Abstract
Cardiac involvement is an important cause of morbidity and mortality in patients with idiopathic inflammatory myopathies (IIMs). Hypertension, an important cardiovascular risk factor for the general population, has a crucial role in heart involvement. However, few studies have focused on the hypertension associated with IIMs. This study aimed to develop and assess the prediction model for incident hypertension in patients with IIMs. A retrospective cohort study was performed on 362 patients with IIMs, of whom 54 (14.9%) were given a diagnosis of new‐onset hypertension from January 2008 to December 2018. The predictors of hypertension in IIMs were selected by least absolute shrinkage and selection operator (LASSO) regression, multivariable logistic regression, and clinically relevance, and then these predictors were used to draw the nomogram. Discrimination, calibration and clinical usefulness of the model were evaluated using the C‐index, calibration plot, and decision curve analysis, respectively. The predicting model was validated by the bootstrapping validation. The nomogram mainly included predictors such as age, diabetes mellitus, triglyceride, low‐density lipoprotein‐cholesterol (LDL‐C), antinuclear antibodies (ANA), and smoking. This prediction model demonstrated good discrimination with a C‐index of 0.754 (95%CI, 0.684 to 0.824) and good calibration. The C‐index of internal validation was 0.728, and decision curve analysis demonstrated that this nomogram was clinically useful. Clinicians can use this prediction model to assess the risk of hypertension in IIMs patients, and early preventive measures should be taken to reduce the incidence of hypertension in high‐risk patients.
Keywords: hypertension, idiopathic inflammatory myopathies, nomogram, prediction model
1. INTRODUCTION
Idiopathic inflammatory myopathies (IIMs) are a group of autoimmune diseases that are characterized by chronic inflammation of muscle and proximal muscle weakness. The best recognized subsets of IIMs are polymyositis and dermatomyositis. As a rare disease, the annual incidence of IIMs ranges from 5.8 to 7.9 per 100 000 person‐years, and the prevalence of which ranges from 14.0 to 17.4 per 100 000 person‐years. 1 IIMs can involve multiple organs, including skin, lungs, joints, heart, and gastrointestinal tract. Previous studies demonstrated that cardiovascular involvement was the important cause of high morbidity and mortality in patients with IIMs. 2 , 3 , 4 , 5 In fact, cardiovascular involvement in patients with polymyositis is higher than the general population. 6 In addition, the reported prevalence of hypertension in patients with IIMs varies between 38.7% and 71%, based on different patient selection and the definition of the disease, which is also higher than the general population. 5 , 7 , 8
The prevalence of hypertension in patients with IIMs is extremely high. However, the physicians overlook the presence and onset of hypertension in IIMs patients. What's more, the underlying pathogenesis and mechanisms of diseases remain unclear. Limited evidence found that the autoimmune mechanism played a vital role in incident hypertension. 7 Antibodies against α1‐adrenergic receptors and angiotensin Ⅱ were considered important in the development of hypertension, which may be related to the reaction of myositis autoantigens and stimulated the inflammatory response in IIMs. 9 Patients with hypertension may lead to serious complications, including stroke, atherosclerosis, kidney damage, myocardial infarction, and so on. Hypertension is a main risk factor for cardiovascular and cerebrovascular events, which can increase the risk of death. 10 Thus, it is important to be able to predict whether patients with IIMs have a high risk of developing hypertension so that preventive measures can be taken early.
So far, many studies have developed or verified the risk models for predicting the incidence of hypertension, and the results of these models show that age, diabetes mellitus, smoking, and dyslipidemia are important predictors of hypertension. 11 , 12 , 13 , 14 However, most of these prediction models of hypertension are based on the general population and rarely involve patients with related diseases. To our knowledge, there is no study focused on the incidence of hypertension in patients with IIMs.
Nomograms can make the results of prediction model readable and help doctors make better clinical decisions. Therefore, the purpose of this study is to develop a valid nomogram to predict the incident hypertension based on baseline features, laboratory‐related indexes, and therapy‐related factors of IIMs patients.
2. MATERIALS AND METHODS
2.1. Study population
The study was conducted by patients with IIMs attending a routine hospital visit at the Third People's Hospital of Chengdu between January 2008 and December 2018. The diagnosis of IIMs was confirmed according to the criteria of Bohan and Peter by skilled clinicians. 15 All participants should be periodical (at least once every three months) follow‐up visits between January 2008 and December 2018. The duration of follow‐up was defined as the duration from the onset of IIMs to the date of diagnosis of hypertension or the last visit, whichever came first. Patients were excluded in the baseline if they had previous history of hypertension, malignant tumor, valvular heart disease, congenital heart disease, myocardial infarction, heart failure, chronic obstructive pulmonary disease, renal failure, overlap syndrome, primary hyperaldosteronism, Cushing's syndrome, infectious diseases, electrolyte imbalances, and severe hypothyroidism. Moreover, patients were also not included who were irregular follow‐up, incomplete data, and subjects with uncertain hypertension status at follow‐up. Finally, a total of 362 patients who met the criteria were included. Ethical approval was obtained from the ethics committee of the Third People's Hospital of Chengdu, and the informed consent requirement was informed.
