Abstract
Background
Low-dose dexamethasone demonstrated clinical improvement in patients with coronavirus disease 2019 (COVID-19) needing oxygen therapy; however, evidence on the efficacy of high-dose dexamethasone is limited.
Methods
We performed a randomised, open-label, controlled trial involving hospitalised patients with confirmed COVID-19 pneumonia needing oxygen therapy. Patients were randomly assigned in a 1:1 ratio to receive low-dose dexamethasone (6 mg once daily for 10 days) or high-dose dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days). The primary outcome was clinical worsening within 11 days since randomisation. Secondary outcomes included 28-day mortality, time to recovery and clinical status at day 5, 11, 14 and 28 on an ordinal scale ranging from 1 (discharged) to 7 (death).
Results
A total of 200 patients (mean±sd age 64±14 years; 62% male) were enrolled. 32 (31.4%) out of 102 patients enrolled in the low-dose group and 16 (16.3%) out of 98 in the high-dose group showed clinical worsening within 11 days since randomisation (rate ratio 0.427, 95% CI 0.216–0.842; p=0.014). The 28-day mortality was 5.9% in the low-dose group and 6.1% in the high-dose group (p=0.844). There was no significant difference in time to recovery, and in the seven-point ordinal scale at days 5, 11, 14 and 28.
Conclusions
Among hospitalised COVID-19 patients needing oxygen therapy, high dose of dexamethasone reduced clinical worsening within 11 days after randomisation, compared with low dose.
Short abstract
This study showed that in hospitalised COVID-19 patients with moderate or severe COVID-19 pneumonia needing oxygen therapy, high dose of dexamethasone reduced clinical worsening within 11 days after randomisation compared with low dose of dexamethasone https://bit.ly/3dBe5Aa
Introduction
Since the emergence of the 2019 novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan, China in December 2019, it has spread rapidly across China and many other countries. The most common presenting symptoms by patients with coronavirus disease 2019 (COVID-19) are fever, cough, myalgia, dyspnoea, headache and diarrhoea [1]. Many of those admitted to hospital show fatigue and dyspnoea, displaying bilateral lung and/or multilobe involvement on radiological imaging and requiring some type of oxygen support. Although most patients are known to have a favourable outcome, in some of them respiratory failure sets in, and may eventually progress to acute respiratory distress syndrome (ARDS). Eventually, respiratory support such as high-flow nasal cannula (HFNC), noninvasive mechanical ventilation (NIMV) or mechanical ventilation (MV) become necessary [2–4]. The unfavourable outcome of these patients has been related to an exaggerated inflammatory response caused by SARS-CoV-2.
Recently, a meta-analysis [5] that included seven randomised clinical trials showed improved outcomes in moderate or severe COVID-19 patients treated with corticosteroids. However, the doses (high versus low dose) and the type of corticosteroids (dexamethasone versus hydrocortisone versus methylprednisolone) used in these trials were different. The most important clinical trial due to the number of patients investigated was RECOVERY [6]. The RECOVERY trial used a fixed dose of 6 mg dexamethasone daily for 10 days showing a clear beneficial effect in patients with COVID-19 who were receiving mechanical ventilation at the time of randomisation. However, only a modest benefit was observed in less severely ill patients receiving oxygen without mechanical ventilation, whereas no benefits were seen in patients without respiratory support. As a result of the RECOVERY trial, numerous studies with different corticosteroids and higher doses were stopped prematurely [7–9], and low-dose dexamethasone was recommended by the Infectious Diseases Society of America [10], the United States National Institutes of Health [11] and the World Health Organization (WHO) [12] for the usual care of hospitalised patients with COVID-19 needing oxygen therapy. At present, it is unclear which would be the optimal drug and dose, and when to initiate corticosteroid therapy. Findings from previous studies involving patients with moderate or severe COVID-19 pneumonia have suggested that the use of higher doses of corticosteroids may be associated with better outcomes [7, 13].
We performed a randomised, open-label, controlled clinical trial (HIGHLOWDEXA-COVID) to evaluate the efficacy of high-dose dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days) versus low-dose dexamethasone (6 mg once daily for 10 days) in patients hospitalised with COVID-19 pneumonia needing oxygen therapy. We hypothesised that a high dose of dexamethasone might reduce the risk of clinical worsening defined as worsening of the patient's condition during treatment (need to increase inspiratory oxygen fraction (FiO2) by >0.2, need for FiO2 >0.5, respiratory rate >25 breaths·min−1) or score >4 on the seven-point WHO Ordinal Scale for Clinical Improvement (CIS).
