Abstract
Gestational diabetes mellitus (GDM) can have adverse effects on pregnancy. GDM is associated with changes in the lipid profile of pregnant women. Finding out the early ways to diagnose GDM can prevent the adverse outcomes. This meta-analysis study aimed to determine the effect of GDM on lipid profile. PubMed, ProQuest, Web of Science, Scopus, Science Direct, Google Scholar, and ClinicalTrial were systematically searched for published articles relating to GDM until 2021 according to PRISMA guidelines. Newcastle Ottawa scale was used to assess the quality of the studies. Thirty-three studies with a sample size of 23,792 met the criteria for entering the meta-analysis. Pooled standardized mean difference (SMD) for total cholesterol (TC) and triglyceride (TG) was 0.23 mg/dL (95% CI: 0.11–0.34) and 1.14 mg/dL (95% CI: 0.91–1.38), respectively. The mean of TC and TG in people with GDM was higher than that in normal pregnant women. A similar pattern was observed for the very low-density lipoprotein (VLDL) and TG/high-density lipoprotein (HDL) ratio, with pooled SMD of 0.99 mg (95% CI: 0.71–1.27) and 0.65 mg (95% CI: 0.36–0.94), respectively. Pooled SMD for HDL was −0.35 mg/dL (95% CI: −0.54 to −0.16), women with GDM had a mean HDL lower than normal pregnant women. Although pooled SMD was higher for low-density lipoprotein (LDL) in the GDM group, this difference was not significant (0.14 [95% CI: −0.04 to 0.32]). Of all the lipid profiles, the largest difference between the GDM and control groups was observed in TG (SMD: 1.14). Elevated serum TG had the strongest effect on GDM. Higher levels of TC, LDL, VLDL, and TG/HDL ratio, and lower level of HDL were exhibited in GDM group. So, these markers can be considered as a reliable marker in the diagnosis of GDM.
Keywords: gestational diabetes mellitus, lipid profile, triglyceride, TG/HDL ratio, total cholesterol, LDL-C, HDL-C, VLDL-C
1. Introduction
Gestational diabetes mellitus (GDM) is the most common metabolic disorder during pregnancy and is defined as diabetes identified in the second or third trimester of pregnancy that was not previously known. A possible cause of GDM is an exacerbation of physiological changes in glucose metabolism during pregnancy [1]. Pregnancy as a complex process leads to physiological changes in the female body. Most pregnant women go through pregnancy safely; however, some of them develop complications such as gestational diabetes. Myo-inositol and d-chiro-inositol are natural compounds involved in many biological pathways and both are currently well tolerated. They are effective alternatives to classical insulin sensitizers and are useful in the prevention and treatment of metabolic and reproductive disorders such as polycystic ovary syndrome and GDM [2,3]. In the last decade, the prevalence of GDM has increased due to inactivity, obesity, and increasing age of mothers. One in ten pregnancies is diagnosed with diabetes, 90% of which is identified as GDM. The prevalence of GDM is estimated at 17% worldwide. It is reported to be 10% in North America and 25% in Southeast Asia, depending on population, region, diagnostic criteria, and methods of data collection [4]. According to the World Health Organization (WHO), diabetes is reported as the seventh cause of human death [5]. GDM is considered as a silent disease that can have adverse effects on the mother and fetus and lead to undesirable consequences such as polyhydramnios, pre-eclampsia, stillbirth, fetal macrosomia, hyperbilirubinemia, hypocalcemia, hypoglycemia, respiratory distress syndrome, and polycythemia on mother and fetus [6]. On the other hand, the risk of developing type 2 diabetes, metabolic syndrome, and cardiovascular problems will increase in the mother with GDM and her child in the future [7]. GDM is also a serious concern for any system with increasing use of health and care resources and adverse outcomes, many of which can be mitigated by early diagnosis and treatment [8]. GDM is associated with physiological changes in the lipid profile of pregnant women [9]. A lipid profile is a direct measure of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C) [10]. During early pregnancy, the increase in maternal fat depots is facilitated by insulin, followed by increased adipose tissue breakdown, and subsequent hypertriglyceridemia, mainly due to insulin resistance and estrogen effects [11]. It is known that many factors affect lipid levels in GDM because carbohydrate metabolism directly affects lipid metabolism. There is still controversy over the association between lipid profile and GDM [12]. Although lipid levels have been extensively studied during pregnancy, there are conflicting results in this regard. There are also few studies on whether fat patterns are different in women with GDM in the first trimester of pregnancy [9]. Since changes in fat metabolism during pregnancy can be associated with adverse pregnancy outcomes such as GDM, this comprehensive systematic review and meta-analysis aimed to determine the effect of GDM on lipid profile and this study was performed to update the previous results and find reliable data in order to complete the existing knowledge.
2. Materials and methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were observed in the report of the study. PRISMA contains 27 items related to the content of a systematic and meta-analysis, and includes abstracts, methods, results, discussions, and financial resources [13,14,15]. This study was approved by ethnical code IR.ABZUMS.REC.1399.140.
3. Information source and search strategy
PubMed, Web of Science, Scopus, Google Scholar ProQuest, and ClinicalTrials were searched until 2021 by MESH keywords and search strategy was as below:
‘Gestational diabetes’[tiab], OR ‘GD’ [tiab], OR ‘Gestational Diabetes Mellitus’ [tiab], OR ‘GDM’[tiab], OR ‘pregnancy induced diabetes’[tiab], ‘Diabetes, Pregnancy-Induced’[tiab], ‘Diabetes, Pregnancy Induced’[tiab], ‘Diabetes Mellitus, Gestational’[tiab]
‘lipid profile’[tiab], OR ‘total cholesterol’[tiab], OR ‘high-density lipoprotein-cholesterol’[tiab], OR ‘low-density lipoprotein-cholesterol’[tiab], OR ‘Very low-density lipoprotein-cholesterol’[tiab], OR ‘triglycerides’[tiab], OR ‘TC’[tiab], OR ‘LDL-C’[tiab], OR ‘HDL-C’[tiab], OR ‘VLDL-C’ [tiab], OR ‘TG’[tiab]
‘Screening’[tiab], OR ‘Predicting’[tiab]
1 AND 2
1 AND 3
1 AND 2 AND 3
4. Eligibility criteria
4.1. Inclusion and exclusion criteria
Studies were included if they were published until 2021, full-text available, and with no language restrictions. Other inclusion criteria were: single pregnancy, GDM based on the criteria, and gestational age considered for each study based on ultrasound. Participation, intervention, comparators, outcomes, and study design (PICOS) criteria including:
Population: pregnant women
Exposure: serum lipid concentration
Comparison: healthy control group
Outcome: GDM
Study design: cohort, case control, and cross sectional
4.2. Exclusion criteria
Multiple pregnancies, smoking and alcohol use, a history of type 1 and type 2 diabetes, a history of pre-pregnancy hyperlipidemia, a history of hypertension/cardiovascular disease, a history of metabolic syndrome, a history of other systemic diseases such as liver failure, chronic renal failure, endocrine disorders, and autoimmune diseases. Case reports, qualitative, and review studies, as well as research with missing data, were also excluded.
4.3. Study selection
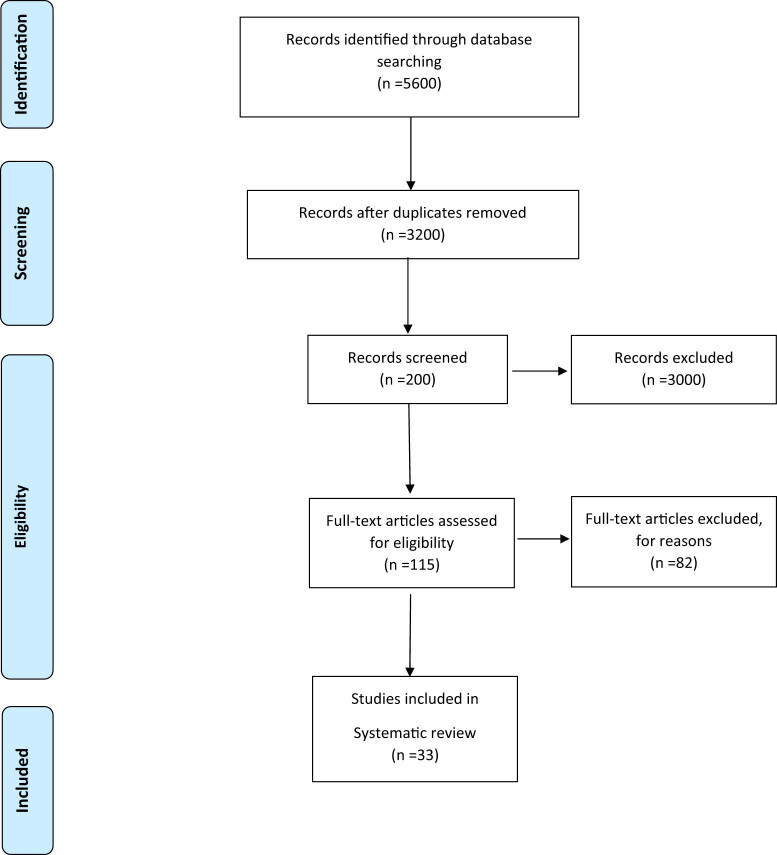
The EndNote reference management software was applied to manage the acquired articles. The initial search yielded 5,600 results. The eligibility of these articles was independently evaluated by two authors and any disagreements were resolved by consensus. In the first stage 2,400 articles were excluded due to being irrelevant or duplicated. After reviewing the titles and abstracts of the remaining articles, 3,000 more papers were excluded. In the evaluation of the full texts, 82 out of the remaining 115 articles were excluded due to being ineligible. Finally, a total of 33 eligible articles were reviewed (Figure 1).
Figure 1.
Flowchart of the study selection process.
4.4. Quality assessment
Newcastle Ottawa scale was used to measure the quality of studies. This scale is used to measure the quality of cohort and case control studies. The validity and reliability of this tool have been proven in various studies [16,17].
5. Data extraction
Two authors independently performed the study selection and validity assessment and resolved any disagreements by consulting a third researcher. The first author name, year, study design, country, sample size, maternal age, maternal BMI or weight, diagnostic criteria, methods of analysis, quality assessment, gestational age at sampling, TC, LDL-C, HDL-C, TG, VLDL-C, TG/HDL-C ratio, and outcomes.
6. Unification of units
All lipid profiles were converted to mg/dL. For conversion of TC, HDL, and LDL from SI units mmol/L to mg/dL, the values were multiplied with 38.67. For conversion of TG from SI units mmol/L to mg/dL, the values were multiplied with 88.57. In order to calculation of VLDL, we used TG/5.
7. Statistical analysis
All analyses were conducted with Stata software version 14.0 (College Station, Texas). For each study, mean value and standard deviation (SD) of lipid profile were extracted and if IQR was reported we changed it to SD with IQR/1.35. Then, standardized mean difference (SMD) of lipids profile for each study was calculated based on Cohen’s d formula:
where M 1, n 1, and SD1, and M 2, n 2, and SD2 are mean values, samples size, and SDs in GDM and control groups, respectively. Some studies reported odds ratio (OR) and for calculating the SMD and standard error (Se), we used below formula:
where log OR and π are the natural logarithm odds ratio and 3.14, respectively. Then, pooled SMD was calculated by “Metan” command [18]. Heterogeneity was determined using Cochran’s Q test of heterogeneity, and the I 2 index was used to quantify heterogeneity. In accordance with Higgins classification approach, I 2 values above 0.7 were considered as high heterogeneity. To estimate the pooled SMD for lipid profile and for subgroup analysis (based on trimester), the fixed-effect model was used, and when the heterogeneity was greater than 0.7, the random effects model was used. The meta-regression analysis was used to examine the effect of age, BMI, sample size, and publication date as factors affecting heterogeneity among studies. The “Meta bias” command [19] was used to check for publication bias, and if there was any publication bias, the pooled SMD was adjusted with the “Meta trim” command using the trim-and- fill method [20]. In all analyses, a significance level of 0.05 was considered [21].
