Abstract
Background
Liver disease is an important cause of morbidity and mortality in people living with human immunodeficiency virus (PLWH), of which nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized cause. There are limited data investigating NAFLD in HIV monoinfection and histologically defined disease. We aimed to identify who is at risk of fibrosis, NAFLD, and nonalcoholic steatohepatitis (NASH) among PLWH and explore the diagnostic accuracy of noninvasive markers of fibrosis.
Methods
This was a retrospective, cross-sectional, international, multicenter study including patients with HIV monoinfection, without chronic viral hepatitis or other known causes of chronic liver disease, who underwent liver biopsy for abnormal liver biochemistry and/or clinical suspicion of liver fibrosis.
Results
A total of 116 patients from 5 centers were included. Sixty-three (54%) had NAFLD, of whom 57 (92%) had NASH. Overall, 36 (31%) had advanced fibrosis (≥F3) and 3 (3%) had cirrhosis. Of the 53 cases without NAFLD, 15 (28%) had advanced fibrosis. Collagen proportionate area was similar between cases with and without NAFLD (3% vs 2%). Body mass index was independently associated with NAFLD (aOR, 1.2; 95% CI, 1.08–1.34), and type 2 diabetes was independently associated with advanced fibrosis (aOR, 3.42; 95% CI, 1.00–11.71). The area under the curve for advanced fibrosis was 0.65 and 0.66 for both NAFLD Fibrosis Score (NFS) and FIB-4. Cutoff values of −1.455 (NFS) and 1.3 (FIB-4) have negative-predictive values of 0.80 and 0.82, respectively.
Conclusions
Advanced fibrosis is strongly associated with type 2 diabetes in PLWH. Serological markers require further optimization.
Keywords: NAFLD, NASH, fibrosis, HIV, histopathology
In patients living with human immunodeficiency virus and without viral hepatitis or alcohol excess, obesity is the main predictor of fatty liver and type 2 diabetes of developing advanced fibrosis. Current noninvasive tests for fibrosis need optimization in this population.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis in the absence of secondary causes such as excessive alcohol consumption. It encompasses a large spectrum of disease from nonalcoholic fatty liver (NAFL; “simple steatosis”) to hepatocyte inflammation/ballooning defined as nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis.
A major limitation in the literature to date on NAFLD in people living with human immunodeficiency virus (HIV; PLWH) has been little available data based on disease stage defined by liver histology. Despite the advances of noninvasive markers, NASH remains a histological diagnosis and the gold standard for fibrosis staging is liver biopsy. Therefore, accurate phenotyping of patients with NAFLD requires a histological diagnosis, and this is particularly needed in special populations such as PLWH in whom noninvasive markers have not been well validated [1] and the pathogenesis of liver disease is potentially more complex.
To facilitate research collaborations within this field, an international consortium of clinical academics with expertise on HIV-NAFLD was established: the Steatohepatitis in HIV Emerging Research (SHIVER) Group. This study represents the inaugural project for the group. The primary objective was to assess the histopathological features of liver biopsies performed in HIV monoinfection and identify risk factors associated with fibrosis, NAFLD, and NASH. The secondary objective was to assess the performance of noninvasive tests for the diagnosis of liver fibrosis in patients with HIV monoinfection using liver histology as a reference standard.
METHODS
Study Population
This study was a retrospective international, multicenter, cross-sectional study. Five centers from the United Kingdom (Imperial College and Royal Free Hospital), Italy (University of Modena), the United States (University of California San Francisco), and Canada (McGill University) collected liver biopsy samples from adult (age ≥18 years) cases with HIV monoinfection. Exclusion criteria were positive hepatitis C antibody or hepatitis B surface antigen; current or recent (within 6 months) alcohol excess defined as 21 units or more per week for men and 14 or more units per week for women; concurrent life-threatening illness, active malignancy, AIDS-defining illness, or evidence of other chronic liver disease at the time of liver biopsy, including biliary disease, autoimmune hepatitis, Wilson’s disease, and hereditary hemochromatosis; and long-term exposure to steroids or amiodarone. Clinical data nearest to the time of liver biopsy and within 6 months were collected, including basic demographics and anthropometrics, liver biochemistry, HIV history including drug exposure and HIV-specific complications, and medical comorbidities. The Triglyceride Glucose Index (TGI) was used as a surrogate marker of insulin resistance: TGI = ln(triglycerides [mg/dL] × fasting glucose [mg/dL]) [2].
Liver Biopsy and Histological Analysis
Liver biopsies were performed as part of the clinical evaluation of patients with unexplained elevations in liver transaminases and/or clinical suspicion of liver fibrosis, either by percutaneous or transjugular approach, with a minimum core of 10 mm.
Liver samples were paraffin fixed and formalin embedded, and stained with hemotoxylin and eosin and Sirius red. Liver biopsy slides were centrally read by an expert liver histopathologist (R. G.) blinded to clinical and biological data, scored according to the NASH Clinical Research Network (CRN) system (Nonalcoholic Steatohepatitis [NAS] score) [3] and classified as NAFLD or NASH according to the Fatty Liver Inhibition of Progression (FLIP) algorithm [4]. Fibrosis stage was defined by the Brunt classification [5].
Collagen proportionate area (CPA) was quantified on picro-Sirius red–stained formalin-fixed, paraffin-embedded sections using previously published methods [6]. Image capture was carried out at 4× objective magnification on a Zeiss Azioskop 50 using a Zeiss Axiocam ICc5 camera. Image analysis was carried out by A. H. in a single center (Royal Free Hospital, London, UK) on the captured images using a custom script for Zeiss Axiovison software: segmentation of tissue and collagen was achieved in an RGB color-space, followed by a manual editing step to remove areas not related to pathological collagen deposition, such as structural collagen in portal tracts and image artefacts. The CPA was calculated as the amount of collagen expressed as a percentage proportion of overall biopsy tissue area, as previously published [6].
Noninvasive Markers of Liver Fibrosis
The following noninvasive markers of fibrosis were calculated: FIB-4 = age (years) × aspartate aminotransferase (AST; IU/L)/platelets (109/L) × √ alanine aminotransferase (ALT; IU/mL) [7]; NAFLD Fibrosis Score (NFS) = −1.675 + 0.037 − age (years) + 0.094 − body mass index (BMI; kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST:ALT ratio − 0.013 × platelet count (×109/L) − 0.66 × albumin (g/dL) [8].
