Abstract
Background
Clostridioides difficile infections (CDIs) are among the most prevalent hospital-associated infections (HAIs), particularly for intensive care unit (ICU) patients. The risks for developing active CDI from asymptomatic carriage of C. difficile are not well understood.
Methods
We identified asymptomatic C. difficile carriage among 1897 ICU patients using rectal swabs from an existing ICU vancomycin-resistant enterococci (VRE) surveillance program. C. difficile isolates from VRE swabs, and from C. difficile–positive stool samples, were genome sequenced. Spatial-temporal data from hospital records assessed genomically identified clusters for potential transmission events.
Results
Genomic analyses identified a diverse set of strains in infected patients and asymptomatic carriers. A total of 7.4% of ICU patients asymptomatically carried C. difficile; 69% of isolates carried an intact toxin locus. In contrast, 96% of C. difficile stool isolates were toxin encoding. CDI rates in asymptomatic carriers of toxin-encoding strains were 5.3% versus 0.57% in noncarriers. The relative risk for CDI with asymptomatic carriage of a toxin-encoding strain was 9.32 (95% confidence interval, 3.25–26.7). Genomic identification of clonal clusters supported analyses for asymptomatic transmission events, with spatial-temporal overlaps identified in 13 of 28 cases.
Conclusions
Our studies provide the first genomically confirmed assessments of CDI relative risk from asymptomatic carriage of toxin-encoding strains and highlight the complex dynamics of asymptomatic transmission in ICUs. Asymptomatic carriers are an active reservoir of C. difficile in the nosocomial environment. C. difficile screening can be implemented within existing HAI surveillance programs and has the potential to support infection-control efforts against this pathogen.
Keywords: Clostridioides difficile, ICU, asymptomatic carriage, genomic epidemiology, screening
Relative risks for C. difficile infections increase to 9.32 in asymptomatic ICU patients carrying toxin-encoding strains. Integrated genomic and epidemiologic analyses illustrate the functional use of C. difficile genomic data to identify asymptomatic transmission events and assist in outbreak investigations.
Clostridioides difficile infection (CDI) is the most prevalent healthcare-associated pathogen [1], costing more than $5 billion annually in the United States, from more than 500 000 infections and more than 29 000 deaths [2]. Risks for CDI include chronic contact with healthcare systems, use of broad-spectrum antibiotics, and underlying medical conditions including inflammatory bowel disease or prior CDI [3]. Asymptomatic carriage has also been linked to increased risks for CDI [4]. Clostridioides difficile infection occurs from pathogen-released toxins, particularly toxins A and B, encoded by its pathogenicity locus [5]. A third toxin, binary toxin (CDT), is associated with more severe disease [6].
Asymptomatic carriers are thought to contribute to CDI in healthcare facilities [7–11], but their activity as pathogen reservoirs, and contributions to their own risks for CDI, are poorly defined [12–14]. One interventional study that placed asymptomatic carriers on contact precautions successfully decreased CDI [15, 16]. Healthcare workers have also been identified as potential carriers of toxin-encoding strains, but at rates reflective of the general population [17–19].
We hypothesized that asymptomatic carriage of C. difficile increased the risks for infections, and that carriers may provide an undetected reservoir for C. difficile transmission. To evaluate this hypothesis, we undertook intensive care unit (ICU) surveillance for C. difficile over an 8-month period. Analyses validated the sensitivity of a culture-based screening method for C. difficile using rectal swabs collected for an existing ICU screening program for vancomycin-resistant enterococci (VRE) [4, 13, 20, 21]. Clostridioide difficile was also isolated from toxin-positive stool samples collected from all hospitalized patients to compare genomic findings in asymptomatic carriers with symptomatic patients. Integrated genomic and epidemiologic analyses identified strain dynamics and supported the development of a platform leveraging clinical infrastructure and nationally available resources to improve surveillance efforts for C. difficile [22].
METHODS
Study Protocol
Brigham and Women’s Hospital (BWH) in Boston, Massachusetts, is a 793-bed hospital providing care to more than 600 000 patients per year, including more than 36 000 inpatient admissions. The hospital includes multiple ICUs. The study was carried out under Institutional Review Board protocol 2011-P-002883 (L. B., Partners Healthcare), which allows access to discarded clinical samples and medical records in support of hospital infection-control investigations and clinical laboratory assay development. The Crimson LIMS [24] was used for retrieval of clinically ordered VRE rectal surveillance swabs and C. difficile–positive stool samples over the 246-day study period. Clinical VRE-swab surveillance is performed only in the hospital ICUs. Swab retrieval occurred from days 48 to 199 from medical, surgical, and neurological ICUs (Figure 1), and C. difficile toxin B–positive stool collection throughout the hospital over the entire study period. Stool toxin B was detected by enzyme immunosorbent assay (EIA; C. diff Quik Chek Complete; Abbott, Abbott Park, IL), Samples with indeterminate results were tested by polymerase chain reaction (PCR) (GeneXpert C. difficile; Cepheid, Sunnyvale, CA). Patients with suspected or confirmed C. difficile infection are isolated under contact precautions, following Infectious Diseases Society of America guidelines [23], with donning of gowns and gloves by personnel upon room entry. Patient demographic and contact data were retrieved from the Partners Research Patient Data Registry and Theradoc [25], and were de-identified for analyses (Table 1, Supplementary Table 1).
