Abstract
Outbreaks of the coral-killing sponge Terpios hoshinota Rützler and Muzik, 1993 have become a threat to corals and result in coral reef deterioration. This species has an increasing distribution in the Indo-Pacific Ocean and thrives in patches on some reefs in Okinawa, Japan. However, the dispersal process and mechanisms involved remain unknown. We observed the self and non-self recognition capabilities of T. hoshinota by performing contact assays in aquarium and in the field. In the contact assays (indirect and direct contact), allogeneic sets did not fuse and showed a rejection reaction as they formed boundaries (approx. 0.2 mm width) between their tissues. Although the initial reaction between individuals involved adhesion in allogeneic sets, the two individuals remained distant from each other. Histological observations showed that soft tissues (such as collagen) were not present in the boundary zones. These boundaries were maintained for more than 2 weeks. Boundary formations were also confirmed at three field sites in Okinawa, Japan. Our results suggest that T. hoshinota can distinguish self and non-self individuals. Contact assays are a useful method for evaluating the spatial distribution and local population structures of T. hoshinota in coral reefs.
Keywords: Self recognition, Tissue reaction, Rejection, Coral reef, Histoincompatibility
BACKGROUND
Sponges play an important role in marine ecosystems as water filters, substratum stabilizers, and hosts of diverse symbionts (Wulff 2012). However, some sponges compete with and are threats to benthic communities in coral reef ecosystems. Aggressive sponges kill coral by overgrowing live coral tissue and some species also secrete harmful chemical substances (Wulff 2012). An abundance of these sponges can remarkably reduce coral populations (Wulff 2016). The distribution of the encrusting sponge Terpios hoshinota Rützler and Muzik, 1993 has increased and become a serious threat to coral reefs (Plucer-Rosario 1987; Liao et al. 2007; Reimer et al. 2010 2011; Fujii et al. 2011; de Voogd et al. 2013). Terpios hoshinota was first reported in Guam, and became widespread on the Ryukyu archipelago. In the 1980s, this sponge was named ‘black disease’ by the local media in Okinawa, Japan (Rützler and Muzik 1993; Hirose and Murakami 2011). The sponge harbors numerous cyanobacteria symbionts and produces multilobed tylostyle spicules in its thin (< 1 mm) tissue (Rützler and Muzik 1993), which are used to identify this species. The sponge surface is covered in tiny particles obtained from the environment; these particles cause the color of the sponge to become gray or black (Rützler and Muzik 1993). Terpios hoshinota also grows quickly, as much as > 2 mm per day (Plucer-Rosario 1987; Aini et al. 2021).
Terpios hoshinota is highly competitive for space in coral reefs and grows over corals and other hard substrata such as rocks and even plastics (Bryan 1973; Plucer-Rosario 1987; Reimer et al. 2011). Toxic chemicals with the potential to kill live coral tissue were found in T. hoshinota (Teruya et al. 2004). However, allelochemicals may not be the major mechanisms used by these organisms to kill corals. Rather, T. hoshinota is thought to compete with live coral by overgrowing it (Wang et al. 2012 2015). This sponge reduces coral populations as it grows from the bottom to the tip of coral, and outbreaks of this sponge are related to the degradation of reef ecosystems (Liao et al. 2007; Plucer-Rosario 1987). Although the sponge is a pervasive threat to coral reefs, few studies have evaluated its distribution in Japan (Rützler and Muzik 1993; Reimer et al. 2011; Yomogida et al. 2017).
Studies on the growth progression and dispersal mechanisms of this sponge and the implementation of appropriate conservation measures for reef ecosystems are urgently required. To date, remarkable reports from Taiwan have revealed that T. hoshinota releases larvae (Wang et al. 2012; Hsu et al. 2013; Nozawa et al. 2016). Although the larvae are negatively buoyant, which should limit extensive dispersal, the sponge can be dispersed into new habitats. However, the spatial distribution and population structures of this species in reefs remain unclear. Allorecognition in other metazoans such as the hydrozoa Hydractinia and the colonial ascidian tunicate Botryllus has been reported (Cadavid 2004; Mukai and Watanabe 1974). In the marine demosponge Halichondria japonica (Kadota, 1922), allogeneic reactions of fusion or rejection were observed (Saito 2013).