Statistical Analysis
Continuous variables are expressed as means (SD) for parametric data or medians (interquartile range [IQR]) for nonparametric data, and ordinal variables as frequencies (%). Groups with and without advanced (≥F3) fibrosis were compared using unpaired t test or Mann-Whitney U and chi-square tests, as appropriate. Multivariate logistic regression models for NAFLD and advanced fibrosis were built using biologically relevant variables, presented as odds ratios (ORs) with 95% confidence intervals (CIs). Noninvasive markers of fibrosis were compared with CPA and the gold standard of NAS fibrosis stage, and the diagnostic accuracy was assessed with area under the receiver operator curve (AUROC), sensitivity, specificity, positive-predictive values and negative-predictive values (NPVs), and positive likelihood ratios and negative likelihood ratios (LR−). Analyses were performed using IBM SPSS Statistics version 20.
RESULTS
Population Characteristics
A total of 116 patients were included in the study between August 2001 and February 2019 (Imperial College, including St Mary’s Hospital and Chelsea and Westminster Hospital: n = 39; Royal Free Hospital: n = 39; University of Modena and Reggio Emilia, Modena, Italy: n = 12; University of California San Francisco, USA: n = 14; McGill University Health Centre, Montreal, Canada: n = 12). The demographic and clinical characteristics of patients are included in Table 1. The mean (±SD) age was 48.4 ± 10.4 years and patients were mainly non-Hispanic white (72.9%) males (93.2%) with suppressed HIV viral load (94.1%). The mean (±SD) BMI was 29.2 ± 5.5 kg/m2, with rates of diabetes, hypertension, and dyslipidemia of 21.2%, 44.9%, and 39.8%, respectively.
Table 1.
Study Population Demographic Data
| Characteristics | Values (N = 116) |
|---|---|
| Age, years | 48.4 (10.9) |
| Male, n (%) | 110 (93.2) |
| Ethnicity, n (%) | |
| White, European | 86 (72.9) |
| White, Hispanic | 5 (4.2) |
| Black | 13 (11.0) |
| Other | 12 (10.3) |
| Body mass index, kg/m2 | 29.2 (5.5) |
| Diabetes, n (%) | 25 (21.2) |
| Hypertension, n (%) | 53 (44.9) |
| Dyslipidemia, n (%) | 47 (39.8) |
| Time since HIV, years | 13.0 (7.0–21.0) |
| CD4 nadir, cells/mm3 | 162.5 (36.8–277.5) |
| Time from diagnosis to ART, months | 11 (1–45) |
| Duration of ART, years | 9.0 (5.0–17.0) |
| NRTIs, months | 158 (63–216) |
| NNRTIs, months | 41 (6–95) |
| PIs, months | 22 (0–116) |
| IIs, months | 0 (0–1) |
| D-drugs, months | 0 (0–54) |
| Platelets, ×109/L | 206 (66) |
| ALT, IU/L | 68 (45–107) |
| AST, IU/L | 46 (32–63) |
| ALP, U/L | 91 (74–111) |
| Bilirubin, µmol/L | 10 (7–17) |
| Albumin, g/L | 43.1 (5.2) |
| Cholesterol, mmol/L | 4.8 (1.1) |
| Triglycerides, mmol/L | 1.9 (1.2–3.1) |
| LDL cholesterol, mmol/L | 2.7 (2.1–3.4) |
| HDL cholesterol, mmol/L | 1.2 (0.5) |
| Fasting glucose, mmol/L | 5.9 (2.1) |
| TGI | 505.6 (445.8–608.2) |
| Detectable HIV viral load, % | 7 (5.9) |
| CD4, cells/µL | 638.1 (297.3) |
| CD8, cells/µL | 875 (586–1209) |
| CD4:CD8 | 1.0 (0.5–1.0) |
Data are presented as n (%), means (SD), or medians (IQR) according to distribution.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; D-drug; dideoxynucleoside analog; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; II, integrase inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TGI, Triglyceride Glucose Index.
Histopathological Characteristics
Sixty-three of 116 patients (54.3%) had at least 5% macrovesicular steatosis consistent with the diagnosis of NAFLD, of whom 57 (90.5% cases with NAFLD, 49.1% of entire cohort) had NASH as defined by the FLIP algorithm (steatosis, ballooning, and lobular inflammation) [4]. Most cases (48/116, 41.4%) without NAFLD had nonspecific mild lobular inflammation, and 5 cases had features most in keeping with a drug reaction. No vascular liver disease (eg, nodular regenerative hyperplasia) was reported.
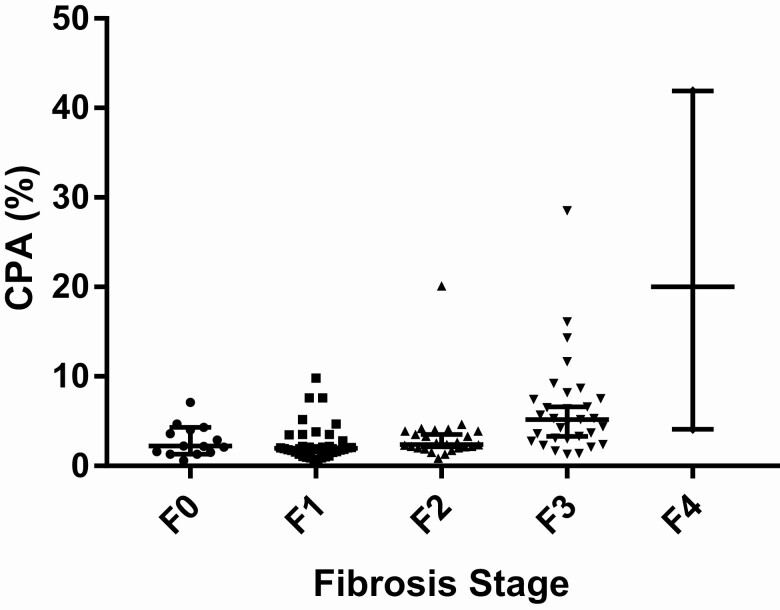
There was liver fibrosis in 102 of 116 (87.9%) cases, including 35 (30.2%) stage 1, 28 (24.1%) stage 2, 36 (31.0%) stage 3, and 3 (2.6%) with cirrhosis (Table 2). The subset of cases (53/116) without steatosis were also reported by the Ishak staging system, and included 21 of 53 (39.6%) stage 0, 9 (17.0%) stage 1, 8 (15.1%) stage 2, 11 (20.8%) stage 3, 1 (1.9%) stage 4, 2 (3.8%) stage 5, and 1 (1.9%) stage 6. The median CPA of all cases was 3.0% (2.0–5.0). The CPA was identical between stages F0 and F2 (2%) but then increased significantly above F3 (F3, 5.0% [IQR, 3.0–7.8]; F4, 20.0% [IQR, 4.0–20.0]) (Figure 1).
Table 2.