2.2. Data collection
Demographics, clinical manifestations, physical signs, laboratory‐related indexes, and therapy‐related factors of patients were systematically extracted from electronic medical records. Data were extricated by two trained physicians and checked by the third experienced physician. After an overnight fast, venous blood was taken in the morning from all cases, and laboratory‐related indexes were determined using standard techniques.
2.3. Hypertension assessment
During the follow‐up period, systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg, and/or self‐reported hypertension was defined as new‐onset hypertension. 16 Patients were checked at the time of diagnosis of hypertension or the last visit, whichever came first. The blood pressure of patients with IIMs was measured three times at the right arm using the digital blood pressure monitor by trained medical students or clinical doctors. The first measurement was made after a five‐minute break. The second and third measurements were performed at three‐minute intervals following the first reading. 17 The average of the second and third results was used for this study.
2.4. Definition
In this study, patients with an overlap syndrome had to meet the criteria of the American Rheumatism Association for systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). 18 , 19 Smokers were defined as subjects who had been regularly smoking for at least one year and were still smoking, whereas nonsmokers were defined as those who had not. 20 The diagnosis criteria of diabetes mellitus were fasting plasma glucose level ≥7.0mmol/L, and/or plasma glucose levels 2 hours after 75 g glucose load (2 hPG) ≥11.1mmol/L, and/or HbA1c ≥6.5%. 21 The normal reference range of some laboratory‐related indexes was determined by the biochemical laboratory of the hospital combined with the reference range provided by reagent manufactures. The specific values were as follows: total protein (TP, 60‐83g/L), albumin (ALB, 35‐55g/L), urea (3.38‐8.57mmol/L), serum creatinine (Scr, 53‐140μmol/L), uric acid (UA, 240‐490μmol/L), triglyceride (TG, 0.29‐1.83mmol/L), total cholesterol (TC, 2.8‐5.7mmol/L), high‐density lipoprotein‐cholesterol (HDL‐C, >0.9mmol/L), low‐density lipoprotein‐cholesterol (LDL‐C, <4.0mmol/L), C‐relative protein (CRP, <5mg/L), and erythrocyte sedimentation rate (ESR, <38mm/h).
2.5. Statistical analysis
All data including baseline features, clinical manifestation, laboratory‐related indexes, and therapy‐related factors were expressed as count (%). All statistical analyses were performed with the R software (Version 3.6.1; https:// www.R‐project.org). All tests were two sided, and P‐value<0.1 was considered to be statistically significant.
The least absolute shrinkage and selection operator (LASSO) regression model was used to select the significant predictive features from the primary data, which was fit for the reduction in high‐dimensional data. 22 We first select the important features related to hypertension in IIMs patients by using LASSO regression. Then, the variables selected by the LASSO regression model were used for multivariable logistic regression. The variables were considered as odds ratio (OR) having 95% confidence interval (CI) and as P‐value. The establishment of predictive model for hypertension was based on the results of the LASSO regression model, multivariable logistic regression, and clinical correlation.
The most relevant factors were used to draw the nomogram. Harrell's C‐index was applied to evaluate the performance of the nomogram. 23 The C‐index estimates the probability of concordance between predicted and observed results. The C‐index of the model ranges from 0.5 to 1.0. The C‐index of 0.5 indicates random chance and this model has no predictive value, and the C‐index of 1.0 shows this model has perfect discrimination and there is at least one threshold that yields a perfect prediction. 23 If the C‐index is between 0.5 and 1.0, it demonstrates the model has a certain predictive value.
The goodness of fit was assessed using a calibration plot, and the area under the curve (AUC) of receiver operating characteristic (ROC) is equal to the C‐index. The clinical usefulness of this prediction model is proved by using the decision curve analysis (DCA), and the DCA curve is obtained by quantifying the net benefits of different threshold probabilities in patients. 24 The relatively correctional C‐index of this nomogram is calculated by using bootstraps with 1000 samples. 25
3. RESULTS
3.1. Patients’ characteristics
A total of 362 patients with IIMs visited the Third People's Hospital of Chengdu from January 2008 to December 2018, and 54 of them developed hypertension, with a mean age of 57 ± 13 years. And 308 patients had normal blood pressure, with a mean age of 47 ± 13 years. The cohort consisted of 97 patients with polymyositis, 253 patients with dermatomyositis, and 12 patients with inclusion body myositis. The median duration of follow‐up in IIMs patients was 6 months (ranging from 1 to 120 months). The mean of baseline SBP and DBP of the included subjects was 118 ± 16 mmHg and 74 ± 12 mmHg. The means of baseline SBP and DBP of the patients with or without hypertension were 134 ± 16 mmHg and 85 ± 13 mmHg, 116 ± 14 mmHg and 72 ± 11 mmHg, respectively. The characteristics of patients including baseline features, clinical manifestation, laboratory‐related indexes, and therapy‐related factors are summarized in Table 1.