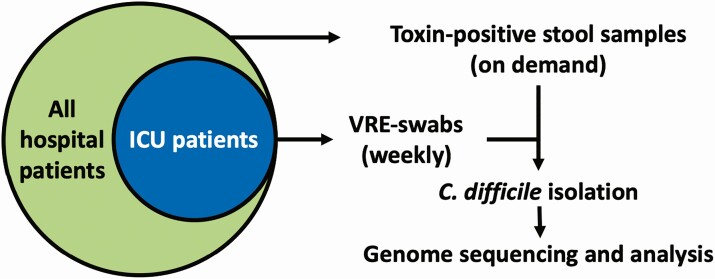
Figure 1.
Patient group and sample collections. ICU patients are a subset of all hospital patients. Clostridioides difficile isolation and strain sequencing were performed on toxin-positive stool samples from all hospital patients and on all VRE swabs collected from ICU patients. Abbreviations: ICU, intensive care unit; VRE, vancomycin-resistant enterococci.
Table 1.
Patient Demographic Data by Clostridioides difficile Colonization and Clostridioides difficile Infection Status
| ICU Patients | Non-ICU Patients | ||||
|---|---|---|---|---|---|
| Parameter | Noncolonized | Asympto-matically Colonized | CDI | CDI | P |
| Number of patients | 1734 | 135 | 28 | 150 | |
| Mean (SD) age, years | 62.5 (16.0) | 62.0 (15.7) | 57.9 (15.9) | 60.7 (15.3) | .317a |
| Sex, % female | 42.3 | 43.2 | 37.0 | 51.9 | .179b |
| Mean (SD) inpatient days across admissions | 13.8 (13.9) | 26.2 (12.1) | 38.8 (27.7) | 14.1 (16.3) | <.001a |
| Mean (SD) no. of inpatient admissions | 1.33 (0.8) | 1.61 (1.05) | 2.04 (1.12) | 1.38 (1.48) | <.001a |
| Mean (SD) inpatient admission length of stay, days | 11.9 (16.4) | 18.9 (15.9) | 26.3 (22.8) | 13.8 (11.8) | <.001a |
| Mean (SD) outpatient hospital visits | 5.03 (7.39) | 4.79 (7.34) | 5.36 (9.14) | 5.33 (7.45) | .013a |
| % Mortality within 30 days of last swab or stool culture | 17.3 | 28.9 | 17.9 | 16.7 | .009b |
Abbreviations: CDI, Clostridioides difficile infection; ICU, intensive care unit; ICU Patients, patients who spent time in an ICU over the study period; Non-ICU Patients, patients not in an ICU over the study period but who had a stool C. difficile isolate.
aHypothesis testing by Kruskall-Wallis test.
bHypothesis testing by chi-square analysis.
Validation of VRE Swabs for Detection of Clostridioides difficile Carriage
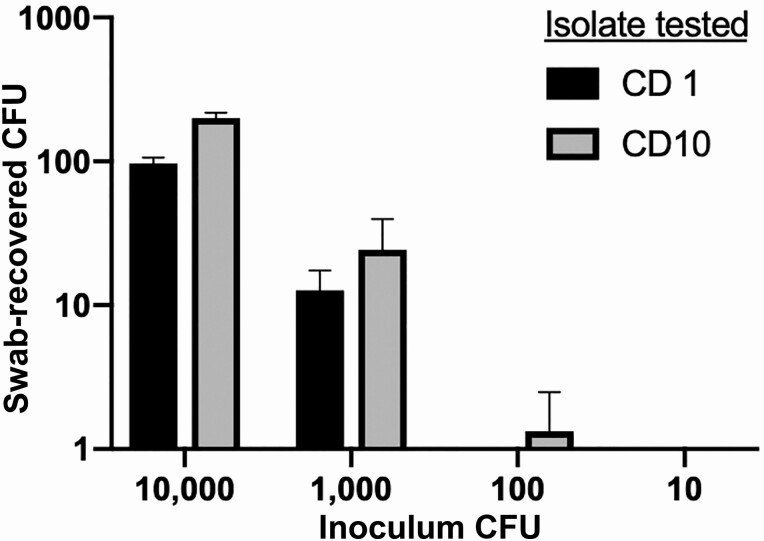
Control C. difficile isolates from prior patient samples were grown in brain-heart infusion broth and serially diluted from 1 × 105 to 102 colony-forming units (CFU)/mL. To follow clinical workflows, BD BBL CultureSwabs (BD, San Jose, CA) were inoculated with 100 μl of dilution, placed in transport tubes, and stored aerobically for 2 hours, then streaked to Spectra VRE agar (Thermo Scientific, Waltham, MA), returned to their transport tubes, and stored for another 2 hours prior to streaking to CHROMID C. difficile agar (Biomerieux, Durham, NC) with anaerobic incubation at 37°C for 24 hours. The input and recovered CFUs were quantified to assess sensitivity of detection and recovery (Figure 2).
Figure 2.
Sensitivity of VRE-swabs for Clostridioides difficile retrieval by culture. The x-axis shows the C. difficile CFU in inocula applied to VRE swabs before plating to VRE media, per standard clinical protocol and, 2 hours later, to CHROMID C. difficile agar. The y-axis shows recovered C. difficile CFU. Abbreviations: CFU, colony-forming units; VRE, vancomycin-resistant enterococci.
Sample Collection
Brigham and Women’s Hospital ICU patients are screened for VRE by rectal swab upon ICU admission and weekly thereafter. C. difficile toxin B–positive stool samples were retrieved after clinical testing. Samples were plated onto CHROMID C. difficile agar. Clostridioides difficile colonies were identified as gray to black colonies on CHROMID agar and were speciated by rapid ANA panels (Biomerieux, Durham, NC).
