Abstract
Background
Hyperbaric oxygen (HBO2) therapy has been proposed to treat hypoxaemia and reduce inflammation in COVID-19. Our objective was to analyse safety and efficacy of HBO2 in treatment of hypoxaemia in patients with COVID-19 and evaluate time to hypoxaemia correction.
Methods
This was a multicentre, open-label randomised controlled trial conducted in Buenos Aires, Argentina, between July and November 2020. Patients with COVID-19 and severe hypoxaemia (SpO2 ≤90% despite oxygen supplementation) were assigned to receive either HBO2 treatment or the standard treatment for respiratory symptoms for 7 days. HBO2 treatment was planned for ≥5 sessions (1 /day) for 90 min at 1.45 atmosphere absolute (ATA). Outcomes were time to normalise oxygen requirement to SpO2 ≥93%, need for mechanical respiratory assistance, development of acute respiratory distress syndrome and mortality within 30 days. A sample size of 80 patients was estimated, with a planned interim analysis after determining outcomes on 50% of patients.
Results
The trial was stopped after the interim analysis. 40 patients were randomised, 20 in each group, age was 55.2±9.2 years. At admission, frequent symptoms were dyspnoea, fever and odynophagia; SpO2 was 85.1%±4.3% for the whole group. Patients in the treatment group received an average of 6.2±1.2 HBO2 sessions. Time to correct hypoxaemia was shorter in treatment group versus control group; median 3 days (IQR 1.0–4.5) versus median 9 days (IQR 5.5–12.5), respectively (p<0.010). OR for recovery from hypoxaemia in the HBO2 group at day 3 compared with the control group was 23.2 (95% CI 1.6 to 329.6; p=0.001) Treatment had no statistically significant effect on acute respiratory distress syndrome, mechanical ventilation or death within 30 days after admission.
Conclusion
Our findings support the safety and efficacy of HBO2 in the treatment of COVID-19 and severe hypoxaemia.
Trial registration number
Keywords: COVID-19, hyperbaric medicine, respiratory
Key messages.
What is already known on this subject
Hypoxaemia in COVID-19 has a pathophysiology not yet fully understood. Many patients have required invasive and expensive treatments for hypoxaemia. Limited literature has suggested that hyperbaric oxygen is a safe and effective oxygenation method for patients with COVID-19, but well-designed studies are needed.
Prior case studies and non-randomised clinical trials have suggested the utility of hyperbaric oxygen for hospitalised patients with COVID-19.
What this study adds
In this multicentre randomised controlled trial of hyperbaric oxygen therapy at 1.45 atmospheres of pressure among patients with COVID-19, hypoxia was resolved in significantly shorter time in those receiving HBO2 therapy than control patients.
Findings suggest hyperbaric oxygen is safe and effective in the treatment of severe hypoxaemia in patients with COVID-19.
Introduction
The COVID-19 pandemic triggered a public health crisis worldwide. The most problematic clinical features of this disease are hypoxaemia and systemic hypoxia, produced by ventilation-perfusion mismatch and alveolar inflammation.1 Hypoxaemia has been independently associated with in-hospital mortality in patients with COVID-19; in moderate to critically ill patients, oxygen saturation (SpO2) of more than 90% with oxygen supplementation indicate a very high likelihood of survival, while values below 90%, despite normobaric oxygen supplementation, have been associated with a high mortality risk.2 Impaired oxygen diffusion leads to a drop in oxygen levels, which produces hypoxaemia and further inflammation.3–5 In severe pneumonia caused by COVID-19, impaired oxygen diffusion leads to hypoxaemia and lower level of oxygen–haemoglobin saturation.3–5
Oxygen administrated at higher pressure increases the partial pressure of oxygen in the haematoalveolar exchange, increasing gas diffusion through an altered alveolar membrane which, under the hyperbaric environment, produces a larger amount of dissolved oxygen in plasma and tissues.6 Moreover, hyperbaric hyperoxia has been associated with reduction of the inflammatory response.7 Hypoxaemia correction and hyperoxia generation could trigger an anti-inflammatory effect that, in turn, could lead to a clinical improvement in patients with hypoxaemic severe pneumonia associated with COVID-19.5 8 In case studies, HBO2 therapy has proved to be effective and safe in patients with COVID-19 pneumonia.9 10 In patients that received HBO2 therapy, SpO2 increased, tachypnoea resolved and inflammatory markers decreased.10 11 Randomised controlled trials investigating HBO2 therapy in patients with COVID-19 have not been published so far.