Summary of Histological Characteristics of Study Population Liver Biopsies
| Histological Feature or Diagnosis | Values |
|---|---|
| Steatosis | |
| None (<5%) | 53 (45.7) |
| Mild (5–33%) | 34 (29.3) |
| Moderate (34–66%) | 28 (24.1) |
| Severe (>66%) | 1 (0.9) |
| NASHa | 57 (49) |
| Ballooning, 0/1/2 | 39 (33.6)/49 (42.2)/28 (24.1) |
| Inflammation, 0/1/2/3 | 14 (12.1)/72 (62.1)/22 (19.0)/8 (6.9) |
| NAS scoreb,c | 4.1 (1.2) |
| NAS <3 | 2 (3.2) |
| NAS 3–4 | 41 (65.1) |
| NAS >4 | 20 (31.7) |
| Drug reaction | 5 (4.3) |
| Fibrosisc | |
| None | 14 (12.1) |
| F1 | 35 (30.2) |
| F1a | 3 (2.6) |
| F1b | 21 (18.1) |
| F1c | 11 (9.5) |
| F2 | 28 (24.1) |
| F3 | 36 (31.0) |
| F4 | 3 (2.6) |
| Nonspecific changes | 48 (41.4) |
Data are presented as n (%).
Abbreviations: CRN, Clinical Research Network; NAFLD, nonalcoholic fatty liver disease; NAS, Nonalcoholic Steatohepatitis (score); NASH, nonalcoholic steatohepatitis.
aNASH is defined as the presence of steatosis, ballooning, and lobular inflammation.
bNAS score is only reported on the subset of cases with NAFLD (n = 63).
cNAS score and fibrosis stage reported according to the NASH CRN system.
Figure 1.
CPA per fibrosis stage (Brunt). Data are presented as medians ± IQRs: F0, 2.0% (1.8–4.0%); F1, 2.0% (1.0–3.3%); F2, 2.0% (2.0–4.0%); F3, 5.0% (3.0–7.8%); F4, 20.0% (4.0–20.0%). Abbreviations: CPA, collagen proportionate area; IQR, interquartile range.
Body Mass Index Is the Key Predictor of Nonalcoholic Fatty Liver Disease in HIV Monoinfection
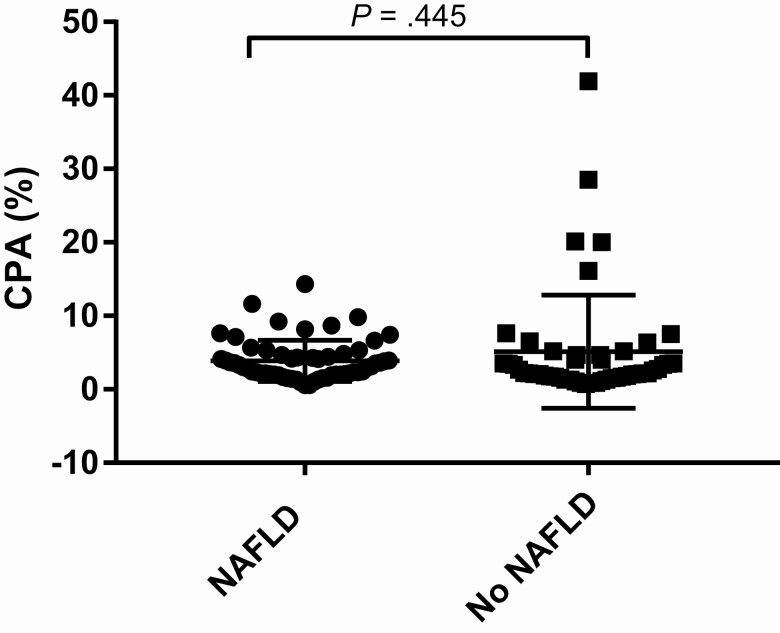
The characteristics of subjects with NAFLD were compared with those with no steatosis on liver biopsy. On univariate analysis, factors significantly associated with NAFLD were increased BMI (OR, 1.15; 95% CI, 1.06–1.25; P = .001), type 2 diabetes (OR, 2.63; 95% CI, 1.00–6.90; P = .050), hypertension (OR, 2.43; 95% CI, 1.14–5.17; P = .021), dyslipidemia (OR, 2.18; 95% CI, 1.01–4.70; P = .047), increased CD4 count (OR, 1.02; 95% CI, 1.01–1.04; P = .002), and increased CD8 count (OR, 1.01; 95% CI, 1.00–1.02; P = .009). Increased AST:ALT ratio (OR, .008; 95% CI, .02–.39; P = .002) and previous use of dideoxynucleoside analogs (D-drugs) (OR, .40; 95% CI, .19–.88; P = .022) were associated with no steatosis (Table 3). Two multivariate models were built to investigate for variables independently associated with NAFLD, both adjusted for age, BMI, and CD4 nadir—model 1 (metabolic): age (years), BMI (kg/m2), type 2 diabetes, hypertension, dyslipidemia, CD4 nadir (cells/µL); model 2 (HIV): age (years), BMI (kg/m2), lifetime D-drug exposure, CD4 (cells/µL), CD8 (cells/µL), CD4 nadir (cells/µL). Body mass index was the only variable independently associated with NAFLD (model 1: adjusted OR [aOR], 1.20; 95% CI, 1.08–1.34; P = .001; model 2: aOR, 1.20; 95% CI, 1.08–1.33; P = .001). Body mass index remained the only independent predictor of NAFLD when TGI replaced type 2 diabetes in the model (Table 4, Supplementary Table 1). More subjects with NAFLD had advanced (≥F3) fibrosis (22.6% vs 42.9%; P = .022), but the CPA was similar between those with and without NAFLD (3.0% [2.0–5.0] vs 2.0% [2.0–5.0]; P = .445) (Figure 2).
Table 3.
