Abstract
This post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) examined the performance of chlorthalidone (C) versus amlodipine (A) monotherapies. ANOVA was used to analyze the differences in systolic blood pressure (SBP) response between C and A. Logistic regression was used to examine monotherapy failure (adding a second antihypertensive agent or switching to a different antihypertensive agent) rates. Four hundred ninety‐one participants were treated with C monotherapy (n = 210, mean dose = 22 mg/day) or A monotherapy (n = 281, mean dose = 7 mg/day). There was a significant difference in mean SBP reduction between the C and A monotherapies at the third visit (higher reduction with A, adjusted p = .018). Unadjusted analysis showed a higher failure with C in the standard treatment group. Although the average SBP at failure was higher and above the 140 mm Hg cutoff that indicated monotherapy failure with A (142.60) compared with C (138.40), more participants on C failed despite having SBP below the 140 cutoff. This was probably due to decisions made by the investigative teams to change the antihypertensive regimen, because, in their opinion, the clinical picture required it. After adjusting for baseline characteristics, C had higher failure than A only in the standard treatment group (1.64 odds ratio [OR], 95% CI 1.06–2.56, p = .028). A sub‐analysis including participants who had never used antihypertensive treatment before randomization had similar results (2.57 OR, 95% CI 1.34–5.02, p = .004). Overall, in SPRINT chlorthalidone was associated with higher monotherapy failure than amlodipine in the standard treatment group because of decisions of the investigative teams.
Keywords: amlodipine, chlorthalidone, hypertension, monotherapy, SPRINT
1. INTRODUCTION
Hypertension is a highly prevalent condition that is associated with an increased risk for cardiovascular (CV), cerebrovascular, renal complications, and death and is responsible for a significant proportion of health care expenditures in the United States.1, 2, 3, 4 Normalizing blood pressure at any stage can reduce these risks.5, 6 For patients with stage 1 hypertension and an estimated 10‐year atherosclerotic cardiovascular disease risk of 10% or higher, the 2017 ACC/AHA guidelines recommend monotherapy with angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, “thiazide or thiazide‐type” diuretics, or calcium channel blockers. 7 Out of the available options, chlorthalidone and amlodipine are two once daily monotherapies that are supported by several studies.8, 9, 10, 11, 12 Chlorthalidone, a thiazide‐type diuretic that acts at the distal convoluted tubule in the nephrons to block the sodium chloride symporter to promote diuresis, has a bioavailability of about 60% and elimination half‐life of 45–60 h.13, 14 Amlodipine, a third‐generation dihydropyridine calcium channel blocker that acts at the voltage‐dependent L‐type calcium channels to inhibit calcium ion influx into vascular smooth muscle and promote vasodilation, has a 60%–80% bioavailability and elimination half‐life of 40‐60 h. 15
However, there are few studies that compared the effectiveness of chlorthalidone and amlodipine monotherapies.8, 16, 17, 18 The Systolic Blood Pressure Intervention Trial (SPRINT) presents an opportunity to compare these two agents. In the trial, “chlorthalidone was encouraged as the primary thiazide‐type diuretic, and amlodipine as the preferred calcium channel blocker.” 19 Furthermore, prior studies are limited by their low numbers of participants and analyses of data that included participants who were no longer on monotherapy. We compared the efficacy and safety of monotherapy with amlodipine and chlorthalidone in the SPRINT trial. 19
2. METHODS
2.1. Data source and participant selection
We used SPRINT data from the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the National Heart, Lung, and Blood Institute. 20 Although this manuscript is based on SPRINT data, it does not represent the SPRINT trial. SPRINT was a NIH sponsored study that examined the effect of intensive treatment (SBP target of <120 mm Hg) versus standard treatment (SBP target of <140 mm Hg) on CV events and mortality. 19 SPRINT participants were at least 50 years old with a SBP of 130–180 mm Hg and had an increased risk of CV events. Participants with diabetes mellitus or prior stroke were excluded. Participants had follow‐up visits once every month for the first 3 months, and every 3 months thereafter. More details on SPRINT inclusion and exclusion criteria along with study design and treatment protocols can be found elsewhere.19, 21, 22 In this study, SPRINT participants were on either amlodipine or chlorthalidone monotherapy at randomization and participants who were on two or more antihypertensive medications or on monotherapy with a medication other than amlodipine or chlorthalidone at randomization were excluded. The Rutgers Robert Wood Johnson Medical School Institutional Review Board has approved this study.
2.2. Outcomes and variables
The primary efficacy outcomes were SBP response and monotherapy failure rate. Monotherapy failure was defined as the addition of a second medication or switching to a different antihypertensive medication. The primary safety outcome was the occurrence of the following SPRINT prespecified adverse events: hypotension, syncope, injurious falls, electrolyte abnormalities, bradycardia, and acute kidney injury/failure. 19
Information on the following variables was included: age, sex, race/ethnicity, baseline SBP, comorbidities, body mass index (BMI), smoking, alcohol use, family history of CV disease (CVD), and history of antihypertensive medication use prior to randomization.