Genomic Analyses
Genome sequencing by Illumina MiSeq was performed as described (Illumina, San Diego, CA) [24]. Genome assembly was done using SPAdes [26] (Supplementary Data File 1). Toxin typing used reference tcdA and tcdB toxin genes from CD630 (AM180355.1) and the cdt toxin gene from strain R20291 (NC_013316.1). Gene calling used cutoffs of 80% reference gene length and 80% amino acid sequence identity. Single nucleotide polymorphism (SNP)–based analyses used the NCBI Pathogen Detection Isolates Browser (https://www.ncbi.nlm.nih.gov/pathogens/isolates) [22].
Clostridioides difficile Phylogenetic Analyses
Clostridioides difficile genomes from the Sequence Reads Archive (SRA) (https://ncbi.nlm.nih.gov/SRA), and NCBI Pathogen Detection Isolates Browser were downloaded for analyses. SPAdes draft assembled genomes passing the following criteria were used: genome sequence length within 3.7–5.0 Mb and <150 contigs, an L90 <30 (fewest number of contigs covering 90% of assembly), and an average coverage >25X. Analyses evaluated 3377 genomes in 173 SNP groups (Supplementary Data File 2).
A core C. difficile genome was created using tblastn with the CD630 reference genome. Feature identification used cutoffs of 80% or higher protein sequence identity and feature length within 20% of the reference protein length. Nucleotide alignments of the extracted genes used MAFFT [27].
A phylogeny of NCBI Pathogen Detection Isolates Browser isolates used up to 3 members from each SNP group. A 95% core genome of 2835 genes resulted in a 422 008b SNP matrix. Of these SNPs, 353 060 occurred in at least 95% of aligned positions, which was used to calculate a phylogeny using RAxML with 1000 bootstraps and GTRCAT [28]. The clade structure concurred with previous analyses [29] and includes 157 SNP clusters, 256 nonclustered single isolates, and several paraphyletic sequence types [30]. The tree is available online at https://itol.embl.de/tree/17022320725465491568233605.
Statistical Analyses
Statistical analyses used the Python package SciPy [31]. Differences in demographic data between patient groups were calculated using the Kruskal-Wallis test and Mann-Whitney post hoc test for continuous variables and χ 2 test for discrete variables. Multi-hypothesis–adjusted P values were calculated using the Benjamini Hochberg procedure [32]. Relative risk ratios were calculated by dividing the probability of developing CDI in the asymptomatically colonized (exposed) group of patients by the probability of developing CDI in the control (noncolonized) group (Table 2).
Table 2.
Relative Risk of Clostridioides difficile Infection per Toxin Status of Carried C. difficile Isolates in Intensive Care Unit Patients
| Relative Risk From Carriage of Toxin-encoding Strains | Developed CDI | Did Not Develop CDI |
|---|---|---|
| ICU patients carrying a toxin-encoding strain | 5 | 89 |
| ICU noncarriers and non–toxin-encoding carriers | 10 | 1742 |
| Relative risk from carriage of non–toxin encoding strains | Developed CDI | Did not develop CDI |
| ICU patients carrying a non–toxin-encoding strain | 0 | 45 |
| ICU noncarriers | 15 | 1786 |
CDI relative risk from carriage of toxin-encoding C. difficile: 9.32; 95% confidence interval: 3.25–26.7; P < .001. CDI relative risk from carriage of non–toxin-encoding C. difficile: 1.26; 95% confidence interval: .08–20.8; P = .87.
Abbreviations: CDI, Clostridioides difficile infection; ICU, intensive care unit.
RESULTS
Clostridioides difficile Surveillance Program
The CDI genomic screening pilot evaluated 2432 VRE swabs from 1897 ICU patients over 152 days, from which 172 C. difficile isolates (7.1% of swabs) were identified in 143 patients (7.5% of ICU patients) (Figure 1, Table 1, Supplementary Table 1). Asymptomatic colonization with C. difficile occurred in 7.4% of ICU patients (n = 140), including 5 who later developed CDI as diagnosed by toxin B stool testing. An additional 3 patients had CDI prior to culture of C. difficile from swabs. A total of 28 ICU patients (1.5% of ICU patients) had CDI diagnosed over the study period, 20 of whom did not have a prior positive swab.
Hospital-wide, toxin B–positive stool samples from ICU and non-ICU patients were cultured for C. difficile to define the genomic diversity of strains causing infections. A total of 178 toxin B–positive stool samples were cultured, with 98.3% of stool samples (n = 174) growing a C. difficile isolate. Of these, 16.1% (28 isolates of 174) were from ICU patients.
Association of Colonization Status With Hospital Admissions and Mortality
The ICU patients with asymptomatic C. difficile colonization or infection had an association with increased inpatient days across all admissions during the study period, as compared with noncolonized ICU patients (Table 1, row 4). ICU patients who developed CDI during the study period averaged 38.8 inpatient days and asymptomatically colonized ICU patients 26.2 days versus 13.8 days for noncolonized ICU patients (P < .001).
Asymptomatic carriage or CDI in ICU patients was also associated with increased lengths-of-stay per hospital admission, defined as days from admission to release (Table 1, row 5). ICU patients diagnosed with CDI demonstrated an average of 26.3 days per admission versus 18.9 for ICU asymptomatic carriers and 11.9 days for noncolonized ICU patients (P < .001).