Demographic and Clinical Characteristics of Subjects According to the Presence of Nonalcoholic Fatty Liver Disease on Liver Biopsy (Defined as Macrovesicular Steatosis ≥5%)
| Characteristics | Values | ||
|---|---|---|---|
| Steatosis <5% (n = 53) |
Steatosis ≥5% (n = 63) |
P | |
| Age, years | 49.3 (10.9) | 47.7 (11.0) | .458 |
| Male, n (%) | 49 (92.5) | 61 (96.8) | .289 |
| Black ethnicity, n (%) | 9 (17.0) | 4 (6.3) | .071 |
| Body mass index, kg/m2 | 27.3 (5.5) | 30.9 (5.0) | <.001 |
| Diabetes, n (%) | 7 (13.2) | 18 (28.6) | .045 |
| Hypertension, n (%) | 18 (34.0) | 35 (55.6) | .020 |
| Dyslipidemia, n (%) | 16 (30.2) | 31 (49.2) | .045 |
| Time since HIV, years | 14.0 (7.0–21.5) | 11.5 (6.8–21.3) | .565 |
| CD4 nadir, cells/mm3 | 143.0 (39.0–238.5) | 189.5 (32.3–294.3) | .419 |
| Time from diagnosis to treatment, months | 11.5 (2.0–37.8) | 11.0 (1.0–50.5) | 1.000 |
| Duration of ART, years | 10.0 (6.0–17.5) | 8.0 (4.8–16.3) | .415 |
| NRTIs, months | 180 (80–253) | 122 (62–204) | .076 |
| NNRTIs, months | 33 (8–94) | 50 (4–107) | .448 |
| PIs, months | 57 (0–130) | 3 (0–106) | .061 |
| IIs, months | 0 (0–0) | 0 (0–12) | .027 |
| Previous D-drugs (yes/no), n (%) | 31 (58.5) | 23 (36.5) | .021 |
| Platelets, ×109/L | 193 (71) | 217 (58) | .047 |
| ALT, U/L | 52 (32–76) | 78 (59–137) | <.001 |
| AST, U/L | 39 (27–62) | 50 (41–63) | .019 |
| AST:ALT | 0.87 (0.41) | 0.65 (0.23) | <.001 |
| ALP, U/L | 96 (75–112) | 89 (72–108) | .174 |
| Bilirubin, µmol/L | 12.0 (7.0–18.0) | 10.0 (7.0–16.3) | .507 |
| Albumin, g/L | 42.9 (6.8) | 43.3 (3.4) | .709 |
| Cholesterol, mmol/L | 4.7 (1.0) | 4.9 (1.1) | .288 |
| Triglycerides, mmol/L | 1.8 (1.1–2.5) | 2.1 (1.4–3.3) | .126 |
| LDL, mmol/L | 2.6 (0.9) | 2.8 (1.0) | .186 |
| HDL, mmol/L | 1.2 (0.4) | 1.1 (0.5) | .265 |
| Fasting glucose, mmol/L | 5.7 (2.5) | 6.1 (1.8) | .367 |
| TGI | 480.5 (405.4–587.0) | 514.5 (460.7–656.8) | .040 |
| Detectable VL, % | 1 (1.9) | 6 (9.5) | .082 |
| CD4, cells/µL | 541 (261) | 720 (303) | .001 |
| CD8, cells/µL | 739 (494–1025) | 900 (673–1339) | .019 |
| CD4:CD8 | 1.0 (0.4–1.0) | 0.9 (0.5–1.0) | .816 |
| CPA, % | 2.0 (2.0–5.0) | 3.0 (2.0–5.0) | .445 |
| Fibrosis | |||
| ≥F2 | 27 (50.9) | 40 (63.5) | .173 |
| ≥F3 | 12 (22.6) | 27 (42.9) | .022 |
Data are presented as n (%), means (SD), or medians (IQR) according to distribution.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; D-drug; dideoxynucleoside analog; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; II, integrase inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TGI, Triglyceride Glucose Index; VL, HIV viral load.
Table 4.
Univariate and Multivariate Analysis of Risk Factors for Nonalcoholic Fatty Liver Disease
| Multivariate | ||||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | ||||
| OR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Age | .99 (.95–1.02) | .455 | .96 (.91–1.01) | .128 | 1.01 (.96–1.06) | .681 |
| BMI | 1.15 (1.06–1.25) | .001 | 1.20 (1.08–1.34) | .001 | 1.20 (1.08–1.33) | .001 |
| Black ethnicity | .33 (.10–1.15) | .081 | … | … | ||
| Diabetes | 2.63 (1.00–6.90) | .050 | 1.07 (.27–4.21) | .928 | … | |
| Hypertension | 2.43 (1.14–5.17) | .021 | 3.14 (.98–10.11) | .055 | … | |
| Dyslipidemia | 2.18 (1.01–4.70) | .047 | 1.28 (.41–3.99) | .668 | … | |
| Duration of ART | .98 (.94–1.03) | .470 | … | … | ||
| D-drugsa | .40 (.19–.88) | .022 | … | .36 (.13–1.02) | .053 | |
| CD4 | 1.02 (1.01–1.04) | .002 | … | 1.00 (1.00–1.00) | .177 | |
| CD8 | 1.01 (1.00–1.02) | .009 | … | 1.00 (1.00–1.00) | .094 | |
| CD4 nadir | 1.02 (.99–1.04) | .226 | 1.00 (1.00–1.00) | .940 | 1.00 (1.00–1.00) | .744 |
Model 1 (Metabolic): age (per year), BMI (per kg/m2), diabetes, hypertension, dyslipidemia, CD4 nadir (per 10 cells/µL). Model 2 (HIV): BMI (per kg/m2), diabetes, lifetime D-drugs, CD4 (per 10 cells/µL), CD8 (per 10 cells/µL), CD4 nadir (per 10 cells/µL).
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; D-drug; dideoxynucleoside analog; HIV, human immunodeficiency virus.
aPast exposure in lifetime.
Figure 2.
CPA in subjects with and without NAFLD. Abbreviations: CPA, collagen proportionate area; NAFLD, nonalcoholic fatty liver disease.
Type 2 Diabetes Is an Independent Predictor of Advanced Fibrosis
A total of 39 of 116 (34%) subjects had advanced (≥F3) fibrosis on liver biopsy (Table 5). On univariate analysis, a diagnosis of NAFLD (OR, 2.56; 95% CI, 1.14–5.78; P = .023), type 2 diabetes (OR, 5.26; 95% CI, 2.05–13.50; P = .001), hypertension (OR, 2.65; 95% CI, 1.20–5.85; P = .016), dyslipidemia (OR, 3.12; 95% CI, 1.40–6.94; P = .005), duration of antiretroviral therapy (ART) (OR, 1.06; 95% CI, 1.00–1.11; P = .036), and time since HIV diagnosis (OR, 1.08; 95% CI, 1.03–1.13; P = .002) were associated with advanced fibrosis (Table 6).
Table 5.