2.3. Statistical analysis
Comparisons of the baseline characteristics of the amlodipine and chlorthalidone groups were made using Pearson's chi‐square test with Yates' continuity correction. For low cell counts, Fisher's exact test was used. Differences in continuous variables were assessed using T tests. Kaplan‐Meier curves for monotherapy failure rates within 105 days from randomization were computed. Logistic regression was used to determine the probability of a participant being on chlorthalidone based on baseline SBP, SPRINT treatment group assignment, BMI, history of myocardial infarction, race, sex, age, Hispanic ethnicity, atrial fibrillation, kidney infection, cancer, history of smoking, and history of antihypertensive medication use. The probability was used as a propensity score. Differences in monotherapy failure rates at Visit 1, 2, and 3 were analyzed for the standard and intensive treatment groups using logistic regression adjusting for the factors in the propensity score, and the model was weighted using inverse propensity score weighting. A sub‐analysis of antihypertensive treatment‐naive participants in the standard and intensive treatment groups was performed with similar analytical methods (excluding history of antihypertensive medication use from the propensity score). Differences in SBP between amlodipine and chlorthalidone were analyzed using standard ANOVA with adjustments for the factors mentioned above and weighted using inverse propensity score weighting.
3. RESULTS
3.1. Participant characteristics
Table 1 displays other baseline demographic and clinical characteristics. Table 2 summarizes the comorbidities in each group. There were no statistically significant differences in baseline comorbidities. There were 491 participants (5.25% of the SPRINT participants) in this study who were on either amlodipine monotherapy (N = 281) or chlorthalidone monotherapy (N = 210) at randomization in the SPRINT trial. In the amlodipine monotherapy group, the mean age was 67.6 years (standard deviation [SD] 9.5 years) and 70% were male. In the chlorthalidone monotherapy group, the mean age (SD) was lower (64.2 [8.9] years, p < .001) and 71% were male. The chlorthalidone monotherapy group had a higher mean baseline SBP than the amlodipine monotherapy group (144 mm Hg vs. 138 mm Hg, p < .001).
TABLE 1.
Differences in demographics and characteristics in amlodipine and chlorthalidone
| Variable | Amlodipine (N = 281) | Chlorthalidone (N = 210) | p‐value |
|---|---|---|---|
| Mean age ± SD in years | 67.6 ± 9.5 | 64.2 ± 8.9 | <.001 |
| Male | 196 (70%) | 150 (71%) | .762 |
| White | 166 (59%) | 131 (62%) | .517 |
| Black | 105 (37%) | 70 (33%) | .408 |
| Other | 10 (4%) | 9 (4%) | .860 |
| Non‐hispanic | 261 (93%) | 204 (97%) | .06 |
| Mean body mass index kg/m2 | 28.1 | 29.6 | .002 |
| Use of alcohol | 14 (5%) | 9 (4%) | .884 |
| Family history of CVD | 161 (57%) | 117 (56%) | .44 |
| History of smoking | 150 (53%) | 129 (61%) | .178 |
| Non‐hypertensive medication | 34 (12%) | 33 (16%) | .319 |
| Mean SBP mm Hg at randomization | 138.2 | 144.1 | <.001 |
| Intensive treatment group | 105 (37%) | 126 (60%) | <.001 |
| History of antihypertensive use a | 211 (75%) | 34 (16%) | <.001 |
Abbreviations: CVD, cardiovascular disease; SBP, systolic blood pressure; SD, standard deviation.
Any hypertensive medication before randomization.
TABLE 2.