When receiving HBO2 treatment, patients breathe oxygen inside a chamber at a pressure higher than the atmospheric pressure at sea level; for clinical use, pressure is usually greater than 1.45 ATA.12 HBO2 therapy at medium pressure (below 2 ATA) has shown that it is neurologically safer than treatment at higher pressures.12–15 However, as the increased pressure of HBO2 therapy may exacerbate an acute lung injury or induce pulmonary oedema among patients with COVID-19, potential complications must be assessed.11 The objective of this study was to analyse the safety and efficacy of HBO2 treatment in patients with COVID-19 with severe hypoxaemia in the reduction of time for recovery from hypoxaemia (defined as SatO2 ≤93%). Also, the study evaluated if HBO2 treatment decreased progression to respiratory distress, mechanical ventilation requirements and mortality.
Materials and methods
This was a multicentre randomised controlled clinical trial of patients with COVID-19 performed at three public hospitals in Buenos Aires, Argentina. When the study was registered, two patients had been enrolled.
Sample size calculation
A sample size of 80 patients (40 in each group) was calculated with a reduction of at least 60% in the time needed to reach SpO2 of 93% (with the app epi https://www.openepi.com/SampleSize/SSCohort.htm). The sample size was based on a reduction of ≥60% in the time needed to reach Sp02 of 93%, using an alpha error of 0.05% and 80% power. This gave a sample size of 80 patients, 40 in each group.
Patient selection
Inclusion criteria were as follows: patients in emergency department (ED) or intensive care unit, over 18 years of age (all sexes), with confirmed diagnosis of COVID-19 by PCR on nasal swab, with pneumonia with oxygen dependence (defined as the need for continuous oxygen supply to maintain pulse oximetry saturation, SpO2 ≥93% or arterial gas with PaO2 ≥60 mm Hg) and no previous hospitalisation within the last 6 months. Exclusion criteria were as follows: patients unable to give consent, were pregnant or breast feeding, required mechanical ventilation, were unable to maintain prolonged sitting position (≥2 hours) or had contraindications for HBO2 therapy (such as acute respiratory distress syndrome (ARDS), emphysema, air cysts or bullae and untreated pneumothorax, or severe chronic obstructive pulmonary disease).
Treatment
All patients were treated following the latest guidelines of the National Ministry of Health of Argentina.16 Standard treatment for COVID-19 consisted of supportive treatment, regular antimicrobial treatment for severe community-acquired pneumonia (ceftriaxone 2 g/day and azithromycin 500 mg/day for 7 days), dexamethasone 8 mg/day, paracetamol 1 g/6 hours in case of high temperature and monitoring for complications. Oxygen was supplied with a reservoir mask.
Procedures
Participants were randomly assigned to receive either HBO2 treatment in addition to the standard treatment (treatment group) or the standard treatment alone (control group) for respiratory symptoms. We used simple randomisation with number assignment by a random number generator (UX App) from 1 to 10 and entered into tables assigning odd or even number to treatment or control.
HBO2 treatment consisted of ≥5 sessions of 90 min of hyperbaric oxygen therapy administered once daily using Revitalair technology (1.45 ATA) with an inspired fraction close to 100% of oxygen. As preventive measures, chambers were cleaned with a disinfectant with quaternary ammonium salts between patients and patients, and operators wore personal protection equipment when patients were transferred to the treatment room. The regimen of ≥5 sessions was chosen based on clinical experience with patients with hypoxaemia associated with COVID-19.10 However, if hypoxaemia was resolved with fewer sessions, that patient would not receive more sessions and would not be included in the study; this did not occur.