Asymptomatically colonized versus noncolonized ICU patients also showed differences in the number of repeat admissions over the study period at 1.61 versus 1.33 admissions (P < .001) (Table 1, row 6). In contrast, ICU patients who developed CDI had higher repeat admissions of 2.04 (P = .017). Mortality rates by 30 days after the end of the rectal swab collection period were also higher in asymptomatically colonized versus noncolonized ICU patients at 28.9% versus 17.3%, respectively (P = .006) (Table 1, row 8).
Genomic Diversity of Hospital Clostridioides difficile Isolates
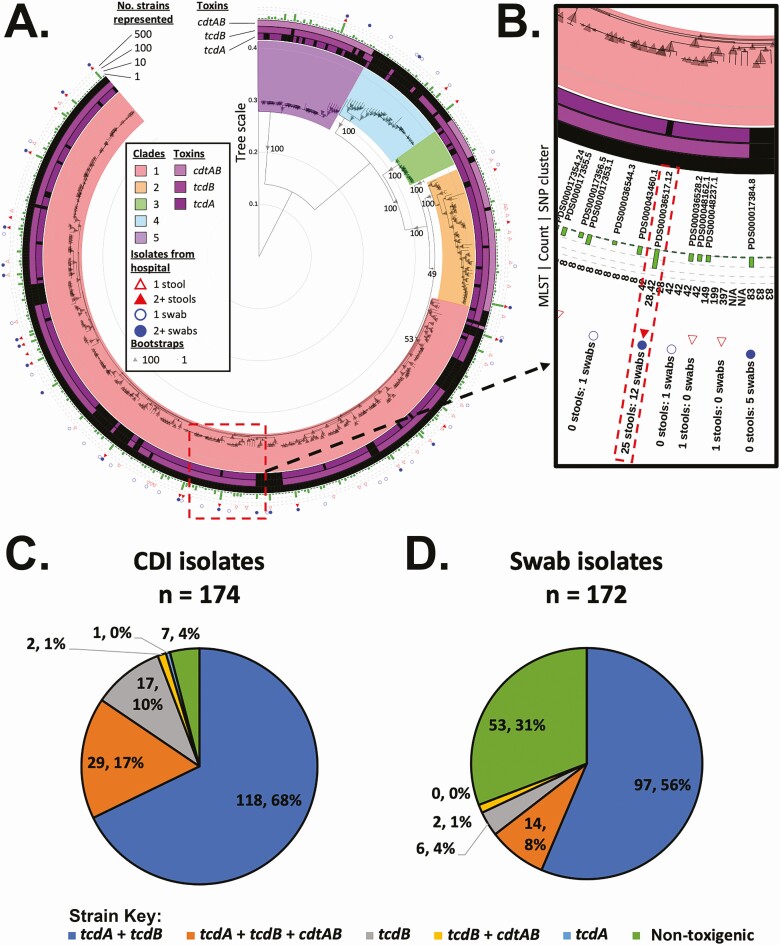
Strain genomic data were submitted to the NCBI Pathogen Detection resource to evaluate C. difficile hospital SNP clusters relative to datasets from other centers. Clusters were analyzed for clade designation and sequence type (ST). The 346 isolates from 309 patients were highly diverse and occurred primarily in clades 1 and 2 (Figure 3A and 3B). Within clade 2, 20 isolates clustered to ST 1 (NAP1/RT027, cdt toxin positive; 5.8% of isolates). Clade 2 strains were more than twice as likely to originate from toxin B–positive stool samples (24 isolates) than from VRE screening swabs (11 isolates; P = .035). The cdt toxin locus was identified in strains from clade 5, primarily in ST11 from SNP cluster PDS000017348 [33]. The CDT-encoding strains occurred more frequently from stool (n = 30) than VRE swabs (Figure 3C and 3D) (n = 16; P = .044).
Figure 3.
BWH patient isolates are genomically diverse. A, Clostridioides difficile SNP cluster tree of 157 SNP clusters, represented by 1 isolate, and 256 nonclustered isolates are shown. The outer ring shows the source of strains present in the branch (stool or VRE swab) and number of isolates. A bar graph in the second ring indicates the number of strains the branch represents in the NCBI Pathogen Detection Isolates Browser. The inner rings indicate presence (purple) or absence (black) of different toxin genes found within each clade. Clades are indicated by a colored box around the interior nodes that compose the clade. B, Inset of panel A showing an interactive view available online with the sequence types and strains represented in each leaf. C and D, Percentage of different toxin types from stool (C) or VRE swabs (D). The difference in non–toxin-encoding strains found in swabs versus stool samples is significant at P < .001. Abbreviations: BWH, Brigham and Women's Hospital; CDI, Clostridioides difficile infection; NCBI, National Center for Biotechnology Information; SNP, single nucleotide polymorphism; VRE, vancomycin-resistant enterococci.
Clade 4 included 2 distinct genetic groups, including ST37 isolates encoding toxin B but not toxin A, of which 7 originated from stool samples and 1 from a VRE swab, a proportion differing significantly from the remaining non–toxin-encoding clade 4 strains (n = 5; P = .01).
Among asymptomactially colonized ICU patients, 31.8% of isolates were non–toxin-encoding lacking both the tcd and cdt loci (53 isolates from 45 patients).