Differences in comorbidities for amlodipine and chlorthalidone
| Variables | Amlodipine (N = 281) | Chlorthalidone (N = 210) | p‐value |
|---|---|---|---|
| Atrial fibrillation | 9 (3.21%) | 4 (1.9%) | .543 |
| Angina | 11 (3.91%) | 3 (1.43%) | .173 |
| Myocardial infarction | 10 (3.56%) | 4 (1.9%) | .415 |
| Ulcer | 18 (6.41%) | 14 (6.67%) | 1 |
| Crohns | 6 (2.14%) | 4 (1.9%) | 1 |
| Diverticulitis | 20 (7.12%) | 9 (4.29%) | .261 |
| Gallbladder | 16 (5.69%) | 10 (4.76%) | .801 |
| Kidney infection | 33 (11.74%) | 21 (10%) | .642 |
| Benign prostatic hyperplasia | 46 (16.37%) | 31 (14.76%) | .719 |
| Osteoarthritis | 69 (24.64%) | 42 (20%) | .269 |
| Rheumatoid arthritis | 24 (8.54%) | 13 (6.19%) | .422 |
| Gout | 28 (9.96%) | 11 (5.24%) | .081 |
| Peripheral vascular disease | 9 (3.2%) | 4 (1.9%) | .547 |
| Transient ischemic attack | 6 (2.14%) | 1 (0.48%) | .25 |
| Anemia | 40 (14.23%) | 22 (10.48%) | .27 |
| Low back pain | 119 (42.35%) | 75 (35.71%) | .163 |
| Cataracts | 83 (29.54%) | 50 (23.81%) | .19 |
| Depression | 59 (21%) | 44 (21.05%) | 1 |
| Anxiety | 22 (7.83%) | 19 (9.05%) | .751 |
A higher proportion of the chlorthalidone participants were in the intensive group compared with the amlodipine participants (60% vs. 37%, p < .001). The chlorthalidone monotherapy group had a higher mean baseline BMI than the amlodipine monotherapy group (30 vs. 28, p < .001). Also, the chlorthalidone monotherapy group had a lower proportion of participants with a history of antihypertensive medication use before randomization (16% vs. 75%, p < .001).
3.2. Blood pressure response
At randomization, amlodipine monotherapy was prescribed at a mean dose of 7 mg/day (median 5 mg with an interquartile range of 5–10 mg) and chlorthalidone monotherapy was prescribed at a mean dose of 22 mg/day (median 25 mg with an interquartile range of 12.5–25 mg). Both monotherapy regimens lead to reductions in mean SBP in all groups, which is shown in Figures 1 and 2. Overall, differences in mean SBP reduction between the amlodipine and chlorthalidone monotherapies were significant at the third visit (adjusted p = .018). In the antihypertensive treatment‐naive participants, however, this difference was not significant (adjusted p = .256).
FIGURE 1.
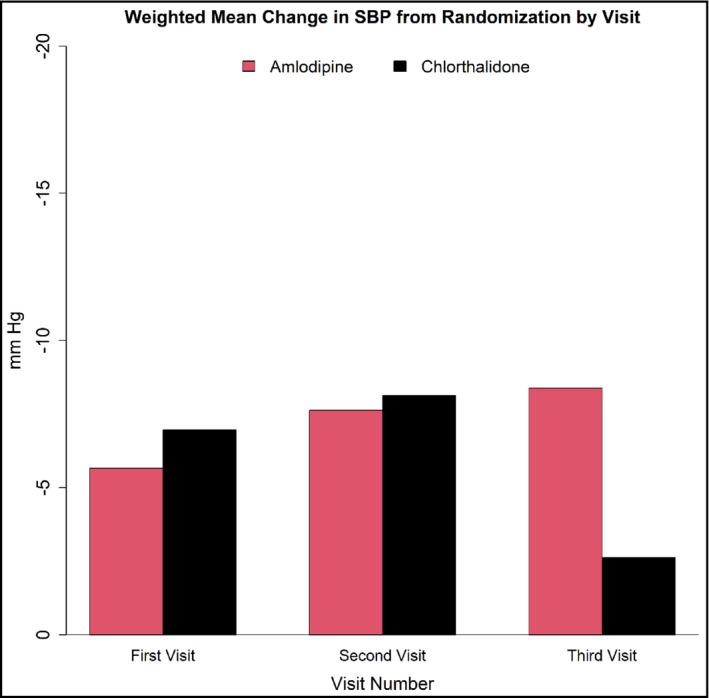
Weighted Mean Change in Systolic Blood Pressure (SBP) from randomization at each of the first three monthly visits in participants while on monotherapy. By the third visit, difference in weighted mean change in SBP between chlorthalidone and amlodipine monotherapies was significant. The following are the differences in weight mean change in SBP between chlorthalidone and amlodipine for visit 1, visit 2, and visit 3:1.30 mm Hg (p = .2130), 0.50 mm Hg (p = .7666), and −5.76 mm Hg (p = .018)
FIGURE 2.