The choice of equipment with pressure at 1.45 ATA was based on multiple benefits: reduced possibility of lung injury, lower cost of the equipment (therefore higher possibility of meeting the demand), ease of installation (important in the context of the pandemic), the possibility of transporting the equipment and ease of operation and disinfection.12 13 15
Prior to and after each treatment, SpO2 evaluation was performed by removing the patient’s oxygen mask and allowing them to breathe room air for ≥5 min while monitoring SpO2 with a pulse oximeter finger probe. For patients in the control group, SpO2 on room air was monitored three times per day, every 8 hours, and the morning measure was used in the study. A fractional inspired oxyge of 21% was used to define room air.
Outcomes
Primary outcomes were proportion of patients that recovered from hypoxaemia and at 3, 5 and 10 days (to assess if patient recovered after two and five sessions) and median time to recovery within 30 days. Normalisation of oxygen requirement (oxygen independence) was defined as pulse oximetry value in ambient air ≥93% and/or arterial blood gas with a partial pressure of oxygen (PaO2) value of ≥60 mm Hg in ambient air. Secondary outcomes were the need for mechanical respiratory assistance, development of ARDS and mortality within 30 days. Adverse events related to HBO2 therapy were recorded, including the presence of ear pain, ear obstruction, significant and constant changes in BP after 4 hours of treatment and HR, barotrauma or other symptoms.
Ethical considerations
All participants provided written informed consent, while all procedures were conducted in accordance with the principles of the World Medical Association Declaration of Helsinki. This study was carried out in compliance with Good Clinical Practice (GCP); personal data were protected and encrypted. Also, a programme of quality monitoring was performed during the clinical trial to adhere to GCP.
Patient and public involvement
No patient involved.
Statistics
Data were analysed with an intention-to-treat approach. Categorical variables were analysed with bivariate analysis using χ2 test, or Fischer’s test and described in percentages with 95% CIs. Continuous variables were analysed with Student’s t-test and or paired t test, as appropriate and described using mean and SD or median with IQR, as appropriate, depending on distribution and analysis with Shapiro-Wilk test. Inferential analyses were performed using a bivariate analysis by calculating adjusted OR with 95% CIs and adjusted OR with a multiple logistic regression model. HRs were used for time to event. Log rank test and Cox regression were used to calculate HR, controlling for age, sex, arterial hypertension, obesity, diabetes, smoking and COPD. Statistical significance was set at p<0.05. Statistical analyses were performed with Stata V.13.0.
Planned interim analysis
An interim analysis of the results was scheduled once 50% of the patients were recruited. An independent investigator presented a report of the analysis to the hospitals' ethics committee, and this authorised the early suspension of the study. Analysis was prespecified in the original protocol; outcomes were the proportion of recovery from hypoxaemia and at 3, 5 and 10 days and time to correct hypoxaemia. Following the methodology presented by Pocock,17 the p values for the interim analysis and the criteria to stop the trial based on clinical benefits were reported with adjusted error type I at 0.0294.
Results
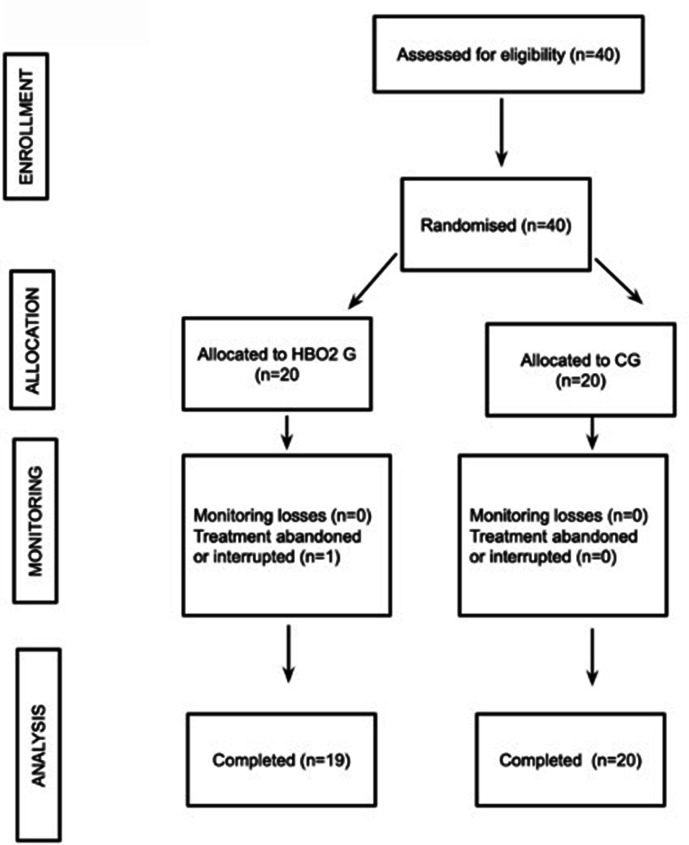
From July to November 2020, 40 patients were included. The study was stopped at interim analysis; analysis occurred after enrolment. The study was interrupted with the approval of each independent ethics committee when interim analysis with 50% of sample size showed a marked benefit of treatment. To that point, the cohort was as follows: 20 (50.0%) were assigned to HBO2 treatment group and 20 (50.0%) to the control group. Of the 40 randomised, 39 completed the protocol (19 in HBO2 group and 20 in the control group). One patient in the HBO2 withdrew after the second session because of ear discomfort (figure 1).