In this study, we performed contact assays in an aquarium to confirm whether T. hoshinota possesses self and non-self recognition capabilities. We also conducted field observations on Okinawa Island, Japan to evaluate the dispersal process of the T. hoshinota sponge.
MATERIALS AND METHODS
Study sites and sample collection
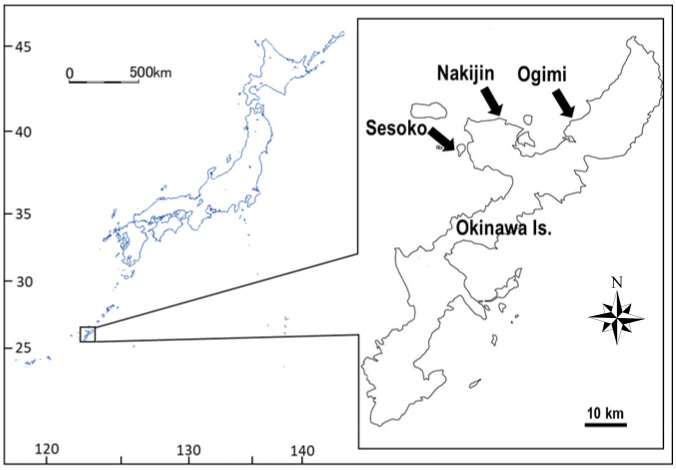
Individuals of Terpios hoshinota overgrowing a branching coral, Montipora digitata (Dana, 1846), were collected from shallow moats containing dense sponge populations at three sites—Sesoko Island (26°39'01.3"N, 127°51'23.6"E), Nakijin (26°42'30.9"N, 127°56'59.2"E), and Ogimi (26°42'04.1"N, 128°06'53.7"E)—in the northern area of Okinawa Island, Japan (Fig. 1). The study sites are located along the western side of Okinawa Island and are more than 15 km apart. At all three sites, the dominant coral was a branching M. digitata forming dense aggregations, followed by foliose Montipora aequituberculata Bernard, 1897. The sampling area was selected based on aerial photographs taken by a drone (Spark, DJI Co., Ltd., Guangdong, China) at a height of 50 m covering a wide area at each site, followed by an intensive snorkeling survey. After selection, coral colonies covered by the sponge were tagged and photographed. Samples were collected using diagonal nippers and transported to the marine laboratory at Sesoko Station, University of the Ryukyus, Sesoko Island, Japan. Sponges were maintained in an outdoor aquarium (28.7 L in volume, shaded by a screen with approximately 25% exterior light intensity) and supplied with running seawater at a flow rate of 7.5 L min-1.
Fig. 1.
Map of the study sites (Sesoko, Nakijin, and Ogimi) in the northern area of Okinawa Island, Japan.
Contact assays
Coral branches covered by the sponge were cut into 2–3 cm fragments for the contact assays. To investigate the contact reactions between sponges, two types of contact assays were conducted: (1) growing-edge contact assays and (2) direct contact assays (Fig. 2a). For growing-edge contact assays, two small individuals (fragments) were placed on a 4.5 × 4.5 cm ceramic tile and separated by a distance of 1–1.5 cm. The samples were observed until they touched each other (Fig. 2b). For direct-contact assays, two individuals (fragments) were placed in contact with each other on a ceramic tile and observed as they grew. Two individuals derived from different patches and collected from the same reef at each study site were paired. The experiment included five replicates of both the growing-edge and direct contact assays. The control treatment paired two fragments derived from the same individuals with five replications performed for both assays. Eleven individuals were used in this study (N1–O4), with 17 sets examined in the allogeneic contact assays, and 11 sets were used as controls.
