear association (Figure 2c). Overall, the model explained 41.3% of the variance in fast walking speed obtained (Table 4). Age, sex, amputation level, TSAmp and TSAmp2 significantly contributed to the model. Age, sex, amputation level and etiology explained 29.2% of the total variance. TSAmp and TSAmp2 individually explained 9.6% and 2.5% of the total variance, respectively, beyond that explained by the other variables.
Figure 2.
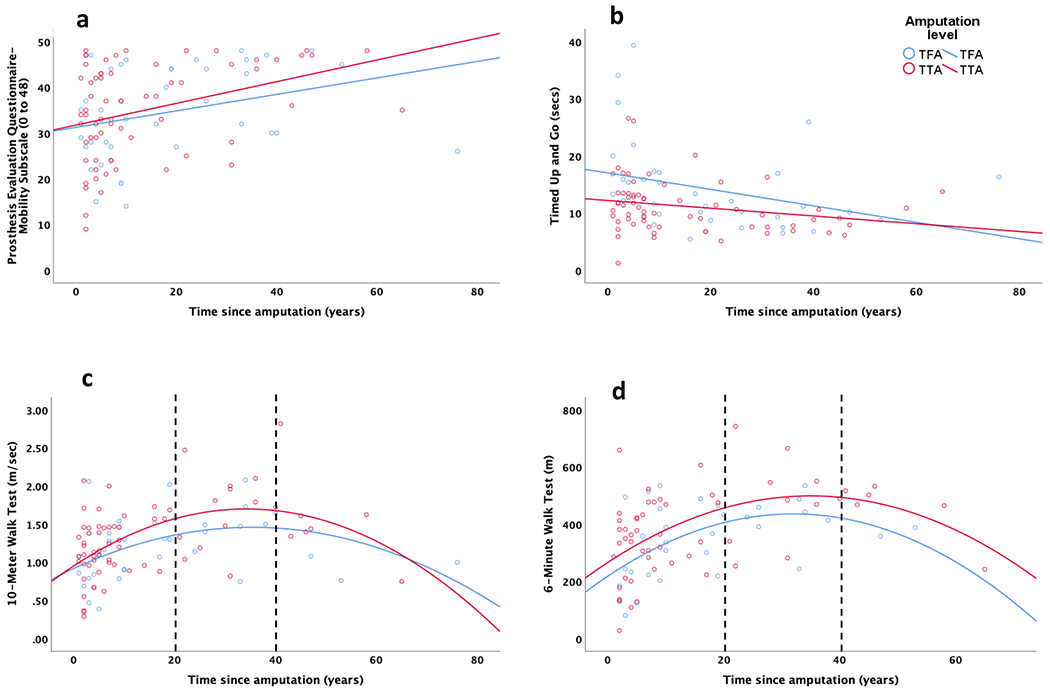
Association of time since amputation with the selected outcome measures based on amputation level: (a) Prosthesis Evaluation Questionnaire-Mobility Subscale, (b) Timed Up and Go, (3) 10-Meter Walk Test, and (4) Six-Minute Walk Test. The best fit lines show the nature of association, i.e. linear or non-linear. The dotted lines in (c) and (d) divide participant data into 3 time since amputation windows: <20, 20 to 40 and >40 years post-amputation. TFA: Transfemoral amputation; TTA: Transtibial amputation.
6MWT and TSAmp had a curvilinear association (Figure 2d). Overall, the model explained 47.4% of the variance in 6MWT distances (Table 4). Age, amputation level, TSAmp, and TSAmp2 significantly contributed to the model. Age, sex, amputation level and etiology explained 34.2% of the total variance. TSAmp and TSAmp2 individually explained 9.9% and 3.3% of the total variance, respectively, beyond that explained by the other variables.
