Abstract
Background
Typical and atypical antipsychotics are widely used to treat agitation and psychosis in dementia. However, whether or not they are beneficial is uncertain. Some trials have yielded negative results and effectiveness may be outweighed by harms.
Objectives
To assess the efficacy and safety of antipsychotics for the treatment of agitation and psychosis in people with Alzheimer's disease and vascular dementia.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's register, MEDLINE (Ovid Sp), Embase (Ovid SP), PsycINFO (Ovid SP), CINAHL (EBSCOhost), Web of Science Core Collection (ISI Web of Science), LILACS (BIREME), ClinicalTrials.gov and the World Health Organization's meta‐register, and the International Clinical Trials Registry Portal on 7 January 2021. Two review authors independently screened the title and abstract of the hits, and two review authors assessed the full text of studies that got through this screening.
Selection criteria
We included randomised, placebo‐controlled, parallel‐arm trials comparing the effects of antipsychotics and placebo for the treatment of agitation or psychosis in people with dementia due to Alzheimer's disease or vascular dementia, or both, irrespective of age, severity of cognitive impairment, and setting. (The majority of) participants had to have clinically significant agitation (including aggression) or psychosis or both at baseline. We excluded studies about antipsychotics that are no longer available in the USA or EU, or that are used for emergency short‐term sedation. We also excluded head‐to‐head trials and antipsychotic withdrawal trials.
Data collection and analysis
The primary outcomes were (1) reduction in agitation or psychosis in participants with agitation or psychosis, respectively at baseline, and (2) the number of participants with adverse events: somnolence, extrapyramidal symptoms, any adverse event, any serious adverse event (SAE), and death.
Two review authors independently extracted the necessary data and assessed risk of bias with the Cochrane risk of bias tool. We calculated the pooled effect on agitation and psychosis for typical and atypical antipsychotics separately, and the pooled risk of adverse effects independent of the target symptom (agitation or psychosis). We used RevMan Web for the analyses.
Main results
The search yielded 8233 separate hits. After assessing the full‐text of 35 studies, we included 24 trials that met the eligibility criteria. Six trials tested a typical antipsychotic, four for agitation and two for psychosis. Twenty trials tested an atypical antipsychotic, eight for agitation and 12 for psychosis. Two trials tested both drug types. Seventeen of 26 comparisons were performed in patients with Alzheimer’s disease specifically. The other nine comparisons also included patients with vascular dementia or mixed dementia. Together, the studies included 6090 participants (12 to 652 per study). The trials were performed in institutionalised, hospitalised and community‐dwelling patients, or a combination of those.
For typical antipsychotics (e.g. haloperidol, thiothixene), we are uncertain whether these drugs improve agitation compared with placebo (standardised mean difference (SMD) ‐0.36, 95% confidence interval (CI) ‐0.57 to ‐0.15, 4 studies, n = 361); very low‐certainty evidence, but typical antipsychotics may improve psychosis slightly (SMD ‐0.29, 95% CI ‐0.55 to ‐0.03, 2studies, n= 240; low‐certainty evidence) compared with placebo. These drugs probably increase the risk of somnolence (risk ratio (RR) 2.62, 95% CI 1.51 to 4.56, 3 studies, n = 466; moderate‐certainty evidence) and increase extrapyramidal symptoms (RR 2.26, 95% CI 1.58 to 3.23, 3 studies, n = 467; high‐certainty) evidence. There was no evidence regarding the risk of any adverse event. The risks of SAEs (RR 1.32, 95% CI 0.65 to 2.66, 1 study, n = 193) and death (RR 1.46, 95% CI 0.54 to 4.00, 6 studies, n = 578) may be increased slightly, but these estimates were very imprecise, and the certainty was low. The effect estimates for haloperidol from five trials were in line with those of the drug class.
Atypical antipsychotics (e.g. risperidone, olanzapine, aripiprazole, quetiapine) probably reduce agitation slightly (SMD ‐0.21, 95% CI ‐0.30 to ‐0.12, 7 studies, n = 1971; moderate‐certainty evidence), but probably have a negligible effect on psychosis (SMD ‐0.11, 95% CI ‐0.18 to ‐0.03, 12 studies, n = 3364; moderate‐certainty evidence). These drugs increase the risk of somnolence (RR 1.93, 95% CI 1.57 to 2.39, 13 studies, n ‐ 3878; high‐certainty evidence) and are probably also associated with slightly increased risk of extrapyramidal symptoms (RR 1.39, 95% CI 1.14 to 1.68, 15 studies, n = 4180; moderate‐certainty evidence), serious adverse events (RR 1.32, 95% CI 1.09 to 1.61, 15 studies, n= 4316; moderate‐certainty evidence) and death (RR 1.36, 95% CI 0.90 to 2.05, 17 studies, n= 5032; moderate‐certainty evidence), although the latter estimate was imprecise. The drugs probably have a negligible effect on the risk of any adverse event (RR 1.05, 95% CI 1.02 to 1.09, 11 studies, n = 2785; moderate‐certainty evidence). The findings from seven trials for risperidone were in line with those for the drug class.
Authors' conclusions
There is some evidence that typical antipsychotics might decrease agitation and psychosis slightly in patients with dementia. Atypical antipsychotics reduce agitation in dementia slightly, but their effect on psychosis in dementia is negligible. The apparent effectiveness of the drugs seen in daily practice may be explained by a favourable natural course of the symptoms, as observed in the placebo groups. Both drug classes increase the risk of somnolence and other adverse events. If antipsychotics are considered for sedation in patients with severe and dangerous symptoms, this should be discussed openly with the patient and legal representative.
Keywords: Humans; Alzheimer Disease; Alzheimer Disease/complications; Alzheimer Disease/drug therapy; Antipsychotic Agents; Antipsychotic Agents/adverse effects; Dementia, Vascular; Dementia, Vascular/drug therapy; Psychotic Disorders; Psychotic Disorders/complications; Psychotic Disorders/drug therapy; Randomized Controlled Trials as Topic; Risperidone; Risperidone/adverse effects
Plain language summary
Do antipsychotic medicines reduce agitated behaviour and psychotic symptoms in people with Alzheimer's disease and vascular dementia?
Key messages
It is uncertain whether older, first‐generation or ‘typical’ antipsychotic medicines such as haloperidol have an effect on agitated behaviour (for example, restlessness and aggression); the effect is moderate at best. Typical antipsychotic medicines may decrease delusions and hallucinations slightly in people with dementia.
Newer, second‐generation ‘atypical’ antipsychotic medicines, such as risperidone, probably reduce agitated behaviour slightly. Atypical antipsychotic medicines probably have no effect on psychotic symptoms.
Both first‐ and second‐generation antipsychotic medicines increase the risk of drowsiness and other unwanted events. When patients’ symptoms improve after antipsychotics have been prescribed, this is probably largely due to natural improvement in symptoms over time.
What are antipsychotic medicines?
Antipsychotics are medicines prescribed to treat psychotic symptoms and severely disturbed behaviour in some mental illnesses, such as schizophrenia, bipolar disorder and severe depression. Psychotic symptoms are delusions (very strongly held beliefs in something which is not true) and hallucinations (sensing – usually seeing or hearing ‐ things which are not really there).
Antipsychotic medicines are often divided into two groups:
1. first‐generation (older) or ‘typical’ antipsychotics, for example haloperidol;
2. second‐generation (newer) or ‘atypical’ antipsychotics, for example risperidone.
Both types can cause unwanted effects, such as drowsiness, movement disorders (for example, involuntary or uncontrollable movements, tremors, muscle contractions) and weight gain.
Why do people with dementia need antipsychotics?
People with dementia quite often experience hallucinations and delusions during their illness for some time. Particularly in the later stages of the illness, they may also show agitated behaviours such as restlessness, shouting out or aggression towards others. It is important to try to understand what is driving these behaviours and there are many ways to manage them which do not involve drugs. However, antipsychotic medicines have often been prescribed to people with dementia for these problems. In many countries, they are prescribed less often than in the past but are still used when the symptoms are severe.
What did we want to find out?
We wanted to know how well antipsychotic medicines reduce the severity of agitation and psychotic symptoms in people with the two commonest types of dementia, namely dementia due to Alzheimer’s disease and vascular dementia. We also wanted to know how many people experienced unwanted effects.
What did we do?
We searched for studies that investigated antipsychotic medicines currently available in the USA or European Union by comparing them with placebo (a ’dummy’ pill), for treatment of persistent agitation or psychotic symptoms. People in the studies had to have Alzheimer’s disease or vascular dementia. They could be any age and reside in a care home, a hospital, or the community. Most of the people in the studies had to be experiencing agitation (including aggression) or psychotic symptoms, or both, at the start of the study.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 24 studies with a total of 6090 people:
‐ six studies tested typical antipsychotics, mostly haloperidol;
‐ 20 studies tested atypical antipsychotics, such as risperidone, olanzapine, and aripiprazole; and
‐ two studies tested both typical and atypical antipsychotics.
All the studies compared antipsychotics with placebo. The people were living in institutions, hospitals, the community, or a combination of these settings.
Main results
Typical antipsychotics (haloperidol, thiothixene) compared with placebo:
‐ may improve symptoms of psychosis slightly (2 studies, 240 people), but we are uncertain about their effect on agitation (4 studies, 361 people);
‐ probably increase the risk of drowsiness (3 studies, 466 people), and movement disorders (3 studies, 467 people);
‐ may slightly increase the risk of serious unwanted effects (1 study, 193 people) and of death (6 studies, 578 people).
There was no evidence about the risk of non‐serious and serious unwanted effects combined.
Atypical antipsychotics (risperidone, olanzapine, aripiprazole, quetiapine) compared with placebo:
‐ probably slightly reduce agitation (7 studies, 1971 people) and may slightly reduce aggression (1 study, 301 people), but probably make no important difference to symptoms of psychosis (12 studies, 3364 people);
‐ increase the risk of drowsiness (13 studies, 2878 people) and probably slightly increase movement disorders (15 studies, 4180 people);
‐ probably slightly increase the risk of experiencing any non‐serious or serious unwanted effect combined, the risk of serious unwanted effects, and the risk of death (17 studies, 5032 people).
What are the limitations of the evidence?
Overall, our confidence in the evidence about typical antipsychotics is limited and our confidence in the evidence about atypical antipsychotics moderate. Typical antipsychotics have been investigated in just a few studies. In addition, the studies about typical and atypical antipsychotics did not always use the best methods to carry out their investigations, or did not report the results. Consequently, the effects on agitation or psychosis may have been overestimated, and the effects on adverse events underestimated.
How up to date is this evidence?
The evidence is up‐to‐date to 7 January 2021.
Summary of findings
Summary of findings 1. Summary of findings ‐ Typical antipsychotics compared to placebo in people with Alzheimer's disease and vascular dementia.
| Outcomes |
Absolute mean change from baseline or absolute risk in each group |
Comparison of mean changes or risks between groups (treatment effect) |
Certainty of the evidence (GRADE) |
Comments | ||
| Placebo group |
Antipsychotics group (95%CI) |
Relative effect, RR (95% CI) |
Absolute effect, MD or RD (95% CI) |
|||
|
Agitation ‐ presented in units on CMAI (higher is worse)a ‐ 361 persons in 4 RCTs of 3 to 16 weeks |
15.0 decrease | 20.2 decrease (17.2 to 23.3)b |
NA | 5.2 greater decrease (2.2 to 8.2) |
⊕⊝⊝⊝ Very lowc,d,e |
Baseline mean on CMAI was 58.8; SMD 0.36 less (0.57 to 0.15) |
|
Response for agitation ‐ as defined by authors of RCTs ‐ 367 persons in 4 RCTs of 3 to 16 weeks |
52 per 100 | 61 per 100 (52 to 71) |
1.18 (1.01 to 1.38) |
9 more per 100 (0 to 19) |
⊕⊕⊕⊝ Moderatec |
Example of response: improvement on CGIS |
|
Psychosis ‐ presented in units of NPI‐NH P (higher is worse)f ‐ 240 persons in 2 RCTs of 6 to 10 weeks |
4.7 decrease | 6.3 decrease (4.9 to 7.7)g |
NA | 1.6 greater decrease (0.2 to 3.0) |
⊕⊕⊝⊝ Lowe,h |
Baseline mean on NPH‐NH P was 11.2; SMD 0.29 less (0.55 to 0.03) |
|
Response for psychosis ‐ as defined by authors of RCTs ‐ 259 persons in 2 RCTs of 6 to 10 weeks |
27 per 100 | 35 per 100 (24 to 52) |
1.31 (0.90 to 1.92) |
8 more per 100 (3 less to 35 more) |
⊕⊕⊝⊝ Lowe,h |
Example of response: improvement on CGIS |
|
Extrapyramidal symptoms ‐ assessed with different instruments ‐ 467 persons in 3 RCTs of 3 to 16 weeks |
15 per 100 | 33 per 100 (23 to 48) |
2.26 (1.58 to 3.23) |
18 more per 100 (8 to 33 more) |
⊕⊕⊕⊕ High |
‐ |
|
Somnolence ‐ assessed with different instruments ‐ 466 persons in 3 RCTs of 3 to 16 weeks |
7 per 100 | 19 per 100 (11 to 33) |
2.62 (1.51 to 4.56) |
12 more per 100 (4 to 26 more) |
⊕⊕⊕⊝ Moderated |
‐ |
|
Death ‐ 578 persons in 6 RCTs of 3 to 16 weeks _________________________________________ |
25 per 1000 ______________ |
36 per 1000 (13 to 98) ____________________ |
1.46 (0.54 to 4.00) ________________ |
11 more per 1000 (12 less to 73 more) __________________ |
⊕⊕⊝⊝ Lowi _____________ |
‐ _____________________________ |
CGIS: Clinical Global Impression scale; CI: confidence interval; CMAI: Cohen‐Mansfield Agitation Inventory; MD: mean difference; NA: not applicable (to changes from baseline); NPH‐NH P: NPH‐NH psychosis subscale; RR: risk ratio; RD: risk difference; RCT: randomised controlled trial; SMD: standardised mean difference
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
a The CMAI is a well‐known and much used scale for agitation (possible range 29‐203)
b The weighted average SD of change from baseline on the CMAI was 14.4 in the intervention groups
c Downgraded one level for risk of bias: all studies were rated at high risk of bias in at least one of the following domains: selection bias (comparability of study groups), attrition bias (incomplete outcome data), and other bias (use of a run‐in period)
d Downgraded one level for inconsistency: pronounced statistical heterogeneity (I2 > 50%)
e Downgraded one level for imprecision: confidence interval indicates both an important effect and an effect with no clinical relevance
f NPH‐NH P was the most frequently used scale (possible range 0‐24)
g The weighted average SD of change from baseline on the NPI‐NH psychosis subscale was 5.4 in the intervention groups
h Downgraded one level for risk of bias: all studies were rated at high risk of other bias (use of a run‐in period)
i Downgraded two levels for imprecision: confidence interval encompasses an important harmful effect as well as a protective effect
Summary of findings 2. Summary of findings ‐ Atypical antipsychotics compared to placebo in people with Alzheimer's disease and vascular dementia.
| Outcomes |
Absolute mean change from baseline or absolute risk in each group |
Comparison of mean changes or risks between groups (treatment effect) |
Certainty of the evidence (GRADE) |
Comments | ||
| Placebo group |
Antipsychotics group (95%CI) |
Relative effect, RR (95% CI) |
Absolute effect, MD or RD (95% CI) |
|||
|
Agitation ‐ presented in units on CMAI (higher is worse)a ‐ 1971 persons in 9 RCTs of 3 to 12 weeks |
15.0 decrease | 18.0 decrease (19.3 to 16.7)b |
NA | 3.0 greater decrease (4.3 to 1.7) |
⊕⊕⊕⊝ Moderatec |
Baseline mean on CMAI was 58.8; SMD 0.21 less (0.30 to 0.12 )a |
|
Response for agitation ‐ as defined by authors of RCTs ‐ 1303 persons in 4 RCTs of 3 to 12 weeks |
36 per 100 | 48 per 100 (42 to 54) |
1.31 (1.16 to 1.48) |
12 more per 100 (6 to 18 more) |
⊕⊕⊕⊝ Moderatec |
Example of response: improvement on CGIS |
|
Psychosis ‐ presented in units of NPI‐NH P (higher is worse)d ‐ 3364 persons in 12 RCTs of 3 to 12 weeks |
4.7 decrease | 5.3 decrease (5.7 to 4.9)e |
NA | 0.6 greater decrease (1.0 to 0.2) |
⊕⊕⊕⊝ Moderatec |
Baseline mean on NPH‐NH P was 11.2; SMD 0.11 less (0.18 to 0.03) |
|
Response for psychosis ‐ as defined by authors of RCTs ‐ 1958 persons in 7 RCTs of 3 to 12 weeks |
49 per 100 | 56 per 100 (51 to 61) |
1.13 (1.03 to 1.23) |
7 more per 100 (2 to 12 more) |
⊕⊕⊝⊝ Lowc,f |
Example of response: improvement on CGI |
|
Extrapyramidal symptoms ‐ assessed with different instruments ‐ 4180 persons in 15 RCTs of 3 to 12 weeks |
8 per 100 | 11 per 100 (9 to 14) |
1.39 (1.14 to 1.68) |
3 more per 100 (1 to 6 more) |
⊕⊕⊕⊝ Moderatec |
‐ |
|
Somnolence ‐ assessed with different instruments ‐ 3878 persons in 13 RCTs of 3 to 12 weeks |
7 per 100 | 14 per 100 (11 to 17) |
1.93 (1.57 to 2.39) |
7 more per 100 (4 to 10 more) |
⊕⊕⊕⊕ High |
‐ |
|
Death ‐ 5032 persons in 17 RCTs of 3 to 12 weeks ___________________________________________ |
19 per 1000 ______________ |
26 per 1000 (17 to 39) ____________________ |
1.36 (0.90 to 2.05) ________________ |
7 more per 1000 (2 less to 20 more) __________________ |
⊕⊕⊕⊝ Moderateg _______________ |
‐ ______________________________ |
CGIS: Clinical Global Impression scale; CI: confidence interval; CMAI: Cohen‐Mansfield Agitation Inventory; MD: mean difference; NA: not applicable (to changes from baseline); NPH‐NH P: NPH‐NH psychosis subscale; RR: risk ratio; RD: risk difference; RCT: randomised controlled trial; SMD: standardised mean difference
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
a The CMAI is a well‐known and much used scale for agitation (possible range 29‐203)
b The weighted average SD of change from baseline on the CMAI was 14.4 in intervention the groups
c Downgraded one level for risk of bias: all studies were rated at high risk of bias in at least one of the following domains: selection bias (comparability of study groups), attrition bias (incomplete outcome data), and other bias (use of a run‐in period)
d NPH‐NH Psychosis subscale was the most frequently used scale (possible range 0‐24)
e The weighted average SD of change from baseline on the NPI‐NH psychosis subscale was 5.4 in the intervention groups
f Downgraded one level for inconsistency: pronounced statistical heterogeneity (I2 > 50%)
g Downgraded one level for imprecision: confidence interval encompasses a harmful effect as well as a protective effect
Background
Description of the condition
Dementia is a clinical syndrome characterised by cognitive, neuropsychiatric, and functional symptoms. It involves cognitive deterioration, disturbances in language, psychological and psychiatric changes, and impairments in activities of daily living (ADL). Five per cent of people aged over 60 years (Prince 2015), and in 2015, an estimated 47 million people were living with dementia worldwide. The total number of people with dementia will most likely continue to rise as the age of the population increases. Alzheimer's disease is the most common type of dementia (Livingston 2017).
Neuropsychiatric symptoms, also known as behavioural and psychological symptoms of dementia (BPSD), or challenging behaviour are common features of dementia. About 90% of people with dementia experience agitation, psychosis, or other neuropsychiatric symptoms such as anxiety, depression, and apathy at some time during the course of the disease (Borsje 2018). Symptoms often co‐occur. Agitation is difficult to define simply (Cummings 2015). It covers unsettled verbal, vocal, or motor activity that is or is not accompanied by aggression (Cohen‐Mansfield 1996). Common symptoms include restlessness, wandering, verbal insults, and shouting. Agitation is often measured with the Cohen‐Mansfield Agitation Inventory (CMAI), a scale that covers many different types of agitation. In clinical practice, simpler definitions are also used.
Psychosis in dementia is characterised by delusions and hallucinations. Simple delusions about theft or abandonment are typical symptoms in Alzheimer's disease (Murray 2014). Prevalence of psychosis in Alzheimer's disease varies from 25% to 50% and depends on the stage of the disease: the prevalence is lower in the early stage of the disease, and rises as the disease progresses (Murray 2014).
Agitation and psychosis are distressing for people with dementia and their carers, and make it more difficult to care for the patient (Gilley 1991; Livingston 2014a; Schmidt 2012). The symptoms are associated with greater functional impairment and poorer quality of life (Morris 2015; Scarmeas 2005; Wetzels 2010). They frequently trigger placement in residential care or use of psychotropic drugs and are associated with higher care costs (Testad 2010; Toot 2017).
Agitation and psychosis can occur as a result of other causes (superimposed on dementia). Therefore, a comprehensive assessment of possible precipitating somatic, psychosocial and environmental factors such as pain, delirium, unmet needs, and annoying sounds should be performed to rule out other treatable causes, before hypothesising that agitation and psychosis are due to the dementia syndrome and considering the use of antipsychotics.
Description of the intervention
Antipsychotics, also known as neuroleptics, are widely used to treat agitation and psychosis in dementia. Antipsychotic use in Western European nursing home residents ranges from 12% to 59% (Janus 2016). Factors influencing antipsychotic use in people with dementia in nursing homes are nurses' job satisfaction and their belief in positive treatment effects (Janus 2017).
Antipsychotics can be classified into two subgroups: typical (conventional, first‐generation) and atypical (second‐generation) agents. Haloperidol is the most commonly used typical antipsychotic and risperidone the most commonly used atypical antipsychotic for agitation and psychosis in dementia (Yohanna 2017). Other typical agents are chlorpromazine and thiothixene, and other atypical agents include olanzapine, quetiapine, clozapine, or aripiprazole. The US Food and Drug Administration (FDA) has not approved any antipsychotics for use in people with dementia; in the EU, only risperidone is licensed for short‐term use for aggression in this patient population (; Almutairi 2018Tampi 2016).
Despite the wide use of antipsychotics for agitation and psychosis in dementia, their benefit is uncertain because some trials have yielded negative results and effectiveness may be outweighed by harms (Schneider 2006). Antipsychotics have various severe adverse effects such extrapyramidal symptoms (EPS), somnolence, and (further) cognitive decline (Ballard 2005; Kirchner 2001). Less frequent but serious adverse events (SAEs) are malignant neuroleptic syndrome, strokes, falls, and pneumonia (Banerjee 2010; Knol 2008; Lonergan 2002).
Regulatory agencies issued a warning about the use of atypical antipsychotics in people with dementia in the mid‐2000s due to an increased risk of death and stoke in this population (EMA 2008; Kuehn 2005; MHRA 2009; Schneider 2005). Cohort studies have also shown an association between use of typical antipsychotics and an increased risk of mortality in older people (Arai 2016; Kales 2007; Kales 2012). However, it has also been postulated that this the co‐occurrence of the use of typical antipsychotics and deaths might result from "confounding by indication" because many cohort studies included people with terminal illness and delirium, but did not adjust for severity of disease (Luijendijk 2016). This could also explain why mortality is highest during the first month of use (Luijendijk 2016).
Overprescribing of antipsychotics in people with dementia has become a major problem. Antipsychotic drugs are often prescribed inappropriately (unclear indication, presence of contraindications, or chronic use longer than necessary or advocated) and with little monitoring (Furniss 1998; Renom‐Guiteras 2018). The use of antipsychotics in people with dementia has also provoked much debate due to the potential for SAEs. In addition, some consider the use of antipsychotics to be simply a chemical restraint, suggesting that antipsychotics are used to calm people down with the sedative effects rather than to treat agitation and psychosis specifically or searching for and remedying the triggers for these behaviours (Hughes 2008). Furthermore, it has been shown that long‐term use of antipsychotics could be successfully discontinued in people with dementia (Van Leeuwen 2018).
How the intervention might work
Almost all typical antipsychotics are antagonists at the dopamine receptor. This effect is considered to reduce agitation and psychosis, but also cause adverse drug reactions, including motor EPS, sedation, and endocrine changes. Atypical antipsychotics also act on serotonergic, adrenergic, histaminergic and muscarinic receptors (Farah 2005). Serotonergic blockade could reduce negative symptoms of psychosis, but also cause EPS. Adrenergic blockade is related to hypotension and sedation, histaminergic blockade to sedation and weight gain, and muscarinergic blockade to cognitive disorder, urinary retention, and obstipation (anti‐cholinergic effects). Nevertheless, atypical antipsychotics have been marketed on the premise that they offer a better adverse effect profile than conventional antipsychotics, in particular fewer severe EPS (Pierre 2005)
Why it is important to do this review
In the current review, we have updated and combined two previous Cochrane Reviews. Both were published when concern about the use of antipsychotics began to emerge. The first concerned haloperidol for agitation in dementia (Lonergan 2002). This review did not cover psychosis in dementia. The second concerned atypical antipsychotics for neuropsychiatric symptoms (Ballard 2006). The present review will focus on agitation (with or without aggression) and psychosis.
In addition, we wish to present the evidence for atypical and typical antipsychotics in one review so that the reader can make an informed choice between the two types of drugs. This review will support decision‐making for clinicians, carers, and patients. Finally, the widespread use of antipsychotics as well as the potentially unfavourable balance between benefits and harms call for an up‐to‐date review.
Objectives
To assess the efficacy and safety of antipsychotics for the treatment of agitation and psychosis in people with Alzheimer's disease and vascular dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, placebo‐controlled trials comparing the effects of antipsychotics and placebo for the treatment of agitation or psychosis in people with dementia due to Alzheimer's disease or vascular dementia. We included full journal publications, online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts. We also included studies which report insufficient data for analysis and described the results narratively.
We excluded studies that were non‐randomised, case reports, and clinical observations. We also excluded studies using antipsychotics that are no longer available on the US or EU market, studies of antipsychotics that are used for acute short‐term sedation in emergency situations, studies comparing different antipsychotics head to head, and antipsychotic withdrawal trials.
There were no language restrictions.
Types of participants
We included trials in people with a diagnosis of dementia due to Alzheimer's disease, vascular dementia, or both, irrespective of age, severity of cognitive impairment, and setting. Diagnoses of dementia must have been made with established diagnostic criteria for Alzheimer's or vascular dementia. We also included studies with mixed dementia populations if at least 80% of the participants had Alzheimer's or vascular dementia. We excluded trials in people with other types of dementia, or delirium.
Participants must have clinically significant agitation (including aggression) or psychosis or both at baseline. We accepted definitions of clinically significant agitation or psychosis from the included trials based either on scores on validated measurement instruments or on reports of clinical relevance from informal carers or healthcare professionals. Validated measurement instruments often used to assess agitation are the Cohen‐Mansfield Agitation Inventory (CMAI), the agitation subscale of the Behavioral Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD), or the Neuropsychiatric Inventory (NPI). The psychosis subscale of BEHAVE‐AD or NPI are frequently used to assess psychosis.
Types of interventions
We included all studies using typical and atypical antipsychotics that are presently available for use on the US or EU market.
As typical antipsychotics, we considered substances coded in the Anatomical Therapeutic Chemical Classification System (ATC) as N05AA, N05AB, N05AD, N05AF, and N05AG (e.g. chlorpromazine, chlorprothixene, flupentixol, fluphenazine, haloperidol, levomepromazine, perphenazine, pimozide, thiothixene, trifluoperazine, zuclopenthixol). Atypical antipsychotics are ATC coded as N05AE, N05AH, N05AL, and N05AX (e.g. amisulpride, aripiprazole, clozapine, lurasidone, olanzapine, quetiapine, risperidone, sertindole, sulpiride, zotepine, ziprasidone) (WHO 2017).
Types of outcome measures
Primary outcomes
-
Efficacy:
severity of agitation in participants with agitation, or severity of psychosis in participants with psychosis.
-
Adverse effects:
somnolence;
EPS;
any adverse event;
any SAE, which is defined by the FDA and EMA as resulting in death, being life‐threatening, requiring hospitalisation, or causing prolongation of existing hospitalisation, resulting in persistent or significant disability/incapacity or requiring interventions to prevent permanent impairment or damage. This includes stroke, thromboembolism, and pneumonia;
death.
Secondary outcomes
Responders for agitation or psychosis in trials that included participants with agitation or psychosis respectively at baseline (response according to definition of primary study authors, or improvement on Clinical Global Impression scale).
Discontinuation (any reason).
Discontinuation due to adverse events.
Health‐related quality of life.
Functioning in activities of daily living (ADL).
Cognitive functioning;.
Carer burden or carer quality of life.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), which is the Cochrane Dementia and Cognitive Improvement Group's (CDCIG) specialised register on 7 January 2021.
ALOIS is maintained by the Information Specialists for the CDCIG, and contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. The studies are identified through searching:
the Cochrane Library's Central Register of Controlled Trials (CENTRAL);
major healthcare databases: MEDLINE (OvidSP), Embase (OvidSP), CINAHL (EBSCOhost), and PsycINFO (OvidSP);
trial registers: ClinicalTrials.gov and the World Health Organization's (WHO) International Clinical Trials Register Platform (ICTRP) which covers ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register, plus others;
grey literature sources: ISI Web of Science Core Collection.
To view a list of all sources searched, see the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies run in healthcare bibliographic databases, used for the retrieval of reports of dementia, cognitive improvement, and cognitive enhancement trials, can be viewed on the CDCIG's website (dementia.cochrane.org/searches).
We ran additional searches in MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), LILACs (Bireme), ClinicalTrials.gov, and the WHO Portal/ICTRP to ensure that the searches for this review are as comprehensive and up‐to‐date as possible. The search strategies used for the retrieval of reports of trials can be seen in Appendix 1.
Searching other resources
We searched relevant trial registers of pharmaceutical companies such as those listed in Section 6.2.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). In addition, we searched regulatory agency sources European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for relevant clinical study reports (Isojarvi 2018; Schroll 2015).
Data collection and analysis
Selection of studies
After removing duplicates, two review authors independently assessed eligibility of studies identified by the search with the defined inclusion criteria. Both review authors independently reviewed full texts of each study deemed possibly relevant. We used Covidence to facilitate the process. We resolved disagreements in consensus discussions or consultation of a third review author. We reported details of included studies in the Characteristics of included studies table and reasons for exclusion in the Characteristics of excluded studies table.
We collated multiple reports of the same study including retraction statements and errata, and other unpublished key information. We included a PRISMA flow chart in the full review showing the status of identified studies (Moher 2009), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We included studies irrespective of whether measured outcome data are reported in a 'usable' way.
Data extraction and management
Two review authors independently extracted data using Covidence. We collected the following data from the main article and other data sources.
General study characteristics: drug and daily dose tested, setting, type of dementia, number randomised, indication (agitation or psychosis), and commercial funding. One author also extracted the mean age, proportion of women, and severity of dementia of the participants.
Continuous outcomes (severity of agitation or psychosis, health‐related quality of life, functioning in activities of daily living (ADL), cognitive functioning, carer burden or carer quality of life): we extracted mean changes per treatment group for continuous outcomes and accompanying standard deviations (SDs), preferably for all randomised participants or otherwise for all participants available at endpoint assessment. If standard deviations (SDs) were not available for the groups that we compared, we calculated them from reported data if possible.
Binary outcomes (occurrence of somnolence, EPS, any adverse event, any SAE, death, response on agitation or psychosis, discontinuation (any reason), discontinuation due to adverse event): we extracted the number of participants with the outcome per treatment group, and the number of all randomised participants as denominator.
Clinical response was treated as a binary variable (present or not) and we used the definition of the study authors. If a response was not defined but measured with the Clinical Global Impression scale or a comparable instrument, we used the categories 'very much improved', 'much improved' and 'minimally improved'. Patients with missing data were regarded as not having a favourable response.
We resolved any disagreements by discussion and consensus using Covidence. After reaching consensus, we transferred data into RevMan Web (Review Manager 2019).
Assessment of risk of bias in included studies
We assessed risk of bias using the Cochrane risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Two review authors independently assessed and rated the methodological quality of the studies to identify any potential source of bias. We assessed the following aspects of trial design: selection bias (random sequence generation, concealment of allocation, comparability of groups at baseline); performance bias (blinding of personnel and participants); detection bias (blinding of outcome assessors); attrition bias (incomplete reporting of outcome data); reporting bias (selective reporting) and other bias (run‐in period). We found very few protocols of studies that were published, and only assessed protocol deviations in terms of selective reporting. Commercial funding was one of the extracted general study characteristics. We categorised studies as having low, high, or unclear risk of bias. The judgements were compared automatically so that discrepancies could be discussed and resolved.
Measures of treatment effect
Where possible, we expressed the treatment effect on a continuous outcome (change from baseline in psychosis or agitation) as pooled standardised mean difference (SMD) with 95% confidence interval (CIs). We included all reported measurement instruments for agitation and psychosis in these analyses, and ensured that higher scores have the same meaning across instruments.
We expressed the treatment effect on dichotomous outcomes as risk ratio (RR) with 95% CIs. Where informative, we performed meta‐analyses to calculate the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to harm for an additional harmful outcome (NNTH) based on pooled risk differences (RDs).
We performed the statistical analyses using RevMan Web (Review Manager 2019).
Unit of analysis issues
We combined data from multiple active drug groups within a trial if they tested the same drug (multiple dosages). We included cross‐over studies using first‐phase data only to avoid carry‐over effects. We excluded groups treated with more than one drug in the same group or groups treated with other psychotropic drugs. If we included more than one study arm per study in the same analysis, we split the control group following the methods described in the Cochrane Handbook for Systematic Reviews of Interventions to avoid that it counted multiple times (Higgins 2019).
We included studies that had a run‐in period before randomisation, even though some eligible participants who met inclusion criteria for the study at the start of the run‐in period, might have been excluded from participation at the end of the run‐in period (Hulshof 2020).
Dealing with missing data
Where possible, we contacted authors of the included studies to obtain missing data. We used data from intention‐to‐treat (ITT) analyses if available. Otherwise, we planned to also included data from per‐protocol analyses but perform sensitivity analyses to assess for their effect, but this was not the case in any of the included studies.
Assessment of heterogeneity
We assessed heterogeneity of the treatment effect between the trials with the Chi² statistic. We used a fixed‐effect model, unless the I² statistic was greater than 40%, in which case we used a random‐effects model.
Assessment of reporting biases
We assessed reporting bias with a funnel plot if at least 10 studies were available for meta‐analysis.
Data synthesis
We used RevMan Web (Review Manager 2019) to analyse the data. We calculated the pooled effects of typical and atypical antipsychotics on agitation and psychosis separately. When investigating adverse effects, we pooled data from studies independent of the indication investigated (agitation or psychosis). If meta‐analysis was not suitable because of heterogeneity or insufficient data, we presented a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
We reran all analyses including only haloperidol and risperidone studies, as these are the antipsychotics of first choice in many countries in 2019. We did not perform subgroup analyses related to participant characteristics.
We also conducted a post‐hoc subgroup analysis among studies including patients with (any type of) agitation versus one trial that included only patients with physical aggression. In addition, in response to a reviewer's comment, we performed post‐hoc analyses for quetiapine only.
Sensitivity analysis
We did not perform a pre‐planned sensitivity analysis excluding trials with at least one rating of high risk of bias because all studies had a high risk of bias rating in at least one domain. We also did not perform the pre‐planned sensitivity analysis excluding trials that only reported per‐protocol analysis due to the lack of such studies.
Summary of findings and assessment of the certainty of the evidence
We assessed the overall quality of the body of evidence for the most important outcomes using the GRADE approach (Guyatt 2013a; Guyatt 2013b). This includes taking into account: risk of bias, inconsistency, indirectness, imprecision, and publication bias. For efficacy, bias away from the null was considered a threat to validity, and bias to the null for adverse effects. If a 95% confidence interval was so wide that it included no effect or a clinically negligible effect, the evidence would be downgraded one level for imprecision.
We described the results using a standardised wording that incorporates the certainty of evidence and the importance of benefits or harms as described in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020). High‐certainty evidence is described as 'improves/reduces' the outcome (for important benefits or harm), 'improves/reduces slightly' (less important benefits or harm), and 'have little or no effect' (for no or negligible benefits or harm). Moderate‐certainty evidence is described as 'probably improves/reduces' the outcome (for important benefits or harm), 'probably improves/reduces slightly' (less important benefits or harm), and 'probably have little or no effect' (for no or negligible benefits or harm). Low‐certainty evidence is described as 'may improve/reduce' the outcome (for important benefits or harm), 'may improve/reduce slightly' (less important benefits or harm), or 'may have little or no effect' (for no or negligible benefits or harm). Very‐low certainty evidence is described as 'we are uncertain whether...improves/reduces' the outcome.
Based on the available data, we presented the results of the following outcomes in the summary of findings tables for typical and atypical antipsychotics:
agitation in trials that included patients with agitation;
response for agitation in trials that included participants with agitation;
psychosis in trials that included patients with psychosis;
response for psychosis in trials that included patients with psychosis;
EPS;
somnolence;
death.
To present the effects of the drug classes on the continuous outcomes agitation and psychosis in the summary of findings tables, we converted the SMDs to absolute changes from baseline in units of a representative measurement instrument for the placebo and antipsychotics groups (CMAI for agitation and NPI‐NH psychosis subscale for psychosis). We calculated the average absolute change of the placebo groups in studies that used this instrument. We calculated the average absolute change of the antipsychotics group by multiplying the SMD with the weighted average SD of change in the antipsychotics groups of the studies that used this instrument, and adding this figure to the average absolute change of the placebo group. For the dichotomous outcomes response, EPS, somnolence, and death, we present the number of participants with the outcome per 100 patients. Absolute and relative effects of treatment that derive from the differences between the groups, expressed in changes or risks, are also shown in the summary of findings tables.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search retrieved 8233 records. After 1196 duplicates were excluded, we screened 7037 for potential eligibility. 7005 were excluded based on title and abstract. We excluded 9 studies and included 24 studies after full‐text screening (Figure 1).
1.
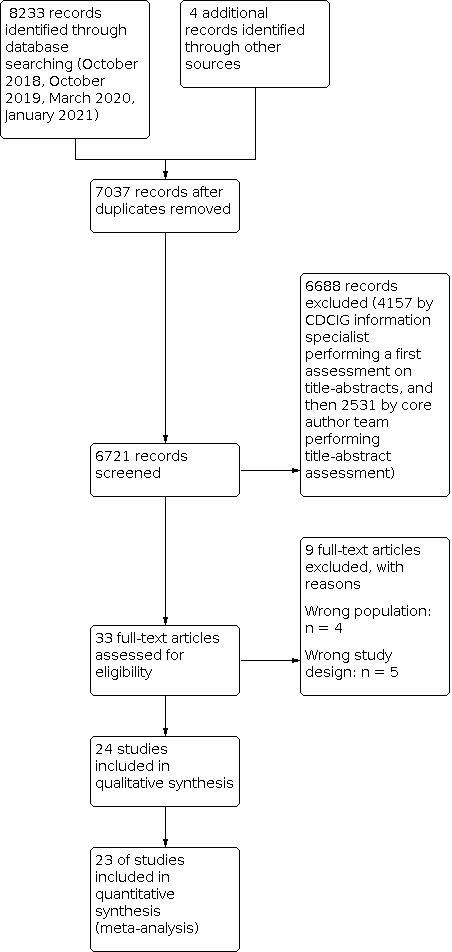
Study flow diagram.
Included studies
We included 24 randomised controlled trials with a total of 6090 participants. Six trials tested a typical antipsychotic (see table 1): five randomised controlled trials (RCTs) with 421 participants investigated the effect on agitation (Allain 2000; Auchus 1997;Devanand 1998; Finkel 1995; Teri 2000), and two trials with 350 participants the effect on psychosis (Devanand 1998; Tariot 2006). Five trials tested haloperidol, and one thiothixene. Mean ages ranged from 72.1 to 85 years and most patients were female (62.9% to 86%). In one study, the participants had mild‐to‐moderate dementia (Allain 2000), in two studies moderate dementia (Auchus 1997; Tariot 2006), and the other three studies moderate‐to‐severe dementia (Devanand 1998; Teri 2000 ; Finkel 1995).
For atypical antipsychotics, there were 20 trials (see table 2): Eight RCTs with 2320 participants tested the effect on agitation (Allain 2000; Ballard 2005; Brodaty 2003 RIS‐AUS‐05; Grossberg 2020a; Grossberg 2020b; Japic CTI 142578 2015; Schneider 2006 CATIE‐AD; Zhong 2007), and 12 studies with 3589 participants the effect on psychosis (Ballard 2018; De Deyn 2005; Deberdt 2005 F1D MC HGGU; De Deyn 2004 F1D MC HGIV; RIS‐INT‐83 2003; Schneider 2003 RIS USA 63; Mintzer 2007; NCT00287742 2006; Paleacu 2008; Mintzer 2006 RIS USA 232; Streim 2008; Tariot 2006). The trials tested aripiprazole (4x), brexpiprazol (2x), olanzapine (3x), pimavanserin (1x), quetiapine (5x), risperidone (7x), and tiapride (1x).
One study included patients with psychosis (71.8%) or psychomotor agitation (78.9%) (Devanand 1998). Of the RIS‐USA 63, only the subgroup of patients with psychosis was included (Schneider 2003 RIS USA 63). Two trials tested both a typical and an atypical antipsychotic drug against placebo (Auchus 1997; Tariot 2006). One publication reported on two RCTs (Grossberg 2020a; Grossberg 2020b).
Mean ages ranged from 73.885.9 years and most patients were female (55.2 to 84.9%). Most studies included patients with moderate dementia (Deberdt 2005 F1D MC HGGU; De Deyn 2005; Mintzer 2006 RIS USA 232; Mintzer 2007; NCT00287742 2006; Paleacu 2008; Schneider 2006 CATIE‐AD; Streim 2008; Tariot 2006) or severe dementia (Ballard 2005; Ballard 2018; Brodaty 2003 RIS‐AUS‐05; Schneider 2003 RIS USA 63; Zhong 2007). In one study, dementia was mild‐to‐moderate (Allain 2000), and in three studies mild‐to‐severe (De Deyn 2004 F1D MC HGIV; Grossberg 2020a; Grossberg 2020b). One study did not mention the severity of dementia (RIS‐INT‐83 2003).
Three studies were non‐commercially funded (Auchus 1997; Devanand 1998; Schneider 2006 CATIE‐AD). Nineteen trials were funded by pharmaceutical companies, and the funding source was unclear in two studies.
We found three studies that have not been published in a journal. A summary of results was reported on a trial registration website for NCT00287742 2006 and RIS‐INT‐83 2003. No results were reported for Japic CTI 142578 2015.
We obtained additional data about one trial (Paleacu 2008). All other data were extracted from published reports.
Table 1: Characteristics of included studies on typical antipsychotics
| Study‐ID | Setting | Condition | Indication | Scale | Drug | Daily dose | Duration, weeks | Number randomised |
| Finkel 1995 | Nursing homes | AD, VDa | Agitation | CMAI | Thiothixene | 0.25 mg to 18 mg | 11 | 33 |
| Auchus 1997 | Community‐dwelling | AD | Agitation | CMAI‐SF | Haloperidol | 3 mg | 6 | 12 |
| Teri 2000 | Community‐dwelling | AD | Agitation | CMAI | Haloperidol | 0.5 mg to 3 mg | 16 | 70 |
| Allain 2000 | Nursing home or hospitalised | AD, VD, mixed type | Agitation | MOSESb | Haloperidol | Up to 6 mg | 3 | 306 |
| Tariot 2006 | Nursing homes | AD | Psychosis | NPI‐NH psychosisc | Haloperidol | 0.5mg to 12 mg | 10 | 284 |
| Devanand 1998 | Community‐dwelling | AD | Psychosis Agitation |
BPRS psychosisc BSSD psychomotor agitation item |
Haloperidol | 0.5 mg to 0.75 mg or 2 mg to 3 mg | 6 | 66 |
AD: Alzheimer's disease; VD: Vascular dementia; CMAI(‐SF): Cohen‐Mansfield Agitation Inventory(‐short form); MOSES: Multidimensional Observation Scale for Elderly Subjects; NPI‐NH: Neuropsychiatric Inventory‐Nursing Home; BPRS: Brief Psychiatric Rating Scale; BSSD: Behavioural Syndromes Scale for Dementia; personal communication; irritability/aggressiveness subscore;subscale.
Table 2: Characteristics of included studies on atypical antipsychotics
| Study‐ID | Setting | Condition | Indication | Scale | Drug | Daily dose | Duration, weeks | Number randomised |
| Allain 2000 | Nursing home or hospitalised | AD, VD, mixed type | Agitation | MOSESa | Tiapride | Up to 300 mg | 3 | 306 |
| Ballard 2005 | Care facilities | AD | Agitation | CMAI | Quetiapine | 50 mg to 100 mg | 6 | 62 |
| Zhong 2007 | Nursing homes, assisted‐living facilities | AD, VD | Agitation | PANSS‐EC | Quetiapine | 100 mg or 200 mg | 10 | 333 |
| Schneider 2006 CATIE‐AD | Community‐dwelling or assisted‐living facilities | AD | Agitation | NPI agitationb | Olanzapine, Quetiapine or Risperidone | Flexible dosec | 12 | 421 |
| Brodaty 2003 RIS‐AUS‐05 | Nursing homes | AD, VD, mixed type | Agitation | CMAI aggressionb | Risperidone | up to 2 mg | 12 | 345 |
| Grossberg 2020a | Community‐dwelling or care facility | AD | Agitation | CMAI | Brexpiprazole | 0.5 mg, 1 mg and 2 mg | 12 | 433 |
| Grossberg 2020b | Community‐dwelling or care facility | AD | Agitation | CMAI | Brexpiprazole | 0.5 mg to 2 mg | 12 | 270 |
| Japic CTI 142578 2015 | Hospital or care facilities | AD | Agitation | Not reported | Aripiprazole | 2, 3 mg or 6 mg | 10 | 150 |
| Tariot 2006 | Nursing homes | AD | Psychosis | NPI‐NH psychosisb | Quetiapine | 25 mg to 600 mg | 10 | 284 |
| Paleacu 2008 | Not reported | AD | Psychosis | NPI‐NH psychosisb | Quetiapine | 50 mg to 300 mg | 6 | 40 |
| Ballard 2018 | Nursing homes | AD | Psychosis | NPI‐NH psychosisb | Pimavanserin | 34 mg | 12 | 181 |
| De Deyn 2004 F1D MC HGIV | Nursing homes, continuing‐care hospitals | AD | Psychosis | NPI‐NH psychosisb | Olanzapine | 1 mg, 2.5 mg, 5 mg or 7.5 mg | 10 | 652 |
| Deberdt 2005 F1D MC HGGU | Outpatients, nursing homes, assisted‐living centres | AD, VD, mixed type | Psychosis | NPI(‐NH) psychosisb | Olanzapine or Risperidone |
2.5 mg to 10 mg, respectively 0.5 mg to 2 mg |
10 | 494 |
| Mintzer 2006 RIS USA 232 | Nursing homes, long‐term care | AD, VD | Psychosis | BEHAVE‐AD psychosisb | Risperidone | 1 mg to 1.5 mg | 8 | 473 |
| NCT00287742 2006 | In‐ or outpatients | AD | Psychosis | BEHAVE‐AD psychosisb | Risperidone | 0.5 mg to ‐2 mg | 8 | 33 |
| RIS‐INT‐83 2003 | Nursing homes or long‐term care | AD | Psychosis | BEHAVE‐AD | Risperidone | 1 mg to 1.5 mg | 8 | 18 |
| Schneider 2003 RIS USA 63 | Nursing homes, hospital | AD, VD, mixed type | Psychosis | BEHAVE‐AD psychosisb | Risperidone | 1 mg, 2 mg or 3 mg | 12 | 463 (psychosis subgroup) |
| Streim 2008 | Institutionalised subjects | AD, VD | Psychosis | NPI‐NH psychosisb | Aripiprazole | 2 mg to 15 mg | 10 | 256 |
| Mintzer 2007 | Nursing homes, assisted‐living facilities | AD | Psychosis | NPI‐NH psychosisb | Aripiprazole | 2, 5 mg or 10 mg | 10 | 487 |
| De Deyn 2005 | Community‐dwelling | AD | Psychosis | NPI Psychosisb | Aripiprazole | 2 mg to 15 mg | 10 | 208 |
AD: Alzheimer's disease; VD: Vascular dementia; MOSES: Multidimensional Observation Scale for Elderly Subjects; CMAI: Cohen‐Mansfield Agitation Inventory; PANSS‐EC: Positive and Negative Syndrome Scale ‐ Excitement Component; NPI: Neuropsychiatric Inventory; NPI‐NH: Neuropsychiatric Inventory‐Nursing Home; BEHAVE‐AD: Behavioural Pathology in Alzheimer's Disease; irritability/aggressiveness subscore; bsubscale; cOlanzapine: mean 5.5mg/day, Quetiapine: mean 56.5mg/day, Risperidone: mean 1.0mg/day.
Excluded studies
Nine studies were excluded. Reasons for exclusion were wrong study design (not placebo controlled Shin 2013; Trequattrini 2003; Holmes 2007; Meguro 2004,or no parallel groups Devanand 1989), wrong population (not Alzheimer's disease or vascular dementia NCT00043849 2002), or wrong indication (not specifically agitation or psychosis Street 2000 F1D MC HGEU; a broad range of neuropsychiatric symptoms (DeDeyn 1999 RIS‐INT‐24) or unclear how many participants were psychotic or agitated Pollock 2002).
Risk of bias in included studies
Most studies were at high risk of bias in at least one domain. Detailed information about the risk of bias in the included studies is presented in the table Characteristics of included studies. An overview is provided in Figure 2 and Figure 3.
2.