TABLE 1.
Clinical and demographic characteristics of IIMs patients
| Characteristics | Hypertension (n=54) | Non‐hypertension (n=308) | Total (n=362) | |
|---|---|---|---|---|
| Age (n, %) (years) | ||||
| <50 | 16(29.6) | 181(58.8) | 197(54.4) | |
| ≥50 | 38(70.4) | 127(41.2) | 165(45.6) | |
| Gender (n,%) | ||||
| Female | 40(74.1) | 205(66.6) | 245(67.7) | |
| Male | 14(25.9) | 103(33.4) | 117(32.3) | |
| SBP (mean±SD) (mmHg) | 134±16 | 116±14 | 118 ±16 | |
| DBP (mean±SD) (mmHg) | 85±13 | 72±11 | 74±12 | |
| Follow‐up (n,%) (months) | ||||
| <6 | 26(48.1) | 143(46.4) | 169(46.7) | |
| ≥6 | 28(51.9) | 165(53.6) | 193(53.3) | |
| Smoking (n,%) | ||||
| Yes | 1(1.9) | 25(8.1) | 26(7.2) | |
| No | 53(98.2) | 283(91.9) | 336(92.8) | |
| Diabetes mellitus (n,%) | ||||
| Yes | 10(18.5) | 25(8.1) | 35(9.7) | |
| No | 44(81.5) | 283(91.9) | 327(90.3) | |
| Dysphagia (n,%) | ||||
| Yes | 19(35.2) | 141(45.8) | 160(44.2) | |
| No | 35(64.8) | 167(54.2) | 202(55.8) | |
| Myalgia (n,%) | ||||
| Yes | 20(37.0) | 157(51.0) | 177(48.9) | |
| No | 34(63.0) | 151(49.0) | 185(51.1) | |
| Arthralgia (n,%) | ||||
| Yes | 16(29.6) | 130(42.2) | 146(40.3) | |
| No | 38(70.4) | 178(57.8) | 216(59.7) | |
| Rash | ||||
| Yes | 34(63.0) | 213(69.2) | 247(68.2) | |
| No | 20(37.0) | 95(30.8) | 115(31.8) | |
| Lung involvement (n,%) | ||||
| Yes | 15(27.8) | 101(32.8) | 116(32.0) | |
| No | 39(72.2) | 207(67.2) | 246(68.0) | |
| Gottron's sign (n,%) | ||||
| Yes | 13(24.1) | 84(27.2) | 97(26.8) | |
| No | 41(75.9) | 224(72.7) | 265(73.2) | |
| Raynaud's phenomenon (n,%) | ||||
| Yes | 3(5.6) | 31(10.1) | 34(9.4) | |
| No | 51(94.4) | 277(89.9) | 328(90.6) | |
| ESR (n,%) | ||||
| Normal | 44(81.5) | 214(69.5) | 258(71.3) | |
| Above normal | 10(18.5) | 94(30.5) | 104(28.7) | |
| CRP (n,%) | ||||
| Normal | 33(61.1) | 166(53.9) | 199(55.0) | |
| Above normal | 21(38.9) | 142(46.1) | 163(45.0) | |
| TP (n,%) | ||||
| Below normal | 41(75.9) | 212(68.8) | 253(69.9) | |
| Normal | 13(24.1) | 96(31.2) | 109(30.1) | |
| ALB (n,%) | ||||
| Below normal | 21(38.9) | 154(50.0) | 175(48.3)) | |
| Normal | 33(61.1) | 154(50.0) | 187(51.7) | |
| Urea (n,%) | ||||
| Below normal | 2(3.7) | 44(14.3) | 46(12.7) | |
| Normal | 47(87.0) | 243(78.9) | 290(80.1) | |
| Above normal | 5(9.3) | 21(6.8) | 26(7.2) | |
| Scr (n,%) | ||||
| Below normal | 21(38.9) | 162(52.6) | 183(50.6) | |
| Normal | 33(61.1) | 146(47.4) | 179(49.4) | |
| UA (n,%) | ||||
| Below normal | 13(24.1) | 109(35.4) | 122(33.7) | |
| Normal | 40(74.1) | 194(63.0) | 234(64.6) | |
| Above normal | 1(1.9) | 5(1.6) | 6(1.7) | |
| TG (n,%) | ||||
| Normal | 19(35.2) | 163(52.9) | 182(50.3) | |
| Above normal | 35(64.8) | 145(47.1) | 180(49.7) | |
| TC (n,%) | ||||
| Below normal | 3(5.6) | 20(6.5) | 23(6.4) | |
| Normal | 35(64.8) | 243(78.9) | 278(76.8) | |
| Above normal | 16(29.6) | 45(14.6) | 61(16.9) | |
| HDL‐C (n,%) | ||||
| Below normal | 12(77.8) | 80(74.0) | 92(74.6) | |
| Normal | 42(22.2) | 228(74.0) | 270(74.6) | |
| LDL‐C (n,%) | ||||
| Normal | 44(81.5) | 293(95.1) | 337(93.1) | |
| Above normal | 10(18.5) | 15(4.9) | 25(6.9) | |
