Abstract
Both morning hypertension (MH) and nocturnal hypertension (NH) are associated with severe target organ damage in patients with chronic kidney disease (CKD). However, the isolated or combined effects of MH and NH on target organ damage are less well‐defined. A cross‐sectional study was conducted among 2386 non‐dialysis CKD patients with ambulatory blood pressure monitoring. The authors categorized patients into four groups based on the presence or absence of MH and NH. Multivariate logistic analyses were used to evaluate the correlation between hypertension subtypes and target organ damage, including left ventricular hypertrophy (LVH), abnormal carotid intima‐media thickness (CIMT), low estimated glomerular filtration rate (eGFR), and albuminuria. The percentages of isolated MH, isolated NH, and combined MH and NH were 2.3%, 24.0%, and 49.3%, respectively. Compared to patients without MH and NH, isolated MH was only related to low eGFR (2.26 [95% confidence interval: 1.00–5.09]) and albuminuria (2.17 [95% CI: 1.03–4.54]). Meanwhile, combined MH and NH group compared to the group without MH and NH had a higher risk of LVH (2.87 [95% CI: 2.01–4.09]), abnormal CIMT (2.01 [95% CI: 1.47–2.75]), low eGFR (3.18 [95% CI: 2.23–4.54]), and albuminuria (1.79 [95% CI: 1.33–2.40]), even in patients without daytime hypertension. The risk of cardiovascular and renal damage was also observed in the isolated NH group. In conclusion, morning hypertension is associated with kidney dysfunction and has combined effects with nocturnal hypertension on cardiovascular damage in chronic kidney disease patients.
Keywords: ambulatory blood pressure monitoring, chronic kidney disease, morning hypertension, nocturnal hypertension, target organ damage
We have highlighted the different risk for target organ damage conferred by isolated and combined morning hypertension and nocturnal hypertension in non‐dialysis CKD patients. Patients with isolated morning hypertension could be related to renal dysfunction. And the combined effect of morning hypertension and nocturnal hypertension on the serious cardiac and carotid damage also apparently deserves attention.

1. INTRODUCTION
Chronic kidney disease (CKD) is a mounting public health problem and substantially increases the risks of death and cardiovascular disease (CVD). 1 Since hypertension is one of the strongest risk factors for CVD, 2 CKD patients with hypertension require more intensive blood pressure (BP) management and comprehensive cardiovascular evaluations. 3 Indeed, the various markers of subclinical target organ damage, including left ventricular hypertrophy (LVH), carotid intima‐media thickness (CIMT), decline of estimated glomerular filtration rate (eGFR), and albuminuria, have been prospectively related to the risk of clinical cardiovascular disease events 2 , 4 , 5 and also are important processes of clinical events and death. Proper management of hypertension has an important role in preventing the decline in renal function and the occurrence of cardiovascular damage. 6 , 7 , 8
To accurately evaluate BP in patients with CKD, ambulatory blood pressure monitoring (ABPM) has to be performed. 9 , 10 Several current guidelines 11 , 12 , 13 recommend routine monitoring of both morning blood pressure and nighttime blood pressure in clinical practice. Large studies have clarified that both morning hypertension and nocturnal hypertension are associated with a higher risk of target organ damage and adverse cardiovascular disease. 14 , 15 , 16 , 17 , 18 However, the results regarding the single or incremental predictive ability of morning hypertension and nocturnal hypertension for subclinical target organ damage were inconsistent in the general population and hypertensive patients. The J‐HOP study, which evaluated outpatients with at least one cardiovascular risk factor, showed that higher morning BP, rather than nocturnal BP, had stand‐alone predictive ability for left ventricular mass index and CIMT, 14 contrary to the study in hypertensive populations. 19 Moreover, the Hisayama Research of the community‐dwelling individuals held another different view that either isolated morning or evening hypertension had a significantly higher mean and maximum CIMT than those with normotension. 20 Probably, the discrepancy in the findings between the studies may indicate that there are differences in the risk of morning BP and nocturnal BP when assessing different populations, races, and evaluation indicators.
As we know, no study had clarified the abovementioned associations in Chinese CKD patients, which is important, as these patients have a high cardiovascular risk. A better estimate of the burden of hypertension subtypes at different time and improved risk stratification from ABPM in CKD patients should be well‐recognized. In this backdrop, we modeled the associations between morning hypertension and nocturnal hypertension, both alone and together, for LVH, abnormal CIMT, and impaired renal damage in Chinese CKD patients. The aim of our study was to assess the differential correlation of hypertension in the morning and in the nighttime with subclinical cardiac, carotid, and renal damage.
2. METHODS
2.1. Participants
The study protocol was approved by the ethics committee of our hospitals and was approved by the Institutional Review Board. All patients signed written informed consent before data collection.
A total of 2551 CKD inpatients aged 18 to 75 years and completed ABPM for this cross‐sectional study. We excluded 165 participants according to the following criteria: dialysis or transplant; changes in eGFR > 30% in the previous 3 months; pregnancy; atrial fibrillation; inadequate ABPM readings; night work or shift‐work employment; and inability to communicate and comply with all of the study requirements. Finally, a total of 2386 CKD patients were included in the current analysis. In terms of causes of renal disease, 1307 patients had chronic glomerulonephritis; 297 patients had diabetic nephropathy; 103 patients had hypertensive nephropathy; and 679 patients had other causes of renal disease.