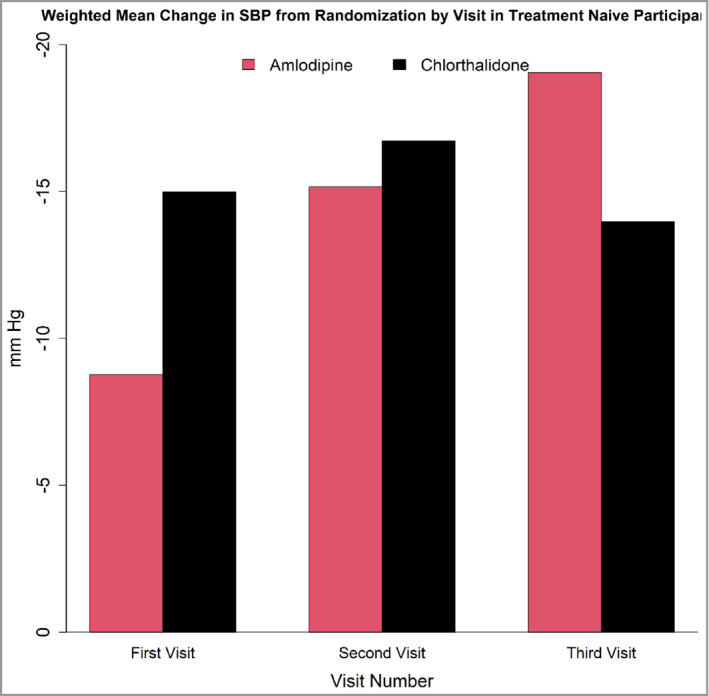
Weighted Mean Change in Systolic Blood Pressure (SBP) from randomization at each of the first three monthly visits in antihypertensive treatment‐naive participants while on monotherapy. By the third visit, difference in mean change in SBP between chlorthalidone and amlodipine monotherapies was not significant. The following are the differences in weight mean change in SBP between chlorthalidone and amlodipine for visit 1, visit 2, and visit 3:6.23 mm Hg (p =< .001), 1.56 mm Hg (p = .586), and −5.07 mm Hg (p = .256)
3.3. Monotherapy failure rates
Table 3 shows the number of participants who were still on monotherapy at the end of each visit. At the end of the third visit, the following percent of participants were still on monotherapy: chlorthalidone standard (56.0%) vs. amlodipine standard (79.5%), and chlorthalidone intensive (26.2%) vs. amlodipine intensive (37.1%). In the antihypertensive treatment‐naive participants, the following percent of participants at the end of the third visit were still on monotherapy: chlorthalidone standard (48.5%) vs. amlodipine standard (73.3%), and chlorthalidone intensive (24.1%) vs. amlodipine intensive (25.0%). In the standard group, majority of the participants were on target SBP for both the amlodipine (A) and chlorthalidone (C) groups. In the standard group at visit 1, 73% (125 of 171) of the A group and 75% (61 of 81) of the C group were reaching their SBP target (<140 mm Hg). During Visit 2, 78% (124 of 158) of the A group and 70% (42 of 60) of the C group were meeting their SBP target. For visit 3, 74% (109 out of 148) of the A group and 66% (35 of 53) of the C group were meeting their SBP target. In the intensive group, majority of the participants were not on target SBP. In the intensive group at visit 1, 20% (21 of 104) of the A group and 30% (37 of 123) of the C group were meeting their SBP target (<120 mmHg). During Visit 2, 40% (25 of 63) of the A group and 39% (28 of 71) of the C group were meeting their SBP target. For visit 3, 50% (21 out of 42) of the A group and 45% (20 of 64) of the C group were meeting their SBP target.
TABLE 3.
Participants still on monotherapy by end of specified visit
| All participants | ||||
|---|---|---|---|---|
| Group | At randomization N = 491 | At visit 1 N = 366 | At visit 2 N = 299 | At visit 3 N = 259 |
| Chlorthalidone standard | 84 | 62 | 54 | 47 |
| Chlorthalidone intensive | 126 | 75 | 47 | 33 |
| Amlodipine standard | 176 | 162 | 152 | 140 |
| Amlodipine intensive | 105 | 67 | 46 | 39 |
| Antihypertensive treatment‐naive participants | ||||
|---|---|---|---|---|
| Group | At randomization N = 246 | At visit 1 N = 162 | At visit 2 N = 117 | At visit 3 N = 91 |
| Chlorthalidone standard | 68 | 47 | 39 | 33 |
| Chlorthalidone intensive | 108 | 62 | 37 | 26 |
| Amlodipine standard | 30 | 28 | 26 | 22 |
| Amlodipine intensive | 40 | 25 | 15 | 10 |
Chlorthalidone monotherapy had higher failure rates than amlodipine monotherapy up to the end of the third month after randomization (at the end of 3 months after randomization, Figure 3). The adjusted odds ratio (OR) comparing monotherapy failure rates of chlorthalidone to amlodipine in the standard treatment group at visit 3 was 1.64 (95% CI [1.06, 2.56], p = .028). The adjusted OR comparing monotherapy failure rates of chlorthalidone to amlodipine in the intensive treatment group at visit 3 was 1.44 (95% CI [0.96, 2.17], p = .076), indicating that there were no significant differences in failure rates between the two medications. The adjusted ORs for failure of chlorthalidone monotherapy versus amlodipine monotherapy at the end of the first visit and second visit were 2.73 (95% CI [1.55, 4.94], p < .001) and 1.56 (95% CI [0.96, 2.53], p = .072), respectively, in the standard group. No differences were seen in the intensive group at visit 1 (p = .787) and visit 2 (p = .765).