| ANA (n,%) | ||||
| Positive | 43(79.6) | 202(65.6) | 245(67.7) | |
| Negative | 11(20.4) | 106(34.4) | 117(32.3) | |
| Anti SSA antibody (n,%) | ||||
| Positive | 2(3.7) | 46(14.9) | 48(13.3) | |
| Negative | 52(96.3) | 262(85.1) | 314(86.7) | |
| Anti SSB antibody (n,%) | ||||
| Positive | 0(0.0) | 16(5.2) | 16(4.4) | |
| Negative | 54(100.0) | 292(94.8) | 346(95.6) | |
| Anti‐SCL‐70 antibody (n,%) | ||||
| Positive | 1(1.9) | 4(1.3) | 5(1.4) | |
| Negative | 53(98.1) | 304(98.7) | 357(98.6) | |
| Anti‐Jo1 antibody (n,%) | ||||
| Positive | 3(5.6) | 23(7.5) | 26(7.2) | |
| Negative | 51(94.4) | 285(92.5) | 336(92.8) | |
| Use of GC (n,%) | ||||
| Yes | 25(46.3) | 131(42.5) | 156(43.1) | |
| No | 29(53.7) | 177(57.5) | 206(56.9) | |
| Use of MTX (n,%) | ||||
| Yes | 7(13.0) | 30(9.7) | 37(10.3) | |
| No | 47(87.0) | 278(90.3) | 325(89.8) | |
| Use of CTX (n,%) | ||||
| Yes | 2(3.7) | 7(2.3) | 9(2.5) | |
| No | 52(96.3) | 301(97.7) | 353(97.5) | |
| Use of HCQ (n,%) | ||||
| Yes | 3(5.6) | 20(6.5) | 23(6.4) | |
| No | 51(94.4) | 288(93.5) | 339(93.6) | |
| Use of AZA (n,%) | ||||
| Yes | 1(1.9) | 7(2.3) | 8(2.2) | |
| No | 53(98.1) | 301(97.7) | 354(97.8) | |
| Use of LEF (n,%) | ||||
| Yes | 1(1.9) | 4(1.3) | 5(1.4) | |
| No | 53(98.1) | 304(98.7) | 357(98.6) | |
| Use of TII (n,%) | ||||
| Yes | 1(1.9) | 8(2.6) | 9(2.5) | |
| No | 53(98.1) | 300(97.4) | 353(97.5) | |
| Use of TGP (n,%) | ||||
| Yes | 1(1.9) | 7(2.3) | 8(2.2) | |
| No | 53(98.1) | 301(97.7) | 354(97.8) | |
Abbreviations: ALB, albumin; ANA, antinuclear antibodies; AZA, azathioprine; CRP, C‐relative protein; CTX, cyclophosphamide; DBP, diastolic blood pressure;ESR, erythrocyte sedimentation rate; GC, glucocorticoid; HCQ, hydroxychloroquine; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; LEF, leflunomide; MTX, methotrexate; SBP, systemic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride; TGP, total glucosides of paeony; TII, tripterygium wilfordii; TP, total protein; UA, uric acid.
3.2. Feature selection
Of baseline features, laboratory‐related indexes and therapy‐related factors, 36 features were reduced to 16 potential predictors on the basis of 362 patients in the cohort (Figure 1), and these features had nonzero coefficients in the LASSO regression model. These features included age, smoking, diabetes mellitus, myalgia, arthralgia, rash, ALB, urea, Scr, UA, TG, LDL‐C, ESR, ANA, anti‐SSA antibody, and anti‐SSB antibody. However, the results of the multivariable logistics regression analysis showed that age, smoking, diabetes mellitus, TG, LDL‐C, and ANA are statistically significant (Table 2). It was worth noting that there was no statistical significance among SBP for every 10 mmHg change in multivariable logistics regression analysis (P = .203), and there was similarity in the discrimination, calibration and clinical usefulness of the prediction models with and without “SBP (per 10 mmHg)” this variable. Based on the results of LASSO regression and multivariable logistic regression, and clinical correlation, we selected age, smoking, diabetes mellitus, TG, LDL‐C, and ANA as predictors of hypertension.