Risk of bias summary
3.

Risk of bias graph
Allocation
The randomisation sequence was adequately generated in five studies (Ballard 2005; Ballard 2018; Grossberg 2020b; Grossberg 2020a; Zhong 2007) and unclear in most studies. Allocation was concealed (non‐predictable) in three studies (Mintzer 2006 RIS USA 232; Schneider 2006 CATIE‐AD; Zhong 2007) and unclear in all other studies.
Despite randomisation in all trials, only three studies were judged to have comparable groups or adequate adjustment for baseline differences (Ballard 2018; Mintzer 2006 RIS USA 232; Teri 2000). Comparability of groups was limited in six studies (Allain 2000; Ballard 2005; De Deyn 2005; De Deyn 2004 F1D MC HGIV; Grossberg 2020a; Zhong 2007) and unclear in 15 studies (the baseline differences were not reported or there were small differences with unclear significance).
Blinding
Participants and personnel were blinded to the treatment status of the participants during the trial in seven studies (Ballard 2005; Ballard 2018; Devanand 1998; Grossberg 2020a; Grossberg 2020b; Japic CTI 142578 2015; Tariot 2006). Outcome assessors were blinded in seven studies (Ballard 2005; Ballard 2018; Devanand 1998; Grossberg 2020a; Grossberg 2020b;Japic CTI 142578 2015; Teri 2000). The persons who were blinded was unclear in the rest of studies.
Incomplete outcome data
Only three studies presented complete outcome data (Grossberg 2020b ;RIS‐INT‐83 2003; Teri 2000). Outcome data were incomplete in 15 studies, and data completeness was unclear in six studies.
Selective reporting
Selective reporting did not seem to be present in five studies (Allain 2000; Auchus 1997; Deberdt 2005 F1D MC HGGU; RIS‐INT‐83 2003; Teri 2000), was unclear in six studies and seemed present in 13 studies.
Funnel plots
Visual inspection of funnel plots for analyses of atypical antipsychotics in Figure 4 (outcome: psychosis); Figure 5 (outcome: somnolence); Figure 6 (outcome: extrapyramidal symptoms); Figure 7 (outcome: any adverse event); Figure 8 (outcome: any serious adverse event); Figure 9 (outcome: death); Figure 10 (outcome: discontinuation due to adverse events); Figure 11 (outcome: discontinuation (any reason)) and Figure 12 (outcome: cognitive function) do not show marked asymmetries. Therefore reporting bias is not clearly indicated, although some funnels plots may show a tendency towards some bias in favour of atypical antipsychotics.
4.

Funnel plot: Analysis 3.2 (Psychosis, atypical antipsychotics)
5.

Funnel plot: Analysis 3.3 (Somnolence, atypical antipsychotics)
6.

Funnel plot: Analysis 3.5 (Extrapyramidal symptoms, atypical antipsychotics)
7.

Funnel plot: Analysis 3.7 (Any adverse event, atypical antipsychotics)
8.

Funnel plot: Analysis 3.9 (Any serious adverse event, atypical antipsychotics)
9.

Funnel plot: Analysis 3.11 (Death, atypical antipsychotics)
10.

Funnel plot: Analysis 3.17 (Discontinuation due to adverse events, atypical antipsychotics)
11.

Funnel plot: Analysis 3.19 (Discontinuation (any reason), atypical antipsychotics)
12.

Funnel plot: Analysis 3.22 (Cognitive function, atypical antipsychotics)
Other potential sources of bias
Nineteen studies used a run‐in period, which may introduce bias due to deselection of (eligible) patients with side effects before randomization. There were only four studies without a run‐in period (Allain 2000; Ballard 2005; Schneider 2006 CATIE‐AD; Zhong 2007), and there was no information about a run‐in period in another study (Japic CTI 142578 2015).
Effects of interventions
Typical antipsychotics versus placebo
Efficacy
Five studies investigated the effect of typical antipsychotics on agitation and four could be pooled. Given the very low‐certainty evidence, we are uncertain whether typical antipsychotics improve agitation compared with placebo (standardised mean difference (SMD) ‐0.36, 95% confidence interval (CI ) ‐0.57 to ‐0.15; I2 = 58%, n = 361; Analysis 1.1; Figure 13). The study that was not included in the meta‐analysis reported no clinically meaningful difference between the antipsychotic and placebo group at the end of the study (the mean difference in decrease on the CMAI between the groups was ‐1.0, with a mean at baseline of 35.2 (range 25 to 44); Auchus 1997).
1.1. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 1: Agitation
13.

Forest plot (1.1 Agitation)
Two studies evaluated the effect of typical antipsychotics on psychosis. Low‐certainty evidence showed that typical antipsychotics may improve psychosis slightly compared with placebo (SMD ‐0.29, 95% CI ‐0.55 to ‐0.03, n = 240; Analysis 1.2; Figure 14).
1.2. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 2: Psychosis
14.