Methods
Study design
This is a randomised, controlled, parallel-group, open-label clinical trial aimed to assess the efficacy of high versus low dose of dexamethasone in hospitalised COVID-19 patients with respiratory failure requiring oxygen therapy. Patients were randomised using a web-based system with a 1:1 allocation ratio. The trial was approved on 13 January 2021, by the ethics committee of Galicia, Spain (CEIm-G, code No. 2020-636), and on 14 January 2021, by the Spanish Agency of Medicines and Health Products (AEMPS, N° EudraCT 2020-005702-25). The study was conducted in accordance with good clinical practice guidelines, the Declaration of Helsinki, local requirements and the institutional protocol for the care of hospitalised COVID-19 patients. The authors designed the trial, collected the data and performed the analysis. All the authors revised the manuscript, testify for its accuracy and the completeness of the data and approved the decision to submit the manuscript for publication.
Participants
Patients were enrolled if they were aged ≥18 years, had SARS-CoV-2 infection confirmed by nasopharyngeal swab PCR and were receiving supplemental oxygen in order to maintain an oxygen saturation >92% (level 4 using the seven-point WHO-CIS). Scores on the seven-point WHO-CIS were defined as follows: 1) not hospitalised; 2) hospitalised, not requiring supplemental oxygen, no longer requires ongoing medical care (independent); 3) hospitalised, not requiring supplemental oxygen, but in need of ongoing medical care (COVID-19 related or otherwise); 4) hospitalised, requiring supplemental oxygen; 5) hospitalised, requiring noninvasive ventilation or HFNC; 6) hospitalised, requiring intensive care unit (ICU) admission and invasive mechanical ventilation or extracorporeal membrane oxygenation; 7) death. Exclusion criteria were pregnancy or active lactation, known history of dexamethasone allergy or known contraindication to the use of corticosteroids, indication for corticosteroids use for other clinical conditions (e.g. refractory septic shock), daily use of oral or intravenous corticosteroids in the past 15 days, expected death within the next 48 h, level other than 4 on the seven-point WHO-CIS, need for supplemental oxygen with FiO2 >0.5 in order to maintain an oxygen saturation >92%, and refusal of consent to participate in the trial. All the patients provided informed consent before randomisation.
Randomisation
Patients were randomly assigned in a 1:1 ratio to receive standard care plus dexamethasone 6 mg once daily for 10 days (low-dose group) or dexamethasone 20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days (high-dose group). The randomisation sequence was performed by a computer programme in blocks of six and was not stratified. Physicians, patients and individuals who assessed the outcomes were not blinded to the assigned treatment. All clinical interventions, such as use of antiviral agents, antibiotics, other immunomodulators, anticoagulants and laboratory testing, were left at the discretion of the medical team for both groups in accordance with the hospital's own protocol, previously agreed upon by the pneumology, internal medicine (infectious diseases unit), anaesthesiology, intensive medicine and hospital pharmacy departments. Patients who suffered clinical worsening could receive a high dose of dexamethasone as a rescue therapy. Protocol adherence was assessed daily until day 11.
Procedures
The following data were collected on all patients upon hospital admission: demographic characteristics, coexisting disorders, home treatments, time from initial symptoms to randomisation, time from hospital admission to randomisation and laboratory values on randomisation (biochemical parameters, leukocytes, lymphocytes, serum ferritin, procalcitonin, lactate dehydrogenase, D-dimer, C-reactive protein). During the hospital stay we assessed the patients’ overall outcome, clinical status using the seven-point WHO-CIS, medication (antibiotic agents, antiviral agents, anticoagulants), need for respiratory support (nasal cannula, simple mask, HFNC, NIMV, invasive mechanical ventilation), the need for ICU admission and complications (hospital-acquired infection, hyperglycaemia, thromboembolic complications, acute kidney injury needing kidney replacement, gastrointestinal bleeding, death).
Outcomes
The goal of the investigation was to evaluate the efficacy of high versus low dose of dexamethasone in patients with respiratory failure by COVID-19. The primary outcome was clinical worsening within 11 days since randomisation, defined as worsening of the patient's condition during treatment (need to increase FiO2 >0.2, need for FiO2 >0.5, respiratory rate >25 breaths·min−1) or score >4 on the seven-point WHO-CIS. Secondary outcomes included time to recovery (defined as the first day after enrolment on which a patient attained category 1, 2 or 3 on the seven-point WHO-CIS), clinical status of patients using the seven-point WHO-CIS at day 5, 11, 14, 28 and 60 days after randomisation, adverse drug reactions, number of patients admitted to the ICU, number of patients who needed mechanical ventilation, duration of mechanical ventilation, length of ICU and hospital stay, number of patients discharged from hospital at day 28, mortality during hospitalisation and mortality at days 28 and 60.
Statistical analysis
We assumed, by clinical experience during the pandemic, that the risk of clinical worsening (primary outcome) within 11 days after randomisation in patients with COVID-19 needing oxygen therapy would be 35% in the low-dose dexamethasone group and that the risk with a high dose would be ∼15%. It was decided to set a limit of 5% superiority. We found that a minimum of 98 patients would need to be included in each group of the test to ensure a power of 80% and to be able to conclude with a significance level of 5%.