Demographic and Clinical Characteristics of Subjects According to the Presence of Advanced Fibrosis (Defined as ≥F3 Fibrosis by Brunt Classification)
| Characteristics | <F3 (n = 77) |
≥F3 (n = 39) |
P |
|---|---|---|---|
| Age, years | 47.2 (10.5) | 50.9 (11.5) | .082 |
| Male, n (%) | 73 (94.8) | 37 (94.8) | .988 |
| Black ethnicity, n (%) | 12 (15.6) | 1 (2.6) | |
| Body mass index, kg/m2 | 28.8 (5.5) | 30.1 (5.5) | .242 |
| Diabetes, n (%) | 9 (11.7) | 16 (41.0) | <.001 |
| Hypertension, n (%) | 29 (37.7) | 24 (61.5) | .015 |
| Dyslipidemia, n (%) | 24 (31.2) | 23 (59.0) | .005 |
| Time since HIV, years | 10.5 (5.0–18.8) | 20.0 (11.0–24.0) | .001 |
| CD4 nadir, cells/mm3 | 171.0 (48.0–299.3) | 137.0 (19.5–216.0) | .175 |
| Time from diagnosis to treatment, months | 6.5 (1.0–37.8) | 14.0 (2.0–78.0) | .157 |
| Duration of ART, years | 8.0 (4.0–15.0) | 13.0 (6.0–20.0) | .038 |
| NRTIs, months | 132 (52–226) | 172 (72–211) | .377 |
| NNRTIs months | 26 (2–88) | 64 (28–112) | .054 |
| PIs, months | 23 (0–110) | 22 (0–120) | .594 |
| Previous D-drugs (yes/no), n (%) | 33 (45.2) | 21 (53.8) | .150 |
| Platelets, ×109/L | 213 (57) | 193 (144–252) | .122 |
| ALT, U/L | 67 (41–101) | 73 (52–137) | .176 |
| AST, U/L | 44 (30–62) | 54 (42–72) | .017 |
| AST:ALT | 0.74 (0.36) | 0.75 (0.31) | .852 |
| ALP, U/L | 93 (74–111) | 90 (75–102) | .377 |
| Bilirubin, µmol/L | 10.0 (6.9–17.1) | 11.0 (7.5–18.6) | .484 |
| Albumin, g/L | 43.2 (5.7) | 42.8 (4.2) | .699 |
| Cholesterol, mmol/L | 4.9 (1.0) | 4.6 (1.2) | .172 |
| Triglycerides, mmol/L | 1.8 (1.2–3.1) | 2.0 (1.2–3.3) | .711 |
| LDL, mmol/L | 2.8 (0.8) | 2.4 (1.2) | .044 |
| HDL, mmol/L | 1.2 (0.4) | 1.1 (0.7) | .626 |
| Fasting glucose, mmol/L | 5.4 (1.1) | 7.1 (3.1) | <.001 |
| TGI | 475.4 (431.4–574.3) | 534.0 (505.0–764.6) | .001 |
| Detectable VL, % | 3 (3.9) | 4 (10.3) | .174 |
| CD4, cells/µL | 626 (262) | 661 (357) | .555 |
| CD8, cells/µL | 902 (683–1264) | 764 (514–1044) | .050 |
| CD4:CD8 | 1.0 (0.4–1.0) | 1.0 (0.7–1.0) | .539 |
| NAFLD | 36 (46.8) | 27 (69.2) | .022 |
| NFS | −1.9 (−3.0 to 1.0) | −0.7 (−2.2 to 0.2) | .002 |
| FIB-4 | 1.1 (0.8–1.8) | 1.7 (1.0–2.1) | .008 |
Data are presented as n (%), means (SD), or medians (IQR) according to distribution. Groups were compared by Mann-Whitney test (discrete data) and chi-square (categorical). P < .05 was considered statistically significant.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; D-drug; dideoxynucleoside analog; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; II, integrase inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD Fibrosis Score; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TGI, Triglyceride Glucose Index; VL, HIV viral load.
Table 6.
Univariate and Multivariate Analysis of Risk Factors for Advanced Fibrosis
| Univariate | Multivariate Model 1 | |||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Age | 1.03 (1.00–1.07) | .085 | … | |
| BMI | 1.04 (.97–1.12) | .242 | … | |
| Black ethnicity | .14 (.02–1.14) | .066 | … | |
| NAFLD | 2.56 (1.14–5.78) | .023 | 2.47 (.96–6.39) | .062 |
| Type 2 diabetes | 5.26 (2.05–13.50) | .001 | 3.42 (1.00–11.71) | .050 |
| Hypertension | 2.65 (1.20–5.85) | .016 | .99 (.34–2.89) | .983 |
| Dyslipidemia | 3.12 (1.40–6.94) | .005 | 1.88 (.69–5.13) | .221 |
| Duration of ART | 1.06 (1.00–1.11) | .036 | 1.03 (.97–1.09) | .394 |
| D-drugsa | 1.82 (.80–4.12) | .152 | … | |
| CD4 | 1.71 (.76–3.86) | .195 | … | |
| CD8 | 1.00 (1.00–1.00) | .124 | … | |
| CD4 nadir | 1.00 (1.00–1.00) | .205 | … | |
| Time since HIV infection | 1.08 (1.03–1.13) | .002 | … | |
Multivariate model: BMI (per kg/m2), NAFLD (≥5% steatosis on liver biopsy), type 2 diabetes, hypertension, dyslipidemia, duration of ART (per year).
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; D-drug; dideoxynucleoside analog; HIV, human immunodeficiency virus; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
aPast exposure in lifetime.
A multivariate model was built using the significant variables from multivariate analysis. Duration of ART and time since HIV infection could not be included together due to collinearity; therefore, duration of ART was selected due to uncertainty between time of diagnosis and duration of infection. In the model, type 2 diabetes had the strongest association with advanced fibrosis (aOR, 3.42; 95% CI, 1.00–11.71; P = .050). When the TGI was used in place of type 2 diabetes in the model, it was the only variable significantly associated with advanced fibrosis (aOR per 10-unit increase, 1.04; 95% CI, 1.00–1.07; P = .033) (Supplementary Table 2).
In a subanalysis on subjects without NAFLD comparing those with (15/53) and without (38/53) advanced fibrosis (≥ Ishak stage 3), the only significant difference between the groups on univariate analysis was longer time since HIV diagnosis (11.5 [6.8–18.3] vs 21.0 [13.0–26.0] years; P = .005) but age was similar (49.0 ± 12.0 vs 50.0 ± 8.0 years; P = .761).