2.2. Blood pressure measurement
Ambulatory blood pressure monitoring was performed via calibrated devices in our clinic centers and programmed at 15‐min intervals during the daytime and 30‐min intervals at night. An appropriate ABPM cuff was placed on the nondominant arm. Valid measurement was regarded as successful recording of a minimum 20 valid daytime and at least 7 valid nighttime measurements, and at least 70% of the expected 24‐h readings. 11 , 21 Day and night periods were defined according to sleeping and waking times reported by the patient. Morning period was defined as the first 2 h after the wake‐up time. 22
Using ambulatory thresholds, 11 regardless of antihypertensive drugs, morning hypertension (MH) was defined as morning systolic BP (SBP) ≥135 mm Hg or/and diastolic BP (DBP) ≥85 mm Hg 22 , 23 ; daytime hypertension was defined as daytime SBP ≥ 135 mm Hg or/and DBP ≥ 85 mm Hg; nocturnal hypertension (NH) was defined as nighttime SBP ≥ 120 mm Hg or/and DBP ≥ 70 mm Hg. Isolated MH was defined as the presence of MH without NH. Isolated NH was defined as the presence of NH without MH. Combined MH and NH was defined as the presence of both MH and NH.
2.3. Cardiac assessment
Echocardiography was performed in accordance with the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. 24
Linear measurements of end‐diastolic interventricular septal wall thickness, left ventricular end‐diastolic diameter, and end‐diastolic posterior wall thickness were assessed using M‐mode tracings to calculate left ventricular mass (LVM). 25 LVH was defined as >115 g/m2 in men and >95 g/m2 in women, after LVM normalized to body surface area. 26
2.4. Carotid ultrasonography
B‐mode ultrasonography (SonoSite) was used to assess the CIMT. It was achieved by averaging three measurements taken on the points proximal to the bilateral carotid bulb at end diastole and in a plaque‐free segment and measuring the distance between the leading edge of the lumen‐intima interface and the leading edge of the collagenous upper layer of the adventitia. 27 Abnormal CIMT referred to CIMT > 0.9 mm. 28
2.5. Renal assessment
An isotope dilution mass spectrometry‐traceable methodology was utilized to determine serum creatinine, and eGFR was estimated using the CKD‐Epidemiology Collaboration (CKD‐EPI) formula. Patients were divided into five stages according to the level of eGFR. A first morning urine sample was also collected on the day of ambulatory blood pressure measurement to measure the concentration of urinary albumin and creatinine by immunoturbidimetry. Impaired renal function included low eGFR (eGFR < 60 ml/min/1.73 m2) and albuminuria (urinary albumin creatinine ratio [UACR] >300 mg/g). 2
2.6. Other measurements
Patient data including sociodemographic and clinical characteristics, medical history, and current therapy were obtained from interviews and physical examinations at the initial study visit and from clinical records. Body mass index was defined as weight in kilograms divided by the height in meters squared. Diabetes mellitus was defined as self‐reported history of a physician's diagnosis, diabetes medication use, or a fasting blood glucose level of 126 mg/dl or higher or a non‐fasting glucose level of 200 mg/dl or higher. 29 History of CVD including angina pectoris, myocardial infarction, and stroke was ascertained that were previously diagnosed. Additionally, A fasting blood sample was collected to measure hemoglobin, albumin, calcium, phosphorus, intact parathyroid hormone, serum fasting glucose, cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, homocysteine, uric acid, serum creatinine, and blood urea nitrogen in our central laboratory.
2.7. Statistical analysis
Data were tested for normal distribution using the Kolmogorov‐Smirnov test. Continuous variables are presented as the mean ± standard deviation (SD) for normally distributed variables and as the median (interquartile range) for non‐normally distributed variables. Frequency and proportions were used for categorical variables. We used one‐way ANOVA or nonparametric tests to compare continuous variables and the chi‐square test to compare categorical variables. The Bonferroni method was used for post hoc pairwise comparisons. Univariate and multivariate logistic regression analyses were used to explore the risk of target organ damage associated with BP types. In a sensitivity analysis, participants with daytime hypertension were excluded. p values < .05 were considered statistically significant. All statistical analyses were performed using SPSS version 25 (IBM Corp.) and R Version 3.6.0. Graphs were generated with GraphPad Prism 8 (GraphPad Software Inc).
3. RESULTS
3.1. Baseline characteristics
Of the 2386 non‐dialysis CKD participants, the mean age was 46.6 ± 15.1 years, and 55.4% were male. The average number of readings in 24 h, morning, daytime, and nighttime was 64.5 ± 7.6, 4.0 ± 0.8, 52.3 ± 7.3, and 12.2 ± 1.5, respectively. The prevalence of patients without MH and NH, with isolated MH, with isolated NH, and with combined MH and NH among all patients was 24.4%, 2.3%, 24.0%, and 49.3%, respectively. Compared with patients without MH and NH, patients with isolated or combined MH and NH were all significantly older, had a higher prevalence of diabetes mellitus and antihypertensive treatment, and had a higher blood urea nitrogen, serum creatinine, serum fasting glucose, phosphate, intact parathyroid hormone, and UACR, but a lower eGFR and hemoglobin (p < .001) (Table 1).
TABLE 1.