For the primary outcome and the secondary outcomes recovery, hospital discharge and death at 28 days, Kaplan–Meier survival curves were used to show time-to-event analysis between the two patterns of treatment. In addition, by the Kaplan–Meier analysis, the number of patients at risk at specific time points in each group was estimated. The two treatments strategies were compared with the use of log-rank tests. For the pre-specified primary outcome and all pre-specified secondary outcomes stated in the protocol, a logistic regression model was used to estimate the risk ratios and their 95% confidence intervals. Risk ratios were estimated for the outcomes of complications and adverse events (nosocomial infection, insulin use for hyperglycaemia, thrombosis, death at day 28 and death at day 60). To consider the age as an important prognostic factor, estimates of risk ratios were adjusted for the baseline age as a continuous variable.
All p-values are two-sided and are shown without adjustment for multiple testing. All analyses were performed according to the intention-to-treat principle.
Finally, we examined whether the treatment effect on the primary outcome varied within subgroups defined as clinical risk factors for poor evolution of COVID-19 such as advanced age, obesity, low arterial oxygen tension (PaO2)/FiO2 ratio and oxygen saturation (SpO2)/FiO2 ratio, and time from symptom onset to randomisation. Although these analyses were not stated in the study protocol, we analysed a low number of subgroups, and treatment-effect modification was assessed in separate interaction models for each risk factor to assess heterogeneity between groups. The statistical significance of the interactions will allow to determine if the differences between the subgroups are random or are due to the effect of the treatment studied.
Results
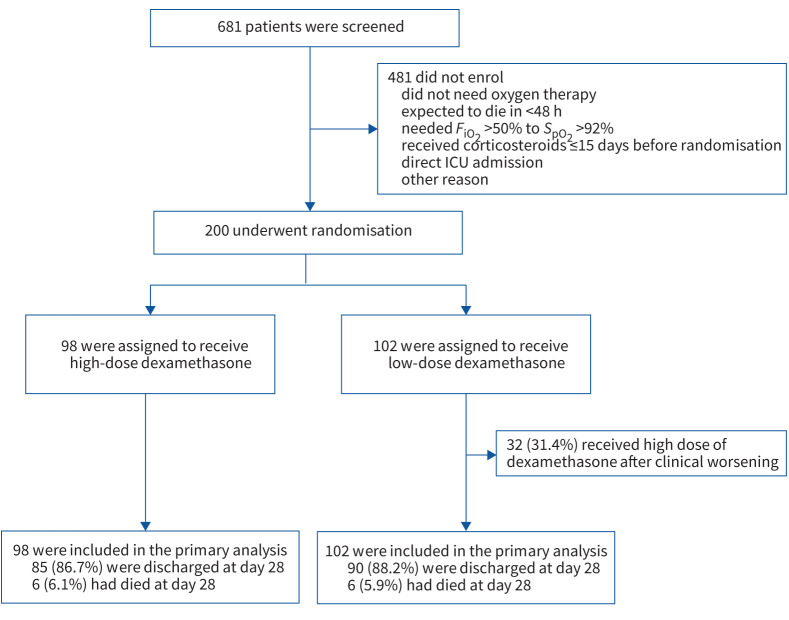
Between 15 January and 26 May 2021, a total of 200 patients were enrolled; 102 patients were randomly assigned to the low-dose group and 98 patients to the high-dose group (figure 1). The number of patients randomised weekly during the study period is presented in supplementary table S1. Follow-up was completed on 5 July 2021, and at that time no patient remained hospitalised. Demographic, baseline clinical and biological characteristics of patients and details of the treatments received during hospitalisation in the two groups are summarised in table 1. One patient in the low-dose group and three patients in the high-dose group did not receive supplemental oxygen during the study period. The mean±sd age was 64.3±14.3 years and 62% were male. The median time for symptoms onset to randomisation was 8 days (interquartile range (IQR) 7–10 days).
FIGURE 1.
Screening, randomisation and outcomes. FiO2: inspiratory oxygen fraction; SpO2: peripheral oxygen saturation; ICU: intensive care unit.
TABLE 1.