8. Results
Finally, 33 studies with a sample size of 23,792 met the criteria for entering the meta-analysis (Table 1). Figure 1 also shows the flowchart of the study selection process. Serum lipid concentration between the groups with and without GDM of included studies is given in Table 2.
Table 1.
The characteristics of included studies
| Author (year) | SD | Country | SS | Maternal age (year) | BMI (kg/m2) or Weight (kg) | Diagnostic criteria of GDM | Method of analysis test | QAS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM | Control | GDM | Control | GDM | Control | ||||||
| Farsangi et al., 2020 [41] | CC | Iran | 42 | 42 | 29.62 ± 0.80 | 27.54 ± 0.95 | 23.51 ± 3.37 | 22.80 ± 3.18 | ADA | Enzymatic assay using commercial kits (Pars Azmun Inc, Iran). | 8 |
| Hossain et al., 2020 [42] | CS | Bangladesh | 31 | 31 | 26.5 | 26.3 | WHO | Enzymatic-colorimetric method | 8 | ||
| Saumya 2020 [43] | CS | India | 51 | 149 | 32.29 ± 4.42 | 30.15 ± 4.33 | — | IADPSG | HDL and TG were estimated by glucose oxidase–peroxidase method, cholesterol oxidase–peroxidase method (CHOD–POD), cholesterol oxidase–cholesterol esterase method, and glycerol phosphate oxidase method. Plasma LDL-C was calculated using Friedewald’s formula. | 7 | |
| Wang et al., 2019 [9] | C | China | 300 | 1,283 | 32.65 ± 3.92 | 31.53 ± 3.68 | 23.22 ± 3.49 | 21.87 ± 2.97 | ADA | Automatic biochemical analyzer | 8 |
| Layton et al., 2019 [38] | C | Canada | 67 | 739 | 30 | 31 | 27.0 (22.0–32.4)* | 23.9 (21.5–27.5)* | IADPSG | Colorimetric method (Johnson & Johnson Clinical Diagnostics) | 9 |
| Correa et al., 2019 [26] | CC | Chile | 16 | 80 | 32.63 ± 6.36 | 29.88 ± 5.75 | 26.55 (6.29)* | 24.9 (4.2)* | IADPSG | VITROS Chemistry Products CHOL Slides, ©Ortho-Clinical Diagnostics, Inc., Buckinghamshire, UK | 9 |
| Aydemir et al., 2019 [40] | CC | Turkey | 99 | 98 | 33.39 ± 4.92 | 32.29 ± 4.62 | 31.09 ± 4.841 | 27.91 ± 3.99 | Department of Obstetrics and Gynecology, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey | Automated latex-enhanced immunoassay | 8 |
| Anjum et al., 2019 [28] | CS | Saudi Arabia | 25 | 50 | 31.4 ± 6.06 | 29.7 ± 6.12 | NR | NR | ADA | Colorimetric method | 7 |
| Alyas et al., 2019 [44] | CC | Pakistan | 58 | 100 | 18–40 | NR | NR | ADA | Clinical chemistry analyzer | 7 | |
| Yue and Ying, 2019 [45] | CC | China | 88 | 456 | 31.86 ± 0.47 | 29.98 ± 0.21 | 23.97 ± 0.5 | 21.22 ± 0.14 | ACOG | Automatic biochemical analyzer | 9 |
| Zebunnesa et al., 2018 [46] | CS | Bangladesh | 30 | 30 | 28.70 ± 3.95 | 28.76 ± 5.47 | NR | NR | IADPSG | Multisystem automatic analyzer | 8 |
| Cao et al., 2018 [47] | CC | China | 33 | 33 | 29.20 ± 1.03 | 28.70 ± 1.16 | 34 | 29 | ADA | Automatic biochemistry analyzer | 8 |
| Bukowiecka-Matusiak et al., 2018 [39] | CC | Poland | 32 | 11 | 31.0 (28–35)* | 29.0 (28–30) | 23.7 (21.4–26.3)* | 20.9 (20.4–21.3)* | WHO | NR | 8 |
| Bugatto et al., 2018 [48] | C | Spain | 22 | 23 | 31.4 + 6.0 | 30.5 + 4.5 | 26.6 + 6.0 | 25.2 + 6.5 | National Diabetes Data Group | Modular DPD biochemical auto-analyzer | 9 |
| Barat et al., 2018 [49] | CS | Iran | 250 | 87 | 30.49 ± 4.00 | 27.33 ± 4.87 | 28.5 ± 3.73 | 25.72 ± 4.33 | ADA | Ziestchem Diagnostic Tehran | 8 |
| Bao et al., 2018 [50] | CC | USA | 107 | 214 | 18–40 | 19–45 | ACOG | Enzymatic assays using Roche COBAS 6000 Chemistry Analyzer | 8 | ||
| Pazhohan, et al., 2017 [51] | C | Iran | 176 | 778 | 27.47 ± 3.54 | 24.23 ± 3.21 | 26.84 ± 3.87 | 24.28 ± 3.04 | IADPSG | NR | 8 |
| Wang et al., 2017 [52] | C | China | 5,218 | — | 28.52 ± 3.86 | — | 21.59 ± 3.23 | — | Chinese criteria | NR | 8 |
| Ghodke et al., 2017 [53] | C | India | 200 | — | 24.87 ± 2.7 | — | NR | NR | NR | AU480 biochemistry auto analyzer by CHOD–POD method | 7 |
| Chen et al., 2017 [24] | CC | China | 28 | 56 | 33.0 (30.3, 36.0)* | 30.0 (28.0, 33.0)* | 20.6 ± 2.5 | 20.1 ± 2.2 | IADPSG | Particle number analysis method | 8 |
| Wang et al., 2016 [54] | CC | China | 1,062 | 4,203 | 29.46 ± 3.96 | 28.29 ± 3.79 | 22.52 ± 3.36 | 21.33 ± 3.03 | IADPSG | NR | 8 |
| Shen et al., 2016 [55] | C | China | 188 | 1,122 | 30.56 ± 3.47 | 29.55 ± 3.13 | 22.07 ± 2.93 | 20.79 ± 2.9 | IADPSG | Automatic biochemical analyzer | 9 |
| Liang et al., 2016 [56] | CC | China | 55 | 50 | 28.2 ± 5.1 | 27.1 ± 5.4 | 22.7 ± 1.7 | 22.1 ± 2.1 | National Diabetes Data Group | Tinder enzymatic method | 8 |
| Khosrowbeygi et al., 2016 [57] | CS | Iran | 30 | 30 | 32.63 ± 0.72 | 28.53 ± 0.94 | 25.00 ± 0.23 | 24.84 ± 0.28 | ADA | Available photometric methods | 8 |
| Jin et al., 2016 [58] | CS | China | 934 | — | 29.21 ± 3.76 | — | 20.66 ± 2.70 | — | IADPSG | Automatic biochemical analyzer | 8 |
| Han et al., 2016 [59] | CC | USA | 254 | 490 | 27.8 ± 5.5 | 27.9 ± 5.2 | 26.1 ± 6.5 | 23.7 ± 4.6 | Carpenter and Coustan | Kodak Ektachem Chemistry analyzer | 7 |
| Ertug et al., 2016 [60] | CS | Turkey | 29 | 20 | 32 ± 4 | 27 ± 5 | 27.6 (25.5–29.9) | 26.0 (23.5 – 28.0) | Carpenter and Coustan | Standard enzymatic colorimetric methods | 8 |
| Wang et al., 2015 [61] | CS | China | 110 | 526 | 31 (29–34)* | 29 (27–31)* | 21.02 (19.24–22.56)* | 20.03 (18.59–21.55)* | Ministry of Health China | Automatic chemistry analyzer | 8 |
| Li et al., 2015 [62] | C | China | 379 | 2,166 | 31.60 ± 4.25 | 30.40 ± 7.36 | 22.57 + 4.75 | 20.81 + 5.45 | ADA | End-point colorimetric method | 7 |
| dos Santos-Weiss et al., 2013 [63] | CC | Brazil | 288 | 288 | 33.1 (30.0–37.0)* | 32.5 (28.0–34.0)* | 33.4 ± 6.4 | 26.1 ± 4.7 | ADA | Automated system Architect Ci8200 | 9 |
| Khan et al., 2012 [64] | CS | Pakistan | 103 | 97 | ≥30 | ≥30 | ≥25 | IADPSG | Enzymatic methods, enzymatic analysis in supernatant fraction, Friedewald’s equation | 7 | |
| Caglar et al., 2012 [65] | CC | Turkey | 19 | 15 | 30.3 ± 5.4 | 30.0 ± 4.7 | 65.7 ± 9.1 | 64.5 ± 9.3 | ADA | Enzymatic colorimetric assays | 8 |
| Wiznitzer et al., 2009 [66] | CS | Israel | 1,209 | 8,700 | 30.9 ± 6.5 | 29.5 ± 5.8 | NR | NR | Universal screening | NR | 7 |
| McGrowder et al., 2009 [67] | CC | India | 84 | 94 | 30.18 ± 0.88 | 29.61 ± 1.03 | NR | NR | WHO | Multichannel auto analyzer | 7 |
*Median (IQR), Abbreviations: SD: study design, SS: sample size, QAS: quality assessment, CC: case control, CS: cross sectional, C: cohort, BMI: body mass index, GDM: gestational diabetes mellitus, ADA: American Diabetes Association, IADPSG: International Association of Diabetes and Pregnancy Study Groups, WHO, World Health Organization, ACOG: American College of Obstetricians and Gynecologists, NR: not reported.
Table 2.