Clinical and ambulatory BP characteristics of patients
| Without NH | With NH | p value | |||
|---|---|---|---|---|---|
| Without MH (N = 581) | With MH (N = 56) | Without MH (N = 572) | With MH (N = 1177) | ||
| Age, y | 39.2 ± 14.6 | 47.6 ± 14.1 | 46.9 ± 14.9 | 50.2 ± 14.2 | <.001 |
| Male, N (%) | 264 (45.4) | 32 (57.1) | 307 (53.7) | 719 (61.1) | <.001 |
| BMI, kg/m2 | 22.6 ± 4.0 | 22.8 ± 2.9 | 23.8 ± 4.2 | 24.1 ± 3.9 | <.001 |
| Current smoker, N (%) | 95 (16.4) | 14 (25.0) | 126 (22.0) | 300 (25.5) | <.001 |
| Alcohol intake, N (%) | 61 (10.5) | 7 (12.5) | 83 (14.5) | 188 (16.0) | .021 |
| Diabetes mellitus, N (%) | 44 (7.6) | 10 (17.9) | 122 (21.3) | 302 (25.7) | <.001 |
| CVD history, N (%) | 26 (4.5) | 5 (8.9) | 58 (10.1) | 189 (16.1) | <.001 |
| Hypertension, N (%) | 97 (16.7) | 22 (39.3) | 260 (45.5) | 897 (76.2) | <.001 |
| Antihypertension drugs, N (%) | 311 (53.5) | 40 (71.4) | 369 (64.5) | 1007 (85.6) | <.001 |
| ACEI, N (%) | 36 (6.2) | 3 (5.4) | 37 (6.5) | 67 (5.7) | .91 |
| ARB, N (%) | 225 (38.7) | 26 (46.4) | 194 (33.9) | 467 (39.7) | .06 |
| β‐blockers, N (%) | 35 (6.0) | 7 (12.5) | 94 (16.4) | 374 (31.8) | <.01 |
| Calcium channel blockers, N (%) | 67 (11.5) | 16 (28.6) | 197 (34.4) | 754 (64.1) | <.01 |
| α‐blocker, N (%) | 9 (1.5) | 2 (3.6) | 21 (3.7) | 192 (16.3) | <.01 |
| Statins, N (%) | 87 (15.0) | 16 (28.6) | 109 (19.1) | 269 (22.9) | <.001 |
| FBG, mmol/L | 4.7 (4.3–5.2) | 4.9 (4.4–5.8) | 4.9 (4.4–5.6) | 5.0 (4.5–5.8) | <.001 |
| Hemoglobin, g/L | 128.6 ± 23.4 | 126.4 ± 22.6 | 124.6 ± 25.7 | 114.6 ± 29.1 | <.001 |
| Serum albumin, g/L | 37.3 ± 8.1 | 35.5 ± 8.6 | 37.4 ± 7.3 | 35.9 ± 7.2 | <.001 |
| Homocysteine, µmol/L | 11.7 (8.7–16.0) | 13.8 (9.5–19.1) | 14.2 (10.5–18.4) | 16.6 (12.6–21.3) | <.001 |
| Uric acid, mmol/L | 390.4 ± 120.8 | 429.1 ± 137.5 | 434.8 ± 134.9 | 475.9 ± 137.3 | <.001 |
| Cholesterol, mmol/L | 5.3 ± 2.3 | 5.1 ± 2.5 | 5.3 ± 2.1 | 5.3 ± 2.1 | .945 |
| HDL‐C, mmol/L | 1.2 ± 0.4 | 1.3 ± 0.6 | 1.1 ± 0.3 | 1.1 ± 0.4 | <.001 |
| LDL‐C, mmol/L | 2.8 (2.2–3.6) | 3.0 (2.4–4.6) | 2.8 (2.2–3.5) | 2.9 (2.2–3.5) | .289 |
| Serum calcium, mmol/L | 2.1 (2.0–2.2) | 2.2 (2.0–2.3) | 2.1 (2.0–2.2) | 2.1 (2.0–2.2) | .005 |
| Serum phosphate, mmol/L | 1.1 (0.9–1.2) | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | <.001 |
| iPTH, pmol/L | 5.3 (3.6–9.5) | 6.0 (3.2–20.2) | 6.0 (3.8–9.8) | 8.9 (4.9–20.2) | <.001 |
| Blood urea nitrogen, mmol/L | 4.9 (3.9–6.5) | 6.3 (4.0–12.2) | 6.3 (4.6–10.4) | 9.6 (6.0–18.4) | <.001 |
| Serum creatinine, µmol/L | 76.0 (59.0–104.0) | 99.0 (62.5–162.2) | 97.5 (69.0–179.7) | 170.0 (95.0–471.0) | <.001 |
| eGFR, ml/min/1.73 m2 | 98.0 (71.0–118.0) | 63.0 (37.9–102.5) | 69.0 (33.0–103.0) | 36.2 (10.9–73.1) | <.001 |
| UACR, mg/g | 269.9 (42.0–987.6) | 705.5 (112.5–1392.2) | 438.4 (83.0–1260.0) | 987.6 (252.3–2065.8) | <.001 |
| Clinic‐SBP, mm Hg | 121.2 ± 17.8 | 131.7 ± 17.1 | 132.3 ± 20.5 | 149.8 ± 23.2 | <.001 |
| Clinic‐DBP, mm Hg | 76.9 ± 10.8 | 80.6 ± 10.3 | 83.7 ± 12.1 | 91.1 ± 14.6 | <.001 |
| 24 h‐SBP, mm Hg | 110.4 ± 8.5 | 120.7 ± 9.8 | 121.9 ± 9.5 | 141.1 ± 14.9 | <.001 |