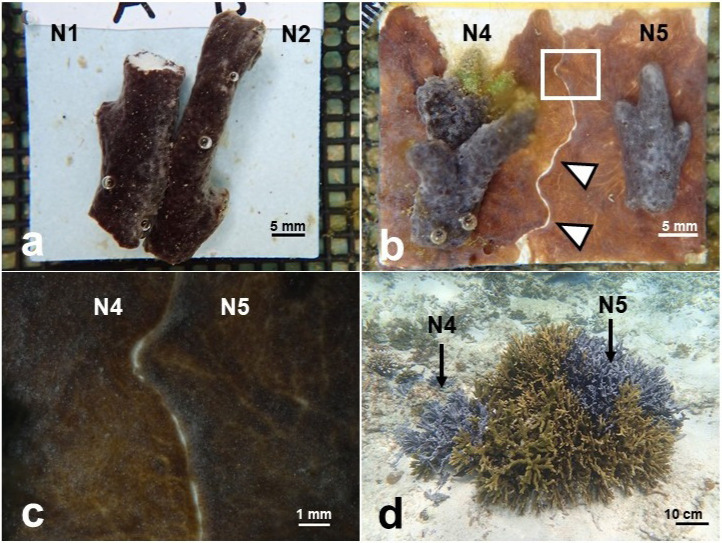
Fig. 2.
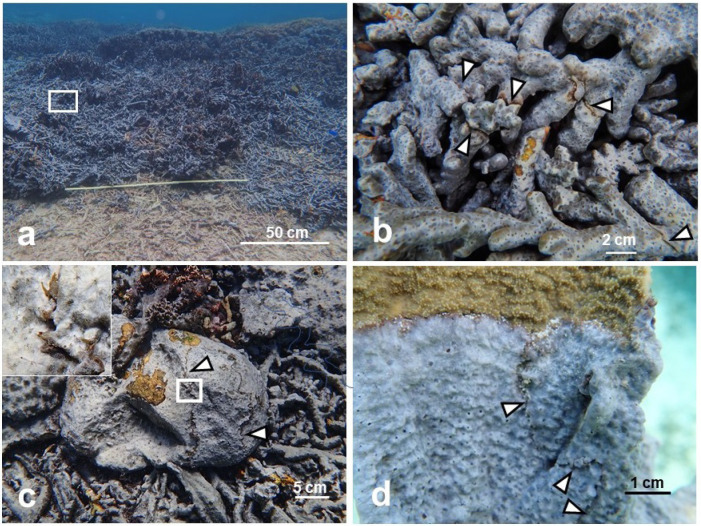
Contact experiments with Terpios hoshinota sponge. a, Two fragments from different individuals (N1 × N2) in the direct contact assay (at day 0). b, Boundary zone (arrow heads) between two individuals (N4 × N5) in the growing-edge contact assay (at day 19). c, Enlarged view of b. d, Colony of Montipora coral (81 cm in diameter) which was covered by sponge patches of N4 and N5 in Nakijin.
We also conducted an additional aquarium experiment to determine how the sponges react to other individuals from distant reefs (> 15 km apart). One individual from each site at Nakijin, Ogimi, and Sesoko was collected and maintained in the outdoor aquarium at Sesoko station. Following the same growing-edge contact assay, one paired set of Nakijin with Ogimi and two control sets were examined with five replicates. The direct contact assay involved three sets of allogeneic contacts (individuals from Nakijin, Ogimi, and Sesoko) and three control sets using sponge fragments from a single location.
For all contact assays, each set was observed and photographed daily. Contact experiments were performed from June 25, 2019 to January 6, 2020 with overgrown fragments divided when required. The experimental duration was dependent on the growth rates and growth directions of the sponge. Most experiments continued for 10–21 days after contact.
Field surveys
In conjunction with the aquarium experiments, field surveys were performed at all three sites (Sesoko, Nakijin, and Ogimi) to investigate whether the contact reaction patterns exist in situ. Surveys were performed regularly (at least once a month at each site) from June to December 2019 by snorkeling to a depth of 1–2 m and photographing samples with a digital camera (Olympus TG-5, Tokyo, Japan). When contact reaction patterns (i.e., boundary formations) were observed, the boundary coral branches were collected and transported to Sesoko Station for microscopic and histological observations.