Serum lipid concentration between the groups with and without GDM of included studies
| Author, year | GA at sampling (week) | TC | LDL-C | HDL-C | TG | VLDL | TG/HDL-C ratio | Out come | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM | Control | GDM | Control | GDM | Control | GDM | Control | GDM | Control | GDM | Control | |||
| Farsangi et al., 2020 [41] | T3 | 228.96 ± 52.03 mg/dL | 211.59 ± 41.83 mg/dL | 122.41 ± 4.82 mg/dL | 144.54 ± 26.01 mg/dL | 53.10 ± 1.72 mg/dL | 46.64 ± 1.70 mg/dL | 225.58 ± 89.849 mg/dL | 208.38 ± 80.66 mg/dL | NR | NR | NR | NR | Significant for HDL |
| Hossain et al., 2020 [42] | T2–T3 | 194.21 ± 42.18 mg/dL | 208.52 ± 42.18 mg/dL | 109.25 ± 28.80 mg/dL | 119.30 ± 34.76 mg/dL | 47.50 ± 16.17 mg/dL | 47.18 ± 11.71 mg/dL | 204.78 ± 58.50 mg/dL | 202.34 ± 79.18 mg/dL | NR | NR | NR | NR | NS |
| Saumya, 2020 [43] | T1 | 0.07 [ 0.04, 0.11]* | 0.12[0.09,0.16]* | NR | 0.21[0.18,0.24]* | NR | NR | Significant | ||||||
| Wang et al., 2019 | T1,T2, and T3 | T1: 157.36 ± 25.90, T2: 218.45 ± 38.66, T3: 233.53 ± 41.37 mg/dL | T1: 156.21 ± 23.41, T2: 223.87 ± 35.41, T3: 238.95 ± 41.37 mg/dL | T1: 85.46 ± 21.27, T2: 114.46 ± 31.71, T3: 237.05 ± 34.42 mg/dL | T1: 82.75 ± 21.65,T2: 117.94 ± 29.78,T3: 128.77 ± 35.58 mg/dL | T1: 52.98 ± 10.05,T2: 67.67 ± 12.76,T3: 63.42 ± 11.60 mg/dL | T1: 55.30 ± 10.44,T2: 72.31 ± 13.35,T3: 66.90 ± 12.76 mg/dL | T1: 95.62 ± 50.46,T2: 227.55 ± 100.05,T3: 297.50 ± 133.70 mg/dL | T1: 80.57 ± 44.27, T2: 197.44 ± 82.34, T3: 272.70 ± 108.90 mg/dL | T1: 19.12 ± 10.09, T2: 45.51 ± 20.01, T3: 59.50 ± 26.74 mg/dL | T1: 16.11 ± 8.85, T2: 39.49 ± 16.47, T3: 26.74 ± 54.54 mg/dL | T1: 0.84 ± 0.54,T2: 1.58 ± 0.96,T3: 2.20 ± 1.45 | T1: 0.66 ± 0.44, T2: 1.27 ± 0.80, T3: 1.90 ± 1.08 | Significant for TG, HDL, and TG/HDL-C ratio |
| Layton et al., 2019 | T2 | 235.85 ± 26.34 mg/dL | 238.95 ± 40.38 mg/dL | 126.06 ± 17.18 mg/dL | 131.86 ± 34.37 mg/dL | 85.07 ± 14.60 mg/dL | 73.47 ± 15.75 mg/dL | 154.95 ± 16.40 mg/dL | 161.14 ± 49.62 mg/dL | 30.99 ± 3.28 mg/dL | 32.23 ± 9.92 mg/dL | NR | NR | Significant for TG |
| Correa et al., 2019 | T1 | 193.01 ± 38.74 mg/dL | 165.50 19.11 mg/dL | 116.40 ± 35.08 mg/dL | 91.80 ± 20.37 mg/dL | 60 ± 8.52 mg/dL | 66 ± 12.96 mg/dL | 137.50 ± 48.15 mg/dL | 96.50 ± 32.22 mg/dL | 27.50 ± 9.63 mg/dL | 19.30 ± 6.44 mg/dL | NR | NR | Significant for TC, TG, and LDL |
| Aydemir et al., 2019 | T3 | 242.86 ± 37.57 mg/dL | 229.14 ± 44.22 mg/dL | 140.82 ± 37.04 mg/dL | 143.86 ± 30.07 mg/dL | 62.48 ± 13.54 mg/dL | 62.34 ± 12.30 mg/dL | 203.30 ± 75.09 mg/dL | 197.13 ± 74.6 mg/dL | 40.66 ± 15.02 mg/dL | 39.43 ± 14.92 mg/dL | NR | NR | Significant for TC |
| Anjum et al., 2019 | T2 | 185.12 ± 22.78 mg/dL | 197.40 ± 40.53 mg/dL | 111.73 ± 17.26 mg/dL | 114.22 ± 35.64 mg/dL | 49.00 ± 8.54 mg/dL | 62.12 ± 15.32 mg/dL | 122.52 ± 51.50 mg/dL | 105.26 ± 41.70 mg/dL | 24.50 ± 10.30 mg/dL | 21.05 ± 8.34 mg/dL | 2.70 ± 1.6 | 1.76 ± 0.90 | Significant for HDL-C and TG/HDL |
| Alyas et al., 2019 | T1 and T2 | T1: 308.91 ± 1.27,T2: 367.86 ± 2.39 mg/dL | T1: 287.71 ± 1.67,T2: 340.43 ± 1.58 mg/dL | T1: 165.62 ± 2.02,T2: 227.13 ± 3.43 mg/dL | T1: 131.16 ± 1.02,T2: 201.60 ± 2.75 mg/dL | T1: 45.71 ± 0.74,T2: 33.42 ± 1.93 mg/dL | T1: 59.80 ± 0.78,T2: 41.63 ± 0.87 mg/dL | T1: 369.52 ± 3.34,T2: 450.45 ± 4.21 mg/dL | T1: 346.42 ± 3.52,T2: 423.94 ± 3.38 mg/dL | T1: 39.95 ± 0.95,T2: 54.39 ± 1.11 mg/dL | T1: 31.88 ± 0.30,T2: 47.38 ± 0.48 mg/dL | NR | NR | Significant |
| Yue and Ying, 2019 | T2 | 239.72 ± 44.80 mg/dL | 238.56 ± 23.20 mg/dL | 141.53 ± 1.55 mg/dL | 143.08 ± 1.55 mg/dL | 53.36 ± 0.77 mg/dL | 53.36 ± 0.39 mg/dL | 397.54 ± 14.16mg/dL | 332.02 ± 7.97 mg/dL | 79.51 ± 2.83 mg/dL | 66.40 ± 1.59 mg/dL | 3.25 ± 0.12 | 2.77 ± 0.07 | Significant for TG and TG/HDL |
| Zebunnesa et al., 2018 | T3 | 209.53 ± 34.66 mg/dL | 230.45 ± 45.25 mg/dL | 119.86 ± 31.56 mg/dL | 110.22 ± 24.79 mg/dL | 55.63 ± 34.26 mg/dL | 53.02 ± 6.81 mg/dL | 267.96 ± 56.34 mg/dL | 232.88 ± 58.43 mg/dL | 53.59 ± 11.27 mg/dL | 46.58 ± 11.69 mg/dL | NR | NR | Significant for TG and TC |
| Cao et al., 2018 | T3 | 146.92 ± 19.15 mg/dL | 80.42 ± 41.64 mg/dL | 146.95 ± 16.92 mg/dL | 108.28 ± 15.14 mg/dL | 65.74 ± 14.63 mg/dL | 81.20 ± 13.64 mg/dL | 557.80 ± 16.41 mg/dL | 283.33 ± 19.21 mg/dL | 44.07 ± 5.41 mg/dL | 46.4 ± 6.14 mg/dL | NR | NR | Significant |
| Bukowiecka-Matusiak et al., 2018 | T2 | 259.9 ± 37.11 mg/dL | 219.5 ± 37.40 mg/dL | 141.0 ± 42.22 mg/dL | 119.0 ± 25.18 mg/dL | 74.10 ± 21.40 mg/dL | 61.4 ± 9.77 mg/dL | 215.9 ± 63.70 (mg/dL) | 157.6 ± 64.74 mg/dL | 43.18 ± 12.74 mg/dL | 31.52 ± 12.95 mg/dL | NR | NR | Significant for TG and TC |
| Bugatto et al., 2018 | T3 | 249.4 + 44.8 mg/dL | 256.9 + 42.8 mg/dL | 143.1 + 38.0 mg/dL | 146.1 + 35.8 mg/dL | 65.4 + 18.6 mg/dL | 70.8 + 21.9 mg/dL | 252.0 + 82.7 mg/dL | 191.4 + 68.8 mg/dL | 50.40 ± 16.54 mg/dL | 38.28 ± 13.76 mg/dL | NR | NR | Significant for TG |
| Barat et al., 2018 | T3 | 228.82 ± 41.10 mg/dL | 234.41 ± 132.01 mg/dL | 122.82 ± 31.47 mg/dL | 122.57 ± 43.35 mg/dL | 53.30 ± 14.88 mg/dL | 66.28 ± 25.78 mg/dL | 275.43 ± 69.33 mg/dL | 205.53 ± 72.51 mg/dL | 55.09 ± 13.87 mg/dL | 41.11 ± 14.50 (mg/dL) | 5.37 ± 1.56 | 3.38 ± 1.54 | Significant for TG, HDL, and TG/HDL |
| Bao et al., 2018 | T1 and T2 | T1: 185.01 ± 16.14, T2: 195.10 ± 22.41 mg/dL | T1: 179 ± 19.54, T2: 208 ± 18.41 mg/dL | T1: 90 ± 0.41, T2: 98 ± 10.24 mg/dL | T1: 88 ± 10.41, T2: 105 ± 11.67 mg/dL | T1: 57.3 ± 9.87, T2: 63.3 ± 13.89 mg/dL | T1: 62.3 ± 21.71, T2: 72.3 ± 13.04 mg/dL | T1: 155 ± 11.41, T2: 198 ± 13.20 mg/dL | T1: 119 ± 19.10, T2: 207 ± 25.19 mg/dL | T1: 31 ± 2.28, T2: 39.60 ± 2.64 mg/dL | T1: 23.80 ± 3.82, T2: 41.40 ± 5.04 mg/dL | NR | NR | Significant for TG and HDL |
| Pazhohan, 2017 | T1 | 202.9 ± 31.83 mg/dL | 195.9 ± 30.0 mg/dL | NR | NR | NR | NR | 198.3 ± 105.6 mg/dL | 164.1 ± 44.3 mg/dL | 39.66 ± 21.12 mg/dL | 32.82 ± 8.86 mg/dL | 3.84 ± 0.83 | 3.14 ± 0.44 | Significant for TG and TG/HDL |
| Wang et al., 2017 | T1 | 177.50 ± 33.26 mg/dL | 171.70 ± 30.16 mg/dL | 92.80 ± 27.84 mg/dL | 88.55 ± 25.13 mg/dL | 65.35 ± 20.50 mg/dL | 67.29 ± 16.63 mg/dL | 117.80 ± 63.77 mg/dL | 103.63 ± 59.34 mg/dL | 23.56 ± 12.75 mg/dL | 20.73 ± 11.87 mg/dL | NR | NR | Significant |
| Ghodke et al., 2017 | T2 and T3 | T2: 223.50 ± 25.16, T3: 242.83 ± 27.14 mg/dL | T2: 214.60 ± 14.11, T3: 242.65 ± 14.19 mg/dL | T2: 96.83 ± 31.39, T3: 150.16 ± 9.88 mg/dL | T2: 92.41 ± 14.41, T3: 137.82 ± 10.41 mg/dL | T2: 52.00 ± 7.07, T3: 41.16 ± 7.27 mg/dL | T2: 49 ± 6.14, T3: 43.07 ± 5.74 mg/dL | T2: 214.33 ± 18.64, T3: 230.50 ± 17.03 mg/dL | T2: 186.68 ± 12.41, T3: 216.78 ± 16.44 mg/dL | T2: 34 ± 5.65, T3: 30.58 ± 5.83 mg/dL | T2: 36.27 ± 3.98, T2: 32.25 ± 4.02 mg/dL | NR | NR | Significant for TG |