Patient characteristics at baseline
| All patients | Low-dose dexamethasone | High-dose dexamethasone | |
| Patients | 200 | 102 | 98 |
| Age years | 64.3±14.3 | 64.8±14.1 | 63.9±14.5 |
| <50 | 37 (18.5) | 18 (17.6) | 19 (19.4) |
| 50–70 | 91 (45.5) | 46 (45.1) | 45 (45.9) |
| >70 | 72 (36.0) | 38 (37.3) | 34 (34.7) |
| Male | 123 (61.8) | 61 (60.4) | 62 (63.3) |
| Weight kg | 85.0 (74.0–95.5) | 84.0 (72.0–95.5) | 87.9 (79.6–95.7) |
| BMI kg·m−2 | 31.0 (27.1–34.1) | 29.8 (27.0–34.0) | 31.2 (27.8–34.2) |
| Coexisting condition | |||
| Hypertension | 96 (48.0) | 49 (48.0) | 47 (48.0) |
| Hyperlipidaemia | 83 (39.8) | 44 (43.1) | 39 (39.8) |
| Obesity (BMI ≥30 kg·m−2) | 106 (53.0) | 48 (47.1) | 58 (59.2) |
| Diabetes | 38 (19.0) | 20 (19.6) | 18 (18.4) |
| Chronic pulmonary disease | 14 (7.0) | 6 (5.9) | 8 (8.0) |
| Asthma | 10 (5.0) | 7 (6.9) | 3 (3.1) |
| Cardiovascular disease | 27 (13.5) | 15 (14.8) | 12 (12.2) |
| History of cancer | 10 (5.0) | 6 (5.9) | 4 (4.1) |
| Chronic kidney disease | 7 (3.5) | 4 (3.9) | 3 (3.1) |
| Previous medication use | |||
| ACE inhibitors | 28 (14.0) | 13 (12.7) | 15 (15.3) |
| Antihypertensive therapy | 52 (26.1) | 25 (24.8) | 27 (27.6) |
| Anticoagulants | 11 (5.5) | 7 (6.9) | 4 (4.1) |
| Antiplatelet therapy | 26 (13.0) | 14, 13.7 | 12 (12.2) |
| Inhaled corticosteroids | 15 (7.5) | 9 (8.8) | 6 (6.1) |
| Statins | 64 (32.0) | 32 (31.4) | 32 (32.7) |
| Immunosuppressants | 4 (2.0) | 1 (1.0) | 3 (3.1) |
| Insulin | 14 (7.0) | 8 (7.8) | 6 (6.1) |
| Laboratory parameters | |||
| Absolute leukocyte count cells·mm−3 | 5210 (3975–6625) | 5190 (3980–6542) | 5290 (3960–7077) |
| Absolute lymphocyte count cells·mm−3 | 860 (612–1080) | 905 (680– 1145) | 780 (507–1010) |
| Lactate dehydrogenase U·L−1 | 384 (284–509)) | 357.00 (242–497) | 415 (310–535) |
| D-dimer ng·mL−1 | 742 (490–1181) | 664 (457– 1160) | 763 (499–1244) |
| C-reactive protein mg·L−1 | 7.0 (2.9–11.3) | 6.1(2.8–9.6) | 8.3 (4.6– 12.9) |
| Procalcitonin ng·mL−1 | 0.10 (0.06–0.16) | 0.09 (0.05–0.15) | 0.11 (0.07–0.17) |
| Ferritin level ng·mL−1 | 599 (284–1226) | 539 (293–1203) | 623 (269–1244) |
| Interleukin-6# pg·mL−1 | 13.1 (6.8–25.1) | 14.5 (7.1–33.8) | 10.8 (6.4–21.6) |
| Symptoms | |||
| Dyspnoea | 128 (64.0) | 61 (59.8) | 67 (68.4) |
| Cough | 155 (77.5) | 84 (82.4) | 71 (72.4) |
| Fever | 146 (73.0) | 76 (74.5) | 70 (71.0) |
| Asthaenia | 46 (23.0) | 21 (20.6) | 25 (25.5) |
| Diarrhoea | 40 (20.3) | 17 (16.8) | 32 (24.0) |
| Disease | |||
| Temperature °C | 36.6 (36.0–37.5) | 36.6 (36.0–37.5) | 36.6 (36.0–37.4) |
| Respiratory rate breaths·min−1 | 16.0 (15.0–17.0) | 16.0 (15.0–18.5) | 16.0 (15.0–16.0) |
| Oxygen saturation % | 92.8 (90.3–94.0) | 93.0 (90.0–94.0) | 92.7 (90.0–94.0) |
| PaCO2 on admission | 34.0 (31.3–37.2) | 34.1 (31.9–37.4) | 34.0 (31.0–37.2) |
| PaO2/FiO2 ratio | 294.0 (264.9–317.7) | 295.2 (266.3–314.3) | 291.4 (262.9–321.1) |
| Days from symptom onset to randomisation | 8 (7–10) | 8 (7–9) | 8 (7–10) |
| Days from hospital admission to randomisation | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Type of oxygen therapy on randomisation | |||
| Nasal cannula | 150 (75.0) | 80 (79.2) | 70 (73.7) |
| Simple mask | 46 (23.0) | 21 (20.8) | 25 (26.3) |
| FiO2 on randomisation | 0.28 (0.28–0.32) | 0.28 (0.28–0.32) | 0.28 (0.28–0.32) |
| SpO2/FiO2 ratio on randomisation | 339.3 (300.0–350.0) | 342.8 (302.3–350.0) | 335.7 (300.0–350.0) |
| Hospital medical treatments | |||
| Remdesivir | 20 (10.0) | 11 (10.8) | 9 (9.2) |
| Prophylactic anticoagulant dose | 140 (70.0) | 76 (74.5) | 64 (65.3) |
| Intermediate anticoagulant dose | 53 (26.5) | 22 (21.6) | 31 (31.6) |
| High anticoagulant dose | 28 (14.0) | 16 (15.7) | 12 (12.2) |
| Tocilizumab | 24 (12.0) | 12 (11.8) | 12 (12.2) |
| Antibiotics | 182 (91.0) | 95 (93.1) | 87 (88.8) |
Data are presented as n, mean±sd, n (%) or median (interquartile range). Percentages may not total 100 because of rounding. There was a significant difference in four laboratory parameters (absolute lymphocyte count, lactate dehydrogenase, D-dimer, procalcitonin) between patients in the low-dose dexamethasone group and those in the high-dose dexamethasone group. There were no significant differences between the groups in any other baseline characteristic. BMI: body mass index; ACE: angiotensin-converting enzyme; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of arterial oxygen; FiO2: inspiratory oxygen fraction; SpO2: peripheral oxygen saturation. #: interleukin-6 levels were missing for 14 patients (six patients in the low-dose dexamethasone group and eight patients in the high-dose dexamethasone group).