Histological observation
To observe sponge tissue, particularly the collagenous matrix in the boundary areas between two individuals, 10 paired coral branches showing distinct boundaries were collected from Nakijin and Ogimi during the field surveys. Fused tissues of the same individual in the aquarium culture experiments were also investigated in the histological analysis to evaluate the fusion process. Samples were fixed in 10% formalin solution in seawater for 8 h and preserved in 80% ethanol solution. The samples were mounted in a 2% agarose gel solution to hold the sponge tissue and preserve the boundaries during the decalcification of the coral skeleton. The boundaries mounted in agarose gel were immersed in decalcifying solution (final concentrations were 12% acetic acid and 6% formalin) for 14–21 days depending on the fragment size. The sponge tissues were dehydrated in a graded series of ethanol-xylene solution and embedded in paraffin. The paraffin block was sliced (7 μm thickness) to prepare the vertical thin sections and stained with a Picro-sirius red staining kit (Scy TeK Laboratories, Inc., Logan, UT, USA) to detect the collagen. The samples were observed using a light microscope (Nikon Eclipse Ci, Tokyo, Japan) equipped with a digital camera (Nikon 1-J1).
RESULTS
Contact assays
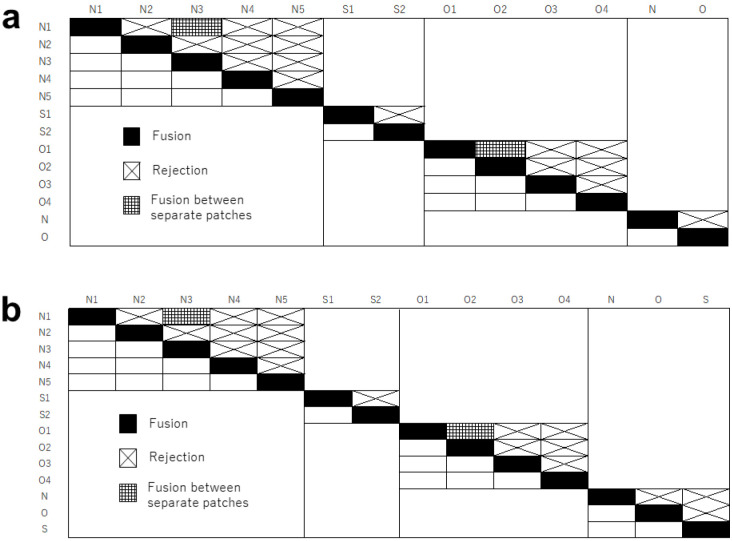
Figure 3 presents the results of the contact assays (a: growing-edge contact assays, b: direct contact assays). Only two sets fused in both experiments (16 of 18 did not fuse in the growing edge contact assays and 18 of 20 did not fuse in the direct contact assays) (Fig. 3a, b). Two sets from Nakijin and Ogimi (N1 × N3 and O1 × O2) fused in all 5 replicates in both contact assays. The distance between the patches of N1 and N3 was approximately 5.0 m. The distance between the patches of O1 and O2 was approximately 40 cm. Intensive field surveys in Ogimi in December 2019 confirmed tissue connection (fusion) between O1 and O2 individuals at the base of dead coral patches.
Fig. 3.
Results of a, growing-edge contact assays and b, direct contact assays. Five replicated sets were examined for each pair. Individuals are from Nakijin (N1–N5, and N), Sesoko (S1–S2, and S), and Ogimi (O1–O4, and O). Assays between distant reefs are provided as N, O, and S.
In direct contact assays (Fig. 2a), some sets from different individuals showed temporary adhesion within 24 h of contact, making it difficult to confirm whether the two individuals had fused (Video S1). However, at 24–48 h after initial contact, a rejection reaction was clearly confirmed, and the samples did not fuse within a 10-day experimental period. In both contact assays, there was extended thread tissue that slightly covered another individual tissue at the contact interface, and the tissue color at the interface was darker (image available upon request). In the growing-edge contact assays, boundary zones approximately 0.2 mm wide were formed at the contact interface between the two individuals (Fig. 2b, c). The sponge did not show overgrowth of the other individual, and the boundary zones were maintained for more than 2 weeks after formation. Non-boundary tissues continued growing on the tile. The sets containing individuals from distant reefs did not fuse in either contact assay (Fig. 3a, b).