| Chen et al., 2017 | T2 | 222.96 ± 36.21 mg/dL | 240.59 ± 42.69 mg/dL | 96.61 ± 28.65 mg/dL | 115.00 ± 35.78 mg/dL | 79.43 ± 17.35 mg/dL | 84.79 ± 18.96 mg/dL | 219.5 (175.8, 285.3) mg/dL | 185.0 (146.5, 236.0) mg/dL | 43.90 ± 16.22 mg/dL | 37 ± 13.26 mg/dL | 2.96 (2.14, 3.84) | 2.16 (1.64, 3.10) | Significant for LDL and TG/HDL |
| Wang et al., 2016 | T1 | 176.69 ± 32.09 mg/dL | 171.67 ± 30.16 mg/dL | NR | NR | NR | NR | 191.32 ± 1.22 mg/dL | 103.60 ± 80.57 mg/dL | 30.26 ± 0.24 mg/dL | 20.72 ± 16.11 mg/dL | 0.92 ± 1.61 | 0.71 ± 0.46 | Significant |
| Shen et al., 2016 | T1,T2, and T3 | T1: 196.41 ± 15.41, T2: 239.72 ± 16.47, T3: 259.83 ± 16.97 mg/dL | T1: 190.61 ± 12.64, T2: 239.72 ± 10.75, T3: 268.72 ± 13.64 mg/dL | T1: 134.18 ± 19.41, T2: 157 ± 13.64, T3: 169.37 ± 20.97 mg/dL | T1: 129.16 ± 17.85, T2: 159.70 ± 16.95, T3: 177.50 ± 17.68 mg/dL | T1: 65.35 ± 19.65, T2: 72.70 ± 13.17, T3: 71.54 ± 18.32 mg/dL | T1: 65.74 ± 15.65, T2: 73.47 ± 16.98, T3: 73.09 ± 19.54 mg/dL | T1: 136.35 ± 16.39, T2: 233.74 ± 18.31, T3: 285.98 ± 21.39 mg/dL | T1: 115.98 ± 16.74, T2: 201.87 ± 9.47, T3: 264.73 ± 9.87, mg/dL | T1: 27.27 ± 3.28, T2: 46.75 ± 3.66, T3: 57.20 ± 4.28 mg/dL | T1: 23.20 ± 3.35, T2: 40.37 ± 1.89, T3: 52.95 ± 1.97 mg/dL | NR | NR | Higher TG and LDL-C at T1, but lower at T2 and T3 |
| Liang et al., 2016 | T2 | 266.78 ± 81.19 mg/dL | 177.86 ± 65.73 mg/dL | NR | NR | NR | NR | 513.33 ± 123.95 mg/dL | 239.06 ± 61.98 mg/dL | 102.71 ± 24.79 mg/dL | 47.81 ± 12.40 mg/dL | NR | NR | Significant |
| Khosrowbeygi et al., 2016 | T2 | 234.90 ± 11.51 mg/dL | 256.13 ± 12.56 mg/dL | 142.25 ± 12.66 mg/dL | 149.27 ± 9.70 mg/dL | 36.90 ± 3.25 mg/dL | 62.07 ± 2.18 mg/dL | 278.73 ± 23.17 mg/dL | 223.97 ± 18.51 mg/dL | 55.75 ± 4.63 mg/dL | 44.79 ± 3.70 mg/dL | 8.64 ± 0.76 | 3.65 ± 0.31 | Significant for HDL-C and TG/HDL-C |
| Jin et al., 2016 | T1,T2, and T3 | T1: 152.72 ± 26.92, T2: 179.79 ± 25.20, T3: 242.42 ± 43.24 mg/dL | — | T1: 87 ± 10.60, T2: 95.13 ± 1.67, T3: 110.98 ± 32.36 mg/dL | — | T1: 64.19 ± 22.50, T2: 64.58 ± 9.63, T3: 69.60 ± 13.33 mg/dL | — | T1: 194.79 ± 62.96, T2: 216.92 ± 51.15, T3: 270.93 ± 105.59 mg/dL | — | T1: 38.96 ± 12.59, T2: 43.38 ± 10.23, T3: 54.19 ± 21.12 mg/dL | NR | NR | NR | Significant for TG, LDL-C, and HDL-C |
| Han et al., 2016 | T2–T3 | 182.9 ± 33.3 mg/dL | 176 ± 32.6 mg/dL | 371.7 ± 125.5 mg/dL | 386.8 ± 119.9 mg/dL | 4180.4 ± 1524.9 mg/dL | 4650.8 ± 1605.5 mg/dL | NR | NR | 134.4 ± 44.5 mg/dL | 130.3 ± 43.5 (mg/dL) | NR | NR | Significant for HDL and TC |
| Ertug et al., 2016 | T2 | 234 ± 46 mg/dL | 241 ± 54 mg/dL | 124 ± 41 mg/dL | 141 ± 52 mg/dL | 64 ± 13 mg/dL | 69 ± 16 mg/dL | 220 ± 78 mg/dL | 160 ± 49 mg/dL | 44 ± 15.60 mg/dL | 32 ± 9.80 mg/dL | NR | NR | Significant for TG and HDL |
| Wang et al., 2015 | T3 | NR | NR | NR | NR | 69.99 ± 16.90 mg/dL | 72.70 ± 13.46 mg/dL | 193.01 ± 64.27 mg/dL | 172.65 ± 54.44 mg/dL | 38.60 ± 12.85 mg/dL | 34.53 ± 10.89 mg/dL | 1.24 ± 0.63 | 1.04 ± 0.43 | Significant for TG and TG/HDL |
| Li et al., 2015 | T1 | 185.20 ± 41.75 mg/dL | 176.31 ± 31.70 mg/dL | 84.30 ± 27.84 mg/dL | 80.82 ± 22.81 mg/dL | 71.15 ± 17.79 mg/dL | 76.18 ± 19.33 mg/dL | 142.54 ± 77.91 mg/dL | 111.56 ± 55.78 mg/dL | 28.51 ± 15.58 mg/dL | 22.31 ± 11.16 | NR | NR | Significant |
| dos Santos-Weiss et al., 2013 | T1,T2, and T3 | T1: 193.32 ± 38.66, T2: 216.52 ± 46.39, T3: 233.92 ± 39.51 mg/dL | T1: 185.59 ± 34.80, T2: 228.12 ± 46.40, T3: 241.65 ± 50.26 mg/dL | T1: 96.67 ± 34.37, T2: 100.54 ± 37.22, T3: 129.54 ± 35.80 mg/dL | T1: 108.28 ± 25.77, T2: 143.08 ± 40.10, T3: 137.27 ± 32.94 mg/dL | T1: 46.40 ± 11.60, T2: 58.01 ± 11.60, T3: 56.07 ± 11.60 mg/dL | T1: 54.14 ± 15.47, T2: 61.87 ± 15.47, T3: 61.87 ± 17.40 mg/dL | T1: 221.35 ± 146.60, T2: 194.79 ± 59.26, T3: 230.20 ± 72.22 mg/dL | T1: 97.39 ± 32.59, T2: 150.51 ± 51.85, T3: 172.65 ± 58.82 mg/dL | T1: 44.27 ± 29.32, T2: 38.96 ± 11.85, T3: 46.01 ± 14.44 mg/dL | T1: 19.48 ± 6.52, T2: 30.10 ± 10.37, T3: 34.53 ± 11.76 mg/dL | NR | NR | Significant |
| Khan et al., 2012 | T3 | 206 ± 18.79 mg/dL | 195 ± 24.15 mg/dL | 93 ± 18.71 mg/dL | 88 ± 16.35 mg/dL | 55 ± 8.20 mg/dL | 56 ± 8.82 mg/dL | 190 ± 19.83 mg/dL | 172 ± 21.66 mg/dL | 38 ± 3.97 mg/dL | 34.40 ± 4.33 mg/dL | NR | NR | Significant for TC and TG |
| Caglar et al., 2012 | T2 | 239.8 ± 39.7 mg/dL | 232.2 ± 36.7 mg/dL | 138.9 ± 42.1 | 135.6 ± 31.0 mg/dL | 67.5 ± 13.7 mg/dL | 75.3 ± 20.3 mg/dL | 207.9 ± 66.8 mg/dL | 191.1 ± 60.7 mg/dL | 41.58 ± 13.36 mg/dL | 38.22 ± 12.14 mg/dL | NR | NR | Not significant |
| McGrowder et al., 2009 | T3 | 220.77 ± 9.28 mg/dL | 193.71 ± 12.37 mg/dL | 128.38 ± 9.28 mg/dL | 117.94 ± 13.15 mg/dL | 48.34 ± 3.10 mg/dL | 56.07 ± 3.10 mg/dL | 162.02 ± 8.85 mg/dL | 126.61 ± 17.71 mg/dL | 13.14 ± 0.77 mg/dL | 12.75 ± 1.93 mg/dL | 1.24 ± 0.08 | 1.21 ± 0.20 | Significant for TC and TG |
*Odds ratio (OR). Abbreviations: GA, gestational age; GDM, gestational diabetes mellitus; HDL-C, high-density lipid cholesterol; LDL-C, low-density lipid cholesterol; TC, total cholesterol; TG, triglycerides; NS, not significant, NR: not reported.
9. Pooled SMD
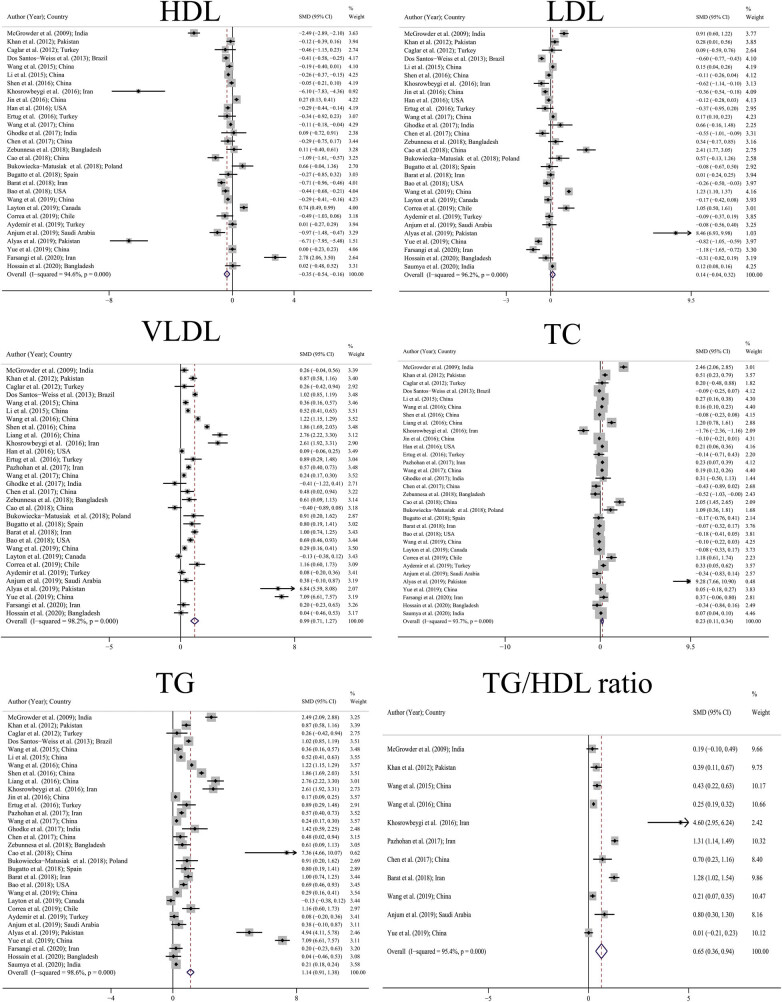
Table 3 shows the pooled SMD and Figure 2 shows the forest plot for the pooled SMD including TC, LDL, HDL, TG, VLDL, and TG/HDL ratio. Accordingly, there were 32 studies for TC, 29 studies for LDL, 29 studies for HDL, 32 studies for TG, 31 studies for VLDL, and 11 studies for TG/HDL ratio. As is clear from the forest plot, pooled SMD for TC and TG was 0.23 mg/dL (95% CI: 0.11–0.34) and 1.14 mg/dL (95% CI: 0.91–1.38). In other words, the mean values of TC and TG in people with GDM were higher than that in normal people. A similar pattern was observed for the VLDL and TG/HDL ratio, with pooled SMD for the VLDL and TG/HDL ratios 0.99 mg/dL (95% CI: 0.71–1.27) and 0.65 mg/dL (95% CI: 0.36–0.94), respectively, which indicates that the average of these indices was higher in the GDM group. Pooled SMD for HDL was also −0.35 mg/dL (95% CI: −0.54 to −0.16). In other words, in general, people with GDM had a mean HDL lower than normal people. Although pooled SMD was higher for LDL in the GDM group, this difference was not significant (0.14 [95% CI: −0.04 to 0.32]). Of all lipid profiles, the biggest difference between the GDM and control groups was observed in TG (SMD: 1.14).