Performance of Noninvasive Markers for Advanced Fibrosis
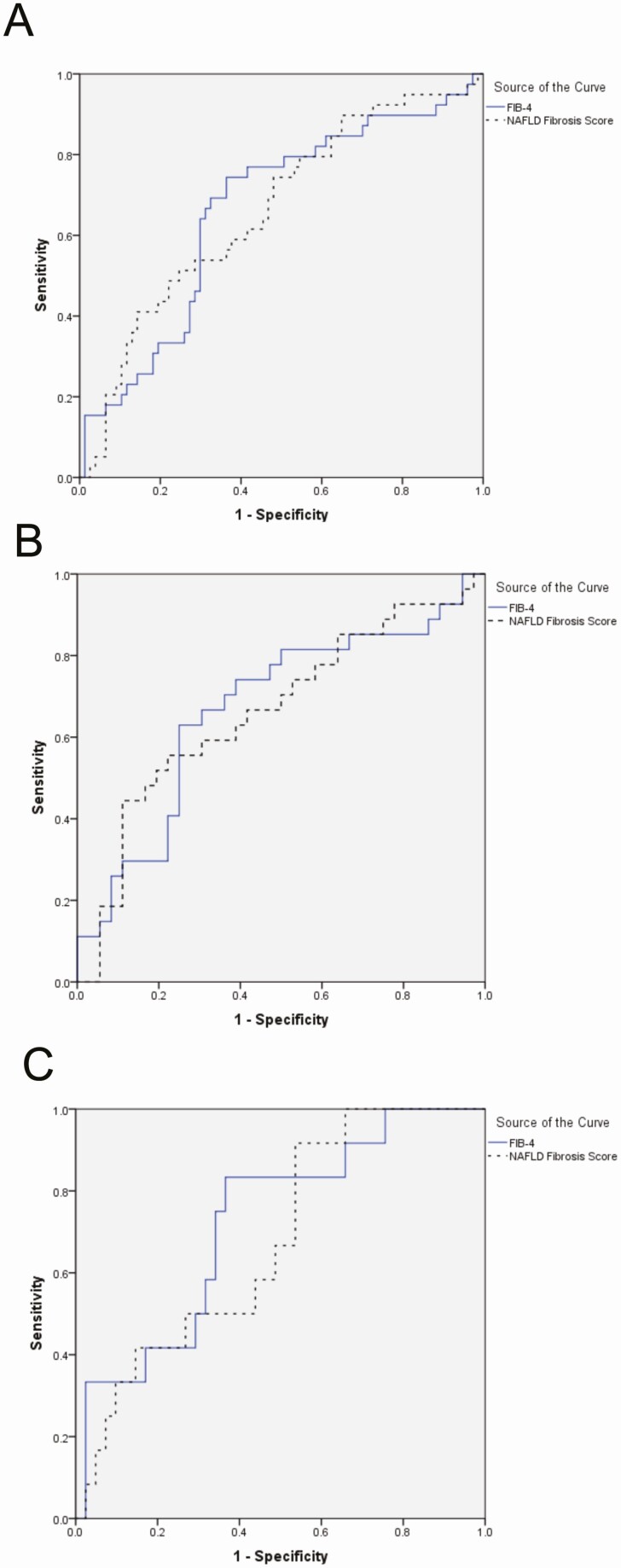
The diagnostic accuracy of noninvasive markers of fibrosis was evaluated using liver histology as a reference. FIB-4 and NFS had poor diagnostic accuracy for advanced liver fibrosis, with AUROCs for FIB-4 and NFS to detect advanced fibrosis of .65 (95% CI, .53–.76) and .66 (95% CI, .56–.80) (all subjects), .64 (95% CI, .49–.79) and .64 (.49–.79) (subjects with NAFLD only), and .72 (95% CI, .55–.88) and .73 (95% CI, .57–.89) (subjects without NAFLD only), respectively (Figure 3).
Figure 3.
ROC curves assessing the performance of the noninvasive markers for detecting advanced (≥F3) fibrosis. A, All cases. AUROC FIB-4, 0.659; NFS, 0.688; combined, 0.716. B, NAFLD cases only. AUROC FIB-4, 0.655; NFS, 0.684; combined, 0.662. C, Non-NAFLD cases only. AUROC FIB-4, 0.722; NFS, 0.679. Abbreviations: AUROC, area under the receiver operator curve; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD Fibrosis Score; ROC, Receiver Operating Characteristic.
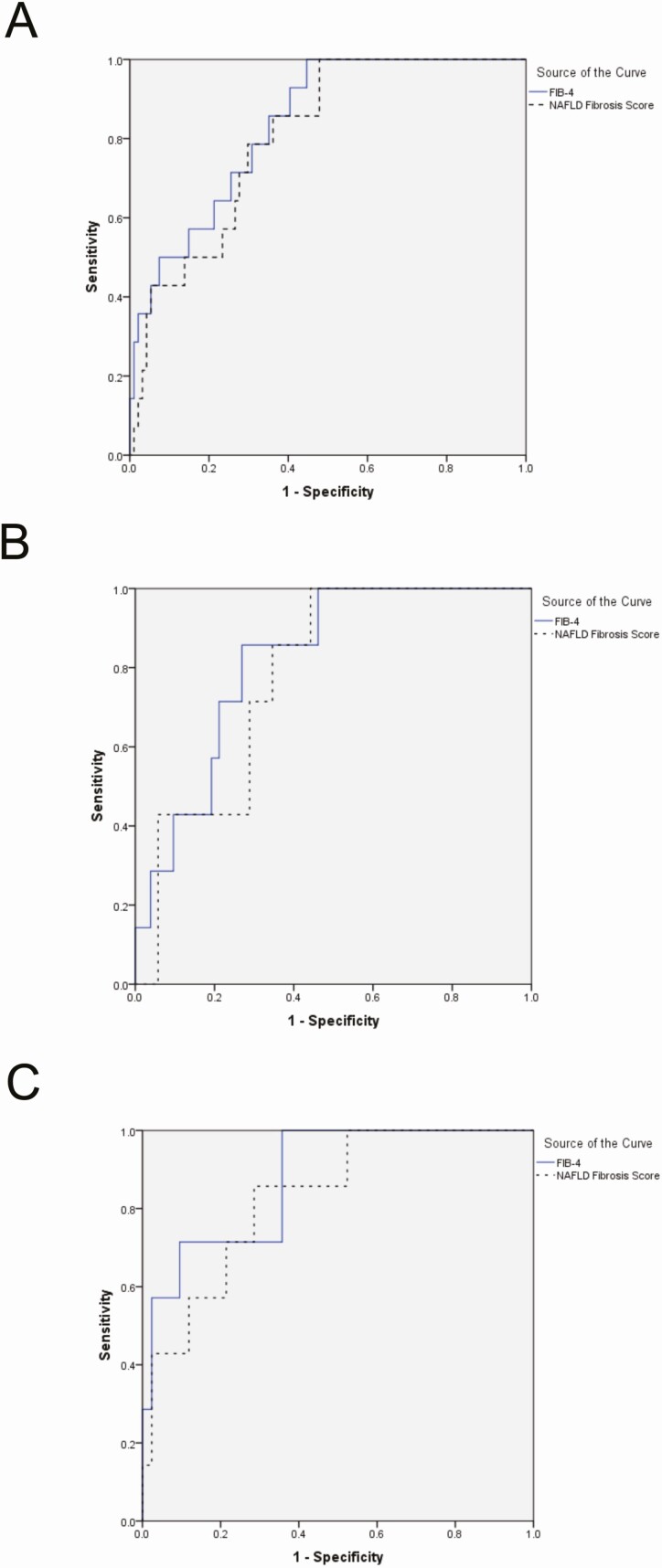
A CPA value of 7.6% or greater is predictive of long-term adverse outcomes [6]. Both FIB-4 and NFS performed more robustly at identifying cases with a CPA of 7.6% or greater than advanced fibrosis as defined by NAS CRN staging, where the AUROCs of FIB-4 and NFS for CPA of 7.6% or greater were .84 (95% CI, .74–.93) and .81 (95% CI, .70–.91) (all subjects), .82 (95% CI, .69–.95) and .78 (95% CI, .64–.92) (subjects with NAFLD only), and .88 (95% CI, .75–1.00) and .83 (95% CI, .68–.98) (subjects without NAFLD only), respectively (Figure 4).