| 24 h‐DBP, mm Hg | 68.5 ± 5.5 | 74.1 ± 5.6 | 78.8 ± 5.9 | 88.6 ± 9.5 | <.001 |
| Daytime‐SBP, mm Hg | 112.4 ± 9.0 | 124.0 ± 10.4 | 122.9 ± 9.8 | 141.9 ± 15.0 | <.001 |
| Daytime‐DBP, mm Hg | 70.3 ± 6.2 | 76.5 ± 6.1 | 79.6 ± 6.5 | 89.4 ± 9.8 | <.001 |
| Nighttime‐SBP, mm Hg | 102.3 ± 7.7 | 105.9 ± 8.5 | 118.7 ± 10.6 | 137.6 ± 17.0 | <.001 |
| Nighttime‐DBP, mm Hg | 62.0 ± 5.2 | 63.3 ± 4.7 | 75.8 ± 5.8 | 85.5 ± 10.7 | <.001 |
| Morning‐SBP, mm Hg | 109.8 ± 10.1 | 138.4 ± 12.2 | 118.4 ± 9.0 | 144.9 ± 16.3 | <.001 |
| Morning‐DBP, mm Hg | 68.2 ± 7.6 | 87.4 ± 9.6 | 76.0 ± 6.1 | 92.0 ± 10.2 | <.001 |
Data are presented as numbers and percentages, means and standard deviations, or median and quartile ranges.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, serum fasting glucose; HDL‐C, high‐density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL‐C, low‐density lipoprotein cholesterol; MH, morning hypertension; NH, nocturnal hypertension; SBP, systolic blood pressure; UACR, urinary albumin‐to‐creatinine ratio.
3.2. Prevalence of target organ damage
The prevalence of LVH, abnormal CIMT, low eGFR, and albuminuria in patients without MH and NH was 10.3%, 14.3%, 19.1%, and 47.5%, respectively. Comparing to the patients without MH and NH, patients with isolated MH had similar prevalence of LVH (17.9%), abnormal CIMT (21.4%), and albuminuria (69.6%), but a higher prevalence of low eGFR (44.6%). Meanwhile, the prevalence of LVH (20.3%), abnormal CIMT (30.8%), low eGFR (43.0%), and albuminuria (58.4%) in patients with isolated NH was higher than that in patients without MH and NH. There was significant difference in the proportion of target organ damage between the isolated NH group and the combined MH and NH group except for albuminuria. The difference in the proportion of target organ damage between the four BP groups showed a linear trend (p < .001) (Figure 1).
FIGURE 1.
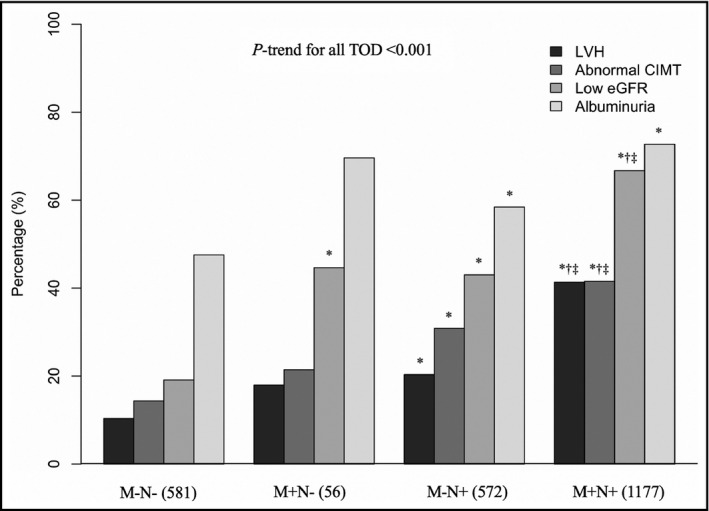
Comparison of target organ damages in four blood pressure groups. LVH, left ventricular hypertrophy; CIMT, carotid intima‐media thickness; eGFR, estimated glomerular filtration rate; TOD, target organ damage; M+, with morning hypertension; M−, without morning hypertension; N+, with nocturnal hypertension; N−, without nocturnal hypertension
3.3. BP types in different CKD stages
The prevalence of patients without MH and NH decreased with the advancement of CKD (44.4% and 7.6% in stage 1 and stage 5, respectively). And similar trend was found in the prevalence of patients with isolated NH (28.5% and 15.4% in stage 1 and stage 5, respectively). In contrast, there was a stepwise increase in the prevalence of patients with combined MH and NH by CKD stage (24.6% and 75.2% in stage 1 and stage 5, respectively). Instead, the prevalence of isolated MH increased from 2.5% to 3.1% from stages 1 to 3, respectively, and then decreased to 0.9% and 1.8% in stages 4 and 5, respectively. We found a linear trend in the proportion of BP types across the CKD stages (p < .001) except for patients with isolated MH (p = .299) (Table 2).
TABLE 2.