Longitudinal Strain Dynamics in Asymptomatic Intensive Care Unit Carriers
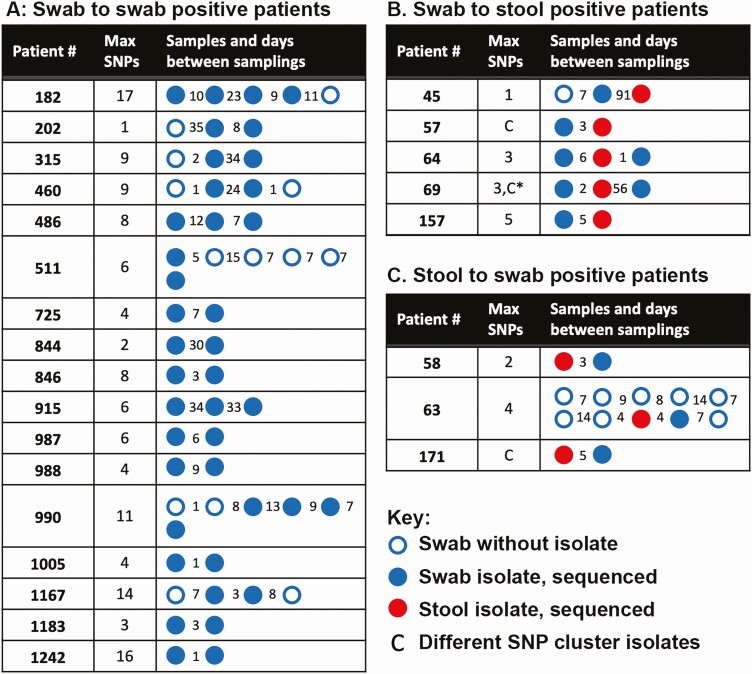
Twenty-five ICU patients (15.3% of all swab-positive patients), including 17 asymptomatic carriers, demonstrated longitudinal C. difficile carriage (Figure 4A). Within these isolate genomes, the maximum number of SNPs was 17, with an average of 7.5 SNPs per asymptomatic patient.
Figure 4.
Longitudinal Clostridioides difficile carriage. Patient samples are indicated with circles. Blue indicates a VRE swab and red a toxin-positive stool sample. Filled circles are samples that produced isolates for sequencing. The number of days between samples is indicated by the number between the circles. A, Asymptomatic carriers. Carried isolates remained in the same SNP cluster for all patients. B, Asymptomatic carriers who developed CDI. In patient 57, a strain from a different cluster was cultured from the positive stool sample. In patient 69, a strain different from the previously carried and CDI-causing strain was detected 56 days after CDI diagnosis and treatment. C, Patients with CDI with subsequent asymptomatic carriage. In patient 171, a different strain was identified 5 days after CDI diagnosis and start of therapy. Abbreviations: CDI, Clostridioides difficile infection; SNP, single nucleotide polymorphism; VRE, vancomycin-resistant enterococci.
Clostridioides difficile Infection Occurrence in Asymptomatic Carriers
Five asymptomatic ICU carriers developed active CDI (Figure 4B), representing 3.5% (5 of 140) of all asymptomatic carriers and 18% (5 of 28) of ICU patients who developed CDI. In 4 cases, the VRE-swab isolate was within 5 SNPs of the stool isolate and was considered clonally related. The fifth case, patient 57, developed CDI with a different epidemic ST37 strain, which occurred 3 days after detection of asymptomatic carriage. Four of five CDI diagnoses occurred within 1 week of asymptomatic carriage detection, while patient 45 was diagnosed 91 days after detection. Five separate ICU patients also had C. difficile–positive VRE swabs after CDI diagnosis. In patients 69 and 171, the swab isolates were distinct from the stool isolate, suggesting potential acquisition after clearance from treatment.
Relative Risks for Clostridioides difficile Infection From Asymptomatic Carriage of Toxin-encoding C. difficile
The relative risk for developing CDI from asymptomatic carriage of a toxin-encoding strain was 9.32 (95% confidence interval [CI], 3.25–26.7; P < .001). The 5 CDI cases from 140 asymptomatic ICU patients represented 3.6% of asymptomatic carriers. In contrast, carriage of a non–toxin-encoding strain did not increase risks for CDI (relative risk, 1.26; 95% CI, .08–20.8, P = .87); no carriers of non–toxin-encoding strains developed CDI over the study period (Table 2).
Genomic-epidemiological Investigations of Asymptomatic Transmission
Genomic cluster analyses identified related subclusters of isolates for spatial-temporal analyses to assess potential transmission events (Figure 4). Per the relatedness of longitudinal isolates from the same patient, thresholds of fewer than 17 SNPs and individual branch lengths within clusters of fewer than 15 SNPs were used to define clusters for analyses. Analyses identified 28 subclusters across 20 SNP groups, involving 76 isolates from 65 patients (Supplementary Data File 3). Analyses evaluated spatial-temporal linkages among hospital floors, wings, rooms, and bed spaces.
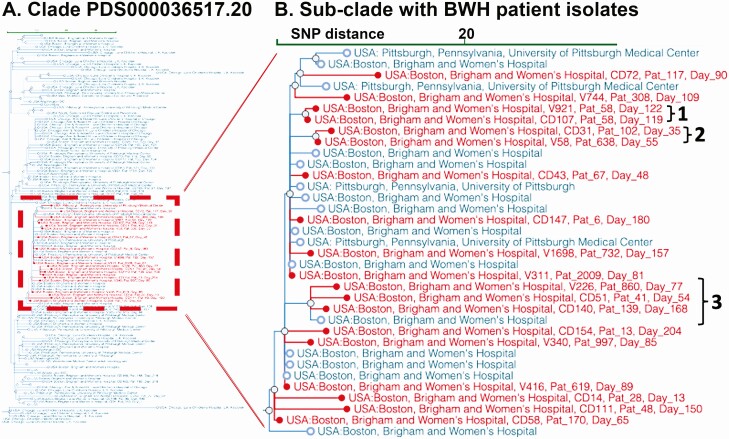
Within the 28 clusters, the largest group of genomically related strains occurred in SNP cluster PDS000036517 (Figure 5A), which included 19 isolates from the present study, 12 prior isolates from BWH, and 4 from the University of Pittsburgh (Figure 5B). Isolates within this subcluster were 2–34 SNPs apart, ruling out a single outbreak cluster. Three groups of BWH isolates within this subcluster met criteria for investigation but did not show subsequent spatial-temporal links among patients.