FIGURE 1.

Demographic and clinical features selection using the LASSO regression model
TABLE 2.
Multivariable logistic regression model for features of hypertension
| Intercept and variable | Prediction model | ||
|---|---|---|---|
| β | Odds ratio (95%CI) | P‐value | |
| Intercept | ‐3.6670 | 0.0256 (0.0028‐0.1528) | 0.0002 |
| Age, years | |||
| ≥50 | 0.8936 | 2.4440 (1.1886‐5.1874) | 0.0168 |
| <50 | – | 1.0000(Reference) | – |
| Diabetes mellitus | |||
| Yes | 1.1402 | 3.1275 (1.1636‐8.2591) | 0.0214 |
| No | – | 1.0000(Reference) | – |
| TG | |||
| Above normal | 0.8243 | 2.2803 (1.1278‐4.7292) | 0.0235 |
| Normal | – | 1.0000(Reference) | – |
| LDL‐C | |||
| Above normal | 1.3073 | 3.6962 (1.2682‐10.6914) | 0.0154 |
| Normal | – | 1.0000(Reference) | – |
| ANA | |||
| Positive | 0.9832 | 2.6731 (1.2306‐6.2820) | 0.0172 |
| Negative | – | 1.0000(Reference) | – |
| Smoking | |||
| Yes | ‐2.1558 | 0.1158(0.0053‐0.7712) | 0.0674 |
| No | – | 1.0000(Reference) | – |
Abbreviations: ANA, antinuclear antibodies; LDL‐C, low‐density lipoprotein‐cholesterol; TG, triglyceride.
3.3. Development of an individualized prediction model
Based on the results of LASSO regression and multivariable logistic regression, and clinical correlation, the prediction model was developed and presented as the nomogram (Figure 2). The higher the sum of the assigned number of points of each related factor in the nomogram, the higher the risk of developing hypertension.
FIGURE 2.
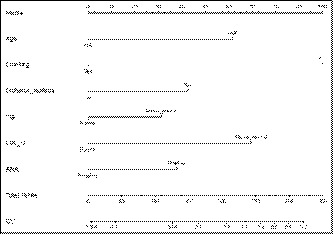
Developed prediction hypertension nomogram
3.4. Apparent performance of the prediction hypertension nomogram in IIMs patients
The calibration curve of the prediction hypertension nomogram in these patients revealed good calibration in this cohort (Figure 3). The C‐index of the prediction hypertension nomogram was 0.754 (95%CI, 0.684 to 0.824), and the C‐index was confirmed to be 0.728 by bootstrapping validation. These results suggested this model had good discrimination.
FIGURE 3.

Calibration curves of the prediction hypertension nomogram in the cohort
3.5. Clinical use
The decision curve analysis of the prediction hypertension nomogram is presented in Figure 4. The decision curve showed that if the threshold probability of a patient and a doctor is >1% and <50%, using this nomogram to predict hypertension in patients with IIMs adds more benefit than the scheme. With this range, net benefit was comparable with several overlaps, on the basis of the prediction hypertension nomogram.
FIGURE 4.

Decision curve analysis for the prediction hypertension nomogram
4. DISCUSSION
Cardiac involvement in IIMs patients is often clinically silent, which rarely attracts the attention of clinicians. 26 However, the causes of death in IIMs patients were mostly attributed to cardiovascular complications. 26 Therefore, it is urgent to take measures to identify the risk factors of cardiovascular events in IIMs patients. Hypertension, an important cardiovascular risk factor, has a crucial role in heart involvement. The nomogram is widely applied to predict the risk of certain complications, disease, death, and so on. Our study was the first to use the nomogram to predict and validate the risk of hypertension in IIMs patients. The nomogram showed that age ≥50 years, diabetes mellitus, high levels of TG and LDL‐C, positive ANA and nonsmokers were more likely to develop hypertension in IIMs patients. In addition, the C‐index of the nomogram and the internal validation was good, which indicated this predictive model had good discriminative ability and calibration. Meanwhile, the decision curve analysis revealed this nomogram had good clinical usefulness. Clinicians can use these features to assess the risk of hypertension in IIMs patients, and early preventive measures should be taken to reduce the incidence of hypertension in high‐risk patients.