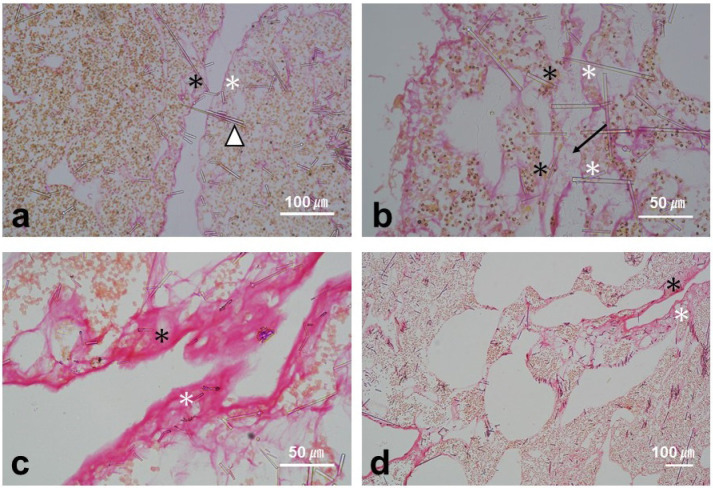
Histological sections of the boundary tissue between two individuals showed that no collagen or other sponge tissue remained in the boundary zones. However, spicule tips were frequently oriented toward and stuck into the opposite sponge beyond the boundary zones (Fig. 4a). When the boundary zone was clear with empty space, the outermost layer (ectopinacoderm) of the two individuals was not disintegrated (Fig. 4a). However, in a few sections, there were obscure outer layers with gathering mesohyl where the two individuals had contacted each other in some sections of the interfaces (indicated by an arrow in Fig. 4b), although the rest of the boundary zone remained clear.
Fig. 4.
Vertical sections of the boundary zone stained with Picro-sirius red. a and b, Rejection reactions between two individuals. c and d, Fusion between two fragments of the same individual. a, Boundary formation between two individuals and a spicule stuck into another individual (arrowhead). b, Gathered mesohyl between two individuals (arrow). c, A collagenous structure (stained pink) formed at the contact interface. d, Fusion of the sponge tissue. Asterisks indicate the dermal-like layer to indicate the boundary region between two sponges.
In the control experiments of the growing-edge contact assays, all paired sets (13 sets) were derived from the same individual and fused (Fig. 3a). In the direct contact assays (Fig. 3b), all paired sets (14 sets) also fused within 24 h (except for one set that fused within 48 h). Some sponge fragments from the same patches had already fused within a few hours, during transport to the marine laboratory for the aquarium contact assays. Fusion involved an accumulation of collagenous tissue (stained pink by Picro-sirius red) at the growing-edge contact interface (Fig. 4c, d).
Field surveys
We conducted field surveys at all three sites to confirm the results obtained in the aquarium experiments. As observed in the aquarium experiments, boundary zone formation between different individuals occurred at all sites (Ogimi, August 27; Sesoko, September 12; Nakijin, September 26, 2019, were the first recorded dates) (Fig. 5). In some cases, we observed extended thread-like tissues in contact with another individual’s tissue (Fig. 5c), as observed in the aquarium experiments. Boundaries were formed on the dead coral surface, regardless of the coral form (e.g., massive, branching, encrusting, or foliose coral), coral genera (Montipora and Porites), and rock substratum (Fig. 5b–d). We also observed Terpios hoshinota encrusting other sympatric demosponges (Ceratodictyon spongiosum Zanardini, 1878, and two other unidentified species).
Fig. 5.