Blood pressure types in different CKD stages
| Without NH | With NH | |||
|---|---|---|---|---|
| Without MH (N = 581) | With MH (N = 56) | Without MH (N = 572) | With MH (N = 1177) | |
| CKD 1 | 343 (44.4) | 19 (2.5) | 220 (28.5) | 190 (24.6) |
| CKD 2 | 127 (28.4) | 12 (2.7) | 106 (23.7) | 202 (45.2) |
| CKD 3 | 56 (12.5) | 14 (3.1) | 115 (25.7) | 262 (58.6) |
| CKD 4 | 18 (7.7) | 2 (0.9) | 56 (24.0) | 157 (67.4) |
| CKD 5 | 37 (7.6) | 9 (1.8) | 75 (15.4) | 366 (75.2) |
| p‐trend value | <.001 | .299 | <.001 | <.001 |
Abbreviations: CKD, chronic kidney disease; MH, morning hypertension; NH, nocturnal hypertension.
3.4. Association between BP types and target organ damage
In the multivariate logistic regression analyses, after adjusting for other risk factors, both morning BP and nocturnal BP, as continuous variables, were risk factors for subclinical target organ damage (Supplemental Table S1). Compared to the patients without MH and NH, isolated MH was associated only with higher risk of low eGFR (2.26 [95% CI: 1.00–5.09]) and albuminuria (2.17 [95% CI: 1.03–4.54]). There were no relationships between isolated MH with LVH (1.36 [95% CI: 0.60–3.09]) and abnormal CIMT (0.89 [95% CI: 0.42–1.85]). Patients with isolated NH, and combined MH and NH had clearly higher odds of target organ damage including LVH, abnormal CIMT, low eGFR, and albuminuria compared to those without MH and NH. The OR of the isolated NH was 1.63 (95% CI: 1.10–2.41) for LVH, 1.60 (95% CI: 1.15–2.22) for abnormal CIMT, 2.12 (95% CI: 1.45–3.11) for low eGFR, and 1.52 (95% CI: 1.12–2.05) for albuminuria. The OR of the combined MH and NH was 2.87 (95% CI: 2.01–4.09) for LVH, 2.01 (95% CI: 1.47–2.75) for abnormal CIMT, 3.18 (95% CI: 2.23–4.54) for low eGFR, and 1.79 (95% CI: 1.33–2.40) for albuminuria (Figure 2).
FIGURE 2.
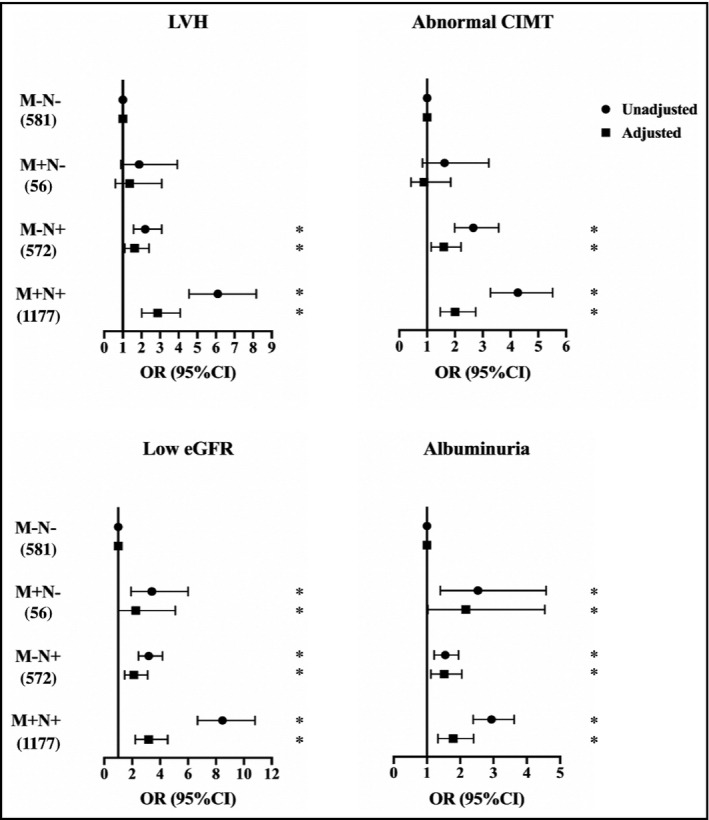
Multivariable logistic regression analysis for blood pressure types and target organ damage. Unadjusted model only contains blood pressure types. Additional adjustment variables for LVH include age, gender, alcohol intake, diabetes mellitus, cardiovascular disease history, antihypertensive drugs, hemoglobin, serum albumin, uric acid, homocysteine, serum fasting glucose, cholesterol, HDL‐C, LDL‐C, serum phosphate, iPTH, and eGFR; additional adjustment variables for abnormal CIMT include age, gender, BMI, current smoking, diabetes mellitus, cardiovascular disease history, antihypertensive drugs, statin, hemoglobin, serum albumin, uric acid, homocysteine, serum fasting glucose, and HDL‐C, iPTH, eGFR; additional adjustment variables for low eGFR include age, gender, current smoking, diabetes mellitus, cardiovascular disease history, antihypertensive drugs, hemoglobin, uric acid, homocysteine, cholesterol, HDL‐C, LDL‐C, serum phosphate, and iPTH; and additional adjustment variables for albuminuria include age, gender, alcohol intake, diabetes mellitus, cardiovascular disease history, antihypertensive drugs, statin, hemoglobin, serum albumin, homocysteine, uric acid, cholesterol, HDL‐C, LDL‐C, serum phosphate, iPTH, and eGFR. BMI, body mass index; CIMT, carotid intima‐media thickness; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; M−, without morning hypertension; M+, with morning hypertension; N−, without nocturnal hypertension; N+, with nocturnal hypertension
3.5. Sensitivity analysis
When we excluded 1145 participants with daytime hypertension, the association of hypertension subtypes with target organ damage remained similar to that observed in the total population, except that isolated MH was not associated with albuminuria in multivariate analysis (Supplemental Figure S1).