Most of the results of the current study are consistent with previous results in the other autoimmune diseases or general population. For example, there is an increased risk of hypertension in IIMs patients older than 50 years old, and it may be associated with the increase in peripheral vascular resistance and vascular stiffness with aging. 27 , 28 Similarly, studies from patients with SLE and RA showed that the prevalence of hypertension in patients was higher than in the general population, and the development of hypertension was associated with age and renal involvement. 29 , 30 In addition, SLE patients with hypertension were significantly older than those without hypertension (median age 58.5 years vs. 46.0 years; P < .001). 30 Metabolic syndrome is a cluster of classical cardiovascular risk factors characterized by hypertension, diabetes mellitus, dyslipidemia, central obesity and so on, and its presence is a strong predictor for cardiovascular diseases. 31 A cross‐sectional study on adult dermatomyositis found that the prevalence of metabolic syndrome was higher than healthy controls (41.7% vs. 7.0%; P < .001), and prior hypertension may be closely related to the development of metabolic syndrome. 32 Diabetes mellitus, higher levels of TG, and LDL‐C are common in IIMs patients with hypertension, which are in accordance with the characteristics of hypertensive subjects with connective tissue diseases in previous study. 33 A multicenter study demonstrated that adult polymyositis and dermatomyositis had a higher prevalence of diabetes mellitus (13% vs. 0%; P < .007). 7 Diabetes mellitus significantly increases the risk of developing hypertension in patients with polymyositis and dermatomyositis compared to controls (OR = 2.94, 95%CI: 2.04–4.23). 5 , 34 Insulin resistance is an important pathological mechanism of patients with diabetes mellitus. The increase in body fluid volume is closely associated with hyperinsulinemia and hyperglycemia caused by insulin resistance, which plays a crucial role in the development of hypertension. 35 In the general population, each unit increases in fasting blood glucose; the risk of developing hypertension increases by 11.6 to 12.3%. 12 Our previous studies had reported altered lipid levels in untreated patients with dermatomyositis and polymyositis, and the results indicated that the frequency of elevated TG, elevated TC, elevated LDL‐C, and reduced HDL‐C was 70.7%, 9.6%, 4.8%, and 41.5% in dermatomyositis patients and 47%, 5%, 0%, and 50% in polymyositis patients, respectively. 9 , 36 However, the precise relationship between dyslipidemia and hypertension in patients with IIMs remains unknown. Limited evidence has demonstrated that hypertension and dyslipidemia often coexist and interact with each other, and their pathogenesis may be related to inflammation and insulin resistance. 37 , 38 Inflammation, mediated through cytokines and acute phase reactants, may be the driving force of dyslipidemia. And it also results in increased arterial stiffness, vascular endothelial dysfunction, and increased oxidative stress, which are closely relevant to hypertension. 29 In addition, insulin resistance and associated hyperinsulinemia in hypertensive population are associated with increased TG levels and decreased HDL‐C concentrations. 39 ANA are the hallmark autoantibodies in autoimmune connective tissue diseases, including IIMs. The studies have shown that ANA is probably secondary to the vascular damage, and positive ANA is an important predictor of cardiovascular events and mortality in patients with rheumatic diseases. 40 , 41 Patients with essential hypertension are more likely to have positive antibodies than individuals with normal blood pressure, especially ANA. 40 , 42 Patel et al reported that positive ANA was more common in patients with hypertension than age‐ and sex‐matched normotensive controls (11% vs 2%, P <.01). 43 However, ANA exerts effects on the development of hypertension with unknown mechanisms in IIMs patients. Quite a few studies pointed out that smoking had a higher risk of developing hypertension than non‐current smoking, but the results of present study were not consistent with these studies. 11 , 44 The reasons may be related to the following two aspects. On the one hand, patients with IIMs are more common in women, and women are less likely to smoke. On the other hand, estrogens are generally considered to have a protective effect on cardiovascular risk factors like hypertension, which may reduce the effect of smoking on hypertension. 37 The mean age of IIMs patients in the current study is 48 ± 14 years, indicating that most patients are premenopausal. Estrogens have immunomodulatory function, and it is often associated with the strong female gender bias related to autoimmune diseases like SLE. 45 Furthermore, women have lower arterial pressure than age‐matched men prior to menopause. 46 In this retrospective cohort study, 40 of 245 female (16.3%) patients with IIMs develop hypertension, and 14 of 117 male (12.0%) patients with IIMs develop hypertension. Although patients with IIMs occur mostly in females, gender has no significant difference among IIMs patients with and without hypertension (P = .442), which is consistent with the study by Souza et al 8 Notably, although the baseline of SBP has differences in IIMs patients, the present study finds no statistical significance among “systolic BP (per 10 mmHg)” in the multivariable regression model (P = .203), which is not in line with the results of the previous studies. 12 , 13 It may be related to the type of the current study and the relatively small sample size. This study is essentially a retrospective study that is hard to get causal relationship. Thus, we should further increase the sample size to explore the relationship between SBP and hypertension in prospective study. In addition, it is worth mentioning that little is known regarding the relationship between clinical manifestations and the development of hypertension in IIMs patients. Recent studies suggest that corticosteroids and nonsteroid immunomodulators (methotrexate, cyclophosphamide, azathioprine, etc) may reduce the risk of arterial events in IIMs, SLE and RA, possibly by lowering disease activity and inflammation. 47 The above findings are also helpful to explain this prediction model.