Post Hoc Analyses
To better describe the curvilinear associations, 10MWT and 6MWT data were split into 3 TSAmp windows based on corresponding curve direction changes (Figure 2c & d). The first, second, and third windows approximately represent an increase, plateau, and decrease, respectively, in 10MWT fast walking speed and 6MWT distance (Table 5). A 1-year increase in TSAmp was associated with a 0.02 m/sec and 0.01 m/sec increase in 10MWT-based fast walking speed, for participants <20 years and 20- to 40-years post-LLA, respectively. Conversely, each 1-year increase in TSAmp was associated with a 0.02 m/sec decrease in walking speed for participants >40-years post-LLA. A 1-year increase in TSAmp was associated with a 6.9 m increase in 6MWT distance for participants <20-years post-LLA. Conversely, each 1-year increase in TSAmp was associated with a 2.2 m and 4.3 m decrease in 6MWT distance for participants 20- to 40- and >40-years post-LLA, respectively.
Table 5.
Estimated 1-year change for curvilinear relationships
| TSAmp | 10MWT (m/sec) | 6MWT (m) |
|---|---|---|
| <20 years | 0.02 | 6.9 |
| 21 to 40 years | 0.01 | -2.2 |
| >40 years | -0.02 | -4.3 |
Discussion
To date, the impact of TSAmp on self-report and performance-based mobility outcomes among adults after LLA has received limited attention. This cross-sectional study provides evidence that TSAmp is an important factor in mobility outcomes following unilateral TFA and TTA, above and beyond demographic factors, including age and amputation level. Specifically, TSAmp is significantly associated with self-reported mobility as assessed with the PEQ-MS and performance-based mobility as assessed with the TUG, 10MWT, and 6MWT. Findings highlight the significance of TSAmp as an important factor in future studies evaluating mobility outcomes for patients with LLA. For example, TSAmp should be systematically obtained, reported, and considered as a covariate in outcome measure studies among patients following LLA. Further, studies seeking to predict long-term mobility outcomes, i.e., 10MWT or 6MWT, after LLA, should consider that performance may not necessarily improve in a linear fashion beyond the first 20 years post-amputation.
TSAmp was linearly associated with participant self-reported mobility, as assessed through the PEQ-MS, suggesting with increase in years following amputation there may be incremental improvement in self-reported mobility. Data from this study aligns with previous research among a similarly aged sample (i.e., 58.5±12.0 years), where mean PEQ-MS scores of 35.0±9.6 points, 12.7±14.5 years after TTA, were reported.21 Wong and colleagues reported PEQ-MS scores of 28.1±12.7 points among adults, aged 55.5±16.0 years, with predominantly TTAs, who were 7.1±13.1 years post-amputation.22 Collectively, results suggest after LLA, self-reported mobility outcomes improve over time in the post-acute period that typically follows concentrated acute rehabilitation.
Similarly, TSAmp was linearly associated with TUG performance and appears to improve over time beyond the first year post-amputation. Recently, Sawer and colleagues reported average TUG times (10.4±2.8 sec) similar to this study’s findings among adults aged 55.1±21.3 years, with predominantly TTA, an average of 16.0±14.8 years prior.23 Conversely, Spaan and colleagues reported average TUG times of 17.3±9.1 sec among similarly-aged adults (59.2±13.3 years) more acutely post-amputation (i.e., 3 weeks post-LLA).24 In the Spaan et al. study, level of amputation was found to be a significant predictor of TUG performance at the end of acute rehabilitation.24 Post-acutely, we found TUG performance may be similarly influenced by amputation level (Table 4). For example, two 40-year-old males who are 1- and 15-years post traumatic-TTA, respectively, may take 7.3 secs and 6.9 secs to complete the TUG, respectively. Two males post-TFA (with similar demographics) may take 13.9 secs and 11.5 secs to complete the TUG. Based on the above example, an adult male 15-years post traumatic-TTA may take 0.4 secs less to complete the TUG as compared to his peer 1-year post traumatic-TTA, while, an adult male 15-years post traumatic-TFA may take 2.4 secs less to complete the TUG, as compared to his peer 1-year post trauma-TFA. Hence, even though individuals with TFA (as compared to peers with TTA) may take a longer time to complete the TUG, they may have the ability to show improvement on this measure (unlike their peers with TTA), as the TUG’s minimal detectable change (at the 90% confidence level) has been reported to be 3.6 sec16 and the TUG is known to have a ceiling effect.25