All patients were followed for 11 days according to the study protocol (primary outcome) and for 28 days for secondary outcomes. Follow-up at 60 days was completed in 182 (91%) patients.
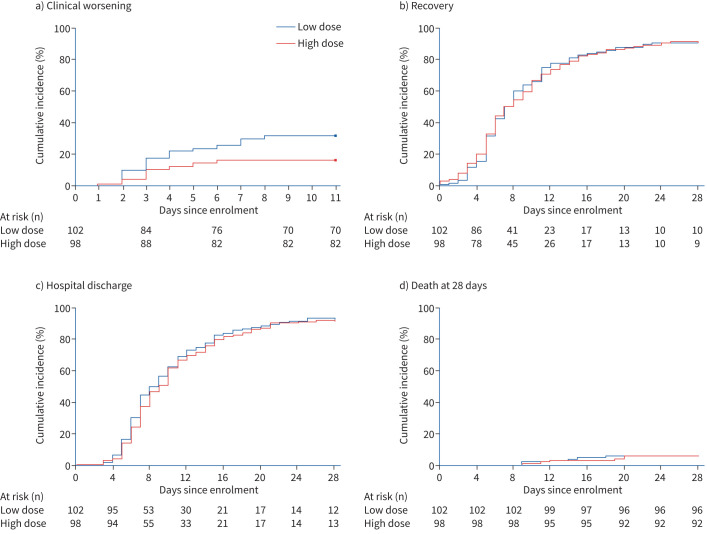
32 (31.4%) out of 102 patients enrolled in the low-dose group and 16 (16.3%) out of 98 in the high-dose group showed clinical worsening within 11 days since randomisation (risk ratio 0.427, 95% CI 0.216–0.842; p=0.014) (table 2). The Kaplan–Meier curves for the time to clinical worsening are shown in figure 2a and in supplementary figure S1. Patients who suffered clinical worsening at the low dose were allowed to receive the high dose of dexamethasone. Table 2 describes the events that led to clinical worsening in the 48 patients. Supplementary tables S4 and S5 describe the evolution of these 48 patients.
TABLE 2.
Primary and secondary outcomes and adverse events
| All patients | Low-dose dexamethasone | High-dose dexamethasone | Risk ratio (95% CI) # | p-value | |
| Patients | 200 | 102 | 98 | ||
| Primary outcome | |||||
| Clinical worsening within 11 days | 48 (24.0) | 32 (31.4) | 16 (16.3) | 0.427 (0.216–0.842) | 0.014 |
| First event | |||||
| Worsening of the patient's condition | 47 (23.5) | 31 (30.4) | 16 (16.3) | ||
| NIV/HFNC (level 5 on the seven-level ordinal scale) | 7 (3.5) | 3 (2.9) | 4 (4.1) | ||
| Mechanical ventilation (level 6 on the seven-level ordinal scale) | 15 (7) | 6 (5.9) | 9 (9.2) | ||
| Death (level 7 on the seven-level ordinal scale) | 1 (0.5) | 1 (1.0) | 0 (0) | ||
| Secondary outcomes | |||||
| Recovery | |||||
| Day 5 | 36 (18.0) | 16 (15.7) | 20 (20.4) | 1.350 (0.648–2.816) | 0.423 |
| Day 11 | 135 (67.5) | 67 (65.7) | 68 (69.3) | 1.164 (0.625–2.170) | 0.632 |
| Day 14 | 156 (78.0) | 79 (77.5) | 77 (78.6) | 1.043 (0.504–2.162) | 0.909 |
| Day 28 | 181 (90.5) | 92 (90.2) | 89 (90.8) | 1.000 (0.369–2.711) | 1.000 |
| Time to recovery days | 7.0 (5.0–11.0) | 7.0 (5.0–11.0) | 7.0 (5.0–11.2) | 0.997 (0.961–1.035) | 0.895 |
| Admission to ICU | 28 (14.0) | 13 (12.7) | 15 (15.3) | 0.995 (0.976–1.015) | 0.622 |
| Length of ICU stay days | 8.0 (6.0–14.7) | 9.0 (6.5–15.0) | 7.0 (6.0–13.0) | 1.020 (0.952–1.093) | 0.577 |
| Mechanical ventilation requirement | 19 (9.5) | 9 (8.8) | 10 (10.2) | 0.995 (0.976–1.015) | 0.629 |
| Duration of mechanical ventilation days | 9.0 (6.0–15.0) | 13.0 (8.0–15.0) | 8.0 (4.7–12.2) | 0.997 (0.915–1.086) | 0.945 |
| Discharged from hospital within 28 days | 175 (87.5) | 90 (88.2) | 85 (86.7) | 1.021 (0.845–1.233) | 0.831 |
| Length of hospital stay days | 9.0 (6.0–14.0) | 8.5 (6.0–13.2) | 9.0 (6.7–14.0) | 0.993 (0.973–1.014) | 0.523 |
| In-hospital mortality | 10 (5) | 6 (5.9) | 4 (4.1) | 0.997 (0.977–1.017) | 0.734 |