Figure 5.
Subclade selection of Clostridioides difficile genomic clusters by institution and region. The NCBI Pathogen Detection Isolates Browser provides a comparison tool for identifying outbreaks and relating them to other submitted isolate genomes. A, Subclade PDS000036517.20 (135 isolates). B, Subcluster of 35 strains within this clade that includes the largest set of related isolates (n = 19) from the present study (red). Blue entries show 16 prior samples from BWH and 4 from the University of Pittsburgh. The de-identified patient identifier and study day of isolate isolation are overlaid on the NCBI tree. “1” Shows 2 isolates from the same patient; “2” shows 2 closely related isolates that occurred within 20 days but with no identifiable patient spatial overlaps; “3” shows 5 isolates forming a related subclade that includes a sample from BWH submitted 2 years prior to the study (blue). Abbreviations: BWH, Brigham and Women's Hospital; NCBI, National Center for Biotechnology Information; SNP, single nucleotide polymorphism.
However, spatial-temporal overlaps among patients were identified in 13 other clonal clusters. These overlaps occurred from 2 to 210 days prior to culture of C. difficile isolates, with a median of 37 days (Figure 6, Supplementary Figure 1). Among cases, 26 of 28 patients shared a floor location, 15 shared a wing location, 3 a room, and 2 a bed space. Using a Poisson cumulative probability cutoff of 0.1, an investigative threshold used by local hospital-infection control teams to flag clusters for evaluation, the period where a repeat observation on the same floor could be considered linked is 258 days, and 447 days for observations from the same wing. The 28 cases flagged by initial genomic analyses of related strains fell within these time constraints.
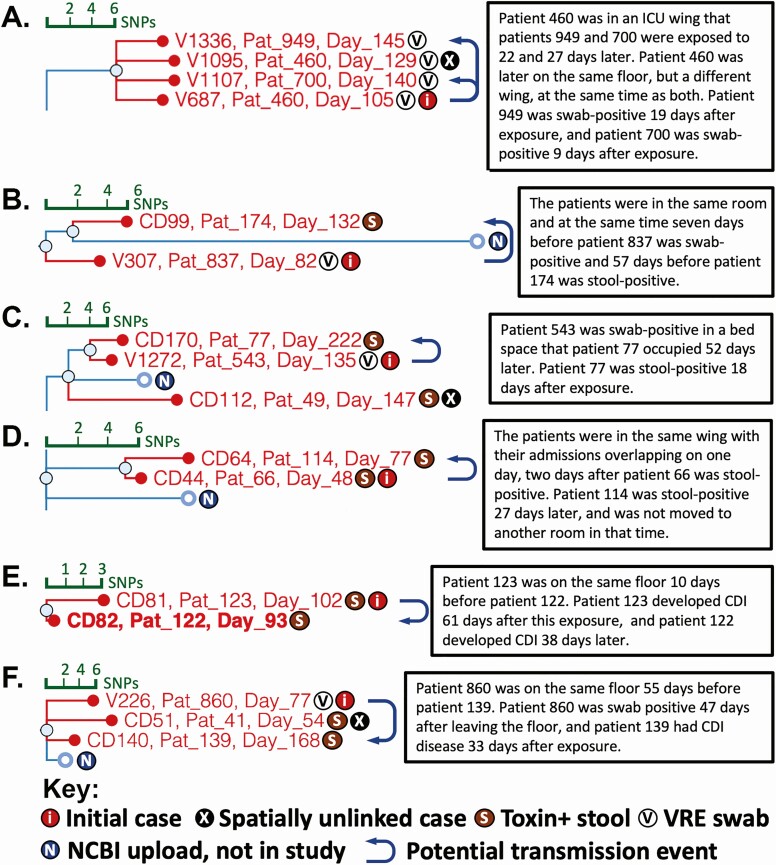
Figure 6.
Cases with genomic and spatial-temporal evidence for nosocomial transmission. SNP tree branches from the NCBI Pathogen Detection Isolates Browser are overlaid with the isolate identifier, de-identified patient number, and day within the study that the sample was collected. The key at the bottom indicates cases and sample types from which Clostridioides difficile was isolated. Arrows indicate potential transmission events based on genomic and hospital epidemiologic analyses of patient hospital location data. A–F, Example cases. Text boxes summarize supporting spatial and temporal information from integrated genomic-epidemiologic analyses. Abbreviations: CDI, Clostridioides difficile infection; ICU, intensive care unit; NCBI, National Center for Biotechnology Information; SNP, single nucleotide polymorphism; VRE, vancomycin-resistant enterococci.
Figure 6A shows an example analysis for asymptomatic transmission in genomically identified strain clusters. Patient 460 produced swab isolate V687 on day 105, 2 days after ICU admission. The patient remained colonized 24 days later during a second hospital admission to a different floor. Patients 949 and 700 had contact with the same ICU bed space and ICU wing, respectively, several weeks after patient 460’s discharge. Patient 949 was confirmed to be noncolonized by VRE swab upon ICU admission. Both patients tested positive for C. difficile by VRE swab after this exposure. Patients 460 and 949 both tested negative for C. difficile by stool EIA. While these patients were showing symptoms that led to CDI testing, the negative toxin EIA results suggest that the biomass of colonizing C. difficile was not elaborating sufficient toxin for detection. The 3 cases thus represent potential asymptomatic transmission events from an initial asymptomatic carrier.