Forest plot (1.2 Psychosis)
Adverse events
Based on the moderate‐certainty evidence, typical antipsychotics probably increase the risk of somnolence compared with placebo (risk ratio (RR) 2.62, 95% CI 1.51 to 4.56; I2 = 78%, n = 466; Analysis 1.3; Figure 15). The corresponding risk difference was 0.12 (95% CI 0.06 to 0.18; NNTH = 8, n = 466; Analysis 1.4). Based on the high‐certainty evidence, typical antipsychotics increase the risk of extrapyramidal symptoms compared with placebo (RR 2.26, 95% CI 1.58 to 3.23, n = 467; Analysis 1.5; Figure 16). The corresponding risk difference was 0.19 (95% CI 0.11 to 0.27, n = 467; NNTH: 5; Analysis 1.6).
1.3. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 3: Somnolence
15.

Forest plot (1.3 Somnolence)
1.4. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 4: Somnolence (RD)
1.5. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 5: Extrapyramidal symptoms
16.

Forest plot (1.5 Extrapyramidal symptoms)
1.6. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 6: Extrapyramidal symptoms (RD)
No study reported the number of participants with at least one adverse event and only one study reported serious adverse events (SAEs). Based on the low‐certainty evidence, typical antipsychotics may increase the risk of SAE slightly compared with placebo (RR 1.32, 95% CI 0.65 to 2.66, n = 193; Analysis 1.7). The corresponding risk difference was 0.04 (95% CI ‐0.06 to 0.14, n = 193; Analysis 1.8).
1.7. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 7: Any serious adverse events
1.8. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 8: Any serious adverse events (RD)
Death was reported in six studies, and in three of those studies no events occurred (Auchus 1997; Devanand 1998; Teri 2000). The low‐certainty evidence suggests that typical antipsychotics may increase the risk of mortality slightly (RR 1.46, 95% CI 0.54 to 4.00, n = 578; Analysis 1.9; Figure 17). The corresponding risk difference was 0.01 (95% CI ‐0.02 to 0.03, n = 578; Analysis 1.10).
1.9. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 9: Death
17.

Forest plot (1.9 Death)
1.10. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 10: Death (RD)
Secondary outcomes
We found moderate‐certainty evidence that typical antipsychotics probably increase the number of responders for agitation slightly (RR 1.18, 95% CI 1.01 to 1.38; I2 = 40%, n = 367; Analysis 1.11; Figure 18). The corresponding risk difference was 0.13 (95% CI 0.04 to 0.22; I2 = 62%, n = 367; NNTB = 7; Analysis 1.12). We found low‐certainty evidence that typical antipsychotics may increase the number of responders for psychosis slightly (RR 1.31, 95% CI 0.90 to 1.92, n = 259; Analysis 1.13; Figure 19). The corresponding risk difference was 0.09 (95% CI ‐0.03 to 0.20, n = 259; NNTB = 11; Analysis 1.14).
1.11. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 11: Number of responders for agitation
18.

Forest plot (1.11 Number of responders for agitation)
1.12. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 12: Number of responders for agitation (RD)
1.13. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 13: Number of responders for psychosis
19.

Forest plot (1.13 Number of responders for psychosis)
1.14. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 14: Number of responders for psychosis (RD)
We found low‐certainty evidence that typical antipsychotics may increase the risk of discontinuation due to adverse events (RR 1.70, 95% CI 1.02 to 2.82, n = 442; Analysis 1.15; Figure 20). The corresponding risk difference was 0.06 (95% CI 0.00 to 0.12; NNTH = 17; I2 = 44%, n = 442; 4 studies; Analysis 1.16). We found moderate‐certainty evidence that typical antipsychotics probably have little or no effect on discontinuation due to any reason (RR 1.16, 95% CI 0.89 to 1.51, n = 578; Analysis 1.17; Figure 21). The corresponding risk difference was 0.01 (95% CI ‐0.06 to 0.07; I2 = 36%, n = 578; Analysis 1.18).
1.15. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 15: Discontinuation due to adverse events
20.

Forest plot (1.15 Discontinuation due to adverse events)
1.16. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 16: Discontinuation due to adverse events (RD)
1.17. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 17: Discontinuation (any reason)
21.

Forest plot (1.17 Discontinuation, any reason)
1.18. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 18: Discontinuation (any reason) (RD)
No studies reported having measured health‐related quality of life. We found low‐certainty evidence that typical antipsychotics may improve functioning slightly (SMD 0.38, 95% CI 0.13 to 0.63, n = 249; Analysis 1.19; Figure 22). We found low‐certainty evidence that typical antipsychotics may have little (harmful) or no effect on cognitive functioning (MD ‐0.25 on Mini Mental State Examination (MMSE), 95% CI ‐1.27 to 0.77, n = 205; Analysis 1.20; Figure 23). For caregiver burden, we found low‐certainty evidence from one study that typical antipsychotics may have little (beneficial) or no effect on caregiver burden (MD 0.70, 95% CI ‐3.65 to 5.05, n = 70; Analysis 1.21). One very small study that was not included in this meta‐analysis (Auchus 1997) found an increase in caregiver stress in the typical antipsychotic and placebo group (14.0 and 18.6 on the Caregiver Strain Index (CSI), respectively, with a baseline mean of 165.4 and 116.2, respectively, n = 9).
1.19. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 19: Functioning (ADL)
22.

Forest plot (1.19 Functioning (ADL))
1.20. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 20: Cognitive function
23.

Forest plot (1.20 Cognitive function)
1.21. Analysis.

Comparison 1: Typical antipsychotics versus placebo, Outcome 21: Carer burden
Subgroup analysis for haloperidol
In the pre‐planned subgroup analysis for haloperidol, we found very low, low‐ and moderate‐certainty evidence for the different outcomes.
Efficacy
Haloperidol may reduce agitation slightly compared with placebo (SMD ‐0.29; 95% CI ‐0.51 to ‐0.07, n = 330; Analysis 2.1; Figure 24). We are uncertain whether typical antipsychotics improve psychosis compared to placebo (SMD ‐0.29; 05% CI ‐0.55 to ‐0.03, n = 240; Analysis 2.2; Figure 25).
2.1. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 1: Agitation
24.

Forest plot (2.1 Agitation)
2.2. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 2: Psychosis
25.

Forest plot (2.2 Psychosis)
Adverse events
We found that haloperidol probably increased the risk of somnolence compared with placebo (RR 2.62, 95% CI 1.51 to 4.56, n = 466; Analysis 2.3; Figure 26). Haloperidol may increase the risk of extrapyramidal symptoms (RR 2.33, 95% CI 0.90 to 6.01, n = 70; Analysis 2.4). No study reported the number of participants with any adverse events. For serious adverse events, we found that haloperidol may increase the risk of serious adverse events slightly compared with placebo (RR 1.32, 95% CI 0.65 to 2.66, n = 193; Analysis 2.5). Death was assessed in five studies and in three of those studies no events occurred (Auchus 1997; Devanand 1998; Teri 2000). Based on the other studies, haloperidol may increase the risk of mortality (RR 1.88, 95% CI 0.65 to 5.48, n = 545; Analysis 2.6; Figure 27).
2.3. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 3: Somnolence
26.

Forest plot (2.3 Somnolence)
2.4. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 4: Extrapyramidal symptoms
2.5. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 5: Serious adverse events
2.6. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 6: Death
27.

Forest plot (2.6 Death)
Secondary outcomes
Haloperidol may have little or no effect on the number of responders for agitation (RR 1.16, 95% CI 0.99 to 1.35, n = 334; Analysis 2.7; Figure 28). Haloperidol may increase the number of responders for psychosis compared with placebo slightly (RR 1.31, 95% CI 0.90 to 1.92, n = 259; Analysis 2.8; Figure 29).
2.7. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 7: Number of responders for agitation
28.

Forest plot (2.7 Number of responders for agitation)
2.8. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 8: Number of responders for psychosis
29.

Forest plot (2.8 Number of responders for psychosis)
Haloperidol may increase discontinuation due to adverse events compared with placebo (RR 1.81, 95% CI 1.08 to 3.03, n = 409; Analysis 2.9; Figure 30). Haloperidol probably has little or no effect on discontinuation due to any reason (RR 1.18, 95% CI 0.90 to 1.54, n = 545; Analysis 2.10; Figure 31).
2.9. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 9: Discontinuation due to adverse events
30.

Forest plot (2.9 Discontinuation due to adverse events)
2.10. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 10: Discontinuation (any reason)
31.

Forest plot (2.10 Discontinuation, any reason)
No study reported health‐related quality of life. Haloperidol may improve functioning slightly (SMD 0.38, 95% CI 0.13 to 0.63, n = 249; Analysis 2.11; Figure 32). Haloperidol may have little or no effect on cognitive functioning (MD ‐0.25 on MMSE, 95% CI ‐1.27 to 0.77, n = 205; Analysis 2.12; Figure 33). For caregiver burden, we are uncertain what the effect of haloperidol is (MD 0.70, 95% CI ‐3.65 to 5.05, n = 70; Analysis 2.13).
2.11. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 11: Functioning (ADL)
32.

Forest plot (2.11 Functioning (ADL))
2.12. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 12: Cognitive function
33.

Forest plot (2.12 Cognitive function)
2.13. Analysis.

Comparison 2: Haloperidol versus placebo, Outcome 13: Carer burden
Atypical antipsychotics versus placebo
Efficacy
Moderate‐certainty evidence indicates that atypical antipsychotics probably reduce agitation slightly compared with placebo (SMD ‐0.21, 95% CI ‐0.30 to ‐0.12, n = 1971; Analysis 3.1; Figure 34). Evidence from one study indicates that atypical antipsychotic (in this study risperidone) may reduce aggression slightly as well (SMD ‐0.38, 95% CI ‐0.61 to ‐0.15, n = 301; Analysis 3.1; Figure 34). Moderate‐certainty evidence indicated that atypical antipsychotics probably have a negligible effect on psychosis (SMD ‐0.11, 95% CI ‐0.18 to ‐0.03, n = 3364; Analysis 3.2; Figure 35).
3.1. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 1: Agitation
34.

Forest plot (3.1 Agitation)
3.2. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 2: Psychosis
35.

Forest plot (3.2 Psychosis)
Adverse events
We found high‐certainty evidence that atypical antipsychotics increase the risk of somnolence compared with placebo (RR 1.93, 95% CI 1.57 to 2.39, n = 3878; Analysis 3.3; Figure 36). The corresponding risk difference was 0.07 (95% CI 0.05 to 0.08; I2 = 65%, n = 3878; Analysis 3.4). We found moderate‐certainty evidence that atypical antipsychotics probably increase extrapyramidal symptoms slightly (RR 1.39, 95% CI 1.14 to 1.68, n = 4180; Analysis 3.5; Figure 37). The corresponding risk difference was 0.03 (95% CI 0.02 to 0.05, n = 4180; Analysis 3.6).
3.3. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 3: Somnolence
36.

Forest plot (3.3 Somnolence)
3.4. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 4: Somnolence (RD)
3.5. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 5: Extrapyramidal symptoms
37.

Forest plot (3.5 Extrapyramidal symptoms)
3.6. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 6: Extrapyramidal symptoms (RD)
We found moderate‐certainty evidence that atypical antipsychotics probably have a negligible effect on the risk of any adverse events (RR 1.05, 95% CI 1.02 to 1.09, n = 2785; Analysis 3.7; Figure 38). The corresponding risk difference was 0.05 (95% CI 0.02 to 0.07, n = 2785; Analysis 3.8). We found moderate‐certainty evidence that atypical antipsychotics probably increase the number of SAE slightly (RR 1.32, 95% CI 1.09 to 1.61, n = 4316; Analysis 3.9; Figure 39). The corresponding risk difference was 0.04 (95% CI 0.02 to 0.05, n = 4316; Analysis 3.10).
3.7. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 7: Any adverse event
38.

Forest plot (3.7 Any adverse event)
3.8. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 8: Any adverse event (RD)
3.9. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 9: Any serious adverse event
39.

Forest plot (3.9 Any serious adverse event)
3.10. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 10: Any serious adverse event (RD)
Moderate‐certainty evidence indicated that atypical antipsychotics probably increase mortality slightly (RR 1.36, 95% CI 0.90 to 2.05, n = 5032; Analysis 3.11; Figure 40). The corresponding risk difference was 0.01 (95% CI ‐0.00 to 0.02, n = 5032; Analysis 3.12).
3.11. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 11: Death
40.

Forest plot (3.11 Death)
3.12. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 12: Death (RD)
Secondary outcomes
Moderate‐certainty evidence indicated that atypical antipsychotics probably increase the number of responders for agitation slightly (RR 1.31, 95% CI 1.16 to 1.48, n = 1304; Analysis 3.13; Figure 41). The corresponding risk difference was 0.13 (95% CI 0.08 to 0.18, n = 1304; Analysis 3.14). Low‐certainty evidence indicated that atypical antipsychotics may increase the risk of response for psychosis slightly compared with placebo (RR 1.13, 95% CI 1.03 to 1.23; I2= 60%, n = 1958; Analysis 3.15; Figure 42). The corresponding risk difference was 0.08 (95% CI 0.04 to 0.13, n = 1958; Analysis 3.16).
3.13. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 13: Number of responders for agitation
41.

Forest plot (3.13 Number of responders for agitation)
3.14. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 14: Number of responders for agitation (RD)
3.15. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 15: Number of responders for psychosis
42.

Forest plot (3.15 Number of responders for psychosis)
3.16. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 16: Number of responders for psychosis (RD)
Moderate‐certainty evidence indicated that atypical antipsychotics probably increase discontinuation due to adverse events slightly (RR 1.41, 95% CI 1.15 to 1.72, n = 5058; Analysis 3.17; Figure 43). The corresponding risk difference was 0.04 (95% CI 0.02 to 0.06, n = 5058; Analysis 3.18). Low‐certainty evidence indicated that atypical antipsychotics may have little or no effect on discontinuation for any reason (RR 0.95, 95% CI 0.89 to 1.01, n = 5095; Analysis 3.19; Figure 44). The corresponding risk difference was ‐0.00 (95% CI ‐0.02 to 0.02, n = 5095; Analysis 3.20).
3.17. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 17: Discontinuation due to adverse events
43.

Forest plot (3.17 Discontinuation due to adverse events)
3.18. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 18: Discontinuation due to adverse events (RD)
3.19. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 19: Discontinuation (any reason)
44.

Forest plot (3.19 Discontinuation, any reason)
3.20. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 20: Discontinuation (any reason) (RD)
We found moderate‐certainty evidence that atypical antipsychotics probably reduce functioning slightly (SMD ‐0.21, 95% CI ‐0.39 to ‐0.03, n = 514; Analysis 3.21; Figure 45), but probably have little or no effect on cognitive function compared with placebo (SMD ‐0.10, 95% CI ‐0.19 to ‐0.02, n = 2698; Analysis 3.22; Figure 46). One small study that was not included in the meta‐analysis also found little or no effect of atypical antipsychotics on cognitive function (MD ‐1.40 on MMSE; 95% CI ‐5.89 to 3.09, n = 38; Analysis 3.23; Paleacu 2008). Based on very‐low‐certainty evidence from another study, we are uncertain whether atypical antipsychotics have an effect on health‐related quality of life (MD 0.95, 95% CI ‐4.14 to 6.04, n = 151 assessed with Alzheimer's Disease‐Related Quality of Life (ADRQL), range 0 to 100; Analysis 3.24; Schneider 2006 CATIE‐AD). For the time that caregivers spend providing care (mean change in hours/day) we found low‐certainty evidence from one study that atypical antipsychotics may have little or no effect compared with placebo (MD 0.08, 95% CI ‐1.39 to 1.55, n = 151; Analysis 3.25; Schneider 2006 CATIE‐AD).
3.21. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 21: Functioning (ADL)
45.

Forest plot (3.21 Functioning (ADL))
3.22. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 22: Cognitive function
46.

Forest plot (3.22 Cognitive function)
3.23. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 23: Cognitive function (single study)
3.24. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 24: Health‐related quality of life
3.25. Analysis.

Comparison 3: Atypical antipsychotics versus placebo, Outcome 25: Time spend providing care (caregiver)
Subgroup analysis for risperidone
In the pre‐planned subgroup analysis for risperidone, we found very low, low‐ and moderate‐certainty evidence for the different outcomes.
Efficacy
Risperidone may slightly reduce agitation compared to placebo (SMD ‐0.26, 95 % CI ‐0.44 to ‐0.09, n = 524; Analysis 4.1; Figure 47). Risperidone probably has little or no effect on psychosis (SMD ‐0.11, 95% CI ‐0.23 to 0.01, n = 1205; Analysis 4.2; Figure 48).
4.1. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 1: Agitation
47.

Forest plot (4.1 Agitation)
4.2. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 2: Psychosis
48.

Forest plot (4.2 Psychosis)
Adverse events
Risperidone probably increases the risk of somnolence compared with placebo (RR 3.35, 95% CI 1.99 to 5.65, n = 700; Analysis 4.3; Figure 49). Risperidone may increase extrapyramidal symptoms (RR 1.75, 95% CI 1.32 to 2.33, n = 1328; Analysis 4.4; Figure 50). Risperidone probably increase the risk of any adverse events slightly (RR 1.19, 95% CI 1.07 to 1.32, n = 700; Analysis 4.5; Figure 51). Risperidone may increase the risk of SAE slightly too (RR 1.21, 95% CI 0.88 to 1.67, n = 1085; Analysis 4.6; Figure 52) and the risk of mortality (RR 1.29, 95% CI 0.64 to 2.60, n = 1298; Analysis 4.7; Figure 53).
4.3. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 3: Somnolence
49.

Forest plot (4.3 Somnolence)
4.4. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 4: Extrapyramidal symptoms
50.

Forest plot (4.4 Extrapyramidal symptoms)
4.5. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 5: Any adverse event
51.

Forest plot (4.5 Any adverse event)
4.6. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 6: Any serious adverse event
52.

Forest plot (4.6 Any serious adverse event)
4.7. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 7: Death
53.

Forest plot (4.7 Death)
Secondary outcomes
Risperidone probably increases the number of responders for agitation slightly compared with placebo (RR 1.61, 95% CI 1.29 to 2.01, n = 572; Analysis 4.8; Figure 54), but may have little or no effect on the number of responders for psychosis compared with placebo (RR 1.05, 95% CI 0.93 to 1.19, n = 781; Analysis 4.9; Figure 55).
4.8. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 8: Number of responders for agitation
54.

Forest plot (4.8 Number of responders for agitation)
4.9. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 9: Number of responders for psychosis
55.

Forest plot (4.9 Number of responders for psychosis)
Risperidone may slightly increase discontinuation due to adverse events compared with placebo (RR 1.60, 95% CI 1.13 to 2.27, n = 1349; Analysis 4.10; Figure 56), but probably has little or no effect on discontinuation for any reason compared with placebo (RR 0.95, 95% CI 0.85 to 1.07, n = 1383; Analysis 4.11; Figure 57).
4.10. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 10: Discontinuation due to adverse events
56.

Forest plot (4.10 Discontinuation due to adverse events)
4.11. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 11: Discontinuation (any reason)
57.

Forest plot (4.11 Discontinuation, any reason)
Risperidone probably has little or no effect on cognitive function compared with placebo (MD ‐0.31 on MMSE, 95% CI ‐1.04 to 0.41, n = 353; Analysis 4.12; Figure 58). Given the evidence from one study, we are uncertain whether risperidone has an effect on functioning (MD ‐1.60, 95% CI ‐5.44 to 2.24, n = 80; Analysis 4.13), on health‐related quality of life (MD ‐2.00, 95% CI ‐8.12 to 4.12, assessed with ADRQL, range 0 to 100, n = 80; Analysis 4.14), or on the time that a caregiver spend on providing care (MD ‐0.50 hours/day, 95% CI ‐2.49 to 1.49, n = 80; Analysis 4.15).
4.12. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 12: Cognitive function
58.

Forest plot (4.12 Cognitive function)
4.13. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 13: Functioning (ADL)
4.14. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 14: Health‐related quality of life
4.15. Analysis.

Comparison 4: Risperidone versus placebo, Outcome 15: Time spend providing care (caregiver)
Subgroup analysis for quetiapine
We performed a post‐hoc subgroup analysis for quetiapine, and we found very‐ low, low‐ and moderate‐certainty evidence for the different outcomes.
Efficacy
Quetiapine may have little or no effect on agitation (SMD ‐0.14, 95% CI ‐0.31 to 0.02, n = 615; Analysis 5.1; Figure 59) and probably has little or no effect on psychosis (SMD 0.05, 95% CI ‐0.22 to 0.31, n = 220; Analysis 5.2; Figure 60).
5.1. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 1: Agitation
59.

Forest plot (5.1 Agitation)
5.2. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 2: Psychosis
60.

Forest plot (5.2 Psychosis)
Adverse events
Quetiapine probably increases the risk of somnolence compared with placebo (RR 4.83, 95% CI 2.73 to 8.57, n = 798; Analysis 5.3; Figure 61). Quetiapine may have little or no effect on extrapyramidal symptoms (RR 0.94, 95% CI 0.52 to 1.70, n = 799; Analysis 5.4; Figure 62). Quetiapine probably has little or no effect on any adverse events (RR 1.03, 95% CI 0.93 to 1.14, n = 609; Analysis 5.5; Figure 63), but may increase the risk of SAE slightly compared with placebo (RR 1.32, 95% CI 0.86 to 2.03, n = 799; Analysis 5.6; Figure 64). Quetiapine may also increase the risk of mortality (RR 1.48, 95% CI 0.67 to 3.31, n = 861; Analysis 5.7; Figure 65).
5.3. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 3: Somnolence
61.

Forest plot (5.3 Somnolence)
5.4. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 4: Extrapyramidal symptoms
62.

Forest plot (5.4 Extrapyramidal symptoms)
5.5. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 5: Any adverse event
63.

Forest plot (5.5 Any adverse event)
5.6. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 6: Any serious adverse event
64.

Forest plot (5.6 Any serious adverse event)
5.7. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 7: Death
65.

Forest plot (5.7 Death)
Secondary outcomes
Quetiapine probably increases the number of responders for agitation slightly compared with placebo (RR 1.35, 95% CI 1.02 to 1.78, n = 569; Analysis 5.8; Figure 66), but may have little or no effect on the number of responders for psychosis (RR 1.03, 95% CI 0.7 to 1.36, n = 230; Analysis 5.9; Figure 67).
5.8. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 8: Number of responders for agitation
66.

Forest plot (5.8 Number of responders for agitation)
5.9. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 9: Number of responders for psychosis
67.

Forest plot (5.9 Number of responders for psychosis)
Quetiapine may slightly increase discontinuation due to adverse events compared with placebo (RR 1.37, 95% CI 0.89 to 2.11, n = 858; Analysis 5.10; Figure 68), but probably has little or no effect on discontinuation for any reason compared with placebo (RR 0.97, 95% 0.88 to 1.08, n = 861; Analysis 5.11; Figure 69).
5.10. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 10: Discontinuation due to adverse events
68.

Forest plot (5.10 Discontinuation due to adverse events)
5.11. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 11: Discontinuation (any reason)
69.

Forest plot (5.11 Discontinuation, any reason)
Quetiapine probably has little or no effect on functioning (SMD ‐0.17, 95% CI ‐0.42 to 0.07, n = 258; Analysis 5.12; Figure 70). Quetiapine probably has little or no effect on cognitive function compared with placebo (SMD ‐0.10, 95% CI ‐0.28 to 0.07, n = 584; Analysis 5.13; Figure 71; and results from one small study that was not included in the meta‐analysis MD ‐1.40 on MMSE; 95% CI ‐5.89 to 3.09, n = 38; Analysis 5.14; Paleacu 2008). Given evidence from one study, we are uncertain whether quetiapine has an effect on health‐related quality of life (MD 2.60, 95% CI ‐3.51 to 8.71, assessed with ADRQL, range 0 to 100, n = 78; Analysis 5.15), or on the time that a caregiver spend on providing care (MD ‐0.10 hours/day, 95% CI ‐2.01 to 1.81, n = 78; Analysis 5.16).
5.12. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 12: Functioning (ADL)
70.

Forest plot (5.12 Functioning (ADL))
5.13. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 13: Cognitive function
71.

Forest plot (5.13 Cognitive function)
5.14. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 14: Cognitive function (single study)
5.15. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 15: Health‐related quality of life
5.16. Analysis.