Boundary zones confirmed in the field. a, Most colonies of Montipora digitata were fragmented and covered by T. hoshinota in Ogimi, Japan. b, Enlarged view of a. Arrowheads indicate boundary zones between individuals. c, Distinct boundary zone (arrow heads) on a massive coral Porites in Ogimi. The insert is an enlarged view of the threads. Yellow patches are brown algae covering the dead coral surface. d, The boundary between two individuals (arrow heads) on foliose coral Montipora aequituberculata in Nakijin.
DISCUSSION
Both contact assays (direct and indirect contact) and field observations demonstrated that Terpios hoshinota can recognize self and non-self, and distinguishes individuals within proximity on the same reef. Although 16 sets (N = 18) in the indirect contact assays showed no growing edge contact and 18 sets in direct contact assays (N = 20) did not fuse, two other sets in each contact assay showed fusion between two individuals from different patches (N1 × N3 and O1 × O2). However, a later intensive field survey revealed fusion (connection) between the individuals of one set, O1 × O2, which were collected from field locations only 40 cm apart. Thus, O1 and O2 are predicted to be the same individual. Although individuals of N1 and N3 were collected from locations 5.0 m apart with no connection between them confirmed by the field surveys, fusion by this set suggests that they originated from the same individual (by fragmentation) or from the same parent (as sexually produced larvae). Mechanical disturbances such as strong wave action during a typhoon and human activities such as fishing or snorkeling may translocate coral branches, particularly the fragile coral M. digitata (Harpeni and David 2011). Furthermore, contact experiments using sponge larvae from both the same and different individuals requires further investigation. Although tissue-compatibility relationships closely reflect clone structure, determining the genealogy of the sponge is crucial for confirming genetic population structures. Fusion may occur between closely related individuals (Ereskovskii 2003), and tissue compatibility between sponge individuals to determine clone identity is an ongoing debate (Wulff 1986). Our results suggest that both contact assays are useful for distinguishing individuals of T. hoshinota sponge. Particularly, the direct contact assay is an easy and rapid approach for determining the histoincompatibility or histocompatibility of this sponge. In addition to asexual propagation (fragmentation), sexual reproduction by larval dispersal is likely to explain their distribution and prevalence patterns. In direct contact assays, tissue adhesion between two individuals in some sets occurred from 24–48 h after contact. Conversely, over 48 h was required to confirm rejection reactions with no additional adhesion and separation observed. In the marine demosponge Halichondria japonica and calcareous sponge Leucandra abratsbo Hôzawa, 1929, allogeneic combinations showed fusion of the ectopinacoderm between two individuals in the early rejection process, although barrier formation and necrotic tissues caused by cytotoxic reactions were the final rejection reactions (Amano 1990; Saito 2013).
In growing-edge contact assays with T. hoshinota, boundary formations were observed between two different individuals. During the growing-edge contact experiment, both sponges continued growing and expanding in other directions; however, the sponge did not overgrow the boundary zones. This result suggests that the sponges recognize each other and avoid excessive contact. Most other sponge species react to allografts by forming a collagen barrier between the grafted tissue or by destroying the foreign tissue at the contact surface via a cytotoxic reaction (Smith 1988; Gaino et al. 1999). According to field observations, T. hoshinota creates amicable relationships by forming a gap between individuals (Fig. 5b–d), but it is aggressive towards other sponge species (overgrowth) at all three study sites.
Histological sections of boundary zone tissues collected in the field revealed no collagen or sponge soft tissue in the boundary (Fig. 4a), suggesting that they recognize each other and remain distant from other sponges. Spicules may play an important role in the interaction between T. hoshinota individuals, including defending self-tissue and avoiding unnecessary contact. In the histological observations, the spicules pointed out and stuck into the ectopinacoderm of the other individual (Fig. 4a), indicating a competitive history for space in the past; it also indicated that the relationship between individuals is not always amicable. Furthermore, in the boundary zone of two individuals, disintegrated and gathered mesohyl was observed in some histological sections (Fig. 4b), indicating an early stage of contact reaction where repetitive contact occurred, although the boundary zones were clearly formed. A time-lapse video showed repeated short-term contact between individuals (Video S1). An adhesion of tissues from different individuals was also observed in the direct contact assays. The mechanism is likely to be by gathering mesohyl, which is the same mechanism used by the sponge to adhere to other individuals, although further histological studies are required to understand the successive processes of fusion and rejection.