Figure 4.
ROC curves assessing the performance of the noninvasive markers for detecting CPA ≥7.6% (n = 14/116). A, All cases. AUROC FIB-4, 0.836; NFS, 0.805; combined, 0.784. B, NAFLD cases only. AUROC FIB-4, 0.819; NFS, 0.780. C, Non-NAFLD cases only. AUROC FIB-4, 0.878; NFS, 0.830. Abbreviations: AUROC, area under the receiver operator curve; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD Fibrosis Score; ROC, Receiver Operating Characteristic.
Validated cutoff values for ruling out advanced fibrosis are less than 1.3 for FIB-4 [9] and less than −1.455 for NFS [8]. Using these values, the sensitivity, NPV, and LR− were 0.72, 0.80, and 0.66 for FIB-4 and 0.84, 0.82, and 0.43 for NFS (Table 7A). These tests were better at ruling out CPA of 7.6% or greater, where the sensitivity, NPV, and LR− were 0.93, 0.98, and 0.16 for FIB-4 and 0.93, 0.97, and 0.20 for NFS (Table 7B).
Table 7.
Diagnostic Accuracy of FIB-4 and NFS for Advanced Fibrosis (A) and Collagen Proportionate Area (B) >7.6%
| Cutoff | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | |
|---|---|---|---|---|---|---|---|
| A | |||||||
| FIB-4 | 1.3 | 0.72 | 0.43 | 0.46 | 0.80 | 1.26 | 0.66 |
| NFS | −1.455 | 0.84 | 0.36 | 0.40 | 0.82 | 1.32 | 0.43 |
| B | |||||||
| FIB-4 | 1.3 | 0.93 | 0.44 | 0.24 | 0.98 | 1.65 | 0.16 |
| NFS | −1.455 | 0.93 | 0.35 | 0.18 | 0.97 | 1.44 | 0.20 |
Abbreviations: LR+, positive likelihood ratio; LR−, negative likelihood ratio; NFS, NAFLD Fibrosis Score; NPV, negative-predictive value; PPV, positive-predictive value.
DISCUSSION
This study reports the largest known sample of liver biopsies in HIV monoinfection, read by a central expert liver pathologist, in which the primary risk factor identified for NAFLD was BMI and for advanced fibrosis was type 2 diabetes.
NAFLD is common in PLWH, but understanding more clearly which patients progress to NASH and advanced fibrosis will help clinicians to appropriately risk-stratify patients for further investigation, such as a liver biopsy, and initiate appropriate management.
In this study, subjects were selected from 5 centers in Europe and North America who had had a liver biopsy without another cause of chronic liver disease. Approximately half (54%) had macrovesicular steatosis consistent with NAFLD, only 4% had a drug reaction, and interestingly, 41% had nonspecific features. Of the 63 subjects with NAFLD, 57 (92%) had NASH, and 58% of the whole cohort had ≥F2 fibrosis, reflecting the selection criteria in centers to biopsy patients with a high pretest probability of more advanced disease.
Collagen proportionate area was used as a quantitative measure of fibrosis, which showed that the quantity of collagen deposition is similar between stages F0 and F2 but then increases steeply from F3 to F4. This helps explain the decline in prognosis with F3 fibrosis, whereas it matches population controls for at least 20 years for F2 and 30 years for F0–F1 fibrosis [10]. The subjects with cirrhosis (n = 3) had a wide range of CPA scores (4.1%, 20.0%, and 41.9%), illustrating an important limitation of current staging systems in which patients with cirrhosis are crudely grouped together despite large differences in collagen content.
Fibrosis quantitation with CPA could have prognostic utility in identifying subjects at high risk of decompensation, which may aid in the identification of patients at risk of hepatic complications [6]. This should also be considered in clinical trial designs for antifibrotic drugs in NASH, both in subjects with and without HIV, where a continuous variable of fibrosis content may be a more sensitive measure of antifibrotic effect compared with semi-quantitative staging, which suffers from significant interobserver variability [11]. A limitation to implementing this technology has been a lack of outcome data for CPA levels, but this has recently been addressed by a study showing that CPA independently predicts clinical outcomes [6].
The main feature differentiating patients with and without NAFLD was BMI, which was significantly increased in patients with NAFLD (30.9 vs 27.3 kg/m2), and this remained independently associated in the multivariate analysis. Clearly, as in the general population, obesity is a hallmark characteristic of NAFLD in PLWH [9, 12, 13]. Some studies have shown an association with nucleoside reverse transcriptase inhibitor (NRTI) exposure and NAFLD [14], possibly through an indirect effect on fat metabolism and redistribution, but this has not consistently been the case [15] and is not supported in this study, where ART exposure was not predictive of NAFLD. It is surprising that D-drug exposure tended towards a protective effect in the univariate and multivariate analysis, but in a cohort with a median ART duration of approximately 10 years and median D-drug exposure of 0 months, the individual exposure of subjects was likely to have been very low towards the end of the era when they were prescribed, so the reported effect may be subject to confounding. There are emerging data on excess weight gain following use of integrase inhibitors, and further evaluation is required to understand if this may result in an increased risk of developing NAFLD [16].
Body mass index was not associated with an increased risk of advanced fibrosis, whereas type 2 diabetes remained independently associated in the multivariate analysis. This was supported by the significant association with increased TGI, a surrogate marker of insulin resistance [2]. A paired biopsy study of subjects with NASH (n = 83) without HIV demonstrated similar results, in which fibrosis progression was independently linked to type 2 diabetes [17], and a study using transient elastography in PLWH has also shown that liver fibrosis is significantly more common in patients with the metabolic syndrome, of which type 2 diabetes is a key feature [18]. Therefore, in PLWH, development of metabolic complications of obesity, particularly diabetes, represents an increased risk for liver fibrosis and the potential for higher liver-related morbidity.