Comparison 5: Quetiapine versus placebo, Outcome 16: Time spend providing care (caregiver)
Discussion
In this systematic review, we included 24 randomised trials that tested the effect of one or more antipsychotics on agitation or psychosis in persons with dementia.
Summary of main results
We identified five trials that tested haloperidol and one that tested thiothixene. The pooled results indicate that typical antipsychotics might improve psychosis slightly compared with placebo, while the effect on agitation is uncertain. These drugs probably increase the risk of somnolence and extrapyramidal symptoms. There was no evidence regarding the risk of at least one adverse event, and a slight increase in the risk of a serious adverse event (SAE) or death. The effect estimates for haloperidol were in line with those of the drug class, although haloperidol may have a small effect on agitation.
In addition, we identified 20 trials that tested one of following atypical antipsychotics: aripiprazole, brexpiprazol, olanzapine, pimavanserin, quetiapine, risperidone, and tiapride. Atypical antipsychotics had a negligible effect on psychosis and a slight effect on agitation. These drugs probably increase the risk of somnolence and extrapyramidal symptoms. They probably increase the risk of any adverse event. The risk of a serious adverse event and the risk of death are slightly increased. The findings from seven trials for risperidone were in line with those for the drug class.
Overall completeness and applicability of evidence
There is low certainty about the effect of typical antipsychotics on psychosis in dementia, due to a small number of studies (only two studies). The number of studies and the number of participants included in the studies about the effect of typical antipsychotics on agitation were too small to provide a precise estimate (four studies). These drugs were developed when trials were not performed and published as often as nowadays. It is unlikely that new trials with haloperidol will be performed soon. The lack of evidence is unfortunate because the current estimates with high upper limit of the confidence intervals does not preclude the possibility that typical antipsychotics have a larger/more substantial effect on psychosis and moderate effect on agitation. Moreover, old studies used high doses of haloperidol that may have negatively affected the balance between beneficial and harmful effects. Compared to studies on atypical antipsychotics, studies on typical antipsychotics often lack elaborate documentation of adverse events. This is most probably due to the fact that most studies are fairly old and were conducted when reporting of adverse events was less regulated by marketing authorities.
In contrast, there was a large number of studies that tested the effect of atypical antipsychotics on psychosis and agitation in dementia (12 and eight studies, respectively), and most studies were relatively large. As a result, the effect estimates are very precise and give certainty that these drugs only have a small effect on agitation and little or no effect on psychosis. With mean SMDs of ‐0.21 and ‐0.11 for agitation and psychosis, respectively, one could seriously question the clinical relevance, as SMDs of 0.20 are defined by Cohen (Cohen 1988; Cohen 1992) as so small that they are not visible to the naked eye.
The negative results of this review for antipsychotics in general and more specific for haloperidol, risperidon, and quetiapine seem to contradict the widespread use of antipsychotics. There may be a number of explanations. First, patients treated with antipsychotics in daily practice may have more severe symptoms than patients enrolled in the trials in this review. In one trial among patients with aggression, i.e. severe agitation, the effect of risperidone was somewhat greater (SMD ‐0.38: ‐0.61 to ‐0.15) (Brodaty 2003 RIS‐AUS‐05) than that on agitation in the other six trials (SMD ‐0.18; ‐0.28 to ‐0.08). In these six trials, the severity of agitation at baseline varied. In the two trials among populations with the highest baseline agitation (given the range of the scale used), tiapride showed a somewhat greater effect as well (SMD ‐0.39: ‐0.66 to ‐0.11), but quetiapine did not (SMD ‐0.15; ‐0.40 to 0.09) (Allain 2000; Zhong 2007).
Secondly, hospitalised and institutionalised patients with severe and dangerous symptoms may have delirium, whether or not superimposed on dementia. Delirium is often missed (Wahid 2004), and most of the trials in our review did not exclude patients with delirium explicitly. As efficacy of antipsychotics is very low in delirium (Neufeld 2019), missed deliriums may explain a part of the negative results.
Thirdly, the relationship of dosage with efficacy and AEs is also relevant. Studies of typical antipsychotics allowed doctors to prescribe relatively high doses if deemed necessary. Hence, the doses cannot explain that our review found only a slight effect on agitation and psychosis. On the other hand, studies about atypical antipsychotics sometimes used relatively low maximum (fixed or flexible) doses. This might explain the lack of efficacy, although a previous review did not show dose‐response effects (Ballard 2006). The differences in dosing between the drug classes may explain the apparent difference in risk of AEs between them.
Quality of the evidence
A limitation of the evidence is the patients enrolled in the identified trials were not representative of all patients for which antipsychotics are considered. First, the studies did not include many patients with vascular dementia. In addition, it is unclear whether the studies recruited patients who had not responded to non‐pharmacological measures. Such measures are recommended by many guidelines as the treatment of first choice. Finally, the use of strict exclusion criteria in most trials may have decreased the representativeness of the study populations. For instance, some studies excluded patients with terminal disease.
Another limitation is that none of the included studies scored low risk of bias on all items. Randomisation procedures were frequently described poorly with just five studies reporting the method of sequence generation and three studies the method of concealment allocation. Despite randomisation, five studies showed clinically relevant baseline differences between groups that might have biased the results of the studies. Blinding procedures were not described well either. Just seven studies reported the explicit blinding of patients and personnel and/or outcome assessors. In the other studies, this was implied by the use of placebo. At least half of the studies showed a high risk of bias due to incomplete data, selective reporting, and commercial funding. Given the often unclear or high risk of bias, it cannot be ruled out that the reported effects of the drugs on agitation and psychosis were overestimated, and the risks of adverse events underestimated, and so were the pooled estimates that we present.
Although uncommon in Cochrane Reviews, we assessed the use of a run‐in period, because it can affect the validity of the study results for the population of interest (as defined in the PICO). A run‐in period takes place between screening and randomisation. Prohibited drugs or investigated drugs already in use can be washed out, a placebo can be given to identify placebo‐responders, and sometimes the investigated drug is given. Participants are sometimes blinded, but clinical assessors and investigators are usually not blinded. Hence, participants that respond favourably to wash‐out or placebo or unfavourably to introduction of an active drug can be deselected at the end of a run‐in period. Use of a run‐in period in trials about antipsychotics and antidepressants has been associated with overestimated treatment effects, and especially underestimated risks of adverse events (Bridge 2007; Hulshof 2020; Khan 1989). Most of the studies included in our review used a run‐in period, and so bias may have been introduced.
Potential biases in the review process
We found three studies that have not been published in a journal. Therefore, these studies lacked external peer review as a quality criterion. Although we included many outcomes and did not correct for multiple testing, we do not think that this introduced bias because the conclusions of our review are mostly negative for beneficial effects, and positive for harmful effects.
Agreements and disagreements with other studies or reviews
One prior review assessed the effect of haloperidol on agitation in dementia (Lonergan 2002). The search was updated in 2010 and yielded no new evidence. It reported a negligible effect on agitation (SMD ‐0.12; 95% CI ‐0.31 to 0.08), and a small effect on aggression (SMD ‐0.31; 95% CI‐0.49 to ‐0.13). This review included two trials among patients with diverse neuropsychiatric symptoms (DeDeyn 1999; Devanand 1998), which we excluded. The inclusion of these two trials might have diluted the effect of conventional antipsychotics on agitation. We found no published reviews of conventional antipsychotics on psychosis outcomes to compare to our results.
Another review of trials has addressed the mortality risk of typical antipsychotics in older patients (Hulshof 2015). It included 14 trials among patients with dementia (independent of type of neuropsychiatric symptoms) and three trials among patients with delirium. No increased risk of mortality was found: RR 1.07 (95% CI 0.54 to 2.13) and RD 0.1% (95% CI: ‐1.0% to 1.2%). The higher number of included trials could explain the difference with our results based on three trials: a slightly increased but imprecisely estimated risk of mortality (RR 1.46, 95% CI 0.54 to 4.00).
Two reviews studied the effect of atypical antipsychotics on behavioural symptoms in patients with dementia (Ballard 2006; Ma 2014). These reviews differentiated between individual antipsychotics and doses, which complicates direct comparison with our results. Nevertheless, those reviews reported modest effects on aggression (with or without other forms of agitation) and on psychosis. Around half of the trials pooled in those reviews had been conducted in patients with diverse neuropsychiatric symptoms, which we excluded, and that approach might have led to overestimated efficacy (Smeets 2018). A recent network meta‐analysis found that there was no single most effective and safe atypical drug (Yunusa 2019).
Some reviews of trials about atypical antipsychotics addressed particular serious harms. Two reviews have reported an identical increased risk of mortality with data from 15 trials (OR 1.54; 95% CI 1.06‐2.23) (Schneider 2005; Yeh 2019). According to these results, one in 100 patients treated with an atypical drug dies due to the drug use. We used data from 17 trials, but the estimate was less precise (RR 1.36; 95% CI 0.90‐2.05), probably because we excluded a number of older and larger trials most of which showed an increased risk. These trials did not enrol patients with agitation or psychosis specifically. For the outcome mortality, it seems justifiable to include these trials, and the prior reviews provided valid evidence (Schneider 2005; Yeh 2019).
In response to the 2005 review about the mortality risk of atypical antipsychotics (Schneider 2005), many observational studies about the mortality risk of typical antipsychotics were performed. The studies showed that use of typical antipsychotics and especially haloperidol was related to an even higher increased risk of death (Luijendijk 2016). In these studies, sicker and older patients received typical antipsychotics more often than atypical antipsychotics. The risk of dying was especially high in the first month of use, and when haloperidol was administered per injection or in high doses. However, none of the observational studies, all based on retrospective analyses of administrative data, had been adjusted for terminal illness (Luijendijk 2016). This could have resulted in an overestimated risk of mortality for typical antipsychotics versus atypical antipsychotics or no antipsychotic use.
Authors' conclusions
Implications for practice.
There is some evidence that typical antipsychotics may decrease psychosis somewhat and that haloperidol may decrease agitation somewhat in patients with dementia. The increased risk of somnolence and other possible adverse events, should urge doctors to be reluctant to prescribe these drugs. Atypical antipsychotics are not effective for psychosis in dementia, and should not be used for this indication. The use of these atypical antipsychotic drugs should be kept to a minimum for agitation in dementia as well: these drugs may decrease agitation somewhat, but they also increase the risk of somnolence, extrapyramidal symptoms (EPS), any adverse events and serious adverse events.
Overall, the evidence shows that most of the decrease in agitation and psychosis seen in the drug groups can be attributed to a favourable natural course of these symptoms, as observed in the placebo groups. This finding explains the apparent effectiveness of the drugs seen in daily practice.
Due to the unfavourable balance between beneficial effects and risks of adverse events for both typical and atypical antipsychotics, it is advised to look at alternative therapeutic options. Treatment of the underlying possible psychosocial and somatic causes of agitation or psychosis should always be considered (Leng 2020; Livingston 2014a; Livingston 2014b). If antipsychotics are considered for sedation in patients with severe and dangerous symptoms nonetheless, and somnolence is the intended outcome, this should be discussed openly with the patient and legal representative.
Implications for research.
The precise effect of haloperidol on psychosis and agitation in dementia is still unclear, and should be studied in sufficiently large well‐designed trials. This also applies to some other frequently used typical antipsychotics such as pipamperon and zuclopentixol, and perhaps the relatively new atypical antipsychotic pimavanserin. Future studies should be carefully designed to address the interaction with co‐medications and non‐pharmacological interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2022 | Amended | Funnel plots added |
History
Protocol first published: Issue 4, 2019 Review first published: Issue 12, 2021
Acknowledgements
We are grateful for the support of the Cochrane Dementia and Cognitive Improvement Group, especially Anna Noel‐Storr for her help with the title and abstract screening. Also, we acknowledge the contribution to former versions of this review by K Riemann‐Lorenz, and E Wolschon, and the help of (PhD‐)students MKA Almaghlouth, S Galama, T Hulshof, and C Smeets. We thank Denise Mitchell for her help with the plain language summary.
We would like to thank peer reviewers Donovan Maust and Susie Shenkin and consumer reviewer Kit Byatt for their comments and feedback.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. CENTRAL (the Cochrane Library) http://crso.cochrane.org/SearchSimple.php [most recent search date: 7 January 2021] | #1 MESH DESCRIPTOR Dementia EXPLODE ALL TREES 5139 #2 MESH DESCRIPTOR Delirium 519 #3 MESH DESCRIPTOR Wernicke Encephalopathy 4 #4 MESH DESCRIPTOR Neurocognitive Disorders 151 #5 dement*:TI,AB,KY 11330 #6 alzheimer*:TI,AB,KY 10070 #7 (lewy* adj2 bod*):TI,AB,KY 388 #8 (chronic adj2 cerebrovascular):TI,AB,KY 107 #9 ("organic brain disease" or "organic brain syndrome"):TI,AB,KY 133 #10 ("benign senescent forgetfulness"):TI,AB,KY 2 #11 (cerebr* adj2 deteriorat*):TI,AB,KY 10 #12 (cerebral* adj2 insufficient*):TI,AB,KY 1 #13 ("major neurocognitive disorder"):TI,AB,KY 18 #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 18343 #15 MESH DESCRIPTOR Antipsychotic Agents 4224 #16 (antipsychotic* or neuroleptic*):TI,AB,KY 10065 #17 (neurolept* or antipsychotic* or Amisulpride* or Chormethiazole* or Clomethiazole* or Distraneurin* or Chlorpromazin* or Aminazine* or Chlorazine* or Chlordelazine* or Contomin* or Fenactil* or Largactil* or Propaphenin* or Thorazine* or Flupenthixol decanoate* or Emergil* or Fluanxol* or Flupentixol* or alphaFlupenthixol* or cisFlupenthixol* or Fluphenazin* or Fluphenazine decanoate* or Flufenazin* or Fluphenazine Hydrochloride* or Lyogen* or Prolixin* or Haloperidol* or Haldol* or Levomepromazin* or Levomeprazin* or Levopromazine* or Tisercin* or Tizercine* or Tizertsin* or Methotrimeprazine* or Loxapine* or Loxapinsuccinate* or Oxilapine* or Cloxazepine* or Loxapine Monohydrochloride* or Loxipine Maleate* or Loxipine Succinate* or Loxitane* or Asendin* or Desmethylloxapine* or Amoxapine* or Olanzapine* or Perphenazine* or Chlorpiprazine* or Perfenazine* or Trilafonor* or Pimozide* or Prothipendyl* or Quetiapine* or Fumarate* or Risperidone* or Risperidal* or Sulpiride* or Dogmatil* or Eglonyl* or Sulperide* or Thioridazine* or Meleril* or Mellaril* or Melleril* or Melleryl* or Sonapax* or Thioridazine Hydrochloride* or Tiaprid* or Tiapridal* or Trifluoperazine Hydrochloride* or Trifluoroperazine* or Triftazin* or Stelazine* or "Trifluperazine*or Tripfluoperazine Hydrochloride* or Cisordinol*" or Zuclopenthixol* or Clopenthixol* or Clozapine*or Melperone hydrochloride* or Ziprasidone* or Zotemine*):TI,AB,KY 20326 #18 MESH DESCRIPTOR PIMOZIDE 103 #19 MESH DESCRIPTOR PERPHENAZINE 189 #20 MESH DESCRIPTOR LOXAPINE 60 #21 MESH DESCRIPTOR METHOTRIMEPRAZINE 33 #22 MESH DESCRIPTOR HALOPERIDOL 1312 #23 MESH DESCRIPTOR FLUPHENAZINE 278 #24 MESH DESCRIPTOR FLUPENTHIXOL 105 #25 MESH DESCRIPTOR CHLORPROMAZINE 577 #26 MESH DESCRIPTOR CHLORMETHIAZOLE 58 #27 MESH DESCRIPTOR CLOPENTHIXOL 53 #28 MESH DESCRIPTOR TRIFLUOPERAZINE 110 #29 MESH DESCRIPTOR THIORIDAZINE 187 #30 MESH DESCRIPTOR SULPIRIDE 309 #31 MESH DESCRIPTOR RISPERIDONE 1245 #32 MESH DESCRIPTOR FUMARATES 235 #33 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 20431 #34 MESH DESCRIPTOR Psychotic Disorders EXPLODE ALL TREES 2574 #35 Psychoses:TI,AB,KY 558 #36 Psychosis:TI,AB,KY 5361 #37 MESH DESCRIPTOR VIOLENCE EXPLODE ALL TREES 1492 #38 MESH DESCRIPTOR HOSTILITY EXPLODE ALL TREES 265 #39 MESH DESCRIPTOR Irritable Mood EXPLODE ALL TREES 135 #40 MESH DESCRIPTOR Impulsive Behavior EXPLODE ALL TREES 1040 #41 Paranoid Behavior 7 #42 Agitat*:TI,AB,KY 4112 #43 aggress*:TI,AB,KY 9491 #44 violen*:TI,AB,KY 2668 #45 impuls*:TI,AB,KY 3638 #46 irritabl*:TI,AB,KY 3680 #47 hostil*:TI,AB,KY 1225 #48 anger:TI,AB,KY 1992 #49 angry:TI,AB,KY 418 #50 anti‐social:TI,AB,KY 26 #51 impuls*:TI,AB,KY 3638 #52 Restless*:TI,AB,KY 2146 #53 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 33621 #54 #14 AND #33 AND #53 453 |
Oct 2018: 396 Oct 2019: 453 March 2020: 83 Jan 2021: 37 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [most recent search date: 7 January 2021] |
1 exp Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Neurocognitive Disorders/ 5 dement*.mp. 6 alzheimer*.mp. 7 (lewy* adj2 bod*).mp. 8 (chronic adj2 cerebrovascular).mp. 9 ("organic brain disease" or "organic brain syndrome").mp. 10 "benign senescent forgetfulness".mp. 11 (cerebr* adj2 deteriorat*).mp. 12 (cerebral* adj2 insufficient*).mp. 13 "major neurocognitive disorder".mp. 14 or/1‐13 15 Antipsychotic Agents/ 16 (antipsychotic* or neuroleptic*).ti,ab. 17 (neurolept* or antipsychotic* or alphaFlupenthixol* or Aminazine* or Amisulpride* or Amoxapine* or Aripiprazole or Asenapine maleate or Asendin* or Benperidol or Benzamides or Brexpiprazole or Butyrophenones or Chlorazine* or Chlordelazine* or Chlorpiprazine* or Chlorpromazin* or Chormethiazole* or cisFlupenthixol* or Cisordinol* or Clomethiazole* or Clopenthixol* or Cloxazepine* or Clozapine*OR Contomin* or Desmethylloxapine* or Diphenylbutylpiperidines or Distraneurin* or Dogmatil* or Eglonyl* or Emergil* or Fenactil* or Fluanxol* or Flufenazin* or Flupenthixol decanoate* or Flupentixol decanoate or Flupentixol* or Fluphenazin* or Fluphenazine decanoate* or Fluphenazine Hydrochloride* or Fumarate* or Haldol* or Haloperidol* or Iloperidone or Largactil* or Levomeprazin* or Levomepromazin* or Levopromazine* or Loxapine Monohydrochloride* or Loxapine* or Loxapinsuccinate* or Loxipine Maleate* or Loxipine Succinate* or Loxitane* or Lurasidone or Lyogen* or Meleril* or Mellaril* or Melleril* or Melleryl* or Melperone hydrochloride* or Methotrimeprazine* or Nalotenserin or Norquetiapine or Olanzapine embonate or Olanzapine* or Oxilapine* or Paliperidone or Perfenazine* or Pericyazine or Perphenazine* or Phenothiazine or Pimavanserin or Pimozide* or Prochlorperazine or Prolixin* or Promazine hydrochloride or Propaphenin* or Prothipendyl* or Quetiapine* or Risperidal* or Risperidone* or Sertindole or Sonapax* or Stelazine* or Sulperide* or Sulpiride* or Thioridazine Hydrochloride* or Thioridazine* or Thiothixene or Thioxanthenes or Thorazine* or Tiaprid* or Tiapridal* or Tisercin* or Tizercine* or Tizertsin* or Trifluoperazine or Trifluoroperazine* or Trifluperazine*OR Triftazin* or Trilafonor* or Tripfluoperazine Hydrochloride* or Ziprasidone* or Zotemine* or Zotepine or Zuclopenthixol or Zuclopenthixol*).ti,ab. 18 PIMOZIDE/ 19 PERPHENAZINE/ 20 LOXAPINE/ 21 METHOTRIMEPRAZINE/ 22 HALOPERIDOL/ 23 FLUPHENAZINE/ 24 FLUPENTHIXOL/ 25 CHLORPROMAZINE/ 26 CHLORMETHIAZOLE/ 27 CLOPENTHIXOL/ 28 TRIFLUOPERAZINE/ 29 THIORIDAZINE/ 30 SULPIRIDE/ 31 RISPERIDONE/ 32 FUMARATES/ 33 or/15‐32 34 14 and 33 35 exp Psychotic Disorders/ 36 Psychoses.ti,ab. 37 Psychosis.ti,ab. 38 exp VIOLENCE/ 39 exp HOSTILITY/ 40 exp Irritable Mood/ 41 exp Impulsive Behavior/ 42 exp Paranoid Behavior/ 43 Agitat*.ti,ab. 44 aggress*.ti,ab. 45 violen*.ti,ab. 46 impuls*.ti,ab. 47 irritabl*.ti,ab. 48 hostil*.ti,ab. 49 anger.ti,ab. 50 angry.ti,ab. 51 anti‐social.ti,ab. 52 impuls*.ti,ab. 53 Restless*.ti,ab. 54 or/35‐53 55 34 and 54 56 randomized controlled trial.pt. 57 controlled clinical trial.pt. 58 randomized.ab. 59 placebo.ab. 60 drug therapy.fs. 61 randomly.ab. 62 trial.ab. 63 groups.ab. 64 or/56‐63 65 exp animals/ not humans.sh. 66 64 not 65 67 55 and 66 |
Oct 2018: 1382 Oct 2019: 100 March 2020: 69 Jan 2021:Jan 2021: 50 |
| 3. Embase 1974 to present [most recent search date: 7 January 2021] |
1 Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 6 "major neurocognitive disorder".ti,ab. 7 or/1‐6 8 neuroleptic agent/ 9 (antipsychotic* or neuroleptic*).ti,ab. 10 (neurolept* or antipsychotic* or alphaFlupenthixol* or Aminazine* or Amisulpride* or Amoxapine* or Aripiprazole or Asenapine maleate or Asendin* or Benperidol or Benzamides or Brexpiprazole or Butyrophenones or Chlorazine* or Chlordelazine* or Chlorpiprazine* or Chlorpromazin* or Chormethiazole* or cisFlupenthixol* or Cisordinol* or Clomethiazole* or Clopenthixol* or Cloxazepine* or Clozapine*OR Contomin* or Desmethylloxapine* or Diphenylbutylpiperidines or Distraneurin* or Dogmatil* or Eglonyl* or Emergil* or Fenactil* or Fluanxol* or Flufenazin* or Flupenthixol decanoate* or Flupentixol decanoate or Flupentixol* or Fluphenazin* or Fluphenazine decanoate* or Fluphenazine Hydrochloride* or Fumarate* or Haldol* or Haloperidol* or Iloperidone or Largactil* or Levomeprazin* or Levomepromazin* or Levopromazine* or Loxapine Monohydrochloride* or Loxapine* or Loxapinsuccinate* or Loxipine Maleate* or Loxipine Succinate* or Loxitane* or Lurasidone or Lyogen* or Meleril* or Mellaril* or Melleril* or Melleryl* or Melperone hydrochloride* or Methotrimeprazine* or Nalotenserin or Norquetiapine or Olanzapine embonate or Olanzapine* or Oxilapine* or Paliperidone or Perfenazine* or Pericyazine or Perphenazine* or Phenothiazine or Pimavanserin or Pimozide* or Prochlorperazine or Prolixin* or Promazine hydrochloride or Propaphenin* or Prothipendyl* or Quetiapine* or Risperidal* or Risperidone* or Sertindole or Sonapax* or Stelazine* or Sulperide* or Sulpiride* or Thioridazine Hydrochloride* or Thioridazine* or Thiothixene or Thioxanthenes or Thorazine* or Tiaprid* or Tiapridal* or Tisercin* or Tizercine* or Tizertsin* or Trifluoperazine or Trifluoroperazine* or Trifluperazine*OR Triftazin* or Trilafonor* or Tripfluoperazine Hydrochloride* or Ziprasidone* or Zotemine* or Zotepine or Zuclopenthixol or Zuclopenthixol*).ti,ab. 11 pimozide/ 12 perphenazine/ 13 loxapine/ 14 levomepromazine/ 15 haloperidol/ 16 fluphenazine/ 17 flupentixol/ 18 chlorpromazine/ 19 clomethiazole/ 20 clopenthixol/ 21 trifluoperazine/ 22 thioridazine/ 23 sulpiride/ 24 risperidone/ 25 fumaric acid derivative/ 26 or/8‐25 27 7 and 26 28 exp psychosis/ 29 Psychoses.ti,ab. 30 Psychosis.ti,ab. 31 exp violence/ 32 exp hostility/ 33 exp irritability/ 34 exp impulsiveness/ 35 exp paranoia/ 36 Agitat*.ti,ab. 37 aggress*.ti,ab. 38 violen*.ti,ab. 39 impuls*.ti,ab. 40 irritabl*.ti,ab. 41 hostil*.ti,ab. 42 anger.ti,ab. 43 angry.ti,ab. 44 anti‐social.ti,ab. 45 impuls*.ti,ab. 46 Restless*.ti,ab. 47 or/28‐46 48 27 and 47 49 randomized controlled trial/ 50 controlled clinical trial/ 51 random$.ti,ab. 52 randomization/ 53 intermethod comparison/ 54 placebo.ti,ab. 55 (compare or compared or comparison).ti. 56 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 57 (open adj label).ti,ab. 58 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 59 double blind procedure/ 60 parallel group$1.ti,ab. 61 (crossover or cross over).ti,ab. 62 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 63 (assigned or allocated).ti,ab. 64 (controlled adj7 (study or design or trial)).ti,ab. 65 (volunteer or volunteers).ti,ab. 66 trial.ti. 67 or/49‐66 68 48 and 67 |
Oct 2018: 2303 Oct 2019: 337 March 2020: 199 Jan 2021: 165 |
| 4. PSYCINFO [most recent search date: 7 January 2021] |
1 exp Dementia/ 2 exp Delirium/ 3 exp Wernickes Syndrome/ 4 exp Cognitive Impairment/ 5 dement*.mp. 6 alzheimer*.mp. 7 (chronic adj2 cerebrovascular).mp. 8 ("organic brain disease" or "organic brain syndrome").mp. 9 "benign senescent forgetfulness".mp. 10 (cerebr* adj2 deteriorat*).mp. 11 "major neurocognitive disorder".mp. 12 (lewy* adj2 bod*).mp. 13 (cerebral* adj2 insufficient*).mp. 14 or/1‐13 15 exp Neuroleptic Drugs/ 16 (antipsychotic* or neuroleptic*).ti,ab. 17 (neurolept* or antipsychotic* or alphaFlupenthixol* or Aminazine* or Amisulpride* or Amoxapine* or Aripiprazole or Asenapine maleate or Asendin* or Benperidol or Benzamides or Brexpiprazole or Butyrophenones or Chlorazine* or Chlordelazine* or Chlorpiprazine* or Chlorpromazin* or Chormethiazole* or cisFlupenthixol* or Cisordinol* or Clomethiazole* or Clopenthixol* or Cloxazepine* or Clozapine*OR Contomin* or Desmethylloxapine* or Diphenylbutylpiperidines or Distraneurin* or Dogmatil* or Eglonyl* or Emergil* or Fenactil* or Fluanxol* or Flufenazin* or Flupenthixol decanoate* or Flupentixol decanoate or Flupentixol* or Fluphenazin* or Fluphenazine decanoate* or Fluphenazine Hydrochloride* or Fumarate* or Haldol* or Haloperidol* or Iloperidone or Largactil* or Levomeprazin* or Levomepromazin* or Levopromazine* or Loxapine Monohydrochloride* or Loxapine* or Loxapinsuccinate* or Loxipine Maleate* or Loxipine Succinate* or Loxitane* or Lurasidone or Lyogen* or Meleril* or Mellaril* or Melleril* or Melleryl* or Melperone hydrochloride* or Methotrimeprazine* or Nalotenserin or Norquetiapine or Olanzapine embonate or Olanzapine* or Oxilapine* or Paliperidone or Perfenazine* or Pericyazine or Perphenazine* or Phenothiazine or Pimavanserin or Pimozide* or Prochlorperazine or Prolixin* or Promazine hydrochloride or Propaphenin* or Prothipendyl* or Quetiapine* or Risperidal* or Risperidone* or Sertindole or Sonapax* or Stelazine* or Sulperide* or Sulpiride* or Thioridazine Hydrochloride* or Thioridazine* or Thiothixene or Thioxanthenes or Thorazine* or Tiaprid* or Tiapridal* or Tisercin* or Tizercine* or Tizertsin* or Trifluoperazine or Trifluoroperazine* or Trifluperazine*OR Triftazin* or Trilafonor* or Tripfluoperazine Hydrochloride* or Ziprasidone* or Zotemine* or Zotepine or Zuclopenthixol or Zuclopenthixol*).ti,ab. 18 exp PIMOZIDE/ 19 exp PERPHENAZINE/ 20 exp LOXAPINE/ 21 exp HALOPERIDOL/ 22 exp FLUPHENAZINE/ 23 exp CHLORPROMAZINE/ 24 exp TRIFLUOPERAZINE/ 25 exp THIORIDAZINE/ 26 exp SULPIRIDE/ 27 exp RISPERIDONE/ 28 or/15‐27 29 14 and 28 30 exp Psychosis/ 31 Psychoses.ti,ab. 32 Psychosis.ti,ab. 33 exp VIOLENCE/ 34 exp HOSTILITY/ 35 exp Impulsiveness/ 36 exp Paranoia/ 37 Agitat*.ti,ab. 38 aggress*.ti,ab. 39 violen*.ti,ab. 40 impuls*.ti,ab. 41 irritabl*.ti,ab. 42 hostil*.ti,ab. 43 anger.ti,ab. 44 angry.ti,ab. 45 anti‐social.ti,ab. 46 impuls*.ti,ab. 47 Restless*.ti,ab. 48 or/30‐47 49 29 and 48 50 exp Clinical Trials/ 51 randomly.ab. 52 randomi?ed.ti,ab. 53 placebo.ti,ab. 54 groups.ab. 55 "double‐blind*".ti,ab. 56 "single‐blind*".ti,ab. 57 RCT.ti,ab. 58 or/50‐57 59 49 and 58 |
Oct 2018: 447 Oct 2019: 27 March 2020: 19 Jan 2021: 16 |
| 5. CINAHL (EBSCOhost) [most recent search date: 7 January 2021] |