4. DISCUSSION
In this cross‐sectional study, we first reported the different relationships between isolated and combined morning hypertension and nocturnal hypertension with subclinical target organ damage in Chinese non‐dialysis CKD patients. We demonstrated that morning hypertension, without nocturnal hypertension, was only associated with renal injury. The risks of LVH, abnormal CIMT, and impaired renal damage conferred by isolated nocturnal hypertension and combined morning and nocturnal hypertension were relatively high. These findings were largely consistent in the sensitivity analysis of patients with normal daytime BP. Therefore, morning hypertension might be an important risk factor for renal dysfunction and have combined effects with nocturnal hypertension on the subclinical cardiovascular and renal injury in patients with CKD.
Since morning hypertension and nocturnal hypertension are robust predictors of incident stroke events, incident CVD events, and sudden death, 30 , 31 , 32 numerous guidelines, including the Asian consensus on hypertension, have pointed out that the assessment of ambulatory BP to evaluate morning hypertension and nocturnal hypertension should be monitored and managed regularly in patients at high risk of CVD. In our study, the prevalence of morning hypertension and nocturnal hypertension was high, 51.7% and 73.3%, respectively. But the prevalence of isolated MH and isolated NH was relatively low, corresponding to relatively high proportion of combined morning and nocturnal hypertension. This phenomenon may due to the strong correlation between morning SBP/DBP and nighttime SBP/DBP (Pearson's r = 0.82/0.77), and the high prevalence of non‐dipping and reverse dipping pattern in CKD patients. 33 , 34 Additionally, the proportion of combined morning and nocturnal hypertension increased with the decline of eGFR, which may be associated with the gradually increased cardiovascular risk with the advancement of CKD stages. 35 As such, the different prevalence of hypertension subtypes in patients with CKD reinforces the need to routine measure morning and nighttime BP for a full characterization of the burden of hypertension.
Earlier studies have shown an increased risk of morning hypertension and nocturnal hypertension per se with both target organ damage and adverse cardiovascular outcomes. 36 , 37 In the present analysis, we extended these findings by demonstrating the discrepancy effects of single and coexisting morning hypertension and nocturnal hypertension on subclinical target organ damage in patients with CKD. From the perspective of renal damage, we noticed that CKD patients with isolated morning hypertension had similar ability to discriminate renal damage with isolated nocturnal hypertension. As this was a cross‐sectional clinical analysis, we cannot rule out the mechanisms by why isolated morning hypertension has a strong association with the decline in renal function. In fact, according to a prospective cohort study of Japanese CKD population and the J‐HOME‐Morning Study, elevated morning BP during the follow‐up period is the best predictor of decline in renal function characterized by the change in eGFR among SBP measured at different times in CKD patients, 38 , 39 which agrees with our results. In addition, the Yokohama add‐on inhibitory efficacy of dapagliflozin on albuminuria in Japanese patients with type 2 diabetes study (Y‐AIDA study), a prospective, multicenter, single‐arm study, suggested that improved morning home systolic BP with dapagliflozin was associated with albuminuria reduction. 40 Then, on our point of view, the single elevated morning BP confers serious risk of kidney injury and apparently requires timely identified and proper management.
Instead, we found that CKD patients with single elevated morning BP were less sensitive to discriminate LVH and abnormal CIMT than that of isolated nocturnal hypertension. The reasons for this phenomenon may be multifaceted. First of all, it is well recognized that morning BP increase is a physiological phenomenon resulting from a high nocturnal BP or an exaggerated morning BP surge. 41 , 42 A Chinese study 43 including 287 individuals with both office and ABPM‐based BP values within the normal ranges demonstrated that elevated morning surge is the main cause of morning hypertension in patients without sustained hypertension during sleep, and morning hypertension could not affect LVMI independently of morning surge. However, the relatively low morning BP surge in CKD patients due to the increased proportion of non‐dipping in CKD 33 may not establish a link between isolated morning hypertension and cardiovascular injury, just as an authoritative evidence suggested, only patients with an extreme condition of morning BP surge rates >95th percentile (43.7 mm Hg) can be associated with a harmful effect. 23 Additionally, the fact that patients with isolated morning hypertension had similar prevalence of LVH and abnormal CIMT as compared to patients without morning and nocturnal hypertension may also reflect the poor correlation between isolated morning hypertension and cardiovascular injury. Nevertheless, morning hypertension still cannot be ignored due to its joint effect with nocturnal hypertension on the risk of cardiac and carotid damage. In our study, most of the patients with high nocturnal BP also have high morning BP, resulting in the combined morning and nocturnal hypertension became the dominant type of hypertension. Sustained hypertension represents long‐standing hypertension, an advanced stage of organ damage, or multiple comorbidities. 44 An increasing evidences, 36 , 37 , 45 such as the Jackson Heart Study, 46 the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study, 47 and our previous study in Chinese non‐dialysis CKD patients, 17 all support the importance of sustained hypertension, for the high risk of increased LVM, LVH, and abnormal CIMT. And the same conclusion about impaired renal function have also been confirmed in our previous studies. 17 , 18 Therefore, identifying morning hypertension, which is associated with nocturnal hypertension, might also be clinically relevant in terms of the risk of severe target organ damage.