However, our current study also has some limitations that deserve a mention. Firstly, this study is a retrospective study, which may lead to the incompleteness of the data. Therefore, our findings will require confirmation in prospective studies. Secondly, the sample size can be regarded as relatively small. Nonetheless, as far as we know, this is the largest prediction model concentrated on hypertension in connective tissue diseases. In addition, there is a lack of external validation of this model. Further studies are needed to test the current results and quantify the underlying mechanisms of hypertension in IIMs patients.
In conclusion, patients with IIMs have a high incidence of hypertension; we developed a relatively accurate and reliable nomogram in predicting hypertension in IIMs patients, which is beneficial to both clinicians and patients.
CONFLICT OF INTEREST
None.
Qin L, Zhang Y, Yang X, Wang H. Development of the prediction model for hypertension in patients with idiopathic inflammatory myopathies. J Clin Hypertens. 2021;23:1556–1566. 10.1111/jch.14267
Funding information
This work was supported by the National Natural Science Foundation of China [grant number: 81300243]
REFERENCES
- 1. Furst DE, Amato AA, Iorga SR, Gajria K, Fernandes AW. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve. 2012;45:676‐683. [DOI] [PubMed] [Google Scholar]
- 2. Taborda AL, Azevedo P, Isenberg DA. Retrospective analysis of the outcome of patients with idiopathic inflammatory myopathy: a long‐term follow‐up study. Clin Exp Rheumatol. 2014;32:188‐193. [PubMed] [Google Scholar]
- 3. Jayakumar D, Zhang R, Wasserman A, Ash J. Cardiac manifestations in idiopathic inflammatory myopathies: an overview. Cardiol Rev. 2019;27:131‐137. [DOI] [PubMed] [Google Scholar]
- 4. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429‐432. [DOI] [PubMed] [Google Scholar]
- 5. Limaye VS, Lester S, Blumbergs P, Roberts‐Thomson PJ. Idiopathic inflammatory myositis is associated with a high incidence of hypertension and diabetes mellitus. Int J Rheum Dis. 2010;13:132‐137. [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Cai Y, Cai L, Hu Y, Chen X, Deng J. Altered lipid levels in untreated patients with early polymyositis. PLoS ONE. 2014;9:e89827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diederichsen LP, Diederichsen AC, Simonsen JA, et al. Traditional cardiovascular risk factors and coronary artery calcification in adults with polymyositis and dermatomyositis: a Danish multicenter study. Arthritis Care Res (Hoboken). 2015;67:848‐854. [DOI] [PubMed] [Google Scholar]
- 8. Souza FH, Levy‐Neto M, Shinjo SK. Prevalence of clinical and laboratory manifestations and comorbidities in polymyositis according to gender. Rev Bras Reumatol. 2011;51:428‐483. [PubMed] [Google Scholar]
- 9. Fu ML, Herlitz H, Schulze W, et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J hypertens. 2000;18:945‐953. [DOI] [PubMed] [Google Scholar]
- 10. Kim HC, Ihm SH, Kim GH, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part I‐epidemiology of hypertension. Clin Hypertens. 2019;25:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near‐term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102‐110. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Wang C, Liu Y, et al. Incident hypertension and its prediction model in a prospective northern urban Han Chinese cohort study. J Hum Hypertens. 2016;30:794‐800. [DOI] [PubMed] [Google Scholar]
- 13. Kanegae H, Oikawa T, Suzuki K, Okawara Y, Kario K. Developing and validating a new precise risk‐prediction model for new‐onset hypertension: the Jichi Genki hypertension prediction model (JG model). J Clin Hypertens (Greenwich). 2018;20:880‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu F, Zhu J, Sun N, et al. Development and validation of prediction models for hypertension risks in rural Chinese populations. J Glob Health. 2019;9:020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344‐347. [DOI] [PubMed] [Google Scholar]
- 16. Verdecchia P, Reboldi G, Angeli F. The 2020 International Society of Hypertension global hypertension practice guidelines ‐ key messages and clinical considerations. Eur J Intern Med. 2020;82:1‐6. [DOI] [PubMed] [Google Scholar]
- 17. Volzke H, Fung G, Ittermann T, et al. A new, accurate predictive model for incident hypertension. J Hypertens. 2013;31:2142‐2150. [DOI] [PubMed] [Google Scholar]