S59 S45 AND S58 S58 S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 S57 TX random* S56 MH "Random Assignment" S55 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S54 MH "Crossover Design" S53 MH "Factorial Design" S52 MH "Placebos" S51 MH "Clinical Trials" S50 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S49 TX crossover OR "cross‐over" S48 AB placebo* S47 TX trial* S46 TX "latin square" S45 S26 AND S44 S44 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 S43 TX Restless* S42 TX impuls* S41 TX anti‐social S40 TX angry S39 TX anger S38 TX hostil* S37 TX irritabl* S36 TX impuls* S35 TX violen* S34 TX aggress* S33 TX Agitat* S32 MH "Disruptive Behavior" S31 MH "Aggression+" S30 MH "Violence+" S29 TX Psychosis S28 TX Psychoses S27 MH "Psychotic Disorders+" S26 S13 AND S25 S25 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S24 MH "Risperidone" S23 MH "Thioridazine" S22 MH "Trifluoperazine Hydrochloride" S21 MH "Chlorpromazine" S20 MH "Fluphenazine" S19 MH "Haloperidol" S18 MH "Loxapine" S17 MH "Perphenazine Hydrochloride" S16 TX neurolept* or antipsychotic* or alphaFlupenthixol* OR Aminazine* OR Amisulpride* OR Amoxapine* OR Aripiprazole OR Asenapine maleate OR Asendin* OR Benperidol OR Benzamides OR Brexpiprazole OR Butyrophenones OR Chlorazine* OR Chlordelazine* OR Chlorpiprazine* OR Chlorpromazin* OR Chormethiazole* OR cisFlupenthixol* OR Cisordinol* OR Clomethiazole* OR Clopenthixol* OR Cloxazepine* OR Clozapine*OR Contomin* OR Desmethylloxapine* OR Diphenylbutylpiperidines OR Distraneurin* OR Dogmatil* OR Eglonyl* OR Emergil* OR Fenactil* OR Fluanxol* OR Flufenazin* OR Flupenthixol decanoate* OR Flupentixol decanoate OR Flupentixol* OR Fluphenazin* OR Fluphenazine decanoate* OR Fluphenazine Hydrochloride* OR Fumarate* OR Haldol* OR Haloperidol* OR Iloperidone OR Largactil* OR Levomeprazin* OR Levomepromazin* OR Levopromazine* OR Loxapine Monohydrochloride* OR Loxapine* OR Loxapinsuccinate* OR Loxipine Maleate* OR Loxipine Succinate* OR Loxitane* OR Lurasidone OR Lyogen* OR Meleril* OR Mellaril* OR Melleril* OR Melleryl* OR Melperone hydrochloride* OR Methotrimeprazine* OR Nalotenserin OR Norquetiapine OR Olanzapine embonate OR Olanzapine* OR Oxilapine* OR Paliperidone OR Perfenazine* OR Pericyazine OR Perphenazine* OR Phenothiazine OR Pimavanserin OR Pimozide* OR Prochlorperazine OR Prolixin* OR Promazine hydrochloride OR Propaphenin* OR Prothipendyl* OR Quetiapine* OR Risperidal* OR Risperidone* OR Sertindole OR Sonapax* OR Stelazine* OR Sulperide* OR Sulpiride* OR Thioridazine Hydrochloride* OR Thioridazine* OR Thiothixene OR Thioxanthenes OR Thorazine* OR Tiaprid* OR Tiapridal* OR Tisercin* OR Tizercine* OR Tizertsin* OR Trifluoperazine OR Trifluoroperazine* OR Trifluperazine*OR Triftazin* OR Trilafonor* OR Tripfluoperazine Hydrochloride* OR Ziprasidone* OR Zotemine* OR Zotepine OR Zuclopenthixol OR Zuclopenthixol* S15 TX antipsychotic* or neuroleptic* S14 MH "Antipsychotic Agents" S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 TX "organic brain disease" or "organic brain syndrome" S11 TX "major neurocognitive disorder" S10 TX cerebral* n2 insufficient* S9 TX cerebr* n2 deteriorat* S8 TX "benign senescent forgetfulness" S7 TX chronic n2 cerebrovascular S6 TX lewy* n2 bod* S5 TX alzheimer* S4 TX dement* S3 MH "Wernicke's Encephalopathy" S2 MH "Delirium" S1 MH "Dementia+" |
Oct 2018: 425 Oct 2019: 575 March 2020: 36 Jan 2021: 40 |
| 6. ISI Web of Science – core collection [most recent search date: 7 January 2021] |
TOPIC: (dement* OR alzheimer* OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL OR "cognit* impair*" OR FTD OR FTLD OR "cerebrovascular insufficienc*" OR AD OR VCI "major neurocognitive disorder") AND TOPIC: (Antipsychotic* OR neurolept* OR HALOPERIDOL OR RISPERIDONE) AND TOPIC: (Psychotic OR Psychoses OR Psychosis OR VIOLENCE OR HOSTILITY) AND TOPIC: (randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial) | Oct 2018: 738 Oct 2019: 66 March 2020: 33 Jan 2021: 33 |
| 7. LILACS (BIREME) [most recent search date: 7 January 2021] |
alzheimer OR alzheimers OR alzheimer’s OR dementia OR demenc$ [Words]and Psychotic OR Psychoses OR Psychosis OR VIOLENCE OR HOSTILITY [Words]and randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind$" OR placebo [Words] | Oct 2018: 20 Oct 2019: 0 March 2020: 0 Jan 2021: 22 |
| 8. ClinicalTrials.gov (www.clinicaltrials.gov) [most recent search date: 7 January 2021] |
Psychotic OR Psychoses OR Psychosis OR VIOLENCE OR HOSTILITY | dementia OR alzheimers OR cognition OR cognitive | Antipsychotic* OR neurolept* OR HALOPERIDOL OR RISPERIDONE OR Brexipiprazole OR Nalotenserin OR Pimavanserine | Oct 2018: 65 Oct 2019: 69 March 2020: 2 Jan 2021: 2 |
| 9. ICTRP [most recent search date: 7 January 2021] |
Psychotic OR Psychoses OR Psychosis OR VIOLENCE OR HOSTILITY | dementia OR alzheimers OR cognition OR cognitive | Antipsychotic* OR neurolept* OR HALOPERIDOL OR RISPERIDONE OR Brexipiprazole OR Nalotenserin OR Pimavanserine | Oct 2018: 10 Oct 2019: 11 March 2020: 2 Jan 2021: 1 |
| TOTAL before deduplication | Oct 2018: 5786 Oct 2019: 1638 March 2020: 443 Jan 2021: 366 TOTAL 8233 |
|
| TOTAL after de‐duplication | Oct 2018: 4911 Oct 2019: 1432 March 2020: 378 Jan 2021: 316 TOTAL 7037 |
|
Appendix 2. Other resources searches
| Source | Search strategy | Hits retrieved |
| 1. GlaxoSmithKline register https://www.gsk‐studyregister.com/en/ [Date of most recent search: 17 February 2021] |
alphaflupenthixol amisulpride amoxapine asendin chlorazine chlordelazine chlormethiazole chlorpiprazine chlorpromazin chlorpromazine chormethiazole cisflupenthixol cisordinol clomethiazole clopenthixol cloxazepine clozapine contomin desmethylloxapine distraneurin dogmatil eglonyl emergil fenactil fluanxol flufenazin flupenthixol decanoate flupentixol fluphenazin fluphenazine decanoate fluphenazine hydrochloride fumarate fumarates haldol haloperidol largactil levomeprazin levomepromazin levopromazine loxapine monohydrochloride loxapine loxapinsuccinate loxipine maleate loxipine succinate loxitane lyogen meleril mellaril melleril melleryl melperone hydrochloride methotrimeprazine olanzapine oxilapine perfenazine perphenazine pimozide prolixin propaphenin prothipendyl quetiapine risperidal risperidone sonapax stelazine sulperide sulpiride thioridazine hydrochloride thioridazine thorazine tiaprid tiapridal tisercin tizercine tizertsin trifluoperazine hydrochloride trifluoroperazine trifluperazine triftazin trilafonor tripfluoperazine hydrochloride ziprasidone zotemine zuclopenthixol |
Feb 2021: 4 |
| 2. European Medicines Agency (EMA) https://www.clinicaltrialsregister.eu/ctr‐search/search [Date of most recent search: 17 February 2021] |
alphaflupenthixol amisulpride amoxapine asendin chlorazine chlordelazine chlormethiazole chlorpiprazine chlorpromazin chlorpromazine chormethiazole cisflupenthixol cisordinol clomethiazole clopenthixol cloxazepine clozapine contomin desmethylloxapine distraneurin dogmatil eglonyl emergil fenactil fluanxol flufenazin flupenthixol decanoate flupentixol fluphenazin fluphenazine decanoate fluphenazine hydrochloride fumarate fumarates haldol haloperidol largactil levomeprazin levomepromazin levopromazine loxapine monohydrochloride loxapine loxapinsuccinate loxipine maleate loxipine succinate loxitane lyogen meleril mellaril melleril melleryl melperone hydrochloride methotrimeprazine olanzapine oxilapine perfenazine perphenazine pimozide prolixin propaphenin prothipendyl quetiapine risperidal risperidone sonapax stelazine sulperide sulpiride thioridazine hydrochloride thioridazine thorazine tiaprid tiapridal tisercin tizercine tizertsin trifluoperazine hydrochloride trifluoroperazine trifluperazine triftazin trilafonor tripfluoperazine hydrochloride ziprasidone zotemine zuclopenthixol |
Feb 2021: 0 |
| 3. Food and Drug administration (FDA) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/Erleada_210951_toc.cfm [Date of most recent search: 17 February 2021] |
alphaflupenthixol amisulpride amoxapine asendin chlorazine chlordelazine chlormethiazole chlorpiprazine chlorpromazin chlorpromazine chormethiazole cisflupenthixol cisordinol clomethiazole clopenthixol cloxazepine clozapine contomin desmethylloxapine distraneurin dogmatil eglonyl emergil fenactil fluanxol flufenazin flupenthixol decanoate flupentixol fluphenazin fluphenazine decanoate fluphenazine hydrochloride fumarate fumarates haldol haloperidol largactil levomeprazin levomepromazin levopromazine loxapine monohydrochloride loxapine loxapinsuccinate loxipine maleate loxipine succinate loxitane lyogen meleril mellaril melleril melleryl melperone hydrochloride methotrimeprazine olanzapine oxilapine perfenazine perphenazine pimozide prolixin propaphenin prothipendyl quetiapine risperidal risperidone sonapax stelazine sulperide sulpiride thioridazine hydrochloride thioridazine thorazine tiaprid tiapridal tisercin tizercine tizertsin trifluoperazine hydrochloride trifluoroperazine trifluperazine triftazin trilafonor tripfluoperazine hydrochloride ziprasidone zotemine zuclopenthixol |
Feb 2021: 0 |
Data and analyses
Comparison 1. Typical antipsychotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Agitation | 4 | 361 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.57, ‐0.15] |
| 1.2 Psychosis | 2 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.55, ‐0.03] |
| 1.3 Somnolence | 3 | 466 | Risk Ratio (IV, Fixed, 95% CI) | 2.62 [1.51, 4.56] |
| 1.4 Somnolence (RD) | 3 | 466 | Risk Difference (IV, Fixed, 95% CI) | 0.12 [0.06, 0.18] |
| 1.5 Extrapyramidal symptoms | 3 | 467 | Risk Ratio (IV, Fixed, 95% CI) | 2.26 [1.58, 3.23] |
| 1.6 Extrapyramidal symptoms (RD) | 3 | 467 | Risk Difference (IV, Fixed, 95% CI) | 0.19 [0.11, 0.27] |
| 1.7 Any serious adverse events | 1 | 193 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [0.65, 2.66] |
| 1.8 Any serious adverse events (RD) | 1 | 193 | Risk Difference (IV, Fixed, 95% CI) | 0.04 [‐0.06, 0.14] |
| 1.9 Death | 6 | 578 | Risk Ratio (IV, Fixed, 95% CI) | 1.46 [0.54, 4.00] |
| 1.10 Death (RD) | 6 | 578 | Risk Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| 1.11 Number of responders for agitation | 4 | 367 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [1.01, 1.38] |
| 1.12 Number of responders for agitation (RD) | 4 | 367 | Risk Difference (IV, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
| 1.13 Number of responders for psychosis | 2 | 259 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.90, 1.92] |
| 1.14 Number of responders for psychosis (RD) | 2 | 259 | Risk Difference (IV, Fixed, 95% CI) | 0.09 [‐0.03, 0.20] |
| 1.15 Discontinuation due to adverse events | 4 | 442 | Risk Ratio (IV, Fixed, 95% CI) | 1.70 [1.02, 2.82] |
| 1.16 Discontinuation due to adverse events (RD) | 4 | 442 | Risk Difference (IV, Fixed, 95% CI) | 0.06 [0.00, 0.12] |
| 1.17 Discontinuation (any reason) | 6 | 578 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [0.89, 1.51] |
| 1.18 Discontinuation (any reason) (RD) | 6 | 578 | Risk Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.07] |
| 1.19 Functioning (ADL) | 2 | 249 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.13, 0.63] |
| 1.20 Cognitive function | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.27, 0.77] |
| 1.21 Carer burden | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Haloperidol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Agitation | 3 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 2.2 Psychosis | 2 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.55, ‐0.03] |
| 2.3 Somnolence | 3 | 466 | Risk Ratio (IV, Fixed, 95% CI) | 2.62 [1.51, 4.56] |
| 2.4 Extrapyramidal symptoms | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Serious adverse events | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 Death | 5 | 545 | Risk Ratio (IV, Fixed, 95% CI) | 1.88 [0.65, 5.48] |
| 2.7 Number of responders for agitation | 3 | 334 | Risk Ratio (IV, Fixed, 95% CI) | 1.15 [0.99, 1.35] |
| 2.8 Number of responders for psychosis | 2 | 259 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [0.90, 1.92] |
| 2.9 Discontinuation due to adverse events | 3 | 409 | Risk Ratio (IV, Fixed, 95% CI) | 1.81 [1.08, 3.03] |
| 2.10 Discontinuation (any reason) | 5 | 545 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.90, 1.54] |
| 2.11 Functioning (ADL) | 2 | 249 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.13, 0.63] |
| 2.12 Cognitive function | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.27, 0.77] |
| 2.13 Carer burden | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Atypical antipsychotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Agitation | 7 | 1971 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.30, ‐0.12] |
| 3.1.1 Patients with agitation | 6 | 1670 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.28, ‐0.08] |
| 3.1.2 Patients with aggression | 1 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.61, ‐0.15] |
| 3.2 Psychosis | 12 | 3364 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.18, ‐0.03] |
| 3.3 Somnolence | 13 | 3878 | Risk Ratio (IV, Fixed, 95% CI) | 1.93 [1.57, 2.39] |
| 3.4 Somnolence (RD) | 13 | 3878 | Risk Difference (IV, Fixed, 95% CI) | 0.07 [0.05, 0.08] |
| 3.5 Extrapyramidal symptoms | 15 | 4180 | Risk Ratio (IV, Fixed, 95% CI) | 1.39 [1.14, 1.68] |
| 3.6 Extrapyramidal symptoms (RD) | 15 | 4180 | Risk Difference (IV, Fixed, 95% CI) | 0.03 [0.02, 0.05] |
| 3.7 Any adverse event | 11 | 2785 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [1.02, 1.09] |
| 3.8 Any adverse event (RD) | 11 | 2785 | Risk Difference (IV, Fixed, 95% CI) | 0.05 [0.02, 0.07] |
| 3.9 Any serious adverse event | 15 | 4316 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.09, 1.61] |
| 3.10 Any serious adverse event (RD) | 15 | 4316 | Risk Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.05] |
| 3.11 Death | 17 | 5032 | Risk Ratio (IV, Fixed, 95% CI) | 1.36 [0.90, 2.05] |
| 3.12 Death (RD) | 17 | 5032 | Risk Difference (IV, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 3.13 Number of responders for agitation | 4 | 1304 | Risk Ratio (IV, Fixed, 95% CI) | 1.31 [1.16, 1.48] |
| 3.13.1 Assessments not including physical aggression | 3 | 959 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 3.13.2 Assessments including physical aggression | 1 | 345 | Risk Ratio (IV, Fixed, 95% CI) | 1.69 [1.31, 2.17] |
| 3.14 Number of responders for agitation (RD) | 4 | 1304 | Risk Difference (IV, Fixed, 95% CI) | 0.13 [0.08, 0.18] |
| 3.14.1 Assessments not including physical aggression | 3 | 959 | Risk Difference (IV, Fixed, 95% CI) | 0.10 [0.04, 0.16] |
| 3.14.2 Assessments including physical aggression | 1 | 345 | Risk Difference (IV, Fixed, 95% CI) | 0.22 [0.12, 0.33] |
| 3.15 Number of responders for psychosis | 7 | 1958 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [1.03, 1.23] |
| 3.16 Number of responders for psychosis (RD) | 7 | 1958 | Risk Difference (IV, Fixed, 95% CI) | 0.08 [0.04, 0.13] |
| 3.17 Discontinuation due to adverse events | 17 | 5058 | Risk Ratio (IV, Fixed, 95% CI) | 1.41 [1.15, 1.72] |
| 3.18 Discontinuation due to adverse events (RD) | 17 | 5058 | Risk Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
| 3.19 Discontinuation (any reason) | 18 | 5095 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 3.20 Discontinuation (any reason) (RD) | 18 | 5095 | Risk Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 3.21 Functioning (ADL) | 3 | 514 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 3.22 Cognitive function | 11 | 2698 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.02] |
| 3.23 Cognitive function (single study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.24 Health‐related quality of life | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.95 [‐4.14, 6.04] |
| 3.25 Time spend providing care (caregiver) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐1.39, 1.55] |
Comparison 4. Risperidone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Agitation | 2 | 524 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.44, ‐0.09] |
| 4.2 Psychosis | 5 | 1205 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.23, 0.01] |
| 4.3 Somnolence | 2 | 700 | Risk Ratio (IV, Fixed, 95% CI) | 3.35 [1.99, 5.65] |
| 4.4 Extrapyramidal symptoms | 6 | 1328 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [1.32, 2.33] |
| 4.5 Any adverse event | 2 | 700 | Risk Ratio (IV, Fixed, 95% CI) | 1.19 [1.07, 1.32] |
| 4.6 Any serious adverse event | 5 | 1085 | Risk Ratio (IV, Fixed, 95% CI) | 1.21 [0.88, 1.67] |
| 4.7 Death | 5 | 1298 | Risk Ratio (IV, Fixed, 95% CI) | 1.29 [0.64, 2.60] |
| 4.8 Number of responders for agitation | 2 | 572 | Risk Ratio (IV, Fixed, 95% CI) | 1.61 [1.29, 2.01] |
| 4.9 Number of responders for psychosis | 3 | 781 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.93, 1.19] |
| 4.10 Discontinuation due to adverse events | 5 | 1349 | Risk Ratio (IV, Fixed, 95% CI) | 1.60 [1.13, 2.27] |
| 4.11 Discontinuation (any reason) | 6 | 1383 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 4.12 Cognitive function | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.04, 0.41] |
| 4.13 Functioning (ADL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.14 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.15 Time spend providing care (caregiver) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Quetiapine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Agitation | 3 | 615 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.31, 0.02] |
| 5.2 Psychosis | 2 | 220 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.22, 0.31] |
| 5.3 Somnolence | 4 | 798 | Risk Ratio (IV, Fixed, 95% CI) | 4.83 [2.73, 8.57] |
| 5.4 Extrapyramidal symptoms | 4 | 799 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.52, 1.70] |
| 5.5 Any adverse event | 3 | 609 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 5.6 Any serious adverse event | 4 | 799 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [0.86, 2.03] |
| 5.7 Death | 5 | 861 | Risk Ratio (IV, Fixed, 95% CI) | 1.48 [0.67, 3.31] |
| 5.8 Number of responders for agitation | 2 | 569 | Risk Ratio (IV, Fixed, 95% CI) | 1.35 [1.02, 1.78] |
| 5.9 Number of responders for psychosis | 2 | 230 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.78, 1.36] |
| 5.10 Discontinuation due to adverse events | 5 | 858 | Risk Ratio (IV, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| 5.11 Discontinuation (any reason) | 5 | 861 | Risk Ratio (IV, Fixed, 95% CI) | 0.97 [0.88, 1.08] |
| 5.12 Functioning (ADL) | 2 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.42, 0.07] |
| 5.13 Cognitive function | 4 | 584 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.07] |
| 5.14 Cognitive function (single study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.15 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.16 Time spend providing care (caregiver) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allain 2000.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 3 weeks Rescue medication: not allowed; quote:"All psychotropics drug were excluded except benzodiazepines prescribed as hypnotics, zopiclone and zolpidem and antidepressants prescribed at low doses (less than a third of the usual dose for major depression). Such drugs could be continued during the study under the condition that doses remained unchanged for a month and during the period of the study." |
|
| Participants |
Number randomised: 306 (101 haloperidol, 102 tiapride, 103 placebo) Mean age: 79.6 years Sex (female): 64% Type of dementia: Alzheimer's disease, vascular dementia and mixed type dementia. Severity of dementia: mild‐moderate Indication: Agitation (baseline MOSES: 20.2) Setting: nursing home or otherwise hospitalised patients Country: France, the Netherlands, Germany, Latvia and Portugal |
|
| Interventions |
Intervention characteristics Haloperidol
Tiapride
Placebo The active drug‐treated patients received 175.45 ± 44.70 mg/day of tiapride or 3.53 ± 1.05 mg/day of haloperidol. Seven tiapride‐treated patients (7%) and 13 haloperidol‐treated patients (13%) received the maximum dose planned in the protocol (300 mg/day and 6 mg/day, respectively). Most of the patients received the recommended dose: 68 patients (67%) in the tiapride group (200 mg/day) and 58 patients (57%) in the haloperidol group (4 mg/day). |
|
| Outcomes | Agitation: Multidimensional Observation Scale for the Elderly Subjects (MOSES) Number of responders for agitation Extrapyramidal symptoms: “Udvalg for Kliniske Undersøgelser” (Task force for clinical investigations) (UKU scale) Somnolence: “Udvalg for Kliniske Undersøgelser” (Task force for clinical investigations) (UKU scale) Death Discontinuation due to adverse events Discontinuation (any reason) |
|
| Identification | ||
| Notes | Sponsorship source: Unclear, but Laboratoires Synthélabo involved | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated to tiapride 100 mg/day (50 mg twice a day), haloperidol 2 mg/day (1 mg twice a day) or placebo. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in study |
| Comparability of groups (selection bias) | High risk | There were group differences in age, sex, and agitation at baseline, which were not adjusted for. Baseline extrapyramidal symptoms and other UKU adverse events were not reported. Mean ages were, respectively, for the placebo, tiapride and haloperidol groups 78.6±7.3 years; 80.3±7.6 years; 79.9±7.9 years and sex distributions were 71 (69%) female and 32 (31%) male patients; 63 (62%) female and 39 (38%) male patients and 63 (62%) female and 38 (38%) male patients. MOSES irritability/aggressiveness subscores were: Placebo 20.28±2.85; tiapride 19.90±2.92; haloperidol 20.52±3.27. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind but no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind but no further information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Forty‐seven patients (15%) dropped out from the study, ten in the tiapride group (adverse event five; lack of efficacy one; uncooperativeness three; recovery one), 21 in the haloperidol group (adverse event 17; lack of efficacy one; uncooperativeness two; concomitant medication one) and 16 in the placebo group (adverse event six; lack of efficacy eight; uncooperativeness two). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section are reported in the results section. |
| Other bias | Low risk | No run‐in period. |
Auchus 1997.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 6 weeks Rescue medication: no information provided |
|
| Participants |
Number randomised: 12 (6 haloperidol 6 placebo) Mean age: 75.6 years Sex (female): 66% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: Agitation (baseline CMAI 35.9) Setting: community‐dwelling Country: USA |
|
| Interventions |
Intervention characteristics Haloperidol
Placebo |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Death Discontinuation due to adverse events Discontinuation (any reason) Carer burden or carer quality of life: Caregiver Strain Index (CSI) |
|
| Identification | ||
| Notes | Sponsorship source: Grant from Emory University Research Council (226‐93) and Grant Alzheimer’s Disease Core Center from the National Institute on Aging (P3OAG1O13O) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Comparability of groups (selection bias) | Unclear risk | Baseline characteristics are not presented per group for all randomized. Baseline outcome score differ greatly but direction of bias unclear. Unclear whether the difference has been adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "double blind", but it is not described if haloperidol and placebo tablets did look the same and no other actions to secure blinding of personnel and participants are described. Patients probably blinded but unclear if blinding of personnel was performed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of drop‐out: 2/6 haloperidol (33%) and 1/6 (17%) placebo treated patients drop‐out. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section are reported in the results section. |
| Other bias | High risk | Subjects in each group completed a 2‐week washout period during which any current psychotropic medications were carefully withdrawn. |
Ballard 2005.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 6 weeks Rescue medication: not mentioned |
|
| Participants |
Number randomised: 62; 31 quetiapine 31 placebo Mean age: 83.6 years Sex (female): 82.3% Type of dementia: Alzheimer's disease Severity of dementia: severe Indication: = agitation (baseline CMAI 58.8) Setting: care facilities Country: UK |
|
| Interventions |
Intervention characteristics Quetiapine
Placebo |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Death Discontinuation due to adverse events Discontinuation (any reason) Cognitive function: Severe Impairment Battery (SIB) |
|
| Identification | ||
| Notes | Sponsorship source: general donations to CB’s research programme and profits from previously completed commercially funded CT (by Astra Zeneca and Novartis), with additional support from the Alzheimer’s Research Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocations were computer generated with block randomisation (block sizes of three and six)." |
| Allocation concealment (selection bias) | Unclear risk | It is reported that: "The study statistician randomly assigned patients." "The randomising clinician faxed a form to the statistician, who communicated allocation to the pharmacy, ensuring concealment."The procedure is not clearly described because it first suggests that the statistician performs the randomisation, and later that the clinician randomises (which would be wrong). |
| Comparability of groups (selection bias) | High risk | Clear differences between groups at baseline, f.i. in % women (87.1 versus 77.4) and 5 with EPS (12.9 versus 6.5), and mean CMAI (59.1 versus 56.4) for quetiapine versus placebo. Only the latter was adjusted for in the analyses. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Design: Randomised double blind (clinician, patient, outcomes assessor) placebo controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessors were blind to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out was: 8/31 in quetiapine group and 1/31 in placebo group. Modified ITT analysis (those who dropped out not included, and LOCF) |
| Selective reporting (reporting bias) | High risk | Few outcomes compared to what is common in these trials. ECG and full blood count not reported. Adverse events/ side effects missing in general. |
| Other bias | Low risk | No run‐in period. |
Ballard 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 12 weeks Rescue medication: was allowed. |
|
| Participants |
Number randomised: 181 (90 pimavanserin 91 placebo) Mean age: 85.9 years Sex (female): 80.9% Type of dementia: Alzheimer's disease Severity of dementia: severe Indication: Psychosis (baseline Psychosis NPI‐NH: 9.75) Setting: nursing homes Country: UK |
|
| Interventions |
Intervention characteristics Pimavanserin
Placebo |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory ‐ Nursing Home Version (NPI‐NH) Somnolence (Notes: reported only on clinicaltrials.gov) Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: ACADIA Pharmaceuticals Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistician without any other involvement in the study generated the randomisation sequence with use of permuted block sizes of four, which was implemented using Trident software (version 1.2)." |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Comparability of groups (selection bias) | Low risk | Small baseline differences, which seem to be in favour of placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "We masked participants, caregivers, the study sponsor, and study personnel at the clinic site to treatment assignment. We achieved masking of active treatment and placebo by using identical‐appearing tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | According to clinicaltrials.gov: Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | A lot of analyses with subscores and various weeks of follow‐up. Primary outcome was reduction of psychotic symptoms at 12 weeks according to protocol in clinicaltrials.gov. In article, the primary outcome was reduction of psychotic symptoms at 6 weeks. There was not difference in symptoms at 12 weeks. |
| Other bias | High risk | This period also allowed for washout in participants taking antipsychotic medication. During screening, participants entered a 3‐week period in which BPST was used to ensure that only individuals who required a pharmacological treatment progressed to randomisation in the study, to minimise subsequent placebo response. |
Brodaty 2003 RIS‐AUS‐05.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 12 weeks Rescue medication: anticholinergic medication was allowed to treat EPS only if a reduction in trial medication dose was not effective. Treatment of urinary incontinence with low‐dose tricyclic antidepressants or anticholinergic medication was allowed to continue. Low‐dose oxazepam was permitted to treat agitation, provided that usage did not exceed 4 days in a 7‐day period. Short‐acting sedative/hypnotic agents prescribed chronically for insomnia at baseline were permitted if the clinician judged that they could not be discontinued. Under exceptional circumstances, initiation of night sedation for insomnia was allowed using a short‐acting benzodiazepine (preferably oxazepam at the lowest effective dose). Narcotic analgesics were permitted, provided that the dosage had been stable for at least 3 months and that they were not prescribed to control agitation or aggression. |
|
| Participants |
Number randomised: 345 (173 risperidone 172 placebo) Mean age: 83.5 years Sex (female): 84.9% Type of dementia: Alzheimer's disease, vascular dementia, mixed type dementia Severity of dementia: severe Indication: aggression (is subtype of agitation); subgroup analysis in patients with psychosis (additionally) was reported (Baseline CMAI total aggression: 33.5) Setting: nursing home Country: Australia and New Zealand |
|
| Interventions |
Intervention characteristics Risperidone
Placebo Mean risperidone dosage was 1.03 ± 0.61 mg/day. |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) total aggression subscale Number of responders for agitation Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Janssen‐Cilag Australia and Johnson & Johnson, L.L.C. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No baseline characteristics for all randomized per group are shown (n=156 instead of 172 for placebo; n=153 instead of 173 for risperidone). Small differences in the reported baseline characteristics, e.g. more aggression and NPS in risperidone group. Most are not adjusted for in the analyses. Unclear how the differences could have affected the estimated effects. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Agitation: In the article, 152 for placebo and 149 for risperidone were reported instead of 170 and 167 who received at least once the study medication. |