FIGURE 3.
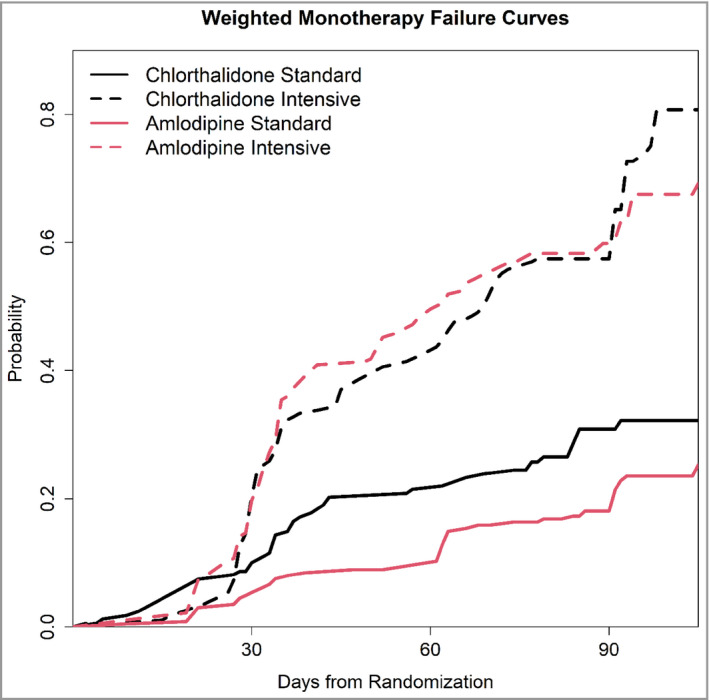
Probability of Monotherapy Failure by Monotherapy and SPRINT Treatment Group Assignment. In the standard treatment groups, participants in the chlorthalidone group were more likely to fail monotherapy than participants in the amlodipine treatment group from day 45. This difference persisted throughout the follow‐up. In the intensive treatment groups, there was no significant difference in monotherapy failure between chlorthalidone and amlodipine throughout the duration of follow‐up
Figure 4 displays the distribution of the SBPs at each of the first three monthly visits of all participants who failed monotherapy. The following are the percent of monotherapy failing participants who had SBP below 140 mm Hg for chlorthalidone standard vs. amlodipine standard at visits 1, 2, and 3:38.1% vs. 35.7%, 62.5% vs. 60.0%, and 42.9% vs. 41.7%, respectively. The following are the percent of monotherapy failing participants who had SBP below 120 mm Hg for chlorthalidone intensive vs. amlodipine intensive at visits 1, 2, and 3:7.8% vs. 0.0%, 10.7% vs. 9.5%, and 14.29% vs. 0.0%, respectively. This indicates that in the standard groups for both chlorthalidone and amlodipine monotherapies there were large proportions of monotherapy failing participants who were reaching the target SBP (<140 mm Hg). Furthermore, although the average SBP at failure was higher and above the 140 mm Hg cutoff that indicated monotherapy failure with amlodipine (142.60) compared with chlorthalidone (138.40), more participants on chlorthalidone failed in spite of having SBP below the 140 mm Hg cutoff.
FIGURE 4.