Table 3.
Result of meta-analysis for calculation of lipid profile SMD; publication bias and fill and trim method
| Lipids profile | Meta-analysis | Egger’s test for publication bias | Fill and trim | ||||
|---|---|---|---|---|---|---|---|
| Number | I2% | SMD | Coefficient (95% CI) | P-value | SMD | 95% CI | |
| TC | 32 | 93.7 | 0.23 (0.11–0.34) | 1.24 (−0.60–3.10) | 0.179 | — | — |
| LDL | 29 | 96.2 | 0.14 (−0.04 to 0.32)* | −0.05 (−2.75–2.66) | 0.972 | — | — |
| HDL | 29 | 94.6 | −0.35 (−0.54 to −0.16) | −1.77 (−4.37–0.08) | 0.173 | — | — |
| TG | 32 | 98.6 | 1.14 (0.91–1.38) | 5.21 (1.70–8.71) | 0.005 | 1.13 | (0.92–1.39) |
| VLDL | 31 | 98.2 | 0.99 (0.71–1.27) | 2.04 (−2.23–6.31) | 0.337 | — | — |
| TG/HDL ratio | 11 | 95.4 | 0.65 (0.36–0.94) | 3.58 (−1.29–8.46) | 0.130 | — | — |
*No significance; SMD: standardized mean difference; TC: total cholesterol; TG: triglyceride; CI: confidence interval; LDL: low-density lipoproteins; HDL: high-density lipoproteins; VLDL: very low-density lipoproteins.
Figure 2.
Pooled SMD of lipid profile based on random effects model. The midpoint of each line segment shows the SMD, the length of the line segment indicates 95% confidence interval in each study, and the diamond mark illustrates the pooled SMD for different lipid profile.
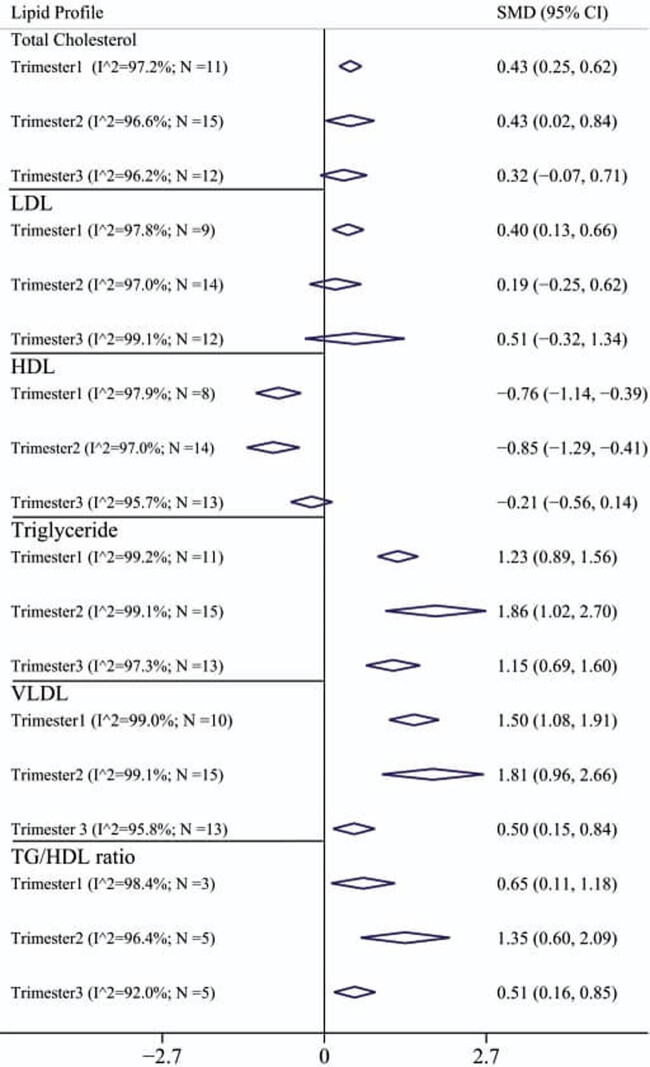
10. Pooled SMD based on different trimesters
Figure 3 shows the pooled SMD values for the lipid profile in terms of trimester. Accordingly, pooled SMD for TG, VLDL, and TG/HDL ratio at different trimesters in GDM group was significantly higher than that in normal individuals. In contrast, pooled SMD for HDL in 1st trimester (−0.76 [95% CI: −1.14 to −0.39]) and 2nd trimester (0.85 [95% CI: −1.29 to −0.41]) in the GDM group were significantly lower than that in normal group, and in the 3rd trimester no difference was observed between the two groups. Pooled SMD for LDL was significantly different only in the 1st trimester (0.40 [95% CI: 0.13–0.66]) so that in the GDM group the mean LDL was higher than that in the control group, and for the 2nd trimester (0.19 [95% CI: −0.25 to 0.62]) and for the third trimester (0.51 [95% CI: −0.32 to 1.34]), no significant difference was observed. Also, pooled SMD for TC only in the 1st trimester (0.43 [95% CI: 0.25–0.62]) and the second trimester (0.43 [95% CI: 0.02–0.84]), there was a significant difference between the two groups and in the 3rd trimester, a significant difference was not observed.
Figure 3.
Pooled SMD and 95% confidence interval of lipid profile based on different trimesters.
11. Publication bias
Table 3 shows the publication bias results based on the Egger’s test and the fill and trim method. As it turns out, there was a significant publication bias for TG (coefficient; 5.21; P: 0.005). According to the fill and trim method, the value of adjusted pooled SMD for TG was 1.13 (95% CI: 0.92–1.39), which was not significantly different from the pooled SMD calculated for TG (1.14 [95% CI: 0.91–1.38]). No publication bias was observed for other lipid profiles including TC, LDL, HDL, VLDL, and TG/HDL ratio.
12. Heterogeneity and meta-regression results
As shown in Table 3, there was significant heterogeneity between different studies for lipid profiles (Cochran’s Q test P-value < 0.001 for all lipid profiles) so that the I 2 index was above 90% for all lipid profiles. Table 4 shows the meta-regression results to investigate the effect of publication year, sample size, age, and BMI on heterogeneity between studies. Accordingly, none of the variables had a significant role on heterogeneity between studies (P > 0.05 for all of them).
Table 4.
Results of the univariate meta-regression analysis on the heterogeneity of the determinants
| Lipids profile | Publication year | Sample size | Mean age | BMI mean | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| TC | −0.015 (−0.25 to 0.22) | 0.898 | 0.00 (−0.01 to 0.01) | 0.642 | −0.04 (−0.26 to 0.18) | 0.701 | 0.00 (−0.04 to 0.04) | 0.999 |
| LDL | 0.05 (−0.18 to 0.27) | 0.688 | 0.01 (−0.01 to 0.01) | 0.815 | −0.06 (−0.29 to 0.17) | 0.609 | 0.01 (−0.04 to 0.05) | 0.750 |
| HDL | 0.11 (−0.14 to 0.35) | 0.392 | 0.00 (−0.01 to 0.01) | 0.551 | 0.00 (−0.25 to 0.25) | 0.995 | −0.02 (−0.09 to 0.05) | 0.547 |
| Triglyceride | 0.00 (−0.25 to 0.25) | 0.991 | 0.01 (−0.01 to 0.01) | 0.551 | −0.03 (−0.28 to 0.22) | 0.794 | −0.02 (−0.11 to 0.07) | 0.631 |
| VLDL | 0.09 (−0.16 to 0.34) | 0.464 | 0.00 (0.00 to 0.00) | 0.736 | 0.01 (−0.23 to 0.24) | 0.935 | −0.03 (−0.11 to 0.04) | 0.352 |
| TG/HDL ratio | 0.04 (−0.20 to 0.27) | 0.742 | 0.01 (−0.01 to 0.01) | 0.492 | −0.06 (−0.26 to 0.15) | 0.558 | 0.26 (−0.05 to 0.56) | 0.086 |
CI: confidence interval; TC: total cholesterol; LDL: low-density lipoproteins; TG: triglyceride; HDL: high-density lipoproteins; VLDL: very low-density lipoproteins; BMI: body mass index.
13. Discussion
The aim of this comprehensive systematic review and meta-analysis was to determine the effect of GDM on lipid profile. In this study we have concluded the following: (1) the levels of TC, LDL-C, VLDL-C, and TG were higher in women with GDM than in normal pregnant women, (2) the level of HDL-C was lower in women with GDM than in normal pregnant women, and (3) of all lipid profiles, the largest difference between the GDM and control groups was observed in TG.
Studies have shown that even mild hyperglycemia during pregnancy is associated with an increase in perinatal complications [22,23]. Although the adverse effects of GDM on the mother and fetus are widely known, there are still many unresolved issues regarding GDM [24]. Therefore, the WHO states that there are many ambiguities about the various strategies for screening for GDM. However, despite recent research, there is still no general international agreement on the best way to screen for GDM, and screening for diabetes during pregnancy is essential because with timely diagnosis, appropriate treatment can be provided, and thereby, maternal and fetal complications, especially pre-eclampsia, macrosomia, and shoulder dystocia can be reduced [25]. In this regard, many researchers are interested in studying different markers in pregnant women so that they can detect the adverse effects of pregnancy, including diabetes, with the changes in these markers and reduce the complications [26]. Various markers including C-reactive protein, Interleukin-6, Unconjugated Estriol, Pregnancy-associated plasma protein, Hemoglobin A1C (HbA1C), and sex hormone binding globulin have been examined in diagnosis of GDM [5,6].