Unlike the PEQ-MS and TUG, mobility as assessed with the 10MWT and 6MWT was observed to have a curvilinear relationship with TSAmp. As can be observed in Figures 2c & d, greater TSAmp is associated with better performance on the 10MWT or 6MWT until approximately 20-years post-LLA, suggesting better performance with longer TSAmp from 1-20 years post-LLA. After approximately 20-years post-LLA, however, performance appears to plateau until approximately 40-years post-LLA, after which greater TSAmp is associated with worse 10MWT and 6MWT performance. Given TSAmp and age may be closely related (i.e., as one increases with the other), a large portion of adults post-LLA may be older adults by the time they are ~40-years post-LLA,26 For example, our participants with TFA had a mean age of 54 and were on average 18 years post-TFA at the time of the Amputee Clinic evaluation. After 40 years post-TTA (18+22 TSAmp years) their mean age would be 76 years. Similarly, participants with TTA had a mean age of 58 and were on average 14 years post-TTA, and after 40 years post-TTA (14 +26 TSAmp years) their mean age would be 84 years. Hence, any mobility-decline may also be age-related and expected.
Conceptually, it may be logical to assume a curvilinear association is more appropriate between TSAmp and mobility outcomes (rather than a linear association as found with PEQ-MS and TUG), as it accounts for mobility changes due to age-related decline. The PEQ-MS, however, assesses self-perceived mobility based on ability to perform a task,10 not efficiency in task performance; therefore, PEQ-MS scores may not decline with increasing TSAmp if an individual maintains the ability to complete said tasks, regardless of age-related changes in efficiency. It is, noteworthy, however, that participants with the longest TSAmp (>60 years since amputation), had relatively lower PEQ-MS scores (Figure 2a). With the TUG (see Figure 2b), it is possible that the various mobility and balance related tasks (sit-to-stand and stand-to-sit transfers, a brief walking bout at self-selected speed, and a 180º turn) assessed by the TUG may not reflect age-related decline as well as fast-paced walking tests. Perhaps for this reason, unlike fast gait speed 27 and 6MWT distance,28 minimal age-related declines have been reported for TUG among older adults without LLA.29 For example, TUG reference values of 8.1 secs (95% CI: 7.1-9.0) and 9.2 secs (95% CI: 8.2, 10.2) have been reported for adults without LLA aged 60-69 years and 70-79 years, respectively.29 Nevertheless, as evidenced by an observable decline in TUG performance in participants with TSAmp >50years, a curvilinear relationship between TUG and TSAmp is possible and warrants further exploration in future studies with larger sample sizes.
While, TSAmp was significantly associated with each mobility outcome, it is noteworthy, that TSAmp’s impact may vary across the selected mobility outcomes. For example, given the variance explained, TSAmp may have a weaker impact on TUG scores as compared to self-perceived mobility, fast walking speed and 6MWT distance (Table 4: 5.3% variance explained for TUG versus 10.6% for PEQ-MS, 9.6% for 10mwt, and 9.9% for 6MWT). On the other hand, unlike performance-based mobility, TSAmp may have a larger impact on PEQ-MS scores as compared to patient demographic factors (Table 4, PEQ-MS: 10.6% variance explained by TSAmp versus 6.9% by demographics). Thus, a patient’s perception of their mobility may be more influenced by their time or experience with an amputation and less by their demographic characteristics.
Biological sex was significantly associated with fast gait speed, aligning with previous research in the general population (without LLA).28 Age and sex explained a total of 26.1% of the variance in gait speed (Table 4). Thus, findings suggest not only age-related differences in gait speed, but also sex-related differences, post-LLA. In fact, fast gait speeds of females with LLA are approximately 0.19m/sec slower than males peers. As walking speed is indicative of community ambulation potential,15 females with LLA may be more challenged in achieving unrestricted community ambulation, when compared to their male counterparts.