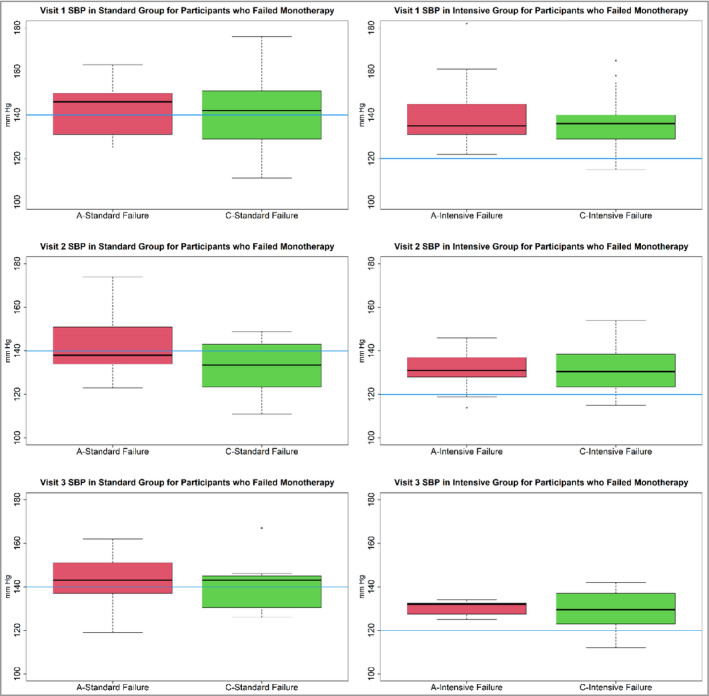
Distribution of systolic blood pressure (SBP) at each of the first three monthly visits of all participants on amlodipine (A) or chlorthalidone (C) monotherapies who failed monotherapy. The first‐row panels are for Visit 1. The second‐row panels are for Visit 2. The third‐row panels are for visit 3. The first column is for the standard group participants on A or C monotherapies. The second column is for the intensive group participants on A or C monotherapies. For the standard group participants on A or C monotherapies, at each visit, the interquartile range (IQR) crosses below the target of 140 mm Hg (blue bar). This indicates that many participants who failed monotherapy had SBPs that were at target. However, for the intensive group, this was not the case as the IQRs are above the 120 mm Hg target (blue bar)
Figure 5 shows the monotherapy failure rate curves for both standard and intensive groups in antihypertensive treatment‐naive participants. The sub‐analysis found a higher monotherapy failure rate in the chlorthalidone group (2.57 OR, 95% CI [1.34, 5.02], p = .004) at the end of 3 months after randomization, in the standard group. In contrast, in the intensive treatment group there was no statistically significant difference (1.10 OR, 95% CI [0.64, 1.88], p = .732) in the failure rates.
FIGURE 5.
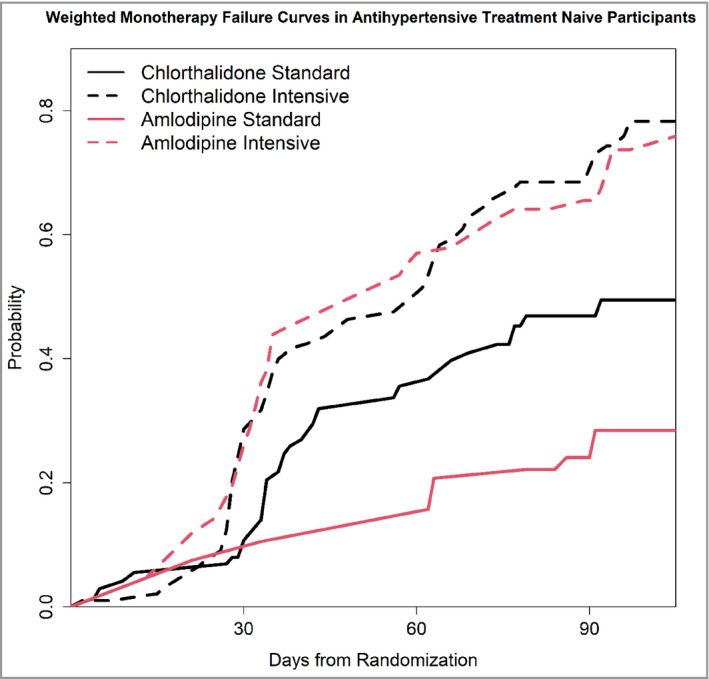
Probability of Monotherapy Failure by Monotherapy and SPRINT Treatment Group Assignment in antihypertensive treatment‐naive participants. In the treatment‐naive participants, the effects were similar to those observed in the whole group of participants. However, in the standard treatment groups, the difference in monotherapy failure rate between chlorthalidone (higher failure rate) compared with amlodipine was magnified. This difference persisted throughout the follow‐up. In the intensive treatment groups, there was no significant difference in monotherapy failure between chlorthalidone and amlodipine throughout the duration of follow‐up
3.4. Adverse events
There was a total of 4 prespecified adverse events. In the amlodipine monotherapy group, there were one acute kidney injury and one injurious fall. In the chlorthalidone monotherapy group, there were two cases of hypotension.
4. DISCUSSION
This study showed that in SPRINT chlorthalidone was associated with a higher monotherapy failure rate than amlodipine in the standard treatment group. Although the average SBP at failure was higher and above the 140 mm Hg cutoff that indicated monotherapy failure with amlodipine (142.60) compared to chlorthalidone (138.40), more participants on chlorthalidone failed in spite of having SBP below the 140 mm Hg cutoff. The investigative teams decided to add a second antihypertensive agent despite the participants achieving the target SBP goal of the trial. The investigative teams probably made these decisions because, in their opinion, the clinical picture required it.