| Complications and adverse events | |||||
| Nosocomial infection | 20 (10.0) | 10 (9.8) | 10 (10.2) | 1.081 (0.425–2.750) | 0.870 |
| Pneumonia | 12 (6.0) | 7 (6.9) | 5 (5.1) | ||
| Catheter-related bloodstream infection | 1 (0.5) | 1 (0.9) | 0 (0.0) | ||
| Bacteraemia | 5 (2.5) | 4 (3.9) | 1 (1.0) | ||
| Urinary tract infection | 6 (3.0) | 1 (0.9) | 5 (5.1) | ||
| Insulin use for hyperglycaemia | 96 (48.0) | 49 (48.0) | 47 (48.0) | 0.997 (0.572–1.736) | 0.991 |
| Thrombosis | 7 (3.5) | 6 (5.9) | 1 (1.0) | 0.169 (0.020–1.434) | 0.103 |
| Death at day 28 | 12 (6.0) | 6 (5.9) | 6 (6.1) | 1.129 (0.338–3.772) | 0.844 |
| Death at day 60¶ | 15 (8.2) | 8 (8.3) | 7 (8.0) | 1.012 (0.333–3.080) | 0.983 |
Data are presented as n, n (%) or median (interquartile range), unless otherwise stated. NIV: noninvasive ventilation; HFNC: high-flow nasal cannula; ICU: intensive care unit. #: rate ratios have been adjusted for age with respect to the outcomes studied; ¶: follow-up at 60 days was completed in 184 (92.0%) patients. Data regarding death at day 60 were missing for 10 in the low-dose dexamethasone group and six patients in the high-dose dexamethasone group.
FIGURE 2.
Kaplan–Meier analysis of efficacy outcomes: Kaplan–Meier curves for the time-to-event analyses of a) clinical worsening (primary outcome); b) recovery, defined as the first day after enrolment on which a patient attained category 1, 2 or 3 on the seven-point ordinal scale (scores range from 1 to 7, with higher scores indicating worse clinical condition); c) hospital discharge; d) death.
Secondary outcomes are shown in table 2 and figures 1–3. The median time to recovery was 7.0 days (interquartile range (IQR) 5.0–11.0 days) in the low-dose group and 7.0 days (IQR 5.0–11.2 days) in the high-dose group (risk ratio 0.997, 95% CI 0.961–1.035; p=0.895). The Kaplan–Meier curves for the time to recovery are shown in figure 2b and in supplementary figure S2. At 14 days, 78.6% of patients in the high-dose group and 77.5% in the low-dose group were no longer receiving supplemental oxygen. At 28 days, the percentages were 90.8% and 90.2%, respectively (table 2).
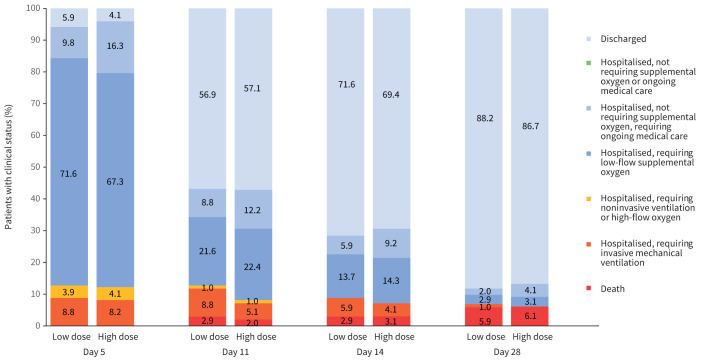
FIGURE 3.
Clinical status on a seven-point ordinal scale on study days 5, 11, 14 and 28 by treatment group. All percentage values in each point category are provided in supplementary table S3. At day 5, p=0.885 for comparison of the distribution of the high-dose group versus low-dose group. At day 11, p=0.666 for comparison of the distribution of the high-dose group versus low-dose group. At day 14, p=0.870 for comparison of the distribution of the high-dose group versus low-dose group. At day 28, p=0.831 for comparison of the distribution of the high-dose group versus low-dose group.