Figure 1.
Flow chart of included patients. CG, control group; HBO2, hyperbaric oxygen treatment group.
Mean age was 55.2±9.2 years, and 26 (65.0%) patients were male. Demographic and clinical characteristics are shown in table 1. Baseline demographics and clinical variables between the two groups were comparable (table 1). Baseline SpO2 was 85.1%±4.3% for the whole group, 86.5%±3.9% in the treatment group and 84.1%±4.5% in the control group, with no significant difference between the two groups (p=0.150) (table 2).
Table 1.
Demographic and clinical characteristics
| Characteristic | Total, n=40 | HBO2, n=20 | Control, n=20 |
| Male, n (%) | 26(65) | 13(65) | 13(65) |
| Age, years* | 55.2±9.2 | 52.8±8.5 | 57.7±9.3 |
| Weight, kg† | 82.0 (90.0; 75.0) | 79.5 (74.0; 90.0) | 86.0 (79.0; 100.0) |
| Height, cm† | 172.5 (169.5; 179.0) | 171.0 (169.5; 174.0) | 175.0 (170.5; 179.5) |
| BMI, kg/m2† | 27.7 (25.1; 30.3) | 27.9 (24.7; 29.0) | 27.2 (25.8; 32.1) |
| SAP, mm Hg† | 120.0 (120.0; 130.0) | 120.0 (115.0; 127.5) | 125.0 (120.0;130.0) |
| DAP, mm Hg* | 77.8±10.4 | 75.9±10.7 | 79.7±9.9 |
| HR, bpm* | 88.8±13.4 | 85.9±14.6 | 91.7±11.7 |
| Respiratory rate, rpm* | 26.4±4.6 | 25.3±0.9 | 27.4±1.0 |
| Temperature, °C† | 36.7 (36.4; 37.0) | 36.8 (36.4; 37.2) | 36.7 (36.4; 36.8) |
| Haematocrit %* | 38.3±4.8 | 39±3,99 | 37.7±5.4 |
| White cell count, /mm3 * | 9,008±3456 | 9,214±3209 | 8,803±3758 |
| Lymphocytes, /mm3* | 1,319±489 | 1,403±563 | 1,245±415 |
| Platelets, ×1000/mm3† | 281.5 (212.0; 352.5) | 299.5 (268.1–372.0) | 268.0 (105.5; 310.0) |
| Blood glucose, mg/dL† | 109.0 (99.0; 119.5) | 109.0 (101.5; 115.5) | 107.0 (97.0; 199.5) |
| Urea, mg/dL† | 39.0 (26.0; 46.0) | 34.0 (24.0; 46.0) | 40.5 (32.4; 45.5) |
| Creatinine, mg/dL† | 0.88 (0.7; 1.1) | 0.9 (0.7; 1.0) | 0.9 (0.7; 1.1) |
| Sodium, mEq/L* | 136.4±4.8 | 136.7±3.2 | 136.4±4.8 |
| Potassium, mEq/L* | 4.0±0.4 | 3.9±0.4 | 4.0±0.3 |
| Comorbidity | |||
| Obesity, n (%) | 14 (35.0) | 6 (30.0) | 8 (40.0) |
| Hypertension, n (%) | 13 (32.5) | 8 (40.0) | 5 (25.0) |
| Diabetes, n (%) | 7 (17.5) | 3 (15.0) | 4 (20.0) |
| COPD, n (%), | 2 (5.0) | 0 (0.0) | 2 (10.0) |