Other potential asymptomatic transmission cases demonstrated longer periods between spatial overlaps, including up to 52 days apart (Figure 6B–F, Supplementary Figure 1).
DISCUSSION
This study provides the first detailed genomic and epidemiologic analyses of asymptomatic C. difficile carriage in ICU patients. The relative risk for developing active infection from asymptomatic carriage of a toxin-encoding strain was 9.32. Carriage was also associated with increased hospital lengths of stay and re-admissions during the study period, as well as 67% increased mortality in carriers when compared with mortality rates in noncolonized ICU patients. However, these findings are not definitively causal relative to other clinical factors, including that asymptomatic carriers had higher overall exposure to the healthcare system, a factor that increases risks for C. difficile colonization. The potential for asymptomatic carriage to cause subclinical disease in patients with underlying comorbidities nonetheless raises a critical question on benefits of ICU screening for C. difficile to identify carriers, not only to reduce reservoirs for transmission but to also reduce longer-term comorbidities and mortality in carriers. Screening of vulnerable patient populations for C. difficile carriage also has the potential to inform use of antibiotics and other clinical interventions to reduce risks for CDI [34].
Integrated genomic and epidemiologic analyses identified multiple potential transmission events from patients with CDI and asymptomatic carriers to other patients. Analyses identified a 258-day window in which patient spatial overlaps were significantly associated with potential transmission.
Asymptomatic carriers longitudinally carried the same strain, sometimes over months [35, 36]. Furthermore, asymptomatically carried strains caused active CDI in 4 of the 5 cases identified. In cases of extended periods between identification of asymptomatic carriage and CDI, we note that our findings cannot rule out infection from direct carriage versus strain re-introduction from spores that persist in the patient’s environment [37].
We validated a culture-based method for C. difficile screening, leveraging rectal swab samples from an existing VRE surveillance program to reduce the complexity of implementation. For centers with anaerobic culturing capabilities, screening costs include the selective agar, species and toxin confirmation, quality programs for testing, and efforts of clinical laboratory and infection-control personnel to perform the testing and act upon results. The majority of cultures are negative and can be reported within 24 hours, offering a more cost-effective screening option over molecular methods [13, 20, 21]. As 30% of asymptomatic carriers were colonized with non–toxin-encoding strains, a finding that did not elevate risks for CDI, confirmation of toxin production or carriage by EIA or PCR is warranted. Swab-based detection of toxin-encoding C. difficile preceded 18% of ICU CDI cases, providing an opportunity for early interventions, while also potentially preventing further asymptomatic transmission. Hospital-onset CDI costs an average of more than $34 000 per patient. Thus, prevention of even a subset of cases can bring significant savings [38].
A study placing C. difficile–colonized patients on contact precautions saw significant reductions in CDI [15]. As 5.1% of ICU-admitted patients were found to carry a toxin-encoding strain of C. difficile, the number of patients put on contact precautions would increase, potentially introducing burdens on clinical infrastructure. Informed by local rates of C. difficile carriage and CDI, healthcare facilities can assess the utility of screening by incorporating logistical and economic costs, as well as clinical actions to take upon identifying asymptomatic carriage [39, 40].
Our epidemiologic analyses used an SNP cutoff of up to 17 SNPs to flag potential clonal clusters, a cutoff defined from analyses of longitudinal isolates from the same patient. Incorporation of strain genomic and patient spatial-temporal information identified potential C. difficile transmission events among 65 patients, 21% of the 315 patients who produced isolates during the study. Analyses also validated the use of publicly available SNP calling tools for C. difficile in the NCBI Pathogen Detection Isolates Browser. As more institutions contribute C. difficile genomic data, higher resolution analyses may be undertaken, particularly given the widespread nature of CDI across healthcare institutions.
Asymptomatic carriers of C. difficile provide a significant and hidden pathogen reservoir that can have adverse effects for carriers, other patients, and healthcare workers. We demonstrate constructive use of existing hospital surveillance programs and nationally available genomic tools and resources for C. difficile surveillance within an ICU setting. Leveraging this model, institutions can make informed decisions regarding the utility of screening to reduce CDI incidence.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Andy Onderdonk and Jessica Allegretti for comments on the findings and their clinical relevance.
Financial support. This work was supported by the Hatch Family Foundation, the BWH Precision Medicine Institute, and NIDDK grant P30-DK034854. The work of J. W. was supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health.