The median time to home discharge was 9.0 days (IQR 6.7–14 days) in the high-dose group and 8.5 days (IQR 6.0–13.2 days) in the low-dose group (risk ratio 0.993, 95% CI 0.973–1.014; p=0.523). The Kaplan–Meier curves for time to home discharge are shown in figure 2c and in supplementary figure S3. At 14 days, 69.4% of patients in the high-dose group and 71.6% in the low-dose group were discharged home. At 28 days, the percentages were 86.7% and 88.2%, respectively.
There was no significant difference between the two groups in need for ICU admission, need for mechanical ventilation, in-hospital mortality and all-cause mortality at 28 days (table 2). The Kaplan–Meier curves for the time to death are shown in figure 2d and in supplementary figure S4.
There was no significant difference between the two groups in the seven-point WHO-CIS at days 5, 11, 14 and 28 (figure 3 and supplementary table S2).
In the subgroup analyses performed according to age, sex, obesity, PaO2/FiO2 ratio, SpO2/FiO2 ratio and time from symptoms onset to randomisation, we cannot establish difference for the treatment effect. These results are shown in supplementary table S3.
Adverse events are shown in table 2. Both groups had a comparable need for insulin use for hyperglycaemia: 47 (48%) patients in the high-dose group versus 49 (48%) in the low-dose group. There was no significant difference in complications and adverse events between the two groups studied (table 2).
Discussion
In this randomised clinical trial involving 200 hospitalised patients with respiratory failure caused by COVID-19 needing oxygen therapy, high dose compared with low dose of dexamethasone significantly decreased clinical worsening within 11 days after randomisation. In addition, high dose of dexamethasone was not associated with increased risk of adverse events in this population of COVID-19 patients.
Clinical studies in which corticosteroids have been used to treat patients with COVID-19 have a high heterogeneity regarding the type of corticosteroids, dose, course for which the medication was given and which patients are suitable for the drug [6–9, 13–16]. Since the publication of the RECOVERY trial, all studies comparing different corticosteroids and regimens have been discontinued. Since then, the corticosteroid and recommended dose for COVID-19 needing oxygen therapy is 6 mg dexamethasone [10–12]. However, we do not know if a higher dose can improve outcome of COVID-19 patients. The high dose of dexamethasone used in the present trial was chosen based on previous trials showing the benefit of this dose in patients with COVID-19 [7] and non-COVID-19 ARDS [14].
In the present trial we decided to include only hospitalised patients with moderate or severe COVID-19 pneumonia, needing oxygen therapy at the time of randomisation. HFNC, NIMV, mechanical ventilation or ICU patients were not included. Clinical worsening in these patients was chosen as the primary outcome to investigate whether a higher dose of dexamethasone than the one recommended in the RECOVERY trial, administered in an early phase of the disease, when the inflammation is starting and the patient needs oxygen therapy, but is not sick enough to need more respiratory support or intensive care, may be more effective in reducing progression to more-severe disease. This primary outcome was also used in trials of other drugs such as remdesivir, tocilizumab or hydroxychloroquine in non-ICU patients with moderate-to-severe COVID-19 pneumonia [17–23]. Our data suggest that an early intervention with a high dose of dexamethasone may have prevented the progression to a more severe respiratory disease, as shown by the lower proportion of clinical worsening in patients who received a high dose of dexamethasone compared to those who received the low dose (16.4% versus 31.4%). In a recent multicentre observational cohort study in patients with severe respiratory disease admitted to the ICU, the authors observed that early use of a moderate-to-high dose of corticosteroids was associated with better outcomes such as shorter length of ICU stay, decreased organ dysfunction, fewer days on mechanical ventilation and no increase in medical or infection complications [13]. In a trial involving severely ill COVID-19 patients, the administration of high dose of methylprednisolone compared with standard care in the early pulmonary phase of the disease decreased the mortality rate and improved pulmonary involvement, oxygen saturation and inflammatory markers [15]. The results of these studies [13, 15] and the present trial should lead us to believe that ideally, corticosteroids should be started in the early stages of inflammation to avoid onset of severe inflammation.
Although in present study we observed a decrease on clinical worsening within 11 days in the group of patients who received high doses of corticosteroids (primary outcome), we did not observe differences between the two groups in secondary outcomes such as the time of recovery, clinical status at day 5, 11, 14 and 28 or mortality. This may be mainly because in the low-dose group, all patients who suffered a clinical worsening were administered a high dose of dexamethasone. Many of these patients improved after administration of high-dose dexamethasone, avoiding further clinical deterioration or the need for HFNC, NIV or mechanical ventilation (supplementary table S4).
The lower mortality rate seen in present study compared with the RECOVERY trial might be explained by the type of patients studied, and the higher doses administered. In the RECOVERY trial, patients were divided into those who needed oxygen therapy and mechanical ventilation with a mortality of 29.3%, and those who did not need mechanical ventilation with a mortality of 23.3%. However, in this last group the authors included patients with different severity levels of disease. In the present trial we included only patients who needed oxygen therapy and we excluded patients requiring HFNC, NIMV or mechanical ventilation. Consequently, this might have excluded patients with comorbidities and high risk of mortality.