| Asthma, n (%) | 2 (5.0) | 2 (10.0) | 0 (0.0) |
| CKD, n (%) | 2 (5.0) | 1 (5.0) | 1 (5.0) |
| Cancer, n (%) | 2 (5.0) | 0 (0.0) | 2 (10.0) |
| Symptoms, n (%) | |||
| Fever | 35 (87.5) | 17 (85.0) | 18 (90.0) |
| Odynophagia | 17 (42.5) | 10 (50.0) | 7 (35.0) |
| Anosmia | 6 (15.0) | 4 (20.0) | 2 (10.0) |
| Dysgeusia | 7 (17.5) | 4 (20.0) | 3 (15.0) |
| Dyspnoea | 37 (92.5) | 19 (95.0) | 18 (90.0) |
| Headache | 4 (10.0) | 2 (10.0) | 2 (10.0) |
Data are described as
*mean±SD.
†median (IQR).
BMI, body mass index; bpm, beats per minute; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DAP, diastolic arterial pressure; rpm, rate per minute; SAP, systolic arterial pressure.
Table 2.
Outcomes within 30 days of diagnosis
| Outcomes | Total (n=40) | HBO2
(n=20) |
Control (n=20) | P value | Effect size (95% CI) |
| Days to normalisation* | 5.0 (3.0; 9.0) | 3.0 (1.0; 4.5) | 9.0 (5.5; 12.5) | <0.010 | HR 6.9 (2.9 to 16.2) |
| SpO2 ≥93% at 3 days, n (%) | 12 (30) | 11 (55) | 1 (8) | 0.001 | OR 23.2 (1.6 to 329.6) |
| SpO2 ≥93% at 5 days, n (%) | 27 (68) | 19 (95) | 8 (40) | <0.001 | OR 28.5 (1.8 to 447.4) |
| SpO2 ≥93% at 10 days, n (%) | 33 (83) | 20 (100) | 13(65) | 0.004 | ∞ |
| SpO2 ≥93% at 15 days, n (%) | 36 (90) | 20 (100) | 16 (80) | 0.106 | ∞ |
| Acute respiratory distress, n (%) | 4 (10) | 1 (5) | 3 (15) | 0.605 | OR 0.3 (0.0 to 3.4) |
| Mechanical ventilation, n (%) | 4 (10) | 1 (5) | 3 (15) | 0.605 | OR 0.3 (0.0 to 3.4) |
| Death, n (%) | 2 (5) | 1 (5) | 1 (5) | 1.000 | OR 1.0 (0.1 to 17.8) |
* Median (25th percentile; 75th percentile).
SpO2, oxygen saturation.
Four (10.0%) of 40 patients had a poor clinical course (composite outcome of acute respiratory distress syndrome, mechanical ventilation requirements or death) within 30 days of admission, with 3 (15.0%) patients in the control group and 1 (5.0%) patient in the treatment group, with no statistically significant difference. Two (5.0%) patients died within 30 days after admission, one in each group. The use of HBO2 treatment had no statistically significant effect on the incidence of ARDS, mechanical ventilation or death within 30 days after admission (table 2).