Potential conflicts of interest. M. K. reports grants from Rx Foundation, during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol 2011; 8:17–26. [DOI] [PubMed] [Google Scholar]
- 4. Meltzer E, Smollan G, Huppert A, et al. ; SHIC Research Group . Universal screening for Clostridioides difficile in a tertiary hospital: risk factors for carriage and clinical disease. Clin Microbiol Infect 2019; 25:1127–32. [DOI] [PubMed] [Google Scholar]
- 5. Monot M, Eckert C, Lemire A, et al. Clostridium difficile: new insights into the evolution of the pathogenicity locus. Sci Rep 2015; 5:15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerding DN, Johnson S, Rupnik M, Aktories K. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014; 5:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheth PM, Douchant K, Uyanwune Y, et al. Evidence of transmission of Clostridium difficile in asymptomatic patients following admission screening in a tertiary care hospital. PLoS One 2019; 14:e0207138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 2014; 59:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ponnada S, Guerrero DM, Jury LA, et al. Acquisition of Clostridium difficile colonization and infection after transfer from a Veterans Affairs Hospital to an affiliated long-term care facility. Infect Control Hosp Epidemiol 2017; 38:1070–6. [DOI] [PubMed] [Google Scholar]
- 10. Guerrero DM, Becker JC, Eckstein EC, et al. Asymptomatic carriage of toxigenic Clostridium difficile by hospitalized patients. J Hosp Infect 2013; 85:155–8. [DOI] [PubMed] [Google Scholar]
- 11. Terveer EM, Crobach MJ, Sanders IM, Vos MC, Verduin CM, Kuijper EJ. Detection of Clostridium difficile in feces of asymptomatic patients admitted to the hospital. J Clin Microbiol 2017; 55:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kong LY, Eyre DW, Corbeil J, et al. Clostridium difficile: investigating transmission patterns between infected and colonized patients using whole genome sequencing. Clin Infect Dis 2019; 68:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curry SR, Schlackman JL, Hamilton TM, et al. Perirectal swab surveillance for Clostridium difficile by use of selective broth preamplification and real-time PCR detection of tcdB. J Clin Microbiol 2011; 49:3788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donskey CJ, Sunkesula VCK, Stone ND, et al. Transmission of Clostridium difficile from asymptomatically colonized or infected long-term care facility residents. Infect Control Hosp Epidemiol 2018; 39:909–16. [DOI] [PubMed] [Google Scholar]
- 15. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med 2016; 176:796–804. [DOI] [PubMed] [Google Scholar]
- 16. Xiao Y, Paquet-Bolduc B, Garenc C, et al. Impact of isolating Clostridium difficile carriers on the burden of isolation precautions: a time series analysis. Clin Infect Dis 2018; 66:1377–82. [DOI] [PubMed] [Google Scholar]
- 17. Kato H, Kita H, Karasawa T, et al. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol 2001; 50:720–7. [DOI] [PubMed] [Google Scholar]
- 18. van Nood E, van Dijk K, Hegeman Z, Speelman P, Visser CE. Asymptomatic carriage of Clostridium difficile among HCWs: do we disregard the doctor? Infect Control Hosp Epidemiol 2009; 30:924–5. [DOI] [PubMed] [Google Scholar]
- 19. Hell M, Sickau K, Chmelizek G, et al. Absence of Clostridium difficile in asymptomatic hospital staff. Am J Infect Control 2012; 40:1023–4. [DOI] [PubMed] [Google Scholar]
- 20. Jazmati N, Kirpal E, Piepenbrock E, Stelzer Y, Vehreschild MJGT, Seifert H. Evaluation of the use of rectal swabs for laboratory diagnosis of Clostridium difficile infection. J Clin Microbiol 2018; 56. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6018322/. Accessed 4 October 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers DS, Kundrapu S, Sunkesula VC, Donskey CJ. Comparison of perirectal versus rectal swabs for detection of asymptomatic carriers of toxigenic Clostridium difficile. J Clin Microbiol 2013; 51:3421–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The NCBI Pathogen Detection Project. 2016. Available at: https://www.ncbi.nlm.nih.gov/pathogens/.
- 23. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nudel K, Zhao X, Basu S, et al. Genomics of Corynebacterium striatum, an emerging multi-drug resistant pathogen of immunocompromised patients. Clin Microbiol Infect 2018; 24:1016.e7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy S, Churchill S, Bry L, et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 2009; 19:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013; 30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dingle KE, Elliott B, Robinson E, et al. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol Evol 2014; 6:36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williamson CHD, Stone NE, Nunnally AE, et al. A global to local genomics analysis of Clostridioides difficile ST1/RT027 identifies cryptic transmission events in a northern Arizona healthcare network. Microb Genom 2019; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0—fundamental algorithms for scientific computing in Python. arXiv 2019. [DOI] [PMC free article] [PubMed]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57:289–300. [Google Scholar]
- 33. Knight DR, Kullin B, Androga GO, et al. Evolutionary and genomic insights into Clostridioides difficile sequence type 11: a diverse zoonotic and antimicrobial-resistant lineage of global one health importance. mBio 2019; 10. Available at: https://mbio.asm.org/content/10/2/e00446-19. Accessed 7 January 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allegretti JR, Mullish BH, Kelly C, Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019; 394:420–31. [DOI] [PubMed] [Google Scholar]
- 35. Mac Aogáin M, Moloney G, Kilkenny S, et al. Whole-genome sequencing improves discrimination of relapse from reinfection and identifies transmission events among patients with recurrent Clostridium difficile infections. J Hosp Infect 2015; 90:108–16. [DOI] [PubMed] [Google Scholar]
- 36. Sim JH, Truong C, Minot SS, et al. Determining the cause of recurrent Clostridium difficile infection using whole genome sequencing. Diagn Microbiol Infect Dis 2017; 87:11–6. [DOI] [PubMed] [Google Scholar]
- 37. Shaughnessy MK, Bobr A, Kuskowski MA, et al. Environmental Contamination in Households of Patients with Recurrent Clostridium difficile Infection. Appl Environ Microbiol 2016; 82:2686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis 2016; 16. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5000548/. Accessed 8 October 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar N, Miyajima F, He M, et al. Genome-based infection tracking reveals dynamics of Clostridium difficile transmission and disease recurrence. Clin Infect Dis 2016; 62:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eyre DW, Cule ML, Wilson DJ, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013; 369. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3868928/. Accessed 7 October 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.