An interesting observation in our study was the high rate of nonspecific findings in cases with no evidence of significant steatosis, including 60% with some evidence of fibrosis and 28% with Ishak stage 3 or higher fibrosis, representing a group with significant underlying liver damage but no known cause of chronic liver disease. This included the patients with cirrhosis, possibly through “burnt-out” NASH, but this observation does raise further questions about the natural history of liver fibrosis in patients with HIV, particularly since the time since HIV diagnosis was the main variable associated with fibrosis in subjects without steatosis. There were insufficient cases to conduct a multivariate analysis in this subgroup, which warrants further investigation, particularly with longitudinal follow-up.
How, therefore, should we select patients to send for further evaluation including liver biopsy? Targeted screening for NAFLD should certainly be considered in PLWH who are obese, a practice that becomes even more important in those with accompanying metabolic complications, especially type 2 diabetes. Current guidelines for NAFLD recommend risk stratification of patients at risk of NAFLD and liver fibrosis with noninvasive markers, including FIB-4 and NFS [9]. The accuracy of these serological markers of fibrosis were evaluated in this population of PLWH and the AUROC values were poor. A recent large (n = 452) cross-sectional study by Boursier et al [19] in subjects with NAFLD in the general population validating these markers against a liver biopsy gold standard also showed these tests only have a modest AUROC for diagnosing advanced fibrosis: 0.732 for NAFLD and 0.780 for FIB-4. However, these scores are primarily applied using cutoff values designed to optimize the NPV of the test, a practice that has been successfully applied to stratify patients for referral to secondary care from the community [20]. Using previously validated cutoffs for FIB-4 [21] and NFS [8] in this study, there was an NPV of 0.80 and 0.82 for FIB-4 and NFS. The performance of these markers was similar to the Boursier et al study where the optimized cutoffs of −1.036 (NFS) and 1.515 (FIB-4) had NPVs of 0.81 and 0.82, respectively [19]. The performance improved in the prediction of CPA of 7.6% or greater (NPVs of 0.98 for FIB-4 and 0.97 for NFS), but overall, about one-quarter of cases with advanced fibrosis could be mis-classified as low risk, supporting concerns about the accuracy of these markers in HIV-associated NAFLD [1].
The main limitations of the study are its retrospective design with data from a selected population and heterogeneous indications for liver biopsy, and a lack of elastography data. However, the strength of the study is the large, multicenter international collection of centrally reviewed liver biopsy data in a field where, to date, only a few, small studies with liver biopsy in patients with HIV monoinfection have been published. Although significant alcohol excess was an exclusion criterion, we did not have data on moderate alcohol use. However, our cutoff approximates that used in NAFLD trials.
Conclusions
In HIV monoinfection, advanced liver fibrosis is strongly associated with type 2 diabetes and NAFLD with elevated BMI. Liver fibrosis may be evident in PLWH and with no known established cause of chronic liver disease. The biochemical markers of fibrosis (FIB-4 and NFS) require further validation in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Jill Callard for her help in preparing the histology slides.
Financial support. The Department of Metabolism, Digestion and Reproduction at Imperial College London, receives funding from the National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) based at Imperial College London and Imperial College Healthcare NHS Trust. H. P. has received funding support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)–Brazil—Universal 2016 (grant number 405.211/2016-3). G. S. is supported by a Junior 1 and 2 Salary Award from Fonds de Recherche Santé Québec (grant numbers 27127 and 267806) and research salary from the Department of Medicine of McGill University. J. C. P. has received support from the ACG Junior Faculty Development Award from the American College of Gastroenterology and UCSF Liver Center National Institute of Health (grant number P30 DK026743).
Potential conflicts of interest. J. B. M. has received consulting fees and conference support from Intercept Pharma and Norgine, and research funding from ViiV. G. S. has acted as speaker for Merck, Gilead, AbbVie, and Novo Nordisk; served as an advisory board member for Merck, Gilead, Intercept, Allergan, and Novartis; and has received research funding from Merck, Theratec, and Pfizer. P. I. has received speaker’s or consultancy fees from Gilead, AbbVie, MSD, and ViiV and holds a research grant from Gilead (NoCo). L. G. has received advisory/speaker fees or conference support from Gilead and Janssen. G. G. has acted as speaker and served as an advisory board member for Merck, Gilead, Jansen, and ViiV. M. L. has received research funding from ViiV and Gilead. J. C. P. reports grants from Merck and Gilead Sciences, and advisory board fees from Theratechnologies, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lemoine M, Assoumou L, De Wit S. Diagnostic accuracy of noninvasive markers of steatosis, NASH, and liver fibrosis in HIV-monoinfected individuals at risk of nonalcoholic fatty liver disease (NAFLD): results from the ECHAM study. J Acquir Immune Defic Syndr 2019; 80:86–94. [DOI] [PubMed] [Google Scholar]
- 2. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity: comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95:3347–51. [DOI] [PubMed] [Google Scholar]
- 3. Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–21. [DOI] [PubMed] [Google Scholar]
- 4. Bedossa P; FLIP Pathology Consortium . Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014; 60:565–75. [DOI] [PubMed] [Google Scholar]
- 5. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94:2467–74. [DOI] [PubMed] [Google Scholar]
- 6. Buzzetti E, Hall A, Ekstedt M, et al. Collagen proportionate area is an independent predictor of long-term outcome in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2019; 49:1214–22. [DOI] [PubMed] [Google Scholar]
- 7. Sterling RK, Lissen E, Clumeck N, et al. ; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 8. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–54. [DOI] [PubMed] [Google Scholar]
- 9. Byrne CD, Targher G. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: is universal screening appropriate? Diabetologia 2016; 59:1141–4. [DOI] [PubMed] [Google Scholar]
- 10. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017; 67:1265–73. [DOI] [PubMed] [Google Scholar]
- 11. Juluri R, Vuppalanchi R, Olson J, et al. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol 2011; 45:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pembroke T, Deschenes M, Lebouché B, et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J Hepatol 2017; 67:801–8. [DOI] [PubMed] [Google Scholar]
- 13. Perazzo H, Cardoso SW, Yanavich C, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc 2018; 21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV‐infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis 2008; 47:250–7. [DOI] [PubMed] [Google Scholar]
- 15. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 16. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment naïve persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. doi: 10.1093/cid/ciz407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol 2015; 62:1148–55. [DOI] [PubMed] [Google Scholar]
- 18. Lemoine M, Lacombe K, Bastard JP, et al. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients. Aids 2017; 44:1. [DOI] [PubMed] [Google Scholar]
- 19. Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016; 65:570–8. [DOI] [PubMed] [Google Scholar]
- 20. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019; 71:371–8. [DOI] [PubMed] [Google Scholar]
- 21. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.