CONCLUSIONS
The population structures of Terpios hoshinota are likely complex, as both asexual fragmentation and sexual reproduction by larval release occur. Boundaries between allogenic sponge individuals can be identified in the field, and the direct contact method is a simple and effective approach for distinguishing sponge individuals. Although intermittent observations are required to evaluate fusion and rejection reactions in T. hoshinota, contact experiments are convenient for evaluating microscale antagonism and important processes in reefs at risk from this coral-killing sponge.
Supplementary materials
Time-lapse video of the contact reaction between different individuals (Sesoko vs Nakijin samples, from October 22–25, 2019) over three days. They had separated by October 27.
Acknowledgments
Coral sampling was performed with the approval in Okinawa Prefecture, Japan (No. 2-2). Culture experiments were conducted at Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus with the kind support of the staff. This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 19K06091).
Footnotes
Authors’ contributions: All authors contributed to the study conception and design. Sampling was
done by YH. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no conflicts of interests.
Availability of data and materials: The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Aini SN, Tang S-L, Yamashiro H. 2021. Monthly progression rates of the coral-killing sponge Terpios hoshinota in Sesoko Island, Okinawa, Japan. Coral Reefs 40:973–981 doi:10.1007/s00338-021-02099-6.
- Amano S. 1990. Self and non-self recognition in a calcareous sponge, Leucandra abratsbo. Biol Bull 179:272–278. doi:10.2307/1542318. [DOI] [PubMed]
- Bryan PG. 1973. Growth rate, toxicity and distribution of the encrusting sponge Terpios sp. (Hadromerida: Suberitidae) in Guam, Mariana Islands. Micronesica 9:237–242.
- Cadavid LF. 2004. Self-discrimination in colonial invertebrates: genetic control of allorecognition in the hydroid Hydractinia. Dev Comp Immunol 28:871–879. doi:10.1016/j.dci.2004.01.007. [DOI] [PubMed]
- Ereskovskii AV. 2003. Problems of coloniality, modularity, and individuality in sponges and special features of their morphogenesis during growth and asexual reproduction. Rus J Mar Biol 29:S46–S56. doi:10.1023/B:RUMB.0000011716.90730. ac.
- Fujii T, Keshavmurthy S, Zhou W, Hirose E, Chen CA, Reimer JD. 2011. Coral-killing cyanobacteriosponge (Terpios hoshinota) on the Great Barrier Reef. Coral Reefs 30:483. doi:10.1007/s00338-011-0734-6.
- Gaino E, Bavestrello G, Magino G. 1999. Self/non-self recognition in sponges. Ital J Zool 66:299–315. doi:10.1080/112500099093562 70.
- Harpeni E, David AL. 2011. Life history studies of Montipora digitata in Pioneer Bay, North Queensland, Australia. J Coast Dev 15:72–81.
- Hirose E, Murakami A. 2011. Microscopic anatomy and pigment characterization of coral-encrusting black sponge with cyanobacterial symbiont, Terpios hoshinota. Zool Sci 28:199–205. doi:10.2108/zsj.28.199. [DOI] [PubMed]
- Hsu C-M, Wang J-T, Chen CA. 2013. Larval release and rapid settlement of the coral-killing sponge, Terpios hoshinota, at Green Island, Taiwan. Mar Biodiv 43:259–260. doi:10.1007/s12526-013-0176-1.
- Liao M-H, Tang S-L, Hsu C-M, Wen K-C, Wu H, Chen W-M, Wang J-T, Meng P-J, Twan W-H, Lu C-K, Dai C-F, Soong K, Chen CA. 2007. The “black disease” of reef-building corals at Green Island, Taiwan -outbreak of a cyanobacteriosponge, Terpios hoshinota (Suberitidae; Hadromerida). Zool Stud 46:520.