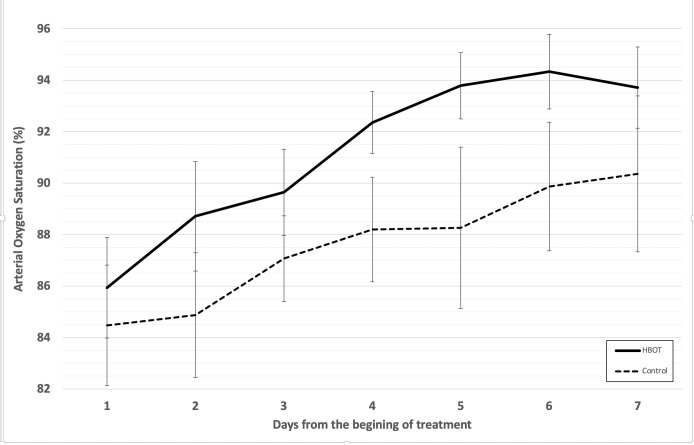
Patients included in the treatment group (n=20), including the patient who abandoned treatment, received an average of 6.2±1.2 hyperbaric oxygen sessions. The difference between arterial saturation at pre-HBO2 and post-HBO2 treatment session was significant for most sessions (table 3). SpO2 showed an immediate and successive daily improvement at a higher slope compared with the patients who did not receive HBO2 (table 3 and figure 2).
Table 3.
Oxygen saturation response
| Saturation assessment | Total (n=40) | HBO2 (n=20) | Control (n=20) | P value |
| SpO2 on day 1, %* | 85.1±4.3 | 86.5±3.9 | 84.1±4.5 | 0.150 |
| SpO2 on day 2, %* | 86.7±4.6 | 89.0±4.1 | 84.4±3.9 | <0.010‡ |
| SpO2, on day 3, %* | 87.9±3.1 | 89.6±2.8 | 86.4±2.5 | <0.010‡ |
| SpO2 on day 4, %† | 89.0 (88.0; 92.0) | 93.0 (91.0; 94.0) | 88.0 (82.0; 89.0) | <0.010‡ |
| SpO2 on day 5, %† | 91.0 (88.0; 93.0) | 93.0 (92.0; 95.0) | 88.0 (87.0; 91.0) | <0.010‡ |
| SpO2 on day 6, %† | 93.0 (89.0; 94.0) | 94.0 (93.5; 95.5) | 90.0 (88.0; 92.0) | <0.010‡ |
| SpO2 on day 7, %† | 92.0 (90.0; 94.0) | 94.0 (93.0; 95.0) | 91.0 (89.5; 92.0) | 0.020‡ |
Data are described as
*mean±SD.
†median (IQR).
‡Statistically significant.
SpO2, oxygen saturation.
Figure 2.
Improvement of arterial oxygen saturation over the course of treatment.
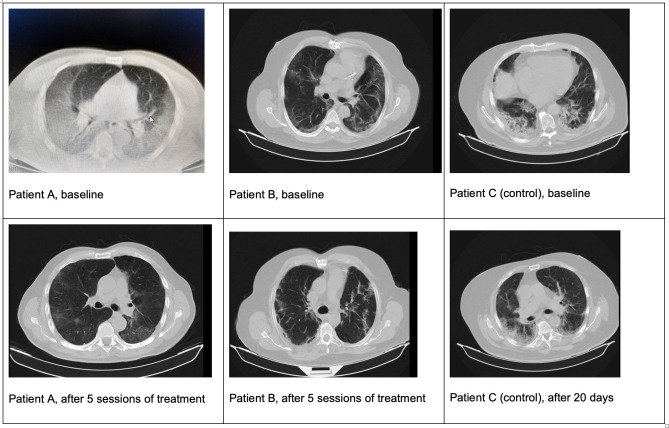
Hypoxaemia correction (defined as SpO2 ≥93%) was reached in the HBO2 treatment group, with a greater upward slope of arterial saturation compared with control group. Time to correct hypoxaemia was shorter in the treatment group compared with the control group: median (IQR) 3 (1.0–4.5) days versus 9 (5.5–12.5) days, respectively (p<0.010), log rank χ2 28.31, p<0.001; HR 6.9 (95% CI 2.9 to 16.2); adjusted HR was 7.7 (95% CI 3.1 to 19.5) (z 4,3 p<0.001). The OR of recovery from hypoxaemia in the HBO2 group compared with the control group was 23.2 (95% CI 1.6 to 329.6; p=0.001) at day 3 and 28.5 (95% CI 1.8 to 447.4; p<0.001) at day 5 (table 2). Figure 3 shows computed tomography (CT) images of two patients at the beginning of HBO2 treatment (figure 3A) and after receiving five sessions (figure 3B) and one patient in the control group at day 1 and 20 days later (figure 3C).