It is less likely that the distribution of baseline characteristics of chlorthalidone and amlodipine monotherapy participants and SPRINT randomization and protocol were major contributors to the differences in monotherapy failure rates. The differences in monotherapy failure rates persisted in the standard treatment groups in the adjusted analysis. In the Treatment of Mild Hypertension study, a lower percentage of patients on chlorthalidone monotherapy than amlodipine monotherapy was still on their initial regimen at 48 months (67.5% vs. 82.5%). 17
Although the differences in mean SBP reduction were statistically significant at the third visit, the decisions of the investigative teams were more important than the observed differences in SBP reduction in explaining the differences in monotherapy failure rates. The SBP responses of the chlorthalidone monotherapy group and amlodipine monotherapy group are consistent with the literature.8, 9, 10, 16, 17, 23 Furthermore, in the sub‐analysis of antihypertensive treatment‐naive participants in which chlorthalidone still had a higher failure rate, the differences in SBP reduction were not statistically significant. This is consistent with the study by Grimm Jr et al, which compared chlorthalidone versus amlodipine monotherapies versus placebo in patients with isolated systolic hypertension, and a study by Moes et al, which examined chlorthalidone versus amlodipine monotherapies in a randomized crossover trial.8, 16 In both studies, the differences in BP responses between chlorthalidone and amlodipine were not statistically significant. The differences in results could be due to differences in study population, study design, starting dose, and dose titration.
The differences in monotherapy failure rates between chlorthalidone and amlodipine monotherapies were not statistically significant in the intensive treatment group probably, because the range of SBP lowering did not allow large SBP differences to occur. In the SPRINT trial, despite an average of 2.8 BP medications, the intensive treatment group had a mean SBP of 121.5 mm Hg throughout the 3.26 years of follow‐up. 19 This highlights the difficulty of achieving a SBP lower than 120 mm Hg.
Adverse events of chlorthalidone and amlodipine are more common at higher doses than those used in the SPRINT trial and those recommended by guidelines.7, 9, 10, 24 As a result, in this study, each monotherapy group had only two adverse events.
This study has limitations that are inherent to post‐hoc analyses. SPRINT participants were randomized to standard or intensive SBP targets, and the investigative teams were able to choose any of the protocol approved medications. Investigative teams decided how to titrate the dose to achieve the SBP target goal. Data for dose titration are not available. A small percentage (5.25%) of the SPRINT participants met criteria for this study. Although this manuscript is based on SPRINT data, it does not represent the SPRINT trial. However, this study has numerous strengths. This study had a significantly higher number of participants on chlorthalidone or amlodipine monotherapies compared with the other studies mentioned above. These other studies required participants to have a minimum SBP of 140 mm Hg, which is Stage 2 hypertension where treatment with combination therapy is recommended. Comparing monotherapy failure rates in that setting is suboptimal given that these patients are more effectively managed with combination therapy. However, SPRINT included participants with a SBP of 130‐180 mm Hg, which allows for the inclusion of participants with Stage 1 hypertension for whom monotherapy is recommended. The other studies mentioned above included participants who were not on monotherapy. This SPRINT study focused on the comparison of the efficacy and safety of chlorthalidone and amlodipine as monotherapies. Most important, to account for the impact of the SPRINT randomization and protocol and variations in baseline characteristics, we compared the standard and intensive groups and adjusted for a variety of baseline characteristics.
5. CONCLUSION
In this study comparing chlorthalidone and amlodipine monotherapies, SPRINT participants on chlorthalidone were more likely to fail monotherapy than those on amlodipine because of decisions of the investigative teams. Randomized trials need to be conducted to better compare the effectiveness of monotherapy with chlorthalidone, amlodipine, and other antihypertensive agents and to determine the generalizability of the reported findings. Research in optimizing monotherapy drug selection will improve population level control of hypertension and the associated mortality, morbidity, and health care expenditures.
ACKNOWLEDGEMENTS
This study was supported in part by the New Jersey Health Foundation.
CONFLICT OF INTEREST
Drs. John B. Kostis, William J. Kostis, and Abel E. Moreyra were investigators in the Systolic Blood Pressure Intervention Trial. The other investigators declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the Department of Veterans Affairs, or the US Government.
AUTHOR CONTRIBUTIONS
DV‐ contributed to conception and design of the study, interpretation of the data, and draft of manuscript. SZ‐ contributed to acquisition of data, statistical analysis and interpretation of data, and revision of manuscript. JK‐ contributed to conception and design of the study, interpretation of data, and revisions of manuscript. JD‐ contributed to revisions and final approval of the manuscript. NC‐ contributed to acquisition of data, interpretation of data, revisions of manuscript, and final approval. AM‐ contributed to the interpretation of data, revisions for manuscript, and final approval. WK‐ contributed to conception and design of the study, analytic methods, interpretation of the data, revisions for manuscript, and final approval.
Vakil D, Zinonos S, Kostis JB, et al. Monotherapy treatment with chlorthalidone or amlodipine in the systolic blood pressure intervention trial (SPRINT). J Clin Hypertens. 2021;23:1335–1343. 10.1111/jch.14296
Funding information
Supported by contracts (HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C) and an interagency agreement (A‐HL‐13‐002‐001) from the NIH, including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke.