| Selective reporting (reporting bias) | High risk | Results on all reported measures are reported. However, effect on MMSE and FAST not reported in numbers. Post‐hoc subgroup analysis with respect to effect on psychosis.One site excluded. Only adjusted least square means are reported. Crude means are only mentioned in a figure without providing exact numbers. No CI or SD or SE are reported for the change from baseline. Only difference of LS between placebo and risperidone. |
| Other bias | High risk | The double‐blind treatment period was preceded by a maximum 7‐day, single‐blind washout period, during which patients took 0.5 mL of placebo oral solution each evening while existing psychotropic medication was discontinued. |
Deberdt 2005 F1D MC HGGU.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed for EPS as well as benzodiazepines. |
|
| Participants |
Number randomised: 494 (204 olanzapine, 196 risperidone, 94 placebo) Mean age: 78.3 years Sex (female): 65.6% Type of dementia: Alzheimer's disease, vascular dementia, mixed type dementia Severity of dementia: moderate Indication: Psychosis (Baseline Psychosis NPI: 11.2) Setting: outpatients or nursing homes or assisted‐living centres Country: USA |
|
| Interventions |
Intervention characteristics Olanzapine
Risperidone
Placebo Half the patients assigned to olanzapine began treatment with 2.5 mg/day, which was increased to 5 mg/day at the end of the first week; the other half began treatment with 5 mg/day. Those assigned to risperidone began with 0.5 mg/day, which was increased to 1 mg/day at the end of the first week. The mean daily olanzapine dose was 5.2 mg and that for risperidone was 1.0 mg. |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory–Nursing Home version (NPI‐NH) psychosis score Number of responders for psychosis Extrapyramidal symptoms Somnolence Death Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: Lilly Research Laboratories, Indianapolis, IN (WGD, PDF, CAY, DPH, DLL, EKD, AB), the Geriatric Research, Education and Clinical Center Program, Veterans Affairs Medical Center; Minneapolis, MN (MWD), Agewell Ltd., Indianapolis, IN (SAR), and Eli Lilly G.m.b.H., Vienna, Austria (MD). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was high (42%). Analyses not with all randomized. LOCF for missing data. |
| Selective reporting (reporting bias) | Low risk | "Any adverse events" and "serious adverse events" not reported, but reported measurement were all reported. Negative trial. |
| Other bias | High risk | "After a 3‐ to 14‐day placebo/washout period (...)" |
De Deyn 2004 F1D MC HGIV.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed |
|
| Participants |
Number randomised: 652 (520 olanzapine129 placebo) Mean age: 76.6 years Sex (female): 75% Type of dementia: Alzheimer's disease Severity of dementia: mild‐severe Indication: Psychosis (baseline Psychosis NPI‐NH: 9.7) Setting: long‐term nursing homes or continuing‐care hospitals Country: Europe, Australia, Israel, Lebanon, and South Africa |
|
| Interventions |
Intervention characteristics Olanzapine
Placebo Patients randomly assigned to receive olanzapine 1.0mg or olanzapine 2.5mg were respectively given a single 1.0 mg or 2.5 mg capsule of olanzapine daily (at bedtime) throughout the study period. Patients assigned to receive olanzapine 5.0 mg or olanzapine 7.5 mg began therapy on 2.5 mg/day for the first week and were titrated to their final dose by 2.5 mg/week increments. Patients unable to tolerate the assigned olanzapine or placebo dose were discontinued from the study. |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory–Nursing Home version (NPI‐NH) Death Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Eli Lilly and Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | High risk | Baseline characteristics were not reported per group and not for all randomized in the main results article (NPI). A limited set of characteristics (not the primary outcome) was reported in a clinical study report and showed clear differences in f.i. sex and smaller differences in other characteristics. Only baseline scores for outcomes were adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total number randomized 652 does not agree with numbers randomized per group (520 and 129). |
| Selective reporting (reporting bias) | High risk | Many subanalyses, for instance with the individual NPI items. Post‐hoc analyses. Each time for all four drug groups. No adjustment for multiple testing. Much missing data on adverse events (no information f.i. on somnolence, or just summary information, f.i. on cognitive function). |
| Other bias | High risk | "Following a placebo lead‐in phase of up to a maximum of 14 days (...)" |
De Deyn 2005.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed for NPS, not for EPS |
|
| Participants |
Number randomised: 208 (106 aripiprazole 102 placebo) Mean age: 81.5 years Sex (female): 72% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: Psychosis (Baseline Psychosis NPI Psychosis: 12.4) Setting: assisted living facilities, adult communities or living with a caregiver Country: USA |
|
| Interventions |
Intervention characteristics Aripiprazole
Placebo Eligible patients were randomised to aripiprazole 2 mg/d or placebo, administered once daily for 10 weeks, in this multicentre, double‐blind study. Aripiprazole could be titrated to higher doses (5, 10 mg, and 15 mg/day) at 2‐week intervals (or more rapidly based on investigator’s judgement) if the patient showed insufficient clinical response. At end point, the mean daily dose of aripiprazole was 10.0 mg. |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory (NPI) Psychosis subscale Extrapyramidal symptoms Somnolence Death Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: Bristol‐Myers Squibb and Otsuka Pharmaceutical Co., Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comments |
| Allocation concealment (selection bias) | Unclear risk | No comments |
| Comparability of groups (selection bias) | High risk | Judgement Comment: No baseline information in main article. A clinical study report provides data on age, sex, race and weight only: small differences that might or might not be in favour of the drug. They are not adjusted for. Baseline scores for outcomes are given for analysed (not all randomised) patients only, but are adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No comments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement Comment: Drop‐out was just below 20% and did not differ >5% between groups.It seems that not all patients were included in the analyses (see table with results on outcomes). Missing data were imputed with LOCF. |
| Selective reporting (reporting bias) | High risk | Insufficient information about outcomes in the protocol. Lots of secondary outcomes. |
| Other bias | High risk | "Following screening and a minimum 7‐day washout period for previous psychotropic medication (...)" |
Devanand 1998.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Study duration: 6 weeks Rescue medication: not allowed for NPS or EPS. |
|
| Participants |
Number randomised: 66 (42 haloperidol, 24 placebo) Mean age: 72.1 years Sex (female): 64.8% Type of dementia: Alzheimer's disease Severity of dementia: moderate‐severe Indication: diverse NPS, but the majority showed psychosis (71.8%), and psychomotor agitation (78.9%) (baseline psychosis BPRS: 6.8, psychomotor agitation BSS: 3.6) Setting: outpatients Country: USA |
|
| Interventions |
Intervention characteristics Haloperidol
Placebo After the first week the daily dose was raised from two capsules (haloperidol, 2 mg or 0.50 mg, or placebo) to three capsules (haloperidol, 3mg or 0.75 mg, or placebo). If side effects (e.g. extrapyramidal signs) were limiting, on the basis of the psychiatrist’s clinical judgment, the dose was maintained at two capsules daily. Therefore, in both phases, patients were on a stable dose for 5 weeks before the endpoint evaluation of efficacy and side effects. |
|
| Outcomes | Psychosis: Brief Psychiatric Rating Scale (BPRS) psychosis subscale Number of responders for psychosis Death Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Supported in part by grants MH‐44176, MH‐50038, and MH‐55735 from NIMH; grants AG‐07370, AG‐07232, and AG‐08702 from the National Institute on Aging; NIH grant RR‐00645; and the Charles S. Robertson Memorial Gift for Alzheimer’s Disease Research from the Banbury Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Raters and patients were blind to the study design throughout phase A and phase B. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinded research psychiatrist (D.P.D.) who evaluated the patients was also in charge of treatment, including dose adjustment, at all time points in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out was 9% overall. Results of completers analyses were shown in detail. "Intent‐to‐treat analyses were also conducted for the phase A sample, carrying forward the last observation", but results were not shown in detail. Apparently, they were not very different. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | In the initial 1‐week single‐blind phase, all patients received placebo. At the end of this week, the patients who still met the entry criteria were eligible for phase A |
Finkel 1995.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: gross‐over Study duration: 11 weeks (until cross‐over) Rescue medication: benztropine was allowed for 1‐3 weeks, but unclear how many participants used this during the trial. |
|
| Participants |
Number randomised: 33 (16 thiothixene, 17 placebo) Mean age: 85 years Sex (female): 86% Type of dementia: not specified in study, but according to personal communication Alzheimer's and vascular dementia Severity of dementia: severe Indication: agitation Setting: nursing homes Country: USA |
|
| Interventions |
Intervention characteristics Thiothixene
Placebo Twice a day dosage could be adjusted by a maximum of 2 mg every 2 days on the basis of a psychiatrist’s judgement of behavioural improvement or side‐effects after examination of the patient, discussion with the nursing team and review of progress notes |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Number of responders for agitation Death Discontinuation (any reason) Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Weber Pharmaceuticals involved in trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Comparability of groups (selection bias) | Unclear risk | No baseline characteristics of all randomized per group reported, but apparently "unbalanced distributions" at baseline were adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" trial, but persons blinded not specified. "...medication or placebo, which were dispensed twice a day either in 1 mg outwardly identical capsules or in a liquid form at an initial dose of 1 mg." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" trial, but persons blinded not specified. "...medication or placebo, which were dispensed twice a day either in 1 mg outwardly identical capsules or in a liquid form at an initial dose of 1 mg." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 of 33 drop‐out (9%), but all in one group (0% versus 18%). ITT seems to have been performed, but not mentioned explicitly. |
| Selective reporting (reporting bias) | High risk | All reported outcomes were reported, but all secondary outcomes including adverse events were only reported in terms of statistical significance. No means or percentages. |
| Other bias | High risk | "Patients were screened and, if they met criteria, entered a 1‐week medication washout phase, after which a baseline assessment was obtained." |
Grossberg 2020a.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 12 weeks Rescue medication: allowed: antidepressants (if the dose was stable for 30 days prior to randomisation and did not change during the study) and benzodiazepines (during the first 4 weeks of the randomised phase only (limited to 4 days/week with a maximum dose of 2 mg/day of lorazepam or equivalent) |
|
| Participants |
Number randomised: 433 (297 brexpiprazole, 136 placebo) Mean age: 73.8 years Sex (female): 55.2% Type of dementia: Alzheimer's disease Severity of dementia: mild‐severe Indication: Agitation (baseline CMAI 70.9) Setting: care facility or community‐dwelling, provided the patient was not living alone Country: Russia (29.1% of randomised patients), the USA (27.9%), Ukraine (14.8%), Serbia (12.2%), Croatia (8.5%), Spain (4.4%), and Germany (3.0%). Efficacy results of 1 study group (0.5mg/day) not reported |
|
| Interventions | Brexpiprazole: 0.5, 1 mg and 2 mg/day Placebo Doses were titrated over a period of 2−4 weeks (days 1−3, 0.25 mg/day; days 4−14, 0.5mg/day; days 15−28, 1 mg/day; day 29 onwards, assigned dose). Patients unable to tolerate their assigned dose (or matching placebo) were discontinued from the trial. |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Extrapyramidal symptoms Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship Source: Otsuka Pharmaceutical Development & Commercialization Inc (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were assigned by an interactive voice/web response system based on a fixed‐block, computer‐generated randomization code provided by the study sponsor and stratified by study center." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Comparability of groups (selection bias) | High risk | There were various differences between the study groups, including a higher score on the CMAI and on the MMSE in the placebo group than in (all three) drug groups. These differences were not (all) taken into account in the analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment assignments were blinded to patients, investigators, and sponsor personnel..." "Brexpiprazole and matching placebo tablets were provided by the sponsor, packaged in numbered, weekly blister cards." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treatment assignments were blinded to patients, investigators, and sponsor personnel..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No efficacy data for one study group (0.5mg/day). Overall and differential drop‐out low. No ITT analysis of efficacy. |
| Selective reporting (reporting bias) | High risk | Many outcomes reported on clinical trials.gov were not reported |
| Other bias | High risk | Run‐in of 6 weeks to wash out of antipsychotics, and other types of medication |
Grossberg 2020b.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Group: parallel group Study duration: 12 weeks Rescue medication: allowed: antidepressants (if the dose was stable for 30 days prior to randomisation and did not change during the study) and benzodiazepines (during the first 4 weeks of the randomized phase only (limited to 4 days/week with a maximum dose of 2 mg/day of lorazepam or equivalent) |
|
| Participants |
Number randomised: 270 (133 brexpiprazole, 137 placebo) Mean age: 73.8 years Sex (female): 63.0% Type of dementia: Alzheimer's disease Severity of dementia: mild‐severe Indication: agitation (baseline CMAI 69.9) Setting: care facility or community‐dwelling, provided the patient was not living alone Country: Ukraine (28.9% of randomised patients), the USA (22.6%), Russia (19.3%), Bulgaria (17.8%), Canada (4.8%), France (3.3%), Slovenia (2.2%), the UK (0.7%), and Finland (0.4%) |
|
| Interventions | Brexpiprazole: flexible dose 0.5 m to 2mg/day, mean dose 1.54 mg/day. Placebo Brexpiprazole was initiated at 0.25 mg/day (days 1−3), increased to 0.5 mg/day (days 4−14), and further increased to a target dose of 1 mg/day (days 15−28; could be decreased back to 0.5 mg/day). An additional dose increase to 2 mg/day could be initiated from day 29 (week 4 visit) onwards. After week 4, stepwise dose decreases and increases could occur at any time (scheduled or unscheduled visits), based on the investigator’s clinical evaluation of the patient’s response and tolerability. Patients unable to tolerate brexpiprazole 0.5 mg/day (or matching placebo) were discontinued from the trial. |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship Source: Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were assigned by an interactive voice/web response system based on a fixed‐block, computer‐generated randomization code provided by the study sponsor and stratified by study center." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Comparability of groups (selection bias) | Unclear risk | There are several small differences that were not taken into account, and might have influence the results |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment assignments were blinded to patients, investigators, and sponsor personnel..." "Brexpiprazole and matching placebo tablets were provided by the sponsor, packaged in numbered, weekly blister cards." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treatment assignments were blinded to patients, investigators, and sponsor personnel..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall and differential drop‐out low. ITT not used, but given negative study results this was probably not problematic |
| Selective reporting (reporting bias) | High risk | Many outcomes mentioned on clinicaltrials.gov have not been reported |
| Other bias | High risk | 6‐week run‐in for wash‐out |
Japic CTI 142578 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: no information |
|
| Participants |
Number randomised: 150 Type of dementia: Alzheimer's disease Indication: agitation Setting: hospital or care facilities Country: Japan |
|
| Interventions |
Intervention characteristics Aripiprazole
Placebo |
|
| Outcomes | No outcomes reported | |
| Identification | ||
| Notes | Sponsorship source: Otsuka Pharmaceutical Co., Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Use of placebo, but identical tablets not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | The trial was early terminated after enrolment of 150 of 880 participants. The results have not been published. The company did not provide the data when one of the authors (HJL) requested them. |
| Other bias | Unclear risk | No information |
Mintzer 2006 RIS USA 232.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 8 weeks Rescue medication: allowed |
|
| Participants |
Number randomised: 473 (235 risperidone 238 placebo) Mean age: 83.3 years Sex (female): 77.0% Type of dementia: Alzheimer's disease, vascular dementia Severity of dementia: moderate Indication: psychosis (although 50 (included) patients with psychosis at screening were found not to have psychosis anymore at baseline) (baseline BEHAVE‐AD psychosis: 7.4) Setting: nursing homes, long‐term care facilities Country: USA |
|
| Interventions |
Intervention characteristics Risperidone
Placebo Medication was initiated at 0.50 mg (0.25‐mg tablets twice daily) and increased after three days to 1.00 mg (0.5‐mg tablets twice daily). For patients whose clinical response remained inadequate by day 13, medication was increased to 1.50 mg (0.75 mg twice daily). Subsequent adjustments were allowed in patients who experienced adverse events. The minimum treatment dosage was 0.50 mg daily for patients who achieved and maintained efficacy at this dose. After randomisation, the mean daily dosage of risperidone was 1.03 ± 0.24 mg (median: 1.00 mg/day, range: 0.40 mg to 1.90 mg/day), with a maximum daily risperidone dosage of 2.5 mg, which exceeded trial recommendations. |
|
| Outcomes | Psychosis: Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) psychosis subscale Number of responders for psychosis Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Johnson & Johnson Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | "Investigators received sealed envelopes for each patient containing coded details of the treatment in this phase." |
| Comparability of groups (selection bias) | Low risk | (Small) baseline differences, f.i. in sex, type dementia and psychosis, but some are in favour of risperidone and others not. Only psychosis at baseline was adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled with identical tablets, double‐blind, but no reference to blinding of participants and personel specifically. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out was high (>20%). Modified ITT (excluding patients without assessment after baseline). Handling of missing data not reported. |
| Selective reporting (reporting bias) | Unclear risk | Many subanalyses with primary outcome, but adverse events reported in more detail than usual. |
| Other bias | High risk | During the run‐in phase, all patients received placebo for 1 week to wash out previously used psychotropic medications. The run‐in length was reduced for patients not using psychotropic medications and for patients whose psychosis or agitation worsened. |
Mintzer 2007.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed for NPS and EPS |
|
| Participants |
Number randomised: 487 (366 aripiprazole, 121 placebo) Mean age: 82.5 years Sex (female): 79% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: Psychosis (baseline NPI‐NH psychosis: 11.6) Setting: nursing homes or residential assisted‐living facilities Country: USA, Australia, Canada, South Africa, and Argentina |
|
| Interventions |
Intervention characteristics Aripiprazole
Placebo Nodose modification of study medication was allowed for tolerability reasons after titration during the acute phase. Patients unable to tolerate acute‐phase study medication were discontinued from the study. |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory–Nursing Home version (NPI‐NH) psychosis subscale Number of responders for psychosis Extrapyramidal symptoms Somnolence Death Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: Alzheimer’s Research & Clinical Programs, Medical University of South Carolina and the Ralph H. Johnson VA Medical Center, Charleston, SC (JEM); the Department of Psychiatry, Emory University, Atlanta, GA (LET); Bristol‐Myers Squibb Company, Wallingford, CT (CDB, RNM); Bristol‐Myers Squibb Company, Braine l’Alleud, Belgium (RS); and Otsuka Pharmaceutical Development & Commercialization, Princeton, NJ (RDM, AF). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out was very high (42%). Modified ITT with exclusion of patients who did not take at least one dose of study medication, and did not have at least one postbaseline evaluation within 7 days after the last medication was taken. Handling of missing data with LOCF. |
| Selective reporting (reporting bias) | High risk | Outcome was measured with several scales and presented for each drug group separately. Subanalyses of individual NPI items. No correction for multiple testing. % any adverse event per group missing. No study protocol available. |
| Other bias | High risk | Concomitant use of antipsychotics, mood stabilizers or sedatives (except trazodone [25–50 mg], zolpidem tartrate [2.5–5.0 mg] and temazepam [7.5–15.0 mg]) was prohibited during the study treatment period and the seven days prior to randomization. |
NCT00287742 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 8 weeks Rescue medication: no information provided |
|
| Participants |
Number randomised: 33 (13 risperidone, 17 placebo, 3 unclear) Mean age: unclear, around 77 years Sex (female): 77% Type of dementia: Alzheimer's dementia Severity of dementia: moderate Indication: psychosis Setting: In‐ or outpatients Country: Japan |
|
| Interventions |
Intervention characteristics Risperidone
Placebo |
|
| Outcomes | Psychosis: Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) psychotic symptom cluster score Extrapyramidal symptoms Any adverse event Any serious adverse event Discontinuation (any reason) |
|
| Identification | ||
| Notes | Sponsorship source: Janssen Pharmaceutical K.K. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Comparability of groups (selection bias) | Unclear risk | Clear difference in psychosis between groups at baseline. Other characteristics not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" trial, but unclear who was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" trial, but unclear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was 7 of 33 (21%) in total and differed for 18% between groups. Outcomes were provided for all participants at endpoint. |
| Selective reporting (reporting bias) | High risk | Only BEHAVE‐AD Psychotic Symptom Cluster Score reported. Results for ADL and MMSE not reported. Early terminated trial. No full article. |
| Other bias | High risk | Single‐blind (with placebo) run‐in of 1 week. |
Paleacu 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 6 weeks Rescue medication: not allowed for NPS or EPS |
|
| Participants |
Number randomised: 40 (20 quetiapine, 20 placebo) Mean age: 82.2 years Sex (female): 65% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: diverse NPS, but 68% had delusions, so majority was psychotic. Setting: not reported Country: Israel |
|
| Interventions |
Intervention characteristics Quetiapine
Placebo After initial screening visit, patients were given at visit 2 (baseline) quetiapine at a dose of 25 mg twice daily during the first week, and then increased to a target dose of 150 mg/day by increments of 50 mg every week. If at the target dose no NPI improvement was measured additional increments of 50 mg per week were continued until a maximal dose of 300 mg/day or until side effects were reported by the patient. Median total daily dose of quetiapine in the treatment group was 200 mg at week 6 (range: 75 mg to 300 mg/day). |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory (NPI) items delusions and hallucinations only) Number of responders for psychosis Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: Grant from AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out was 15%. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. But high discontinuation rate with a very small sample with a high rate of imputed data. Results included patients that dropped‐out (ITT analysis), with LOCF for missing data. |
| Selective reporting (reporting bias) | Unclear risk | No detailed data for NPI total baseline to endpoint. For CGI‐C no comparison data with placebo. The study report fails to include results for a key outcome (NPI total) that would be expected to have been reported for in the usual way (baseline to endpoint). No study protocol available. |
| Other bias | High risk | For those patients who received other antipsychotics before the trial a wash‐out period of 2 weeks was mandatory. |
RIS‐INT‐83 2003.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 8 weeks Rescue medication: no information provided |
|
| Participants |
Number randomised: 18 (10 risperidone, 8 placebo) Mean age: unclear Sex (female): 72.2% Type of dementia: Alzheimer's disease Severity of dementia: unclear Indication: Psychosis (Baseline BEHAVE‐AD Psychosis: 7.1) Setting: nursing homes or long‐term care facilities Country: Israel |
|
| Interventions |
Intervention characteristics Risperidone
Placebo |
|
| Outcomes | Psychosis: Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) Number of responders for psychosis Extrapyramidal symptoms Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events |
|
| Identification | ||
| Notes | Sponsorship source: Johnson & Johnson Pharmaceutical Research& Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out was 22%, but results were reported for all randomized. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported. |
| Other bias | High risk | All eligible subjects first participated in a single‐blind, placebo‐controlled run‐in phase of 7 days. |
Schneider 2003 RIS USA 63.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 12 weeks Rescue medication: allowed Country: USA |
|
| Participants |
Number randomised: 463 (of 625 in main trial) Mean age: 83.3 years Sex (female): 72% Type of dementia: Alzheimer's disease, vascular dementia, mixed type dementia Severity of dementia: severe Indication: mixed for main trial, but results were reported for the subgroup with psychosis at baseline. Setting: nursing home or chronic disease hospital |
|
| Interventions |
Intervention characteristics Risperidone
Placebo |
|
| Outcomes | Psychosis: Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) psychosis subscale | |
| Identification | ||
| Notes | Sponsorship source: Janssen Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information for subgroup cohort |
| Allocation concealment (selection bias) | Unclear risk | No information for subgroup cohort |
| Comparability of groups (selection bias) | Unclear risk | No information for subgroup cohort |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Identically appearing risperidone (0.25, 0.5, and 1.0 mg) and placebo tablets were supplied by the sponsor. Each patient received 2 tablets twice daily. All trial drugs were appropriately labelled with a 2‐part la‐ bel containing the visit, protocol, patient numbers, and directions for administration. The first part of the label remained attached to the medication carton and the second part (double‐blind portion) was detached and placed in the case report form.Only says double blind but doesn't mention the persons who were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information if trained raters were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Modified (all patients who received at least one dose and one post baseline assessment) ITT analysis performed. Imputation LOCF. Discontinuation rates imbalanced across groups (range from 27% in the placebo group to 42% in the group receiving 2mg risperidone). |
| Selective reporting (reporting bias) | High risk | This is a secondary report of the trial based on the patients with psychosis only. Primary results are presented for the 1.0 and 2.0 group combined. Unclear why. Many outcomes that were reported in the main results paper based on all participants were not reported here. |