During pregnancy, fat metabolism undergoes physiological changes that increase the production of lipid profiles [27]. Increased estrogen levels and insulin resistance in pregnant women can increase the production of lipids in the liver [28]. These changes in fat metabolism indicate a physiological adaptation in the body of pregnant women that shifts the priority of lipid metabolism over glucose metabolism, and lipids are used as a source of energy for pregnant women so that they can preserve glucose for growth and development of fetal development. Lipids also make it possible to produce embryonic cell membranes, bile acids, and steroid hormones [27]. In early pregnancy, fat accumulation occurs due to increased synthesis of lipids and blood lipids, which increase the level of free fatty acids, especially triglycerides in the blood. On the other hand, increased free fatty acids in the blood can cause insulin resistance [29]. Also, abnormal lipid profile changes are seen in patients with type 2 diabetes [30], so that increasing TG levels above 250 mg/dL and lowering HDL-C levels below 35 mg/dL are considered as a risk factor for type 2 diabetes [31]. Insulin resistance is one of the leading causes of GDM and type 2 diabetes [32]. According to changes in normal pregnancy, insulin resistance occurs due to decreased glucose uptake and increased insulin secretion, and mainly GDM occurs in women whose pancreas does not function sufficiently to compensate for the insulin resistance caused by pregnancy [33]. Also, progesterone plays a role in a way to reset the lipostat in the hypothalamus, leading to increase in the lipids during second trimester of pregnancy [34].
Results similar to present study were observed in a meta-analysis study conducted by Ryckman et al. (2015). TG levels were increased in women with GDM than in women without GDM (95% CI: 25.4–36.4). This finding was consistent in the 1st, 2nd, and 3rd trimesters of pregnancy. HDL-C levels were significantly decreased in women with GDM than in women without GDM in the 2nd (95% CI: −6.2 to −3.1) and 3rd (95% CI: −6.5 to − 1.7) trimesters of pregnancy. No significant difference was shown in TC or LDL-C levels between women with GDM and those without GDM [35].
The present study showed that TG, VLDL-C, and TG/HDL-C ratio were significantly higher in women with gestational diabetes in each trimester of pregnancy than in normal women. HDL in the 1st and 2nd trimesters of pregnancy was lower than the normal group, and TC in the 1st and 2nd trimesters of pregnancy was significantly different in the group of women with GDM and healthy women. But Mankuta et al. observed that TC, LDL-C, and TG decrease in 1st trimesters and increase during 2nd and 3rd trimester. HDL-C levels had no change significantly in the 1st trimester, although it elevated in 2nd trimester and decreased in 3rd trimester [36]. But in other studies it was reported that fat storage increases in the 2nd trimester of pregnancy and causes elevated TG concentration [37].
Correa et al. (2019) evaluated maternal biomarkers in the 1st trimester of pregnancy for early detection of GDM. They showed that there was a significant association between TG, TC, and LDL levels in the 1st trimester of pregnancy with GDM. In this study, lipid profile changes occurred during glycemic normal state and glycosylated hemoglobin [26]. In addition, Layton et al. (2019) conducted a study to determine the lipid profile in women with different sub-groups of GDM. The results of this study showed that there is a significant relationship between TG and GDM. In this study, GDM was grouped into three subgroups, GDM-sensitivity, GDM-secretion, and GDM-mixed, based on measurement of insulin sensitivity and insulin secretion, and there was significant relationship between TG and GDM-sensitivity sub-group compared to the other two groups [38]. In addition, Bukowiecka-Matusiak et al. conducted a study to examine changes in lipid profiles in the membranes of red blood cells in pregnant women with diagnosed GDM. The results showed that TG and TC levels in the group with GDM were significantly higher than that in the group of women with non-GDM [39]. Anjum et al. (2019) investigated the association between HbA1C and lipid profiles with GDM in Saudi Arabian women. The results of this study did not find a significant correlation in terms of TG level between the group with GDM and the non-diabetic group [28]. Besides, the results of Aydemir et al.’s study aimed at examining serum lipoprotein particle levels and its relationship with metabolic status of gestational glucose showed that TG levels were not significantly associated in the two groups of GDM and control group [40]. The reason for the difference in the results of these studies can be considered as not confining the effect of confounding factors on GDM and lipid profiles. On the other hand, these studies measured the levels of lipid profiles using different kits and methods and also different criteria were used for measuring GDM.
Although every attempt to conduct a flawless study was made, this study had some limitations. The authors desired to report age-specific pooled SMD of lipid profile but because most studies did not report age estimate, the authors could not perform the calculations. However, the study had some strong points, as well. For example, it was the first study that reported the overall pooled SMD for lipid profile separated by trimester. In addition, a high number of studies were retrieved in the extensive search and finally 33 studies with a total sample size of 23,792 were analyzed, which provides a sufficient statistical power. Also, we had done unification of units in order to be able to pool the lipid profile. Use of complicate statistical model for unification of SMD and use of fill and trim method for adjustment of publication bias were the strong points of the present study. The other limitations include insufficient studies during the 1st trimester of pregnancy, failure to measure the predictive power of all, studies not examining mothers before pregnancy and during the first trimester of pregnancy in terms of lipid profiles as well as not examining factors such as lifestyle, diet, or other factors involved in increasing the profile of lipids in some studies make it difficult to decide whether to generalize the results.
14. Conclusion
Elevated levels of TG in pregnancy occur significantly more in women with GDM than in healthy pregnant women. Higher levels of TC, LDL, VLDL, and TG/HDL ratio and lower level of HDL were exhibited in GDM group. Therefore, TG and TG/HDL ratio can be considered as a possible risk factor and reliable marker in the diagnosis of GDM. Although more research is needed in this area.
Acknowledgements
We appreciate the Alborz University of Medical Sciences.
Footnotes
Funding information: This study has no funding.
Author contributions: FA is correspondence, conceived the study and approved the final version of the paper. FAR designed the study. RP critically analyzed the data. FAR and RP contributed equally to this article. Other authors interpreted the data.
Conflict of interest: The authors declare no conflict of interest.
Data availability statement: All the data generated or analyzed during this study are included in this published article.
References
- [1].Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S13–28. [DOI] [PubMed]; Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S13–28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- [2].Dinicola S, Unfer V, Facchinetti F, Soulage CO, Greene ND, Bizzarri M, et al. Inositols: from established knowledge to novel approaches. Int J Mol Sci. 2021;22(19):10575. [DOI] [PMC free article] [PubMed]; Dinicola S, Unfer V, Facchinetti F, Soulage CO, Greene ND, Bizzarri M. et al. Inositols: from established knowledge to novel approaches. Int J Mol Sci. 2021;22(19):10575. doi: 10.3390/ijms221910575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gambioli R, Forte G, Buzzaccarini G, Unfer V, Laganà AS. Myo-inositol as a key supporter of fertility and physiological gestation. Pharm (Basel). 2021;14(6):504. [DOI] [PMC free article] [PubMed]; Gambioli R, Forte G, Buzzaccarini G, Unfer V, Laganà AS. Myo-inositol as a key supporter of fertility and physiological gestation. Pharm (Basel) 2021;14(6):504. doi: 10.3390/ph14060504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed]; Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- [5].Amirian A, Kariman N, Hedayati M, Borumandnia N, Sheikhan Z. Predictive power of unconjugated estriol in diagnosis of gestational diabetes: a cohort study. Iran Red Crescent Med J. 2019;21(11):1–15.; Amirian A, Kariman N, Hedayati M, Borumandnia N, Sheikhan Z. Predictive power of unconjugated estriol in diagnosis of gestational diabetes: a cohort study. Iran Red Crescent Med J. 2019;21(11):1–15. [Google Scholar]
- [6].Amirian A, Rahnemaei FA, Abdi F. Role of C-reactive Protein (CRP) or high-sensitivity CRP in predicting gestational diabetes mellitus: systematic review. Diabetes & Metab Syndrome: Clin Res & Rev. 2020;4:229–36. [DOI] [PubMed]; Amirian A, Rahnemaei FA, Abdi F. Role of C-reactive Protein (CRP) or high-sensitivity CRP in predicting gestational diabetes mellitus: systematic review. Diabetes & Metab Syndrome: Clin Res & Rev. 2020;4:229–36. doi: 10.1016/j.dsx.2020.02.004. [DOI] [PubMed] [Google Scholar]
- [7].Sudasinghe B, Wijeyaratne C, Ginige P. Long and short-term outcomes of Gestational Diabetes Mellitus (GDM) among South Asian women–a community-based study. Diabetes Res Clin Pract. 2018;145:93–101. [DOI] [PubMed]; Sudasinghe B, Wijeyaratne C, Ginige P. Long and short-term outcomes of Gestational Diabetes Mellitus (GDM) among South Asian women–a community-based study. Diabetes Res Clin Pract. 2018;145:93–101. doi: 10.1016/j.diabres.2018.04.013. [DOI] [PubMed] [Google Scholar]
- [8].Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care#. Int J Gynecol & Obstet. 2015;131:S173–211. [DOI] [PubMed]; Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC. et al. The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care#. Int J Gynecol & Obstet. 2015;131:S173–211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- [9].Wang J, Li Z, Lin L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine. 2019;98(16):15320. [DOI] [PMC free article] [PubMed]; Wang J, Li Z, Lin L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine. 2019;98(16):15320. doi: 10.1097/MD.0000000000015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Antwi-Baffour S, Kyeremeh R, Boateng SO, Annison L, Seidu MA. Haematological parameters and lipid profile abnormalities among patients with Type-2 diabetes mellitus in Ghana. Lipids health Dis. 2018;17(1):1–9. [DOI] [PMC free article] [PubMed]; Antwi-Baffour S, Kyeremeh R, Boateng SO, Annison L, Seidu MA. Haematological parameters and lipid profile abnormalities among patients with Type-2 diabetes mellitus in Ghana. Lipids health Dis. 2018;17(1):1–9. doi: 10.1186/s12944-018-0926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Hormone Mol Biol Clin investigation. 2016;26(2):109–27. [DOI] [PubMed]; Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Hormone Mol Biol Clin investigation. 2016;26(2):109–27. doi: 10.1515/hmbci-2015-0025. [DOI] [PubMed] [Google Scholar]
- [12].Aghaie Z, Hajian S, Abdi F. The relationship between lipid profiles in pregnancy and preterm delivery: a systematic review. Biomed Res Ther. 2018;5(8):2590–609.; Aghaie Z, Hajian S, Abdi F. The relationship between lipid profiles in pregnancy and preterm delivery: a systematic review. Biomed Res Ther. 2018;5(8):2590–609. [Google Scholar]
- [13].Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of Preeclampsia: a systematic review. Taiwan J Obstet Gynecol. 2020;59(2):173–82. [DOI] [PubMed]; Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of Preeclampsia: a systematic review. Taiwan J Obstet Gynecol. 2020;59(2):173–82. doi: 10.1016/j.tjog.2020.01.002. [DOI] [PubMed] [Google Scholar]
- [14].Amirian A, Mahani MB, Abdi F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet & Gynecol Sci. 2020;63(4):407–16. [DOI] [PMC free article] [PubMed]; Amirian A, Mahani MB, Abdi F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet & Gynecol Sci. 2020;63(4):407–16. doi: 10.5468/ogs.20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abdi F, Mobedi H, Bayat F, Mosaffa N, Dolatian M, Tehrani FR. The effects of transdermal estrogen delivery on bone mineral density in postmenopausal women: a meta-analysis. Iran J Pharm Res: IJPR. 2017;16(1):380–9. [PMC free article] [PubMed]; Abdi F, Mobedi H, Bayat F, Mosaffa N, Dolatian M, Tehrani FR. The effects of transdermal estrogen delivery on bone mineral density in postmenopausal women: a meta-analysis. Iran J Pharm Res: IJPR. 2017;16(1):380–9. [PMC free article] [PubMed] [Google Scholar]