Correction statement: This article was modified on 14 July, after first online publication: Acknowledgment has been added.
REFERENCES
- 1. Guo FJ, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599‐606. [DOI] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kannel WB. Role of blood pressure in cardiovascular morbidity and mortality. Prog Cardiovasc Dis. 1974;17(1):5‐24. [DOI] [PubMed] [Google Scholar]
- 4. Kirkland EB, Heincelman M, Bishu KG, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc. 2018;7(11):2003‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Probstfield JL. Prevention of Stroke by Antihypertensive Drug‐Treatment in Older Persons with Isolated Systolic Hypertension ‐ Final Results of the Systolic Hypertension in the Elderly Program (Shep). Jama‐J Am Med Assoc. 1991;265(24):3255‐3264. [PubMed] [Google Scholar]
- 6. Lee CJ, Ryu J, Kim HC, et al. Clinical benefit of treatment of stage‐1, low‐risk hypertension: Korean National Health Insurance Database Analysis. Hypertension. 2018;72(6):1285‐1293. [DOI] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (vol 71, pg e127, 2018). J Am Coll Cardiol. 2018;71(19):2275‐2279. [DOI] [PubMed] [Google Scholar]
- 8. Grimm RH, Black H, Rowen R, et al. Amlodipine versus Chlorthalidone versus placebo in the treatment of stage I isolated systolic hypertension. Am J Hypertens. 2002;15(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 9. Mehta JL, Lopez LM, Vlachakis ND, et al. Double‐blind evaluation of the dose‐response relationship of amlodipine in essential‐hypertension. Am Heart J. 1993;125(6):1704‐1709. [DOI] [PubMed] [Google Scholar]
- 10. Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side‐effects of a 15‐Mg Chlorthalidone formulation in the treatment of mild hypertension. Jama‐J Am Med Assoc. 1987;258(4):484‐488. [DOI] [PubMed] [Google Scholar]
- 11. Pareek AK, Messerli FH, Chandurkar NB, et al. Efficacy of low‐dose chlorthalidone and hydrochlorothiazide as assessed by 24‐h ambulatory blood pressure monitoring. J Am Coll Cardiol. 2016;67(4):379‐389. [DOI] [PubMed] [Google Scholar]
- 12. Huraib S, Askar A, Abuaisha H, Alwakeel J, Mitwalli A, Almajed S. Efficacy of once‐daily amlodipine in the control of 24‐hour blood‐pressure using ambulatory blood‐pressure monitoring. Curr Ther Res Clin E. 1995;56(11):1125‐1131. [Google Scholar]
- 13. Fleuren HL, Thien TA, Verwey‐van Wissen CP, van Rossum JM. Absolute bioavailability of chlorthalidone in man: a cross‐over study after intravenous and oral administration. Eur J Clin Pharmacol. 1979;15(1):35‐50. [DOI] [PubMed] [Google Scholar]
- 14. Riess W, Dubach UC, Burckhardt D, Theobald W, Vuillard P, Zimmerli M. Pharmacokinetic studies with chlorthalidone (Hygroton) in man. Eur J Clin Pharmacol. 1977;12(5):375‐382. [DOI] [PubMed] [Google Scholar]
- 15. Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 1992;80:31‐36. [DOI] [PubMed] [Google Scholar]
- 16. Moes AD, Hesselink DA, van den Meiracker AH, Zietse R, Hoorn EJ. Chlorthalidone versus amlodipine for hypertension in kidney transplant recipients treated with tacrolimus: a randomized crossover trial. Am J Kidney Dis. 2017;69(6):796‐804. [DOI] [PubMed] [Google Scholar]
- 17. Neaton JD, Grimm RH, Prineas RJ, et al. Treatment of mild hypertension study ‐ Final results. Jama‐J Am Med Assoc. 1993;270(6):713‐724. [PubMed] [Google Scholar]
- 18. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981‐2997. [DOI] [PubMed] [Google Scholar]
- 19. SPRINT Research Group , Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). [DOI] [PMC free article] [PubMed]
- 21. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the systolic blood pressure intervention trial (SPRINT). Clin Trials. 2014;11(5):532‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Systolic Blood Pressure Intervention Trial (SPRINT) . Protocol Version 4.0. http://sprinttrial.org/public/protocol_current.pdf. Accessed January 2, 2021.
- 23. Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure‐lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Db Syst Rev. 2014;5:1‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hulley SB, Furberg CD, Gurland B, et al. Systolic hypertension in the elderly program (Shep) ‐ Antihypertensive efficacy of chlorthalidone. Am J Cardiol. 1985;56(15):913‐920. [DOI] [PubMed] [Google Scholar]