| Other bias | High risk | After a single‐blind placebo washout period of 3 to 7 days, eligible patients were randomly assigned to placebo or to 0.5, 1.0, or 2.0 mg/day of risperidone |
Schneider 2006 CATIE‐AD.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 2 to 36 weeks, but endpoint for outcomes is 12 weeks Rescue medication: allowed |
|
| Participants |
Number randomised: 421 (100 olanzapine 94 quetiapine 85 risperidone 142 placebo) Mean age: 77.9 years Sex (female): 56% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: generic NPS, but 82% had delusions and 86% agitation. Setting: outpatients living at home or assisted‐ living facility with regular contact to caregiver Country: USA |
|
| Interventions |
Intervention characteristics Olanzapine
Quetiapine
Risperidone
Placebo The treating physician could adjust the dosage, as indicated clinically, over the 36 weeks of the trial. The mean initially prescribed doses were 3.2 mg of olanzapine per day, 34.1 mg of quetiapine per day, and 0.7 mg of risperidone per day. The last prescribed mean dose in phase 1 was 5.5 mg of olanzapine per day, 56.5 mg of quetiapine per day, and 1.0 mg of risperidone per day. |
|
| Outcomes | Agitation: Neuropsychiatric Inventory (NPI) agitation Number of responders for agitation Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) Health‐related quality of life: Alzheimer’s Disease Related Quality of Life (ADRQL) Functioning in activities of daily living: Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory (ADCS‐ADL) Carer burden or carer quality of life: The Caregiver Activity Survey (CAS) |
|
| Identification | ||
| Notes | Sponsorship source: Supported by a grant (NO1 MH9001) from the NIMH. AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly provided medications for the studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed with the use of permuted blocks of nine per site without stratification and was implemented with the use of an interactive voice‐response telephone system."The use of blocks diminishes concealment, but the blocks are large and an interactive voice‐response telephone system is used. |
| Comparability of groups (selection bias) | Unclear risk | Many small baseline differences varyingly in favour of a drug or placebo. Analyses were adjusted for differences in age, sex, MMSE score, and total BPRS score. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trials was "double‐blind" and "Medications were dispensed at each visit in the form of identically appearing small and large capsules [...]". Persons blinded has not been specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was "double‐blind" and "Medications were dispensed at each visit in the form of identically appearing small and large capsules [...]". Persons blinded has not been specified. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall rate of discontinuation of treatment at 12 weeks was 63% (primary outcome).Other outcomes are analysed with modified ITT (patients without an assessment after baseline are excluded). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available but no outcomes prespecified. |
| Other bias | Low risk | Washout from previous treatment and run‐in periods were not used because of the patients’ acute clinical symptoms; instead, the study design allowed for rapid assignment and initiation of treatment to be consistent with clinical practice. |
Streim 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed for NPS and EPS. |
|
| Participants |
Number randomised: 256 (131 aripiprazole125 placebo) Mean age: 83.0 years Sex (female): 76% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: psychosis (Psychosis NPI‐NH: 10.6) Setting: institutionalised persons (i.e. residing in a nursing home or residential assisted‐living facility) Country: USA |
|
| Interventions |
Intervention characteristics Aripiprazole
Placebo Aripiprazole dosing was flexible, starting at 2 mg/day, with titration to higher doses (5 mg, 10 mg, and 15 mg/day) depending on the study physicians’ clinical judgment. The recommended titration schedule was 2 mg/day for 1 week, increasing to 5 mg/day for 2 weeks, 10 mg/day for the next 2 weeks, and 15 mg/day for the remainder of the acute phase (Weeks 6 –10). The mean daily aripiprazole dose at Week 10 was 9.0 mg (range: 0.7 mg to 15.0 mg). |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory–Nursing Home version (NPI‐NH) psychosis score Number of responders for psychosis Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) Functioning in activities of daily living: Alzheimer’s Disease Cooperative Study – Activities of Daily Living for Severe impairment (ADCS‐ADL‐SEV) |
|
| Identification | ||
| Notes | Sponsorship source: Bristol‐Myers Squibb Company, Wallingford, CT (CDB, Marcus); Bristol‐Myers Squibb Company, Braine l’Alleud, Belgium (RS); Otsuka America Pharmaceutical Inc., Princeton, NJ (McQuade, WHC) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | Limited set of relevant baseline characteristics shown. Small differences in set shown. Outcome score at baseline is not given for all randomized, but adjusted for in the analyses. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out was high (41%). Modified ITT was used excluding patients that did not receive one dose of study medication or an assessment after baseline (within 7 days after taking medication). Missing data was imputed with LOCF. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. Study protocol is available but no outcomes prespecified. Two coprimary outcomes. Multiple scales for NPS. Many additional analyses including OC comparisons. Unusual cut‐offs f.i. only weight increase of 7% or more included. Correction for multiple testing. |
| Other bias | High risk | Following screening and a minimum 7‐day psychotropic medication washout period (...) |
Tariot 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed for agitation, not for EPS |
|
| Participants |
Number randomised: 284 (94 haloperidol 91 quetiapine 99 placebo) Mean age: 83.1 years Sex (female): 73.2% Type of dementia: Alzheimer's disease Severity of dementia: moderate Indication: Psychosis (Baseline Psychosis NPI‐NH: 10.0) Setting: nursing homes Country: USA |
|
| Interventions |
Intervention characteristics Haloperidol
Quetiapine
Placebo We initiated quetiapine at 25 mg per day, increased by 25 mg every four days to a target dosage of 100 mg per day by day 14. We started haloperidol at 0.5 mg per day, increased by 0.5 mg per day every four days through day 14. We had the ability to adjust dosages thereafter according to clinical response and tolerability to a maximum of 600 mg per day of quetiapine or 12 mg per day of haloperidol. The median of the mean daily dose of quetiapine was 96.9 mg; the median maximum was 125.0 mg. The median of the mean daily dose of haloperidol was 1.9mg; the median maximum was 2.0 mg. |
|
| Outcomes | Psychosis: Neuropsychiatric Inventory–Nursing Home version 2 (NPI‐NH2) (delusion + hallucination items) Number of responders for psychosis Extrapyramidal symptoms Somnolence Deaths (Notes: Reported for all participants, not just for those with AD) Any serious adverse events Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) Functioning in activities of daily living (ADL): Physical Self‐Maintenance Scale (PSMS) |
|
| Identification | ||
| Notes | Sponsorship source: AstraZeneca Pharmaceuticals LP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Comparability of groups (selection bias) | Unclear risk | Some smaller and larger differences, of which it is unclear if and how they affected the results. Only baseline outcome score was adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a "double‐blind" trial. "Identically appearing capsules containing 25 mg quetiapine, 0.5 mg haloperidol, or placebo" were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out was high (29%), analyses were not based on all randomized, LOCF for missing data. However, it is a negative trial. In addition, missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information. 'Any adverse events' and 'serious adverse events' were not reported. |
| Other bias | High risk | Participants underwent a washout of ≥48 hours. |
Teri 2000.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 16 weeks Rescue medication: not allowed for NPS or EPS. |
|
| Participants |
Number randomised: 70 (34 haloperidol, 36 placebo) Mean age: 75.5 years Sex (female): 62.9% Type of dementia: Alzheimer's disease Severity of dementia: moderate‐severe Indication: agitation Setting: community Country: USA |
|
| Interventions |
Intervention characteristics Haloperidol
Placebo Medication was provided in tablets of haloperidol 0.5 mg, treatment began with one tablet each day and increased at the next clinic visit by one tablet a day unless the subject was rated as moderately or markedly improved, significant adverse events were noted, or the maximum dose was reached (3 mg/day for haloperidol).Participants achieved a mean haloperidol dose of 1.861 mg/day (range, 0 to 3 mg/d)ay. |
|
| Outcomes | Agitation: Cohen‐Mansfield Agitation Inventory (CMAI) Number of responders for agitation Extrapyramidal symptoms (Notes: Parkinsonian gait, rigidity, tremor, and bradykinesia are forms of EPS. Rigidity showed the largest contrast to avoid underestimating the risk of EPS: 33% (11) and 13% (5)). Somnolence Death Discontinuation (any reason) Cognitive function: Mini‐Mental State Examination (MMSE) Functioning in activities of daily living (ADL): Instrumental Activities of Daily Living (IADL) Carer burden or carer quality of life: The Screen for Caregiver Burden (SCB) |
|
| Identification | ||
| Notes | Sponsorship source: supported by a grant from the National Institute of Aging (AG‐010483). Active study medications and corresponding placebos were provided by Purpac Pharmaceutical, Elizabeth, NJ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided.It was a "randomized" trial. "Treatments were assigned in randomized blocks of nine (for three arms) or 12 (for four arms)." Restriction methods could potentially affect allocation concealment. |
| Comparability of groups (selection bias) | Low risk | There were small differences between the treatment groups. The authors found that "None of the prestated potential confounding variables [patient age, patient gender, MMSE score, total CMAIscore, and caregiver gender] [...] was significantly related to primary outcome." And the trial was a negative trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not completely blinded, because one arm (behaviour management techniques) could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments were conducted [...] by interviewers blind to treatment assignment." "To insure that interviewers remained blind to treatment assignment, caregivers did not discuss any aspect of their treatment with the interviewer. In no instance was the blinding compromised.""Medication was provided in [...] identically appearing tablets." "The primary outcome measure was [...] completed by a trained clinician blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out was high (36%).ITT‐analysis: "Missing scores at posttreatment were imputed with last observation carried forward from discontinuation visit scores or, if those were not available, from midpoint visit scores. If no ADCS‐CGIC score [primary outcome, rev] was available, a value of “worse” was assigned (n=12). The rationale for doing this was to assume the worst case scenario: subjects who dropped out did so because they became worse. In addition,we reviewed the reasons caregivers cited for dropping out of the study and in each instance our assignment of“worse” was confirmed. " |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to be reported. |
| Other bias | High risk | Subjects receiving psychotropic medications were required to discontinue them at least 2 weeks before study enrolment. |
Zhong 2007.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Study duration: 10 weeks Rescue medication: allowed |
|
| Participants |
Number randomised: 333 (241 quetiapine, 92 placebo) Mean age: 83.2 years Sex (female): 74.2% Type of dementia: Alzheimer's disease and vascular dementia Severity of dementia: severe Indication: Agitation (Baseline PANSS‐EC: 23.0) Setting: nursing homes and assisted living facilities Country: USA |
|
| Interventions |
Intervention characteristics Quetiapine
Placebo Participants randomised to treatment with quetiapine initially received 25 mg/day. The dose was titrated in 25 mg increments every day to reach 100 mg/day on day 4 for both quetiapine treatment groups; those assigned to 100 mg/day were maintained on this dose, those randomised to 200 mg/day continued the titration in 25 mg increments daily to reach the target dose of 200 mg on day 8, after which the dose was held constant. Participants unable to tolerate the assigned treatment were discontinued from the study. |
|
| Outcomes | Agitation: Positive and Negative Syndrome Scale‐Excitement Component (PANSS‐EC) Number of responders for agitation Extrapyramidal symptoms Somnolence Death Any adverse event Any serious adverse event Discontinuation (any reason) Discontinuation due to adverse events Cognitive function: Mini‐Mental State Examination (MMSE) |
|
| Identification | ||
| Notes | Sponsorship source: AstraZeneca Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The centralized randomization schedule was generated using a random block size of 8 and was created using random seed and treatment allocation ratios of 3:3:2 and maintained blinded by the sponsor’s randomization group. |
| Allocation concealment (selection bias) | Low risk | "The centralized randomization schedule was [...] maintained blinded by the sponsor’s randomization group." |
| Comparability of groups (selection bias) | High risk | Limited table of baseline characteristics. Clear baseline differences in sex, race and type of dementia. Also somewhat more agitation at baseline in drug groups. Type of dementia and baseline outcome score were adjusted for. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was a "double‐blind" trial. "Each [medication] kit contained 10 blister wallets with the same number of tablets and the same configuration of color, size, and shape. Study medication was administered twice daily from blister wallets."Personal not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was a "double‐blind" trial. "Each [medication] kit contained 10 blister wallets with the same number of tablets and the same configuration of color, size, and shape. Study medication was administered twice daily from blister wallets."Personal not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out was high (35%). Modified ITT analysis excluding patients that did not receive one dose of study medication or one assessment after baseline. LOCF was used for imputing missing data. |
| Selective reporting (reporting bias) | High risk | Various scale for the outcome have been used. The least commonly used is presented as the primary outcome. Results based on OC analyses and p‐values are given undue attention f.i. in the abstract. Subgroup analyses for type of dementia. Protocol was submitted after publication and does not list pre‐specified outcomes. |
| Other bias | Low risk | No run‐in period |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| DeDeyn 1999 RIS‐INT‐24 | Wrong patient population. Patients with a broad range of neuropsychiatric symptoms enrolled, not specific for agitation or psychosis. |
| Devanand 1989 | Wrong study design. Placebo and haloperidol were not given in parallel groups. Each patient received both drugs consecutively (placebo ‐ haloperidol ‐ placebo). |
| Holmes 2007 | Wrong study design. Wrong comparator, not placebo controlled |
| Meguro 2004 | Wrong study design. Wrong comparator, not placebo controlled |
| NCT00043849 2002 | Wrong patient population (not Alzheimer's or vascular dementia) |
| Pollock 2002 | Wrong population. Unclear how many were psychotic or agitated. Many patients with Lewy body dementia included. |
| Shin 2013 | Wrong study design (one study group only, not placebo controlled) |
| Street 2000 F1D MC HGEU | Wrong population. Description on the patient population far too vague and therefore unclear how many patients were psychotic or agitated |
| Trequattrini 2003 | Wrong study design (one study group only, not placebo controlled) |
Differences between protocol and review
We replaced two secondary outcomes to be displayed in the SoF tables. We presented the number of responders for agitation and the number of responders for psychosis instead of risk of adverse event and serious adverse events since we judged the number of responders to be more important than the unspecific adverse events and serious adverse events ‐ we still display specific adverse events in the SoF tables (i.e. extrapyramidal symptoms, somnolence and death).
There were eight trials that tested an atypical antipsychotic for agitation in dementia. One of those trials focussed specifically on aggression, which is a subtype of agitation. We pooled the trials as planned, but also decided to show the pooled effect of the other trials without this trial in the meta‐analysis.
We did not perform the pre‐planned sensitivity analyses excluding trials with at least one rating of high risk of bias because all studies had a high risk of bias rating in at least one domain. In addition, we did not perform the pre‐planned sensitivity analysis excluding trials that only reported per‐protocol analysis due to the lack of such studies.
We did not find any unpublished studies for which we needed to contact the pharmaceutical company.
Contributions of authors
VM: developing the protocol, screening relevant literature, extracting data, performing the data‐analyses and drafting the review.
RM: screening relevant literature, extracting data, performing the data‐analysis, and drafting the review.
MND: screening relevant literature and drafting the review.
SUZ: developing the protocol and drafting the review.
SK: developing the protocol, screening relevant literature, data‐extraction, and drafting the review.
HJL: developing the protocol, screening relevant literature, data‐extraction, and drafting the review.
Sources of support
Internal sources
-
New Source of support, Other
None
External sources
-
NIHR, UK
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
VM: none known
RM: none known
MND: none known
SUZ: none known
SK: none known
HJL: none known
Edited (no change to conclusions)
References
References to studies included in this review
Allain 2000 {published data only}
- Allain H, Dautzenberg PH, Maurer K, Schuck S, Bonhomme D, Gerard D. Double blind study of tiapride versus haloperidol and placebo in agitation and aggressiveness in elderly patients with cognitive impairment. Psychopharmacology 2000;148(4):361-6. [DOI] [PubMed] [Google Scholar]
Auchus 1997 {published data only}
- Auchus AP, Bissey-Black C. Pilot study of haloperidol, fluoxetine, and placebo for agitation in Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences 1997;9(4):591-3. [DOI: 10.1176/jnp.9.4.591] [DOI] [PubMed] [Google Scholar]
Ballard 2005 {published data only}
- Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874-7. [DOI: 10.1136/bmj.38369.459988.8F] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2018 {published data only}
- Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurology 2018;17(3):213-22. [DOI: 10.1016/S1474-4422%2818%2930039-5] [DOI] [PubMed] [Google Scholar]
Brodaty 2003 RIS‐AUS‐05 {published data only}
- Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. Journal of Clinical Psychiatry 2003;64(2):134143. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, etal. Risperidone for psychosis of Alzheimer's disease and mixed dementia: results of a double-blind, placebo-controlled trial. International Journal of Geriatric Psychiatry 2005;20(12):1153-7. [DOI: 10.1002/gps.1409] [DOI] [PubMed] [Google Scholar]
- Brodaty H, Clarnette R, Ames D, Snowdon J, Woodward M, Kirwan J, et al. Risperidone in the treatment of agitation, aggression and psychosis of dementia. Neurobiology of Aging 2002;23(1 Suppl):AbstractNo289. [Google Scholar]
- Brodaty H, Grossman F, Bruynseels J, Lyons B. Risperidone in the treatment of agitation and psychosis of dementia. International Psychogeriatrics 2001;13(Suppl 2):S108. [Google Scholar]
- Clarnette R, Brodaty H, Ames D, Snowdon J, Lee E, Woodward M, et al. Psychological symptoms are improved with risperidone in patients with dementia-related psychosis. In: Proceedings of the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologicum (International Journal of Neuropsychopharmacology), June 23-27 2002, Montreal, Canada CNO - CN-01000034. Vol. 5. 2002:S91.
- Frank L, Kleinman L, Ciesla G, Rupnow MF, Brodaty H. The effect of risperidone on nursing burden associated with caring for patients with dementia. Journal of the American Geriatrics Society 2004;52(9):1449-55. [DOI: 10.1111/j.1532-5415.2004.52406.x] [DOI] [PubMed] [Google Scholar]
- Kirwan J, Brodaty H, Ames D, Snowdon J, Woodward M, Clarnette R, et al. Risperidone in the treatment of agitation and psychosis of dementia. In: 155th Annual Meeting of the American Psychiatric Association; 2002 may 18-23; Philadelphia, PA, USA. 2002.
- Woodward M, Brodaty H, Ames D, Clarnette R, Kirwan J, Lee E, et al. Risperidone in the treatment of agitation adn psychosis of dementia [abstract]. Internal Medicine Journal 2003;33(5-6):A30. [Google Scholar]
- Woodward M, Brodaty H, Lee E. Risperidone in the treatment of agitation and psychosis of dementia: a multicentre, double-blind, placebo controlled study. In: Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3-6, Geneva CNO - CN-00386102. 2002:255.
Deberdt 2005 F1D MC HGGU {published data only}
- Deberdt WG, Dysken MW, Rappaport SA, Feldman PD, Young CA, Hay DP, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. American Journal of Geriatric Psychiatry 2005;13(8):722-30. [DOI: 10.1176/appi.ajgp.13.8.722] [DOI] [PubMed] [Google Scholar]
- Eli Lilly. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer's disease. Eli Lilly and Company Clinical Trial Registry CNO - CN-00744177 2005:1-52.
- Eli Lilly Inc. Olanzapine versus risperidone and placebo in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Eili Lilly Inc CNO - CN-00548830 2005:1-38.
- No author. Risperidone vs olanzapine vs placebo (ORP): Alzheimer's disease with psychosis. John Hopkins University clinical trials database 2000.
De Deyn 2004 F1D MC HGIV {published data only}
- Deberdt WG, De Deyn PP, Carrasco MM, Jeandel C, Hay DP, Feldman PD, et al. Cognitive function in patients with Alzheimer's dementia during double-blind treatment of psychosis with olanzapine. In: Conference posterEli lilly and CO. 2005.
- De Deyn PP, Carrasco MM, Deberdt W, Jeandel C, Hay DP, Feldman PD, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(2):115-26. [DOI: 10.1002/gps.1032] [DOI] [PubMed] [Google Scholar]
- Hay DP, Deberdt W, DeDeyn PP, Carrasco MM, Jeandel C, Feldman PD, et al. Cognitive function in patients with alzheimers dementia during double-blind treatment of psychosis with olanzapine. International Psychogeriatrics 2003;15(Suppl 2):336. [Google Scholar]
- Hoffmann VP, DeDeyn PP, Carrasco MM, Deberdt W, Jeandel C, Hay DP, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with alzheimer's dementia. International Psychogeriatrics 2003;15(Suppl 2):337. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Deberdt W, DeDeyn PP, Carrasco MM, Jeandel C, Hay DP, et al. Analysis of motor function in patients with Alzheimer's dementia during double-blind treatment of psychosis with olanzapine. International Psychogeriatrics 2003;15(Suppl 2):307. [Google Scholar]
De Deyn 2005 {published data only}
- De Deyn P, Jeste DV, Swanink R, Kostic D, Breder C, Carson WH, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer's disease: a randomized, placebo-controlled study. Journal of Clinical Psychopharmacology 2005;25(5):463-7. [DOI: 10.1097/01.jcp.0000178415.22309.8f] [DOI] [PubMed] [Google Scholar]
Devanand 1998 {published data only}
- Devanand, DP, Michaels K, Sackeim HA, Marder K, Mayeux RP. Antipsychotics in the treatment of dementia complicated by psychosis. In: Conference Proceedings 151st Annual Meeting of the American Psychiatric Association.; 30th May-4th June, 1998; Toronto, Ontario, Canada CNO - CN-00279937. 1998:No. 27D.
- Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, et al. A randomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer's disease. American Journal of Psychiatry 1998;155(11):1512-20. [DOI: 10.1176/ajp.155.11.1512] [DOI] [PubMed] [Google Scholar]
Finkel 1995 {published data only}
- Finkel, SI, Lyons JS, Anderson RL, Sherrell K, Davis J, Cohen-Mansfield, J, et al. A randomized, placebo-controlled trial of thiothixene in agitated, demented nursing home patients. International Journal of Geriatric Psychiatry 1995;10(2):129-36. [DOI: ] [Google Scholar]
Grossberg 2020a {published data only}
- Grossberg GT, Kohegyi E, Mergel V, Josiassen MK, Meulien D, Hobart M, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer's dementia: two12-week, randomized, double-blind, placebo-controlled trials. American Journal of Geriatric Psychiatry 2020;28(4):383-400. [DOI] [PubMed] [Google Scholar]
Grossberg 2020b {published data only}
- Grossberg GT, Kohegyi E, Mergel V, Josiassen MK, Meulien D, Hobart M, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer's Dementia: two12-week, randomized, double-blind, placebo-controlled trials. American Journal of Geriatric Psychiatry 2020;28(4):383-400. [DOI] [PubMed] [Google Scholar]
Japic CTI 142578 2015 {published data only}
- JapicCTI-142578. Aripiprazole for the treatment of patients with agitation associated with dementia of the Alzheimer's type. http://www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-142578 2015.
Mintzer 2006 RIS USA 232 {published data only}
- Colon S, Figueroa C, Thein S, Siegal A, Arias B, De La Ganadara J, et al. Efficacy and safety of a flexible dose of risperidone versus placebo in the treatment of psychosis of Alzheimer's disease. In: Clinical trials journal CNO - CN-00404159. 2002:1-6.
- Mintzer J, Greenspan A, Caers I, Van Hove I, Kushner S, Weiner M, et al. Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. American Journal of Geriatric Psychiatry 2006;14(3):280-91. [DOI: 10.1097/01.JGP.0000194643.63245.8c] [DOI] [PubMed] [Google Scholar]
Mintzer 2007 {published data only}
- Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. American Journal of Geriatric Psychiatry 2007;15(11):918-31. [DOI: 10.1097/JGP.0b013e3181557b47] [DOI] [PubMed] [Google Scholar]
NCT00287742 2006 {published data only}
- A study of the effectiveness and safety of risperidone versus placebo in the treatment of patients with hallucinations and delusions associated with Alzheimer's disease. https://clinicaltrials.gov/show/NCT00287742 2006.
Paleacu 2008 {published data only}
- Paleacu D, Barak Y, Mirecky I, Mazeh D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer's disease patients: a 6-week, double-blind, placebo-controlled study. International Journal of Geriatric Psychiatry 2008;23(4):393-400. [DOI: 10.1002/gps.1892] [DOI] [PubMed] [Google Scholar]
RIS‐INT‐83 2003 {published data only}
- Johnson, Johnson Pharmaceutical R, Development LLC. Efficacy and safety of a flexible dose of risperidone versus placebo in the treatment of psychosis of Alzheimers disease. A double-blind, placebo-controlled, parallel-group study. Yale open data access (YODA) project 2003.
Schneider 2003 RIS USA 63 {published data only}
- Brecher M, Clyde C, Risperidone Study Group. Risperidone in the treatment of psychosis and aggressive behavior in patients with dementia. In: Proceedings of the 8th Congress of the International Psychogeriatric Association: Jerusalem, Israel, August 17-22, 1997 CNO - CN-00713949. 1997:10.
- Brecher M. Follow-up study of risperidone in the treatment of patients with dementia: interim results on tardive dyskinesia and dyskinesia severity. In: 11th European College of NeuropsychoPharmacology Congress. Paris, France. 31st October - 4th November 1998. 1998.
- Grossman F, Gharabawi G. Elderly patients with dementia and psychosis treated with risperidone. In: Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20-25, Stockholm, Sweden CNO - CN-00385944. 2002:AbstractNo299.
- Grossman F, Okamoto A, Turkoz I, Gharabawi G. Risperidone in the treatment of elderly patients with psychosis of Alzheimer's disease and related dementias. Journal of the American Geriatrics Society 2004;52(5):852-3. [DOI] [PubMed] [Google Scholar]
- Katz I, Brecher M, Clyde C, Napolitano J, Risperidone Study Group. Risperidone in the treatment of psychosis and aggressive behavior in patients with dementia. Proceedings of the 9th Biennial Winter Workshop on Schizophrenia; 1998 Feb 7-13, Davos, Switzerland CNO - CN-00856755 1998;29(1-2):145. [Google Scholar]
- Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M, Risperidone Study Group. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Journal of Clinical Psychiatry 1999;60(2):107-15. [DOI] [PubMed] [Google Scholar]
- Katz IR, Rupnow M, Kozma C, Schneider L. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation: secondary analysis of a double-blind, placebo-controlled trial. American Journal of Geriatric Psychiatry 2004;12(5):499-508. [DOI: 10.1176/appi.ajgp.12.5.499] [DOI] [PubMed] [Google Scholar]
- Mintzer J, Ko G. Risperidone in the treatment of nonaggressive agitated symptoms related to dementia: analysis of 617 patients in a randomized, double-blind controlled trial. International Psychogeriatrics 2003;15(Suppl 2):84. [Google Scholar]