4.1. Study limitations
The strengths of this study include its first comprehensive assessment of clinical value of the isolated and combined morning and nocturnal hypertension in Chinese CKD patients, and a relatively large sample size. However, a number of limitations to the current study need to be considered. First, this study is cross‐sectional and observational in nature, which limits the assessment of causality. Second, our findings may not be generalized to other racial/ethnic groups due to the fact that all of the participants were Chinese. Third, some of our recruited participants were on antihypertensive medication, which could potentially impact our observations. But we were unable to assess the effect of changes in BP phenotypes for a small number of patients without antihypertensive drugs. Fourth, it has been shown that taking drugs before sleeping has a certain effect on the control of nocturnal hypertension. 48 However, the lack of data on nocturnal medication in our study made it impossible to determine whether nocturnal medication played any role in the observed association. Fifth, studies in Western countries showed that the reproducibility of ABPM is not perfect, especially when examining nighttime BP status. 49 Due to the changes in season, temperature, emotional state, antihypertensive drugs, and sleep quality, the fluctuation of blood pressure at night may be relatively large. However, the extent that these results could be applied to other populations including Chinese is not known and more research in various ethnicities is needed. Finally, the outcomes assessed in this paper are just surrogates for clinical cardiovascular disease, and longitudinal follow‐up study was needed to determine whether the distinct hypertension subtypes are associated with increased risk of cardiovascular events and kidney disease progression.
5. CONCLUSIONS
We have highlighted the different risk of target organ damage conferred by isolated and combined morning hypertension and nocturnal hypertension in non‐dialysis CKD patients. Patients with isolated morning hypertension could be related to renal dysfunction. And the combined effect of morning hypertension and nocturnal hypertension on the serious cardiac and carotid damage also apparently deserves attention. Further studies are warranted in the clinical setting to investigate whether treating the single or combined hypertension status would reduce the cardiovascular risk and which therapeutic strategy would be effective in treating those forms of high blood pressure.
DISCLOSURE
None.
AUTHORS CONTRIBUTIONS
XL and JTK searched the literature, analyzed the data, interpreted the results, and drafted and revised the manuscript. CW conceived and design the study, organized and supervised the study, interpreted the results, and revised the manuscript. JZ organized and supervised the study, interpreted the results, and revised the manuscript. Other members collected and analyzed the data, and revised the manuscript. All authors read and approved the final manuscript, and agree to be accountable for all aspects of the work.
Supporting information
Fig S1
Table S1
ACKNOWLEDGEMENTS
We would like to thank all patients and their families for participating in this study.
Li X, Ke J, Chen X, et al. Different effects of morning and nocturnal hypertension on target organ damage in chronic kidney disease. J Clin Hypertens. 2021;23:1051–1059. 10.1111/jch.14234
Xue Li and Jianting Ke contributed equally to this work.
Funding information
This work was supported by the Five‐Five Project of the Fifth Affiliated Hospital of Sun Yat‐sen University.
Contributor Information
Jun Zhang, Email: zj_ncjx@163.com.
Cheng Wang, Email: wangch2@mail.sysu.edu.cn.
REFERENCES
- 1. Gansevoort RT, Correa‐Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339‐352. [DOI] [PubMed] [Google Scholar]
- 2. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713‐735. [DOI] [PubMed] [Google Scholar]
- 3. Huang Q‐F, Hoshide S, Cheng H‐M, et al. Management of hypertension in patients with chronic kidney disease in Asia. Curr Hypertens Rev. 2016;12(3):181‐185. [DOI] [PubMed] [Google Scholar]
- 4. Eckardt KU, Scherhag A, Macdougall IC, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol. 2009;20(12):2651‐2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon A, Megnien JL, Chironi G. The value of carotid intima‐media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010;30(2):182‐185. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki H, Moriwaki K, Nakamoto H, Sugahara S, Kanno Y, Okada H. Blood pressure reduction in the morning yields beneficial effects on progression of chronic renal insufficiency with regression of left ventricular hypertrophy. Clin Exp Hypertens. 2002;24(1&2):51‐63. [DOI] [PubMed] [Google Scholar]
- 7. Marfella R, Siniscalchi M, Nappo F, et al. Regression of carotid atherosclerosis by control of morning blood pressure peak in newly diagnosed hypertensive patients. Am J Hypertens. 2005;18(3):308‐318. [DOI] [PubMed] [Google Scholar]
- 8. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease. Can Med Assoc J. 2013;185(11):949‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parati G, Ochoa JE, Bilo G, et al. Hypertension in chronic kidney disease part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension. 2016;67(6):1102‐1110. [DOI] [PubMed] [Google Scholar]
- 10. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163(9):691‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 12. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 13. Wang JG, Kario K, Chen CH, et al. Management of morning hypertension: a consensus statement of an Asian expert panel. J Clin Hypertens (Greenwich, Conn). 2018;20(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens. 2014;27(7):939‐947. [DOI] [PubMed] [Google Scholar]
- 15. Kario K, Saito I, Kushiro T, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large‐scale prospective, real‐world observational study. Hypertension. 2014;64(5):989‐996. [DOI] [PubMed] [Google Scholar]