- [16].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed]; Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- [17].Sedgh G, Bearak J, Singh S, Bankole A, Popinchalk A, Ganatra B, et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet. 2016;388(10041):258–67. [DOI] [PMC free article] [PubMed]; Sedgh G, Bearak J, Singh S, Bankole A, Popinchalk A, Ganatra B. et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet. 2016;388(10041):258–67. doi: 10.1016/S0140-6736(16)30380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Herrera Martinez A, Palomares Ortega R, Bahamondes Opazo R, Moreno-Moreno P, Molina Puerta MF, Galvez-Moreno MA. Hyperlipidemia during gestational diabetes and its relation with maternal and offspring complications. Nutr Hosp. 2018;35(3):698–706. [DOI] [PubMed]; Herrera Martinez A, Palomares Ortega R, Bahamondes Opazo R, Moreno-Moreno P, Molina Puerta MF, Galvez-Moreno MA. Hyperlipidemia during gestational diabetes and its relation with maternal and offspring complications. Nutr Hosp. 2018;35(3):698–706. doi: 10.20960/nh.1539. [DOI] [PubMed] [Google Scholar]
- [19].Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, et al. The association between metabolic syndrome and its components with systemic lupus erythematosus: a comprehensive systematic review and meta-analysis of observational studies. Lupus. 2018;27(6):899–912. [DOI] [PubMed]; Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E. et al. The association between metabolic syndrome and its components with systemic lupus erythematosus: a comprehensive systematic review and meta-analysis of observational studies. Lupus. 2018;27(6):899–912. doi: 10.1177/0961203317751047. [DOI] [PubMed] [Google Scholar]
- [20].Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A, et al. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta-analysis. PLoS One. 2017;12(3):e0170361. [DOI] [PMC free article] [PubMed]; Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A. et al. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta-analysis. PLoS One. 2017;12(3):e0170361. doi: 10.1371/journal.pone.0170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abdi F, Kazemi F, Tehrani FR, Roozbeh N. Protocol for systematic review and meta-analysis: hop (Humulus lupulus L.) for menopausal vasomotor symptoms. BMJ Open. 2016;6(4):e010734. [DOI] [PMC free article] [PubMed]; Abdi F, Kazemi F, Tehrani FR, Roozbeh N. Protocol for systematic review and meta-analysis: hop (Humulus lupulus L.) for menopausal vasomotor symptoms. BMJ Open. 2016;6(4):e010734. doi: 10.1136/bmjopen-2015-010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the US preventive services task force and the national institutes of health office of medical applications of research. Ann Intern Med. 2013;159(2):123–9. [DOI] [PubMed]; Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the US preventive services task force and the national institutes of health office of medical applications of research. Ann Intern Med. 2013;159(2):123–9. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- [23].Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of preeclampsia: a systematic review. Taiwan J Obstet Gynecol. 2020;59(2):173–82. [DOI] [PubMed]; Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of preeclampsia: a systematic review. Taiwan J Obstet Gynecol. 2020;59(2):173–82. doi: 10.1016/j.tjog.2020.01.002. [DOI] [PubMed] [Google Scholar]
- [24].Chen Y, Du M, Xu J, Chen D. The small dense LDL particle/large buoyant LDL particle ratio is associated with glucose metabolic status in pregnancy. Lipids Health Dis. 2017;16(1):244. [DOI] [PMC free article] [PubMed]; Chen Y, Du M, Xu J, Chen D. The small dense LDL particle/large buoyant LDL particle ratio is associated with glucose metabolic status in pregnancy. Lipids Health Dis. 2017;16(1):244. doi: 10.1186/s12944-017-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Durnwald C, Werner EF. Diabetes mellitus in pregnancy: Screening and diagnosis. UpToDate Available at: URL: http://www uptodate com/contents/diabetes-mellitus-in-pregnancy-screening-and-diagnosis; 2018.; Durnwald C, Werner EF. Diabetes mellitus in pregnancy: Screening and diagnosis. 2018. http://www uptodate com/contents/diabetes-mellitus-in-pregnancy-screening-and-diagnosis UpToDate Available at: URL:
- [26].Correa PJ, Venegas P, Palmeiro Y, Albers D, Rice G, Roa J, et al. First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study. J Perinat Med. 2019;47(2):161–8. [DOI] [PubMed]; Correa PJ, Venegas P, Palmeiro Y, Albers D, Rice G, Roa J. et al. First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study. J Perinat Med. 2019;47(2):161–8. doi: 10.1515/jpm-2018-0120. [DOI] [PubMed] [Google Scholar]
- [27].Emet T, Ustüner I, Güven SG, Balık G, Ural UM, Tekin YB, et al. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch Gynecol Obstet. 2013;288(1):49–55. [DOI] [PubMed]; Emet T, Ustüner I, Güven SG, Balık G, Ural UM, Tekin YB. et al. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch Gynecol Obstet. 2013;288(1):49–55. doi: 10.1007/s00404-013-2750-y. [DOI] [PubMed] [Google Scholar]
- [28].Anjum F, Zaini R, Shami A, Rehaili A, Kufia R. Glycated hemoglobin and lipid profile association among pregnant women in Saudi Arabian population. Int J Womens Health Reprod Sci. 2019;7(2):216–22.; Anjum F, Zaini R, Shami A, Rehaili A, Kufia R. Glycated hemoglobin and lipid profile association among pregnant women in Saudi Arabian population. Int J Womens Health Reprod Sci. 2019;7(2):216–22. [Google Scholar]
- [29].Van de Woestijne A, Monajemi H, Kalkhoven E, Visseren F. Adipose tissue dysfunction and hypertriglyceridemia: mechanisms and management. Obes Rev. 2011;12(10):829–40. [DOI] [PubMed]; Van de Woestijne A, Monajemi H, Kalkhoven E, Visseren F. Adipose tissue dysfunction and hypertriglyceridemia: mechanisms and management. Obes Rev. 2011;12(10):829–40. doi: 10.1111/j.1467-789X.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- [30].Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diabetes Vasc Dis Res. 2011;8(1):22–8. [DOI] [PubMed]; Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diabetes Vasc Dis Res. 2011;8(1):22–8. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- [31].Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(11):765–76. [DOI] [PubMed]; Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(11):765–76. doi: 10.7326/M14-2221. [DOI] [PubMed] [Google Scholar]
- [32].Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T, et al. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–9. [DOI] [PubMed]; Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T. et al. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–9. doi: 10.1016/j.jsbmb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [33].Sonagra AD, Biradar SM, Dattatreya K, Ds JM. Normal pregnancy-a state of insulin resistance. J Clin diagnostic research: JCDR. 2014;8(11):CC01–3. [DOI] [PMC free article] [PubMed]; Sonagra AD, Biradar SM, Dattatreya K, Ds JM. Normal pregnancy-a state of insulin resistance. J Clin diagnostic research: JCDR. 2014;8(11):CC01–3. doi: 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith FR. Hyperlipidemia and premature arteriosclerosis. Lipids. 1978;13(5):375–7. [DOI] [PubMed]; Smith FR. Hyperlipidemia and premature arteriosclerosis. Lipids. 1978;13(5):375–7. doi: 10.1007/BF02533734. [DOI] [PubMed] [Google Scholar]
- [35].Ryckman K, Spracklen C, Smith C, Robinson J, Saftlas A. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta‐analysis. Bjog: Int J Obstet Gynaecol. 2015;122(5):643–51. [DOI] [PubMed]; Ryckman K, Spracklen C, Smith C, Robinson J, Saftlas A. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta‐analysis. Bjog: Int J Obstet Gynaecol. 2015;122(5):643–51. doi: 10.1111/1471-0528.13261. [DOI] [PubMed] [Google Scholar]
- [36].Mankuta D, Elami-Suzin M, Elhayani A, Vinker S. Lipid profile in consecutive pregnancies. Lipids Health Dis. 2010;9(1):58. [DOI] [PMC free article] [PubMed]; Mankuta D, Elami-Suzin M, Elhayani A, Vinker S. Lipid profile in consecutive pregnancies. Lipids Health Dis. 2010;9(1):58. doi: 10.1186/1476-511X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Asare-Anane H, Bawah A, Osa-Andrews B, Adanu R, Ofori E, Tagoe SBRAE, et al. Lipid profile in Ghanaian women with gestational diabetes mellitus. Cell. 2013;233:246024002.; Asare-Anane H, Bawah A, Osa-Andrews B, Adanu R, Ofori E, Tagoe SBRAE. et al. Lipid profile in Ghanaian women with gestational diabetes mellitus. Cell. 2013;233:246024002. [Google Scholar]
- [38].Layton J, Powe C, Allard C, Battista MC, Doyon M, Bouchard L, et al. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism-Clinical Exp. 2019;91:39–42. [DOI] [PMC free article] [PubMed]; Layton J, Powe C, Allard C, Battista MC, Doyon M, Bouchard L. et al. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism-Clinical Exp. 2019;91:39–42. doi: 10.1016/j.metabol.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bukowiecka-Matusiak M, Burzynska-Pedziwiatr I, Sansone A, Malachowska B, Zurawska-Klis M, Ferreri C, et al. Lipid profile changes in erythrocyte membranes of women with diagnosed GDM. PLoS One. 2018;13(9):0203799. [DOI] [PMC free article] [PubMed]; Bukowiecka-Matusiak M, Burzynska-Pedziwiatr I, Sansone A, Malachowska B, Zurawska-Klis M, Ferreri C. et al. Lipid profile changes in erythrocyte membranes of women with diagnosed GDM. PLoS One. 2018;13(9):0203799. doi: 10.1371/journal.pone.0203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aydemir B, Behice Serinkan Cinemre F, Cinemre H, Tüten A, Aytaç Yüksel M, Yılmaz N, et al. Paraoxonase 1 (PON1) Q192R and L55M polymorphisms, lipid profile, lipid peroxidation and lipoprotein-a levels in Turkish patients with pregnancy-related disorders. Gynecol Endocrinol. 2019;35(5):417–21. [DOI] [PubMed]; Aydemir B, Behice Serinkan Cinemre F, Cinemre H, Tüten A, Aytaç Yüksel M, Yılmaz N. et al. Paraoxonase 1 (PON1) Q192R and L55M polymorphisms, lipid profile, lipid peroxidation and lipoprotein-a levels in Turkish patients with pregnancy-related disorders. Gynecol Endocrinol. 2019;35(5):417–21. doi: 10.1080/09513590.2018.1532990. [DOI] [PubMed] [Google Scholar]
- [41].Farsangi Z, Zoghi G, Kheirandish M, Shahbazi R, Mahmoudi M, Khayatian M, et al. Lipid profile in pregnant women with and without gestational diabetes mellitus: a case-control study. Hormozgan Med J. 2021;25(1):3–8.; Farsangi Z, Zoghi G, Kheirandish M, Shahbazi R, Mahmoudi M, Khayatian M. et al. Lipid profile in pregnant women with and without gestational diabetes mellitus: a case-control study. Hormozgan Med J. 2021;25(1):3–8. [Google Scholar]
- [42].Hossain M, Rahman AKMS, Mahjabeen S, Zaman M, Abedin M, Mahmood T, et al. Comparison of serum lipid profile between gestational diabetes mellitus and pregnant women with normal glucose tolerance. J Biosci Med. 2020;8(6):148–59.; Hossain M, Rahman AKMS, Mahjabeen S, Zaman M, Abedin M, Mahmood T. et al. Comparison of serum lipid profile between gestational diabetes mellitus and pregnant women with normal glucose tolerance. J Biosci Med. 2020;8(6):148–59. [Google Scholar]
- [43].Saumya V. Evaluation of fasting plasma glucose, lipid profile and thyroid stimulating hormone as predictors of gestational diabetes mellitus. Age (years). 2020;30(4.13):32.29–4.42.; Saumya V. Evaluation of fasting plasma glucose, lipid profile and thyroid stimulating hormone as predictors of gestational diabetes mellitus. Age (years) 2020;30(4.13):32.29–4.42. [Google Scholar]
- [44].Alyas S, Roohi N, Ashraf S, Ilyas S, Ilyas A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab Syndrome-Clinical Res Rev. 2019;13(4):2353–6. [DOI] [PubMed]; Alyas S, Roohi N, Ashraf S, Ilyas S, Ilyas A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM) Diabetes Metab Syndrome-Clinical Res Rev. 2019;13(4):2353–6. doi: 10.1016/j.dsx.2019.06.006. [DOI] [PubMed] [Google Scholar]
- [45].Yue CY, Ying CM. Epidemiological analysis of maternal lipid levels during the second trimester in pregnancy and the risk of adverse pregnancy outcome adjusted by pregnancy BMI. J Clin Laboratory Anal. 2018;32(8):22568. [DOI] [PMC free article] [PubMed]; Yue CY, Ying CM. Epidemiological analysis of maternal lipid levels during the second trimester in pregnancy and the risk of adverse pregnancy outcome adjusted by pregnancy BMI. J Clin Laboratory Anal. 2018;32(8):22568. doi: 10.1002/jcla.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zebunnesa M, Karim MM, Shirin F, Rehena MZ, Dhar S. Evaluation of lipid profile between gestational diabetes mellitus and normal pregnant women. IOSR-JDMS. 2018;17(6):15–21.; Zebunnesa M, Karim MM, Shirin F, Rehena MZ, Dhar S. Evaluation of lipid profile between gestational diabetes mellitus and normal pregnant women. 2018;17(6):15–21. IOSR-JDMS.