Figure 3.
CT images of patients. Patients in treatment group (A and B) and patient in control group (C).
Discussion
Our findings suggest that supplementing oxygen through HBO2 treatment contributed to an increased SpO2 in patients with COVID-19 with severe hypoxaemia, with no significant adverse effects. Cases of severe COVID-19 that need mechanical ventilation have a high mortality risk. Therefore, novel therapeutic strategies are needed, and this study offers evidence supporting HBO2 treatment. Even in the context of the ED, a small number of HBO2 sessions, as shown in our findings, could benefit patients before they are transferred to intensive care.
Dyspnoea and hypoxaemia (SpO2 <90% despite oxygen supplementation) have been added to a list of risk factors for mortality in patients with COVID-19 pneumonia, together with age, sex, comorbidities and inflammatory biomarkers.2 In severe cases of COVID-19, there occurs an acute lung injury, macrophage-neutrophil accumulation in the lungs and elevated proinflammatory serum cytokines.18 HBO2 treatment involves the increasing partial pressure of oxygen in plasma and tissues, with improved diffusion efficiency of oxygen through the alveolar barrier, a higher content of dissolved oxygen in the blood and increased diffusion distance of oxygen.6 HBO2 treatment may modulate the inflammatory response and the cytokine level, appearing to increase FGF production and collagen synthesis and decrease interleukin 1, interleukin 6 and tumour necrosis alpha factor; also, it has been suggested that the treatment may affect the expression levels of transforming growth factor beta-1 and platelet-derived growth factor.6 19–21 Moreover, COVID-19 related stressors exacerbate the effects of the disease by inducing the generation of oxidative stress, affecting the host’s immune response and associated with systemic tissue damage.22 HBO2 treatment below 2.0 ATA increases the activity of antioxidant enzymes, including first-line defence antioxidants superoxide dismutase and catalase.11
Case series have reported that patients with COVID-19 treated with HBO2 showed improved survival and could avoid mechanical ventilation.7 Our findings support case series suggesting the beneficial effect of HBO2 in the correction of hypoxaemia. In case series, as suggested by Paganini et al 7, it is not possible to determine if improved outcomes were the effect of the treatment or time. Comparing our two groups, the effect of HBO2 seems to be significantly more beneficial than receiving no treatment.
We acknowledge that this study has limitations. The interim analysis and early suspension because of clinical benefits (superiority and safety) should be taken into account; this limited conclusions about the secondary outcomes. Indeed, the small number of patients included in the study is an important limitation. Although not statistically significant, there is a trend towards younger patients in the treatment group. Also, data were acquired under emergency conditions; so, information on some variables could not be included, for example, baseline oxygen requirement. However, only patients with requirement of high-flow oxygen were included. It was not possible to determine the moment hypoxaemia had been established, and HBO2 could have been more effective in reducing mortality when hypoxaemia was recently established. Normalisation of oxygen was selected as the primary outcome because long-term hyperpoxia induces more residual multisystemic inflammatory complications.2 In fact, mortality depends on many other variables that could not be controlled in this study, such as previous days of hypoxaemia.
This treatment could be easily available in various settings. Portable hyperbaric chambers offer a fast setup to avoid transferring patients to other hospital areas, attenuating the risk of virus transmission. In conclusion, our findings support the efficacy of HBO2 in the treatment of COVID-19 with severe hypoxaemia; larger trials are needed to further confirm the treatment effects on survival.
Acknowledgments
The authors would like to thank Ms Pilar Maria Emilia Fraga for quality monitorisation of the present study.