- 16. Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease: the HONEST study. J Am Coll Cardiol. 2016;67(13):1519‐1527. [DOI] [PubMed] [Google Scholar]
- 17. Wang C, Deng WJ, Gong WY, et al. High prevalence of isolated nocturnal hypertension in Chinese patients with chronic kidney disease. J Am Heart Assoc. 2015;4(6):e002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Li Y, Zhang J, et al. Prognostic effect of isolated nocturnal hypertension in Chinese patients with nondialysis chronic kidney disease. J Am Heart Assoc. 2016;5(10):e004198. 10.1161/JAHA.116.004198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu K, Xu Y, Gong S, et al. The disadvantage of morning blood pressure management in hypertensive patients. Medicine (Baltimore). 2020;99(8):e19278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakata S, Hata J, Fukuhara M, et al. Morning and evening blood pressures are associated with intima‐media thickness in a general population‐ the Hisayama study. Circ J. 2017;81(11):1647‐1653. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Shin J, Chen CH, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens (Greenwich, Conn). 2019;21(9):1250‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71(3):375‐382. [DOI] [PubMed] [Google Scholar]
- 23. Cheng H‐M, Chung‐Li WU, Sung S‐H, et al. Prognostic utility of morning blood pressure surge for 20‐year all‐cause and cardiovascular mortalities: results of a community‐based study. J Am Heart Assoc. 2017;6:e007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1‐39 e14. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79‐108. [DOI] [PubMed] [Google Scholar]
- 26. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159‐2219. [DOI] [PubMed] [Google Scholar]
- 27. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness and plaque consensus (2004‐2006‐2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naqvi TZ, Lee MS. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025‐1038. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes A . (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8‐S16. [DOI] [PubMed] [Google Scholar]
- 30. Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733‐743. [DOI] [PubMed] [Google Scholar]
- 31. Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12(2):35S‐42S. [DOI] [PubMed] [Google Scholar]
- 32. Elliott WJ. Circadian variation in the timing of stroke onset. Stroke. 1998;29:992‐996. [DOI] [PubMed] [Google Scholar]
- 33. Wang C, Zhang J, Liu X, et al. Reversed dipper blood‐pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013;8(2):e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 35. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375(9731):2073‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuspidi C, Facchetti R, Bombelli M, et al. Nighttime blood pressure and new‐onset left ventricular hypertrophy: findings from the Pamela population. Hypertension. 2013;62(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 37. Kario K, Tomitani N, Matsumoto Y, et al. Research and development of information and communication technology‐based home blood pressure monitoring from morning to nocturnal hypertension. Ann Glob Health. 2016;82(2):254‐273. [DOI] [PubMed] [Google Scholar]
- 38. Ishikura K, Obara T, Kikuya M, et al. Home blood pressure level and decline in renal function among treated hypertensive patients: the J‐HOME‐Morning Study. Hypertens Res. 2016;39(2):107‐112. [DOI] [PubMed] [Google Scholar]
- 39. Okada T, Nakao T, Matsumoto H, Nagaoka Y. Value of morning home blood pressure as a predictor of decline in renal function in patients with chronic kidney disease. Am J Nephrol. 2008;28(6):982‐989. [DOI] [PubMed] [Google Scholar]
- 40. Kinguchi S, Wakui H, Ito Y, et al. Improved home BP profile with dapagliflozin is associated with amelioration of albuminuria in Japanese patients with diabetic nephropathy: the Yokohama add‐on inhibitory efficacy of dapagliflozin on albuminuria in Japanese patients with type 2 diabetes study (Y‐AIDA study). Cardiovasc Diabetol. 2019;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sogunuru GP, Kario K, Shin J, et al. Morning surge in blood pressure and blood pressure variability in Asia: Evidence and statement from the HOPE Asia Network. J Clin Hypertens (Greenwich, Conn). 2019;21(2):324‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731‐1768. [DOI] [PubMed] [Google Scholar]
- 43. Ye R, Liu K, Gong S, Li J, Xu Y, Chen X. The association between morning blood pressure and subclinical target organ damage in the normotensive population. J Hypertens. 2019;37(7):1427‐1436. [DOI] [PubMed] [Google Scholar]
- 44. Redon J, Lurbe E. Nocturnal blood pressure versus nondipping pattern: what do they mean? Hypertension. 2008;51(1):41‐42. [DOI] [PubMed] [Google Scholar]
- 45. Hoshide S, Ishikawa J, Eguchi K, et al. Masked nocturnal hypertension and target organ damage in hypertensives with well‐ controlled self‐measured home blood pressure. Hypertens Res. 2007;30(2):143‐149. [DOI] [PubMed] [Google Scholar]
- 46. Ogedegbe G, Spruill TM, Sarpong DF, et al. Correlates of isolated nocturnal hypertension and target organ damage in a population‐based cohort of African Americans: the Jackson Heart Study. Am J Hypertens. 2013;26(8):1011‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuspidi C, Facchetti R, Bombelli M, et al. Is night‐time hypertension worse than daytime hypertension? A study on cardiac damage in a general population: the PAMELA study. J Hypertens. 2017;35(3):506‐512. [DOI] [PubMed] [Google Scholar]
- 48. Wang C, Ye Y, Liu C, et al. Evening versus morning dosing regimen drug therapy for chronic kidney disease patients with hypertension in blood pressure patterns: a systematic review and meta‐analysis. Intern Med J. 2017;47(8):900‐906. [DOI] [PubMed] [Google Scholar]
- 49. Bo Y, Kwok KO, Chung VC, et al. Short‐term reproducibility of ambulatory blood pressure measurements: a systematic review and meta‐analysis of 35 observational studies. J Hypertens. 2020;38(11):2095‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