- Schneider L, Katz IR, Napolitano J, Martinez R, Azen SP. Psychosis of Alzheimer's disease: validity of the construct, and response to risperidone. Research and Practice in Alzheimer's Disease 2004;9(2004):153-9. [PubMed] [Google Scholar]
- Schneider LS, Katz IR, Park S, Azen S, Martinez RA. Psychosis of alzheimer's disease: validity and response to risperidone. In: 155th annual meeting of the American Psychiatric Association; 2002 May 18-23rd; philadelphia, PA, USA. 2002.
- Schneider LS, Katz IR, Park S, Napolitano J, Martinez RA, Azen SP. Psychosis of Alzheimer disease: validity of the construct and response to risperidone. American Journal of Geriatric Psychiatry 2003;11(4):414-25. [PubMed] [Google Scholar]
Schneider 2006 CATIE‐AD {published data only}
- Davis SM, Koch GG, Davis CE, LaVange LM. Statistical approaches to effectiveness measurement and outcome-driven re-randomizations in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studies. Schizophrenia Bulletin 2003;29(1):73-80. [DOI] [PubMed] [Google Scholar]
- Nagata T, Shinagawa S, Nakajima S, Plitman E, Mihashi Y, Hayashi S, et al. Classification of neuropsychiatric symptoms requiring antipsychotic treatment in patients with Alzheimer's disease: analysis of the CATIE-AD study. Journal of Alzheimer's Disease 2016;50(3):839-45. [DOI: 10.3233/JAD-150869] [DOI] [PubMed] [Google Scholar]
- Ozawa C, Roberts R, Yoshida K, Suzuki T, Lebowitz B, Reeves S, et al. Placebo effects in the treatment of Noncognitive symptoms of Alzheimer's disease: analysis of the CATIE-AD data. Journal of Clinical Psychiatry 2017;78(9):e1204-e1210. [DOI: 10.4088/JCP.17m11461] [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Leslie DL, Sindelar JL, Miller EA, Tariot PN, Dagerman KS, et al Clinical Antipsychotic Trial of Intervention Effectiveness-Alzheimer's Disease (CATIE-AD) investigators. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer disease. Archives of General Psychiatry 2007;64(11):1259-68. [DOI: 10.1001/archpsyc.64.11.1259] [DOI] [PubMed] [Google Scholar]
- Schneider LS, Ismail MS, Dagerman K, Davis S, Olin J, McManus D, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer's disease trial. Schizophrenia Bulletin 2003;29(1):57-72. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Mack W, Dagerman K, Hsiao JK, Lebowitz BD, Lyket CG, et al. Second-generation antipsychotics for Alzheimer's disease patients and metabolic abnormalities: the CATIE-AD study. American Journal of Geriatric Ppsychiatry 2009;17(SUPPL. 13):A75-A76. [DOI: 10.1097/01.JGP.0000346964.46544.ec] [DOI] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. New England Journal of Medicine 2006;355(15):1525-38. [DOI: 10.1056/NEJMoa061240] [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. American Journal of Geriatric Psychiatry 2001;9(4):346-60. [PubMed] [Google Scholar]
- Schneider LS, Vigen CL, Mack WJ, Dagerman KS, Keefe R, Sano M, et al. Cognitive effects of atypical antipsychotics in patients with Alzheimer's disease: outcomes from CATIE-AD. In: American Journal of Geriatric Psychiatry. Vol. Conference: AAGP Annual Meeting 2009 Honolulu, HI United States. Conference Start: 20090305 Conference End: 20090308. Conference Publication:. 2009:A76. [DOI: 10.1097/01.JGP.0000346964.46544.ec] [DOI]
- Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Antipsychotic medication treatment response in Alzheimer's disease (CATIE-AD): patient symptoms, function, and life quality, and caregiver well-being. In: American Journal of Geriatric Psychiatry. Vol. Conference: AAGP Annual Meeting 2009 Honolulu, HI United States. Conference Start: 20090305 Conference End: 20090308. Conference Publication:. 2009:A74-A75. [DOI: 10.1097/01.JGP.0000346964.46544.ec] [DOI]
- Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et alCATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. American Journal of Psychiatry 2008;165(7):844-54. [DOI: 10.1176/appi.ajp.2008.07111779] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen CL, Mack WJ, Keefe RS, Sano M, Sultzer DL, Stroup TS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: outcomes from CATIE-AD. American Journal of Psychiatry 2011;168(8):831-9. [DOI: 10.1176/appi.ajp.2011.08121844] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Roberts R, Suzuki T, Lebowitz B, Reeves S, Howard R, et al. Lack of early improvement with antipsychotics is a marker for subsequent nonresponse in behavioral and psychological symptoms of dementia: analysis of CATIE-AD Data. American jJournal of GeriatricPpsychiatry 2017;25(7):708-16. [DOI: 10.1016/j.jagp.2017.01.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Mack WJ, Dagerman KS, Hsiao JK, Lebowitz BD, Lyketsos CG, et al. Metabolic changes associated with second-generation antipsychotic use in Alzheimer's disease patients: the CATIE-AD study. American Journal of Psychiatry 2009;166(5):583-90. [DOI: 10.1176/appi.ajp.2008.08081218] [DOI] [PMC free article] [PubMed] [Google Scholar]
Streim 2008 {published data only}
- Streim JE, Porsteinsson AP, Breder CD, Swanink R, Marcus R, McQuade R, et al. A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. American Journal of Geriatric Psychiatry 2008;16(7):537-50. [DOI: 10.1097/JGP.0b013e318165db77] [DOI] [PubMed] [Google Scholar]
Tariot 2006 {published data only}
- A Protocol No 5077IL/0039: a multicenter, double-blind comparison of efficacy and safety of Seroquel® (quetiapine fumarate), haloperidol, and placebo in the treatment of elderly subjects residing in nursing homes or assisted care facilities and presenting with Alzheimer's dementia and psychoses or other selected psychoses. www.clinicalstudyresults.org 2006.
- Tariot P, Schneider L, Katz I, Mintzer J, Street J. Quetiapine in nursing home residents with Alzheimer's dementia and psychosis. In: American Journal of Geriatric Psychiatry. Vol. 10. 2002:93.
- Tariot PN, Schneider L, Katz IR, Mintzer JE, Street J Copenhaver M, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial Epub 2006 Aug 11; Erratum in: am J Geriatr Psychiatry 2006 Nov;14(11): 988Tariot PN et al. AmericanJjournal of Geriatric Psychiatry 2006;14(9):767-76. [DOI: 10.1097/01.JGP.0000196628.12010.35] [DOI] [PubMed] [Google Scholar]
Teri 2000 {published data only}
- Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, , et al Alzheimer's Disease Cooperative Study. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology 2000;55(9):1271-8. [DOI] [PubMed] [Google Scholar]
Zhong 2007 {published data only}
- A multicenter, double-blind, randomized comparison of the efficacy and safety of quetiapine fumarate (SEROQUEL®) and placebo in the treatment of agitation associated with dementia. Clinicaltrials.gov [http://clinicaltrials.gov] 2009.
- NCT00621647. [Public title] Seroquel- agitation associated with dementia; [Scientific title] A multicenter, double-blind, randomized, comparison of the efficacy and safety of quetiapine fumarate (SEROQUEL) and placebo in the treatment of agitation associated with dementia. ClinicalTrials.gov [http://clinicaltrials.gov] CNO - CN-00724658 2002.
- Tariot PN. Efficacy and tolerability of atypical antipsychotics in agitation and psychosis: research results. In: 158th Annual Meeting of the American Psychiatric Association; 2005 May 21-26; Atlanta, GA CNO - CN-00547351. 2005:No. 31A.
- Zhong K, Tariot P, Minkwitz MC, Devine NA, Mintzer J. Quetiapine for the treatment of agitation in patients with dementia. 158th Annual Meeting of the American Psychiatric Association, Atlanta, GA, USA, 21-26 May, 2005 CNO - CN-00548828 2005;abstract NR.726:269. [Google Scholar]
- Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Current Alzheimer Research 2007;4(1):81-93. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
DeDeyn 1999 RIS‐INT‐24 {published data only}
- DeDeyn PP, Rabheru K, Rasmussen MD, Bocksberger JP, Dautzenberg PL, Eriksson S, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53(5):946-55. [DOI] [PubMed] [Google Scholar]
Devanand 1989 {published data only}
- Devanand DP, Sackeim HA, Brown RP, Mayeux R. A pilot study of haloperidol treatment of psychosis and behavioral disturbance in Alzheimer's disease. Archives of Neurology 1989;46(8):854-7. [DOI] [PubMed] [Google Scholar]
Holmes 2007 {published data only}
- Holmes C, Wilkinson D, Dean C, Clare C, El-Okl M, Hensford C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer's disease: a randomised double blind placebo controlled study. International Journal ofGgeriatric Psychiatry 2007;22(4):380-1. [DOI: 10.1002/gps.1667] [DOI] [PubMed] [Google Scholar]
Meguro 2004 {published data only}
- Meguro K, Meguro M, Tanaka Y, Akanuma K, Yamaguchi K, Itoh M. Risperidone is effective for wandering and disturbed sleep/wake patterns in Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology 2004;17(2):61-7. [DOI: 10.1177/0891988704264535] [DOI] [PubMed] [Google Scholar]
NCT00043849 2002 {published data only}
- NCT00043849. Treatment of Agitation/Psychosis in Dementia/Parkinsonism (TAP/DAP). https://clinicaltrials.gov/show/NCT00043849 CNO - CN-01496582 2002.
Pollock 2002 {published data only}
- Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. American Journal of Psychiatry 2002;159(3):460-465. [DOI: 10.1176/appi.ajp.159.3.460] [DOI] [PubMed] [Google Scholar]
- Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Research and Practice in Alzheimer's Disease 2003;7:180-6. [DOI] [PubMed] [Google Scholar]
Shin 2013 {published data only}
- Shin CS. A randomized placebo-controlled trial of quetiapine for the treatment of behavioral and psychological symptom of dementia in Alzheimer's disease patients. Journal of the Neurological Sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication: MISC2 - Journal of the Neurological Sciences. Conference: 21st World Congress of Neurology Vienna Austria. Conference(var.pagings):e294-e295. [Google Scholar]
Street 2000 F1D MC HGEU {published data only}
- Clark WS, Street J, Gannon KS, Sanger T, Tollefson GD, Breier A. Olanzapine in the prevention of psychosis among nursing home patients with behavioral disturbances associated with alzheimer's disease. European Journal of Neurology 1999;6(Suppl 3):115. [Google Scholar]
- Clark WS, Street J, Gannon KS, Sanger T, Tollefson GD, Breier A. Olanzapine in the prevention of psychosis among nursing home patients with behavioral disturbances associated with Alzheimer's disease. Journal of the European College of Neuropsychopharmacology 1999;9(suppl 5):S332. [Google Scholar]
- Clark WS, Street JS, Feldman PD, Breier A. The effects of olanzapine in reducing the emergence of psychosis among nursing home patients with Alzheimer's disease. Journal of Clinical Psychiatry 2001;62(1):34-40. [DOI] [PubMed] [Google Scholar]
- Clark WS, Street JS, Gannon KS, Sanger TM, Tollefson GD, Breier A. Reduction of psychotic symptoms in patients with lewy body like symptoms treated with olanzapine. In: Proceedings of the 9th Congress of the International Psychogeriatric Association; 1999 Aug 15-20, Vancouver, Canada CNO - CN-00858739. 1999:172.
- Clark WS, Street JS, Sanger TM, Feldman PD, Breier A. Olanzapine in the prevention of psychosis among nursing home patients with behavioral disturbances associated with Alzheimer's disease. Biological Psychiatry 1999;47(Suppl):S162. [Google Scholar]
- Clark WS, Street JS, Gannon KS, Sanger TM, Tollefson GD, Breier A. Olanzapine in the prevention of psychosis among nursing home patients with behavioral disturbances associated with Alzheimer's disease. InternationalJjournal of Neuropsychopharmacology 2000;3(Suppl 1):S357. [Google Scholar]
- Feldman PD, Clark WS, Breier A, Sanger TM, Street JS. Olanzapine in the prevention of psychosis among nursing home patients with behavioral disturbances associated with alzheimer's disease. Schizophrenia Research 2001;49(1-2 Suppl):227. [Google Scholar]
- Kennedy J, Basson B, Zagar A, Clark W, Sanger T, Street J, et al. The effects of olanzapine on alzheimer's disease assessment scale scores in patients with mild to moderate alzheimer's disease with psychosis and behavioral disturbances. In: Proceedings of the Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17-21, Nashville CNO - CN-00368536. 2000:175.
- Kennedy JS, Basson BB, Zagar AJ, Clark WS, Sanger TM, Street JS, et al. The effects of olanzapine on alzheimer's disease assessment scale scores in patients with mild to moderate alzheimer's disease with psychosis and behavioural disturbances. Clinical Neuropsychological Assessment 2000;1(6):42-43. [Google Scholar]
- Kennedy JS, Basson BR, Zagar AJ, Gilmore JA, Wei H, Kinon BJ. The effects of olanzapine on alzheimer's disease assessment scale scores in patients with mild to moderate alzheimer's disease with psychosis and behavioral disturbances: continued improvement in quality of life despite weight change during olanzapine or haloperidol treatment. In: Proceedings of the 52nd Institute on Psychiatric Services; 2000 oct 25-29, Philadelphia. 2000.
- Kennedy J S, Zagar A, Bymaster F, Nomikos G, Trzepacz P T, Gilmore JA, et al. The central cholinergic system profile of olanzapine compared with placebo in Alzheimer's disease. International Journal of Geriatric Psychiatry 2001;16:S24-S32. [DOI: 10.1002/1099-1166%28200112%2916:1+%3C::AID-GPS570%3E3.0.CO] [DOI] [PubMed] [Google Scholar]
- Mintzer J, Faison W, Street J, Sutton VK, Breier A. Olanzapine in the treatment of anxiety symptoms due to Alzheimer's disease: a post hoc analysis. InternationalJournal of Geriatric Psychiatry 2001;16 Suppl 1(Suppl 1):S71-7. [DOI] [PubMed] [Google Scholar]
- Slawson DC. Is olanzapine effective in the treatment of agitation/aggression and psychosis in patients with Alzheimer disease (AD)? [Commentary on Street JS, Clark WS, Gannon KS et al Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial Archives of General Psychiatry 2000;57: 968-76]. Evidence Based Practice736 2001;4(1):5-6. [DOI] [PubMed] [Google Scholar]
- Street J, Clark SW, Gannon KS, Mitan S, Kadman D, Sanger T, et al. Effect of olanzapine on psychosis and behavioural disturbances associated with alzheimer's disease. Journal of the American Geriatrics Society 1999;47:S89. [Google Scholar]
- Street J, Clark WS, Gannon K, Mitan S, Kadam D, Sanger T, et al. Olanzapine reduces psychosis and behavioral disturbances associated with Alzheimer's disaese. In: Proceedings of the 9th Congress of the International Psychogeriatric Association; 1999 Aug 15-20, Vancouver, Canada CNO - CN-00368751. 1999:123.
- Street J, Clark WS, Gannon KS, Mitan S, Kadman D, Famura R, . Olanzapine in the treatment of psychosis and behavioural disturbances assocated with alzheimer's disease. Schizophrenia Research 1999;1-3:298. [Google Scholar]
- Street J, Clark KS, Gannon KS, Mitan S, Kadam D, Sanger T. Olanzapine reduces psychosis and behavioral disturbances associated with Alzheimer's disease. Journal of the European College of Neuropsychopharmacology 1999;9(suppl 5):S331. [Google Scholar]
- Street JS, Clark WS, Cummings JL, Kadam DL, Mitan SJ, Sanger TM, et al. Olanzapine in the treatment of psychosis and behavioural disturbances associated with alzheimer's disease. Journal of Neurosurgery 2001;187(Suppl 1):S57. [Google Scholar]
- Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry CNO - CN-00322635 2000;57(10):968-76. [DOI] [PubMed] [Google Scholar]
- Street JS, Clark WS, Gannon KS, Cummings JL Bymaster FP, Tamura RN, et al, HGEU Study Group. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Archives of General Psychiatry 2000;57(10):968-96. [DOI] [PubMed] [Google Scholar]
- Street JS, Clark WS, Gannon KS, Mitan S, Sanger TM, Tollefson GD. Olanzapine in the treatment of psychosis and behavioral disturbances associated with alzheimer's disease. In: Proceedings of the 152nd annual meeting of the American Psychiatric Association; 1999 may 15-20, Washington DC. 1999.
- Street JS, Clark WS, Kadam DL, Mitan SJ, Juliar BE, Feldman PD, et al. Long-term efficacy of olanzapine in the control of psychotic and behavioral symptoms in nursing home patients with Alzheimer's dementia. International Journal of Geriatric Psychiatry 2001;16(Suppl 1):S62-70. [DOI] [PubMed] [Google Scholar]
Trequattrini 2003 {published data only}
- Trequattrini A, Attala T, Spadoni L, Ciappi F. Olanzapin in the treatment of psychological and behavioral diseases in patients with Alzheimer-type dementia. Giornale di Neuropsicofarmacologia 2003;3:105-13. [Google Scholar]
Additional references
Almutairi 2018
- Almutairi S, Masters K, Donyai P. The health professional experience of using antipsychotic medication for dementia in care homes: a study using grounded theory and focussing on inappropriate prescribing. Journal of Psychiatric and Mental Health Nursing 2018;25(5-6):307-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arai 2016
- Arai H, Nakamura Y, Taguchi M, Kobayashi H, Yamauchi K, Schneider LS. Mortality risk in current and new antipsychotic Alzheimer's disease users: large scale Japanese study. Alzheimer's & Dementia 2016;12(7):823-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ballard 2005
- Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. BMJ (Clinical Research Ed.) 2005;330(7496):874. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2006
- Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD003476. [DOI: 10.1002/14651858.CD003476.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2010
- Banerjee S. The use of antipsychotic medication for people with dementia: time for action. A report, 2010. webarchive.nationalarchives.gov.uk/20130104175837/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_108302.pdf (accessed prior to 1 April 2019).
Borsje 2018
- Borsje P, Lucassen PL, Wetzels RB, Pot AM, Koopmans RT. Neuropsychiatric symptoms and psychotropic drug use in patients with dementia in general practices. Family Practice 2018;35(1):22-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bridge 2007
- Bridge JA, Iyengar S, Salary CB. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007;297(15):1683-96. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: LEA Publishers, 1988. [Google Scholar]
Cohen 1992
- Cohen J. A power primer. Psychological Bulletin 1992;112(1):155-9. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1996
- Cohen-Mansfield J. Conceptualization of agitation: results based on the Cohen-Mansfield Agitation Inventory and the Agitation Behavior Mapping Instrument. International Psychogeriatrics / IPA 1996;8 Suppl 3:309-15; discussion 351-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence systematic review software. Melbourne (Australia): Veritas Health Innovation, 2013. Available at www.covidence.org.
Cummings 2015
- Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. International Psychogeriatrics / IPA 2015;27(1):7-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeDeyn 1999
- DeDeyn PP, Rabheru K, Rasmussen MD, Bocksberger JP, Dautzenberg PL, Eriksson S, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53(5):946-55. [DOI] [PubMed] [Google Scholar]
EMA 2008
- European Medicines Agency. Questions and answers on the review of the use of conventional antipsychotic medicines in elderly patients with dementia, 2008. www.ema.europa.eu/en/documents/other/questions-answers-review-use-conventional-antipsychotic-medicines-elderly-patients-dementia_en.pdf (accessed prior to 1 April 2019).
Farah 2005
- Farah A. Atypicality of atypical antipsychotics. Primary Care Companion Journal of Clinical Psychiatry 2005;7(6):268-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furniss 1998
- Furniss L, Craig SK, Burns A. Medication use in nursing homes for elderly people. International Journal of Geriatric Psychiatry 1998;13(7):433-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilley 1991
- Gilley DW, Whalen ME, Wilson RS, Bennett DA. Hallucinations and associated factors in Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences 1991;3(4):371-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables – binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles – continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventionsversion 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hughes 2008
- Hughes R. Chemical restraint in nursing older people. Nursing Older People 2008;20(3):33-8; quiz 39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hulshof 2015
- Hulshof TA, Zuidema SU, Ostelo RW, Luijendijk HJ. The Mortality Risk of Conventional Antipsychotics in Elderly Patients: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials.. Journal of the American Medical Directors Association 2015;16(10):817-24. [DOI] [PubMed] [Google Scholar]
Hulshof 2020
- Hulshof TA, Zuidema SU, Gispen-de Wied CC, Luijendijk HJ. Run-in periods and clinical outcomes of antipsychotics in dementia: A meta-epidemiological study of placebo-controlled trials. Pharmacoepidemiology and Drug Safety 2020;29(2):125-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isojarvi 2018
- Isojarvi J, Wood H, Lefebvre C, Glanville J. Challenges of identifying unpublished data from clinical trials: getting the best out of clinical trials registers and other novel sources.. Research Synthesis Methods 2018;9(4):561-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Janus 2016
- Janus SI, Manen JG, IJzerman MJ, Zuidema SU. Psychotropic drug prescriptions in Western European nursing homes. International Psychogeriatrics / IPA 2016;28(11):1775-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Janus 2017
- Janus SI, Manen JG, IJzerman MJ, Bisseling M, Drossaert CH, Zuidema SU. Determinants of the nurses' and nursing assistants' request for antipsychotics for people with dementia. International Psychogeriatrics / IPA 2017;29(3):475-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kales 2007
- Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. American Journal of Psychiatry 2007;164(10):1568-76; quiz 1623. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kales 2012
- Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried LS, Chiang C, et al. Risk of mortality among individual antipsychotics in patients with dementia. American Journal of Psychiatry 2012;169(1):71-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1989
- Khan A, Cohen S, Dager S, Avery D, Dunner D. Onset of response in relation to outcome in depressed outpatients with placebo and imipramine. Journal of Affective Disorders 1989;17(1):33-8. [DOI] [PubMed] [Google Scholar]
Kirchner 2001
- Kirchner V, Kelly CA, Harvey RJ. Thioridazine for dementia. Cochrane Database of Systematic Reviews 2001, Issue 3. Art. No: CD000464. [DOI: 10.1002/14651858.CD000464] [PMID: ] [DOI] [PubMed] [Google Scholar]
Knol 2008
- Knol W, Marum RJ, Jansen PA, Souverein PC, Schobben AF, Egberts AC. Antipsychotic drug use and risk of pneumonia in elderly people. Journal of the American Geriatrics Society 2008;56(4):661-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuehn 2005
- Kuehn BM. FDA warns antipsychotic drugs may be risky for elderly. JAMA 2005;293(20):2462. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leng 2020
- Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: A systematic review and Bayesian network meta-analysis. International Journal of Nursing Studies 2020;102:103489. [DOI: 10.1016/j.ijnurstu.2019.103489.] [DOI] [PubMed] [Google Scholar]
Livingston 2014a
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technology Assessment (Winchester, England) 2014;18(39):1-226, v-vi. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingston 2014b
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. British Journal of Psychiatry 2014;205(6):436-42. [DOI: 10.1192/bjp.bp.113.141119.] [DOI] [PubMed] [Google Scholar]
Livingston 2017
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673-734. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lonergan 2002
- Lonergan E, Luxenberg J, Colford J. Haloperidol for agitation in dementia. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002852. [DOI: 10.1002/14651858.CD002852] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luijendijk 2016
- Luijendijk HJ, Bruin NC, Hulshof TA, Koolman X. Terminal illness and the increased mortality risk of conventional antipsychotics in observational studies: a systematic review. Pharmacoepidemiology and Drug Safety 2016;25(2):113-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ma 2014
- Ma H, Huang Y, Cong Z, Wang Y, Jiang W, Gao S, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. Journal of Alzheimer's disease 2014;42(3):915-37. [DOI] [PubMed] [Google Scholar]
MHRA 2009
- Medicines and Healthcare products Regulatory Agency. Antipsychotics: use in elderly people with dementia. Drug Safety Update 2009;2(8):5-6. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2015
- Morris S, Patel N, Baio G, Kelly L, Lewis-Holmes E, Omar RZ, et al. Monetary costs of agitation in older adults with Alzheimer's disease in the UK: prospective cohort study. BMJ Open 2015;5(3):e007382. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2014
- Murray PS, Kumar S, Demichele-Sweet MA, Sweet RA. Psychosis in Alzheimer's disease. Biological Psychiatry 2014;75(7):542-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neufeld 2019
- Neufeld KJ, Needham DM, Oh ES, Wilson LM, Nikooie R, Zhang A, et al. Antipsychotics for the Prevention and Treatment of Delirium. Comparative Effectiveness Review No. 219. (Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2015-00006-I-2.). AHRQ Publication No. 19-EHC019-EF. Rockville, MD: Agency for Healthcare Research and Quality; September 2019. [DOI: ] [PubMed]
Pierre 2005
- Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Safety 2005;28(3):191-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Prince 2015
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015 - The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends.. London: Alzheimer's Disease International, 2015. [Google Scholar]
Renom‐Guiteras 2018
- Renom-Guiteras A, Thurmann PA, Miralles R, Klaassen-Mielke R, Thiem U, Stephan A, et al. Potentially inappropriate medication among people with dementia in eight European countries. Age and Ageing 2018;47(1):68-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2019 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Scarmeas 2005
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Archives of Neurology 2005;62(10):1601-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2012
- Schmidt SG, Dichter MN, Palm R, Hasselhorn HM. Distress experienced by nurses in response to the challenging behaviour of residents – evidence from German nursing homes. Journal of Clinical Nursing 2012;21(21-22):3134-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schneider 2005
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294(15):1934-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schneider 2006
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. New England Journal of Medicine 2006;355(15):1525-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schroll 2015
- Schroll J, Bero L. Regulatory agencies hold the key to improving Cochrane Reviews of drugs. Cochrane Database of Systematic Reviews (Online) 2015;4:Art. No.: ED000098. [DOI: 10.1002/14651858.ED000098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. [Google Scholar]
Smeets 2018
- Smeets CH, Zuidema SU, Hulshof TA, Smalbrugge M, Gerritsen DL, Koopmans RT, et al. Efficacy of antipsychotics in dementia depended on the definition of patients and outcomes: a meta-epidemiological study. Journal of Clinical Epidemiology 2018;101:17-27. [DOI] [PubMed] [Google Scholar]
Tampi 2016
- Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Therapeutic Advances in Chronic Disease 2016;7(5):229-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Testad 2010
- Testad I, Ballard C, Bronnick K, Aarsland D. The effect of staff training on agitation and use of restraint in nursing home residents with dementia: a single-blind, randomized controlled trial. Journal of Clinical Psychiatry 2010;71(1):80-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Toot 2017
- Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. International Psychogeriatrics / IPA 2017;29(2):195-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leeuwen 2018
- Van Leeuwen E, Petrovic M, Driel ML, De Sutter AI, Vander Stichele R, Declercq T, et al. Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD007726. [DOI: 10.1002/14651858.CD007726.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahid 2004
- Wahid ZU. Delirium in hospitalized patients : underdiagnosed and undertreated missed opportunities in preventive care. Journal of the National Medical Association 2004;96(4, 2010):414. [Google Scholar]
Wetzels 2010
- Wetzels RB, Zuidema SU, Jonghe JF, Verhey FR, Koopmans RT. Determinants of quality of life in nursing home residents with dementia. Dementia and Geriatric Cognitive Disorders 2010;29(3):189-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2017
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2018. Oslo (Norway): World Health Organization, 2017. [Google Scholar]
Yeh 2019
- Yeh TC, Tzeng NS, Li JC, Huang YC, Hsieh HT, Chu CS, et al. Mortality risk of atypical antipsychotics for behavioral and psychological symptoms of dementia: a meta-analysis, meta-regression, and trial sequential analysis of randomized controlled trials. Journal of Clinical Psychopharmacology 2019;39(5):472-8. [DOI] [PubMed] [Google Scholar]
Yohanna 2017
- Yohanna D, Cifu AS. Antipsychotics to treat agitation or psychosis in patients with dementia. JAMA 2017;318(11):1057-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yunusa 2019
- Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Network Open 2019;2(3):e190828. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mühlbauer 2019
- Mühlbauer V, Luijendijk H, Dichter MN, Möhler R, Zuidema SU, Köpke S. Antipsychotics for agitation and psychosis in people with Alzheimer's disease and vascular dementia. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013304. [DOI: 10.1002/14651858.CD013304] [DOI] [PMC free article] [PubMed] [Google Scholar]