The main adverse events related to corticosteroids are hyperglycaemia and new infections. Although higher doses of corticosteroids could be associated with more complications, in the present trial the number of adverse events, new infections and the use of insulin were comparable in both groups, in line with previous studies that did not demonstrate an augmented risk of adverse events with corticosteroids in patients with ARDS and with or without COVID-19 [7, 14, 23].
This trial has several limitations. First, 31.4% of the patients in the low-dexamethasone group received high dose of dexamethasone after clinical worsening (first outcome studied), related to the open-label design, and because it was allowed in the study protocol according to the treatment protocol of our hospital. The decision to allow a rescue therapy with high dose of dexamethasone was motivated by ethical concerns regarding the use of a safe and potentially effective drug as reported by previous clinical trials [7, 14]. Nevertheless, although rescue therapy did not affect the results of the primary end-point, it would have biased the results towards the null in the secondary end-points such as mortality, hospital stay or side-effects. Second, the open-label design and investigator-reported data on adverse events and infections may have led to bias in the description of these events. Third, the trial was underpowered for important secondary outcomes such as mortality and the study was limited to demonstrate benefits in secondary outcomes. Fourth, our study did not include critically ill patients or patients with mild disease. Nevertheless, the study results confirm the beneficial effects of high dose of dexamethasone in a well-defined subset of patients, including those with moderate or severe pneumonia needing oxygen therapy who were at risk of developing ARDS, thus becoming critically ill and requiring mechanical ventilation. To our knowledge, this is the first randomised clinical trial evaluating the effect of two doses of dexamethasone in patients with COVID-19 and pneumonia. Another six randomised clinical trials are underway comparing high versus standard doses (6 mg) of dexamethasone in adult patients with COVID-19 and hypoxia (NCT04509973, NCT04545242, NCT04663555, NCT04726098, NCT04395105, IRCT20100228003449N31).
In conclusion, among hospitalised COVID-19 patients needing oxygen therapy, high dose of dexamethasone reduced clinical worsening within 11 days after randomisation as compared with low dose of dexamethasone. Further studies are necessary for confirming these preliminary results and to compare different types of corticosteroids, doses and regimens in different stages of the disease.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02518-2021.Supplement (680.9KB, pdf)
Shareable PDF
Acknowledgements
The authors are grateful to physicians and nurses of the University Clinical Hospital of Santiago de Compostela.
Footnotes
This trial was prospectively registered at ClinicalTrials.gov with identifier NCT04726098, and with EudraCT identifier 2020-005702-25. Data collected for the study, including deidentified participant data and related documents, including the protocol, statistical analysis plan, will be made available to qualified researchers after publication of the manuscript upon reasonable request via application to the corresponding author.
Author contributions: M. Taboada and V. Caruezo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors contributed equally to this study. Concept and design: all authors. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: M. Taboada, P.M. Varela, N. Rodríguez, M.T. Rodríguez, V. Caruezo and A. Cariñena. Statistical analysis: A. Estany-Gestal. Administrative, technical, or material support: M. Taboada, P.M. Varela, N. Rodríguez, M.T. Rodríguez and V. Caruezo. Supervision: M. Taboada, P.M. Varela, N. Rodríguez, M.T. Rodríguez and V. Caruezo. Editing and approval of the manuscript: all authors
Conflict of interest: The authors declare the absence of conflict of interests.
Support statement: Support was provided solely from institutional and departmental sources.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taboada M, Rama P, Pita-Romero R, et al. Critically ill COVID-19 patients attended by anesthesiologists in northwestern Spain: a multicenter prospective observational study. Rev Esp Anestesiol Reanim 2021; 68: 10–20. doi: 10.1016/j.redar.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323: 2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324: 1307–1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; 324: 1317–1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020; 324: 1298–1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020; in press [ 10.1093/cid/ciaa478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/. Date last accessed: 12 May 2021. [PubMed]
- 12.World Health Organization . Corticosteroids for COVID-19. 2020. Available from: www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1
- 13.Monedero P, Gea A, Castro P, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care 2021; 25: 2. doi: 10.1186/s13054-020-03422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicenter, randomized controlled trial. Lancet Respir Med 2020; 8: 267–276. doi: 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 15.Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalized severe COVID-19 patients: results from a randomized controlled clinical trial. Eur Respir J 2020; 56: 2002808. doi: 10.1183/13993003.02808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2021; 72: e373–e381. doi: 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181: 24–31. doi: 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324: 1048–1057. doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383: 1827–1837. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383: 2333–2344. doi: 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021; 384: 1503–1516. doi: 10.1056/NEJMoa2028700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–1684. doi: 10.1056/NEJMoa051693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02518-2021.Supplement (680.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02518-2021.Shareable (233.8KB, pdf)