In prior research among adults following LLA, TSAmp has largely been reported as a participant demographic. Cut-points of 1-year since amputation have been historically used to identify a theoretically homogeneous group of adults with LLA for inclusion in studies seeking to study outcomes in the post-acute amputation period. An underlying assumption is adults ≥1-year post-amputation have sufficiently acclimated to their amputation or have sufficient experience, such that any potential changes (to the selected outcomes), thereafter, may be directly attributed to an intervention. Based on the findings, however, this assumption may be flawed, as TSAmp is independently associated with mobility outcomes among adults >1-year post-amputation, such that mobility outcomes (e.g., walking speed, endurance) may continue to improve well-beyond the initial years post-amputation. Further, relationships between TSAmp and some outcomes (gait speed, walking endurance) may vary as a result of time elapsed since amputation (e.g., <20 years versus >40 years), confounding interpretation of long-term results when individuals >1-year post-amputation are considered as a homogeneous and stable group.
As previously discussed, estimating mobility over time is important for enhancing clinical care of adults-LLA. Findings of this study indicate that TSAmp, alongside age, sex, and amputation level, may be a potential predictor of future mobility outcomes, but this warrants longitudinal investigation. As the number of factors impacting mobility outcomes extends beyond age and sex, use of prediction models/equations that can accommodate the variability in patient presentations (e.g., age, sex, amputation level, time since amputation), may be an appropriate and accurate means of predicting future mobility of individual patients.
Study Limitations
Causal relationships cannot be determined given the cross-sectional study design. Results may only be generalized to community-dwelling adults who are ≥1-year post-TFA or TTA. Findings may not be generalized to individuals (a) with significant comorbidity burden, (b) classified as home-, but not community-level ambulators, (c) acutely post-amputation, (d) with bilateral amputations, or (e) minors with LLA. Future studies may consider increasing female representation given biological sex may be a factor impacting some mobility outcomes. While the sample size is large when compared to most limb loss studies, lack of sufficient participants with some etiologies (e.g., cancer, infection) required traumatic versus non-traumatic etiology dichotomization and individuals >40 years TSAmp are under-represented. Under-representation for >40 years post-amputation is not unexpected, however, given the high, i.e., 77%, 5-year post-amputation mortality rate in this population.30 Further, we did not have access to years of prosthesis use with concurrent weekly wear to allow calculation of ‘prosthesis experience’. While the majority of our participants, i.e., 62%, reported using their prosthesis during all waking hours (per the Houghton scale), to objectively quantify prosthesis experience, future studies may consider the use of prosthesis-embedded accelerometers. Moreover, additional factors (e.g., psychological, social) impacting mobility outcomes should be considered as there is still a considerable amount of the variance that may be explained.
Conclusions
The TSAmp for an individual following TFA or TTA, along with age and amputation level, are important considerations when evaluating mobility outcomes. Based on the findings, greater TSAmp is associated with better self-reported, prosthesis-enabled mobility and functional mobility. TSAmp also appears to be an important factor for consideration when evaluating patient gait speed and walking endurance, although relationships may differ based on the TSAmp, such that better outcomes may be anticipated only until ~20 years since amputation, after which patient gait speed and endurance may plateau or decline. Collectively, findings suggest future studies should carefully consider TSAmp when evaluating mobility outcomes post-amputation, planning prospective studies, and developing models that may allow clinicians to predict future mobility outcomes.
Supplementary Material
What is known / What is new:
What is known
Adults after lower-limb amputation exhibit persistent impairments in mobility. Demographic factors, such as age, amputation level and etiology, are important factors in mobility outcomes.