- Mukai H, Watanabe H. 1974. On the occurrence of colony specificity in some compound ascidians. Biol Bull 147:411–421. doi:10.2307/1540458. [DOI] [PubMed]
- Nozawa Y, Huang Y-S, Hirose E. 2016. Seasonality and lunar periodicity in the sexual reproduction of the coral-killing sponge, Terpios hoshinota. Coral Reefs 35:1071–1081. doi:10.1007/s00338-016-1417-0.
- Plucer-Rosario G.1987. The effect of substratum on the growth of Terpios, an encrusting sponge which kills corals. Coral Reefs 5:197–200. doi:10.1007/BF00300963.
- Reimer JD, Nozawa Y, Hirose E. 2010. Domination and disappearance of the black sponge: A quarter century after the initial Terpios outbreak in southern Japan. Zool Stud 50:394.
- Reimer JD, Mizuyama M, Nakano M, Fujii T, Hirose E. 2011. Current status of the distribution of the coral-encrusting cyanobacteriosponge Terpios hoshinota in southern Japan. Galaxea, J Coral Reef Stud 13:35–44. doi:10.3755/galaxea.13.35.
- Rützler K, Muzik K. 1993. Terpios hoshinota, a new cyanobacterio-sponge threatening Pacific reefs. Sci Mar 57:395–403.
- Saito, Y. 2013. Self and nonself recognition in a marine sponge, Halichondria japonica (Demospongiae). Zool Sci 30:651–657. doi:10.2108/zsj.30.651. [DOI] [PubMed]
- Smith LC. 1988. The role of mesohyl cells in sponge allograft rejections. In: Grosberg, RK, Hedgecook D, Nelson K. (eds) Invertebrate Historecognition. Springer, Boston, MA, pp. 15–30. doi:10.1007/978-1-4613-1053-2_2.
- Teruya T, Nakagawa S, Koyama T, Arimoto H, Kita M, Uemura D. 2004. Nakiterpiosin and nakiterpiosinone, novel cytotoxic C-nor-D-homosteroids from the Okinawan sponge Terpios hoshinota. Tetrahedron 60:6989–6993. doi:10.1016/j.tet.2003.08.083.
- de Voogd NJ, Cleary DFR, Dekker F. 2013. The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 32:755. doi:10.1007/s00338-013-1030-4.
- Yomogida M, Mizuyama M, Kubomura T, Reimer JD. 2017. Disappearance and return of an outbreak of the coral-killing cyanobacteriosponge Terpios hoshinota in southern Japan. Zool Stud 56:7. doi:10.6620/ZS.2017.56-07. [DOI] [PMC free article] [PubMed]
- Wang J-T, Chen Y-Y, Meng P-J, Sune Y-H, Hsu C-M, Wei K-Y, Chen CA. 2012. Diverse interactions between corals and the coral-killing sponge Terpios hoshinota (Suberitidae: Hadromerida). Zool Stud 51:150–159.
- Wang J-T, Hsu C-M, Kuo C-Y, Meng P-J, Kao S-J, Chen CA. 2015. Physiological outperformance at the morphologically-transformed edge of the cyanobacteriosponge Terpios hoshinota (Suberitidae: Hadromerida) when confronting opponent corals. PLoS ONE 10:e0131509. doi:10.1371/journal.pone.0131509. [DOI] [PMC free article] [PubMed]
- Wulff J. 1986. Variation in clone structure of fragmenting coral reef sponges. Biol J Linn Soc 27:311–330. doi:10.1111/j.1095-8312.1986.tb01740.x.
- Wulff J. 2012. Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344. doi:10.1016/B978-0-12-387787-1.00003-9. [DOI] [PubMed]
- Wulff J. 2016. Sponge contributions to the geology and biology of reefs: past, present, and future. In: Hubbard DK, Lipps JH, Rogers CS, Stanley Jr GD (eds), Coral Reefs of the World 6, Coral Reefs at the crossroads. Springer, pp. 103–126. doi:10.1007/978-94-017-7567-0.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-lapse video of the contact reaction between different individuals (Sesoko vs Nakijin samples, from October 22–25, 2019) over three days. They had separated by October 27.