Footnotes
Handling editor: Lara Nicole Goldstein
Contributors: Concept: MC, GK and EE. Design: MC, GK, LJ-V and DM. Data collection: MD, GK, RL, EC, VC, MM, DMB, EG, HEDS, FV, CD and GDG. Data analysis: MC, MD, GK, LJ-V and EE. Visualisation: LJ-V and JR. Interpretation: MC, MD, GK, RL, DMB, HEDS, FV, LJ-V, GDG, JR and EE. Investigation: MC, MD, GK, LJ-V, JR and EE. Draft writing: LJV, MC and JR. Guarantor: MC. All authors reviewed and commented the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MC is medical director, FV is medical liaison and LJ-V is scientific director and management in clinical research of Biobarica Hyperbaric Medical Centers Net.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Additional information available on request to authors.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
Ethics committee of three participating hospitals: Municipal de San Isidro, Santojanni, Muñiz.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc 2020;95:1138–47. 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is Baffling to physicians. Am J Respir Crit Care Med 2020;202:356–60. 10.1164/rccm.202006-2157CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract 2020;10:24–30. 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solaimanzadeh I. Heterogeneous perfusion in COVID-19 and high altitude pulmonary edema: a review of two cases followed by implications for hypoxic pulmonary vasoconstriction, thrombosis development, ventilation perfusion mismatch and emergence of treatment approaches. Cureus 2020;12:e10230. 10.7759/cureus.10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choudhury R. Hypoxia and hyperbaric oxygen therapy: a review. Int J Gen Med 2018;11:431–42. 10.2147/IJGM.S172460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paganini M, Bosco G, Perozzo FAG, et al. The role of hyperbaric oxygen treatment for COVID-19: a review. Adv Exp Med Biol 2021;1289:27–35. 10.1007/5584_2020_568 [DOI] [PubMed] [Google Scholar]
- 8. Geier MR, Geier DA. Respiratory conditions in coronavirus disease 2019 (COVID-19): important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses 2020;140:109760. 10.1016/j.mehy.2020.109760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thibodeaux K, Speyrer M, Raza A, et al. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J Wound Care 2020;29:S4–8. 10.12968/jowc.2020.29.Sup5a.S4 [DOI] [PubMed] [Google Scholar]
- 11. Gorenstein SA, Castellano ML, Slone ES, et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: treated cases versus propensity-matched controls. Undersea Hyperb Med 2020;47:405–13. [PubMed] [Google Scholar]
- 12. Kot J, Winklewski PJ, Sicko Z, et al. Effect of oxygen on neuronal excitability measured by critical flicker fusion frequency is dose dependent. J Clin Exp Neuropsychol 2015;37:276–84. 10.1080/13803395.2015.1007118 [DOI] [PubMed] [Google Scholar]
- 13. Efrati S, Ben-Jacob E. Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Rev Neurother 2014;14:233–6. 10.1586/14737175.2014.884928 [DOI] [PubMed] [Google Scholar]
- 14. Shah J. Hyperbaric oxygen therapy. J Am Col Certif Wound Spec 2010;2:9–13. 10.1016/j.jcws.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cannellotto M, Romero-Feris D, Pascuccio MM, et al. Aplicaciones médicas de las cámaras de oxigenación hiperbárica de nueva generación. Asoc Med Arg 2018;131:12–20. [Google Scholar]
- 16. National Ministry of Health of Argentina . Recomendaciones para el abordaje terapéutico de COVID-19. Buenos Aires, Argentina, 2020. [Google Scholar]
- 17. Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika 1977;64:191–9. 10.1093/biomet/64.2.191 [DOI] [Google Scholar]
- 18. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020;34:327–31. 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- 19. Strauss MB, Bryant BJ, Hart GB. Transcutaneous oxygen measurements under hyperbaric oxygen conditions as a predictor for healing of problem wounds. Foot Ankle Int 2002;23:933–7. 10.1177/107110070202301008 [DOI] [PubMed] [Google Scholar]
- 20. Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg 2010;112:1080–94. 10.3171/2009.7.JNS09363 [DOI] [PubMed] [Google Scholar]
- 21. Al-Waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. ScientificWorldJournal 2006;6:425–41. 10.1100/tsw.2006.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakadia BM, Boni BOO, Ahmed AAQ, et al. The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci 2021;264:118653. 10.1016/j.lfs.2020.118653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Additional information available on request to authors.