What is new
Beyond age, amputation level and etiology, time since amputation helps explain mobility outcomes among adults greater than 1-year post-amputation. Self-reported and functional mobility may improve with greater time elapsed since amputation, while gait speed and walking endurance may improve or worsen depending on the time elapsed. Findings highlight time since amputation as a significant factor in future studies evaluating mobility outcomes after lower-limb amputation.
Acknowledgements
We would like to thank Dr. Peter C. Coyle, PT, DPT, PhD, for his feedback on this manuscript during the data analysis and drafting phases.
Author disclosure:
The authors have no competing interests to declare. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Data collections and manuscript preparation by Dr. Beisheim, however, were supported by the National Institute of Health (grant T32HD007490) and a Promotion of Doctoral Studies Level I and II scholarships from the Foundation for Physical Therapy Research. Dr. Seth is supported by a Postdoctoral Researcher Fund provided by Independence Prosthetics-Orthotics, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
The material within has been presented as a virtual platform presentation at the 2020 National Assembly of the American Orthotists and Prosthetists Association on September 11, 2020, but has not been previously published.
References
- 1.van Velzen JM, van Bennekom CA, Polomski W, Slootman JR, van der Woude LH, Houdijk H. Physical capacity and walking ability after lower limb amputation: a systematic review. Clinical Rehabilitation. 2006;20(11):999–1016. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, van den Heuvel WJ, Arokiasamy P. Factors affecting quality of life in lower limb amputees. Prosthetics and orthotics international. 2011;35(1):90–96. [DOI] [PubMed] [Google Scholar]
- 3.Sansam K, Neumann V, O’Connor R, Bhakta B. Predicting walking ability following lower limb amputation: A systematic review of the literature. Journal of Rehabilitation Medicine. 2009;41(8):593–603. [DOI] [PubMed] [Google Scholar]
- 4.Kahle JT, Highsmith MJ, Schaepper H, Johannesson A, Orendurff MS, Kaufman K. PREDICTING WALKING ABILITY FOLLOWING LOWER LIMB AMPUTATION: AN UPDATED SYSTEMATIC LITERATURE REVIEW. Technol Innov. 2016;18(2-3):125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services UDoHaHS. HCFA Common Procedure Coding System (HCPCS), 2001. Springfield, VA:. In: US Department of Commerce, National Technical Information Service2001. [Google Scholar]
- 6.Kaluf B Evaluation of Mobility in Persons with Limb Loss Using the Amputee Mobility Predictor and the Prosthesis Evaluation Questionnaire–Mobility Subscale: A Six-Month Retrospective Chart Review. JPO: Journal of Prosthetics and Orthotics. 2014;26(2):70–76. [Google Scholar]
- 7.Hafner BJ, Smith DG. Differences in function and safety between Medicare Functional Classification Level-2 and-3 transfemoral amputees and influence of prosthetic knee joint control. Journal of Rehabilitation Research & Development. 2009;46(3). [PubMed] [Google Scholar]
- 8.Kark L, Odell R, McIntosh AS, Simmons A. Quantifying prosthetic gait deviation using simple outcome measures. World journal of orthopedics. 2016;7(6):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisano G, Hashizume S, Kobayashi Y, et al. Factors associated with a risk of prosthetic knee buckling during walking in unilateral transfemoral amputees. Gait & Posture. 2020;77:69–74. [DOI] [PubMed] [Google Scholar]
- 10.Franchignoni F, Giordano A, Ferriero G, Orlandini D, Amoresano A, Perucca L. Measuring mobility in people with lower limb amputation: Rasch analysis of the mobility section of the prosthesis evaluation questionnaire. J Rehabil Med. 2007;39(2):138–144. [DOI] [PubMed] [Google Scholar]
- 11.Miller WC, Deathe AB, Speechley M. Lower extremity prosthetic mobility: A comparison of 3 self-report scales. Archives of Physical Medicine and Rehabilitation. 2001;82(10):1432–1440. [DOI] [PubMed] [Google Scholar]