- [47].Cao W, Wang X, Chen T, Xu W, Feng F, Zhao S, et al. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp Ther Med. 2018;16(1):427–35. [DOI] [PMC free article] [PubMed]; Cao W, Wang X, Chen T, Xu W, Feng F, Zhao S. et al. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp Ther Med. 2018;16(1):427–35. doi: 10.3892/etm.2018.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bugatto F, Quintero-Prado R, Visiedo FM, Vilar-Sánchez JM, Figueroa-Quiñones A, López-Tinoco C, et al. The influence of lipid and proinflammatory status on maternal uterine blood flow in women with late onset gestational diabetes. Reprod Sci. 2018;25(6):837–43. [DOI] [PubMed]; Bugatto F, Quintero-Prado R, Visiedo FM, Vilar-Sánchez JM, Figueroa-Quiñones A, López-Tinoco C. et al. The influence of lipid and proinflammatory status on maternal uterine blood flow in women with late onset gestational diabetes. Reprod Sci. 2018;25(6):837–43. doi: 10.1177/1933719117698576. [DOI] [PubMed] [Google Scholar]
- [49].Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Casp J Intern Med. 2018;9(4):368–75. [DOI] [PMC free article] [PubMed]; Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Casp J Intern Med. 2018;9(4):368–75. doi: 10.22088/cjim.9.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bao W, Dar S, Zhu Y, Wu J, Rawal S, Li S, et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J diabetes. 2018;10(6):487–95. [DOI] [PMC free article] [PubMed]; Bao W, Dar S, Zhu Y, Wu J, Rawal S, Li S. et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J diabetes. 2018;10(6):487–95. doi: 10.1111/1753-0407.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pazhohan A, Rezaee Moradali M, Pazhohan N, Sadeghyanifar A. Indicators of obesity and serum levels of triglycerides in the first trimester of pregnancy as the predictors of gestational diabetes mellitus among Urmia women. Modern Care J. 2018;15(1):1–18.; Pazhohan A, Rezaee Moradali M, Pazhohan N, Sadeghyanifar A. Indicators of obesity and serum levels of triglycerides in the first trimester of pregnancy as the predictors of gestational diabetes mellitus among Urmia women. Modern Care J. 2018;15(1):1–18. [Google Scholar]
- [52].Wang C, Zhu W, Wei Y, Su R, Feng H, Hadar E, et al. The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatology. 2017;37(2):127–33. [DOI] [PubMed]; Wang C, Zhu W, Wei Y, Su R, Feng H, Hadar E. et al. The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatology. 2017;37(2):127–33. doi: 10.1038/jp.2016.191. [DOI] [PubMed] [Google Scholar]
- [53].Ghodke B, Pusukuru R, Mehta V. Association of lipid profile in pregnancy with preeclampsia, gestational diabetes mellitus, and preterm delivery. Cureus. 2017;9(7):1420. [DOI] [PMC free article] [PubMed]; Ghodke B, Pusukuru R, Mehta V. Association of lipid profile in pregnancy with preeclampsia, gestational diabetes mellitus, and preterm delivery. Cureus. 2017;9(7):1420. doi: 10.7759/cureus.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang C, Zhu W, Wei Y, Su R, Feng H, Lin L, et al. The predictive effects of early pregnancy lipid profiles and fasting glucose on the risk of gestational diabetes mellitus stratified by body mass index. J Diabetes Res. 2016;2016:3013567. [DOI] [PMC free article] [PubMed]; Wang C, Zhu W, Wei Y, Su R, Feng H, Lin L. et al. The predictive effects of early pregnancy lipid profiles and fasting glucose on the risk of gestational diabetes mellitus stratified by body mass index. J Diabetes Res. 2016;2016:3013567. doi: 10.1155/2016/3013567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shen H, Liu X, Chen Y, He B, Cheng W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open. 2016;6(12):e013509. [DOI] [PMC free article] [PubMed]; Shen H, Liu X, Chen Y, He B, Cheng W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open. 2016;6(12):e013509. doi: 10.1136/bmjopen-2016-013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liang Z, Wu Y, Zhu X, Fang Q, Chen D. Insulin resistance and lipid profile during an oral glucose tolerance test in women with and without gestational diabetes mellitus. J Obstet Gynaecol. 2016;36(3):337–9. [DOI] [PubMed]; Liang Z, Wu Y, Zhu X, Fang Q, Chen D. Insulin resistance and lipid profile during an oral glucose tolerance test in women with and without gestational diabetes mellitus. J Obstet Gynaecol. 2016;36(3):337–9. doi: 10.3109/01443615.2015.1060197. [DOI] [PubMed] [Google Scholar]
- [57].Khosrowbeygi A, Shiamizadeh N, Taghizadeh N. Maternal circulating levels of some metabolic syndrome biomarkers in gestational diabetes mellitus. Endocrine. 2016;51(2):245–55. [DOI] [PubMed]; Khosrowbeygi A, Shiamizadeh N, Taghizadeh N. Maternal circulating levels of some metabolic syndrome biomarkers in gestational diabetes mellitus. Endocrine. 2016;51(2):245–55. doi: 10.1007/s12020-015-0697-4. [DOI] [PubMed] [Google Scholar]
- [58].Jin W-Y, Lin S-L, Hou R-L, Chen X-Y, Han T, Jin Y, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC pregnancy childbirth. 2016;16(1):60. [DOI] [PMC free article] [PubMed]; Jin W-Y, Lin S-L, Hou R-L, Chen X-Y, Han T, Jin Y. et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC pregnancy childbirth. 2016;16(1):60. doi: 10.1186/s12884-016-0852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, et al. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol & Metab. 2016;101(7):2721–7. [DOI] [PMC free article] [PubMed]; Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP. et al. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol & Metab. 2016;101(7):2721–7. doi: 10.1210/jc.2015-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ertug EY, Usta M, Baytekin O, Diker VO, Korkmazer E, Ozkaya E. Serum lipid profile and inflammatory status in women with gestational diabetes mellitus. Eur J Gen Med. 2016;13(1):45–52.; Ertug EY, Usta M, Baytekin O, Diker VO, Korkmazer E, Ozkaya E. Serum lipid profile and inflammatory status in women with gestational diabetes mellitus. Eur J Gen Med. 2016;13(1):45–52. [Google Scholar]
- [61].Wang DY, Xu SQ, Chen HT, Zhong LQ, Wang ZL. The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol. 2015;83(4):490–7. [DOI] [PubMed]; Wang DY, Xu SQ, Chen HT, Zhong LQ, Wang ZL. The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol. 2015;83(4):490–7. doi: 10.1111/cen.12742. [DOI] [PubMed] [Google Scholar]
- [62].Li G, Kong L, Zhang L, Fan L, Su Y, Rose JC, et al. Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci. 2015;22(6):712–7. [DOI] [PMC free article] [PubMed]; Li G, Kong L, Zhang L, Fan L, Su Y, Rose JC. et al. Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci. 2015;22(6):712–7. doi: 10.1177/1933719114557896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].dos Santos-Weiss IC, Réa RR, Fadel-Picheth CM, Rego FG, Pedrosa Fde O, Gillery P, et al. The plasma logarithm of the triglyceride/HDL-cholesterol ratio is a predictor of low risk gestational diabetes in early pregnancy. Clinica Chim Acta. 2013;418:1–4. [DOI] [PubMed]; dos Santos-Weiss IC, Réa RR, Fadel-Picheth CM, Rego FG, Pedrosa Fde O, Gillery P. et al. The plasma logarithm of the triglyceride/HDL-cholesterol ratio is a predictor of low risk gestational diabetes in early pregnancy. Clinica Chim Acta. 2013;418:1–4. doi: 10.1016/j.cca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [64].Khan R, Ali K, Khan Z, Ahmad T. Lipid profile and glycosylated hemoglobin status of gestational diabetic patients and healthy pregnant women. Indian J Med Sci. 2012;66(7–8):149–54. [PubMed]; Khan R, Ali K, Khan Z, Ahmad T. Lipid profile and glycosylated hemoglobin status of gestational diabetic patients and healthy pregnant women. Indian J Med Sci. 2012;66(7–8):149–54. [PubMed] [Google Scholar]
- [65].Caglar GS, Cakal GO, Yuce E, Pabuccu R. Evaluation of serum boron levels and lipid profile in pregnancies with or without gestational diabetes. J Perinat Med. 2012;40(2):137–40. [DOI] [PubMed]; Caglar GS, Cakal GO, Yuce E, Pabuccu R. Evaluation of serum boron levels and lipid profile in pregnancies with or without gestational diabetes. J Perinat Med. 2012;40(2):137–40. doi: 10.1515/JPM.2011.121. [DOI] [PubMed] [Google Scholar]
- [66].Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201(5):482. [DOI] [PMC free article] [PubMed]; Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A. et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201(5):482. doi: 10.1016/j.ajog.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McGrowder D, Grant K, Irving R, Gordon L, Crawford T, Alexander-Lindo R, et al. Lipid profile and clinical characteristics of women with gestational diabetes mellitus and preeclampsia. J Med Biochem. 2009;28(2):72–81.; McGrowder D, Grant K, Irving R, Gordon L, Crawford T, Alexander-Lindo R. et al. Lipid profile and clinical characteristics of women with gestational diabetes mellitus and preeclampsia. J Med Biochem. 2009;28(2):72–81. [Google Scholar]