- 18. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569‐2581. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Wu H, Liu Q, et al. Association of CHRNA5‐A3‐B4 variation with esophageal squamous cell carcinoma risk and smoking behaviors in a Chinese population. PLoS ONE. 2013;8:e67664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar R, Nandhini LP, Kamalanathan S, Sahoo J, Vivekanadan M. Evidence for current diagnostic criteria of diabetes mellitus. World J Diabetes. 2016;7:396‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512‐5528. [DOI] [PubMed] [Google Scholar]
- 23. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543‐2546. [PubMed] [Google Scholar]
- 24. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X, Tong DK, Ji F, et al. Predictive nomogram for postoperative delirium in elderly patients with a hip fracture. Injury. 2019;50:392‐397. [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Wang GC, Ma L, Zu N. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol. 2012;35:686‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franklin SS, Wt G, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308‐315. [DOI] [PubMed] [Google Scholar]
- 28. Vincze M, Der H, Kerekes G, et al. Decreased flow‐mediated dilatation with increased arterial stiffness and thickness as early signs of atherosclerosis in polymyositis and dermatomyositis patients. Clin Rheumatol. 2014;33:1635‐1641. [DOI] [PubMed] [Google Scholar]
- 29. Manavathongchai S, Bian A, Rho YH, et al. Inflammation and hypertension in rheumatoid arthritis. J Rheumatol. 2013;40:1806‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabio JM, Vargas‐Hitos JA, Navarrete‐Navarrete N, et al. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026‐1032. [DOI] [PubMed] [Google Scholar]
- 31. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351‐375. [DOI] [PubMed] [Google Scholar]
- 32. de Moraes MT, de Souza FH, de Barros TB, Shinjo SK. Analysis of metabolic syndrome in adult dermatomyositis with a focus on cardiovascular disease. Arthritis Care Res (Hoboken). 2013;65:793‐799. [DOI] [PubMed] [Google Scholar]
- 33. Grygiel‐Gorniak B, Ziolkowska‐Suchanek I, Kaczmarek E, Puszczewicz M, Rozwadowska N. Genetic background of hypertension in connective tissue diseases. J Immunol Res. 2020;2020:7509608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolstad KD, Fiorentino D, Li S, Chakravarty EF, Chung L. Pregnancy outcomes in adult patients with dermatomyositis and polymyositis. Semin Arthritis Rheum. 2018;47:865‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41:389‐393. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Tang J, Chen X, Li F, Luo J. Lipid profiles in untreated patients with dermatomyositis. J Eur Acad Dermatol Venereol. 2013;27:175‐179. [DOI] [PubMed] [Google Scholar]
- 37. Wolf VL, Ryan MJ. Autoimmune disease‐associated hypertension. Curr Hypertens Rep. 2019;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silva MG, Borba EF, Mello SB, Shinjo SK. Serum adipocytokine profile and metabolic syndrome in young adult female dermatomyositis patients. Clinics (Sao Paulo). 2016;71:709‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sowers JR. Insulin resistance, hyperinsulinemia, dyslipidemia, hypertension, and accelerated atherosclerosis. J Clin Pharmacol. 1992;32:529‐535. [DOI] [PubMed] [Google Scholar]
- 40. Kristensen B, Andersen PL, Wiik A. Autoantibodies and vascular events in essential hypertension: a five‐year longitudinal study. J Hypertens. 1984;2:19‐24. [DOI] [PubMed] [Google Scholar]
- 41. Liang KP, Kremers HM, Crowson CS, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36:2462‐2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Editorial: Immunogenetics and essential hypertension. Lancet. 1978;2:409‐410. [PubMed] [Google Scholar]
- 43. Patel R, Johnson J, Ansari A. Immunogenetic studies in essential hypertension among black patients. I. Correlative studies of serum autoantibody formation. Int Arch Allergy Appl Immunol. 1982;67:145‐148. [DOI] [PubMed] [Google Scholar]
- 44. Kivimaki M, Batty GD, Singh‐Manoux A, et al. Validating the Framingham Hypertension Risk Score: results from the Whitehall II study. Hypertension. 2009;54:496‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venegas‐Pont M, Ryan MJ. Can estrogens promote hypertension during systemic lupus erythematosus? Steroids. 2010;75:766‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol. 2013;40:542‐550. [DOI] [PubMed] [Google Scholar]
- 47. Tisseverasinghe A, Bernatsky S, Pineau CA. Arterial events in persons with dermatomyositis and polymyositis. J Rheumatol. 2009;36(9):1943‐1946. [DOI] [PubMed] [Google Scholar]