- 12.Schoppen T, Boonstra A, Groothoff JW, de Vries J, Göeken LNH, Eisma WH. The timed “up and go” test: Reliability and validity in persons with unilateral lower limb amputation. Archives of Physical Medicine and Rehabilitation. 1999;80(7):825–828. [DOI] [PubMed] [Google Scholar]
- 13.van Herk IEH, Arendzen JH, Rispens P. Ten-metre walk, with or without a turn? Clinical Rehabilitation. 1998;12(1):30–35. [DOI] [PubMed] [Google Scholar]
- 14.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. [DOI] [PubMed] [Google Scholar]
- 15.Rosa MC, Marques A, Demain S, Metcalf CD. Fast gait speed and self-perceived balance as valid predictors and discriminators of independent community walking at 6 months post-stroke – a preliminary study. Disability and Rehabilitation. 2015;37(2):129–134. [DOI] [PubMed] [Google Scholar]
- 16.Resnik L, Borgia M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error. Physical therapy. 2011;91(4):555–565. [DOI] [PubMed] [Google Scholar]
- 17.Mukaka MM. A guide to appropriate use of correlation coefficient in medical research. Malawi medical journal. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- 19.Devlin M, Pauley T, Head K, Garfinkel S. Houghton Scale of prosthetic use in people with lower-extremity amputations: Reliability, validity, and responsiveness to change. Arch Phys Med Rehabil. 2004;85(8):1339–1344. [DOI] [PubMed] [Google Scholar]
- 20.Wong CK, Gibbs W, Chen ES. Use of the Houghton Scale to Classify Community and Household Walking Ability in People With Lower-Limb Amputation: Criterion-Related Validity. Arch Phys Med Rehabil. 2016;97(7):1130–1136. [DOI] [PubMed] [Google Scholar]
- 21.Sions JM, Arch ES, Horne JR. Self-reported functional mobility, balance confidence, and prosthetic use are associated with daily step counts among individuals with a unilateral transtibial amputation. Journal of physical activity and health. 2018;15(6):423–429. [DOI] [PubMed] [Google Scholar]
- 22.Wong CK, Gibbs W, Chen ES. Use of the Houghton scale to classify community and household walking ability in people with lower-limb amputation: criterion-related validity. Archives of physical medicine and rehabilitation. 2016;97(7):1130–1136. [DOI] [PubMed] [Google Scholar]
- 23.Sawers A, Kim J, Balkman G, Hafner B. Interrater and Test-Retest Reliability of Performance-Based Clinical Tests Administered to Established Users of Lower Limb Prostheses. Physical Therapy. 2020. [DOI] [PubMed] [Google Scholar]
- 24.Spaan MH, Vrieling AH, van de Berg P, Dijkstra PU, van Keeken HG. Predicting mobility outcome in lower limb amputees with motor ability tests used in early rehabilitation. Prosthetics and orthotics international. 2017;41(2):171–177. [DOI] [PubMed] [Google Scholar]
- 25.Gremeaux V, Damak S, Troisgros O, et al. Selecting a test for the clinical assessment of balance and walking capacity at the definitive fitting state after unilateral amputation: a comparative study. Prosthetics and orthotics international. 2012;36(4):415–422. [DOI] [PubMed] [Google Scholar]
- 26.Sabzi Sarvestani A, Taheri Azam A. Amputation: a ten-year survey. Trauma Mon. 2013;18(3):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohannon RW. Comfortable and maximum walking speed of adults aged 20—79 years: reference values and determinants. Age and ageing. 1997;26(1):15–19. [DOI] [PubMed] [Google Scholar]
- 28.Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60-94. Journal of aging and physical activity. 1999;7:162–181. [Google Scholar]
- 29.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. Journal of geriatric physical therapy. 2006;29(2):64–68. [DOI] [PubMed] [Google Scholar]
- 30.Fortington LV, Geertzen JHB, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and Long Term Mortality Rates after a Lower Limb Amputation. European Journal of Vascular and Endovascular Surgery. 2013;46(1):124–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


